Protein Fractions of Jackfruit Leaf Flour and Protein Concentrate: Amino Acid Profile, Functional Properties and Thermal Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemical Substances
2.3. Jackfruit LPC Extraction
2.4. Obtaining Protein Fractions of Jackfruit Leaf Flour and LPC
2.5. Amino Acid Content
2.6. Functional Properties of Protein Fractions
2.6.1. Hydrosolubility
2.6.2. Foaming Properties
2.6.3. Emulsifying Properties
2.7. Thermal Stability of the Fractions
2.8. Statistical Analysis
3. Results and Discussion
3.1. Protein Fractions of Jackfruit Leaf Flour and Protein Concentrate
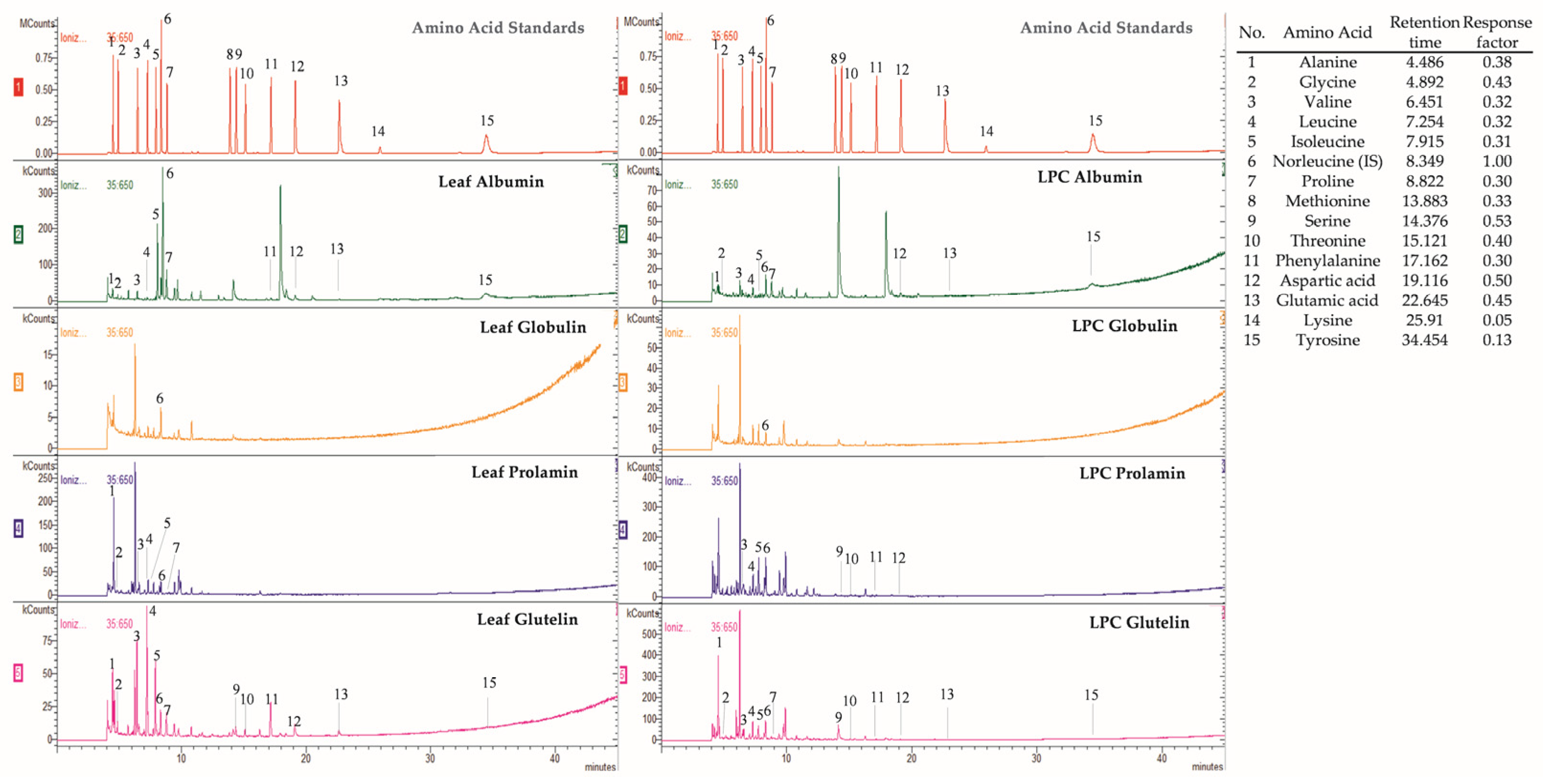
3.2. Amino Acid Profile
| Leaf Flour Fractions | Leaf Protein Concentrate Fractions | WHO/ FAO 7 | |||||
|---|---|---|---|---|---|---|---|
| Amino Acid | Albumin | Prolamin | Glutelin | Albumin | Prolamin | Glutelin | |
| Alanine | 0.34 ± 0.02 a | 0.09 ± 0.02 a | 4.00 ± 1.37 a | 0.34 ± 0.14 a | nd | 6.00 ± 0.40 b | |
| Glycine | 0.20 ± 0.04 a | 0.14 ± 0.01 a | 1.94 ± 1.11 a | 0.26 ± 0.14 a | nd | 3.32 ± 0.90 a | |
| Valine 3* | 0.50 ± 0.02 a | 0.13 ± 0.02 a | 6.54 ± 0.64 a | 0.50 ± 0.18 a | 0.16 ± 0.12 a | 8.44 ± 0.41 b | 3.9 |
| Leucine 3* | 0.13 ± 0.01 a | 0.19 ± 0.02 a | 8.58 ± 0.29 a | 0.31 ± 0.16 a | 0.28 ± 0.06 a | 10.01 ± 0.17 b | 5.9 |
| Isoleucine 3* | 0.21 ± 0.02 a | 0.10 ± 0.02 a | 5.01 ± 0.47 a | 0.33 ± 0.14 a | 0.14 ± 0.06 a | 5.83 ± 0.24 b | 3.0 |
| Proline | 3.07 ± 0.14 a | 0.39 ± 0.02 a | 2.52 ± 0.69 a | 1.82 ± 0.49 b | nd | 2.63 ± 0.45 a | |
| Serine | nd | nd | 0.90 ± 0.03 a | nd | 0.07 ± 0.01 a | 0.89 ± 0.03 a | |
| Threonine 3* | nd | nd | 1.70 ± 0.71 a | nd | 0.02 ± 0.02 a | 2.49 ± 0.24 a | 2.3 |
| Phenylalanine 3* | 0.13 ± 0.02 a | nd | 1.91 ± 0.53 a | nd | 0.03 ± 0.01 a | 3.05 ± 0.07 b | 3.8 ** |
| Aspartic acid | 0.23 ± 0.00 a | nd | 5.08 ± 0.34 a | 0.07 ± 0.08 b | 0.11 ± 0.04 a | 5.17 ± 0.26 a | |
| Glutamic acid | 0.44 ± 0.01 a | nd | 5.59 ± 2.30 a | 0.21 ± 0.02 b | nd | 7.30 ± 0.73 a | |
| Tyrosine | 0.08 ± 0.03 a | nd | 3.62 ± 2.26 a | 0.07 ± 0.00 a | nd | 3.88 ± 0.08 a | 3.8 ** |
| HAA 1 | 4.47 ± 0.19 a | 0.9 ± 0.09 a | 32.18 ± 3.45 a | 3.37 ± 1.04 b | 0.61 ± 0.20 b | 39.85 ± 0.19 b | |
| AAA 2 | 0.21 ± 0.04 a | nd | 5.52 ± 2.78 a | 0.07 ± 0.00 b | 0.03 ± 0.01 a | 6.94 ± 0.16 a | |
| EAA 3* | 0.98 ± 0.05 a | 0.42 ± 0.06 a | 23.74 ± 0.16 a | 1.14 ± 0.49 a | 0.63 ± 0.18 a | 29.82 ± 0.50 b | |
| NCAA 4 | 0.67 ± 0.02 a | nd | 10.67 ± 1.95 a | 0.28 ± 0.03 b | 0.11 ± 0.04 a | 12.47 ± 0.48 b | |
| BCAA 5 | 0.68 ± 0.01 a | 0.39 ± 0.06 a | 17.59 ± 0.61 a | 0.98 ± 0.45 a | 0.42 ± 0.12 a | 21.85 ± 0.01 b | |
| TAA 6 | 5.34 ± 0.25 a | 1.04 ± 0.09 a | 47.39 ± 7.19 a | 3.91 ± 0.89 b | 0.8 ± 0.15 a | 59.03 ± 1.84 b | |
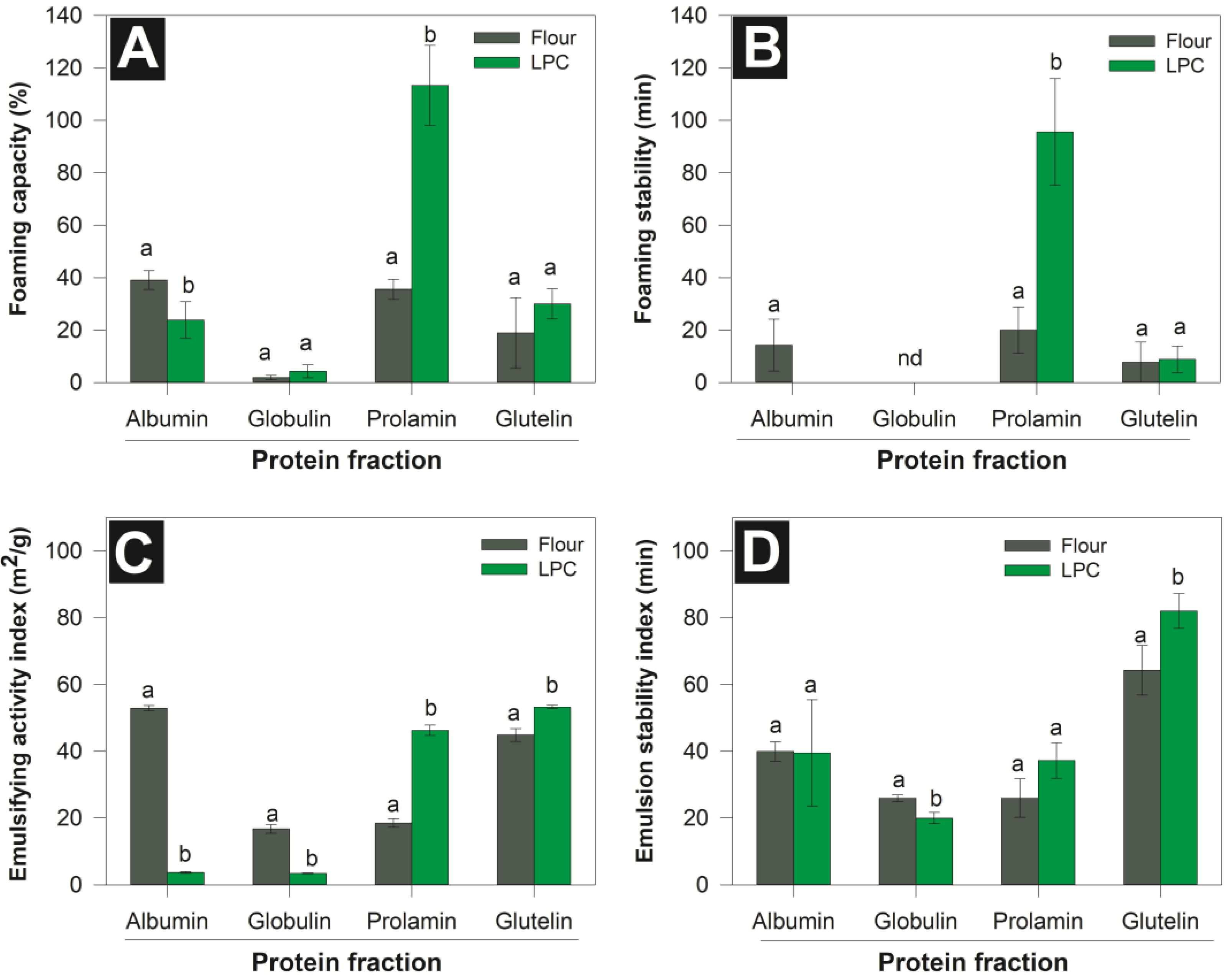
3.3. Functional Properties of Protein Fractions
3.3.1. Hydrosolubility
3.3.2. Foaming and Emulsifying Properties
3.4. Thermal Stability of the Fractions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henchion, M.; Hayes, M.; Mullen, A.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef]
- Ishwarya Shankaran, P.; Kumari, P. Nutritional Analysis of Plant-Based Meat: Current Advances and Future Potential. Appl. Sci. 2024, 14, 4154. [Google Scholar] [CrossRef]
- Duluins, O.; Baret, P.V. A Systematic Review of the Definitions, Narratives and Paths Forwards for a Protein Transition in High-Income Countries. Nat. Food 2024, 5, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Talwar, R.; Freymond, M.; Beesabathuni, K.; Lingala, S. Current and Future Market Opportunities for Alternative Proteins in Low- and Middle-Income Countries. Curr. Dev. Nutr. 2024, 8, 102035. [Google Scholar] [CrossRef] [PubMed]
- Fasolin, L.H.; Pereira, R.N.; Pinheiro, A.C.; Martins, J.T.; Andrade, C.C.P.; Ramos, O.L.; Vicente, A.A. Emergent Food Proteins—Towards Sustainability, Health and Innovation. Food Res. Int. 2019, 125, 108586. [Google Scholar] [CrossRef] [PubMed]
- Samborska, K.; Boostani, S.; Geranpour, M.; Hosseini, H.; Dima, C.; Khoshnoudi-Nia, S.; Rostamabadi, H.; Falsafi, S.R.; Shaddel, R.; Akbari-Alavijeh, S.; et al. Green Biopolymers from By-Products as Wall Materials for Spray Drying Microencapsulation of Phytochemicals. Trends Food Sci. Technol. 2021, 108, 297–325. [Google Scholar] [CrossRef]
- Domokos-Szabolcsy, É.; Yavuz, S.R.; Picoli, E.; Fári, M.G.; Kovács, Z.; Tóth, C.; Kaszás, L.; Alshaal, T.; Elhawat, N. Green Biomass-Based Protein for Sustainable Feed and Food Supply: An Overview of Current and Future Prospective. Life 2023, 13, 307. [Google Scholar] [CrossRef]
- Nynäs, A.-L.; Newson, W.R.; Langton, M.; Wouters, A.G.B.; Johansson, E. Applicability of Leaf Protein Concentrates from Various Sources in Food: Solubility at Food-Relevant PH Values and Air-Water Interfacial Properties. LWT 2023, 184, 114962. [Google Scholar] [CrossRef]
- Avilés-Gaxiola, S.; León-Félix, J.; Jiménez-Nevárez, Y.B.; Angulo-Escalante, M.A.; Ramos-Payán, R.; Colado-Velázquez, J.; Heredia, J.B. Antioxidant and Anti-Inflammatory Properties of Novel Peptides from Moringa oleifera Lam. Leaves. S. Afr. J. Bot. 2021, 141, 466–473. [Google Scholar] [CrossRef]
- Qoms, M.S.; Arulrajah, B.; Shamsudin, R.; Ibadullah, W.Z.W.; Saari, N. Valorization of Green Biomass Azolla Pinnata Fern: Multi-parameter Evaluation of Processing Conditions on Protein Extractability and Their Influence on the Physicochemical, Structural, Techno-functional Properties and Protein Quality. J. Sci. Food Agric. 2022, 102, 6974–6983. [Google Scholar] [CrossRef]
- Calderón-Chiu, C.; Calderón-Santoyo, M.; Herman-Lara, E.; Ragazzo-Sánchez, J.A. Jackfruit (Artocarpus heterophyllus Lam) Leaf as a New Source to Obtain Protein Hydrolysates: Physicochemical Characterization, Techno-Functional Properties and Antioxidant Capacity. Food Hydrocoll. 2021, 112, 106319. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Owolabi, I.O.; Fadairo, O.S.; Ghosal, A.; Coker, O.J.; Soladoye, O.P.; Aluko, R.E.; Bandara, N. Enzymatic Modification of Plant Proteins for Improved Functional and Bioactive Properties. Food Bioprocess. Technol. 2023, 16, 1216–1234. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, L.; Shi, Y.; Akhtar, M.; Chen, J.; Ettelaie, R. Emulsifying and Emulsion Stabilizing Properties of Soy Protein Hydrolysates, Covalently Bonded to Polysaccharides: The Impact of Enzyme Choice and the Degree of Hydrolysis. Food Hydrocoll. 2021, 113, 106519. [Google Scholar] [CrossRef]
- Day, L. Proteins from Land Plants—Potential Resources for Human Nutrition and Food Security. Trends Food Sci. Technol. 2013, 32, 25–42. [Google Scholar] [CrossRef]
- Hojilla-Evangelista, M.P.; Selling, G.W.; Hatfield, R.; Digman, M. Extraction, Composition, and Functional Properties of Dried Alfalfa (Medicago sativa L.) Leaf Protein. J. Sci. Food Agric. 2017, 97, 882–888. [Google Scholar] [CrossRef]
- Teixeira, E.M.B.; Carvalho, M.R.B.; Neves, V.A.; Silva, M.A.; Arantes-Pereira, L. Chemical Characteristics and Fractionation of Proteins from Moringa oleifera Lam. Leaves. Food Chem. 2014, 147, 51–54. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Calderón-Chiu, C.; Calderón-Santoyo, M.; Barros-Castillo, J.C.; Díaz, J.A.; Ragazzo-Sánchez, J.A. Structural Modification of Jackfruit Leaf Protein Concentrate by Enzymatic Hydrolysis and Their Effect on the Emulsifier Properties. Colloids Interfaces 2022, 6, 52. [Google Scholar] [CrossRef]
- Klompong, V.; Benjakul, S.; Kantachote, D.; Shahidi, F. Storage Stability of Protein Hydrolysate from Yellow Stripe Trevally (Selaroides leptolepis). Int. J. Food Prop. 2012, 15, 1042–1053. [Google Scholar] [CrossRef][Green Version]
- Pearce, K.N.; Kinsella, J.E. Emulsifying Properties of Proteins: Evaluation of a Turbidimetric Technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Sedlar, T.; Čakarević, J.; Tomić, J.; Popović, L. Vegetable By-Products as New Sources of Functional Proteins. Plant Foods Hum. Nutr. 2021, 76, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Tenorio, A.T.; Kyriakopoulou, K.E.; Suarez-Garcia, E.; van den Berg, C.; van der Goot, A.J. Understanding Differences in Protein Fractionation from Conventional Crops, and Herbaceous and Aquatic Biomass—Consequences for Industrial Use. Trends Food Sci. Technol. 2018, 71, 235–245. [Google Scholar] [CrossRef]
- Rodríguez, P.; Pérez, E.; Romel, G.; Dufour, D. Characterization of the Proteins Fractions Extracted from Leaves of Amaranthus Dubius (Amaranthus spp.). Afr. J. Food Sci. 2011, 5, 417–424. [Google Scholar]
- Lima, R.R.; Stephani, R.; Perrone, Í.T.; de Carvalho, A.F. Plant-Based Proteins: A Review of Factors Modifying the Protein Structure and Affecting Emulsifying Properties. Food Chem. Adv. 2023, 3, 100397. [Google Scholar] [CrossRef]
- Lamp, A.; Kaltschmitt, M.; Lüdtke, O. Improved HPLC-Method for Estimation and Correction of Amino Acid Losses during Hydrolysis of Unknown Samples. Anal. Biochem. 2018, 543, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Stenerson, K.K. The Derivatization and Analysis of Amino Acids by GC-MS. Rep. US 2011, 25, 1–3. [Google Scholar]
- WHO/FAO. World Health Organization/Food and Agricultural Organization. Report of a Joint WHO/FAO/UNU Expert Consultation 2007; WHO/FAO: Geneva, Switzerland, 2007. [Google Scholar]
- Gao, K.; Rao, J.; Chen, B. Plant Protein Solubility: A Challenge or Insurmountable Obstacle. Adv. Colloid. Interface Sci. 2024, 324, 103074. [Google Scholar] [CrossRef]
- Silva, R.M.d.; Vidigal, M.C.T.R.; Minim, V.P.R.; Minim, L.A. Evaluation of PH, NaCl and CaCl2 Salts on Solubility, Zeta Potential and Air—Water Interfacial Properties of the Protein Isolate from Lupin Seeds. Food Struct. 2023, 38, 100350. [Google Scholar] [CrossRef]
- Amagliani, L.; Silva, J.V.C.; Saffon, M.; Dombrowski, J. On the Foaming Properties of Plant Proteins: Current Status and Future Opportunities. Trends Food Sci. Technol. 2021, 118, 261–272. [Google Scholar] [CrossRef]
- Damodaran, S. Amino Acids, Peptides, and Protein. In Fennema’s Food Chemistry; CRC Press: Boca Raton, FL, USA, 2017; pp. 235–356. ISBN 9781315372914. [Google Scholar]
- Huamaní-Perales, C.; Vidaurre-Ruiz, J.; Salas-Valerio, W.; Cabezas, D.M.; Repo-Carrasco-Valencia, R. A Review of Techno-Functional Properties of Legume Proteins and Their Potential for Development of New Products. Eur. Food Res. Technol. 2024, 250, 2069–2092. [Google Scholar] [CrossRef]
- Quintero, J.; Rojas, J.; Ciro, G. Vegetable Proteins as Potential Encapsulation Agents: A Review. Food Res. 2018, 2, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Joardder, M.U.H.; Bosunia, M.H.; Hasan, M.M.; Ananno, A.A.; Karim, A. Significance of Glass Transition Temperature of Food Material in Selecting Drying Condition: An in-Depth Analysis. Food Rev. Int. 2024, 40, 952–973. [Google Scholar] [CrossRef]
- Ladjal-Ettoumi, Y.; Boudries, H.; Chibane, M.; Romero, A. Pea, Chickpea and Lentil Protein Isolates: Physicochemical Characterization and Emulsifying Properties. Food Biophys. 2016, 11, 43–51. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, C.; Zhang, T.; Ju, X.; He, R. Effects of Acylation and Glycation Treatments on Physicochemical and Gelation Properties of Rapeseed Protein Isolate. RSC Adv. 2018, 8, 40395–40406. [Google Scholar] [CrossRef] [PubMed]


| Protein Concentration (mg/g of Dry Solids) | Yield (%) | |||
|---|---|---|---|---|
| Fraction | Leaf Flour | LPC 1 | Leaf Flour | LPC 1 |
| Albumin | 3.80 ± 0.62 a | 9.77 ± 0.46 b | 1.58 ± 0.26 a | 1.48 ± 0.07 a |
| Globulin | 22.05 ± 0.48 a | 39.87 ± 1.67 b | 9.16 ± 0.20 a | 6.05 ± 0.25 b |
| Prolamin | 65.67 ± 0.23 a | 86.70 ± 0.67 b | 27.29 ± 0.10 a | 13.17 ± 0.1 b |
| Glutelin | 59.13 ± 0.63 a | 346.67 ± 0.96 b | 24.58 ± 0.26 a | 52.64 ± 0.15 b |
| Residue 2 | NA 3 | NA 3 | 37.39 | 26.65 |
| Event 1 | Event 2 | |||||
|---|---|---|---|---|---|---|
| Sample | Fraction | Tg 2 (°C) | Enthalpy (Δh, J/g) | Denaturation Temperature (Td, °C) | Enthalpy (Δh, J/g) | Denaturation Temperature (Td, °C) |
| Leaf flour | Albumin | 138.56 ± 0.01 a | 28.02 ± 0.12 a | 154.67 ± 0.08 a | 74.98 ± 0.02 a | 207.32 ± 0.01 a |
| Globulin | 155.97 ± 0.07 a | 1.63 ± 0.06 a | 196.04 ± 0.02 a | 4.94 ± 0.02 a | 263.23 ± 0.19 a | |
| Prolamin | 157.91 ± 0.01 a | 1.22 ± 0.08 a | 206.5 ± 0.07 a | 19.78 ± 0.26 a | 282.08 ± 0.15 a | |
| Glutelin | 136.50 ± 0.01 a | 14.47 ± 0.79 a | 158.35 ± 0.04 a | 34.89 ± 0.80 a | 271.02 ± 0.19 a | |
| LPC 1 | Albumin | 131.29 ± 0.04 b | 212.21 ± 0.92 b | 153.18 ± 0.01 b | nd | nd |
| Globulin | 152.99 ± 0.09 b | 0.22 ± 0.01 b | 208.86 ± 0.03 b | nd | nd | |
| Prolamin | 134.46 ± 0.01 b | 18.52 ± 0.60 b | 148.03 ± 0.09 b | 45.78 ± 0.25 b | 201.96 ± 0.12 b | |
| Glutelin | 133.25 ± 0.02 b | 40.51 ± 0.01 b | 153.82 ± 0.01 b | 97.83 ± 0.99 b | 200.86 ± 0.10 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderon-Chiu, C.; Calderón-Santoyo, M.; Ragazzo-Sánchez, J.A. Protein Fractions of Jackfruit Leaf Flour and Protein Concentrate: Amino Acid Profile, Functional Properties and Thermal Analysis. Appl. Sci. 2024, 14, 9155. https://doi.org/10.3390/app14209155
Calderon-Chiu C, Calderón-Santoyo M, Ragazzo-Sánchez JA. Protein Fractions of Jackfruit Leaf Flour and Protein Concentrate: Amino Acid Profile, Functional Properties and Thermal Analysis. Applied Sciences. 2024; 14(20):9155. https://doi.org/10.3390/app14209155
Chicago/Turabian StyleCalderon-Chiu, Carolina, Montserrat Calderón-Santoyo, and Juan Arturo Ragazzo-Sánchez. 2024. "Protein Fractions of Jackfruit Leaf Flour and Protein Concentrate: Amino Acid Profile, Functional Properties and Thermal Analysis" Applied Sciences 14, no. 20: 9155. https://doi.org/10.3390/app14209155
APA StyleCalderon-Chiu, C., Calderón-Santoyo, M., & Ragazzo-Sánchez, J. A. (2024). Protein Fractions of Jackfruit Leaf Flour and Protein Concentrate: Amino Acid Profile, Functional Properties and Thermal Analysis. Applied Sciences, 14(20), 9155. https://doi.org/10.3390/app14209155






