Limitations in Maximum Intensity Front Crawl in Swimmers with Down Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
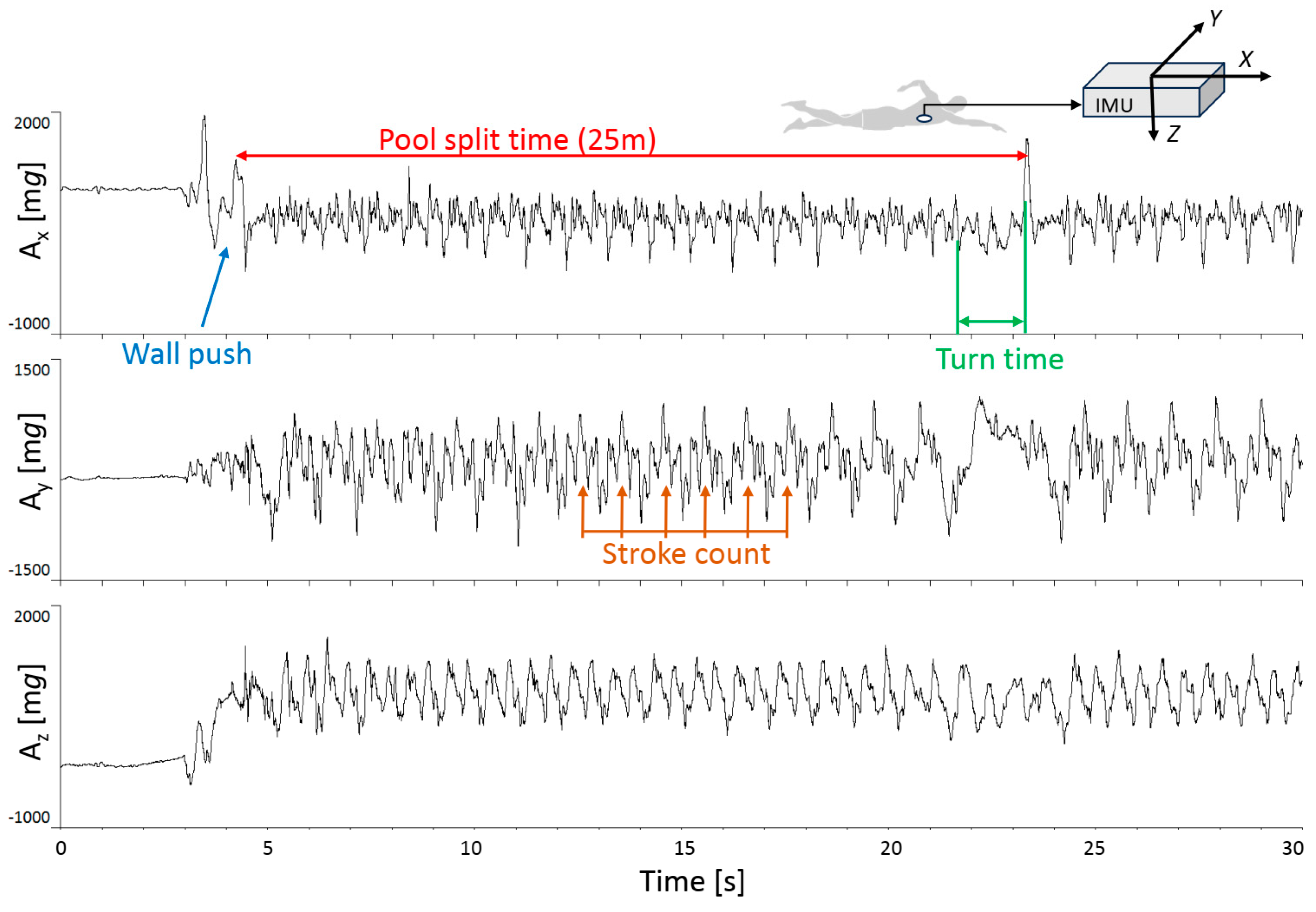
2.2. Measurements and Experimental Procedures
2.3. Data Analysis
2.4. Statistical Analysis
3. Results
3.1. Anthropometric Characteristics and Performance Data
3.2. Exercise Intensity
3.3. Acceleration Regularity
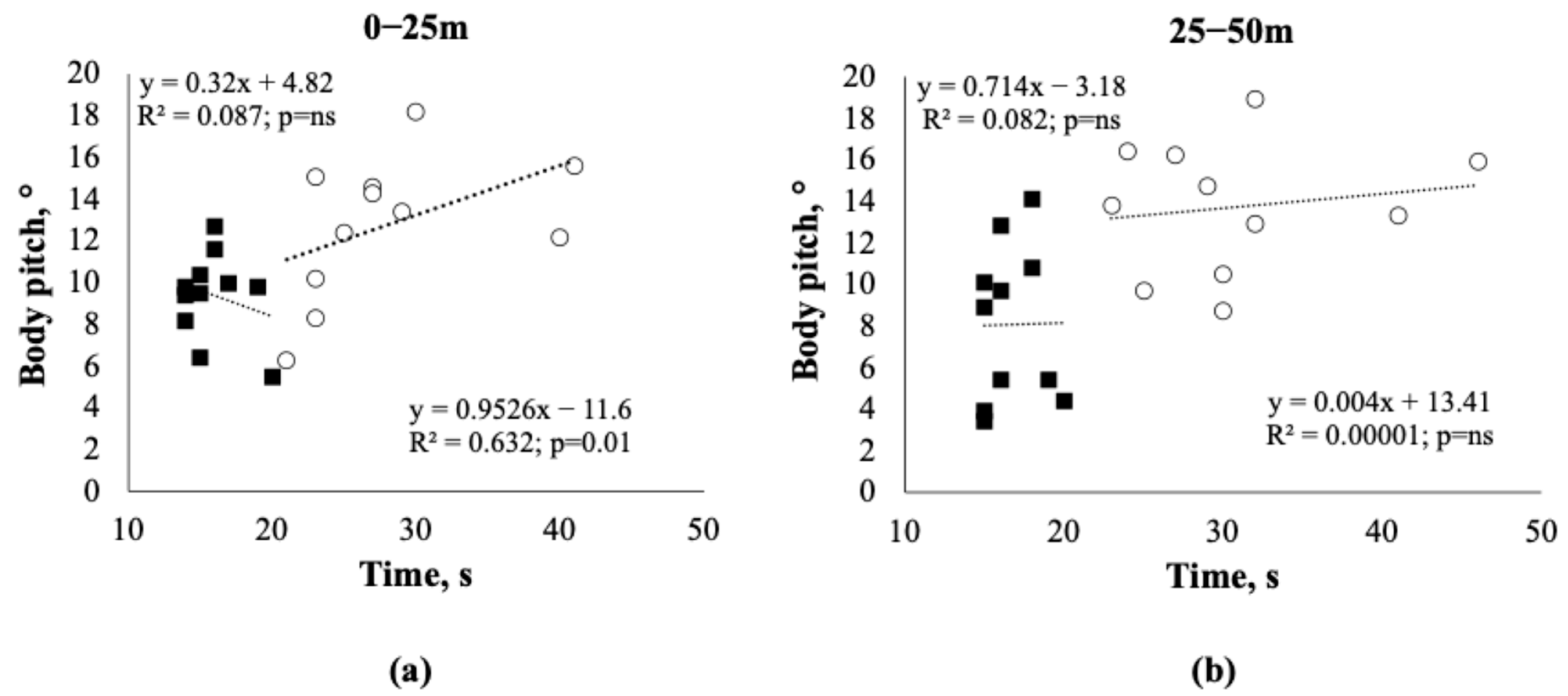
3.4. Body Pitch
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Irving, C.; Basu, A.; Richmond, S.; Burn, J.; Wren, C. Twenty-Year Trends in Prevalence and Survival of Down Syndrome. Eur. J. Hum. Genet. 2008, 16, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, D.; Wellsted, D.; Mengoni, S.E. Definition, Assessment and Management of Frailty for People with Intellectual Disabilities: A Scoping Review. J. Appl. Res. Intellect. Disabil. 2024, 37, e13219. [Google Scholar] [CrossRef] [PubMed]
- Capone, G.T.; Chicoine, B.; Bulova, P.; Stephens, M.; Hart, S.; Crissman, B.; Videlefsky, A.; Myers, K.; Roizen, N.; Esbensen, A.; et al. Co-Occurring Medical Conditions in Adults with Down Syndrome: A Systematic Review toward the Development of Health Care Guidelines. Am. J. Med. Genet. A 2018, 176, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Boer, P.H. The Effect of 8 Weeks of Freestyle Swim Training on the Functional Fitness of Adults with Down Syndrome. J. Intellect. Disabil. Res. 2020, 64, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Querido, A.; Costa, M.J.; Araújo, D.; Sampaio, A.R.; Vilas-Boas, J.P.; Corredeira, R.; Daly, D.J.; Fernandes, R.J. Swimmers with Down Syndrome Are Healthier and Physically Fit than Their Untrained Peers. Healthcare 2023, 11, 482. [Google Scholar] [CrossRef]
- Berghof, R.; Carstens, C. Hip joint problems in patients with Down’s syndrome. Z. Orthop. Ihre Grenzgeb. 1992, 130, 136–141. [Google Scholar] [CrossRef]
- Livingstone, B.; Hirst, P. Orthopedic Disorders in School Children with Down’s Syndrome with Special Reference to the Incidence of Joint Laxity. Clin. Orthop. Relat. Res. 1986, 207, 74–76. [Google Scholar] [CrossRef]
- LaCombe, J.M.; Roper, R.J. Skeletal Dynamics of Down Syndrome: A Developing Perspective. Bone 2020, 133, 115215. [Google Scholar] [CrossRef]
- Brugnaro, B.H.; Pauletti, M.F.; Lima, C.R.G.; Verdério, B.N.; Fonseca-Angulo, R.I.; Romão-Silva, B.; de Campos, A.C.; Rosenbaum, P.; Rocha, N.A.C.F. Relationship between Sensory Processing Patterns and Gross Motor Function of Children and Adolescents with Down Syndrome and Typical Development: A Cross-Sectional Study. J. Intellect. Disabil. Res. 2024, 68, 358–368. [Google Scholar] [CrossRef]
- Büyükçelik, N.M.; Yiğit, S.; Turhan, B. An Investigation of the Effects of Dual-Task Balance Exercises on Balance, Functional Status and Dual-Task Performance in Children with Down Syndrome. Dev. Neurorehabil 2023, 26, 320–327. [Google Scholar] [CrossRef]
- Muñoz-Llerena, A.; Ladrón-de-Guevara, L.; Medina-Rebollo, D.; Alcaraz-Rodríguez, V. Impact of Physical Activity on Autonomy and Quality of Life in Individuals with Down Syndrome: A Systematic Review. Healthcare 2024, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Gomez, R.; Shields, N. Organised and Non-Organised Activities Contribute to Overall Physical Activity Levels in Adolescents and Young Adults with Down Syndrome: A Cross-Sectional Study. J. Intellect. Disabil. Res. 2024, 68, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Merzbach, V.; Jewiss, M.; Scruton, A.; Gordon, D. The Effects of Prescribed Physical and Cognitive Exercise on Life Satisfaction, Self-Efficacy and Mood States in Adults with Down Syndrome: The MinDSets Study. Int. J. Environ. Res. Public Health 2024, 21, 610. [Google Scholar] [CrossRef] [PubMed]
- Marques-Aleixo, I.; Querido, A.; Figueiredo, P.; Vilas-Boas, J.P.; Corredeira, R.; Daly, D.; Fernandes, R.J. Intracyclic Velocity Variation and Arm Coordination Assessment in Swimmers with Down Syndrome. Adapt. Phys. Act. Q. 2013, 30, 70–84. [Google Scholar] [CrossRef]
- Pan, J.; Tompkins, W.J. A Real-Time QRS Detection Algorithm. IEEE Trans. Biomed. Eng. 1985, 32, 230–236. [Google Scholar] [CrossRef]
- Ganzevles, S.; Vullings, R.; Beek, P.J.; Daanen, H.; Truijens, M. Using Tri-Axial Accelerometry in Daily Elite Swim Training Practice. Sensors 2017, 17, 990. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological Time-Series Analysis Using Approximate Entropy and Sample Entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef]
- Silva, L.E.V.; Fazan, R.; Marin-Neto, J.A. PyBioS: A Freeware Computer Software for Analysis of Cardiovascular Signals. Comput. Methods Programs Biomed. 2020, 197, 105718. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; ISBN 978-0-203-77158-7. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Villani, E.R.; Onder, G.; Marzetti, E.; Coelho-Junior, H.; Calvani, R.; Di Paola, A.; Carfì, A. Body Composition Parameters and Sarcopenia in Adults with Down Syndrome: A Case-Control Study. Aging Clin. Exp. Res. 2024, 36, 81. [Google Scholar] [CrossRef]
- Yamanaka, E.; Inayama, T.; Ohkawara, K.; Kojima, M.; Nakada, T.; Kita, I. Effects of Substituting Sedentary Time with Physical Activity on Body Mass Index in Japanese Adults with Down Syndrome: A Cross-Sectional Study. Heliyon 2024, 10, e29294. [Google Scholar] [CrossRef] [PubMed]
- González-Agüero, A.; Vicente-Rodríguez, G.; Moreno, L.A.; Guerra-Balic, M.; Ara, I.; Casajús, J.A. Health-Related Physical Fitness in Children and Adolescents with Down Syndrome and Response to Training. Scand. J. Med. Sci. Sports 2010, 20, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Padia, N.; Bose, M.; Parab, S. Determinants of Hand Function in Children and Adolescent with Down Syndrome—A Scoping Review. J. Hand Ther. 2023, 36, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Kadi, T.; Washino, S.; Tsunokawa, T.; Narita, K.; Mankyu, H.; Murai, A.; Tamaki, H. Role of Kicking Action in Front Crawl: The Inter-Relationships between Swimming Velocity, Hand Propulsive Force and Trunk Inclination. Sports Biomech. 2024, 2303361. [Google Scholar] [CrossRef]
- Fernhall, B.; Pitetti, K.H.; Rimmer, J.H.; McCubbin, J.A.; Rintala, P.; Millar, A.L.; Kittredge, J.; Burkett, L.N. Cardiorespiratory Capacity of Individuals with Mental Retardation Including Down Syndrome. Med. Sci. Sports Exerc. 1996, 28, 366–371. [Google Scholar] [CrossRef]
- Mendonca, G.V.; Pereira, F.D.; Fernhall, B. Reduced Exercise Capacity in Persons with Down Syndrome: Cause, Effect, and Management. Ther. Clin. Risk Manag. 2010, 6, 601–610. [Google Scholar] [CrossRef]
- Iellamo, F.; Galante, A.; Legramante, J.M.; Lippi, M.E.; Condoluci, C.; Albertini, G.; Volterrani, M. Altered Autonomic Cardiac Regulation in Individuals with Down Syndrome. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2387–H2391. [Google Scholar] [CrossRef]
- Boonman, A.J.N.; Schroeder, E.C.; Hopman, M.T.E.; Fernhall, B.O.; Hilgenkamp, T.I.M. Cardiopulmonary Profile of Individuals with Intellectual Disability. Med. Sci. Sports Exerc. 2019, 51, 1802–1808. [Google Scholar] [CrossRef]
- Sinton, J.W.; Cooper, D.S.; Wiley, S. Down Syndrome and the Autonomic Nervous System, an Educational Review for the Anesthesiologist. Paediatr. Anaesth. 2022, 32, 609–616. [Google Scholar] [CrossRef]
- Hilgenkamp, T.I.M.; Baynard, T. Do Individuals with Intellectual Disability Have a Lower Peak Heart Rate and Maximal Oxygen Uptake? J. Appl. Res. Intellect. Disabil. 2018, 31, 785–791. [Google Scholar] [CrossRef]
- Oviedo, G.R.; Carbó-Carreté, M.; Guerra-Balic, M.; Tamulevicius, N.; Esquius, L.; Guàrdia-Olmos, J.; Javierre, C. Hemodynamic and Cardiorespiratory Responses to Submaximal and Maximal Exercise in Adults with Down Syndrome. Front. Physiol. 2022, 13, 905795. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, X.; Sun, Z.; Li, L.; Zuegel, M.; Steinacker, J.M.; Schumann, U. Heart Rate Recovery and Risk of Cardiovascular Events and All-Cause Mortality: A Meta-Analysis of Prospective Cohort Studies. J. Am. Heart Assoc. 2017, 6, e005505. [Google Scholar] [CrossRef] [PubMed]
- Boer, P.H.; Moss, S.J. Effect of Continuous Aerobic vs. Interval Training on Selected Anthropometrical, Physiological and Functional Parameters of Adults with Down Syndrome. J. Intellect. Disabil. Res. 2016, 60, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Rosety-Rodriguez, M.; Bernardi, M.; Elosegui, S.; Rosety, I.; Diaz, A.J.; Rosety, M.A.; Brenes, F.; Oliva-Pascual-Vaca, A.; Alvero-Cruz, J.R.; Ordonez, F.J. A Short-Term Resistance Training Circuit Improved Antioxidants in Sedentary Adults with Down Syndrome. Oxid. Med. Cell. Longev. 2021, 2021, 8811153. [Google Scholar] [CrossRef]
- Chiviacowsky, S.; Wulf, G.; Avila, L.T.G. An External Focus of Attention Enhances Motor Learning in Children with Intellectual Disabilities. J. Intellect. Disabil. Res. 2013, 57, 627–634. [Google Scholar] [CrossRef]
- Cavaggioni, L.; Ardigò, L.P.; Castiglioni, P.; Trecroci, A.; Casalini, L.; Formenti, D.; Merati, G. A Crossover Study on Attentional Focus and Gross Motor Performance in Individuals with Down Syndrome. PLoS ONE 2024, 19, e0305267. [Google Scholar] [CrossRef]
- Cowley, P.M.; Ploutz-Snyder, L.L.; Baynard, T.; Heffernan, K.S.; Jae, S.Y.; Hsu, S.; Lee, M.; Pitetti, K.H.; Reiman, M.P.; Fernhall, B. The Effect of Progressive Resistance Training on Leg Strength, Aerobic Capacity and Functional Tasks of Daily Living in Persons with Down Syndrome. Disabil. Rehabil. 2011, 33, 2229–2236. [Google Scholar] [CrossRef]

| SDS (n = 11) | CNT (n = 11) | p 1 | Effect Size | |
|---|---|---|---|---|
| Age (years) | 26.6 ± 5.6 | 27.1 ± 4.0 | 0.828 | 0.09 |
| Height (cm) | 157 ± 5 | 179 ± 6 | <0.01 | 4.09 |
| Body mass (kg) | 58.0 ± 7.8 | 81.3 ± 13.2 | <0.01 | 2.15 |
| BMI (kg‧m−2) | 23.5 ± 2.7 | 25.1 ± 2.9 | 0.188 | 0.58 |
| Weekly training (hours) | 5.3 ± 1.4 | 4.9 ± 0.7 | 0.400 | 0.37 |
| SDS (n = 11) | CNT (n = 11) | p 1 | Effect Size | |
|---|---|---|---|---|
| Time 0–25 m (s) | 28.09 ± 6.72 | 15.91 ± 2.02 | <0.01 | 2.45 |
| Time 25–50 m (s) | 30.82 ± 7.05 | 16.64 ± 1.80 | <0.01 | 2.75 |
| Total time (s) | 58.91 ± 13.68 | 32.55 ± 3.70 | <0.01 | 2.63 |
| Stroke number 0–25 m | 33.18 ± 4.38 | 25.64 ± 3.47 | <0.01 | 1.91 |
| Stroke number 25–50 m | 32.91 ± 5.43 | 25.82 ± 4.09 | <0.01 | 1.47 |
| Total stroke number | 66.09 ± 9.64 | 51.45 ± 7.41 | <0.01 | 1.70 |
| SDS (n = 11) | CNT (n = 11) | p 1 | |
|---|---|---|---|
| Body pitch 0–25 m (°) | 12.78 ± 3.43 | 9.39 ± 2.08 | 0.016 |
| Body pitch 25–50 m (°) | 13.73 ± 3.14 | 8.08 ± 3.75 | 0.0002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merati, G.; Formenti, D.; Gandola, C.; Castiglioni, P.; Casalini, L.; Trecroci, A.; Cavaggioni, L.; Invernizzi, P.L.; Menichino, U.; Scurati, R. Limitations in Maximum Intensity Front Crawl in Swimmers with Down Syndrome. Appl. Sci. 2024, 14, 8387. https://doi.org/10.3390/app14188387
Merati G, Formenti D, Gandola C, Castiglioni P, Casalini L, Trecroci A, Cavaggioni L, Invernizzi PL, Menichino U, Scurati R. Limitations in Maximum Intensity Front Crawl in Swimmers with Down Syndrome. Applied Sciences. 2024; 14(18):8387. https://doi.org/10.3390/app14188387
Chicago/Turabian StyleMerati, Giampiero, Damiano Formenti, Claudio Gandola, Paolo Castiglioni, Linda Casalini, Athos Trecroci, Luca Cavaggioni, Pietro Luigi Invernizzi, Umberto Menichino, and Raffaele Scurati. 2024. "Limitations in Maximum Intensity Front Crawl in Swimmers with Down Syndrome" Applied Sciences 14, no. 18: 8387. https://doi.org/10.3390/app14188387
APA StyleMerati, G., Formenti, D., Gandola, C., Castiglioni, P., Casalini, L., Trecroci, A., Cavaggioni, L., Invernizzi, P. L., Menichino, U., & Scurati, R. (2024). Limitations in Maximum Intensity Front Crawl in Swimmers with Down Syndrome. Applied Sciences, 14(18), 8387. https://doi.org/10.3390/app14188387













