Cytotoxicity and Microbiological Properties of Ceramic CAD/CAM Materials Subjected to Surface Treatment with Nanometric Copper Layer
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Sample Preparation
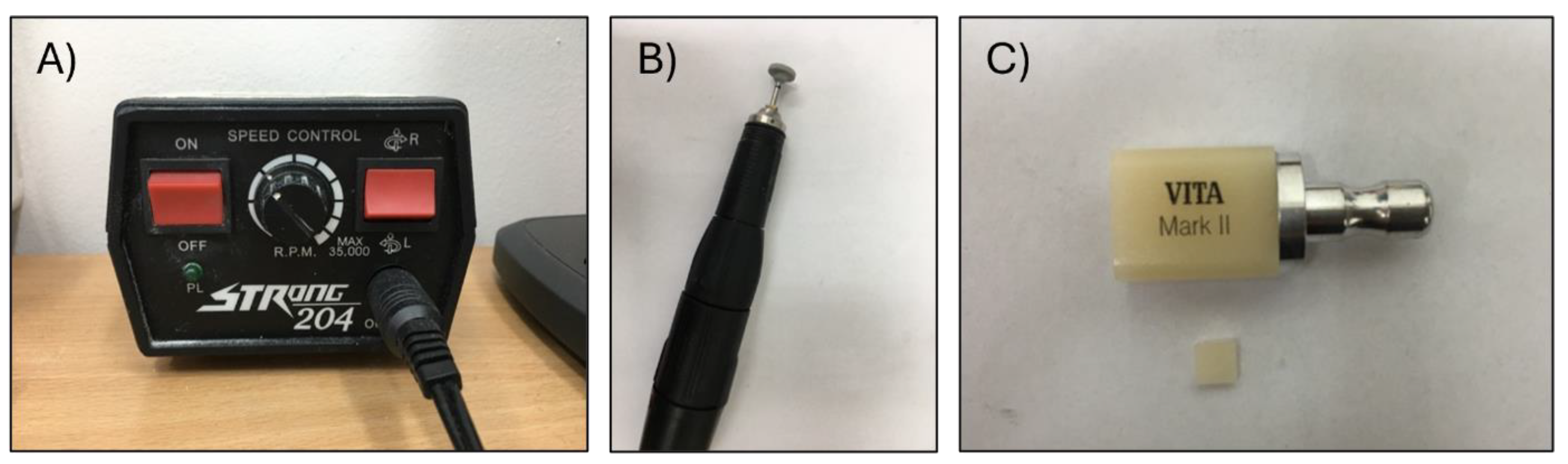
2.2. Copper Coating
2.3. Cytotoxicity and Adhesion
2.3.1. Cell Lines
2.3.2. Cytotoxicity Testing—Direct Contact
2.3.3. Cellular Adhesion to the Surface of the Material
2.4. Adhesion Abilities of Strains
2.5. Water Contact Angle
3. Results
3.1. Direct Contact—Cytotoxicity Evaluation
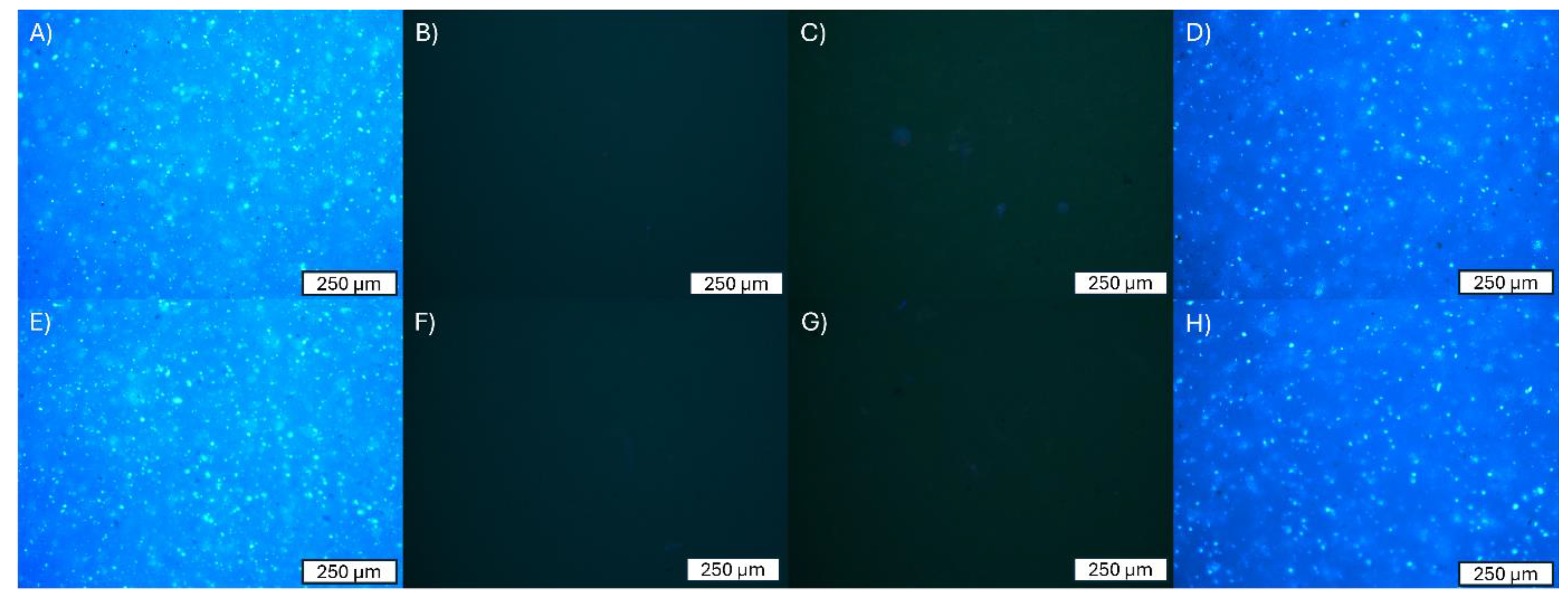
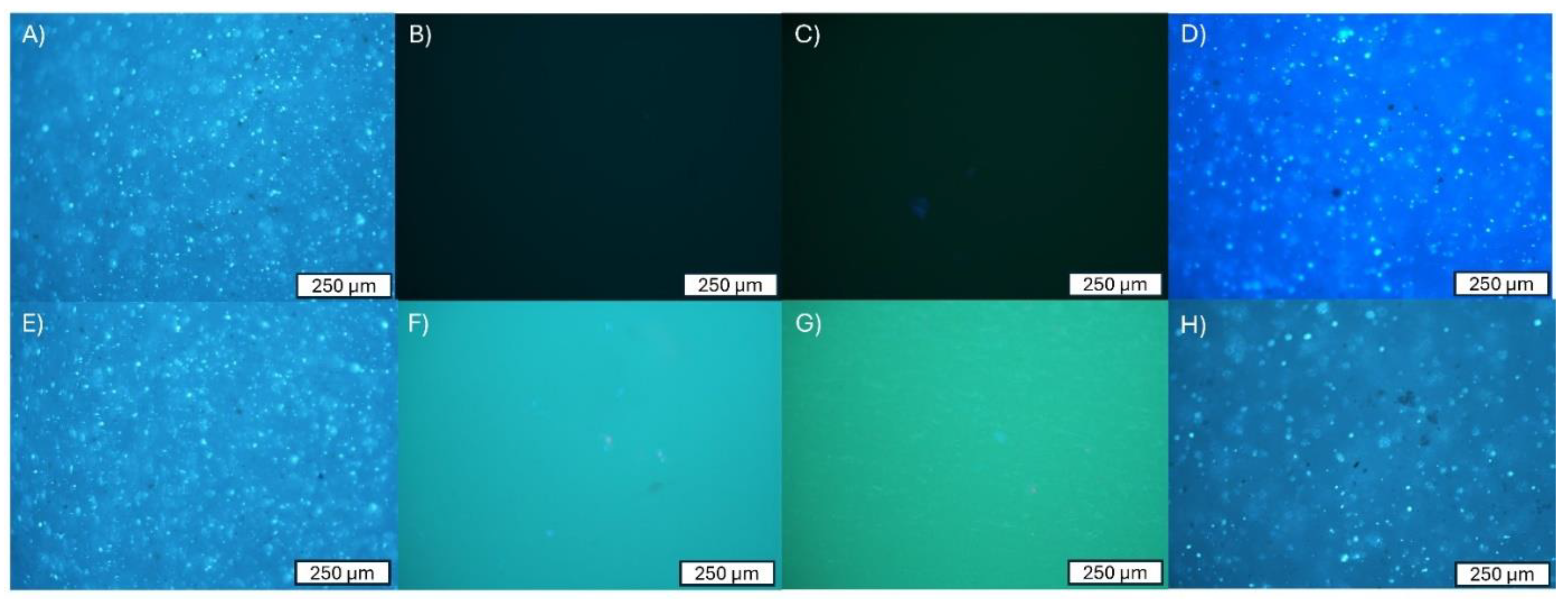
3.2. Adhesion of Cells to the Surface of the Material
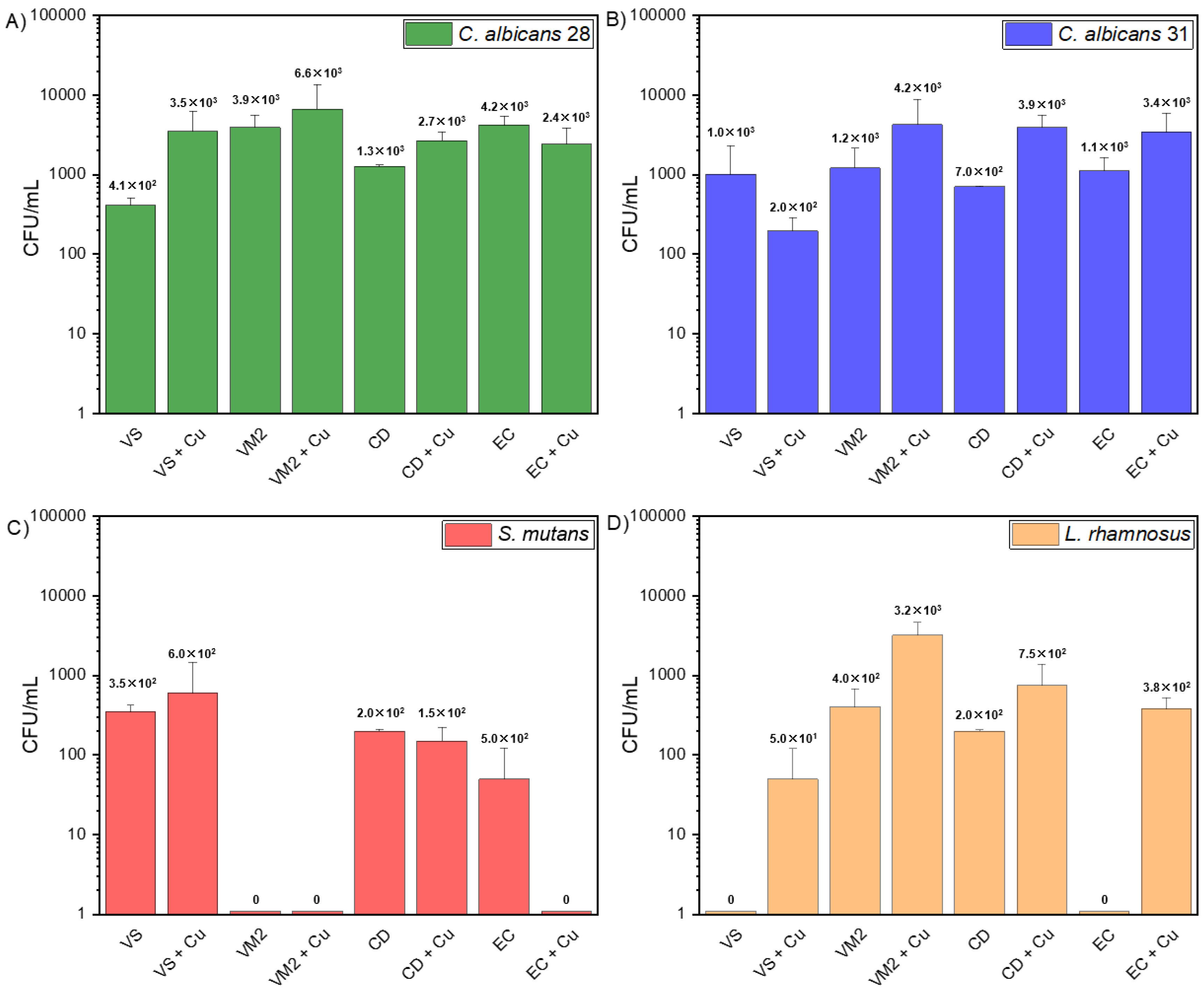
3.3. Adhesion Abilities of Strains
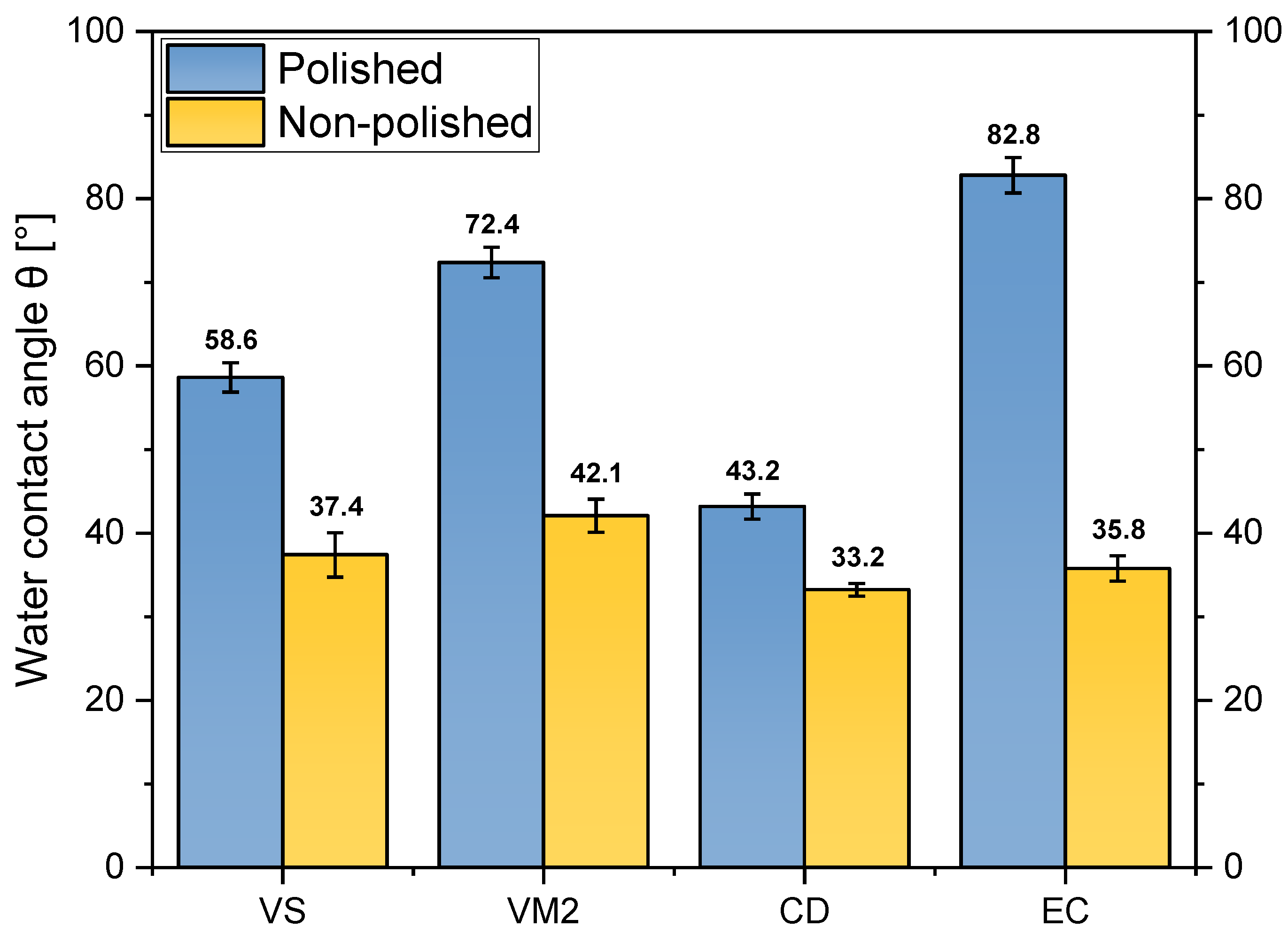
3.4. Water Contact Angle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piszko, A.; Piszko, P.; Rybak, Z.; Szymonowicz, M.; Dobrzyński, M. Review on Polymer, Ceramic and Composite Materials for CAD/CAM Indirect Restorations in Dentistry—Application, Mechanical Characteristics and Comparison. Materials 2021, 14, 1592. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.J.; Trushkowsky, R.D.; Paravina, R.D. Dental Color Matching Instruments and Systems. Review of Clinical and Research Aspects. J. Dent. 2010, 38, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Fasbinder, D.J. Materials for Chairside CAD/CAM Restorations. Compend. Contin. Educ. Dent. 2010, 31, 702–704, 706, 708–709. [Google Scholar]
- Wataha, J.C. Principles of Biocompatibility for Dental Practitioners. J. Prosthet. Dent. 2001, 86, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Wataha, J.C.; Hanks, C.T. Biocompatibility Testing—What Can We Anticipate? Trans. Acad. Dent. Mater. 1997, 10, 109–120. [Google Scholar]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Skośkiewicz-Malinowska, K.; Mysior, M.; Rusak, A.; Kuropka, P.; Kozakiewicz, M.; Jurczyszyn, K. Application of Texture and Fractal Dimension Analysis to Evaluate Subgingival Cement Surfaces in Terms of Biocompatibility. Materials 2021, 14, 5857. [Google Scholar] [CrossRef]
- Kutsch, V.K. Dental Caries: An Updated Medical Model of Risk Assessment. J. Prosthet. Dent. 2014, 111, 280–285. [Google Scholar] [CrossRef]
- Lamont, R.J.; Egland, P.G. Dental Caries. In Molecular Medical Microbiology; Academic Press: Cambridge, MA, USA, 2014; pp. 945–955. [Google Scholar] [CrossRef]
- Bigos, P.; Czerwińska, R.; Pajączkowska, M.; Nowicka, J. Mixed Oral Biofilm. Postępy Mikrobiol.-Adv. Microbiol. 2021, 60, 47–58. [Google Scholar] [CrossRef]
- Dobrzynski, M.; Pajaczkowska, M.; Nowicka, J.; Jaworski, A.; Kosior, P.; Szymonowicz, M.; Kuropka, P.; Rybak, Z.; Bogucki, Z.A.; Filipiak, J.; et al. Study of Surface Structure Changes for Selected Ceramics Used in the CAD/CAM System on the Degree of Microbial Colonization, in Vitro Tests. Biomed Res. Int. 2019, 2019, 9130806. [Google Scholar] [CrossRef]
- Cavalcanti, I.M.G.; Del Bel Cury, A.A.; Jenkinson, H.F.; Nobbs, A.H. Interactions between Streptococcus Oralis, Actinomyces Oris, and Candida Albicans in the Development of Multispecies Oral Microbial Biofilms on Salivary Pellicle. Mol. Oral Microbiol. 2017, 32, 60–73. [Google Scholar] [CrossRef]
- Bhardwaj, S.B. Oral Biofilms. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 643–652. [Google Scholar] [CrossRef]
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the Oral Cavity and Approaches for Biofilm Management by Surface Modifications. Clin. Oral Investig. 2020, 24, 4237–4260. [Google Scholar] [CrossRef] [PubMed]
- Akcalı, A.; Lang, N.P. Dental Calculus: The Calcified Biofilm and Its Role in Disease Development. Periodontol 2000 2018, 76, 109–115. [Google Scholar] [CrossRef]
- Walker, A.W. Microbiota of the Human Body; Schwiertz, A., Ed.; Springer International Publishing: Cham, Switzerland, 2016; Volume 902, ISBN 978-3-319-31246-0. [Google Scholar]
- Tu, Y.; Deng, S.; Wang, Y.; Lin, X.; Yang, Z. Adhesive Ability of Different Oral Pathogens to Various Dental Materials: An In Vitro Study. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 9595067. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Models of Caries Formation around Dental Composite Restorations. J. Dent. Res. 2017, 96, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Cury, M.S.; Silva, C.B.; Nogueira, R.D.; Campos, M.G.D.; Palma-Dibb, R.G.; Geraldo-Martins, V.R. Surface Roughness and Bacterial Adhesion on Root Dentin Treated with Diode Laser and Conventional Desensitizing Agents. Lasers Med. Sci. 2018, 33, 257–262. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Rybak, Z.; Pajączkowska, M.; Nowicka, J.; Szymonowicz, M.; Rusak, A.; Wiglusz, R.J.; Szyszka, K.; Chmielowiec, J.; Chodaczek, G.; et al. Antimicrobial Properties and Cytotoxic Effect Evaluation of Nanosized Hydroxyapatite and Fluorapatite Dedicated for Alveolar Bone Regeneration. Appl. Sci. 2024, 14, 7845. [Google Scholar] [CrossRef]
- Heidenau, F.; Mittelmeier, W.; Detsch, R.; Haenle, M.; Stenzel, F.; Ziegler, G.; Gollwitzer, H. A Novel Antibacterial Titania Coating: Metal Ion Toxicity and in Vitro Surface Colonization. J. Mater. Sci. Mater. Med. 2005, 16, 883–888. [Google Scholar] [CrossRef]
- Ashour, A.A.; Felemban, M.F.; Felemban, N.H.; Enan, E.T.; Basha, S.; Hassan, M.M.; Gad El-Rab, S.M.F. Comparison and Advanced Antimicrobial Strategies of Silver and Copper Nanodrug-Loaded Glass Ionomer Cement against Dental Caries Microbes. Antibiotics 2022, 11, 756. [Google Scholar] [CrossRef]
- Nizami, M.Z.I.; Xu, V.W.; Yin, I.X.; Yu, O.Y.; Chu, C.H. Metal and Metal Oxide Nanoparticles in Caries Prevention: A Review. Nanomaterials 2021, 11, 3446. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzynski, M.; Nowicka, J.; Pajaczkowska, M.; Szymonowicz, M.; Targonska, S.; Sobierajska, P.; Wiglusz, K.; Dobrzynski, W.; Lubojanski, A.; et al. The Influence of Ozonated Olive Oil-Loaded and Copper-Doped Nanohydroxyapatites on Planktonic Forms of Microorganisms. Nanomaterials 2020, 10, 1997. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, M. Antibacterial Property and Biocompatibility of Silver, Copper, and Zinc in Titanium Dioxide Layers Incorporated by One-Step Micro-Arc Oxidation: A Review. Antibiotics 2020, 9, 716. [Google Scholar] [CrossRef]
- Wassmann, T.; Schubert, A.; Malinski, F.; Rosentritt, M.; Krohn, S.; Techmer, K.; Bürgers, R. The Antimicrobial and Cytotoxic Effects of a Copper-Loaded Zinc Oxide Phosphate Cement. Clin. Oral Investig. 2020, 24, 3899–3909. [Google Scholar] [CrossRef]
- Vita Zahnfabrik. VITA SUPRINITY PC. Available online: https://mam.vita-zahnfabrik.com/portal/ecms_mdb_download.php?id=82440&sprache=en&fallback=&cls_session_id=&neuste_version=1 (accessed on 5 July 2024).
- Vita Zahnfabrik. Summary of Safety and Clinical Performance. Available online: https://mam.vita-zahnfabrik.com/portal/ecms_mdb_download.php?id=129503&sprache=en&fallback=&cls_session_id=&neuste_version=1 (accessed on 9 July 2024).
- Vita Dentists Solutions, Catalog for Dentists. Available online: https://www.vita-zahnfabrik.com/datei.php?src=portal/netpaper/dateien/185217/10527E_1_DENTIST_SOLUTIONS_Cataloge_V01.pdf (accessed on 7 July 2024).
- Celtra Duo, CAD Blocks for CEREC and INLAB. Available online: https://www.dentsplysirona.com/content/dam/dentsply/pim/manufacturer/Prosthetics/Fixed/High_strength_glass_ceramic/Celtra_Duo/Celtra%20Duo_DFU.pdf (accessed on 3 July 2024).
- IPS Empress CAD. Available online: https://www.ivoclar.com/en_li/products/digital-processes/ips-empress-cad (accessed on 5 November 2020).
- IVita Suprinity PC. Available online: https://Www.Vita-Zahnfabrik.Com/En/VITA-SUPRINITY-PC-44049.html (accessed on 17 April 2024).
- VITA Mark II for Cerec, Product Information. Available online: https://Www.Dt-Shop.Com/Index.Php?Id=22&L=1&artnr=1021&aw=110&pg=12&geoipredirect=1#:~:Text=SiO%E2%82%82%2056.0%2D64.0%3B%20Al%E2%82%82O%E2%82%83%2020.0,%2D0.6%3B%20TiO%E2%82%82%20%3C%200.1 (accessed on 3 July 2024).
- Celtra Duo. Technical Monograph. Available online: https://assets.dentsplysirona.com/dentsply/microsites/celtra/celtraduo-tech-monograph.pdf (accessed on 19 July 2024).
- IPS Empress CAD. Scientific Documentation. Available online: https://ivodent.hu/__docs/775_d9ea1b27d845c2dd50ef19b4a86c1fdc.pdf (accessed on 5 June 2024).
- Żak, M.; Rusak, A.; Kuropka, P.; Szymonowicz, M.; Pezowicz, C. Mechanical Properties and Osteointegration of the Mesh Structure of a Lumbar Fusion Cage Made by 3D Printing. J. Mech. Behav. Biomed. Mater. 2023, 141, 105762. [Google Scholar] [CrossRef]
- Tomanik, M.; Kobielarz, M.; Filipiak, J.; Szymonowicz, M.; Rusak, A.; Mroczkowska, K.; Antończak, A.; Pezowicz, C. Laser Texturing as a Way of Influencing the Micromechanical and Biological Properties of the Poly(L-Lactide) Surface. Materials 2020, 13, 3786. [Google Scholar] [CrossRef]
- Sztyler, K.; Pajączkowska, M.; Nowicka, J.; Rusak, A.; Chodaczek, G.; Nikodem, A.; Wiglusz, R.J.; Watras, A.; Dobrzyński, M. Evaluation of the Microbial, Cytotoxic and Physico-Chemical Properties of the Stainless Steel Crowns Used in Pediatric Dentistry. Acta Bioeng Biomech 2022, 24, 127–137. [Google Scholar] [CrossRef]
- Law, K.Y. Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef]
- Papadiochou, S.; Pissiotis, A.L. Marginal Adaptation and CAD-CAM Technology: A Systematic Review of Restorative Material and Fabrication Techniques. J. Prosthet. Dent. 2018, 119, 545–551. [Google Scholar] [CrossRef]
- Bose, S.; Robertson, S.F.; Bandyopadhyay, A. Surface Modification of Biomaterials and Biomedical Devices Using Additive Manufacturing. Acta Biomater. 2018, 66, 6–22. [Google Scholar] [CrossRef]
- Siddanna, G.D.; Valcanaia, A.J.; Fierro, P.H.; Neiva, G.F.; Fasbinder, D.J. Surface Evaluation of Resilient CAD/CAM Ceramics after Contouring and Polishing. J. Esthet. Restor. Dent. 2021, 33, 750–763. [Google Scholar] [CrossRef]
- Alao, A.R.; Stoll, R.; Zhang, Y.; Yin, L. Influence of CAD/CAM Milling, Sintering and Surface Treatments on the Fatigue Behavior of Lithium Disilicate Glass Ceramic. J. Mech. Behav. Biomed. Mater. 2021, 113, 104133. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A Review on the Wettability of Dental Implant Surfaces II: Biological and Clinical Aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [PubMed]
- De Jong, H.P.; Van Pelt, A.W.J.; Arends, J. Contact Angle Measurements on Human Enamel-An in Vitro Study of Influence of Pellicle and Storage Period. J. Dent. Res. 1982, 61, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, J.; Janczura, A.; Pajączkowska, M.; Chodaczek, G.; Szymczyk-Ziółkowska, P.; Walczuk, U.; Gościniak, G. Effect of Camel Peptide on the Biofilm of Staphylococcus Epidermidis and Staphylococcus Haemolyticus Formed on Orthopedic Implants. Antibiotics 2023, 12, 1671. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Ren, H.; He, Y.; Ying, J.; Chen, Y. Interaction between Microorganisms and Dental Material Surfaces: General Concepts and Research Progress. J. Oral Microbiol. 2023, 15, 2196897. [Google Scholar] [CrossRef]
- Özarslan, M.; Bilgili Can, D.; Avcioglu, N.H.; Çalışkan, S. Effect of Different Polishing Techniques on Surface Properties and Bacterial Adhesion on Resin-Ceramic CAD/CAM Materials. Clin. Oral Investig. 2022, 26, 5289–5299. [Google Scholar] [CrossRef]
- Vulović, S.; Popovac, A.; Radunović, M.; Petrović, S.; Todorović, M.; Milić-Lemić, A. Microbial Adhesion and Viability on Novel CAD/CAM Framework Materials for Implant-supported Hybrid Prostheses. Eur. J. Oral Sci. 2023, 131, e12911. [Google Scholar] [CrossRef]
- Vulović, S.; Nikolić-Jakoba, N.; Radunović, M.; Petrović, S.; Popovac, A.; Todorović, M.; Milić-Lemić, A. Biofilm Formation on the Surfaces of CAD/CAM Dental Polymers. Polymers 2023, 15, 2140. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, S.; Xu, X.; Du, Q. Copper-Containing Nanoparticles: Mechanism of Antimicrobial Effect and Application in Dentistry-a Narrative Review. Front. Surg. 2022, 9, 905892. [Google Scholar] [CrossRef]
- Fu, Y.; Chang, F.-M.J.; Giedroc, D.P. Copper Transport and Trafficking at the Host–Bacterial Pathogen Interface. Acc. Chem. Res. 2014, 47, 3605–3613. [Google Scholar] [CrossRef]
- Agnihotri, R.; Gaur, S.; Albin, S. Nanometals in Dentistry: Applications and Toxicological Implications—A Systematic Review. Biol. Trace Elem. Res. 2020, 197, 70–88. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.F.; Malaquias, P.; Hass, V.; Matos, T.P.; Lourenço, L.; Reis, A.; Loguercio, A.D.; Farago, P.V. The Role of Copper Nanoparticles in an Etch-and-Rinse Adhesive on Antimicrobial Activity, Mechanical Properties and the Durability of Resin-Dentine Interfaces. J. Dent. 2017, 61, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, J.; Versace, D.L.; Abbad-Andallousi, S.; Pires, R.; Azevedo, C.; Cénédese, P.; Dubot, P. Antibacterial Properties of Nanostructured Cu–TiO2 Surfaces for Dental Implants. Biomater. Sci. 2017, 5, 455–462. [Google Scholar] [CrossRef] [PubMed]


| Material (Abbr.) | Sample Photography | Manufacturer | Classification | Composition | Series Number (lot) | Reference |
|---|---|---|---|---|---|---|
| Vita Suprinity (VS) |  | VITA-Zahnfabrik | Lithium Silicate Ceramics | SiO2 (56–64 wt%), Li2O (15–21 wt%), K2O (1–4 wt%), P2O5 (3–8 wt%), Al2O3 (1–4 wt%), ZrO2 (8–12 wt%), CeO2 (0–4 wt%), La2O3 (0.1 wt%), pigments (0–6 wt%) | 41781 | [32] |
| Vita Mark II (VM2) |  | VITA-Zahnfabrik | Feldspar Ceramics | SiO2 (56.0–64.0 wt%), Al2O3 (20.0–23.0 wt%), Na2O (6.0–9.0 wt%), K2O (6.0–8.0 wt%), CaO (0.3–0.6 wt%), TiO2 (<0.1 wt%) | 80560 | [33] |
| Celtra Duo (CD) |  | Dentsply Sirona | Lithium Silicate Ceramics | SiO2 (58.0 wt%), P2O5 (5.0 wt%), Al2O3 (1.9 wt%), Li2O (18.5 wt%), ZrO2 (10.1 wt%), Tb2O3 (1.0 wt%), CeO2 (2.0 wt%) | 18030920 | [34] |
| Empress Cad (EC) |  | Ivoclar Vivadent | Leucite-Reinforced Glass-Ceramics | SiO2 (60.0–65.0 wt%), Al2O3 (16.0–20.0 wt%), K2O (10.0–14.0 wt%), Na2O (3.5–6.5 wt%), other oxides (0.5–7.0 wt%), pigments (0.2–1.0 wt%) | S50510 | [35] |
| Evaluated Material | Description of Morphological Changes in Cell Culture | Evaluation of Changes in Cell Culture | Cytotoxicity |
|---|---|---|---|
| NP-VS | no changes in the environment of the material and under the material; slight inhibition of cell growth under the sample | none | 0 |
| NP-VM2 | no changes in the environment of the material and under the material; slight inhibition of cell growth under the sample | none | 0 |
| NP-CD | individual cells have degenerated or distorted under the material | low | 1 |
| NP-EC | individual cells have degenerated or distorted under the material | low | 1 |
| NP-VS + Cu | individual cells have degenerated or distorted under the material | low | 1 |
| NP-VM2 + Cu | the zone of changed cells is limited to the surface under the material | moderate | 2 |
| NP-CD + Cu | no changes in the environment of the material and under the material; slight inhibition of cell growth under the sample | none | 0 |
| NP-EC + Cu | the zone of changed cells is limited to the surface under the material | moderate | 2 |
| P-VS | no changes in the environment of the material and under the material; slight inhibition of cell growth under the sample | none | 0 |
| P-VM2 | no changes in the environment of the material and under the material; slight inhibition of cell growth under the sample | none | 0 |
| P-CD | individual cells have degenerated or distorted under the material | low | 1 |
| P-EC | individual cells have degenerated or distorted under the material | low | 1 |
| Material | VS | VS + Cu | VM2 | VM2 + Cu | CD | CD + Cu | EC | EC + Cu |
|---|---|---|---|---|---|---|---|---|
| C. albicans ATCC 90028 [CFU/mL] | 4.1 × 102 ± 9.9 × 101 | 3.5 × 103 ± 2.8 × 103 | 3.9 × 103 ± 1.6 × 103 | 6.6 × 103 ± 6.8 × 103 | 1.3 × 103 ± 7.1 × 101 | 2.7 × 103 ± 7.8 × 102 | 4.2 × 103 ± 1.2 × 103 | 2.4 × 103 ± 1.4 × 103 |
| C. albicans ATCC 10231 [CFU/mL] | 1.0 × 103 ± 1.3 × 103 | 2.0 × 102 ± 9.2 × 101 | 1.2 × 103 ± 9.2 × 102 | 4.2 × 103 ± 4.6 × 103 | 7.0 × 102 ± 0 | 3.9 × 103 ± 1.6 × 103 | 1.1 × 103 ± 5.1 × 102 | 3.4 × 103 ± 2.4 × 103 |
| S. mutans ATCC 25175 [CFU/mL] | 3.5 × 102 ± 7.1 × 101 | 6.0 × 102 ± 8.5 × 102 | 0 | 0 | 2.0 × 102 ± 0 | 1.5 × 102 ± 7.1 × 101 | 5.0 × 101 ± 7.1 × 101 | 0 |
| L. rhamnosus ATCC 9595 [CFU/mL] | 0 | 5.0 × 101 ± 7.1 × 101 | 4.0 × 102 ± 2.8 × 102 | 3.2 × 103 ± 1.4 × 103 | 2.0 × 102 ± 0 | 7.5 × 102 ± 6.4 × 102 | 0 | 3.8 × 102 ± 1.4 × 102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piszko, A.; Grzebieluch, W.; Piszko, P.J.; Rusak, A.; Pajączkowska, M.; Nowicka, J.; Kobielarz, M.; Mikulewicz, M.; Dobrzyński, M. Cytotoxicity and Microbiological Properties of Ceramic CAD/CAM Materials Subjected to Surface Treatment with Nanometric Copper Layer. Appl. Sci. 2024, 14, 9224. https://doi.org/10.3390/app14209224
Piszko A, Grzebieluch W, Piszko PJ, Rusak A, Pajączkowska M, Nowicka J, Kobielarz M, Mikulewicz M, Dobrzyński M. Cytotoxicity and Microbiological Properties of Ceramic CAD/CAM Materials Subjected to Surface Treatment with Nanometric Copper Layer. Applied Sciences. 2024; 14(20):9224. https://doi.org/10.3390/app14209224
Chicago/Turabian StylePiszko, Aleksandra, Wojciech Grzebieluch, Paweł J. Piszko, Agnieszka Rusak, Magdalena Pajączkowska, Joanna Nowicka, Magdalena Kobielarz, Marcin Mikulewicz, and Maciej Dobrzyński. 2024. "Cytotoxicity and Microbiological Properties of Ceramic CAD/CAM Materials Subjected to Surface Treatment with Nanometric Copper Layer" Applied Sciences 14, no. 20: 9224. https://doi.org/10.3390/app14209224
APA StylePiszko, A., Grzebieluch, W., Piszko, P. J., Rusak, A., Pajączkowska, M., Nowicka, J., Kobielarz, M., Mikulewicz, M., & Dobrzyński, M. (2024). Cytotoxicity and Microbiological Properties of Ceramic CAD/CAM Materials Subjected to Surface Treatment with Nanometric Copper Layer. Applied Sciences, 14(20), 9224. https://doi.org/10.3390/app14209224











