Combined Effect of Propyl Gallate and Tert-Butyl Hydroquinone on Biodiesel and Biolubricant Based on Waste Cooking Oil
Abstract
1. Introduction
1.1. Current Environmental Issues
1.2. Waste Cooking Oil
1.3. Biodiesel and Biolubricant Production: Advantages and Challenges
1.4. Antioxidants
1.5. Voltammetry
1.6. Scientific Interest
1.7. Aim and Innovation of This Work
2. Materials and Methods
2.1. Raw Material
2.2. Biodiesel and Biolubricant Production
2.3. Characterization
2.4. Antioxidant Addition
2.5. Voltammetric Analysis
2.6. Extreme Oxidation Conditions
3. Results and Discussion
3.1. Biodiesel and Biolubricant Characterization
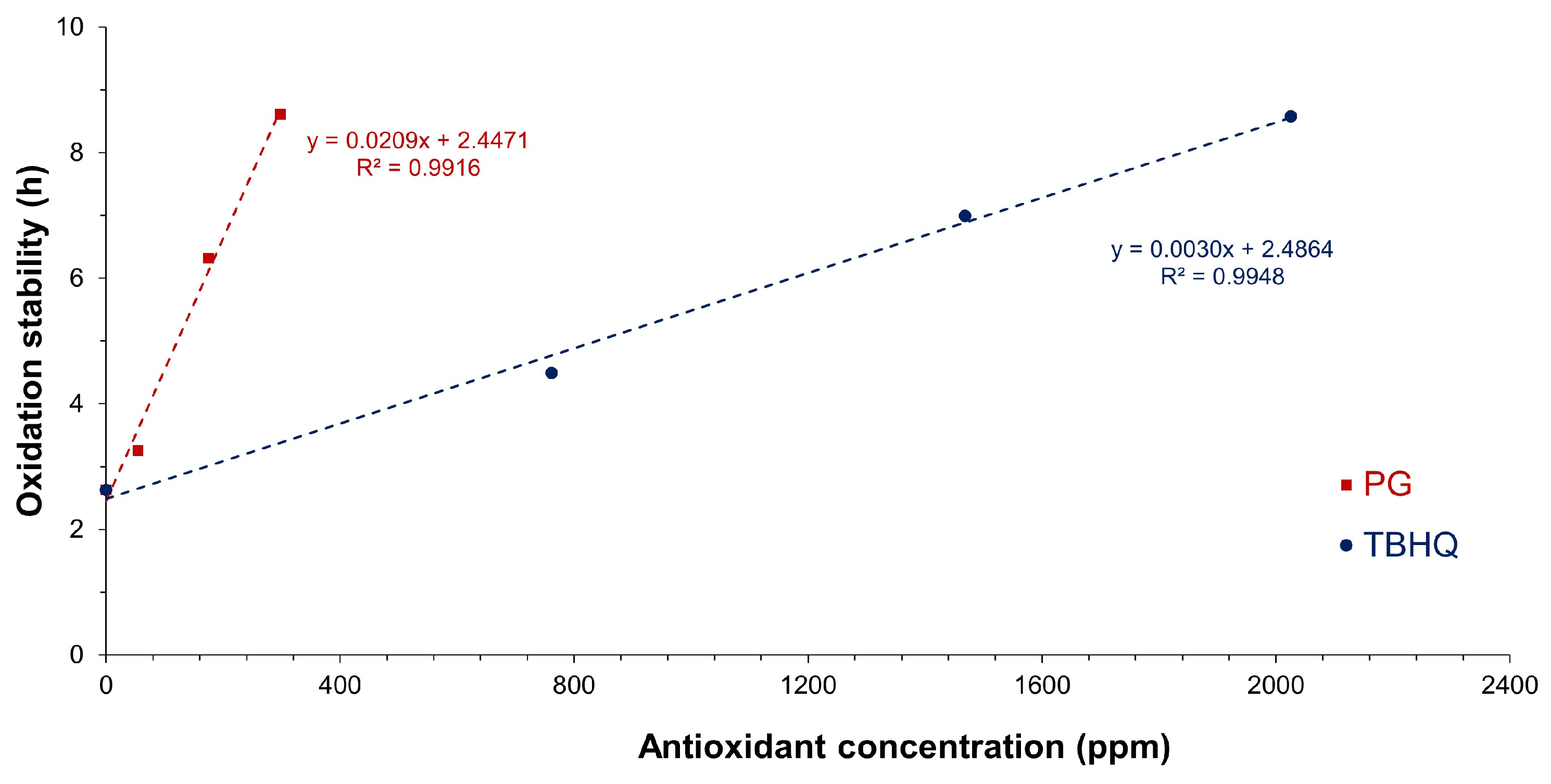
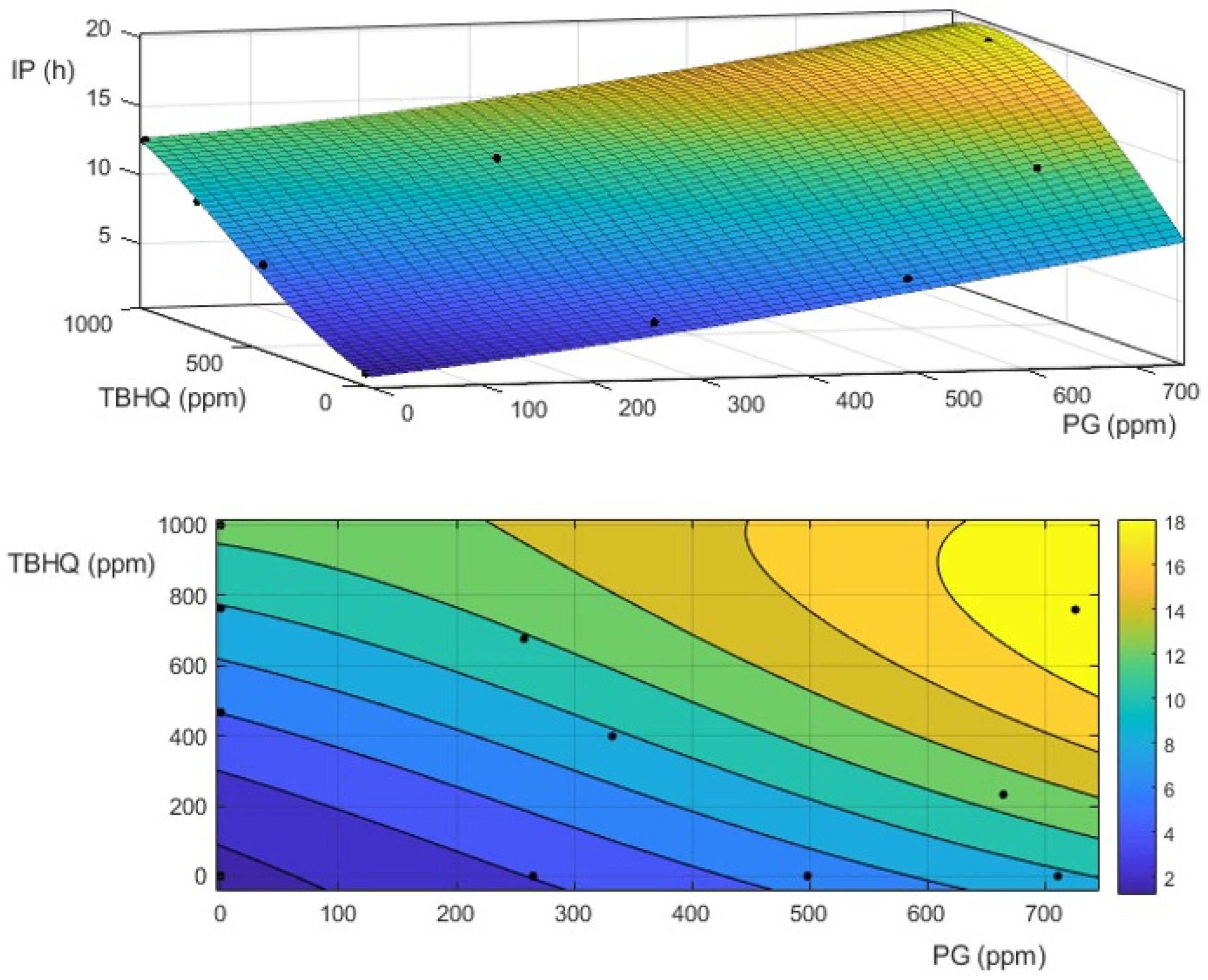
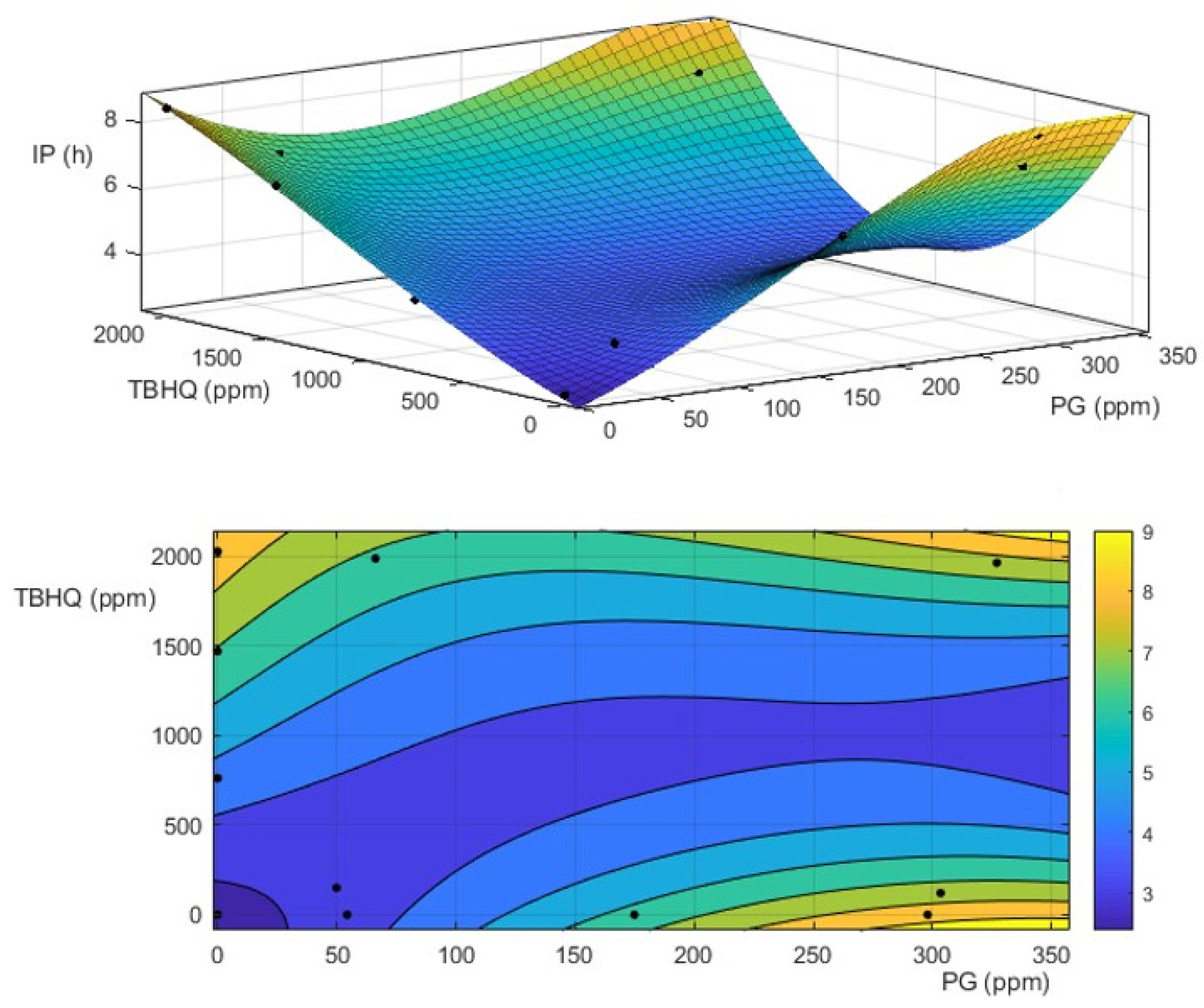
3.2. Effect of Antioxidants on Oxidative Stability of Biodiesel and Biolubricant
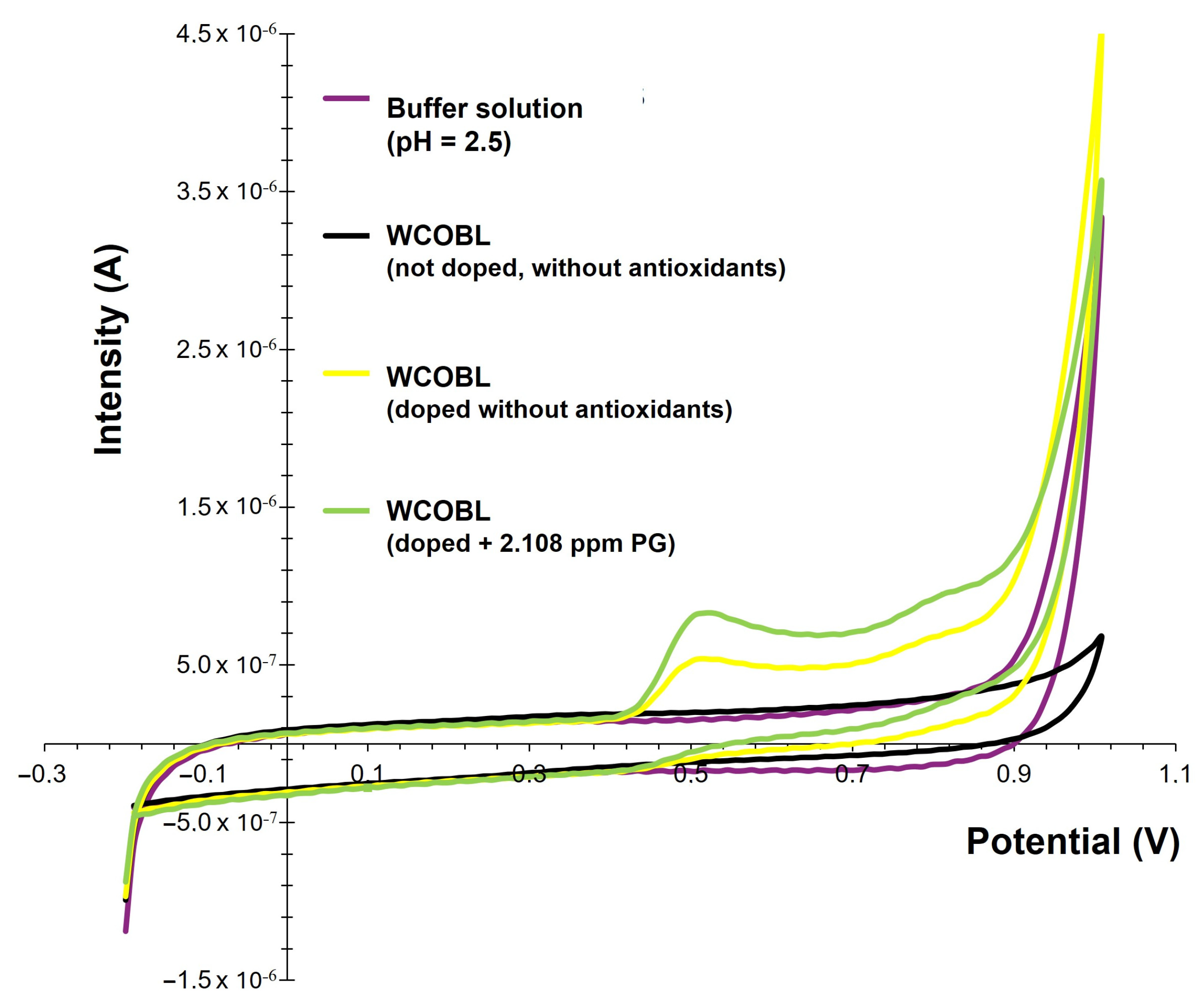
3.3. Voltammetric Quantification of PG and TBHQ
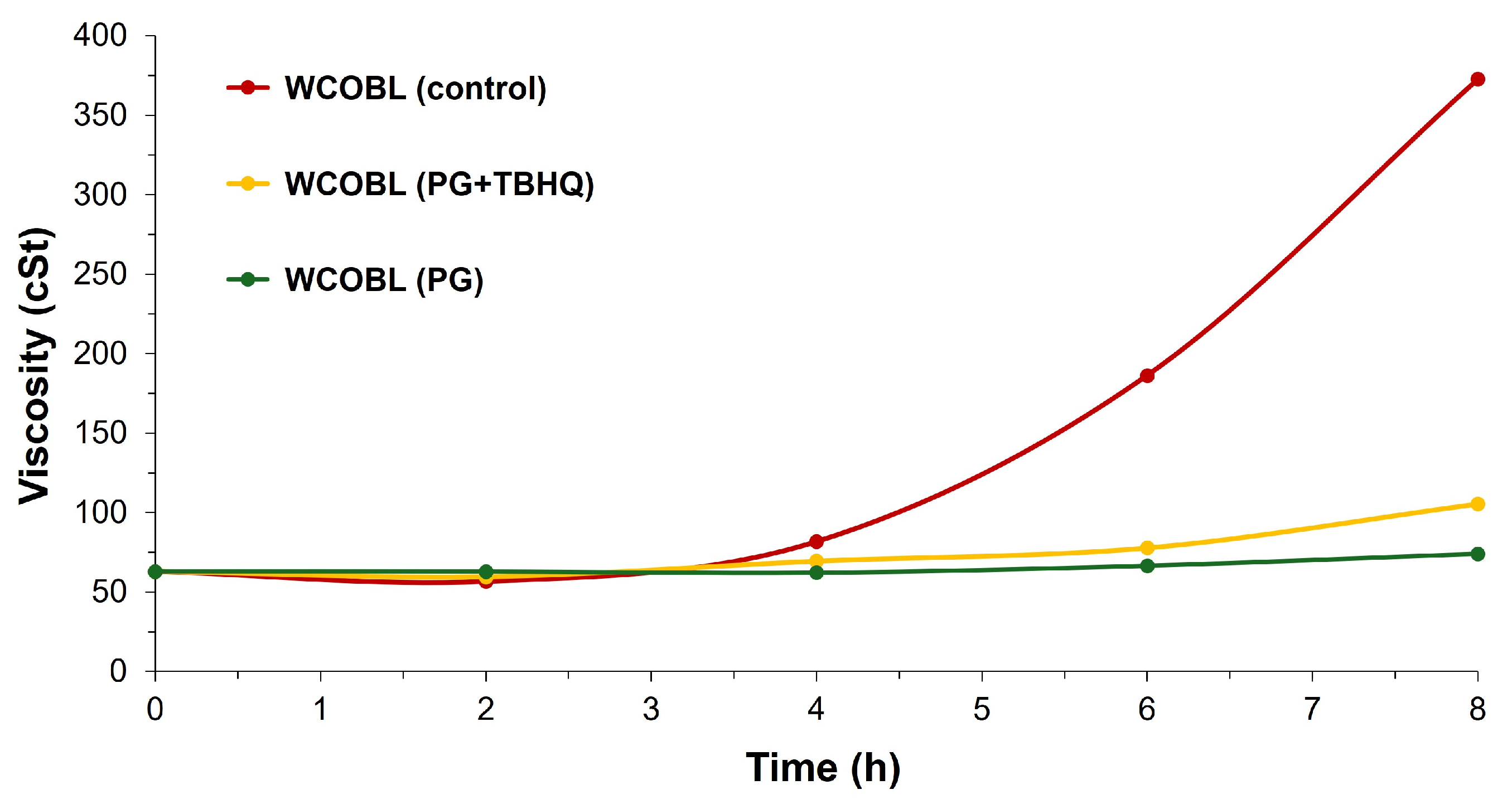
3.4. Effect of Extreme Oxidation Conditions on Biodiesel and Biolubricant Properties
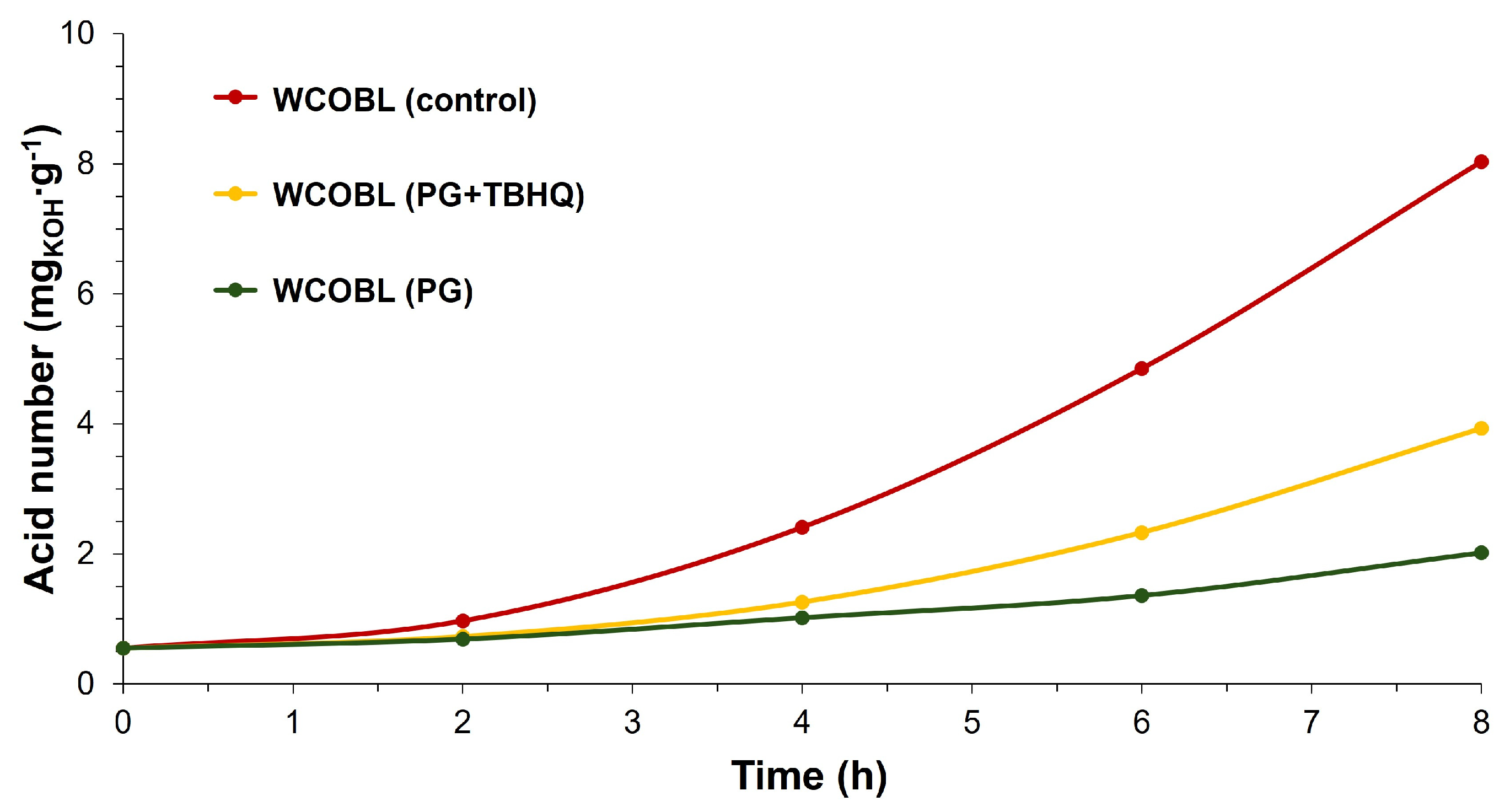
- WCOBL (control). This was WCOBL without antioxidant addition.
- WCOBL (PG + TBHQ). In this case, 300 ppm PG + 1000 ppm TBHQ were added to the sample. This was one of the points where the antagonistic effect (as previously mentioned) was evident, with lower oxidation stability than expected shown according to the additive effect of both antioxidants.
- WCOBL (PG). An addition of 300 ppm PG was carried out.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BHA | Butylated hydroxyanisole |
| BHT | Butylated hydroxytoluene |
| CV | Cyclic voltammetry |
| DPV | Differential pulse voltammetry |
| OG | Octyl gallate |
| PG | Propyl gallate |
| PY | Pyrogallol |
| TBHQ | Tert-butyl hydroquinone |
| WCO | Waste cooking oil |
| WCOBD | Waste cooking oil biodiesel |
| WCOBL | Waste cooking oil biolubricant |
References
- Vakulchuk, R.; Overland, I.; Scholten, D. Renewable Energy and Geopolitics: A Review. Renew. Sustain. Energy Rev. 2020, 122, 109547. [Google Scholar] [CrossRef]
- Palmeros Parada, M.; Osseweijer, P.; Posada Duque, J.A. Sustainable Biorefineries, an Analysis of Practices for Incorporating Sustainability in Biorefinery Design. Ind. Crops Prod. 2017, 106, 105–123. [Google Scholar] [CrossRef]
- Moncada, B.J.; Aristizábal, M.V.; Cardona, A.C.A. Design Strategies for Sustainable Biorefineries. Biochem. Eng. J. 2016, 116, 122–134. [Google Scholar] [CrossRef]
- Kokkinos, N.C.; Emmanouilidou, E.; Sharma, S.K. Waste-To-Biofuel Production for the Transportation Sector. In Intelligent Transportation System and Advanced Technology; Springer: Berlin/Heidelberg, Germany, 2024; pp. 99–122. [Google Scholar]
- Emmanouilidou, E.; Lazaridou, A.; Mitkidou, S.; Kokkinos, N.C. A Comparative Study on Biodiesel Production from Edible and Non-Edible Biomasses. J. Mol. Struct. 2024, 1306, 137870. [Google Scholar] [CrossRef]
- Emmanouilidou, E.; Mitkidou, S.; Agapiou, A.; Kokkinos, N.C. Solid Waste Biomass as a Potential Feedstock for Producing Sustainable Aviation Fuel: A Systematic Review. Renew. Energy 2023, 206, 897–907. [Google Scholar] [CrossRef]
- Suzihaque, M.U.H.; Alwi, H.; Kalthum Ibrahim, U.; Abdullah, S.; Haron, N. Biodiesel Production from Waste Cooking Oil: A Brief Review. Mater. Today Proc. 2022, 63, S490–S495. [Google Scholar] [CrossRef]
- Joshi, J.R.; Bhanderi, K.K.; Patel, J.V. Waste Cooking Oil as a Promising Source for Bio Lubricants—A Review. J. Indian Chem. Soc. 2023, 100, 100820. [Google Scholar] [CrossRef]
- Foo, W.H.; Koay, S.S.N.; Chia, S.R.; Chia, W.Y.; Tang, D.Y.Y.; Nomanbhay, S.; Chew, K.W. Recent Advances in the Conversion of Waste Cooking Oil into Value-Added Products: A Review. Fuel 2022, 324, 124539. [Google Scholar] [CrossRef]
- Gobierno de España, Ministerio de Agricultura, Pesca y Alimentación. Food Consumption in Spain (Report). Available online: https://www.mapa.gob.es/es/estadistica/temas/default.aspx (accessed on 23 October 2024).
- Hanif, M.A.; Nisar, S.; Akhtar, M.N.; Nisar, N.; Rashid, N. Optimized Production and Advanced Assessment of Biodiesel: A Review. Int. J. Energy Res. 2018, 42, 2070–2083. [Google Scholar] [CrossRef]
- Ahmad, U.; Naqvi, S.R.; Ali, I.; Naqvi, M.; Asif, S.; Bokhari, A.; Juchelková, D.; Klemeš, J.J. A Review on Properties, Challenges and Commercial Aspects of Eco-Friendly Biolubricants Productions. Chemosphere 2022, 309, 136622. [Google Scholar] [CrossRef]
- Owuna, F.J.; Dabai, M.U.; Sokoto, M.A.; Dangoggo, S.M.; Bagudo, B.U.; Birnin-Yauri, U.A.; Hassan, L.G.; Sada, I.; Abubakar, A.L.; Jibrin, M.S. Chemical Modification of Vegetable Oils for the Production of Biolubricants Using Trimethylolpropane: A Review. Egypt. J. Pet. 2020, 29, 75–82. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; González, J.F. A Review on Biolubricants Based on Vegetable Oils through Transesterification and the Role of Catalysts: Current Status and Future Trends. Catalysts 2023, 13, 1299. [Google Scholar] [CrossRef]
- Kumar, N. Oxidative Stability of Biodiesel: Causes, Effects and Prevention. Fuel 2017, 190, 328–350. [Google Scholar] [CrossRef]
- Rizwanul Fattah, I.M.; Masjuki, H.H.; Kalam, M.A.; Hazrat, M.A.; Masum, B.M.; Imtenan, S.; Ashraful, A.M. Effect of Antioxidants on Oxidation Stability of Biodiesel Derived from Vegetable and Animal Based Feedstocks. Renew. Sustain. Energy Rev. 2014, 30, 356–370. [Google Scholar] [CrossRef]
- Varatharajan, K.; Pushparani, D.S. Screening of Antioxidant Additives for Biodiesel Fuels. Renew. Sustain. Energy Rev. 2018, 82, 2017–2028. [Google Scholar] [CrossRef]
- Ramalingam, S.; Rajendran, S.; Ganesan, P.; Govindasamy, M. Effect of Operating Parameters and Antioxidant Additives with Biodiesels to Improve the Performance and Reducing the Emissions in a Compression Ignition Engine—A Review. Renew. Sustain. Energy Rev. 2018, 81, 775–788. [Google Scholar] [CrossRef]
- Hosseinzadeh-Bandbafha, H.; Kumar, D.; Singh, B.; Shahbeig, H.; Lam, S.S.; Aghbashlo, M.; Tabatabaei, M. Biodiesel Antioxidants and Their Impact on the Behavior of Diesel Engines: A Comprehensive Review. Fuel Process. Technol. 2022, 232, 107264. [Google Scholar] [CrossRef]
- De Sousa, L.S.; De Moura, C.V.R.; De Oliveira, J.E.; De Moura, E.M. Use of Natural Antioxidants in Soybean Biodiesel. Fuel 2014, 134, 420–428. [Google Scholar] [CrossRef]
- Silva de Sousa, L.; Verônica Rodarte de Moura, C.; Miranda de Moura, E. Action of Natural Antioxidants on the Oxidative Stability of Soy Biodiesel during Storage. Fuel 2020, 288, 119632. [Google Scholar] [CrossRef]
- Ramos, T.C.P.M.; Santos, E.P.S.; Ventura, M.; Pina, J.C.; Cavalheiro, A.A.; Fiorucci, A.R.; Silva, M.S. Eugenol and TBHQ Antioxidant Actions in Commercial Biodiesel Obtained by Soybean Oil and Animal Fat. Fuel 2021, 286, 119374. [Google Scholar] [CrossRef]
- Schaumlöffel, L.S.; Fontoura, L.A.M.; Santos, S.J.; Pontes, L.F.; Gutterres, M. Vegetable Tannins-Based Additive as Antioxidant for Biodiesel. Fuel 2021, 292, 120198. [Google Scholar] [CrossRef]
- Jain, S.; Purohit, S.; Kumar, D.; Goud, V.V. Passion Fruit Seed Extract as an Antioxidant Additive for Biodiesel; Shelf Life and Consumption Kinetics. Fuel 2021, 289, 119906. [Google Scholar] [CrossRef]
- Karishma, S.M.; Rajak, U.; Naik, B.K.; Dasore, A.; Konijeti, R. Performance and Emission Characteristics Assessment of Compression Ignition Engine Fuelled with the Blends of Novel Antioxidant Catechol-Daok Biodiesel. Energy 2022, 245, 123304. [Google Scholar] [CrossRef]
- Dueso, C.; Muñoz, M.; Moreno, F.; Arroyo, J.; Gil-Lalaguna, N.; Bautista, A.; Gonzalo, A.; Sánchez, J.L. Performance and Emissions of a Diesel Engine Using Sunflower Biodiesel with a Renewable Antioxidant Additive from Bio-Oil. Fuel 2018, 234, 276–285. [Google Scholar] [CrossRef]
- García, M.; Botella, L.; Gil-Lalaguna, N.; Arauzo, J.; Gonzalo, A.; Sánchez, J.L. Antioxidants for Biodiesel: Additives Prepared from Extracted Fractions of Bio-Oil. Fuel Process. Technol. 2017, 156, 407–414. [Google Scholar] [CrossRef]
- Delgado, M.A.; García-Rico, C.; Franco, J.M. The Use of Rosemary Extracts in Vegetable Oil-Based Lubricants. Ind. Crops Prod. 2014, 62, 474–480. [Google Scholar] [CrossRef]
- Samuel, J.; Kaisan, M.U.; Sanusi, Y.S.; Narayan, S.; Menacer, B.; Valenzuela, M.; Salas, A.; Oñate, A.; Mahroogi, F.O.; Tuninetti, V. Assessing Antioxidant and Pour Point Depressant Capacity of Turmeric Rhizome Extract in Biolubricants. Lubricants 2024, 12, 282. [Google Scholar] [CrossRef]
- Singh, N.; Agarwal, P.; Porwal, S.K. Natural Antioxidant Extracted Waste Cooking Oil as Sustainable Biolubricant Formulation in Tribological and Rheological Applications. Waste Biomass Valorization 2022, 13, 3127–3137. [Google Scholar] [CrossRef]
- Ahmad, I.; Ullah, J.; Khan, R.; Ahmad, W. Antioxidant Performance of Bio-Oils in Oxidative Stability of Base Lubricating Oil Determined through TGA and PDSC Techniques. Thermochim. Acta 2022, 713, 179241. [Google Scholar] [CrossRef]
- Jemima Romola, C.V.; Meganaharshini, M.; Rigby, S.P.; Ganesh Moorthy, I.; Shyam Kumar, R.; Karthikumar, S. A Comprehensive Review of the Selection of Natural and Synthetic Antioxidants to Enhance the Oxidative Stability of Biodiesel. Renew. Sustain. Energy Rev. 2021, 145, 111109. [Google Scholar] [CrossRef]
- UNE-EN 14214:2013 V2+A1:2018; Liquid Petroleum Products—Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications—Requirements and Test Methods. Asociacion Espanola de Normalizacion: Madrid, Spain, 2018.
- Skoog, D.A.; West, D.M.; Holler, F.G.; Crouch, S.R. Fundamentals of Analytical Chemistry, 10th ed.; Brooks/Cole ISE: Devon, UK, 2021. [Google Scholar]
- Nogales-Delgado, S.; Guiberteau, A.; Encinar, J.M. Effect of Tert-Butylhydroquinone on Biodiesel Properties during Extreme Oxidation Conditions. Fuel 2022, 310, 122339. [Google Scholar] [CrossRef]
- Squissato, A.L.; Richter, E.M.; Munoz, R.A.A. Voltammetric Determination of Copper and Tert-Butylhydroquinone in Biodiesel: A Rapid Quality Control Protocol. Talanta 2019, 201, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Nogales-Delgado, S.; Guiberteau Cabanillas, A.; Moro, J.P.; Encinar Martín, J.M. Use of Propyl Gallate in Cardoon Biodiesel to Keep Its Main Properties during Oxidation. Clean Technol. 2023, 5, 569–583. [Google Scholar] [CrossRef]
- Caramit, R.P.; De Freitas Andrade, A.G.; Gomes De Souza, J.B.; De Araujo, T.A.; Viana, L.H.; Trindade, M.A.G.; Ferreira, V.S. A New Voltammetric Method for the Simultaneous Determination of the Antioxidants TBHQ and BHA in Biodiesel Using Multi-Walled Carbon Nanotube Screen-Printed Electrodes. Fuel 2013, 105, 306–313. [Google Scholar] [CrossRef]
- Tormin, T.F.; Cunha, R.R.; Richter, E.M.; Munoz, R.A.A. Fast Simultaneous Determination of BHA and TBHQ Antioxidants in Biodiesel by Batch Injection Analysis Using Pulsed-Amperometric Detection. Talanta 2012, 99, 527–531. [Google Scholar] [CrossRef]
- Schaumlöffel, L.D.S.; Bolognese Fernandes, P.R.; Sartori Piatnicki, C.M.; Gutterres, M. A Chemometric-Assisted Voltammetric Method for Simultaneous Determination of Four Antioxidants in Biodiesel Samples. Energy Fuels 2020, 34, 412–418. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Cabanillas, A.G.; Romero, Á.G.; Encinar Martín, J.M. Monitoring Tert-Butylhydroquinone Content and Its Effect on a Biolubricant during Oxidation. Molecules 2022, 27, 8931. [Google Scholar] [CrossRef]
- Encinar, J.M.; Nogales-Delgado, S.; Álvez-Medina, C.M. High Oleic Safflower Biolubricant through Double Transesterification with Methanol and Pentaerythritol: Production, Characterization, and Antioxidant Addition. Arab. J. Chem. 2022, 15, 103796. [Google Scholar] [CrossRef]
- Quinchia, L.A.; Delgado, M.A.; Valencia, C.; Franco, J.M.; Gallegos, C. Natural and Synthetic Antioxidant Additives for Improving the Performance of New Biolubricant Formulations. J. Agric. Food Chem. 2011, 59, 12917–12924. [Google Scholar] [CrossRef]
- Jedrzejczyk, M.A.; Van den Bosch, S.; Van Aelst, J.; Van Aelst, K.; Kouris, P.D.; Moalin, M.; Haenen, G.R.M.M.; Boot, M.D.; Hensen, E.J.M.; Lagrain, B.; et al. Lignin-Based Additives for Improved Thermo-Oxidative Stability of Biolubricants. ACS Sustain. Chem. Eng. 2021, 9, 12548–12559. [Google Scholar] [CrossRef]
- Navada, M.K.; Rai, R.; A, G.; Patil, S. Synthesis and Characterization of Size Controlled Nano Copper Oxide Structures for Antioxidant Study and as Eco-Friendly Lubricant Additive for Bio-Oils. Ceram. Int. 2023, 49, 10402–10410. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; González, J.F. Safflower Biodiesel: Improvement of Its Oxidative Stability by Using BHA and TBHQ. Energies 2019, 12, 1940. [Google Scholar] [CrossRef]
- Encinar, J.M.; Nogales, S.; González, J.F. Biorefinery Based on Different Vegetable Oils: Characterization of Biodiesel and Biolubricants. In Proceedings of the 3rd International Conference in Engineering Applications (ICEA), Sao Miguel, Portugal, 8–11 July 2019. [Google Scholar]
- Nogales-Delgado, S.; Encinar Martín, J.M. Cardoon Biolubricant through Double Transesterification: Assessment of Its Oxidative, Thermal and Storage Stability. Mater. Lett. 2021, 302, 130454. [Google Scholar] [CrossRef]
- UNE-EN ISO 3104/AC:1999; Petroleum Products. Transparent and Opaque Liquids. Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity (ISO 3104:1994). Asociacion Espanola de Normalizacion: Madrid, Spain, 1999.
- UNE-EN 116:2015; Diesel and Domestic Heating Fuels—Determination of Cold Filter Plugging Point-Stepwise Cooling Bath Method. Asociacion Espanola de Normalizacion: Madrid, Spain, 2015.
- UNE-EN-ISO 3675; Crude Petroleum and Liquid Petroleum Products. Laboratory Determination of Density. Hydrometer Method. Asociacion Espanola de Normalizacion: Madrid, Spain, 1999.
- UNE-EN ISO 12966-2:2011; Animal and Vegetable Fats and Oils–Gas Chromatography of Fatty Acid Methyl Esters–Part 2: Preparation of Methyl Esters of Fatty Acids. Asociacion Espanola de Normalizacion: Madrid, Spain, 2011.
- UNE-EN 14104:2003; Oil and Fat Derivatives. Fatty Acid Methyl Esters (FAME). Determination of Acid Value. Asociacion Espanola de Normalizacion: Madrid, Spain, 2003.
- UNE-EN 14111:2003; Fat and Oil Derivatives. Fatty Acid Methyl Esters (FAME). Determination of Iodine Value. Asociacion Espanola de Normalizacion: Madrid, Spain, 2003.
- UNE-EN 14112; Fat and Oil Derivatives-Fatty Acid Methyl Esters (FAME)-Determination of Oxidation Stability (Accelerated Oxidation Test). Asociacion Espanola de Normalizacion: Madrid, Spain, 2017.
- Focke, W.W.; Van Der Westhuizen, I.; Oosthuysen, X. Biodiesel Oxidative Stability from Rancimat Data. Thermochim. Acta 2016, 633, 116–121. [Google Scholar] [CrossRef]
- UNE-EN 51023:1990; Petroleum Products. Determination of Flash and Fire Points. Cleveland Open Cup Method. Asociacion Espanola de Normalizacion: Madrid, Spain, 1990.
- Gao, M.; Xu, Y.; Liu, Y.; Wang, S.; Wang, C.; Dong, Y.; Song, Z. Effect of Polystyrene on Di-Butyl Phthalate (DBP) Bioavailability and DBP-Induced Phytotoxicity in Lettuce. Environ. Pollut. 2021, 268, 115870. [Google Scholar] [CrossRef]
- Wu, J.; Wang, G.; Vijver, M.G.; Bosker, T.; Peijnenburg, W.J.G.M. Foliar versus Root Exposure of AgNPs to Lettuce: Phytotoxicity, Antioxidant Responses and Internal Translocation. Environ. Pollut. 2020, 261, 114117. [Google Scholar] [CrossRef] [PubMed]
- Nogales-Delgado, S.; Sánchez, N.; Encinar, J.M. Valorization of Cynara Cardunculus L. Oil as the Basis of a Biorefinery for Biodiesel and Biolubricant Production. Energies 2020, 13, 5085. [Google Scholar] [CrossRef]
- Caldeira, C.; Freire, F.; Olivetti, E.A.; Kirchain, R. Fatty Acid Based Prediction Models for Biodiesel Properties Incorporating Compositional Uncertainty. Fuel 2017, 196, 13–20. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar Martín, J.M.; Sánchez Ocaña, M. Use of Mild Reaction Conditions to Improve Quality Parameters and Sustainability during Biolubricant Production. Biomass Bioenergy 2022, 161, 106456. [Google Scholar] [CrossRef]
- Zhou, J.; Xiong, Y.; Liu, X. Evaluation of the Oxidation Stability of Biodiesel Stabilized with Antioxidants Using the Rancimat and PDSC Methods. Fuel 2017, 188, 61–68. [Google Scholar] [CrossRef]
- Zhang, F.; Li, J.; Yang, S.; Bi, Y. Inhibitory Effect of Antioxidants on Biodiesel Crystallization: Revealing the Role of Antioxidants. Fuel 2021, 297, 120782. [Google Scholar] [CrossRef]
- Sutanto, H.; Pramastiani, A.J.; Yusri, S.; Darmawan, A.; Legowo, E.H.; Nasikin, M. Modification of Tert-Butylhydroquinone with Palmitic Acid as a Soluble Antioxidant for Biodiesel Additive. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Sozopol, Bulgaria, 10–13 September 2020; Institute of Physics Publishing: Bristol, UK, 2020; Volume 742. [Google Scholar]
- Orives, J.R.; Galvan, D.; Pereira, J.L.; Coppo, R.L.; Borsato, D. Experimental Design Applied for Cost and Efficiency of Antioxidants in Biodiesel. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 1805–1811. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; Guiberteau, A.; Márquez, S. The Effect of Antioxidants on Corn and Sunflower Biodiesel Properties under Extreme Oxidation Conditions. JAOCS J. Am. Oil Chem. Soc. 2019, 97, 201–212. [Google Scholar] [CrossRef]

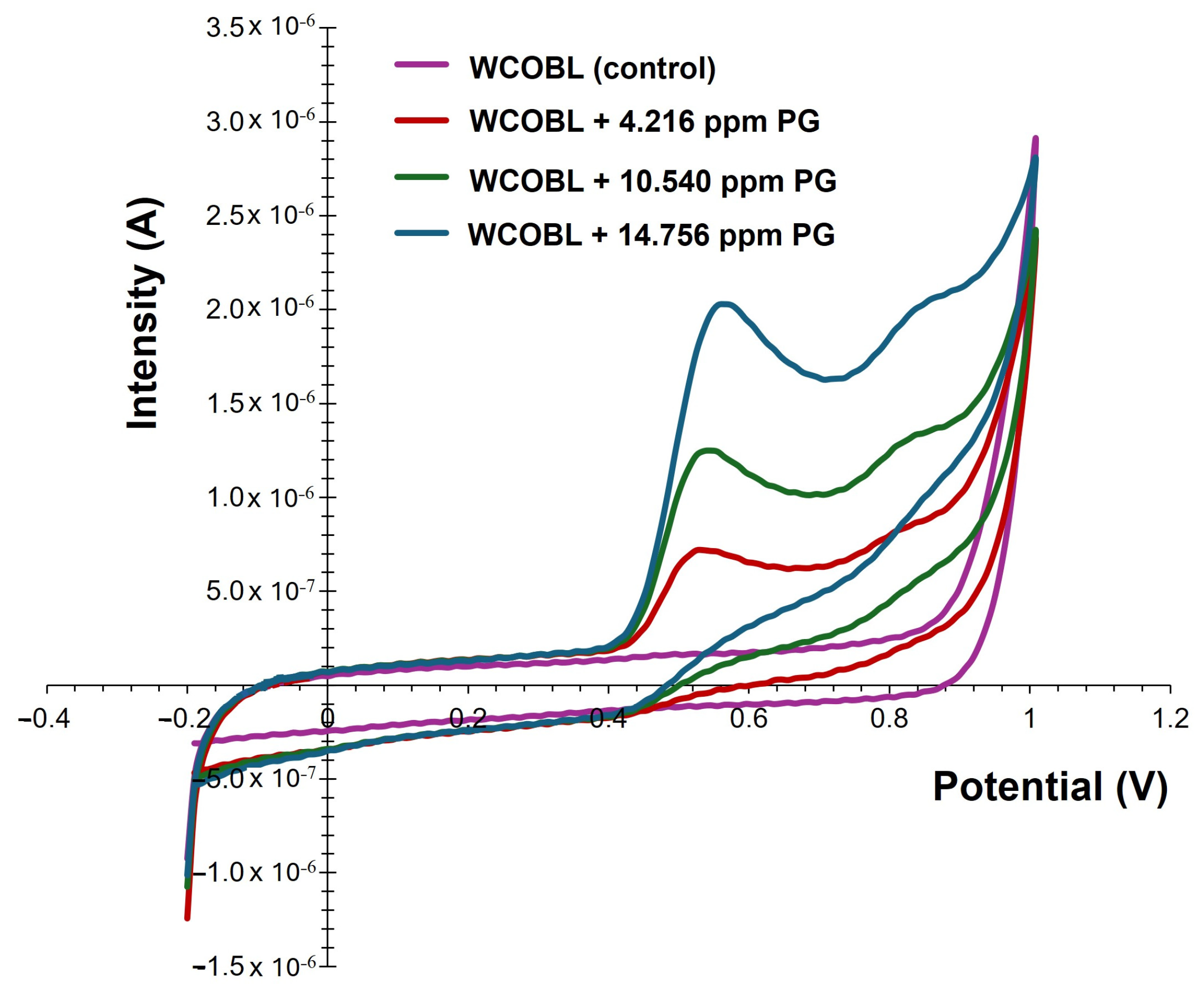
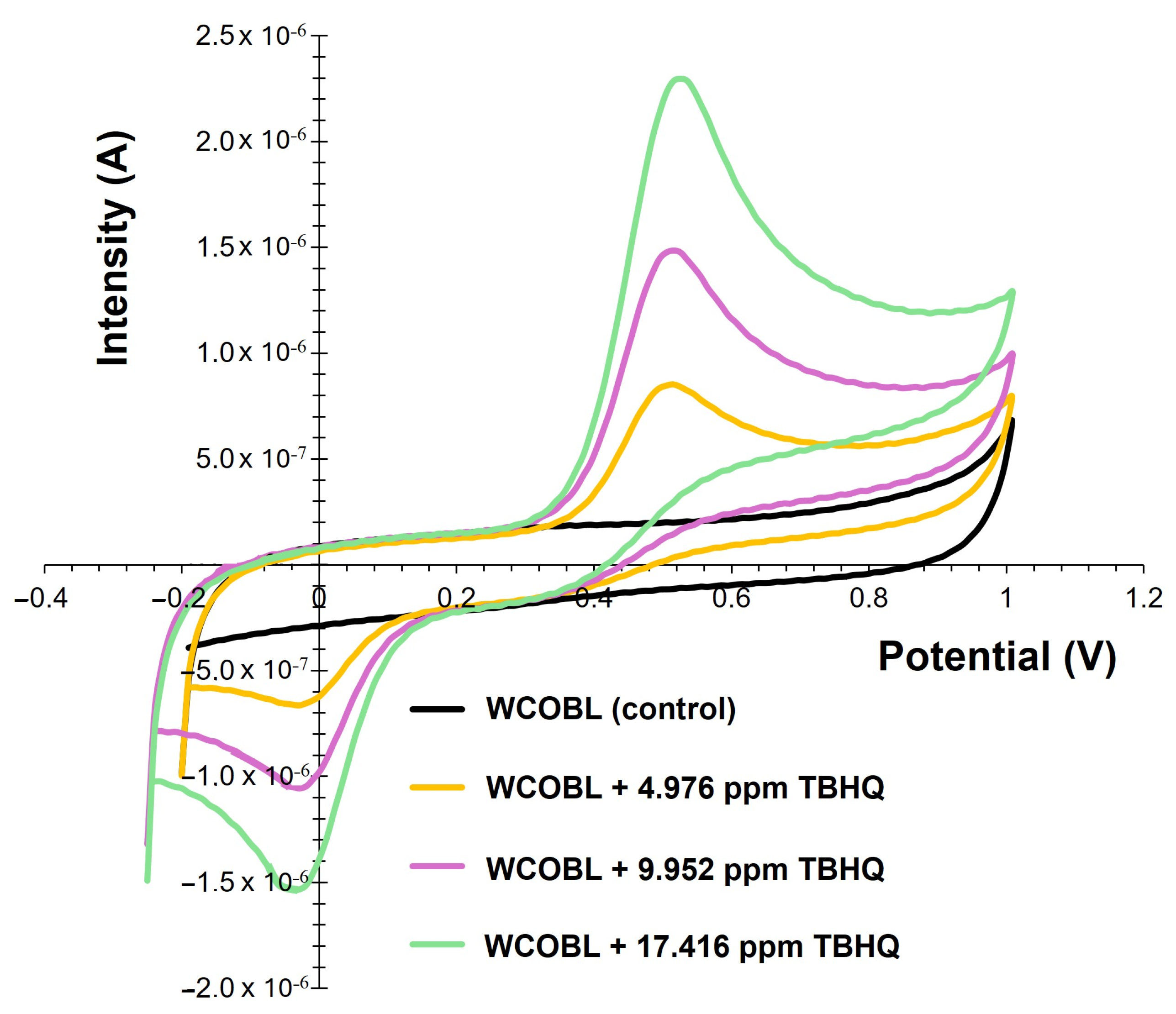
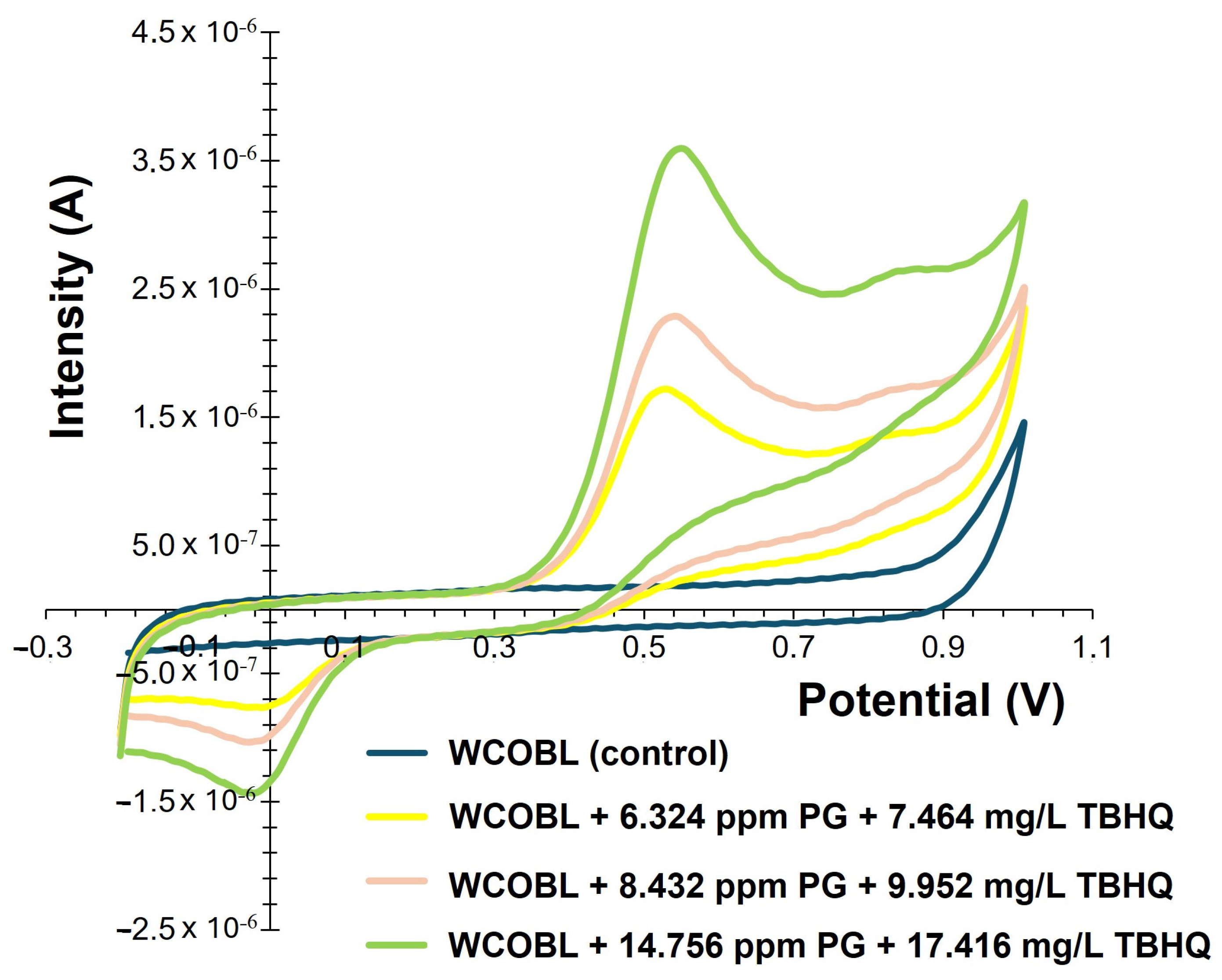

| Details | Ref. |
|---|---|
| TBHQ was quantified in biodiesel samples (WCO, cardoon and canola) by using cyclic voltammetry (CV). | [35] |
| TBHQ and Cu were quantified in biodiesel using squarewave voltammetry (SWV). | [36] |
| PG was quantified in cardoon biodiesel, improving its oxidation stability. PG concentration decreased during oxidation. | [37] |
| Different methods (including CV and DPV) were compared to HPLC, to simultaneously quantify TBHQ and BHA in biodiesel. | [38] |
| TBHQ and BHA were determined in biodiesel through a batch injection analysis and pulsed-amperometric detection. | [39] |
| Different antioxidants applied to biodiesel, including TBHQ and PG, were simultaneously determined by using DPV and artificial neural network. | [40] |
| TBHQ was quantified in a biolubricant from WCO, through CV and DPV methods. | [41] |
| TBHQ was added to a high-oleic safflower biolubricant produced through double transesterification with methanol and pentaerythritol, showing a high efficiency at low concentrations (500 ppm) and keeping the main properties of this biolubricant (mainly viscosity and acidity). | [42] |
| Different antioxidants, including PG, were applied to several biolubricants, proving the highest efficiency of propyl gallate. | [43] |
| Lignin-based additives were applied to castor oil in biolubricant formulations, increasing oxidation induction time. | [44] |
| CuO nanostructures were used as additives for biolubricants (Pongamia oil), with high antioxidant efficiency (above 70%) at low concentrations (50 µg·mL−1) | [45] |
| First Transesterification | ||
|---|---|---|
| Parameter | Conditions | Details |
| Alcohol used | Methanol | Pure, pharma grade, Panreac Applichem, (Castellar del Valles, Barcelona, Spain) |
| Reaction time, min | 120 | -- |
| Reaction temperature, °C | 65 | Higher temperatures were not recommended to avoid methanol boiling |
| Oil/methanol ratio | 1:6 | Excess methanol ratio was used to ensure high conversion |
| Catalyst, % | MeONa, 0.5 | 30% in methanol, Merck (Darmstadt, Germany) |
| Purification | Separation funnel | Through decantation to remove glycerol and catalyst (by washing treatments) |
| Second Transesterification | ||
|---|---|---|
| Parameter | Conditions | Details |
| Alcohol used | Pentaerythritol (PE) | Pure, Merck (Darmstadt, Germany) |
| Reaction time, min | 120 | -- |
| Reaction temperature, °C | 160 | -- |
| FAME/PE ratio | 1:1/3 | A slight excess in pentaerythritol was used to avoid problems during filtering |
| Catalyst, % | MeONa, 1% | 30% in methanol, Merck (Darmstadt, Germany) |
| Pressure, mmHg | 260 | To promote methanol removal from the reaction medium |
| Purification | Filtration | A first gravity filtration was used, followed by different vacuum filtrations |
| Property | Details | Ref. |
|---|---|---|
| Viscosity and Cold Filter Plugging Point (CFPP) | A Cannon-Fenske viscometer was used, controlling temperature at 40 °C. For CFPP, the corresponding standard was used. | [49,50] |
| Density | A densimeter was used for this determination. | [51] |
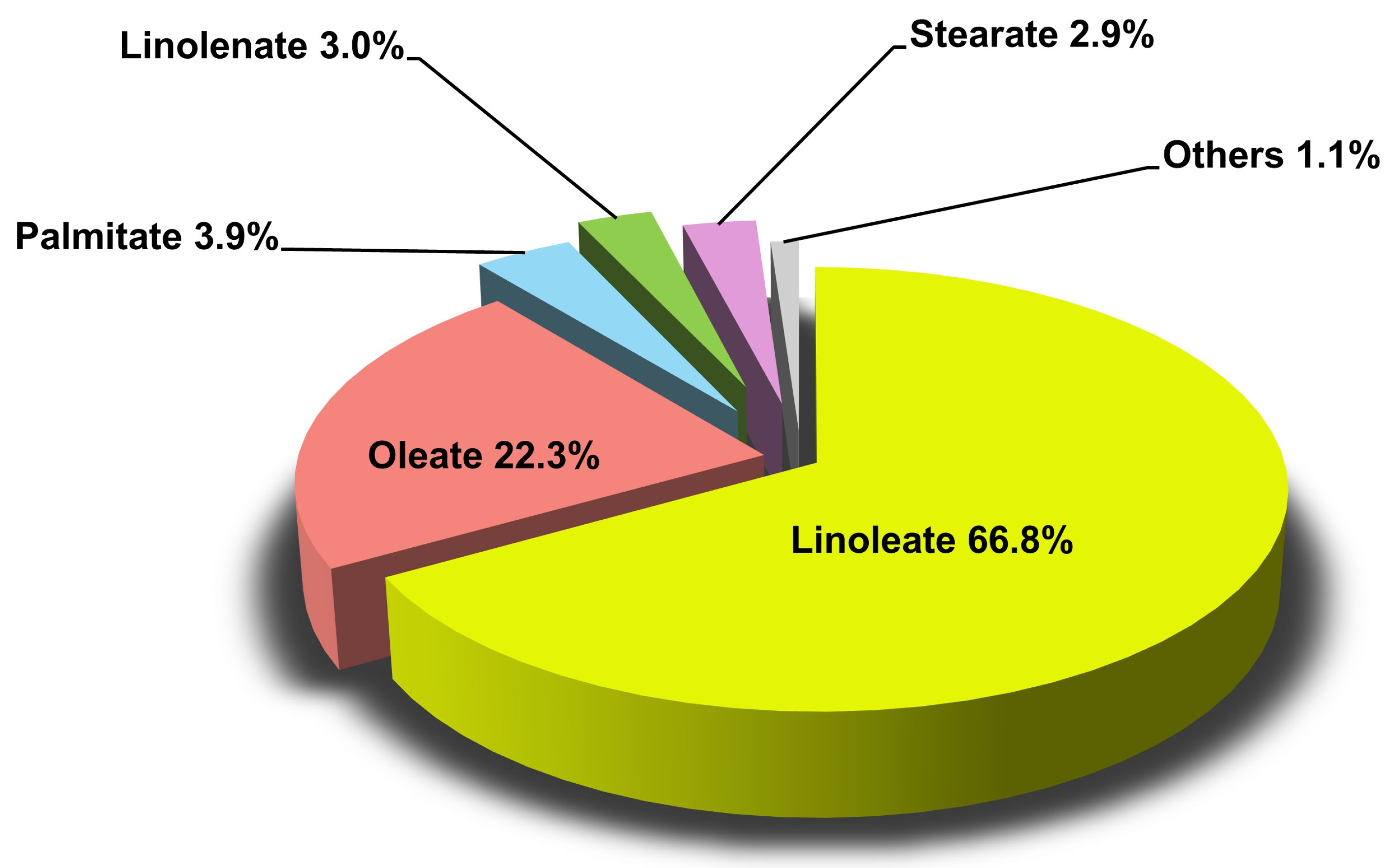
| FAME content | FAME content was analyzed by using a gas chromatograph (Varian 3900, Varian, Palo Alto, CA, USA) coupled to a flame ionization detector (FID). Main FAMEs, such as methyl oleate, linoleate, linolenate, palmitate, and stearate. | [52] |
| Acid value | According to UNE-EN 14104 standard. | [53] |
| Iodine value | According to UNE-EN 14111 standard. | [54] |
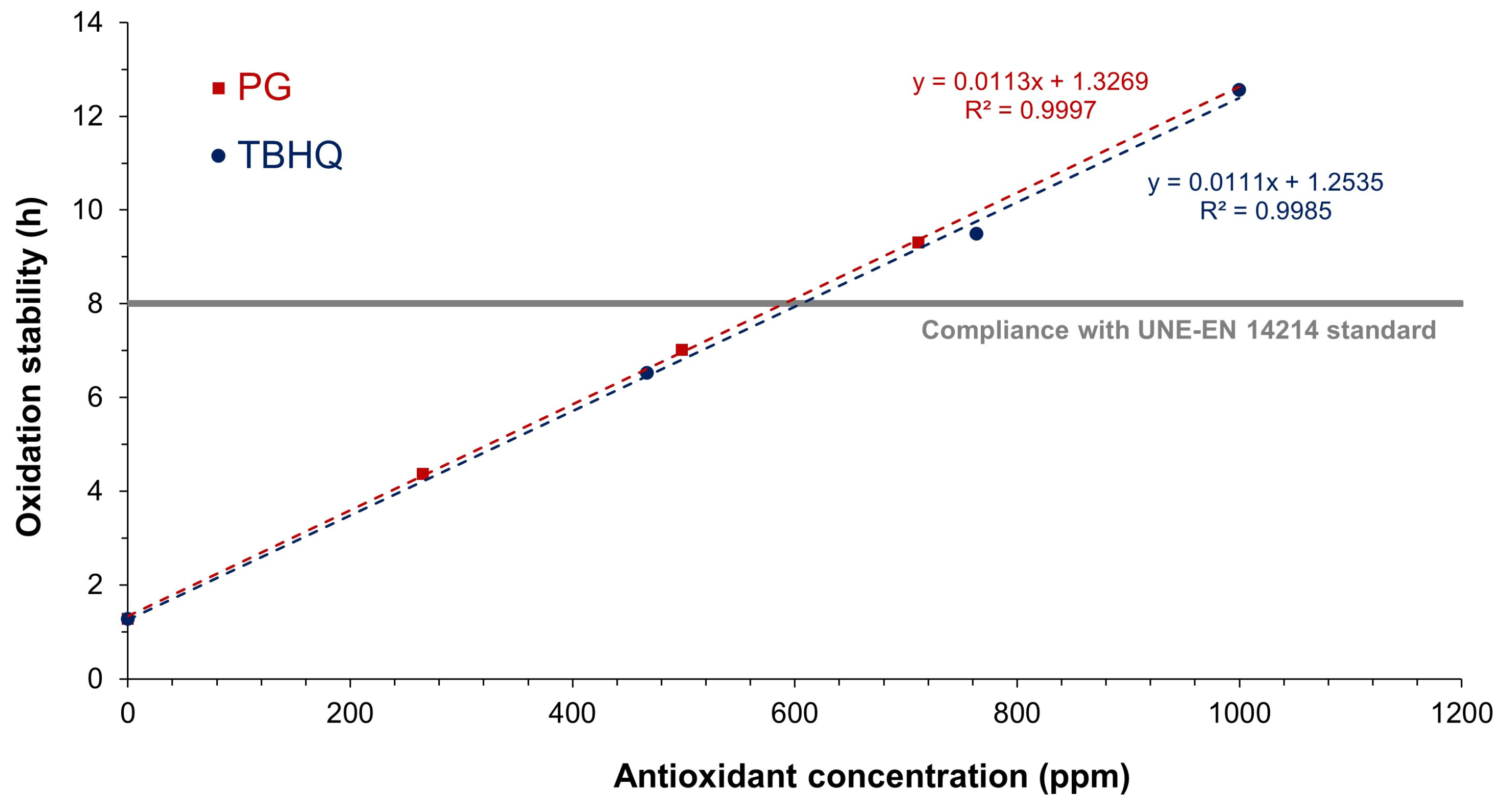
| Oxidation stability | Rancimat method was used, at 110 °C. | [55,56] |
| Flash and fire points | Cleveland open cup method was used. | [57] |
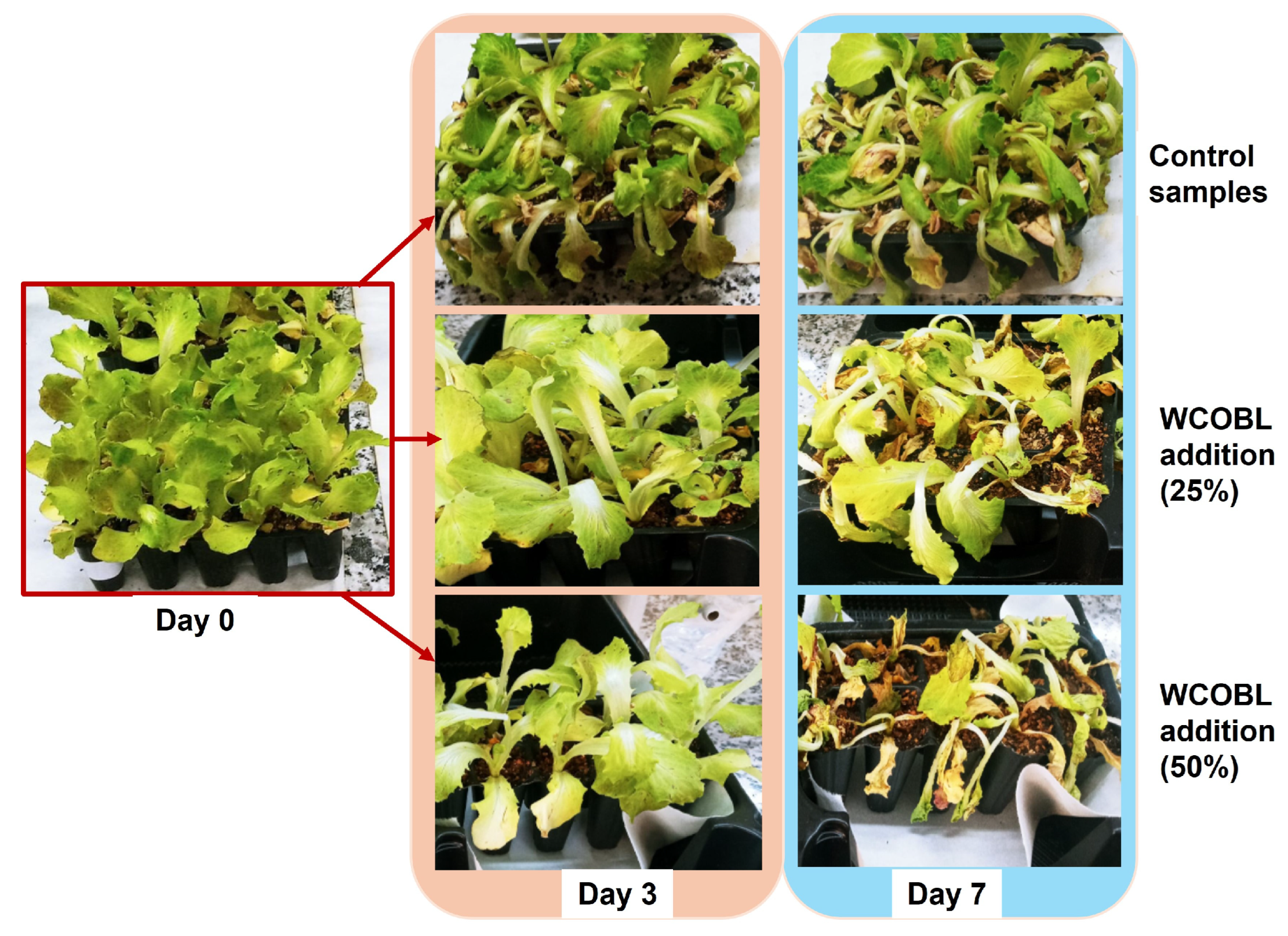
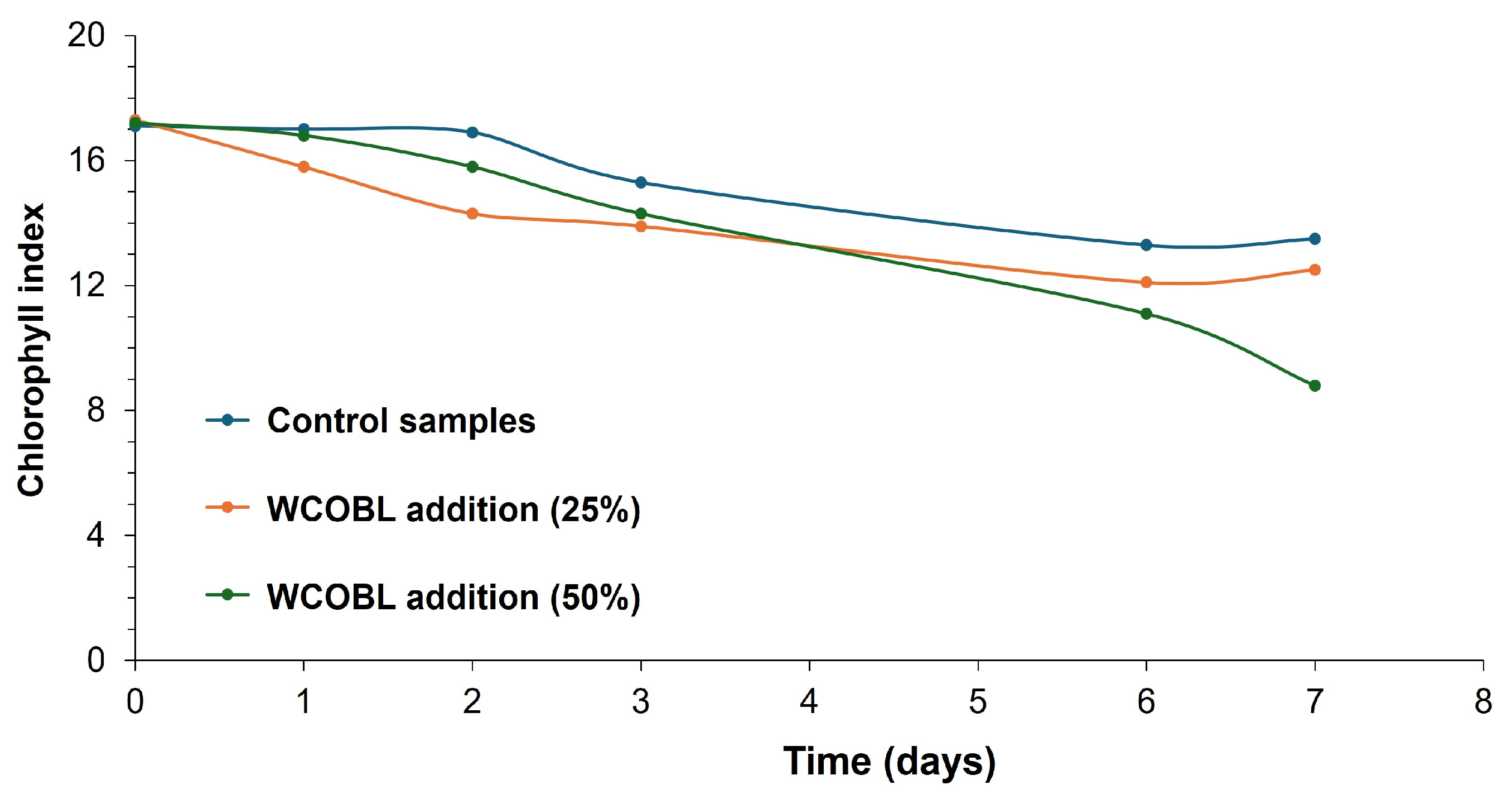
| Phytotoxicity test | Marvel of four seasons lettuce (Lactuca sativa) was selected for this test (lettuce is a recurring species in this kind of tests, as observed in references), adding 20 mL of water as control sample, 25% and 50% solution of WCO biolubricant. Afterwards, the lettuce samples were visually assessed, including chlorophyll content by using a chlorophyll meter (SPAD 502 Plus, Konica Minolta, Tokyo, Japan). Measurements were carried out in different leaves on different days for one week. | [58,59] |
| Property | WCOBD | WCOBL |
|---|---|---|
| Viscosity at 40 °C, cSt | 4.50 | 62.89 |
| Density, kg∙m−3 | 881 | 904 |
| Yield, % | 98.9 | 90.18 |
| Acid value, mgKOH∙g−1 | 0.22 | 0.55 |
| Iodine value, gI2∙100 g−1 | 98 | Not determined |
| Oxidation stability, h | 1.28 | 2.63 |
| Flash and fire points, °C | 173–179 | 242–249 |
| Parameter | Units | PG | TBHQ (Anodic Peak) | TBHQ (Cathodic Peak) |
|---|---|---|---|---|
| Number of standards | -- | 7 | 7 | 7 |
| Slope | nA·L·mg−1 | 74.1421 | 104.2100 | 52.5280 |
| Standard deviation (slope) | nA·L·mg−1 | 3.7465 | 5.5110 | 3.0450 |
| Intercept | nA | 23.6430 | 28.2500 | 31.6660 |
| Standard deviation (intercept) | nA | 35.3190 | 61.3200 | 33.8810 |
| R2 | -- | 0.9727 | 0.9675 | 0.9612 |
| Linearity | % | 94.2030 | 94.1820 | 94.9700 |
| Analytical sensitivity | γ−1 | 1.0790 | 0.5485 | 0.7900 |
| Detection limit (Long–Winefordner) | ppm | 1.94 | 1.22 | 1.42 |
| Detection limit (Clayton) | ppm | 3.15 | 1.83 | 2.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogales-Delgado, S.; Guiberteau Cabanillas, A.; Catela Rodríguez, A. Combined Effect of Propyl Gallate and Tert-Butyl Hydroquinone on Biodiesel and Biolubricant Based on Waste Cooking Oil. Appl. Sci. 2024, 14, 9767. https://doi.org/10.3390/app14219767
Nogales-Delgado S, Guiberteau Cabanillas A, Catela Rodríguez A. Combined Effect of Propyl Gallate and Tert-Butyl Hydroquinone on Biodiesel and Biolubricant Based on Waste Cooking Oil. Applied Sciences. 2024; 14(21):9767. https://doi.org/10.3390/app14219767
Chicago/Turabian StyleNogales-Delgado, Sergio, Agustina Guiberteau Cabanillas, and Antonio Catela Rodríguez. 2024. "Combined Effect of Propyl Gallate and Tert-Butyl Hydroquinone on Biodiesel and Biolubricant Based on Waste Cooking Oil" Applied Sciences 14, no. 21: 9767. https://doi.org/10.3390/app14219767
APA StyleNogales-Delgado, S., Guiberteau Cabanillas, A., & Catela Rodríguez, A. (2024). Combined Effect of Propyl Gallate and Tert-Butyl Hydroquinone on Biodiesel and Biolubricant Based on Waste Cooking Oil. Applied Sciences, 14(21), 9767. https://doi.org/10.3390/app14219767







