Abstract
A new method is presented for the simultaneous determination of 13 multiclass pesticides along with glyphosate. The multiclass pesticides were extracted by creating a soil slurry with 2% ethanol in water (v/v), and then, applying direct-immersion solid-phase microextraction (DI-SPME) with a new type of semi-disposable SPME fiber configuration called LC-Tips. The fibers were then retroextracted to ethanol, and aqueous ammonia was added to the slurry to extract glyphosate. Derivatization of the glyphosate extract was accomplished with a mixture of trifluoroacetic anhydride and trifluoroethanol, after which the reaction mixture was dried and resuspended with the SPME ethanol extract. To this, a mixture of analyte protectants was added, and it was analyzed by GC-MS/MS in multiple-reaction-monitoring mode. All analytes showed a coefficient of determination greater than 0.95 in the 0.1–100 µg/kg calibrated range, and the limits of detection were between 0.1 and 1 µg/kg, except for glyphosate, which was 0.01 µg/kg. The method shows relatively high replicate relative standard deviation (as much as 37% for five extractions at 20 µg/kg), but the isotopically labeled internal standard was effective at mitigating this effect for some analytes.
1. Introduction
The use of pesticides in modern industrial agriculture is nearly ubiquitous, as they are essential in maintaining food security for the increasingly urban world population [1]. However, a large number of currently used pesticides have been found to be detrimental to ecosystem health and prosperity. Furthermore, several pesticides with adverse effects are known to persist in soils for a long time after use, such as some banned organochlorines [2]. Recent studies have pointed to the large amount of pesticides present in agricultural soils, including the very commonly used herbicide glyphosate [3,4,5]. Due to its permanently ionic nature, glyphosate (Figure 1) cannot be analyzed in multiresidue methods, and is often quantified separately [3]. To our knowledge, no method has ever been published which could determine permanently ionic pesticides and their degradation products (glyphosate, aminomethylphosphonic acid, glufosinate) along with other pesticide residues: This work presents a first attempt.
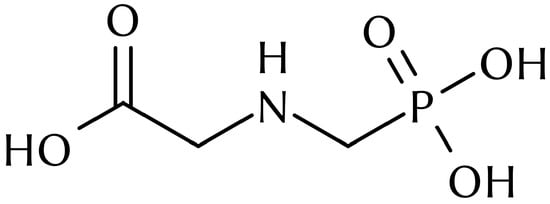
Figure 1.
Glyphosate in its (theoretical) neutral form. At every pH the molecule either has a net charge or is a zwitterion.
In general, methods for the analysis of pesticides in soil need to reach very low detection limits, due to the small quantities of these analytes often present in agricultural soils (commonly in the µg/kg range) [6], and also need to have a certain robustness in order to be able to extract the analytes from soils with different physical and chemical properties, the most important of which are soil texture, pH, and organic matter content [6]. In terms of GC-MS analysis, it is important to obtain an extract which is relatively free from matrix interferents, most notably those which are not detected by the technique, e.g., non-volatile compounds such as inorganic salts, which can compromise the instrument over many runs, causing among other things adsorption of the pesticides and eventual poor peak shape. Also, moderately volatile and/or polar matrix components can cause matrix-induced response enhancement, which results in the sample peaks being significantly larger and more symmetrical than those injected in pure solvent. Generally, matrix-matching calibration has been used to mitigate this problem, but the use of analyte protectants has also been proposed, in which moderately volatile compounds such as some sugars are added to the sample in order to “protect” the analytes from chemical adsorption, resulting in the same effect as matrix-induced response enhancement [7], both in the samples and calibration solutions.
When designing a method which could quantify glyphosate along with other commonly used pesticides, several avenues were considered. Since the extraction of glyphosate from soil is always performed with an aqueous solution [8], two serial extractions would always be needed, since low-polarity analytes will not readily extract onto aqueous phases. Furthermore, since glyphosate extraction is commonly performed with very basic solutions (either with KOH or aqueous NH3), and some pesticides are known to degrade under basic pH [9], it was thought that the aqueous extraction would have to occur last. Thus, the idea was to modify a previously existent multiresidue method which can extract organochlorines, organophosphates, triazoles, among others, and then, perform the glyphosate extraction. The most important condition would be that the first extraction did not degrade or modify the sample to an extent that the glyphosate extraction would be impaired. Thus, QuEChERS was ruled out, since it requires the addition of salts, which would have made the aqueous extraction impossible.
The original conception was to perform ultrasound-assisted extraction (UAE) with an organic solvent, remove the entirety of the extract volume, and then, add the aqueous solution for glyphosate extraction. However, UAE has several drawbacks and does not inherently provide significant concentration factors [6]. Also, the removal of the entire extract solvent volume is operationally challenging, even after centrifugation, and could induce repeatability problems. Since a method for the multiresidue analysis of pesticides from soil using direct-immersion solid-phase microextraction (DI-SPME) was being developed [10], it was thought that it could be adapted to provide the extraction for this combined methodology.
The employment of direct-immersion SPME for soil analysis is made possible by a new type of semi-disposable fiber called SPME LC-Tips [11,12]. These fibers present one major advantage over traditional SPME, namely, their much reduced cost. Whereas commonly used SPME assemblies would quickly degrade when in contact with a stirred soil slurry (via abrasion), and thus, only be usable for a few samples, resulting in extremely high per-sample costs, these new fibers, although they can be used only one to three times, still provide a cost-effective analysis which rivals commonly used methodologies such as QuEChERS [13]. The major downside of this technique is that, unlike traditional SPME where the fiber can undergo direct desorption onto the GC inlet [14], SPME LC-Tips require solvent desorption prior to injection onto the GC or LC. This greatly reduces the concentration factor of the extraction, because only a small percentage of the solvent extract is injected onto the chromatographic system, whereas for direct thermal desorption nearly the entire analyte mass on the fiber can be introduced onto the column. An attractive (but costly) possibility for direct desorption using LC-Tips is to use direct analysis in real time coupled with mass spectrometry (DART-MS), especially using a high-resolution mass analyzer to differentiate analytes with similar masses, where the pesticides trapped in the fiber are desorbed and ionized by a heated stream of excited and ionized helium or nitrogen, allowing their analysis with increased sensitivity and without the need for solvent retroextraction, chromatographic separation, or the increase in soil sample mass [15]. Nevertheless, for the proposed combined method, solvent desorption is ideal, as the SPME extract can easily be combined with the glyphosate extract.
2. Materials and Methods
2.1. Soil Sampling
The soil sample was collected from Idanha-a-Nova Municipality in Portugal (39′8454º N, 7′2544º W), at a depth of 25–30 cm. The land where the soil was sampled used to grow tobacco. Once in the laboratory, samples were sieved through a 2 mm mesh and allowed to air-dry at 22 ºC. The soil had a sandy-loam texture (25% coarse sand, 40.2% fine sand, 17.7% silt, and 17.1% clay), and 3.3% organic matter (st. deviation = 0.3, n = 5). Soil pH was 7.73 ± 0.06 (4 g with 10 mL of milli-Q water, shaken for 1 h, n = 3) and 6.94 ± 0.05 (4 g with 10 mL of 1 M KCl in milli-Q water, shaken for 1 h, n = 3).
2.2. Standards and Chemicals
Individual standards for 2-methyl-4-chlorophenoxyacetic acid (MCPA), buprofezin, 2,4-dichlorophenoxyacetic acid (2,4-D), aminomethylphosphonic acid (AMPA), boscalid, chlorpyrifos, diflufenican, epoxiconazole, glyphosate, malathion, metalaxyl, metazachlor, metolachlor, penconazole, prosulfocarb tebuconazole, tefluthrin, tetraconazole, and terbuthylazine were of analytical grade, obtained from Sigma-Aldrich (Steinheim, Germany). A multiresidue standard for organochlorine pesticides (at 100 µg/mL each) was obtained from Restek (Bellefonte, PA, USA). Penconazole-d7 was obtained from Toronto Research Chemicals (Toronto, ON, Canada). Glyphosate 1,2-13C2-15N was obtained from Dr. Ehrenstorfer (Augsburg, Germany). Water used in the extraction and dilutions was ultrapure, produced in a Milli-Q plus system from Millipore (Bedford, MA, USA). Absolute ethanol (EtOH) and methanol were of HPLC grade, obtained from Honeywell (Charlotte, NC, USA). Dichloromethane was GC-MS grade, purchased from Carlo-Erba (Emmendingen, Germany). The remaining materials were all purchased from Sigma-Aldrich, namely, C18 SPME LC-Tips, trifluoroacetic anhydride and trifluoroethanol (≥99% purity), 25% aqueous ammonia solution (p. a. grade), 3-ethoxy-1,2-propanediol, gulonolactone, and D-sorbitol (analyte protectants) of purities 98%, 95%, and 99%, respectively. Stock solutions for the pesticides were prepared in methanol at 250 µg/mL, and kept refrigerated at −20 ºC, for at most one month. Dilutions for injection and soil spiking were performed in methanol. A stock solution of the three analyte protectants was prepared in a 50/50 (v/v) solution of ethanol–methanol at 1000 µg/mL each [7]. The internal standard solution (penconazole-d7 and glyphosate 1,2-13C2-15N) was prepared with 80/20 (v/v) methanol–water at 800 ng/mL each.
2.3. GC-MS/MS Analysis
The analyses were performed by gas chromatography–tandem mass spectrometry (GC-MS/MS) on a Bruker GC 456 and a Bruker Scion TQ (Triple Quadrupole) system equipped with a CTC CombiPAL automatic injector and a programmable temperature vaporizer (PTV) inlet (Bruker 1079). Data were acquired with Bruker MSWS 8.2 and analyzed with Bruker MS Data Review 8.0. Chromatographic separation was achieved on a ZB-5MS Plus capillary column (20 m × 0.18 mm i.d., 0.18 µm df). The oven temperature program began at 40 ºC, where it was held for 3 min, increased at 20 ºC/min to 140 ºC, then 4 ºC/min to 250 ºC, and finally, 20 ºC/min to 300 ºC, where it was held for 1 min. Helium of 99.9999% purity was used as the carrier gas at a constant flow rate of 0.7 mL/min. The injection volume was 5 µL, performed in PTV large-volume mode, starting at 80 ºC with a split ratio of 1:120, held for 30 s, then splitless and with a temperature increase of 200 ºC/min to 270 ºC. At 3 min, the split valve was opened at a ratio of 1:60, and after 3 more minutes reduced to 15 mL/min and held for the entire run. The mass spectrometer system was operated in multiple-reaction monitoring (MRM), with argon as collision gas at 2.4 mTorr. The transfer line was held at 290 ºC, and the ion source at 270 ºC. The solvent delay was set to 7 min. MRM transitions associated with the selected precursor and product ion pairs of the analytes can be found in the Supplementary Material.
2.4. Final Method
A mass of 2 g of soil sample was weighed onto the extraction vial, to which was added 50 µL of internal standard solution, and it was allowed to air-dry for 1 h. C18 LC-Tip SPME fibers were conditioned by being inserted into a 2 mL glass vial containing 50/50 (v/v) ethanol–water for 20 min, followed by re-equilibration in another 2 mL glass vial containing water with 10% ethanol (v/v) for 10 min, under constant agitation at 300 rpm.
DI-SPME extraction: A volume of 10 mL of 2% ethanol in water (v/v) was added to the extraction vial, then the conditioned fiber was inserted into this solution, which was agitated for 3 h at 1000 rpm with a magnetic stir-bar. Then, the fiber was removed and immediately inserted into a 300 µL vial containing 120 µL of ethanol, and retroextracted for 30 min at 300 rpm, after which it was removed and the extract was stored in the fridge.
Glyphosate extraction: As soon as the fiber was removed from the extraction vial, 1.7 mL of 25% aqueous ammonia solution was added to it, and the soil was stirred at 1200 rpm for 1 h, at the same time that the fiber was being retroextracted. Afterwards, 1 mL of the aqueous solution from the extraction vial was added to a 2 mL Eppendorf tube, and 50 µL of dichloromethane was added, vortexed for 10 s, and centrifuged for 5 min at 4000 rpm. Then, 500 µL of the supernatant were transferred to a 1 mL reaction vial (Thermo Scienfitic, Waltham MA, USA), and completely dried under a nitrogen stream at 60 ºC. Then, 20 µL of 18% (m/m) aqueous hydrochloric acid was added to the reaction vial, and re-dried under nitrogen at 60 ºC. Afterwards, 200 µL of trifluoroacetic acid and 100 µL of trifluoroethanol were added to the reaction vials, and they were maintained at 100 ºC for 1 h. After being allowed to cool to room temperature, the vials were opened and gently dried under nitrogen.
Final extract: The ethanolic fiber extracts were removed from the fridge and allowed to return to room temperature, after which 80 µL of the extract was added to the dry reaction vial, which was then closed, vortexed for 10 s, and sonicated for 1 min. Finally, 20 µL of the analyte protectant solution was added to the reaction vial and the mixture was removed onto an autosampler vial and analyzed by GC-MS/MS. A graphical representation of the method can be seen in Figure 2.
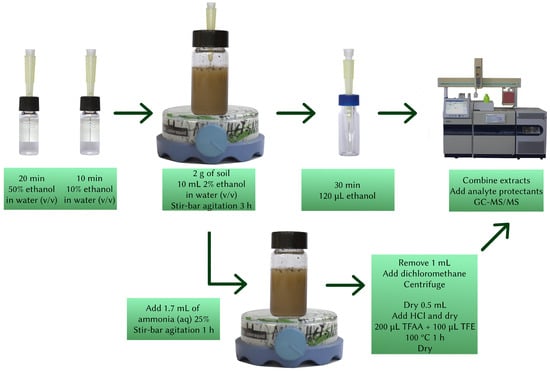
Figure 2.
Schematic representation of the full method. SPME tip retroextraction happens at the same time as the extraction with ammonia solution.
2.5. Method Performance
Calibration curves were performed by spiking various 2 g samples with the appropriate amount of standard containing all the analytes to obtain seven different concentrations in the range of 0.1–100 µg per kg of soil, and then, drying the soil under a very gentle nitrogen stream, before following the full method described above. Each concentration was extracted in triplicate, except for 20 µg/kg, which was extracted five times, for the repeatability and recovery calculations. In order to determine recovery, a calibration was performed for glyphosate without internal standard, in the range of 0.1–10 ng, added directly to the derivatization vial, and then, dried under a gentle nitrogen stream.
3. Results and Discussion
3.1. Multiclass Pesticide Extraction by Immersion SPME
The pesticide selection chosen for the method development included several pesticide classes: Chloroacetamides (metazachlor and metolachor), organochlorines (aldrin, lindane and pentachloroanisole), organophosphates (malathion and chlorpyrifos), triazoles (tetraconazole, penconazole and tebuconazole), as well as one pyrethroid, thiocarbamate, and unclassified pesticides, namely, tefluthrin, prosulfocarb, and buprofezin. The aim was to gauge the applicability of the developed method towards different chemical properties.
The original multiclass extraction method which was adapted used 6% methanol in water (v/v) as a solvent for immersion SPME, followed by desorption of the fiber in methanol and subsequent injection [10]. In the present work, ethanol was used instead, mainly because it has a much smaller toxicity (negligible for humans considering normal laboratory exposure levels), can be produced more easily from renewable feedstocks, and is slightly less polar, which is relevant for the extraction of organochlorines, especially. Furthermore, most salts have lower solubility in ethanol than methanol [16], which is important if in the future this method is applied to soils with greater salt content, where the use of methanol for redissolution of the derivatized glyphosate extract might introduce salts into the chromatographic system. From the two fiber chemistries available, PDMS/DVB and C18, the latter was chosen because the method introduces the analysis of organochlorines, not contemplated in the previous work [10].
Initially, a three factor Box–Behnken design was used as an attempt to optimize the immersion SPME extraction It modeled the percentage of ethanol in the extraction solvent (2%, 6%, and 10%, v/v), the extraction time (60, 90, and 120 min), and the retroextraction volume (100, 120, and 140 µL). However, the results showed that for most analytes, the extraction time was by far the most relevant factor, as it seemed that even 120 min were insufficient to attain equilibrium. Thus, this factor was far outside the values at which a response surface might show a maximum. As a result, the retroextraction volume was fixed to 120 µL (as a compromise between obtaining a good concentration factor and enough working volume), and a two-factor, three-level full-factorial experimental design was constructed (nine experiments), where the percentages of ethanol in the extraction solvent were 2, 6, and 10%, and the extraction times were 120, 150, and 180 min. Each experiment was repeated three times, for a total of 27 runs. The results (in terms of maximum average signal) are presented in Table 1.

Table 1.
Results of the two-factor, three-level full-factorial design. Signals were normalized, with the largest signal for each analyte being 100 and the others scaled accordingly.
From the results, 180 min was selected as the extraction time. A long extraction time is not ideal, because the full method including glyphosate extraction and derivatization takes several hours, and at worst can be longer than a single 8 h shift, which would compromise its applicability in routine laboratories without the implementation of process automation. The results for just 180 min of extraction can be seen in Figure 3.
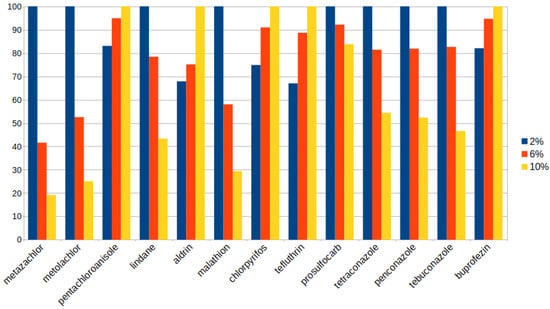
Figure 3.
Relative signal values for 180 min extraction time under 2, 6, and 10% ethanol extractions.
From these results, 2% ethanol in water was chosen as the extraction solvent. Although the more nonpolar compounds were favored by the greater percentage of ethanol, most had the highest signals at 2%. Furthermore, a smaller amount of ethanol would be favorable for the subsequent glyphosate extraction, as the organic modifier would not help in the dissolution of glyphosate. Interestingly, no compound had a maximum at 6% ethanol, which seems to suggest that the ideal percentage for each pesticide lies either closer to 2% or 10%, or outside the evaluated range.
3.2. Glyphosate Extraction
The extraction of glyphosate from the soil matrix had to be carried out via some modifications to the extraction solvent for immersion SPME. After the three hours of extraction, the SPME fiber was removed from the soil slurry and retroextracted onto ethanol. Concurrently, the glyphosate extraction was performed. There were two possibilities explored: addition of potassium hydroxide (KOH) [3] or ammonia solution [17]. The ammonia was chosen because it could be almost entirely removed by drying the extract. KOH, on the other hand, could not be removed from the extract, and although it has a relatively low solubility in ethanol (as does KCl, which is formed after neutralization with hydrochloric acid), it is still sufficient to cause accumulation in the GC liner and eventual clogging and chemisorption. Thus, when an extraction with KOH was assayed (0.6 molar, added dry to the extraction slurry), an extra step of extraction after the derivatization was required. Furthermore, whether neutralization with hydrochloric acid was performed or not, the large amount of salts interfered with the derivatization step, especially at low concentrations of glyphosate.
The sample-to-extraction solvent ratio was slightly modified from a previous work [17], except only one extraction was performed instead of two. Using a larger sample size (e.g., 3 or 4 g) while retaining a similar extraction solvent volume could have been employed in an attempt to improve detection and quantification limits for the multiresidue pesticides extracted with DI-SPME, but would have been operationally more challenging (especially in terms of shaking), and might have compromised repeatability and extractability in terms of the soil–solvent equilibrium, even though it would ultimately result in a larger analyte mass being extracted onto the SPME fiber. Also, this could compromise the glyphosate recovery, unless a larger extraction solvent volume was added after DI-SPME.
After the extraction, a small amount of dichloromethane had to be added to the extract because it was found that it contained non-negligible amounts of the other analytes, and their presence in the aqueous solvent could compromise repeatability. The simple centrifugation of the extract both removed suspended soil particles, thus eliminating the need for filtration, and settled the dichloromethane layer. However, for finer soils (notably clay), it may be required to centrifuge at higher speeds for longer. Neutralization of the extract with hydrochloric acid before centrifugation (in order to precipitate some soil matrix components) was not feasible because of the formation of non-volatile salts which could not be removed in the drying step.
For derivatization of the glyphosate extract, two methods were tested: silylation with N-(tert-butyldimethylsilyl)-N-methyltrifluoroacetamide (MTBSTFA) co-dissolved with acetonitrile [18,19], and simultaneous acylation and esterification with trifluoroacetic anhydride (TFAA) and trifluoroethanol (TFE) [17,20]. The silylation with MTBSTFA suffers from several drawbacks, including high reactivity of the reagent with water (more problematic than for TFAA), high cost per sample, and poor reaction yields for trace amounts of glyphosate [21], possibly caused by low solubility of the underivatized compound and adsorption to the glass vial. Furthermore, neither MTBSTFA nor the other reaction products are sufficiently volatile to allow drying of the mixture after derivatization, which compromises the subsequent combination with the SPME extract. Thus, derivatization with TFAA + TFE was chosen.
One of the drawbacks of this approach was that the glyphosate derivative was much more volatile than the other pesticides analyzed. This was even more pronounced for gylphosate’s degradation product aminomethylphosphonic acid (AMPA), which was also tested in this method. When a starting GC oven temperature of 50 ºC was used, AMPA’s derivative showed poor peak shapes with significant variability between injections of the same extract. As a consequence, the initial oven temperature was reduced to 40 ºC to permit better focusing of this analyte’s band at the top of the column.
3.3. Combined Method and Performance
For the combined method, it was imperative to ensure proper dissolution of the derivative in the ethanol SPME extract. Sonication was employed in an attempt to ensure full dissolution, and thus, good repeatability. The full method was also tested for two phenoxy herbicides: 2-methyl-4-chlorophenoxyacetic acid (MCPA) and 2,4-dichlorophenoxyacetic acid (2,4-D). It was possible to extract these compounds along with glyphosate due to the fact that at the extraction pH (around 12) the carboxylate is dominant, with almost no neutral carboxylic acid molecules present (calculated from the phenoxyacetic acid pKa). However, the repeatability was poor at every concentration (20–100 µg/kg), with coefficients of determination of 0.87 for MCPA and 0.88 for 2,4-D. This was likely caused by the extraction itself, or by the fact that a certain amount of the analytes might have been dissolved in the dichloromethane phase. Naturally, the isotopically labeled glyphosate was very poor at correcting extraction variability, but it may be possible to analyze these compounds with this methodology by using another internal standard which is chemically similar to them, although adding more isotopically labeled compounds to a method will significantly increase its cost per sample. For AMPA, there was also significant lack of repeatability (R2 = 0.91), but this was probably also due to the poor chromatographic performance, since the volatile AMPA derivative is not compatible with the programmed temperature volatilization injection technique used.
Table 2 presents the method performance parameters. The extraction recovery for glyphosate was 92%, with a relative standard deviation of 12% (n = 3). Although for some compounds the internal standard is essential, for others (such as tebuconazole and tefluthrin) it did not increase repeatability. Glyphosate had a lower limit of detection, likely because of the greater concentration factor. Even though SPME is known for greatly concentrating samples, that effect is not present in this method, because instead of direct desorption onto the GC inlet (which transfers the whole extracted analyte mass onto the GC-MS), it employs solvent desorption onto 120 µL of ethanol, of which only 5 µL is injected. Nevertheless, in the future an increase in soil sample mass (e.g., to 4 g) might be an interesting avenue for exploration; it could lower limits of detection, although it could also increase RSDs. Another weakness of the method is that it does not take advantage of the inherent clean-up performed by SPME, since the SPME extract is then mixed with the glyphosate extract, whose only clean-up was the addition of dichloromethane. This results in a final sample with much more matrix interferents than the SPME extract alone, although these were not noticeable in the MRM chromatogram.

Table 2.
Method performance parameters. Limit of detection (LoD) was the lowest calibration concentration with a signal-to-noise ratio greater than 3, and limit of quantitation (LoQ) was that which was greater than 10. Values for LoD and LoQ in µg/kg. %RSD with and without internal standard (IS) refers to 20 µg/kg concentration, extracted five times.
The method was also tested for other currently used pesticides, namely, boscalid, diflufenican, epoxiconazole, metalaxyl, and terbutylazine, but was found lacking in terms of precision (R2 = 0.899–0.94; RSD as high as 40% for 20 µg/kg extractions; n = 5). In the future, an isotopically labeled internal standard which better mimics the chemical properties of these compounds should be used to mitigate such problems. Further optimization of the method in order to achieve better concentration factors from the immersion-SPME extraction would be desirable. In terms of the GC-MS/MS determination, it was possible to isolate every analytes’ signal, and thus, a combined method involving more compounds could be viable. A chromatogram from a spiked sample can be seen in Figure 4.
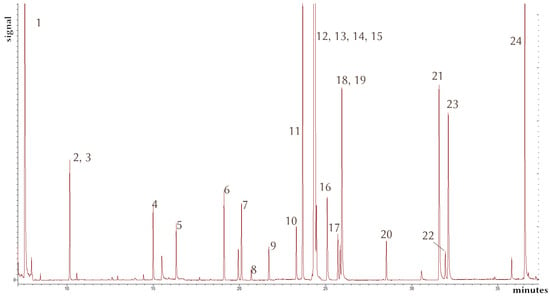
Figure 4.
Sample GC-MS/MS (MRM) chromatogram from an extract of spiked soil at 60 µg/kg. 1—aminomethylphosphonic acid; 2, 3—glyphosate and isotopically labeled glyphosate (co-eluting); 4—MCPA; 5—2,4-D; 6—pentachloroanisole; 7—lindane; 8—terbutylazine; 9—tefluthrin; 10—metalaxyl; 11—prosulfocarb; 12, 13, 14, 15—aldrin, metolachlor, malathion, and chlorpyrifos; 16—tetraconazole; 17—metazachlor; 18, 19—isotopically labeled penconazole and penconazole; 20—buprofezin; 21—tebuconazole; 22—diflufenican; 23—epoxiconazole; 24—boscalid.
The limit of quantification obtained for glyphosate was significantly lower than other published methods (e.g., Ref. [3], LoQ of 50 µg/kg), likely due to the low extraction solvent volume used and high concentration factor generated by drying the aqueous extract, which nonetheless is a difficult and time-consuming process, often avoided. It is possible that a different soil, particularly of finer texture, might require a larger extraction solvent volume to obtain an adequate recovery for glyphosate, which would compromise the detection limit, unless an even larger concentration is performed. In terms of the other analytes, detection and quantification limits are within normal ranges for most multiresidue methods, but not significantly better [6].
4. Conclusions
In this work, a new method for the simultaneous determination of glyphosate and 13 pesticides of different chemical classes was developed. The method shows acceptable performance parameters, and is relatively straightforward in terms of operation. In the future, different ratios of sample to extraction solvent will be tested and optimized in order to achieve a better concentration factor for the SPME extraction. In soils with greater matrix interferents, it may be necessary to employ some form of clean-up of the glyphosate extract prior to derivatization. The use of SPME LC-Tips permitted the development of this combined method in a way that would have been difficult otherwise (by performing serial extractions) because the extraction of multiclass pesticides is itself performed in an aqueous solvent, whereas it normally uses an organic solvent. This new SPME configuration appears to have interesting properties and opens several avenues for further exploration, such as direct screening of soils without prior sample preparation by the insertion of the fibers onto wet soil, and then, transporting them to the laboratory.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app14198584/s1, Table S1: Multiple-reaction monitoring transitions for MS/MS experiments.
Author Contributions
Conceptualization P.G., E.P.M., and A.B.R.; data curation J.B.; formal analysis J.B.; funding acquisition A.B.R., P.G., E.P.M., and M.G.d.S.; investigation J.B.; methodology J.B.; project administration A.B.R., P.G., E.P.M., and M.G.d.S.; resources A.B.R., P.G., E.P.M., and M.G.d.S.; software J.B., P.G., and E.P.M.; supervision M.G.d.S., A.B.R., E.P.M., and P.G.; validation J.B.; writing—original draft J.B.; writing—review and editing A.B.R., P.G., E.P.M., and M.G.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work received national funds through Fundação para a Ciência e a Tecnologia (FCT) through the Research units CENSE “Center for Environmental and Sustainability Research”, (PTDC/CTA-AMB/6587/2020) and LAQV/REQUIMTE (UID/QUI/50006/2020). FCT is also acknowledged for J. Brinco (UI/BD/150867/2021) fellowship, and P. GuedesContract established under Individual Call to Scientific EmploymentStimulus (CEECIND/01969/2020). This research is anchored at RESOLUTION LAB, an infrastructure at NOVA School of Science and Technology.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Thiombane, M.; Petrik, A.; Di Bonito, M.; Albanese, S.; Zuzolo, D.; Cicchella, D.; Lima, A.; Qu, C.; Qi, S.; De Vivo, B. Status, sources and contamination levels of organochlorine pesticide residues in urban and agricultural areas: A preliminary review in central–southern Italian soils. Environ. Sci. Pollut. Res. 2018, 25, 26361–26382. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Mol, H.G.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total. Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Hvězdová, M.; Kosubová, P.; Košíková, M.; Scherr, K.E.; Šimek, Z.; Brodský, L.; Šudoma, M.; Škulcová, L.; Sáňka, M.; Svobodová, M.; et al. Currently and recently used pesticides in Central European arable soils. Sci. Total Environ. 2018, 613–614, 361–370. [Google Scholar] [CrossRef]
- Pose-Juan, E.; Sánchez-Martín, M.J.; Andrades, M.S.; Rodríguez-Cruz, M.S.; Herrero-Hernández, E. Pesticide residues in vineyard soils from Spain: Spatial and temporal distributions. Sci. Total Environ. 2015, 514, 351–358. [Google Scholar] [CrossRef]
- Brinco, J.; Guedes, P.; Gomes da Silva, M.; Mateus, E.P.; Ribeiro, A.B. Analysis of pesticide residues in soil: A review and comparison of methodologies. Microchem. J. 2023, 195, 109465. [Google Scholar] [CrossRef]
- Anastassiades, M.; Maštovská, K.; Lehotay, S.J. Evaluation of analyte protectants to improve gas chromatographic analysis of pesticides. J. Chromatogr. A 2003, 1015, 163–184. [Google Scholar] [CrossRef]
- Koskinen, W.C.; Marek, L.J.; Hall, K.E. Analysis of glyphosate and aminomethylphosphonic acid in water, plant materials and soil. Pest Manag. Sci. 2016, 72, 423–432. [Google Scholar] [CrossRef]
- Maštovská, K.; Lehotay, S.J. Evaluation of common organic solvents for gas chromatographic analysis and stability of multiclass pesticide residues. J. Chromatogr. A 2004, 1040, 259–272. [Google Scholar] [CrossRef]
- Brinco, J.; Carvalho, R.; da Silva, M.R.G.; Guedes, P.; Ribeiro, A.B.; Mateus, E.P. Extraction of Pesticides from Soil Using Direct-Immersion SPME LC-Tips Followed by GC-MS/MS: Evaluation and Proof-of-Concept. J. Chromatogr. A 2024, 1735, 465295. [Google Scholar] [CrossRef]
- Lizot, L.d.L.F.; da Silva, A.C.C.; Bastiani, M.F.; Maurer, T.F.; Hahn, R.Z.; Perassolo, M.S.; Antunes, M.V.; Linden, R. Simultaneous Determination of Cocaine and Metabolites in Human Plasma Using Solid Phase Micro-Extraction Fiber Tips C18 and UPLC–MS/MS. J. Anal. Toxicol. 2019, 44, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Alsenedi, K.A.; Morrison, C. Determination of amphetamine-type stimulants (ATSs) and synthetic cathinones in urine using solid phase micro-extraction fibre tips and gas chromatography-mass spectrometry. Anal. Methods 2018, 10, 1431–1440. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.; Silva, C.; Medina, S.; Câmara, J.S. QuEChERS—Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Gionfriddo, E.; Gruszecka, D.; Li, X.; Pawliszyn, J. Direct-immersion SPME in soy milk for pesticide analysis at trace levels by means of a matrix-compatible coating. Talanta 2020, 211, 120746. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, P.; Zhang, F.; Li, Y.; Pan, C. Direct analysis in real time mass spectrometry for the rapid identification of four highly hazardous pesticides in agrochemicals. Rapid Commun. Mass Spectrom. 2012, 26, 1859–1867. [Google Scholar] [CrossRef]
- Pinho, S.P.; Macedo, E.A. Solubility of NaCl, NaBr, and KCl in Water, Methanol, Ethanol, and Their Mixed Solvents. J. Chem. Eng. Data 2005, 50, 29–32. [Google Scholar] [CrossRef]
- Hu, J.Y.; Chen, C.L.; Li, J.Z. A simple method for the determination of glyphosate residues in soil by capillary gas chromatography with nitrogen phosphorus. J. Anal. Chem. 2008, 63, 371–375. [Google Scholar] [CrossRef]
- Motojyuku, M.; Saito, T.; Akieda, K.; Otsuka, H.; Yamamoto, I.; Inokuchi, S. Determination of glyphosate, glyphosate metabolites, and glufosinate in human serum by gas chromatography–mass spectrometry. J. Chromatogr. B 2008, 875, 509–514. [Google Scholar] [CrossRef]
- Arkan, T.; Molnár-Perl, I. The role of derivatization techniques in the analysis of glyphosate and aminomethyl-phosphonic acid by chromatography. Microchem. J. 2015, 121, 99–106. [Google Scholar] [CrossRef]
- Börjesson, E.; Torstensson, L. New methods for determination of glyphosate and (aminomethyl)phosphonic acid in water and soil. J. Chromatogr. A 2000, 886, 207–216. [Google Scholar] [CrossRef]
- Deyrup, C.L.; Chang, S.M.; Weintraub, R.A.; Moye, H.A. Simultaneous esterification and acylation of pesticides for analysis by gas chromatography. 1. Derivatization of glyphosate and (aminomethyl)phosphonic acid with fluorinated alcohols-perfluorinated anhydrides. J. Agric. Food Chem. 1985, 33, 944–947. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).