A Method to Obtain the Transducers Impulse Response (TIR) in Photoacoustic Imaging
Abstract
1. Introduction
2. Basic Principles of Solving TIR
3. Algorithms
4. Tests and Discussion
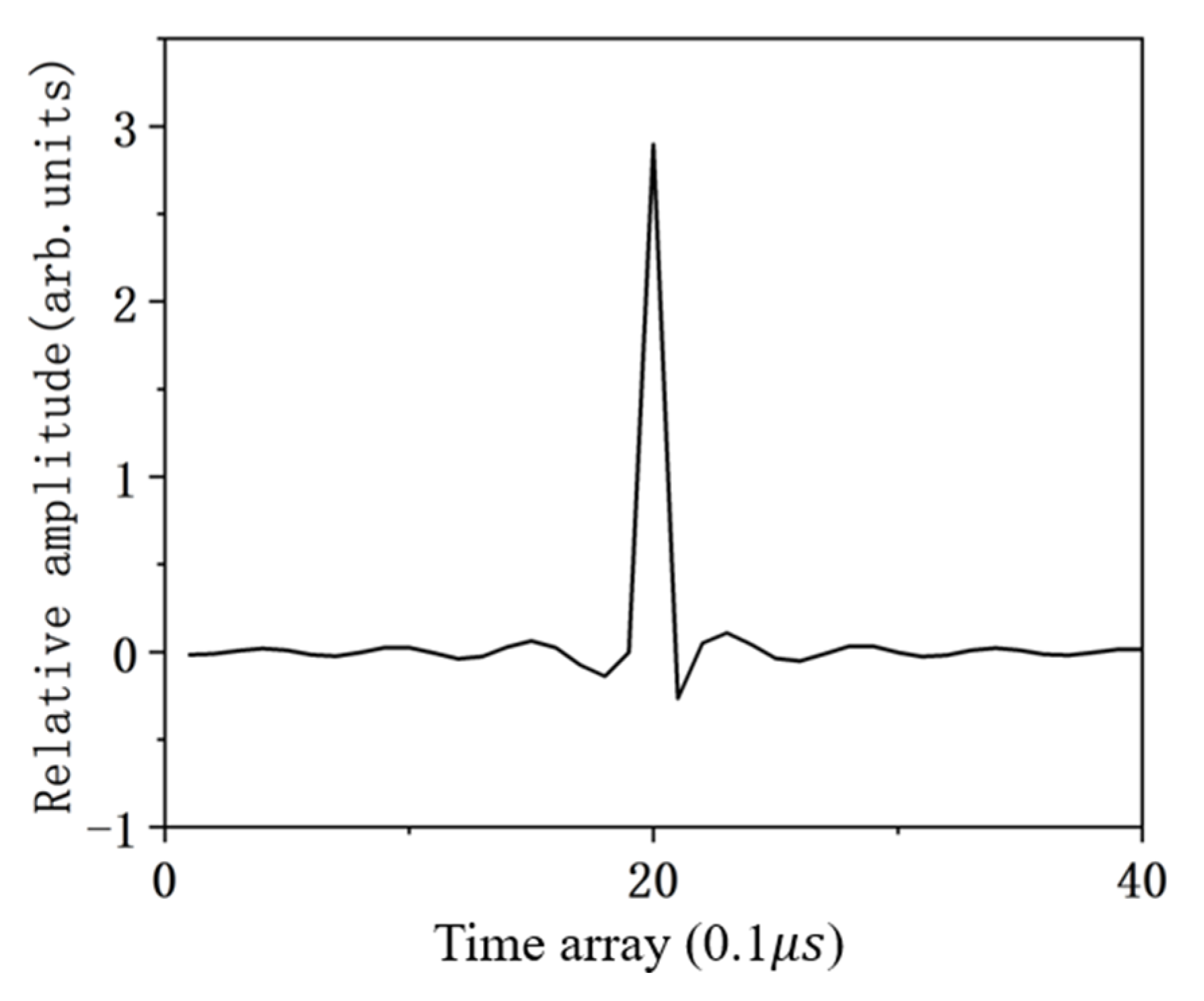
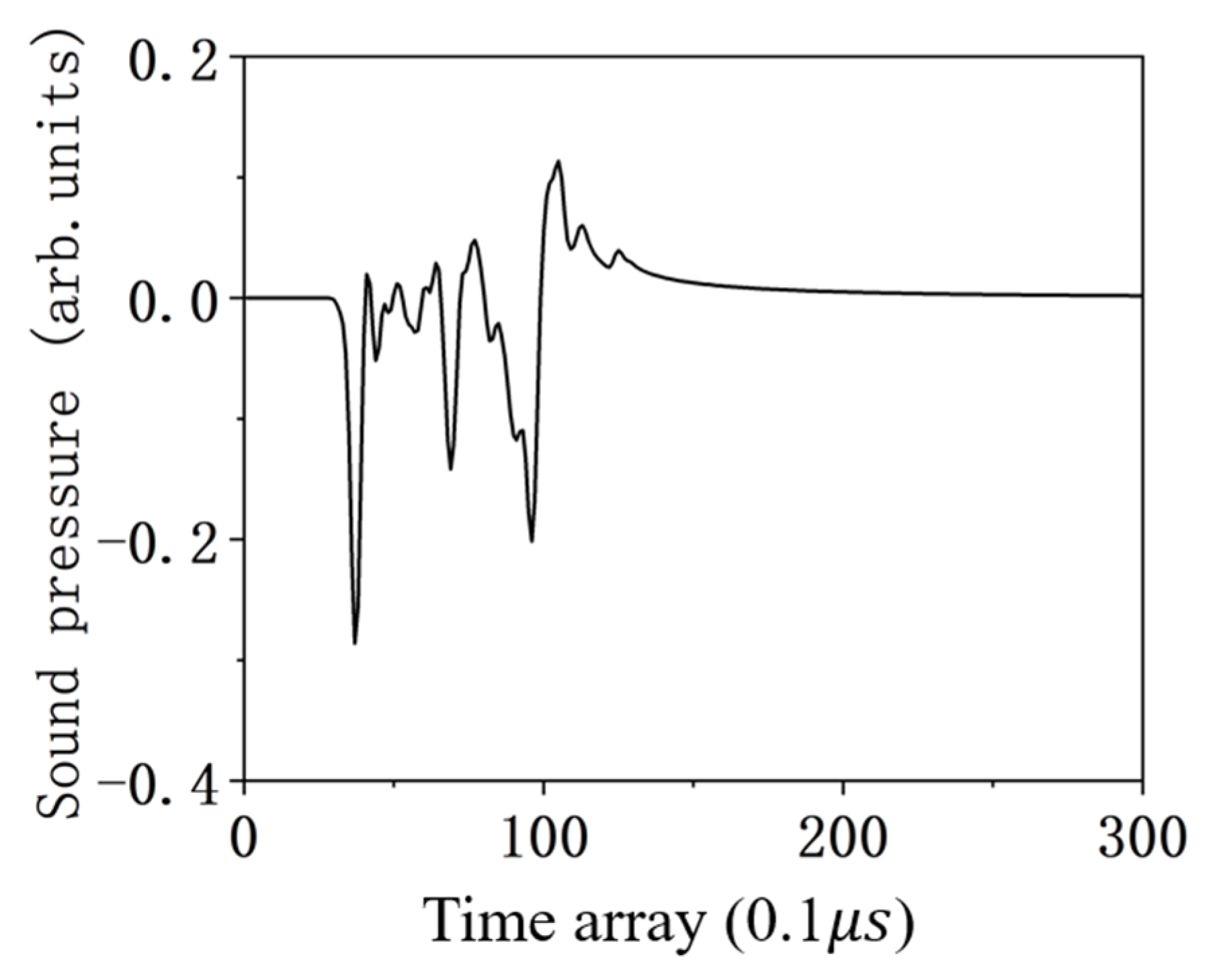
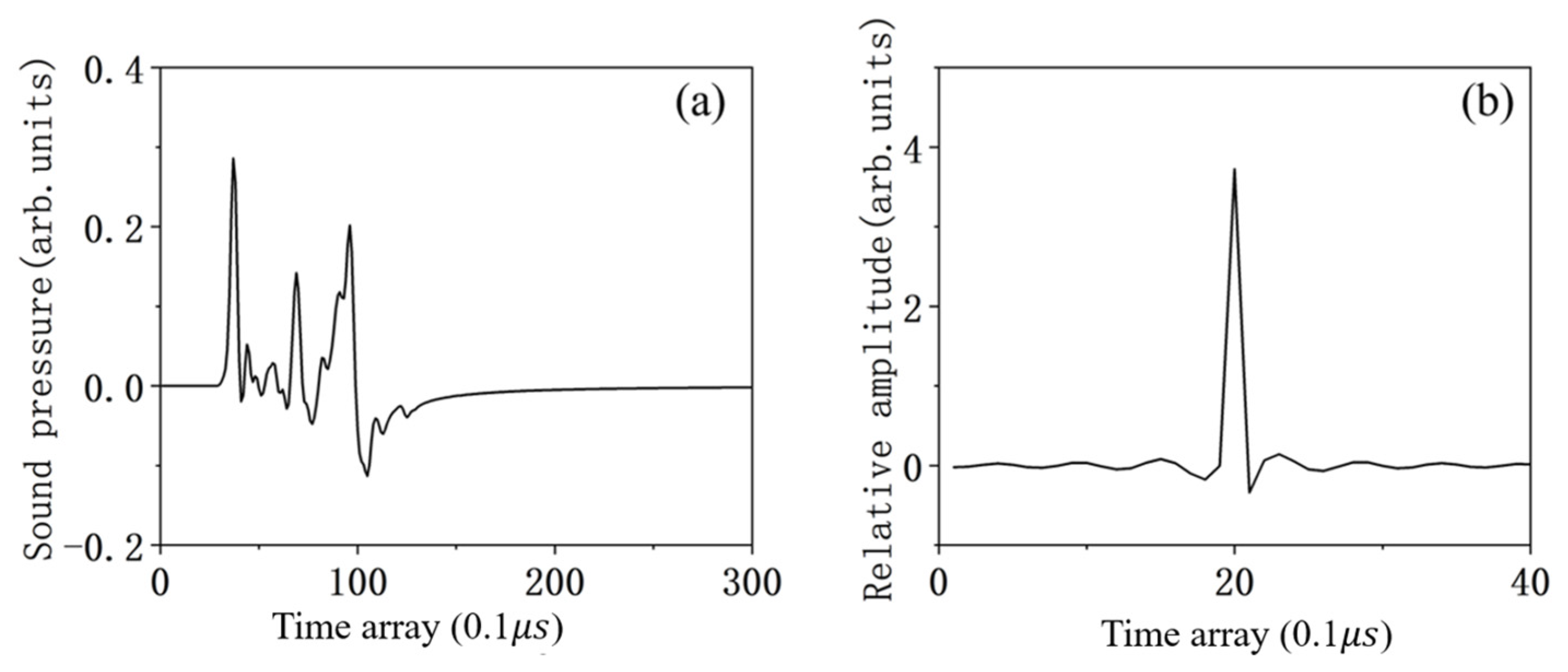
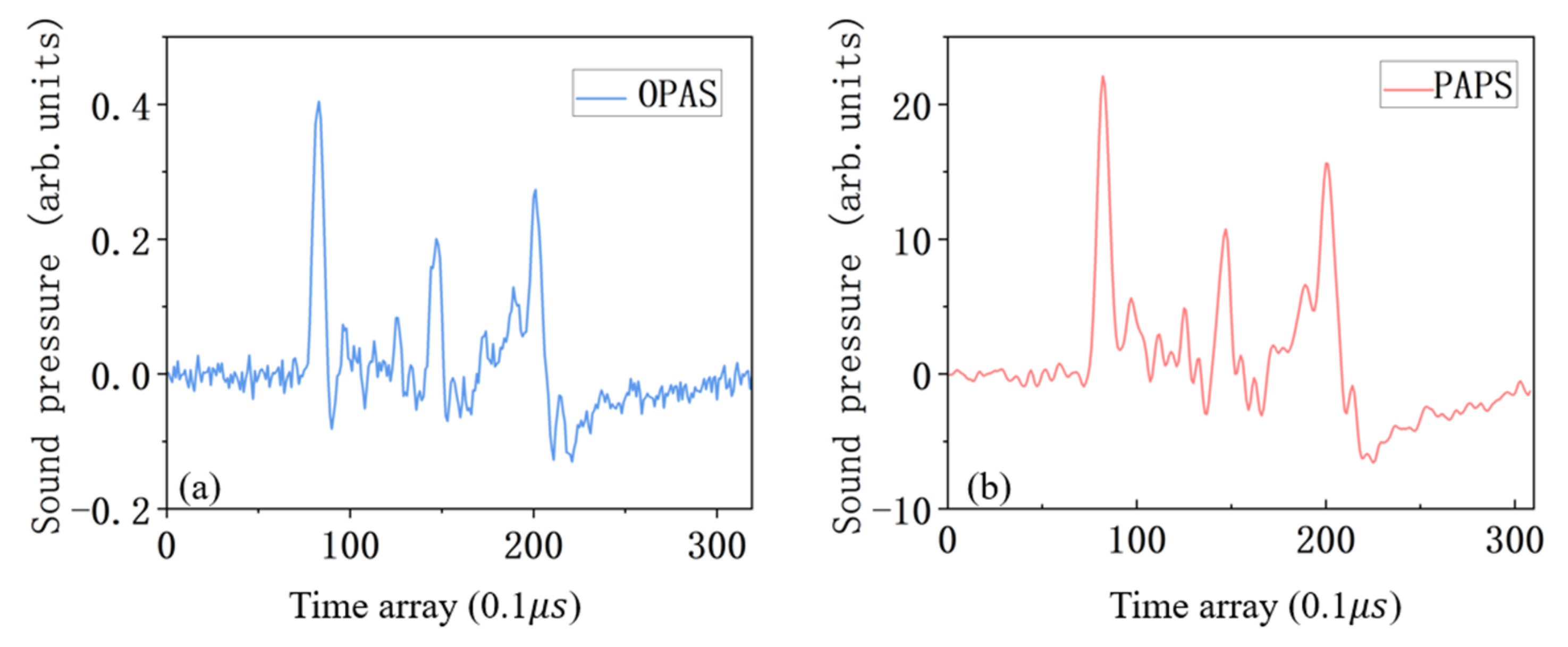
4.1. The Ability to Solve the TIR
4.2. The Effect of the Number of Transducers on the Results
4.3. The Effect of OPAS Length of the Transducer on Results
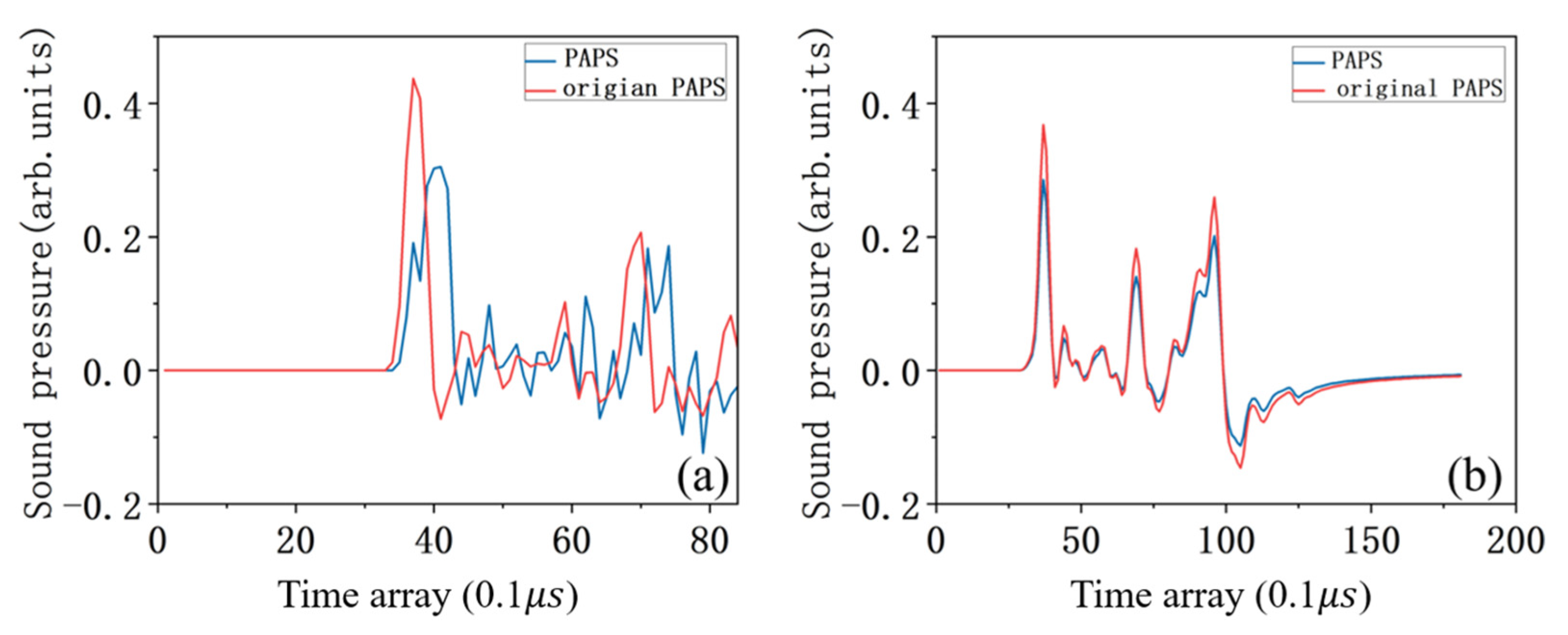
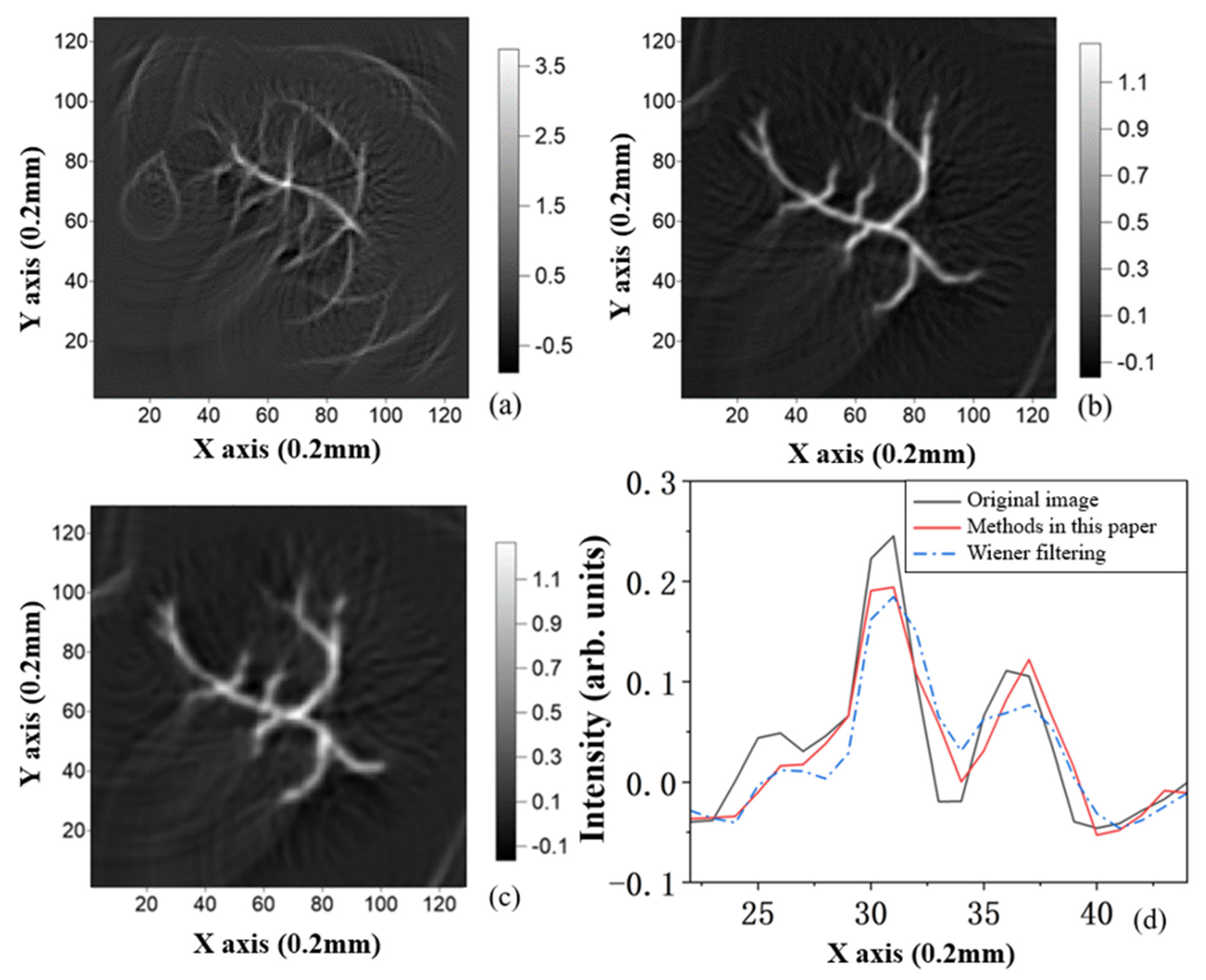
4.4. Comparison with Classical Wiener Filtering
5. Simulation of OPAS with Noise
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.V.; Hu, S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Paltauf, G.; Nuster, R.; Frenz, M. Progress in biomedical photoacoustic imaging instrumentation toward clinical application. J. Appl. Phys. 2020, 128, 180907. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.F.; Tao, Z.; Shung, K.K.; Zhu, B. Recent Advancements in Ultrasound Transducer: From Material Strategies to Biomedical Applications. BME Front. 2022, 2022. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Lee, J.E.-Y.; Lee, C. Thin-film PMUTs: A review of over 40 years of research. Microsyst. Nanoeng. 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- Herickhoff, C.D.; van Schaijk, R. cMUT technology developments. Z. Für Med. Phys. 2023, 33, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Oraevsky, A.A.; Savateeva, E.V.; Solomatin, S.V.; Karabutov, A.A.; Andreev, V.G.; Gatalica, Z.; Khamapirad, T.; Henrichs, P.M. Optoacoustic imaging of blood for visualization and diagnostics of breast cancer. Biomed. Optoacoust. 2002, 4618, 81–94. [Google Scholar]
- Hoelen, C.G.A.; de Mul, F.F.M.; Pongers, R.; Dekker, A. Three-dimensional photoacoustic imaging of blood vessels in tissue. Opt. Lett. 1998, 23 8, 648–650. [Google Scholar] [CrossRef]
- Siphanto, R.I.; Thumma, K.K.; Kolkman, R.G.M.; Leeuwen, T.G.v.; Mul, F.F.M.d.; Neck, J.W.v.; Adrichem, L.N.A.v.; Steenbergen, W. Serial noninvasive photoacoustic imaging of neovascularization in tumor angiogenesis. Opt. Express 2005, 13, 89–95. [Google Scholar] [CrossRef]
- Wang, X.; Pang, Y.; Ku, G.; Xie, X.; Stoica, G.; Wang, L.V. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat. Biotechnol. 2003, 21, 803–806. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.V. Rhesus monkey brain imaging through intact skull with thermoacoustic tomography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 53, 542–548. [Google Scholar]
- Li, C.; Wang, L.V. Photoacoustic tomography of the mouse cerebral cortex with a high-numerical-aperture-based virtual point detector. J. Biomed. Opt. 2009, 14, 024047. [Google Scholar] [CrossRef] [PubMed]
- Dangi, A.; Roy, K.; Agrawal, S.K.; Chen, H.; Ashok, A.; Wible, C.; Osman, M.; Pratap, R.; Kothapalli, S.-R. A modular approach to neonatal whole-brain photoacoustic imaging. In Photons Plus Ultrasound: Imaging and Sensing 2020; BiOS: Sydney, Australia, 2020. [Google Scholar]
- Pramanik, M.; Ku, G.; Li, C.; Wang, L.V. Design and evaluation of a novel breast cancer detection system combining both thermoacoustic (TA) and photoacoustic (PA) tomography. Med. Phys. 2008, 35, 2218–2223. [Google Scholar] [CrossRef] [PubMed]
- Ermilov, S.A.; Khamapirad, T.; Conjusteau, A.; Leonard, M.H.; Lacewell, R.D.; Mehta, K.; Miller, T.; Oraevsky, A.A. Laser optoacoustic imaging system for detection of breast cancer. J. Biomed. Opt. 2009, 14, 024007. [Google Scholar] [CrossRef] [PubMed]
- Kruger, R.A.; Miller, K.D.; Reynolds, H.; Kiser, W.L.; Reinecke, D.R.; Kruger, G.A. Breast cancer in vivo: Contrast enhancement with thermoacoustic CT at 434 MHz-feasibility study. Radiology 2000, 216, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Vaartjes, S.; van Hespen, J.C.G.; Klaase, J.M.; van den Engh, F.M.; Steenbergen, W.; van Leeuwen, T.G. Initial results of in vivo non-invasive cancer imaging in the human breast using near-infrared photoacoustics. Opt. Express 2007, 15, 12277–12285. [Google Scholar] [CrossRef]
- Piras, D.; Xia, W.; Steenbergen, W.; van Leeuwen, T.G.; Manohar, S. Photoacoustic Imaging of the Breast Using the Twente Photoacoustic Mammoscope: Present Status and Future Perspectives. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 730–739. [Google Scholar] [CrossRef]
- Song, K.H.; Stein, E.W.; Margenthaler, J.A.; Wang, L.V. Noninvasive photoacoustic identification of sentinel lymph nodes containing methylene blue in vivo in a rat model. J. Biomed. Opt. 2008, 13, 054033. [Google Scholar] [CrossRef]
- Erpelding, T.N.; Kim, C.; Pramanik, M.; Janković, L.; Maslov, K.; Guo, Z.; Margenthaler, J.A.; Pashley, M.D.; Wang, L.V. Sentinel lymph nodes in the rat: Noninvasive photoacoustic and US imaging with a clinical US system. Radiology 2010, 256, 102–110. [Google Scholar] [CrossRef]
- Pan, D.; Pramanik, M.; Senpan, A.; Ghosh, S.; Wickline, S.A.; Wang, L.V.; Lanza, G.M. Near infrared photoacoustic detection of sentinel lymph nodes with gold nanobeacons. Biomaterials 2010, 31, 4088–4093. [Google Scholar] [CrossRef]
- Pramanik, M.; Song, K.H.; Swierczewska, M.; Green, D.E.; Sitharaman, B.; Wang, L.V. In vivo carbon nanotube-enhanced non-invasive photoacoustic mapping of the sentinel lymph node. Phys. Med. Biol. 2009, 54, 3291–3301. [Google Scholar] [CrossRef]
- Lu, Q.-B.; Liu, T.; Ding, L.; Lu, M.-H.; Zhu, J.; Chen, Y.-F. Probing the Spatial Impulse Response of Ultrahigh-Frequency Ultrasonic Transducers with Photoacoustic Waves. Phys. Rev. Appl. 2020, 14, 034026. [Google Scholar] [CrossRef]
- Seeger, M.R.; Soliman, D.; Aguirre, J.; Diot, G.; Wierzbowski, J.; Ntziachristos, V. Pushing the boundaries of optoacoustic microscopy by total impulse response characterization. Nat. Commun. 2020, 11, 2910. [Google Scholar] [CrossRef] [PubMed]
- Ku, G.; Wang, X.; Stoica, G.; Wang, L.V. Multiple-bandwidth photoacoustic tomography. Phys. Med. Biol. 2004, 49, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Sheu, Y.-L.; Chou, C.-Y.; Hsieh, B.-Y.; Li, P.-C. Image reconstruction in intravascular photoacoustic imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Jiao, Y.; Jian, X.; Cui, Y. Image Deconvolution with Hybrid Reweighted Adaptive Total Variation (HRATV) for Optoacoustic Tomography. Photonics 2021, 8, 25. [Google Scholar] [CrossRef]
- Rathi, N.; Sinha, S.; Chinni, B.; Dogra, V.S.; Rao, N. Computation of Photoacoustic Absorber Size from Deconvolved Photoacoustic Signal Using Estimated System Impulse Response. Ultrason. Imaging 2021, 43, 46–56. [Google Scholar] [CrossRef]
- Kruger, R.A.; Reinecke, D.R.; Kruger, G.A. Thermoacoustic computed tomography—Technical considerations. Med. Phys. 1999, 26, 1832–1837. [Google Scholar] [CrossRef]
- Xu, M.; Wang, L.V. Time-domain reconstruction for thermoacoustic tomography in a spherical geometry. IEEE Trans. Med. Imaging 2002, 21, 814–822. [Google Scholar]
- Wang, Y.; Xing, D.; Zeng, Y.; Chen, Q. Photoacoustic imaging with deconvolution algorithm. Phys. Med. Biol. 2004, 49, 3117–3124. [Google Scholar] [CrossRef]
- Lu, T.; Mao, H. Deconvolution Algorithm with LTI Wiener Filter in Photoacousic Tomography. In Proceedings of the 2009 Symposium on Photonics and Optoelectronics, Wuhan, China, 14–16 August 2009; pp. 1–4. [Google Scholar]
- Qi, L.; Wu, J.; Li, X.; Zhang, S.; Huang, S.; Feng, Q.; Chen, W. Photoacoustic Tomography Image Restoration With Measured Spatially Variant Point Spread Functions. IEEE Trans. Med. Imaging 2021, 40, 2318–2328. [Google Scholar] [CrossRef]
- Bravo-Miranda, C.A.; Gonzalez-Vega, A.; Gutiérrez-Juárez, G. On the sensor influence in photoacoustic signal produced by point-like source. Biomed. Opt. 2013, 8581, 858144. [Google Scholar]
- Bravo-Miranda, C.A.; Gonzalez-Vega, A.; Gutiérrez-Juárez, G. Influence of the size, geometry and temporal response of the finite piezoelectric sensor on the photoacoustic signal: The case of the point-like source. Appl. Phys. B 2014, 115, 471–482. [Google Scholar] [CrossRef]
- Bravo-Miranda, C.A.; Gonzalez-Vega, A.; Gutiérrez-Juárez, G. Dependence of photoacoustic signal generation on the transducer and source type. Biomed. Opt. 2014. [Google Scholar]
- Rietsch, E. Euclid and the art of wavelet estimation, Part I: Basic algorithm for noise-free data. Geophysics 1997, 62, 1931–1938. [Google Scholar] [CrossRef]
- Koay, C.G.; Chang, L.-C.; Carew, J.D.; Pierpaoli, C.; Basser, P.J. A unifying theoretical and algorithmic framework for least squares methods of estimation in diffusion tensor imaging. J. Magn. Reson. 2006, 182, 115–125. [Google Scholar] [CrossRef]
- Veraart, J.; Sijbers, J.; Sunaert, S.; Leemans, A.; Jeurissen, B. Weighted linear least squares estimation of diffusion MRI parameters: Strengths, limitations, and pitfalls. NeuroImage 2013, 81, 335–346. [Google Scholar] [CrossRef]
- Treeby, B.E.; Cox, B.T. k-Wave: MATLAB toolbox for the simulation and reconstruction of photoacoustic wave fields. J. Biomed. Opt. 2010, 15, 021314. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Jing, X.; Yin, Z.; Chen, S.; Wang, C. A Method to Obtain the Transducers Impulse Response (TIR) in Photoacoustic Imaging. Appl. Sci. 2024, 14, 920. https://doi.org/10.3390/app14020920
Yang H, Jing X, Yin Z, Chen S, Wang C. A Method to Obtain the Transducers Impulse Response (TIR) in Photoacoustic Imaging. Applied Sciences. 2024; 14(2):920. https://doi.org/10.3390/app14020920
Chicago/Turabian StyleYang, Huan, Xili Jing, Zhiyong Yin, Shuoyu Chen, and Chun Wang. 2024. "A Method to Obtain the Transducers Impulse Response (TIR) in Photoacoustic Imaging" Applied Sciences 14, no. 2: 920. https://doi.org/10.3390/app14020920
APA StyleYang, H., Jing, X., Yin, Z., Chen, S., & Wang, C. (2024). A Method to Obtain the Transducers Impulse Response (TIR) in Photoacoustic Imaging. Applied Sciences, 14(2), 920. https://doi.org/10.3390/app14020920




