Abstract
The therapeutic potential of combining 5-FU with repurposed drugs such as Sildenafil, Tezosentan, Levosimendan, and Resveratrol was investigated in lung cancer treatment using the A549 cell line. This study aimed to enhance 5-FU efficacy while mitigating side effects and overcoming drug resistance. The cytotoxic effects of 5-FU were assessed via MTT assay, with an IC50 value of 5.03 µM for A549 cells. Subsequent experiments evaluated the impact of combining 5-FU with the aforementioned drugs on cell viability, clonogenic potential, and morphology. The results demonstrated that while Sildenafil and Tezosentan modestly improved 5-FU efficacy, Levosimendan reduced cell viability by 40% (p < 0.01) and Resveratrol by over 50% (p < 0.001), with clonogenicity reduced by up to 60% (p < 0.001). These findings suggest that combining 5-FU with Levosimendan or Resveratrol offers promising approaches for lung cancer therapy, potentially reducing the need for higher doses of 5-FU and minimizing associated toxicity. Future studies are warranted to elucidate the mechanisms underlying these interactions and assess their clinical relevance.
1. Introduction
Lung cancer ranks as the foremost cause of cancer-related deaths globally, with an alarming average five-year survival rate of only 15%. The disease’s incidence and mortality rates vary significantly worldwide due to differences in tobacco smoking habits, environmental risk factors, and genetic predispositions. Smoking remains the predominant risk factor associated with this severe illness. However, genetic mutations and alterations play a critical role in lung cancer development and progression, influencing both the onset of the disease and responses to treatment. Key genetic mutations frequently observed in lung cancer include alterations in the Epidermal Growth Factor Receptor (EGFR) gene, which is particularly prevalent in non-smokers, women, and certain ethnic groups, as well as mutations in the KRAS gene, more commonly associated with smokers [1,2,3]. Other significant genetic changes include ALK (Anaplastic Lymphoma Kinase) rearrangements [4,5], ROS1 fusions [6,7], and BRAF mutations [8,9], each contributing to tumor growth and therapeutic resistance. Additionally, TP53, a tumor suppressor gene, is often found mutated in lung cancer, further exacerbating the disease’s aggressiveness [10]. Lung cancer is broadly divided into two primary histological categories: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) [11,12]. NSCLC, the most common type, encompasses subtypes such as squamous cell carcinoma, adenocarcinoma, and large cell carcinoma. SCLC is less common, but tends to be more aggressive. Treatment approaches for lung cancer typically involve a combination of strategies, including surgery [13], radiation therapy [14], chemotherapy [15], targeted therapy [16], and immunotherapy [17]. In certain cases, hormone therapy may also be considered [18]. The specific treatment plan is tailored to the individual, taking into account factors such as the cancer’s stage, tumor location, the patient’s overall health, and personalized treatment goals [19].
The compound 5-Fluorouracil (5-FU) is a cornerstone chemotherapeutic agent utilized in the therapy of multiple cancer types [20]. Since its introduction into clinical practice, 5-FU has been a cornerstone in oncology, playing a critical role in the management of malignant neoplasms, both in adjuvant and palliative chemotherapy regimens [21,22]. Its effectiveness is largely attributed to its mechanism of action as a pyrimidine analog, where 5-FU disrupts the synthesis of DNA and RNA in tumor cells, leading to the disruption of cell replication and eventually apoptosis [23]. Despite its proven efficacy, the use of 5-FU is accompanied by several limitations. Drug resistance, which can occur due to alterations in the drug’s metabolic pathway or changes in the expression of genes related to the cell cycle, is one of the major challenges in cancer treatment with this agent [24,25]. Furthermore, the adverse side effects, such as myelosuppression [26], gastrointestinal toxicity [27,28,29], and cardiotoxicity [30], limit its application, particularly in patients with comorbidities or clinical frailty. These challenges underscore the need for therapeutic strategies that can enhance the efficacy of 5-FU while minimizing its adverse effects, such as combining it with other therapeutic agents that could overcome drug resistance or mitigate its toxic effects.
In recent years, the strategy of combining chemotherapeutic agents has gained significant traction as a means to enhance the effectiveness of cancer treatment [31,32]. The rationale behind drug combination therapy lies in the ability to target multiple pathways simultaneously, thereby enhancing the cytotoxic effects on cancer cells while minimizing the chances of developing drug resistance [33]. By using lower doses of each drug in combination, it is often possible to achieve a synergistic effect, where the combined effect exceeds the total of the individual impacts [34]. This approach not only increases therapeutic efficacy, but also helps to minimize the adverse side effects typically associated with higher doses of single-agent chemotherapy. Numerous studies have shown the advantages of synergistic combinations of drugs in cancer therapy. For instance, combinations of 5-FU with other agents such as leucovorin, oxaliplatin, and irinotecan have been shown to significantly improve outcomes in colorectal cancer patients [35]. Similarly, the combination of 5-FU with newer targeted therapies and natural compounds, like Resveratrol, has shown promise in preclinical studies, suggesting that these combinations can enhance anticancer activity while potentially reducing toxicity [36]. These findings support the ongoing exploration of novel drug combinations to optimize cancer therapy, particularly in challenging malignancies like lung cancer.
Given the persistent challenges in treating lung cancer, especially the emergence of drug resistance and significant side effects linked to standard treatments, the selection of these drugs is based on their potential to target key pathways involved in tumor survival and proliferation. Sildenafil, for instance, has been shown to improve drug delivery by enhancing tumor perfusion, while Levosimendan’s effects on cellular signaling pathways may inhibit cancer cell proliferation. Resveratrol’s ability to modulate apoptotic pathways further supports its use as an adjuvant therapy. This multi-targeted approach could offer a promising strategy for overcoming the limitations of current lung cancer treatments.
Sildenafil, a phosphodiesterase type 5 (PDE5) inhibitor, is traditionally used to treat erectile dysfunction, but has also demonstrated potential in improving the delivery and efficacy of chemotherapeutic agents by modulating blood flow and vascular permeability in tumors [37,38]. Tezosentan, an endothelin (ET) receptor antagonist, exhibits anticancer properties by targeting ET receptors, which are crucial in processes such as cell proliferation, survival, neovascularization, immune response, and drug resistance [39]. Levosimendan, mainly employed in the management of heart failure due to its calcium sensitizing and vasodilatory properties, has shown promising anticancer potential through drug repurposing. Its effects include inhibition of cancer cell migration, enhancement of hypoxic cell sensitivity to radiation, and organ protection via mitochondrial potassium channels [40]. Levosimendan has demonstrated synergistic effects when used in combination with traditional anticancer drugs like 5-FU, particularly in bladder cancer cells [41]. Finally, Resveratrol, a natural polyphenol present in grapes and berries, is renowned for its wide range of anticancer activities, including the suppression of cell proliferation [42], induction of apoptosis [43], and modulation of various signaling pathways involved in tumor growth and metastasis [44,45]. The selection of these compounds was based on their potential to complement the action of 5-FU through different mechanisms, with the hypothesis that they might enhance its cytotoxic effects while allowing for a reduction in the required dosage and associated side effects. Although this synergy is yet to be confirmed, targeting multiple pathways simultaneously utilizing these combinations shows potential for overcoming drug resistance and possibly enhancing therapeutic outcomes in lung cancer treatment.
This research holds significant social and scientific relevance, as lung cancer continues to be one of the top causes of cancer-related deaths globally. The results of this study could aid in the creation of novel therapeutic strategies aimed at improving patient outcomes by providing more effective and targeted treatments for lung cancer. Investigating these drug combinations not only aims to boost the effectiveness of current therapies, but also tackles the persistent issue of drug resistance in cancer treatment, which poses a significant obstacle to successful long-term disease management.
2. Materials and Methods
2.1. Drugs
The drugs 5-FU, Sildenafil, Tezosentan, Levosimendan, and Resveratrol were purchased from Merck Life Sciences (Algés, Portugal).
2.2. Cell Culture
A549 adenocarcinomic human alveolar basal epithelial cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco®, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco®, Grand Island, NY, USA) and 1% (v/v) penicillin/streptomycin (Sigma-Aldrich®, Steinheim, Germany). Cells were maintained in a 37 °C environment with 95% humidified air and 5% CO2. Cultures were grown in monolayers in T25 cm2 flasks (Thermo-Scientific®, Waltham, MA, USA), with the medium being replaced every 2–3 days. When the cells reached 80% confluence, they were subcultured using 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA, Sigma-Aldrich®, Steinheim, Germany).
2.3. In Vitro Drug Treatment
The antineoplastic potential of 5-FU and three repurposed drugs (Sildenafil, Tezosentan, and Levosimendan) was evaluated in the A549 cell line. Cells were treated with drug concentrations varying from 0.1 to 100 μM for 48 h and cell viability was assessed using the MTT assay. Based on these results, a dose–response curve was generated, and the half-maximal inhibitory concentration (IC50) value was determined. IC50 values greater than 100 μM were excluded from consideration.
2.4. Cell Viability Assay
For the experiments, A549 cells were seeded into 96-well plates at a density of 5000 cells/well, with a total volume of 200 µL per well. The cells were allowed to adhere overnight to ensure proper attachment to the well surface. After 24 h, the culture medium was carefully aspirated from each well. Then, 200 µL of medium containing the desired drug was added to each well, exposing the cells to the treatment. The cells were incubated in the drug-containing medium for 48 h to assess cytotoxic effects.
Following the 48 h incubation, an MTT assay was conducted to evaluate the impact of the treatments on both protein synthesis and cell viability. In this assay, an MTT reagent solution was added to each well and the cells were incubated further to enable viable cells to convert MTT into a purple formazan compound. The formazan crystals were subsequently dissolved and the absorbance of the solution was measured at 570 nm using a spectrophotometer (Sinergy HT, Biotek Instruments Inc., Winooski, VT, USA). The absorbance readings correspond to the level of cell viability.
2.5. Microscopic Observation
Prior to conducting the MTT assays, the cells were observed under a Leica DMI 6000B microscope equipped with a Leica DFC350 FX camera (Leica Microsystems, Wetzlar, Germany). Images were captured and analyzed using the Leica LAS X imaging software (v3.7.4, Leica Microsystems, Wetzlar, Germany).
2.6. Wound Healing Assay
To evaluate cell motility, a wound-healing assay was performed. Silicone ibidi inserts were placed in a 12-well plate, creating a defined gap between the cell layers. Around 9 × 105 cells were plated into each side of the insert, with a total volume of 70 µL per well, allowing for 24 h of cell adhesion. Once the insert was removed, a gap or “wound” was formed between the cell layers. The wells were rinsed twice with PBS to remove any non-adherent cells and the remaining cells were treated with drugs for 48 h under controlled conditions (37 °C, 95% humidity, and 5% CO2). Images of the wound area were taken at 0, 24, and 48 h under 100× magnification. The percentage of wound closure was calculated by measuring the remaining gap at each time point and normalizing it to the initial wound area (0 h time point) using ImageJ software version 1.53 (FIJI, National Institutes of Health, Bethesda, MD, USA).
2.7. Clonogenic Assay
For the clonogenic assay, A549 cells were plated at a density of 100 cells per well in 6-well plates, with three replicates, once they had reached around 80% confluence. After an overnight incubation, the cells were treated with or without drugs for 48 h. The cultures were then maintained at 37 °C with 5% CO2 for a total of 14 days, with the culture medium being refreshed every 2 days, excluding the drug treatments. At the end of the incubation period, the cells were fixed and stained with 0.5% (v/v) crystal violet solution. Colonies were counted only if they were visible, had a diameter greater than 0.5 mm and did not overlap with other colonies.
2.8. Statistical Analysis
The data presented were derived from a minimum of three independent experiments. The results are reported as the mean ± standard deviation (M ± SD). Statistical analysis was conducted using one-way ANOVA, followed by Student’s t-test for comparing control and treated cells. Differences were considered statistically significant at p ≤ 0.05, with a 95% confidence level. All analyses were performed using GraphPad Prism version 9 (GraphPad Software, San Diego, CA, USA). Significant results are indicated with the following symbols: * Statistically significant at p < 0.1. ** Statistically significant at p < 0.01. *** Statistically significant at p < 0.001. **** Statistically significant at p < 0.0001.
3. Results
3.1. The Effect of 5-FU as Single Agent on A549 Cellular Viability
In the previous studies of our group, we developed a new combination model involving both antineoplastic and repurposed drugs [46,47,48].
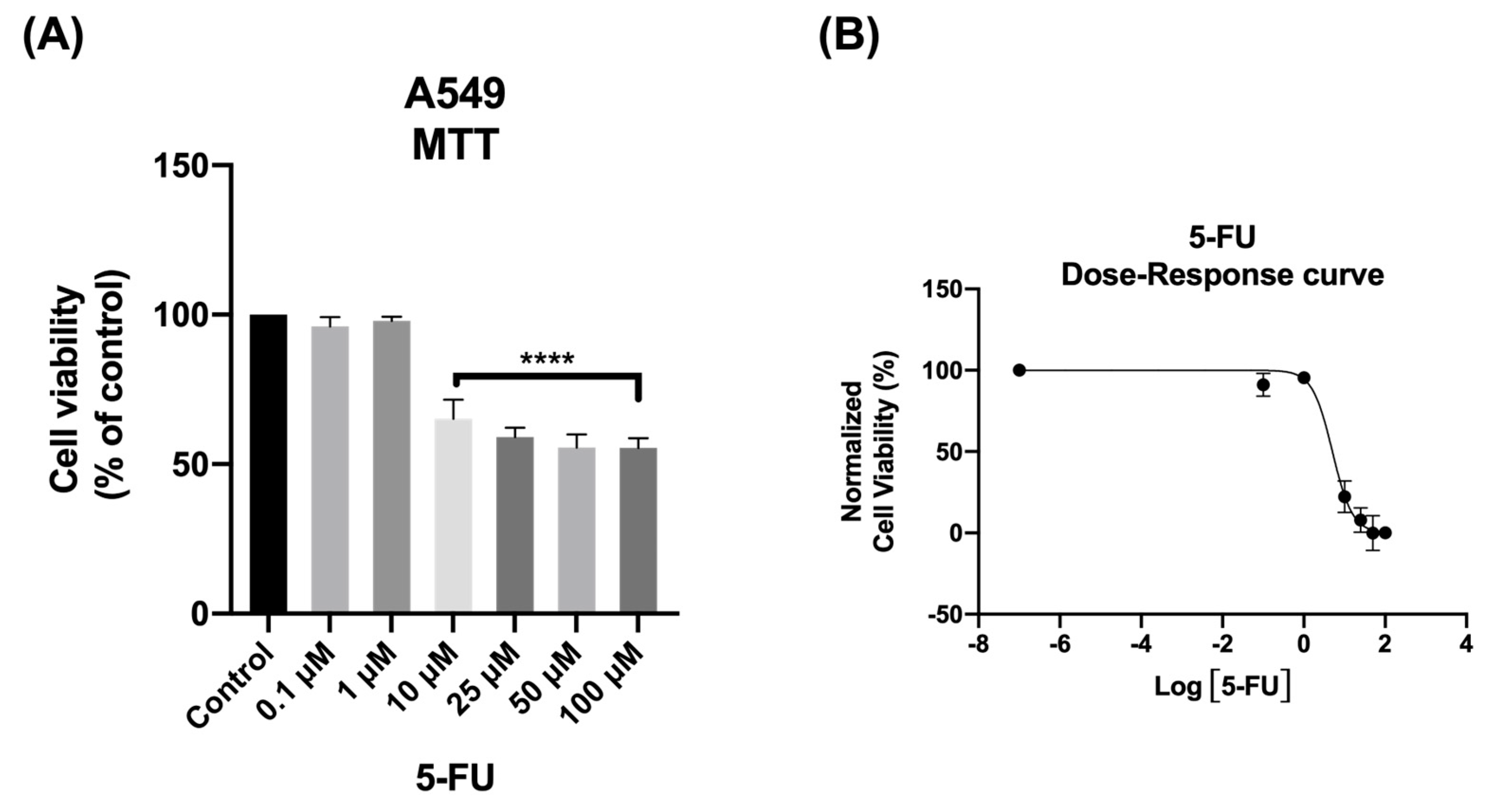
We start by evaluating the cytotoxic effect of the antineoplastic drug 5-FU on A549 cells, to verify its effectiveness against this type of cancer. The cells were exposed with different concentrations of 5-FU, ranging from 0.1 μM to 100 μM, for a duration of 48 h. The cell survival was measured using the MTT assay, which assesses mitochondrial activity, and is commonly used to determine cell viability. Cellular morphology was also observed after 48 h.
As shown in Figure 1A, the MTT assay results demonstrated that 5-FU exhibited significant anticancer activity at concentrations above 10 μM. The morphological analysis further confirmed the toxic profile of 5-FU. Consistent with the results obtained from the MTT assay, the analysis of cellular morphology revealed significant changes in the phenotype of A549 cells when exposed to concentrations above 10 μM, as well as a decrease in cell density upon 5-FU treatment.
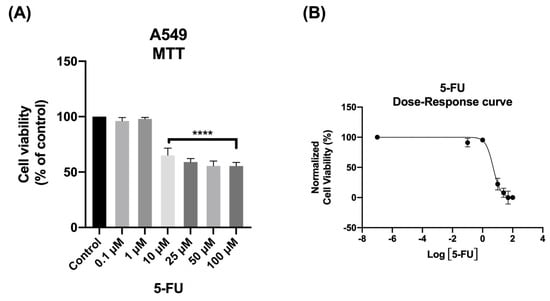
Figure 1.
Viability of A549 cells following treatment with 5−FU. The cells were exposed to different concentrations of 5−FU (0.1−100 μM) and after 48 h, the MTT assay was performed to assess cell viability. (A) Cell viability and (B) dose–response curve. Data are presented as percentages relative to the control group and represent the mean ± SD. Each experiment was performed independently three times (n = 3); **** statistically significant vs. control at p < 0.0001.
To gain a clearer understanding of the dose–response relationship of 5-FU, a dose–response curve was generated using the data from the MTT assay (Figure 1B). From this curve, the IC50 value, representing the concentration of 5-FU needed to reduce cell growth of A549 cells by 50%, was calculated. This value offers a quantitative measure of the drug’s potency in inhibiting cancer cell proliferation. Interestingly, even at concentrations below 6 μM, approximately 50% of the cells were already affected by 5-FU’s cytotoxicity. Based on these results, a concentration of 5.03 µM of 5-FU for 48 h was selected for further experiments with A549 cells (Table 1).

Table 1.
IC50 values of 5-FU, Sildenafil, Tezosentan, and Levosimendan in A549 cells.
The IC50 value obtained in our study is consistent with the variability observed in the literature, where different studies have reported varying IC50 values for the same cell line [49,50,51,52]. This variability may be due to differences in experimental conditions, assay techniques, and the characteristics of the cell lines.
3.2. The Effect of Sildenafil as a Single Agent and in Various Combinations with 5-FU
In this study, A549 cells were treated with increasing concentrations of Sildenafil (1 µM to 100 µM), either alone or in combination with 5-FU at various concentrations. According to Table 1, the IC50 of 5-FU was tested in combination with increasing concentrations of the vasodilator (0.1, 1, 10, 25, 50, and 100 μM). After 48 h, cell viability was measured using the MTT assay, as described previously, and the results were recorded. Additionally, morphological evaluations were performed to observe any visible changes in the cells.
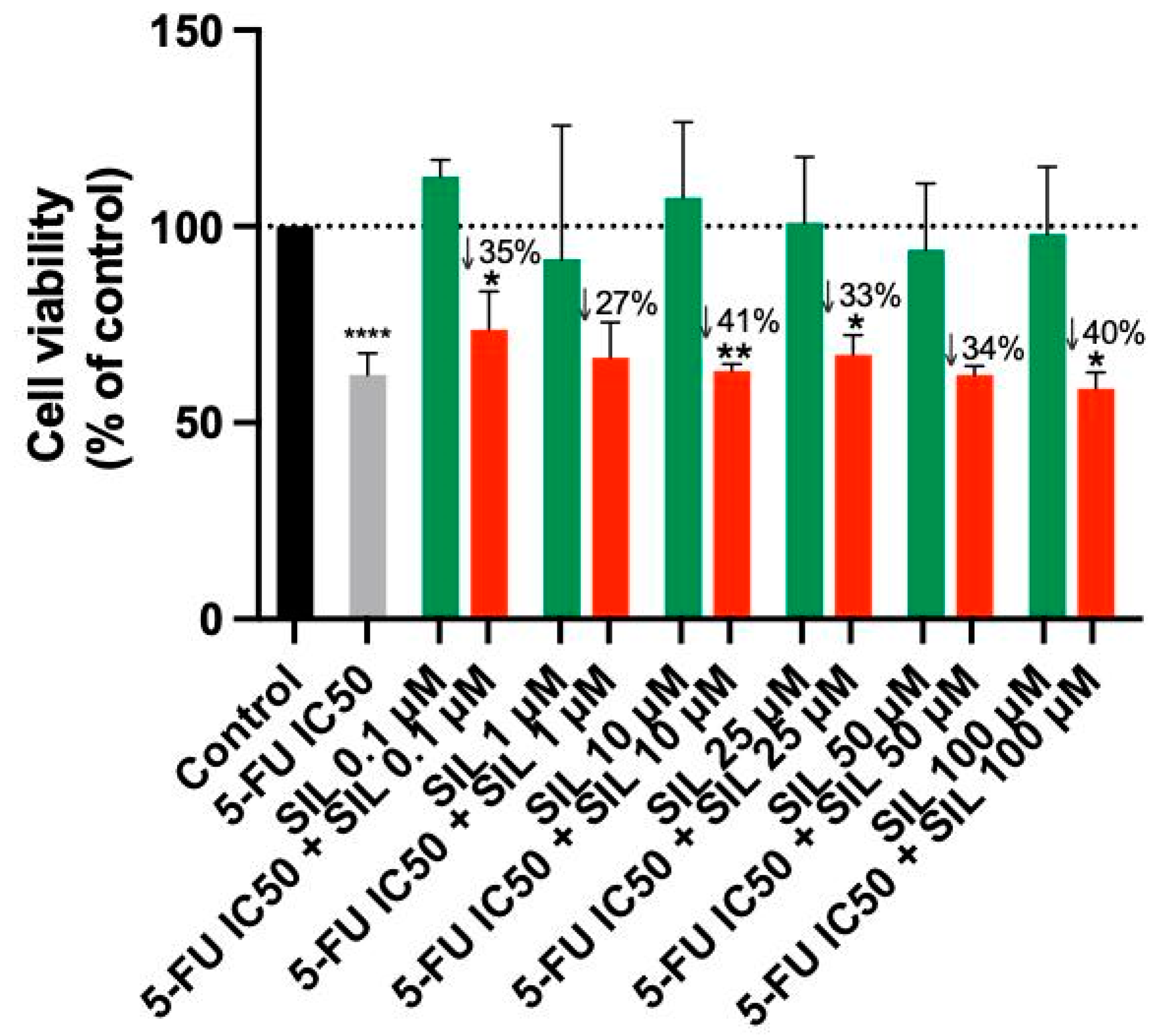
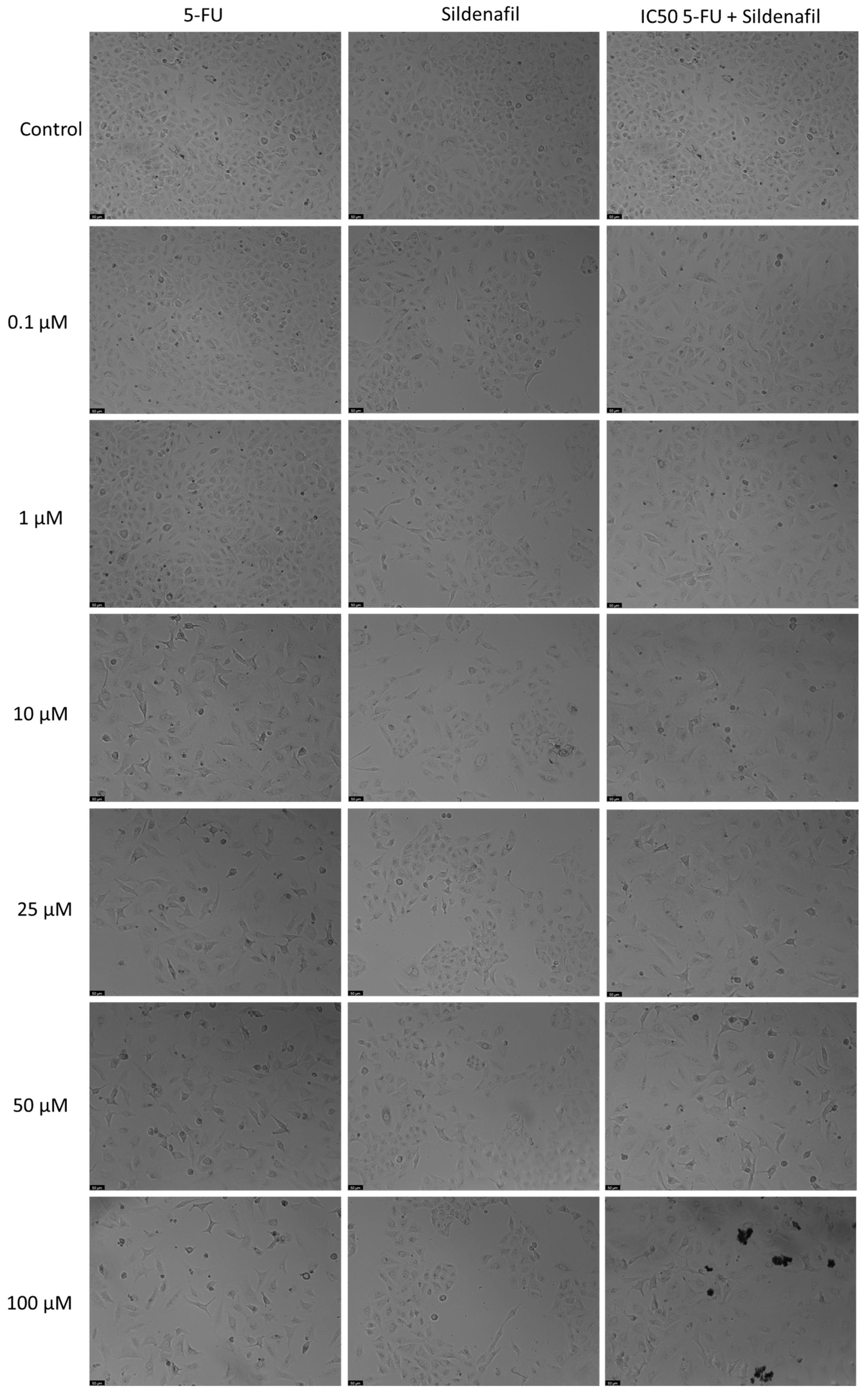
Sildenafil alone showed no significant anticancer activity at any of the tested concentration, as shown by the MTT assay (Figure 2). However, combining 5-FU with Sildenafil at 0.1 µM, 10 µM, 25 µM, and 100 µM resulted in a notable reduction in cell viability compared to Sildenafil alone, with up to a 41% decrease observed. Furthermore, the morphological analysis confirmed that only 5-FU was active in this cell line, as Sildenafil alone had no impact on the appearance of the cells (Figure 3). Morphological changes were only evident when 5-FU was combined with Sildenafil, suggesting that these changes were caused by the antineoplastic activity of 5-FU (Figure 3).
Figure 2.
Cell viability of A549 cells following treatment with Sildenafil alone and in combination with 5-FU. Data are presented as percentages relative to the control group and represent the mean ± SD. Each experiment was performed independently three times (n = 3); * statistically significant vs. drug alone at p < 0.05; ** statistically significant vs. drug alone at p < 0.01; **** statistically significant vs. control at p < 0.0001. The green, red, black and gray bars are used solely to differentiate between data groups and do not represent specific experimental variables.
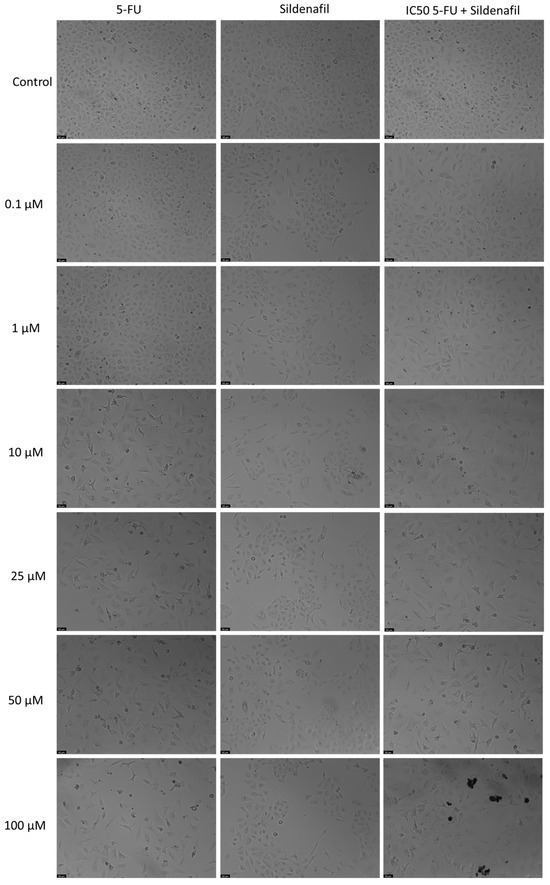
Figure 3.
Morphological assessment of A549 cells treated with 5-FU, Sildenafil alone, and their combination. Cells were exposed to the vehicle (0.1% DMSO) as a control. The results are representative of three separate experiments. Scale bar: 50 µm.
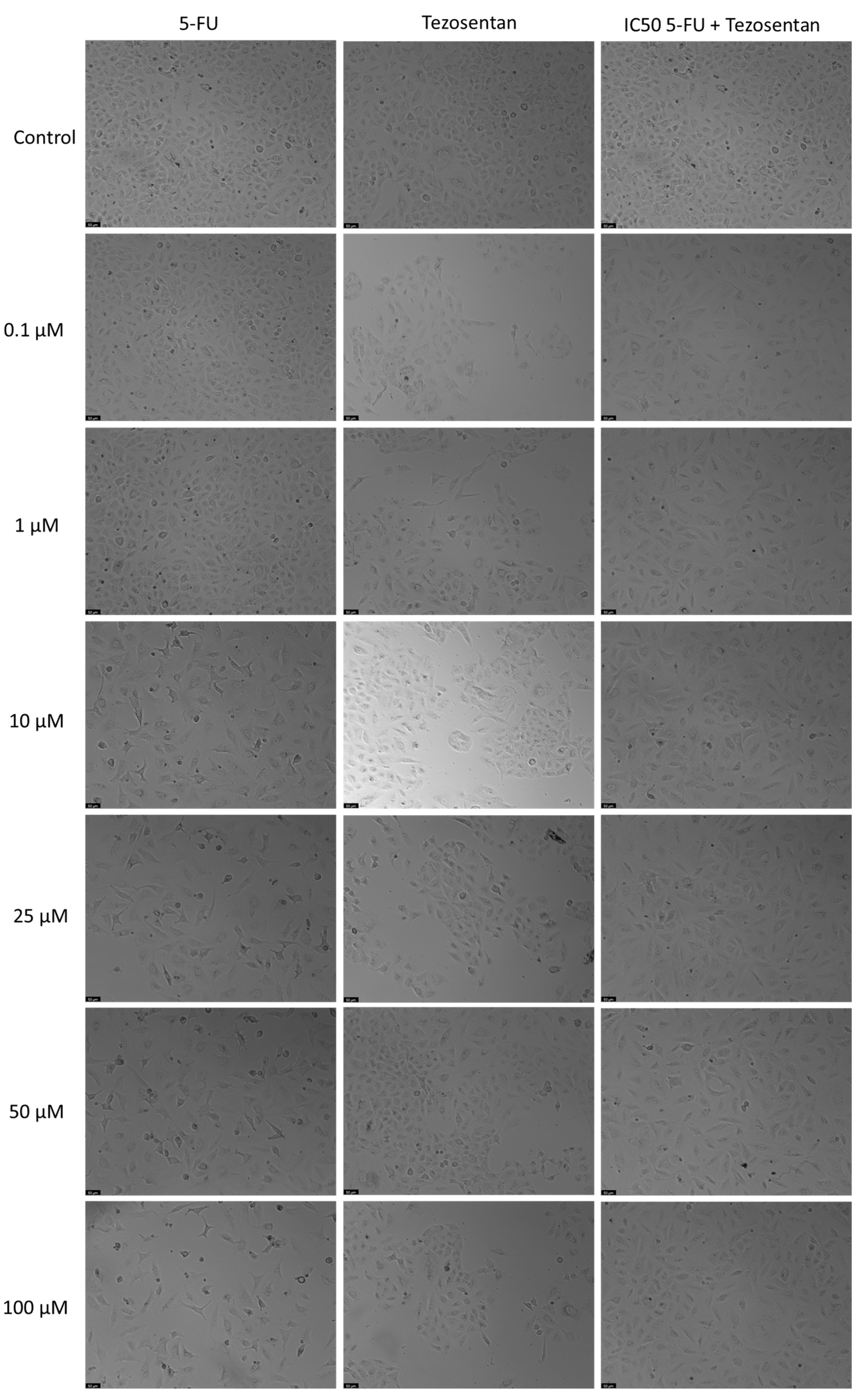
3.3. The Effect of Tezosentan as a Single Agent and in Various Combinations with 5-FU
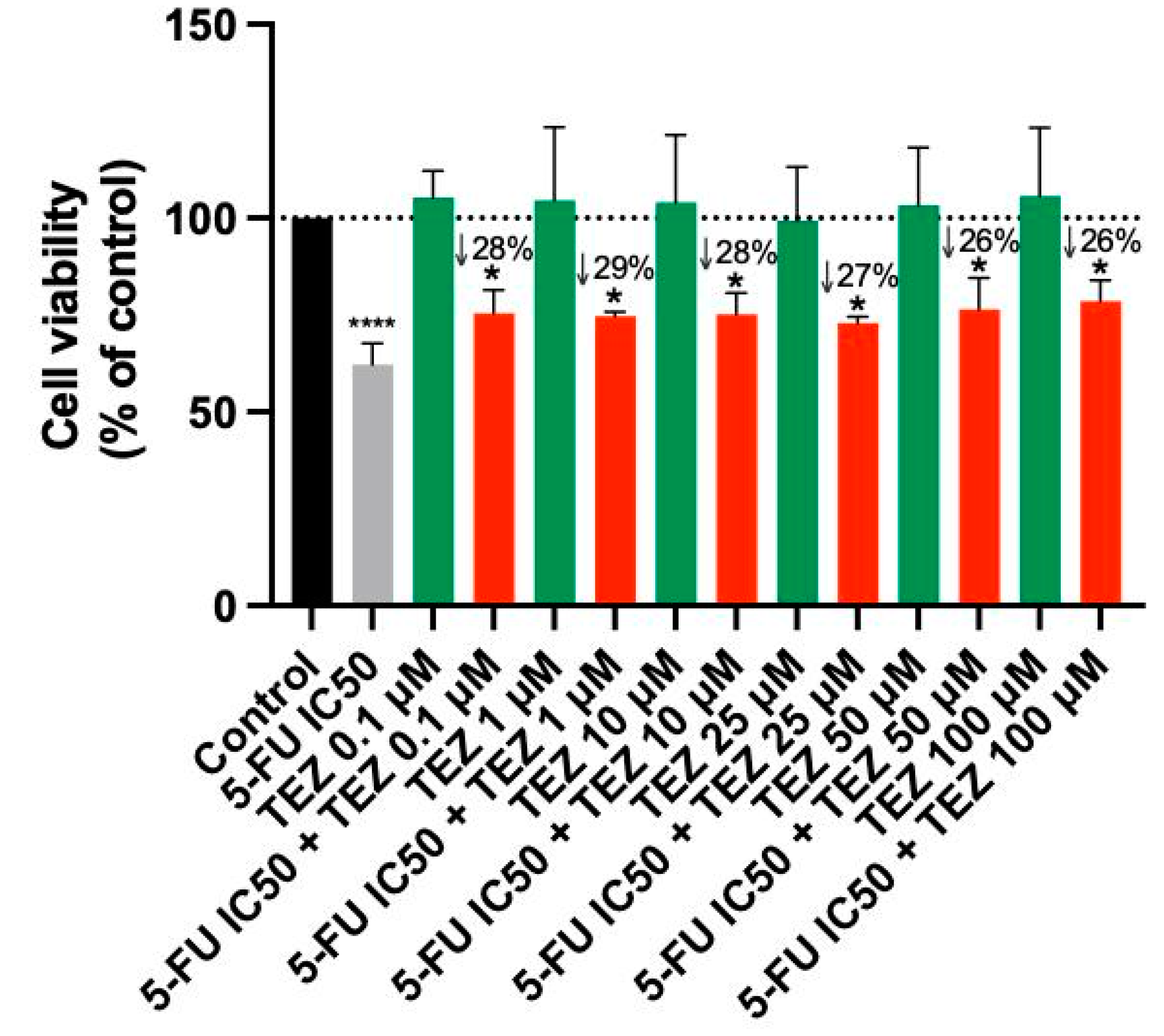
Treatment of A549 cells with Tezosentan did not exhibit any significant anticancer effects at any concentration, according to the MTT assay results (Figure 4). The combination of Tezosentan with 5-FU also failed to show notable cytotoxicity compared to 5-FU alone. However, the combination did result in significant reductions in cell viability across all tested concentrations, with decreases ranging from 26% to 28% compared to Tezosentan alone. Furthermore, morphological evaluations revealed that 5-FU was the sole active agent in this cell line, as Tezosentan did not induce any changes in cell phenotype. Observable morphological alterations were only seen in the combinations that included 5-FU, implying that these changes were attributed to the effects of the antineoplastic drug 5-FU (Figure 5).
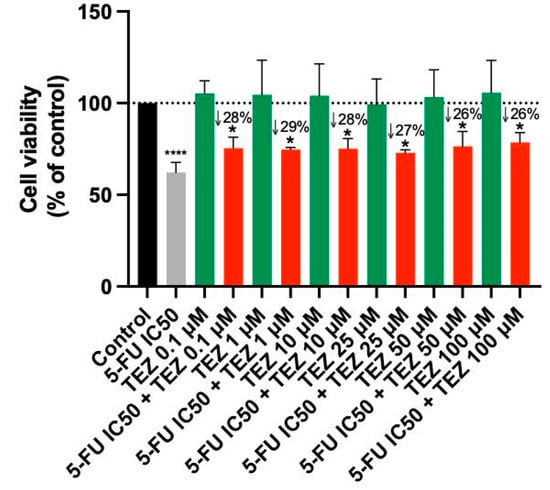
Figure 4.
Cell viability of A549 cells following treatment with Tezosentan alone and in combination with 5-FU. Data are presented as percentages relative to the control group and represent the mean ± SD. Each experiment was performed independently three times (n = 3); * statistically significant vs. drug alone at p < 0.05; **** statistically significant vs. control at p < 0.0001. The green, red, black and gray bars are used solely to differentiate between data groups and do not represent specific experimental variables.
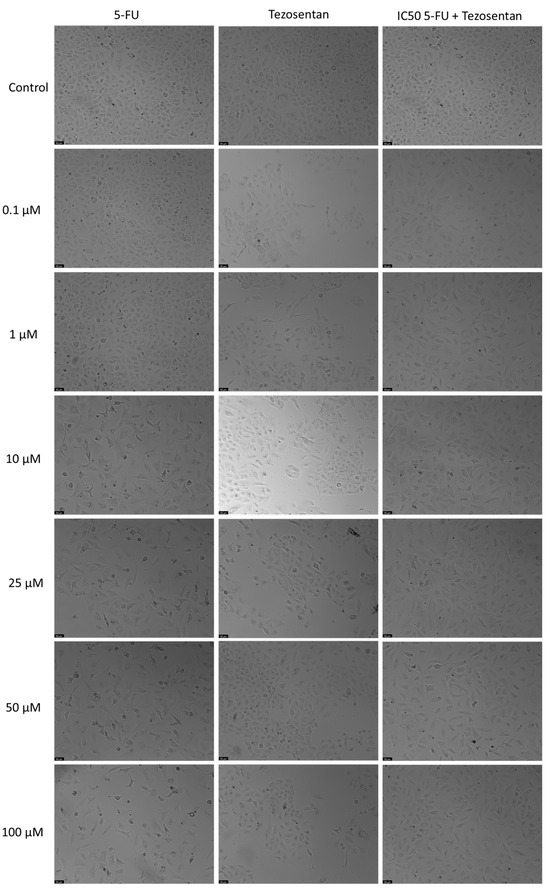
Figure 5.
Morphological assessment of A549 cells treated with 5-FU, Tezosentan alone, and their combination. Cells were exposed to the vehicle (0.1% DMSO) as a control. The results are representative of three separate experiments. Scale bar: 50 µm.
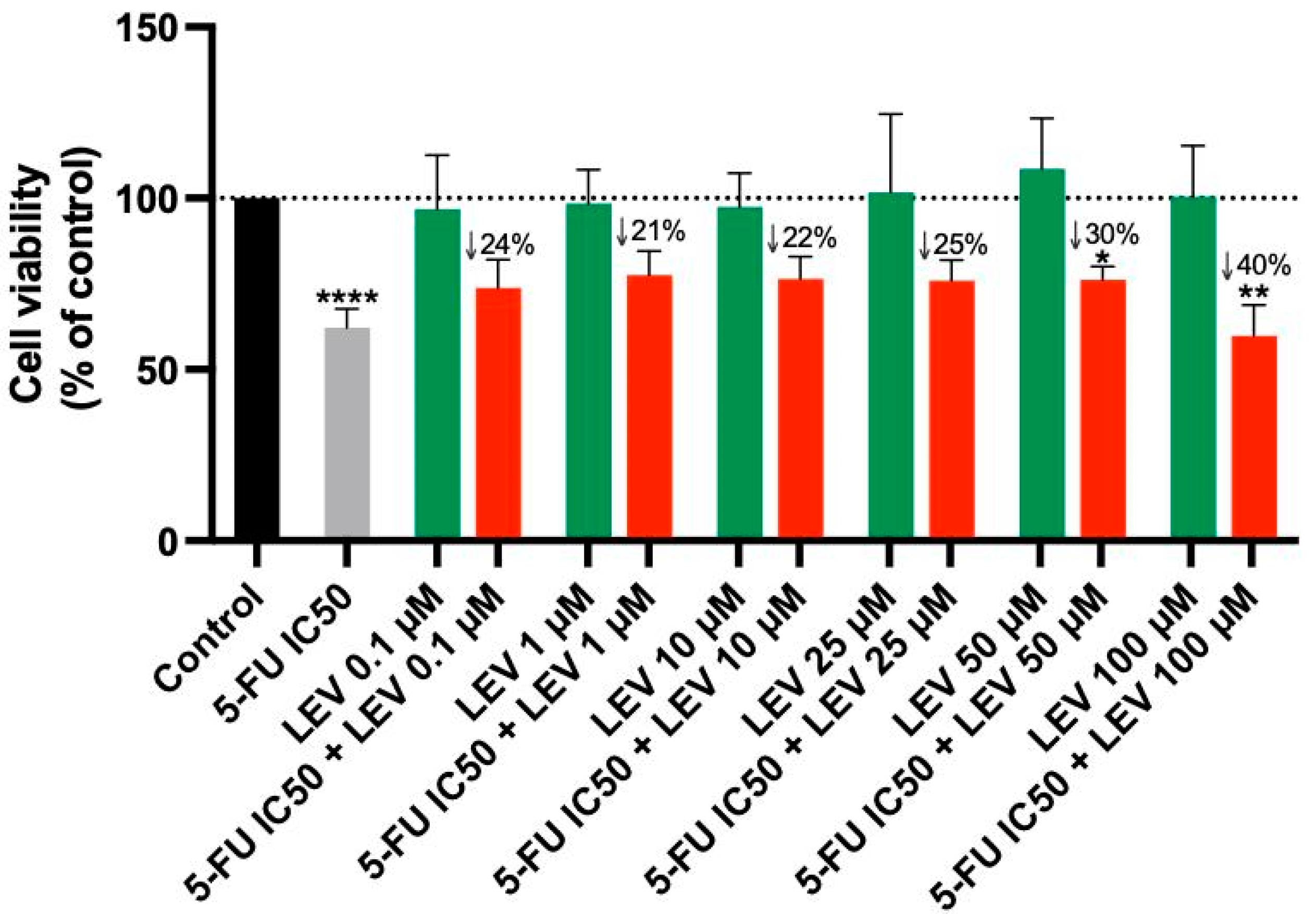
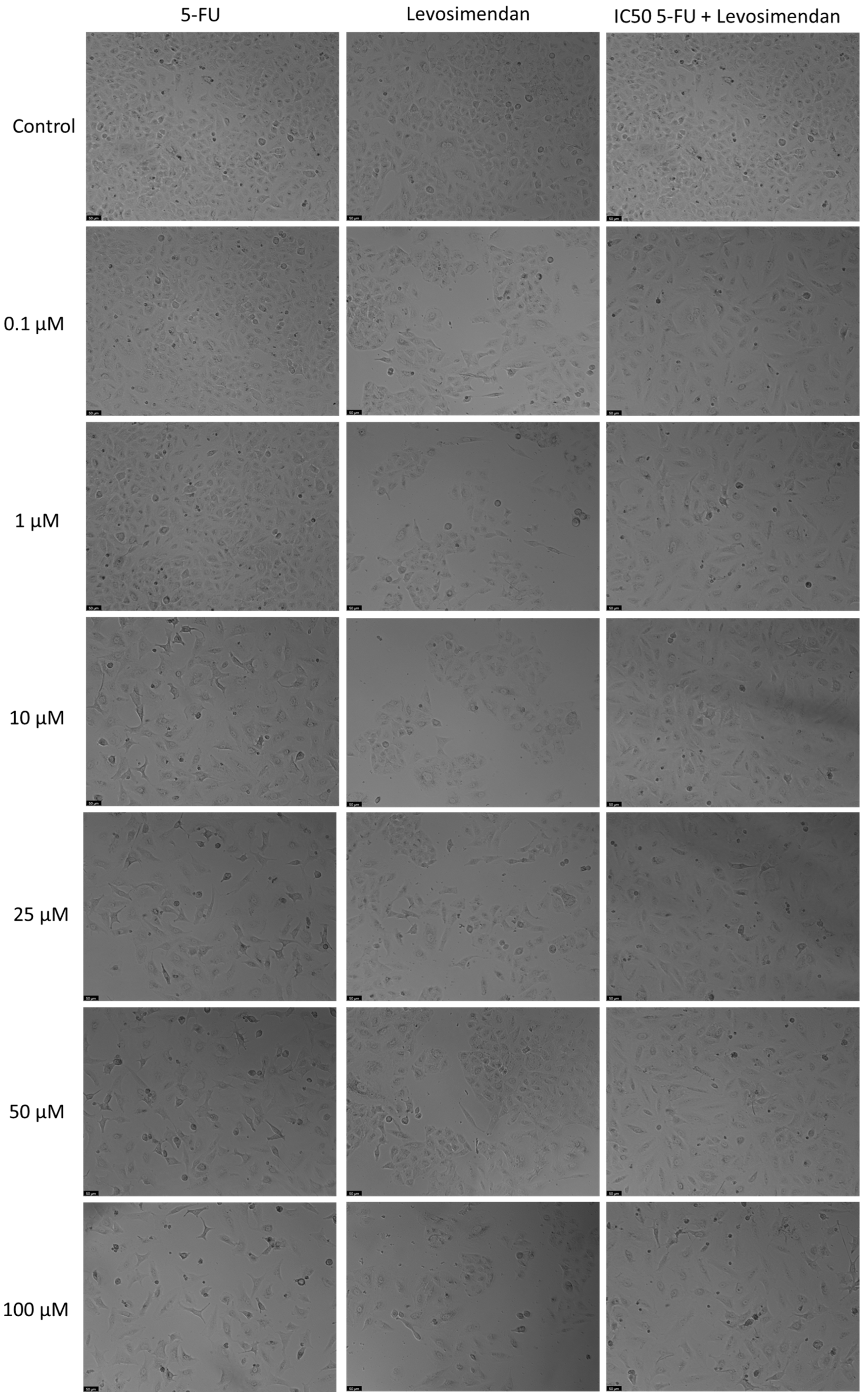
3.4. The Effect of Levosimendan as a Single Agent and in Various Combinations with 5-FU
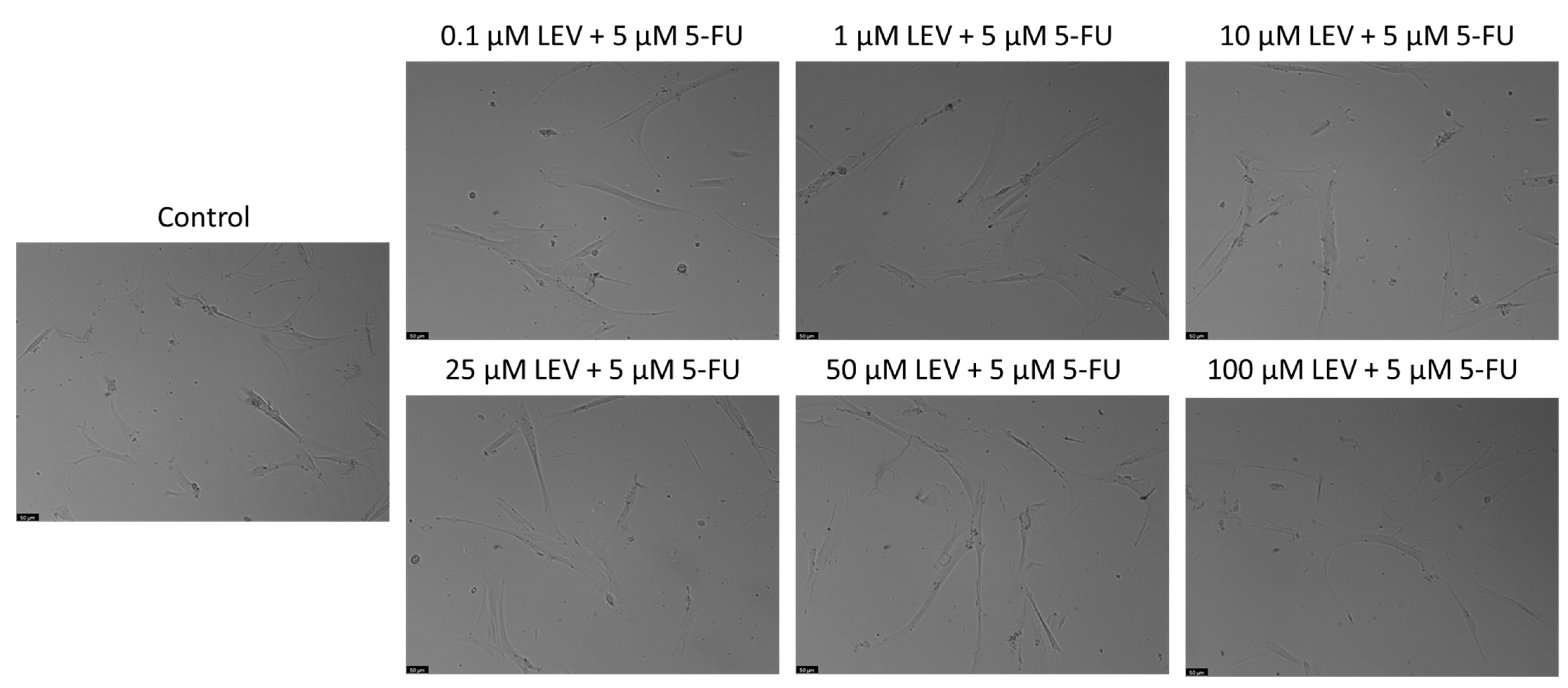
For the A549 cell line, Levosimendan did not exhibit significant anticancer activity at any concentration, as indicated by the MTT assay results (Figure 6). When combined with 5-FU, Levosimendan also did not show significant cytotoxic effects compared to its effects alone, except at the highest concentrations (50 and 100 µM), where there was a notable reduction of up to 40% in cell viability compared to Levosimendan alone. Morphological assessments revealed that these changes were primarily due to the effects of the antineoplastic drug 5-FU, since Levosimendan alone did not impact the cell phenotype (Figure 7).
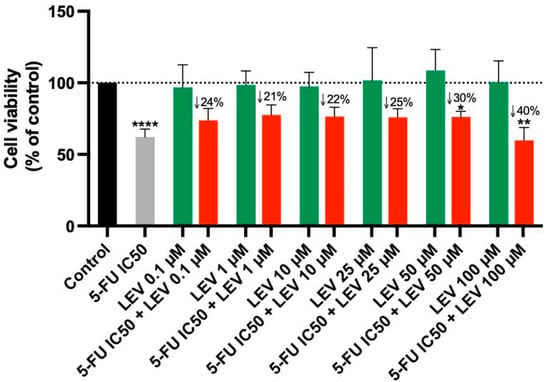
Figure 6.
Cell viability of A549 cells following treatment with Levosimendan alone and in combination with 5-FU. Data are presented as percentages relative to the control group and represent the mean ± SD. Each experiment was performed independently three times (n = 3); * statistically significant vs. drug alone at p < 0.05; ** statistically significant vs. drug alone at p < 0.01; **** statistically significant vs. control at p < 0.0001. The green, red, black and gray bars are used solely to differentiate between data groups and do not represent specific experimental variables.
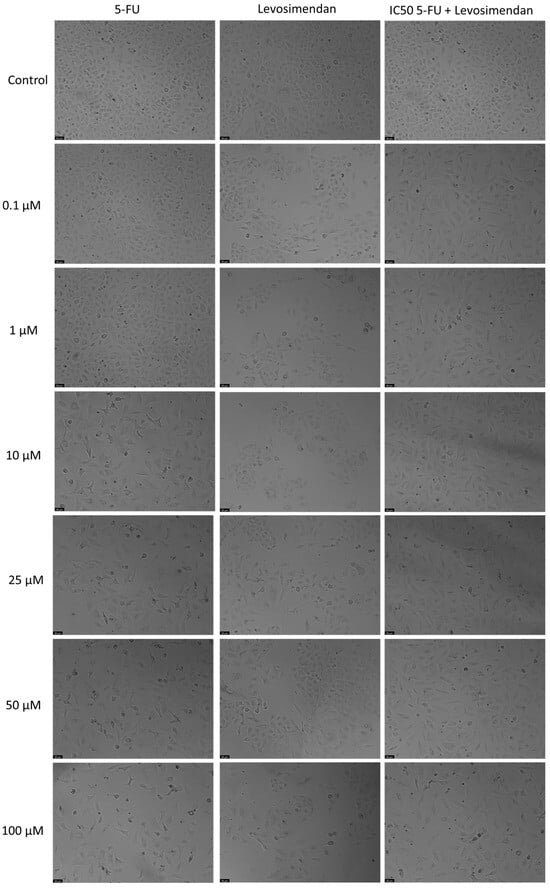
Figure 7.
Morphological assessment of A549 cells treated with 5-FU, Levosimendan alone, and their combination. Cells were exposed to the vehicle (0.1% DMSO) as a control. The results are representative of three separate experiments. Scale bar: 50 µm.
3.5. Safety Assessment of 5-FU and Levosimendan and Their Combination in Non-Cancerous MRC-5 Cells
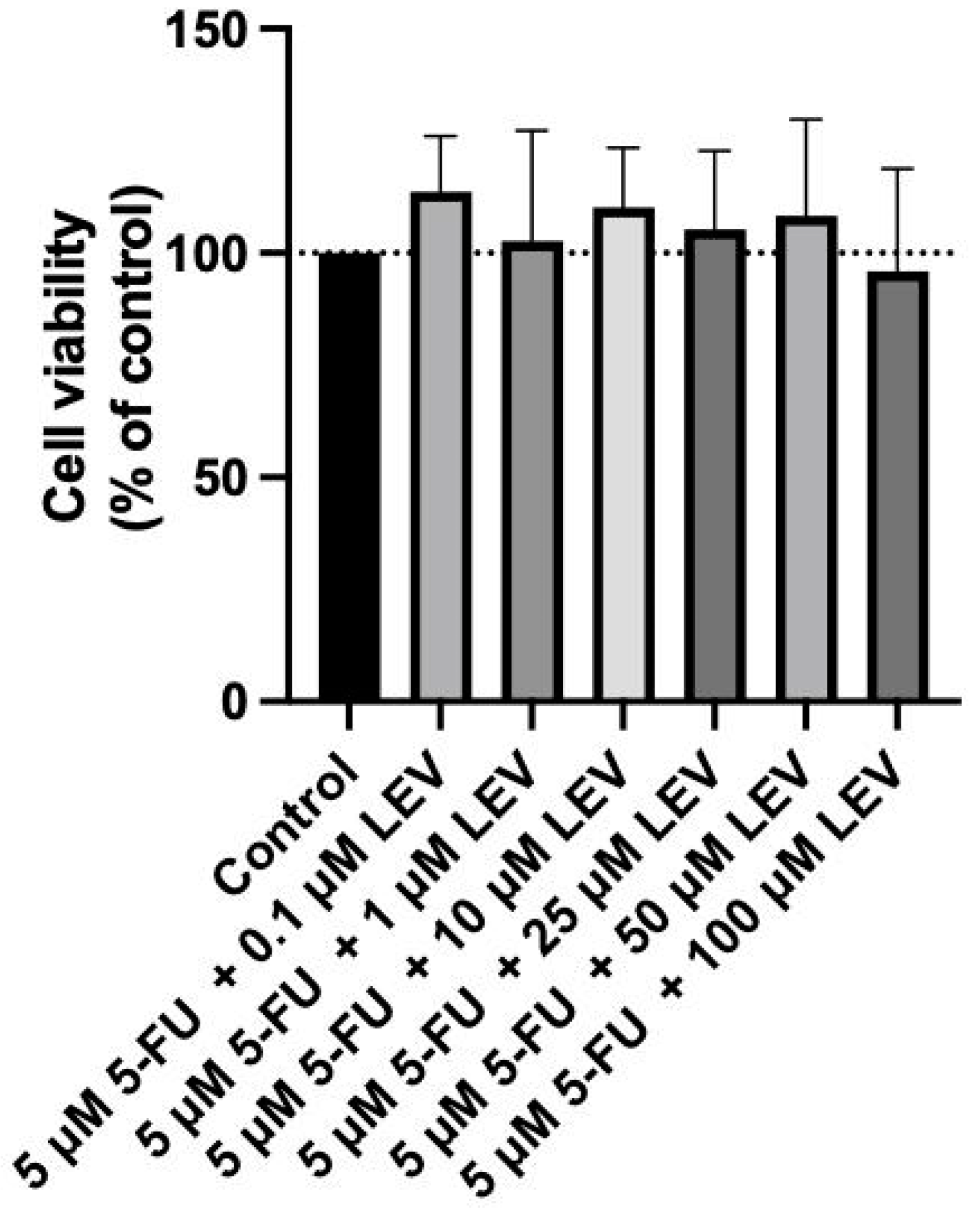
In a previous study of our group [41], we thoroughly evaluated the safety of 5-FU and Levosimendan over 24, 48, and 72 h, assessing their cytotoxicity both as single agents and in combination, using non-cancerous MRC-5 cells. The results demonstrated that neither 5-FU alone nor its combination with Levosimendan at the IC50 concentration for the A549 cell line exhibited any significant cytotoxic effects on normal cells. Specifically, no changes in cell viability or morphology were observed, even at higher concentrations (up to 50 µM) and across all tested time points. However, it is noteworthy that Levosimendan alone, at a concentration of 100 µM, caused approximately 25% toxicity in MRC-5 cells after 48 h of exposure. Despite this level of cytotoxicity, the morphology of the MRC-5 cells remained unaffected at these time points.
For the current study, we extended this investigation by testing the combination of the IC50 value of 5-FU determined for A549 cells with increasing concentrations of Levosimendan in MRC-5 cells. This assessment aims to further confirm the non-toxic nature of this drug combination in non-cancerous cells, reinforcing the potential of Levosimendan as a safe candidate for combination therapy with 5-FU. As indicated in Figure 8, the combination of the IC50 5-FU for the A549 cell line with increasing concentrations of Levosimendan did not indicate cytotoxicity towards normal MRC-5 cells and did not result in any morphological changes in these cells, as corroborated by the observations in Figure 9.
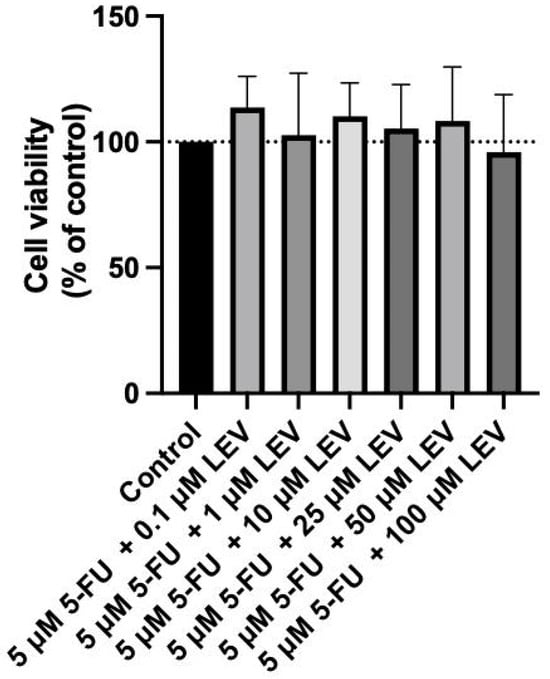
Figure 8.
Biosafety assessment of 5 µM 5-FU combined with Levosimendan in the MRC-5 cell line. MRC-5 cells were treated with 0.1% DMSO (control group), and with increasing concentrations (0.1–100 µM) of Levosimendan combined with the IC50 5-FU for 48 h. Results are presented as percentages of control and represent the mean ± SD. Each experiment was conducted independently three times (n = 3).
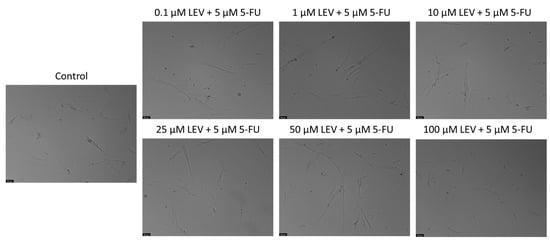
Figure 9.
Morphological assessment of A549 cells treated with increasing concentrations of Levosimendan combined with IC50 5-FU. Cells were exposed to the vehicle (0.1% DMSO) as a control. The results are representative of three separate experiments. Scale bar: 50 µm.
3.6. Transient Inhibition of Lung Cancer Cell Migration by 5-FU and Levosimendan Combination
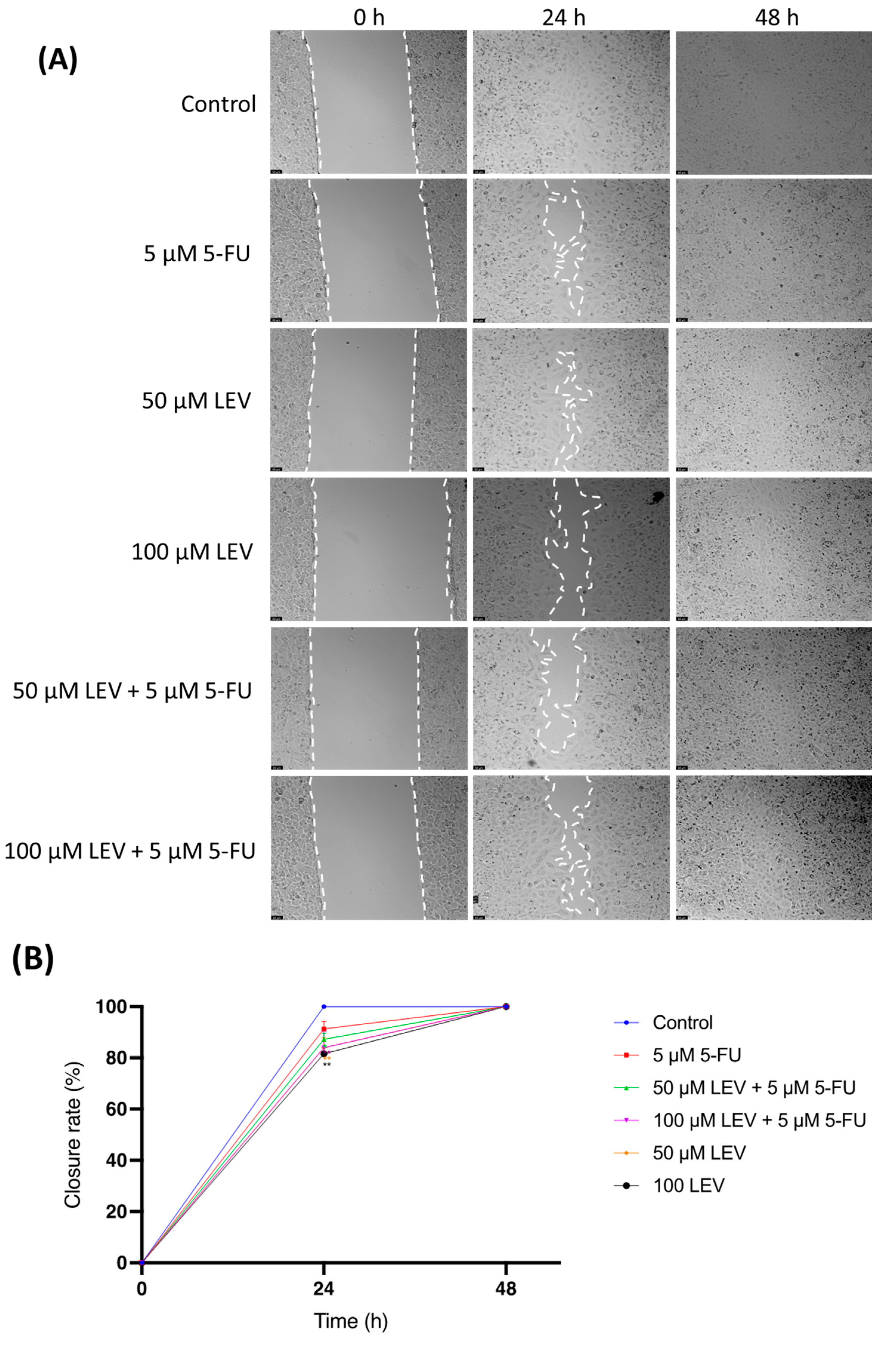
The impact of Levosimendan on cell motility was assessed using a wound-healing assay (Figure 10A,B). As illustrated in Figure 10, after 24 h, A549 cells treated with a combination of IC50 5-FU and Levosimendan showed a significant reduction in migration compared to untreated cells. However, by 48 h, this inhibitory effect on cell migration was no longer apparent, with the treated cells displaying a similar level of wound closure as the control group (Figure 10A). This indicates that while the combination of 5-FU and Levosimendan effectively impairs cell migration in the short term, this effect diminishes over time.
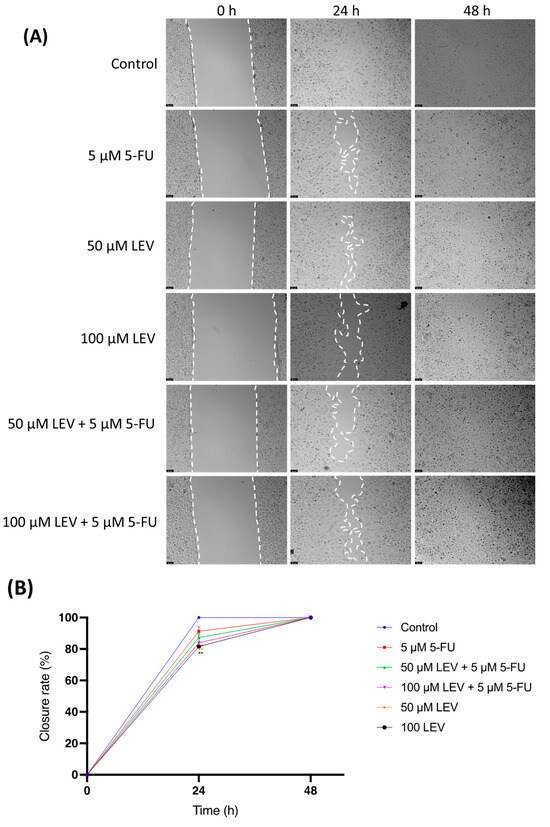
Figure 10.
Wound-healing assay for assessing cellular migration in human lung cancer cell line A549. (A) Representative images from in vitro wound healing assays, captured after drug treatment (0 h) and at 24 h and 48 h. These images are representative of three separate experiments conducted in duplicate. Scale bar: 50 µm. (B) Statistical analysis of the migration results from the images. Data are presented as percentages of the control group and represent the mean ± SD. Each experiment was performed independently three times (n = 3); * statistically significant vs. control at p < 0.5; ** statistically significant vs. control at p < 0.01. Dashed lines in (A) are used only to guide the identification of cell growth trends and do not represent specific values.
3.7. Levosimendan Enhances the Clonogenic Inhibition of 5-FU in Lung Cancer Cells
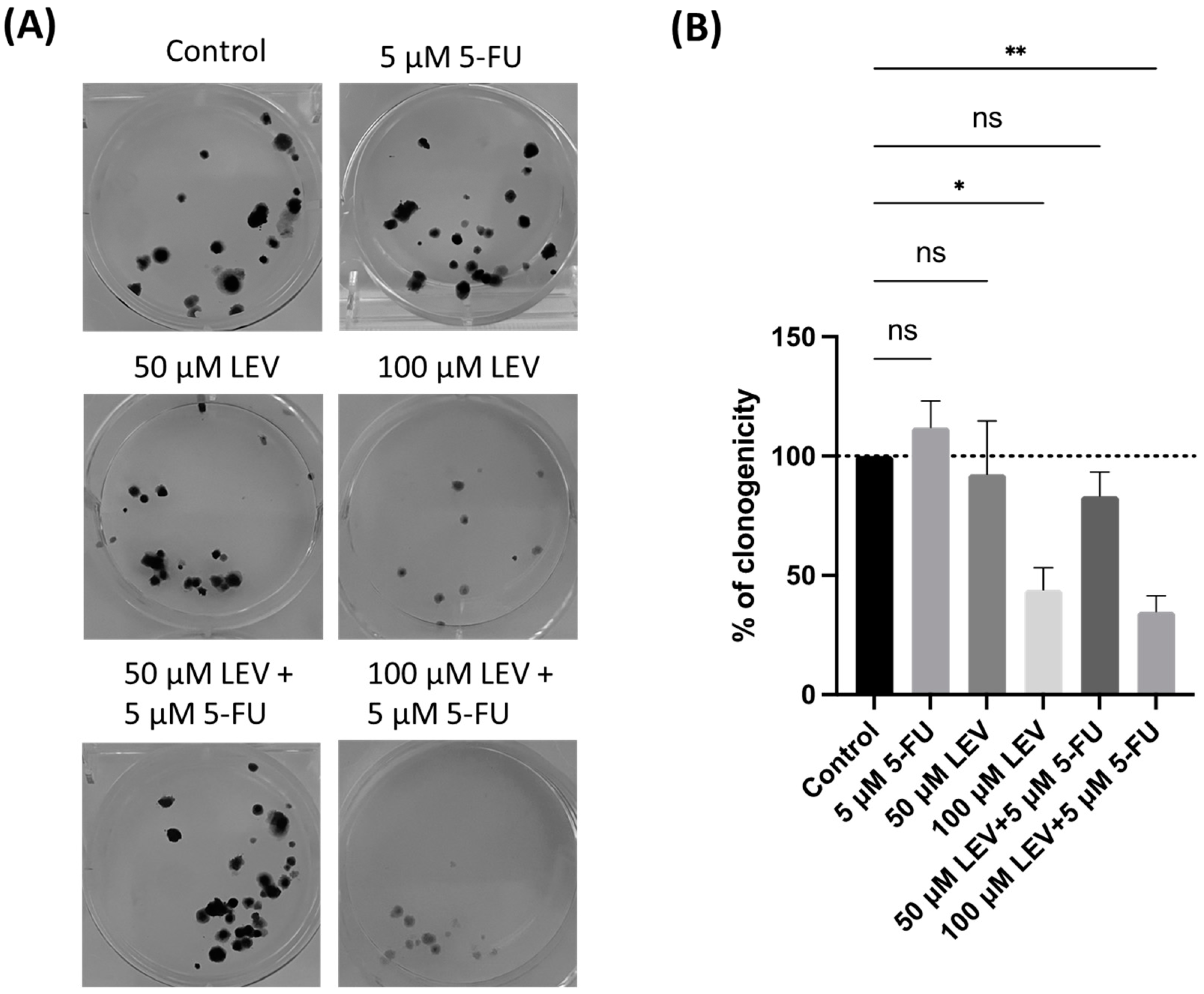
To further evaluate the ability of A549 cells for “unlimited” divisions, a clonogenic assay was conducted. In this assay, 100 A549 cancer cells per well were incubated with or without 5-FU and Levosimendan for 48 h. The results revealed that the combination of 5-FU and Levosimendan significantly decreased the clonogenic potential of A549 cells (Figure 11). This suggests that Levosimendan may have a more pronounced antitumor effect under certain conditions of cell proliferation. The reduction in colony formation underscores Levosimendan’s potential to enhance the effectiveness of 5-FU in targeting cancer cell growth.
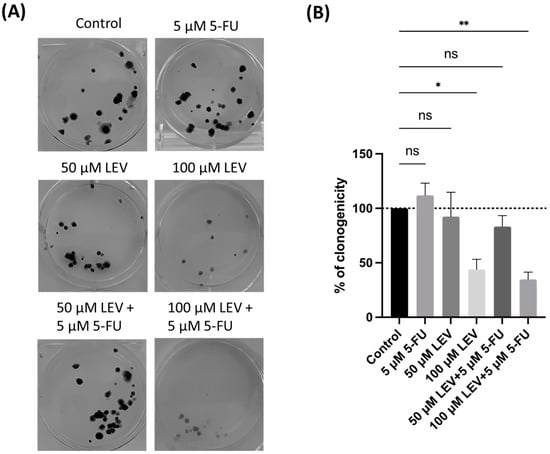
Figure 11.
Clonogenic assay of the human lung cancer cell line treated with IC50 concentration of 5-FU and Levosimendan, both alone and in combination. (A) Image displaying the colonies formed by the human cancer cell line after plating 100 cells and incubating for 14 days. (B) The number of colonies was quantified, with error bars representing the mean ± SD from three independent experiments. ns: non-significant; * statistically significant vs. control at p < 0.05; ** statistically significant vs. control at p < 0.01.
3.8. Impact of Resveratrol on the Cytotoxicity of Levosimendan and 5-FU in Lung Cancer Cells
In our previous experiments, Levosimendan did not exhibit significant anticancer effects in the A549 lung cancer cell line as it did in bladder and prostate cancer cell lines [41]. To enhance its antitumor activity, we explored the combination of Levosimendan with Resveratrol, a compound known for its potent anticancer properties. Resveratrol was specifically studied in the A549 cell line because it shares common receptors with this line, including B2 adrenergic [53,54] and EGFR receptors [55,56], which are implicated in cancer progression and treatment response. Additionally, given the established efficacy of 5-FU, we investigated whether Resveratrol could also augment the effects of 5-FU at its IC50 concentration in A549 cells.
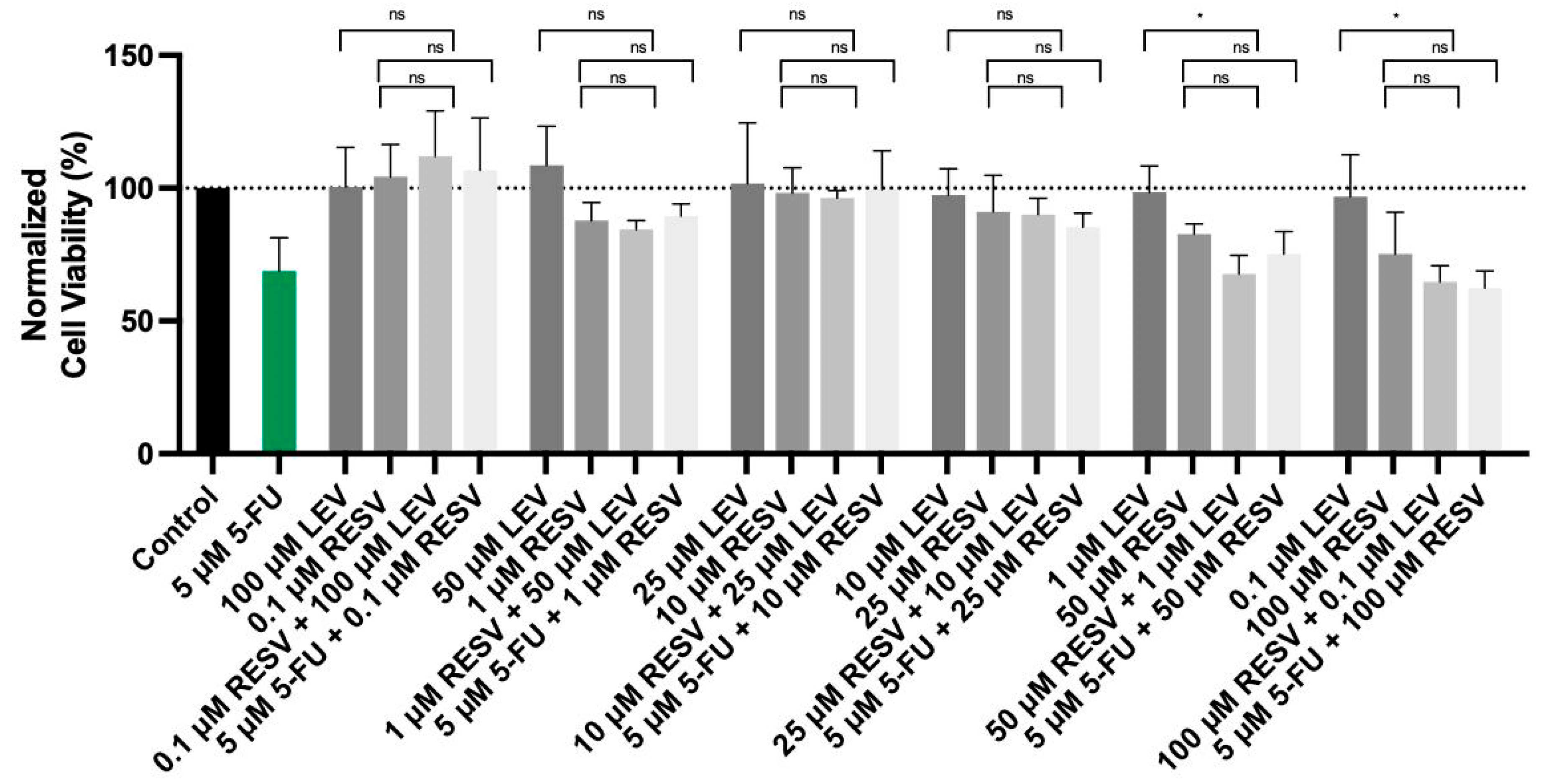
As shown in the accompanying data (Figure 12), the combination of Resveratrol with Levosimendan demonstrated a significant reduction in cell viability. Specifically, the combination of 50 µM Resveratrol with 1 µM Levosimendan led to a 31% decrease in cell viability compared to Levosimendan alone, while 100 µM Resveratrol combined with 0.1 µM Levosimendan resulting in a 33% reduction in cell viability. Moreover, when combining Resveratrol with the IC50 concentration of 5-FU, similar trends were observed. These findings suggest that Resveratrol enhances the cytotoxic effects of both Levosimendan and 5-FU, reinforcing its potential role as a synergistic agent in cancer therapy.
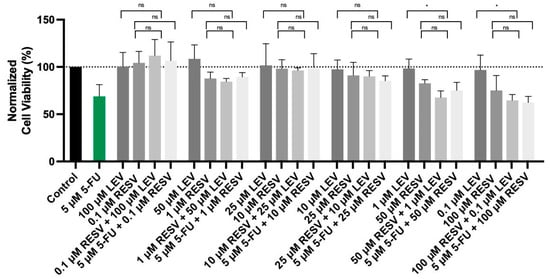
Figure 12.
Cell viability of A549 cells treated with the drugs Levosimendan and Resveratrol alone, in a combination of Levosimendan with Resveratrol, and Resveratrol with IC50 5-FU. Values are expressed as percentages of control and represent means ± SD. Each experiment was done three times independently (n = 3); ns: non-significant; * statistically significant vs. drug alone at p < 0.05. The different gray bars are used solely to differentiate between data groups and do not represent specific experimental variables.
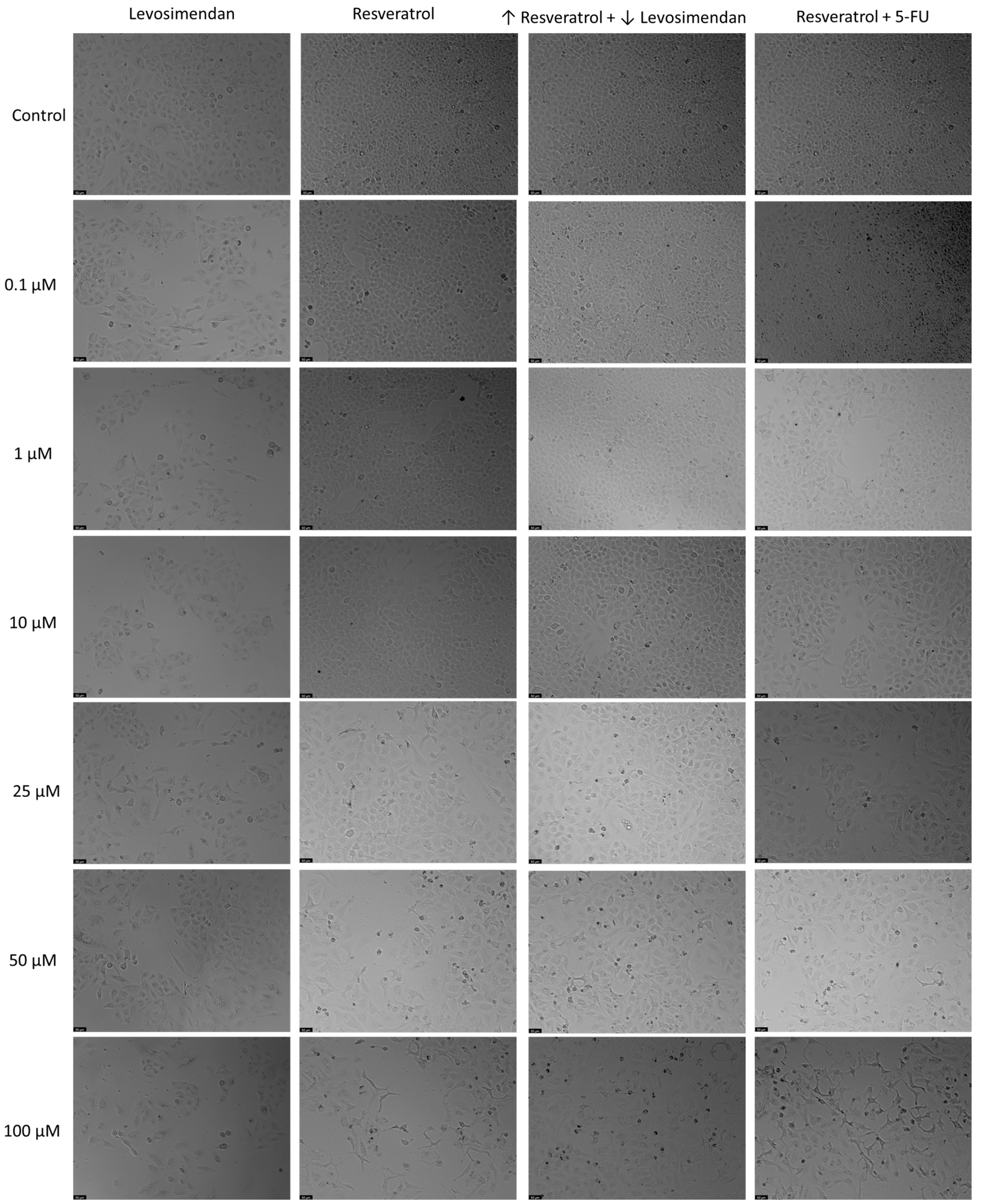
In relation to the morphological analysis (Figure 13), treatments with Resveratrol alone showed a visible reduction in the number of cells as the drug concentration increased, with noticeable alterations in cell phenotype at the highest concentration of Resveratrol. Similar observations were made with the combination of Resveratrol and the IC50 concentration of 5-FU. Furthermore, in the combination of Levosimendan with Resveratrol, a reduction in the number of cells was also evident as the concentration of Resveratrol increased and that of Levosimendan decreased.
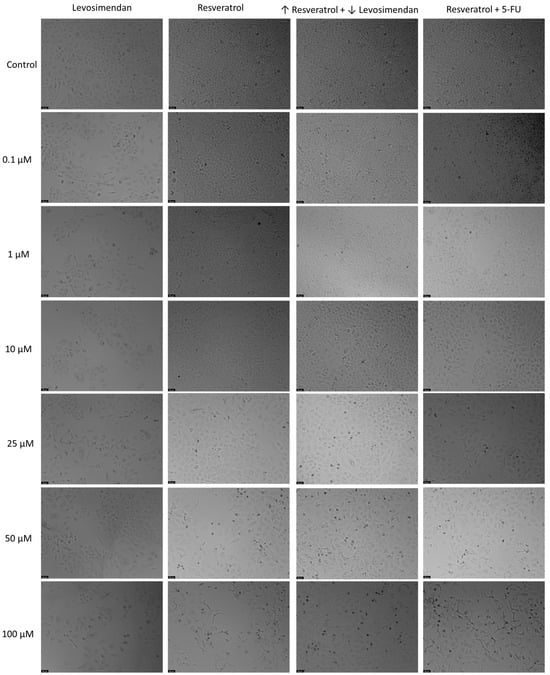
Figure 13.
Morphological analysis of Levosimendan and Resveratrol alone and in combination in A549 cells. Cells were treated with vehicle (0.1% DMSO). Results are representative of three independent experiments. Scale bar: 50 µm.
3.9. Synergistic Inhibition of Colony Formation by Resveratrol and 5-FU in A549 Cells
Based on the initial cell viability assays, Resveratrol significantly enhanced the cytotoxic effects of both 5-FU and Levosimendan in A549 cells. However, the combination of Resveratrol with 5-FU showed a more consistent and pronounced reduction in cell viability compared to its combination with Levosimendan. Given the well-established efficacy of 5-FU as a chemotherapeutic agent, we hypothesized that combining it with Resveratrol would offer a stronger foundation for investigating potential improvements in cancer treatment.
Levosimendan alone exhibited limited cytotoxicity in A549 cells. While the addition of Resveratrol enhanced this effect, the synergistic impact was not as significant as that observed with 5-FU. To maximize the potential therapeutic impact and explore the most promising combination, we decided to focus subsequent experiments, including the clonogenic assay, on the Resveratrol and 5-FU combination. This approach allowed us to better understand Resveratrol’s capacity to enhance the antitumor effects of a well-characterized chemotherapeutic drug, offering valuable insights into potential combination therapies for lung cancer.
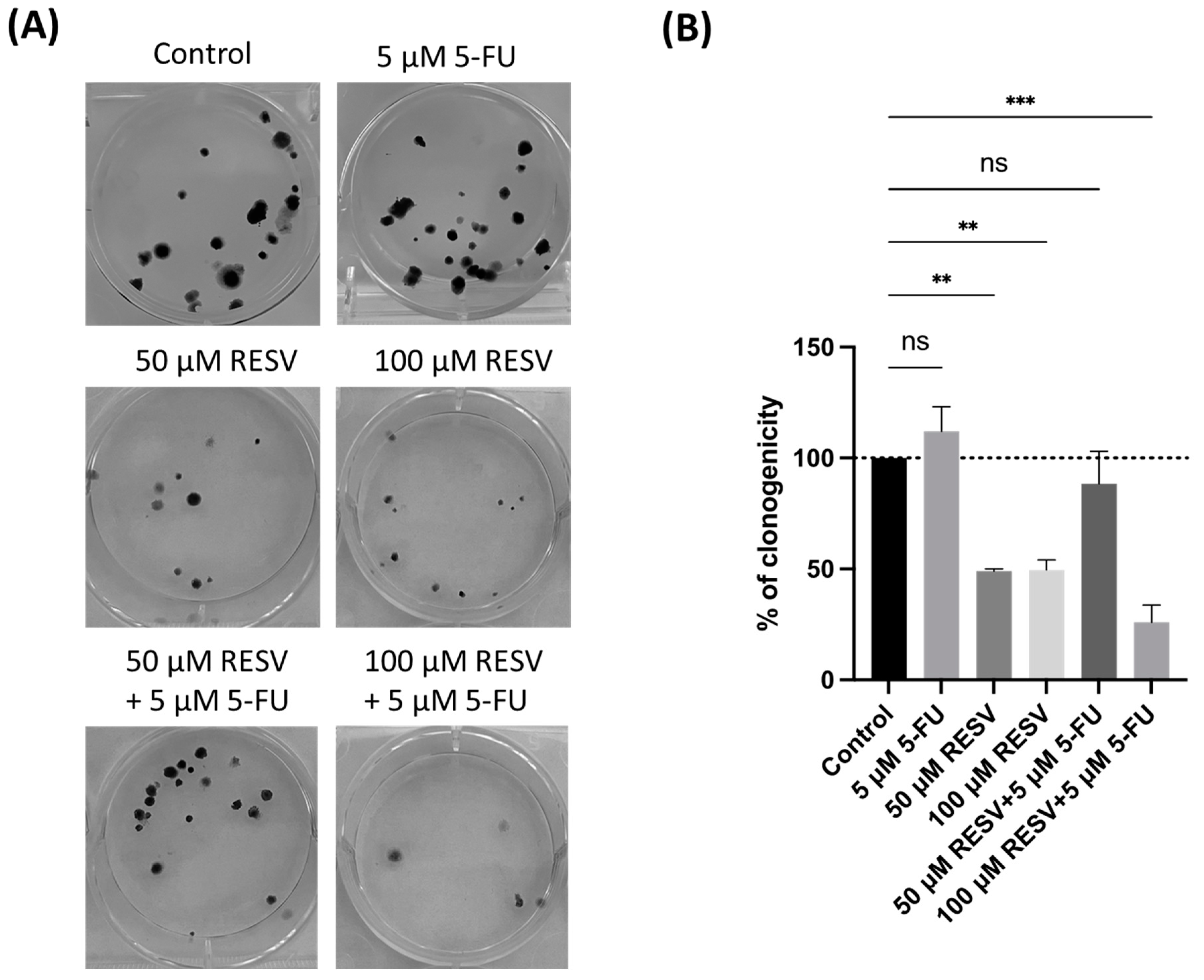
To further investigate Resveratrol’s potential in enhancing 5-FU antitumor effects, a clonogenic assay was conducted, focusing on the combination of Resveratrol with the IC50 concentration of 5-FU. The results demonstrated that Resveratrol significantly suppressed colony formation in A549 cells, particularly when combined with IC50 5-FU (Figure 14). Resveratrol alone reduced the number of colonies in a dose-dependent manner, indicating its pronounced effect on long-term cell proliferation.
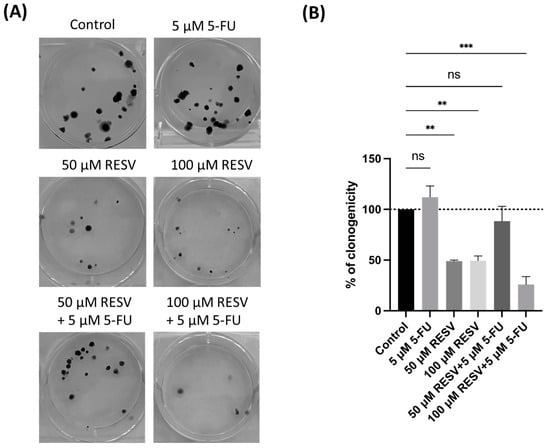
Figure 14.
Clonogenic assay of human lung cancer cell line treated with IC50 concentration of 5-FU and Resveratrol, both alone and in combination. (A) Image displaying the colonies formed by the human cancer cell line following plating 100 cells and incubation for 14 days. (B) Cell colonies were quantified, with errors bar representing the mean ± SD from three independent experiments; ns: non-significant; ** statistically significant vs. control at p < 0.01; *** statistically significant vs. control at p < 0.001.
Interestingly, the combination of Resveratrol with IC50 5-FU resulted in a more substantial reduction in clonogenicity compared to either treatment alone. This highlights Resveratrol’s potential to not only enhance the immediate cytotoxic effects of 5-FU, but to also inhibit the long-term survival and proliferation of lung cancer cells. These findings emphasize the importance of selecting effective drug combinations to optimize therapeutic outcomes, suggesting that Resveratrol could be a valuable addition to 5-FU-based treatments in lung cancer therapy.
4. Discussion
This study provides valuable insights into the therapeutic potential of combining 5-FU with Sildenafil, Tezosentan, Levosimendan, and Resveratrol for treating lung cancer, specifically in the A549 cell line. Our findings indicate that while 5-FU is effective against A549 cells, combining it with other compounds may offer new strategies to overcome limitations associated with 5-FU, such as side effects and drug resistance. Our calculated IC50 value of 5.03 µM for 5-FU in the A549 cell line aligns with the range of values reported in the literature. The differences observed between studies can be attributed to various factors, including differences in experimental protocols, cell line handling, and assay methods. Such variability highlights the importance of standardizing experimental conditions to achieve more comparable results across studies. The mechanism of action of 5-FU is well-known, involving the inhibition of DNA and RNA synthesis, leading to cell death [23]. However, the clinical efficacy of 5-FU can be limited by severe side effects and the development of resistance [24,25]. Thus, exploring combinations of 5-FU with other drugs may help address these limitations.
Sildenafil alone showed minimal anticancer activity, but modestly reduced cell viability when combined with 5-FU. This suggests that Sildenafil may enhance 5-FU efficacy by improving tumor perfusion and drug delivery, consistent with reports of Sildenafil improving tumor vasculature [37,38]. Similarly, Tezosentan, an endothelin receptor antagonist, also exhibited limited anticancer activity individually, but showed a moderate reduction in cell viability when combined with 5-FU. While Tezosentan as a monotherapy was not highly effective, its ability to modulate endothelin receptors—key regulators of tumor growth and resistance—suggests that it could contribute to enhancing the efficacy of chemotherapeutic combinations [39].
Levosimendan demonstrated a more pronounced effect when combined with 5-FU. While Levosimendan alone showed limited anticancer activity, its combination with 5-FU resulted in a significant reduction in cell viability, up to 40%, and notably inhibited the clonogenic potential of A549 cells. This enhancement of 5-FU efficacy by Levosimendan is particularly evident in the clonogenic assays, where the reduction in the number and size of colonies suggests that Levosimendan exerts a more substantial effect on cell proliferation rather than merely affecting cell viability. This impact on clonogenicity is crucial, as it can reduce the likelihood of tumor recurrence and improve long-term cancer control [57,58]. Tumors with high clonogenic potential are more likely to regrow after initial treatment, so Levosimendan’s action of hindering this potential is particularly valuable in cancer therapy. This suggests that Levosimendan might offer clinical benefits by preventing tumor relapse and improving patient outcomes, in addition to its role as a cytotoxic agent.
Additionally, the fact that Levosimendan did not significantly affect non-cancerous MRC-5 cells supports its favorable safety profile. This selective impact on cancer cells compared to normal cells enhances its potential for clinical use, as it reduces the risk of adverse effects on healthy tissue. The differential effects observed also highlight the potential of Levosimendan as an adjuvant therapy, particularly in complex tumor environments were enhancing the efficacy of established chemotherapeutic agents like 5-FU is crucial.
Resveratrol demonstrated a consistent and pronounced reduction in cell viability when combined with 5-FU, surpassing the effect observed with Levosimendan. Resveratrol’s anticancer properties, such as inducing apoptosis [43], inhibiting cell proliferation [42], and suppressing angiogenesis [59], likely contribute to this enhanced effect. Morphological analysis further supported these findings, revealing that higher concentrations of Resveratrol led to visible changes in cell phenotype, such as a reduction in cell number and alterations in cellular morphology. These effects were especially pronounced when Resveratrol was combined with the IC50 concentration of 5-FU. The clonogenic assays provided additional insight, showing that Resveratrol significantly reduced the clonogenicity of A549 cells, particularly when combined with 5-FU. This indicates that Resveratrol not only enhances the immediate cytotoxic effects of 5-FU, but also impairs the long-term survival and proliferative capacity of lung cancer cells.
Interestingly, the cell viability assays revealed that the combination of IC50 5-FU with Resveratrol did not lead to a significant further reduction in cell viability compared to IC50 5-FU alone. This suggests that while Resveratrol has strong anticancer properties, it may not enhance the immediate cytotoxic effects of 5-FU in this specific context. However, considering the severe side effects associated with 5-FU, the finding that combinations of Resveratrol with Levosimendan (50 µM Resveratrol + 1 µM Levosimendan and 100 µM Resveratrol + 0.1 µM Levosimendan) achieved similar reductions in cell viability is particularly promising. This suggests that the Resveratrol and Levosimendan combination could be a viable alternative treatment strategy, potentially offering similar anticancer efficacy with a reduced risk of side effects.
The differential effects of Levosimendan and Resveratrol across various cancer cell lines have been studied. For instance, Levosimendan demonstrated significant anticancer activity in bladder cancer cells (UM-UC-5) at low concentrations (0.1 µM), where its combination with 5-FU resulted in a 59% reduction in cell viability [41]. However, in prostate cancer cells (PC-3), higher concentrations of Levosimendan (above 10 µM) were required to observe a substantial reduction in cell viability, indicating a dose-dependent effect that varies across cancer types. These findings highlight the importance of considering cancer subtype and drug concentration when evaluating therapeutic efficacy. Recent research on cervical cancer further elucidates Levosimendan’s potential [60]. The study found that Levosimendan had a selective anticancer effect on HPV-negative C33A cells, with an IC50 value of 58.42 µM. Although its potency was lower compared to cisplatin, a standard therapy for cervical cancer, Levosimendan selectivity and its ability to induce apoptosis in C33A cells make it a promising candidate for use as an adjuvant to standard treatments. The study also revealed that Levosimendan significantly reduced the migration and invasion of C33A cells, suggesting potential antimetastatic properties.
The discrepancy in Levosimendan’s effects across different cell lines may be attributed to variations in molecular pathways active in each type. Recent findings that Levosimendan activates nitric oxide (NO) production and induces cell-cycle disturbances, particularly the elevation of cells in the G1 phase and reduction in the S phase, provide insights into its mechanism of action [40,60]. NO is a signaling molecule that plays a critical role in the regulation of blood flow, vascular permeability, and immune responses within the tumor microenvironment. By increasing NO production, Levosimendan may enhance apoptosis and promote cell cycle arrest, which reduces cancer cell proliferation. Additionally, Levosimendan inhibits phosphodiesterase 3 (PDE3), leading to an increase in intracellular cyclic AMP (cAMP) levels. Elevated cAMP levels are associated with antiproliferative effects and the inhibition of metastatic processes in several cancer models, which may explain the reduced clonogenic potential observed in A549 cells [40]. These mechanisms, coupled with Levosimendan’s favorable safety profile observed in non-cancerous cells, underscore its potential as a therapeutic adjunct to 5-FU and other standard treatments.
Resveratrol, on the other hand, exhibits potent anticancer effects through the downregulation of nuclear factor kappa B (NF-κB), a key transcription factor involved in promoting inflammation, cell proliferation, and survival [44]. The inhibition of NF-κB signaling by Resveratrol leads to enhanced apoptosis and decreased tumor cell proliferation. Furthermore, Resveratrol interferes with the PI3K/Akt and mTOR pathways, both of which are often implicated in chemotherapy resistance [45,61,62]. By disrupting these pathways, Resveratrol not only sensitizes cancer cells to chemotherapeutic agents like 5-FU, but also limits their long-term survival and ability to evade treatment.
Similar morphological changes in lung cancer have been documented in other studies where Resveratrol induced apoptosis and cell-cycle arrest, further validating our observations [63,64]. In previous studies, Resveratrol has shown consistent anticancer activity in several cancer models. In renal cell carcinoma [65] and pancreatic cancer cells [66], Resveratrol was effective in reducing clonogenic potential through pathways involving Akt and ERK1/2 signaling and FOXO transcription factor activation. The observed reduction in cell viability and clonogenicity in A549 cells, particularly when combined with 5-FU, aligns with these broader findings, reinforcing Resveratrol’s broad anticancer potential across different cancer types.
These results underscore the importance of carefully selecting drug combinations in cancer therapy, where agents like Resveratrol could be prioritized for their ability to enhance the efficacy of standard treatments like 5-FU. The comparable efficacy of the Resveratrol and Levosimendan combination to IC50 5-FU in reducing cell viability (by 40% with Levosimendan, p < 0.01) and clonogenic potential suggests that this combination could be a promising alternative, particularly for patients who may not tolerate standard chemotherapy well. These drug combinations could be especially beneficial for patients with advanced-stage lung cancer, those who have developed resistance to conventional treatments, or those unable to tolerate high doses of 5-FU due to severe side effects. By reducing the dosage of 5-FU while maintaining therapeutic efficacy through combination therapies, it is possible to mitigate some of the associated toxicities, offering a safer alternative for certain patient populations. Furthermore, the reduction in clonogenicity observed with these combinations highlights their potential in targeting tumor recurrence, a significant challenge in long-term cancer management. However, several limitations must be acknowledged. First, the drug concentrations used in this in vitro study may not directly correspond to those achievable in vivo. Factors such as drug metabolism, distribution, and clearance in the body could influence the efficacy and safety profiles of these combinations. Additionally, the use of a single lung cancer cell line (A549) may limit the generalizability of our findings to other lung cancer subtypes, such as squamous cell carcinoma or SCLC, which could respond differently to these treatments. Future studies incorporating a wider range of lung cancer cell lines will be essential to determine whether the observed effects are applicable across different subtypes. Furthermore, while the in vitro results are promising, translating these findings into clinical practice presents challenges. Tumor microenvironments in vivo, variability in patient responses, and potential drug interactions could affect the efficacy of these combinations. Before advancing to clinical trials, it is crucial to further investigate the pharmacokinetics, optimal dosing regimens, and potential toxicities of these drug combinations in more complex biological systems.
While this study provides promising in vitro results, future research will focus on validating these findings in vivo. Preclinical studies using relevant animal models will be essential to assess the therapeutic potential of these drug combinations in more complex biological environments. Additionally, identifying key biomarkers, such as markers of apoptosis, proliferation, or angiogenesis, will be crucial for evaluating treatment response. Further investigation into combinatory regimens involving other drugs could help optimize therapeutic strategies for lung cancer. Future clinical investigations, including phase I/II studies, will also be necessary to assess the safety and efficacy of these combinations in human populations. Identifying the most responsive patient groups and determining optimal dosing regimens will be key to translating these findings into clinical practice. These studies will lay the groundwork for future clinical trials and guide the integration of combination therapies into current treatment protocols, ultimately contributing to more targeted and effective approaches to lung cancer therapy.
Author Contributions
Conceptualization, N.V.; methodology, E.R.; formal analysis, E.R. and N.V.; investigation, E.R.; writing—original draft preparation, E.R.; writing—review and editing, N.V.; supervision, N.V.; project administration, N.V.; funding acquisition, N.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed by Fundo Europeu de Desenvolvimento Regional (FEDER) funds through the COMPETE 2020 Operational Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and by Portuguese funds through Fundação para a Ciência e a Tecnologia (FCT) in the framework of the projects IF/00092/2014/CP1255/CT0004 and CHAIR in Onco-Innovation from the Faculty of Medicine, University of Porto (FMUP).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
E.R. acknowledges CHAIR in Onco-Innovation/FMUP for funding her grant.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Obradović, J.; Djordjević, N.; Tošic, N.; Mrdjanović, J.; Stanković, B.; Stanić, J.; Zarić, B.; Perin, B.; Pavlović, S.; Jurišić, V. Frequencies of EGFR single nucleotide polymorphisms in non-small cell lung cancer patients and healthy individuals in the Republic of Serbia: A preliminary study. Tumour Biol. 2016, 37, 10479–10486. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.; Tomizawa, K.; Mitsudomi, T. Biological and clinical significance of KRAS mutations in lung cancer: An oncogenic driver that contrasts with EGFR mutation. Cancer Metastasis Rev. 2010, 29, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Soh, J.; Toyooka, S.; Matsuo, K.; Yamamoto, H.; Wistuba, I.I.; Lam, S.; Fong, K.M.; Gazdar, A.F.; Miyoshi, S. Ethnicity affects EGFR and KRAS gene alterations of lung adenocarcinoma. Oncol. Lett. 2015, 10, 1775–1782. [Google Scholar] [CrossRef]
- Du, X.; Shao, Y.; Qin, H.F.; Tai, Y.H.; Gao, H.J. ALK-rearrangement in non-small-cell lung cancer (NSCLC). Thorac. Cancer 2018, 9, 423–430. [Google Scholar] [CrossRef]
- Arbour, K.C.; Riely, G.J. Diagnosis and Treatment of Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer. Hematol. Oncol. Clin. N. Am. 2017, 31, 101–111. [Google Scholar] [CrossRef]
- Gendarme, S.; Bylicki, O.; Chouaid, C.; Guisier, F. ROS-1 Fusions in Non-Small-Cell Lung Cancer: Evidence to Date. Curr. Oncol. 2022, 29, 641–658. [Google Scholar] [CrossRef] [PubMed]
- Muminovic, M.; Carracedo Uribe, C.R.; Alvarez-Pinzon, A.; Shan, K.; Raez, L.E. Importance of ROS1 gene fusions in non-small cell lung cancer. Cancer Drug Resist. 2023, 6, 332–344. [Google Scholar] [CrossRef]
- O’Leary, C.G.; Andelkovic, V.; Ladwa, R.; Pavlakis, N.; Zhou, C.; Hirsch, F.; Richard, D.; O’Byrne, K. Targeting BRAF mutations in non-small cell lung cancer. Transl. Lung Cancer Res. 2019, 8, 1119–1124. [Google Scholar] [CrossRef]
- Yan, N.; Guo, S.; Zhang, H.; Zhang, Z.; Shen, S.; Li, X. BRAF-Mutated Non-Small Cell Lung Cancer: Current Treatment Status and Future Perspective. Front. Oncol. 2022, 12, 863043. [Google Scholar] [CrossRef]
- Mogi, A.; Kuwano, H. TP53 mutations in nonsmall cell lung cancer. J. Biomed. Biotechnol. 2011, 2011, 583929. [Google Scholar] [CrossRef]
- Collins, L.G.; Haines, C.; Perkel, R.; Enck, R.E. Lung cancer: Diagnosis and management. Am. Fam. Physician 2007, 75, 56–63. [Google Scholar] [PubMed]
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef] [PubMed]
- Howington, J.A.; Blum, M.G.; Chang, A.C.; Balekian, A.A.; Murthy, S.C. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e278S–e313S. [Google Scholar] [CrossRef]
- Vinod, S.K.; Hau, E. Radiotherapy treatment for lung cancer: Current status and future directions. Respirology 2020, 25 (Suppl. 2), 61–71. [Google Scholar] [CrossRef]
- Nagasaka, M.; Gadgeel, S.M. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev. Anticancer Ther. 2018, 18, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Singhal, S.; Kulkarni, P.; Horne, D.; Malhotra, J.; Salgia, R.; Singhal, S.S. Advances in Non-Small Cell Lung Cancer: Current Insights and Future Directions. J. Clin. Med. 2024, 13, 4189. [Google Scholar] [CrossRef]
- Reck, M.; Remon, J.; Hellmann, M.D. First-Line Immunotherapy for Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 586–597. [Google Scholar] [CrossRef]
- Li, W.; Lin, X.; Wang, R.; Wang, F.; Xie, S.; Tse, L.A. Hormone therapy and lung cancer mortality in women: Systematic review and meta-analysis. Steroids 2017, 118, 47–54. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Acevedo, A.; Davidoff, E.J.; Timmins, L.M.; Marrero-Berrios, I.; Patel, M.; White, C.; Lowe, C.; Sherba, J.J.; Hartmanshenn, C.; et al. The growing role of precision and personalized medicine for cancer treatment. Technol. Singap World Sci. 2018, 6, 79–100. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Abak, A.; Tondro Anamag, F.; Shoorei, H.; Fattahi, F.; Javadinia, S.A.; Basiri, A.; Taheri, M. 5-Fluorouracil: A Narrative Review on the Role of Regulatory Mechanisms in Driving Resistance to This Chemotherapeutic Agent. Front. Oncol. 2021, 11, 658636. [Google Scholar] [CrossRef]
- Jee, S.H.; Moon, S.M.; Shin, U.S.; Yang, H.M.; Hwang, D.Y. Effectiveness of Adjuvant Chemotherapy with 5-FU/Leucovorin and Prognosis in Stage II Colon Cancer. J. Korean Soc. Coloproctol. 2011, 27, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Magné, N.; François, E.; Broisin, L.; Guardiola, E.; Ramaïoli, A.; Ferrero, J.M.; Namer, M. Palliative 5-fluorouracil-based chemotherapy for advanced colorectal cancer in the elderly: Results of a 10-year experience. Am. J. Clin. Oncol. 2002, 25, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Sethy, C.; Kundu, C.N. 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: Implication of DNA repair inhibition. Biomed. Pharmacother. 2021, 137, 111285. [Google Scholar] [CrossRef]
- Azwar, S.; Seow, H.F.; Abdullah, M.; Faisal Jabar, M.; Mohtarrudin, N. Recent Updates on Mechanisms of Resistance to 5-Fluorouracil and Reversal Strategies in Colon Cancer Treatment. Biology 2021, 10, 854. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Goessl, E.; Jin, G.; Collie-Duguid, E.S.; Cassidy, J.; Wang, W.; O’Brien, V. Cell cycle perturbation and acquired 5-fluorouracil chemoresistance. Anticancer Res. 2008, 28, 9–14. [Google Scholar]
- Garg, M.B.; Lincz, L.F.; Adler, K.; Scorgie, F.E.; Ackland, S.P.; Sakoff, J.A. Predicting 5-fluorouracil toxicity in colorectal cancer patients from peripheral blood cell telomere length: A multivariate analysis. Br. J. Cancer 2012, 107, 1525–1533. [Google Scholar] [CrossRef]
- Chang, C.-W.; Liu, C.-Y.; Lee, H.-C.; Huang, Y.-H.; Li, L.-H.; Chiau, J.-S.C.; Wang, T.-E.; Chu, C.-H.; Shih, S.-C.; Tsai, T.-H. Lactobacillus casei variety rhamnosus probiotic preventively attenuates 5-fluorouracil/oxaliplatin-induced intestinal injury in a syngeneic colorectal cancer model. Front. Microbiol. 2018, 9, 983. [Google Scholar] [CrossRef]
- Bano, N.; Najam, R.; Qazi, F.; Mateen, A. Gastrointestinal adverse effects in advanced colorectal carcinoma patients treated with different schedules of FOLFOX. Asian Pac. J. Cancer Prev. 2014, 15, 8089–8093. [Google Scholar] [CrossRef]
- Matsusaka, S.; Lenz, H.-J. Pharmacogenomics of fluorouracil-based chemotherapy toxicity. Expert Opin. Drug Metab. Toxicol. 2015, 11, 811–821. [Google Scholar] [CrossRef]
- Sara, J.D.; Kaur, J.; Khodadadi, R.; Rehman, M.; Lobo, R.; Chakrabarti, S.; Herrmann, J.; Lerman, A.; Grothey, A. 5-fluorouracil and cardiotoxicity: A review. Ther. Adv. Med. Oncol. 2018, 10, 1758835918780140. [Google Scholar] [CrossRef]
- Qin, S.-Y.; Cheng, Y.-J.; Lei, Q.; Zhang, A.-Q.; Zhang, X.-Z. Combinational strategy for high-performance cancer chemotherapy. Biomaterials 2018, 171, 178–197. [Google Scholar] [CrossRef] [PubMed]
- Gilad, Y.; Gellerman, G.; Lonard, D.M.; O’Malley, B.W. Drug Combination in Cancer Treatment—From Cocktails to Conjugated Combinations. Cancers 2021, 13, 669. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G.R.; Lehár, J.; Keith, C.T. Multi-target therapeutics: When the whole is greater than the sum of the parts. Drug Discov. Today 2007, 12, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Tallarida, R.J. Quantitative methods for assessing drug synergism. Genes Cancer 2011, 2, 1003–1008. [Google Scholar] [CrossRef]
- Kalofonos, H.P.; Aravantinos, G.; Kosmidis, P.; Papakostas, P.; Economopoulos, T.; Dimopoulos, M.; Skarlos, D.; Bamias, A.; Pectasides, D.; Chalkidou, S.; et al. Irinotecan or oxaliplatin combined with leucovorin and 5-fluorouracil as first-line treatment in advanced colorectal cancer: A multicenter, randomized, phase II study. Ann. Oncol. 2005, 16, 869–877. [Google Scholar] [CrossRef]
- Moutabian, H.; Majdaeen, M.; Ghahramani-Asl, R.; Yadollahi, M.; Gharepapagh, E.; Ataei, G.; Falahatpour, Z.; Bagheri, H.; Farhood, B. A systematic review of the therapeutic effects of resveratrol in combination with 5-fluorouracil during colorectal cancer treatment: With a special focus on the oxidant, apoptotic, and anti-inflammatory activities. Cancer Cell Int. 2022, 22, 142. [Google Scholar] [CrossRef]
- Huang, S.A.; Lie, J.D. Phosphodiesterase-5 (PDE5) Inhibitors In the Management of Erectile Dysfunction. Pharm. Ther. 2013, 38, 407–419. [Google Scholar]
- Mauro, N.; Cillari, R.; Andrea Utzeri, M.; Costa, S.; Giammona, G.; Nicosia, A.; Cavallaro, G. Controlled delivery of sildenafil by β-Cyclodextrin-decorated sulfur-doped carbon nanodots: A synergistic activation of ROS signaling in tumors overexpressing PDE-5. Int. J. Pharm. 2023, 645, 123409. [Google Scholar] [CrossRef]
- Ribeiro, E.; Vale, N. Repurposing of the Drug Tezosentan for Cancer Therapy. Curr. Issues Mol. Biol. 2023, 45, 5118–5131. [Google Scholar] [CrossRef]
- Ribeiro, E.; Vale, N. Understanding the Clinical Use of Levosimendan and Perspectives on its Future in Oncology. Biomolecules 2023, 13, 1296. [Google Scholar] [CrossRef]
- Ribeiro, E.; Costa, B.; Marques, L.; Vasques-Nóvoa, F.; Vale, N. Enhancing Urological Cancer Treatment: Leveraging Vasodilator Synergistic Potential with 5-FU for Improved Therapeutic Outcomes. J. Clin. Med. 2024, 13, 4113. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Enríquez, S.; Pacheco-Velázquez, S.C.; Marín-Hernández, Á.; Gallardo-Pérez, J.C.; Robledo-Cadena, D.X.; Hernández-Reséndiz, I.; García-García, J.D.; Belmont-Díaz, J.; López-Marure, R.; Hernández-Esquivel, L.; et al. Resveratrol inhibits cancer cell proliferation by impairing oxidative phosphorylation and inducing oxidative stress. Toxicol. Appl. Pharmacol. 2019, 370, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Brockmueller, A.; Buhrmann, C.; Shayan, P.; Shakibaei, M. Resveratrol induces apoptosis by modulating the reciprocal crosstalk between p53 and Sirt-1 in the CRC tumor microenvironment. Front. Immunol. 2023, 14, 1225530. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Wang, L.; Cui, J.; Huoc, Z.; Xue, J.; Cui, H.; Mao, Q.; Yang, R. Resveratrol inhibits NF-kB signaling through suppression of p65 and IkappaB kinase activities. Pharmazie 2013, 68, 689–694. [Google Scholar]
- Jiang, H.; Shang, X.; Wu, H.; Gautam, S.C.; Al-Holou, S.; Li, C.; Kuo, J.; Zhang, L.; Chopp, M. Resveratrol downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma cells. J. Exp. Ther. Oncol. 2009, 8, 25–33. [Google Scholar]
- Duarte, D.; Guerreiro, I.; Vale, N. Novel Strategies for Cancer Combat: Drug Combination Using Repurposed Drugs Induces Synergistic Growth Inhibition of MCF-7 Breast and HT-29 Colon Cancer Cells. Curr. Issues Mol. Biol. 2022, 44, 4930–4949. [Google Scholar] [CrossRef]
- Duarte, D.; Vale, N. Combining repurposed drugs to treat colorectal cancer. Drug Discov. Today 2022, 27, 165–184. [Google Scholar] [CrossRef]
- Duarte, D.; Nunes, M.; Ricardo, S.; Vale, N. Combination of Antimalarial and CNS Drugs with Antineoplastic Agents in MCF-7 Breast and HT-29 Colon Cancer Cells: Biosafety Evaluation and Mechanism of Action. Biomolecules 2022, 12, 1490. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhang, X.; Sun, H.; Zhang, J.; Yan, M.; Zhang, H. Autophagy inhibition promotes 5-fluorouraci-induced apoptosis by stimulating ROS formation in human non-small cell lung cancer A549 cells. PLoS ONE 2013, 8, e56679. [Google Scholar] [CrossRef]
- Cao, F.; Shi, M.; Yu, B.; Cheng, X.; Li, X.; Jia, X. Epigenetic Mechanism of Enrichment of A549 Lung Cancer Stem Cells with 5-Fu. Onco Targets Ther. 2021, 14, 3783–3794. [Google Scholar] [CrossRef]
- Huang, C.; Li, N.M.; Gao, P.; Yang, S.; Ning, Q.; Huang, W.; Li, Z.P.; Ye, P.J.; Xiang, L.; He, D.X.; et al. In vitro and in vivo evaluation of macromolecular prodrug GC-FUA based nanoparticle for hepatocellular carcinoma chemotherapy. Drug Deliv. 2017, 24, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.R.; Yuen, J.G.; Fesler, A.; Farley, H.; Haley, J.D.; Ju, J. Development of a 5-FU modified miR-129 mimic as a therapeutic for non-small cell lung cancer. Mol. Ther. Oncolytics 2023, 28, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.B.; Le, X.; Heymach, J.V. β-Adrenergic Signaling in Lung Cancer: A Potential Role for Beta-Blockers. J. Neuroimmune Pharmacol. 2020, 15, 27–36. [Google Scholar] [CrossRef]
- Ma, J.; Xue, M.; Zhang, S.; Cheng, L.; Qian, W.; Duan, W.; Shen, X. Resveratrol inhibits the growth of tumor cells under chronic stress via the ADRB-2-HIF-1α axis. Oncol. Rep. 2019, 41, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Feng, W.; Ji, Y.; Jin, L. Resveratrol mediates cell cycle arrest and cell death in human esophageal squamous cell carcinoma by directly targeting the EGFR signaling pathway. Oncol. Lett. 2017, 13, 347–355. [Google Scholar] [CrossRef]
- Zhou, P.; Hu, J.; Wang, X.; Wang, J.; Zhang, Y.; Wang, C. Epidermal growth factor receptor expression affects proliferation and apoptosis in non-small cell lung cancer cells via the extracellular signal-regulated kinase/microRNA 200a signaling pathway. Oncol. Lett. 2018, 15, 5201–5207. [Google Scholar] [CrossRef]
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Marino, M.; Martinez-Chantar, M.L.; Nawroth, R.; Sanchez-Garcia, I.; et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015, 35 (Suppl.), S25–S54. [Google Scholar] [CrossRef] [PubMed]
- Adan, A.; Kiraz, Y.; Baran, Y. Cell Proliferation and Cytotoxicity Assays. Curr. Pharm. Biotechnol. 2016, 17, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.C.; Fiscus, R.R. Resveratrol at anti-angiogenesis/anticancer concentrations suppresses protein kinase G signaling and decreases IAPs expression in HUVECs. Anticancer Res. 2015, 35, 273–281. [Google Scholar]
- Schelz, Z.; Muddather, H.F.; Jaski, F.S.; Bózsity, N.; Zupkó, I. An In Vitro Investigation of the Antiproliferative and Antimetastatic Effects of Levosimendan: Potential Drug Repurposing for Cervical Cancer. Curr. Issues Mol. Biol. 2024, 46, 6566–6579. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, B.; Peng, J.; Tang, H.; Wang, S.; Peng, S.; Ye, F.; Wang, J.; Ouyang, K.; Li, J.; et al. Inhibition of NF-κB signaling unveils novel strategies to overcome drug resistance in cancers. Drug Resist. Updates 2024, 73, 101042. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Tsai, K.W.; Shee, J.J.; Li, Y.Z.; Chen, C.H.; Chuang, J.J.; Liu, Y.W. 4′-Chloro-3,5-dihydroxystilbene, a resveratrol derivative, induces lung cancer cell death. Acta Pharmacol. Sin. 2010, 31, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhang, Y.; Xia, J.; Liu, B.; Zhang, Q.; Liu, J.; Luo, L.; Peng, Z.; Song, Z.; Zhu, R. Resveratrol induces cell cycle arrest via a p53-independent pathway in A549 cells. Mol. Med. Rep. 2015, 11, 2459–2464. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, H.; Zeng, X.; Ye, D.; Liu, J. Resveratrol inhibits proliferation, migration and invasion via Akt and ERK1/2 signaling pathways in renal cell carcinoma cells. Biomed. Pharmacother. 2018, 98, 36–44. [Google Scholar] [CrossRef]
- Roy, S.K.; Chen, Q.; Fu, J.; Shankar, S.; Srivastava, R.K. Resveratrol inhibits growth of orthotopic pancreatic tumors through activation of FOXO transcription factors. PLoS ONE 2011, 6, e25166. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).