Mesenchymal Stromal/Stem Cells Isolated by Explant Culture Method from Wharton’s Jelly and Subamnion Possess Similar Biological Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of MSCs from Human UC
2.2. Cell Viability: MTT Assay
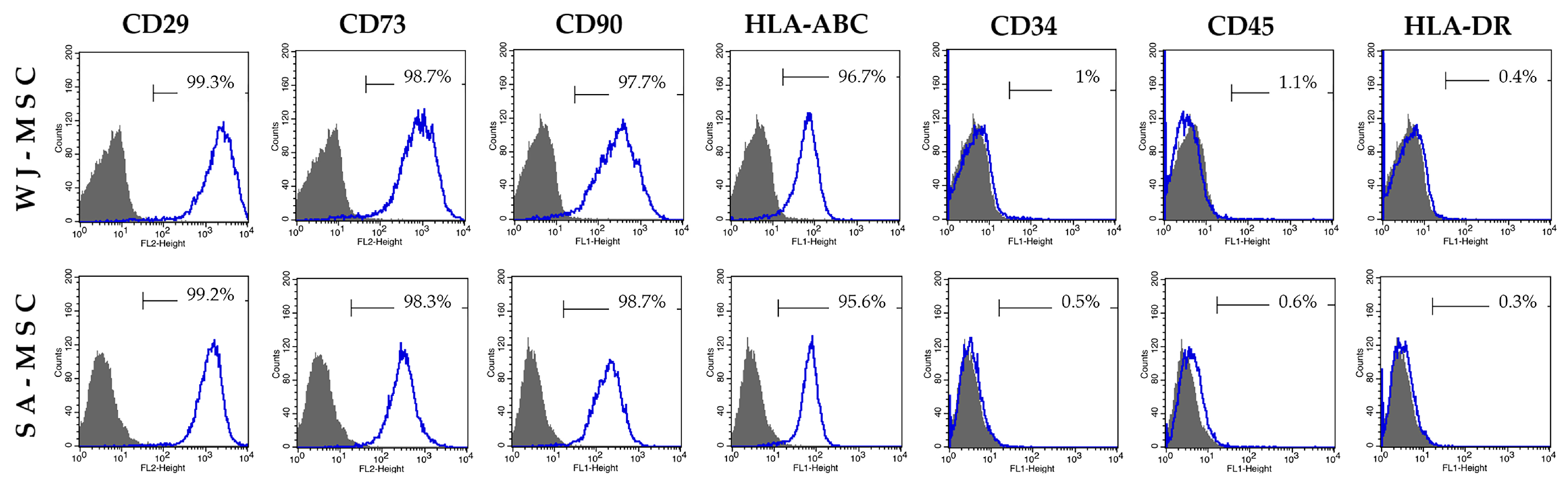
2.3. Flow Cytometry Immunophenotyping
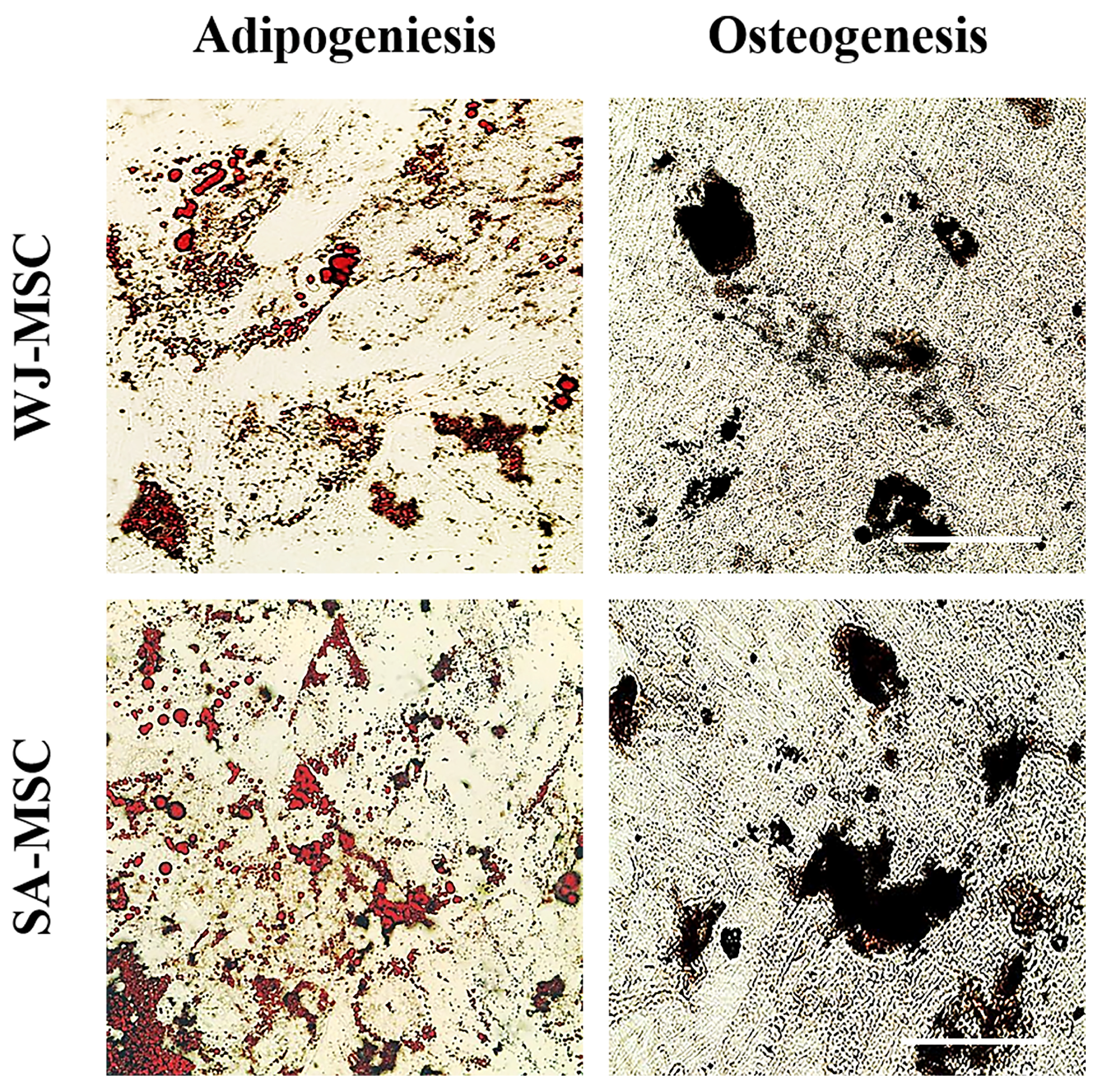
2.4. Cell Differentiation
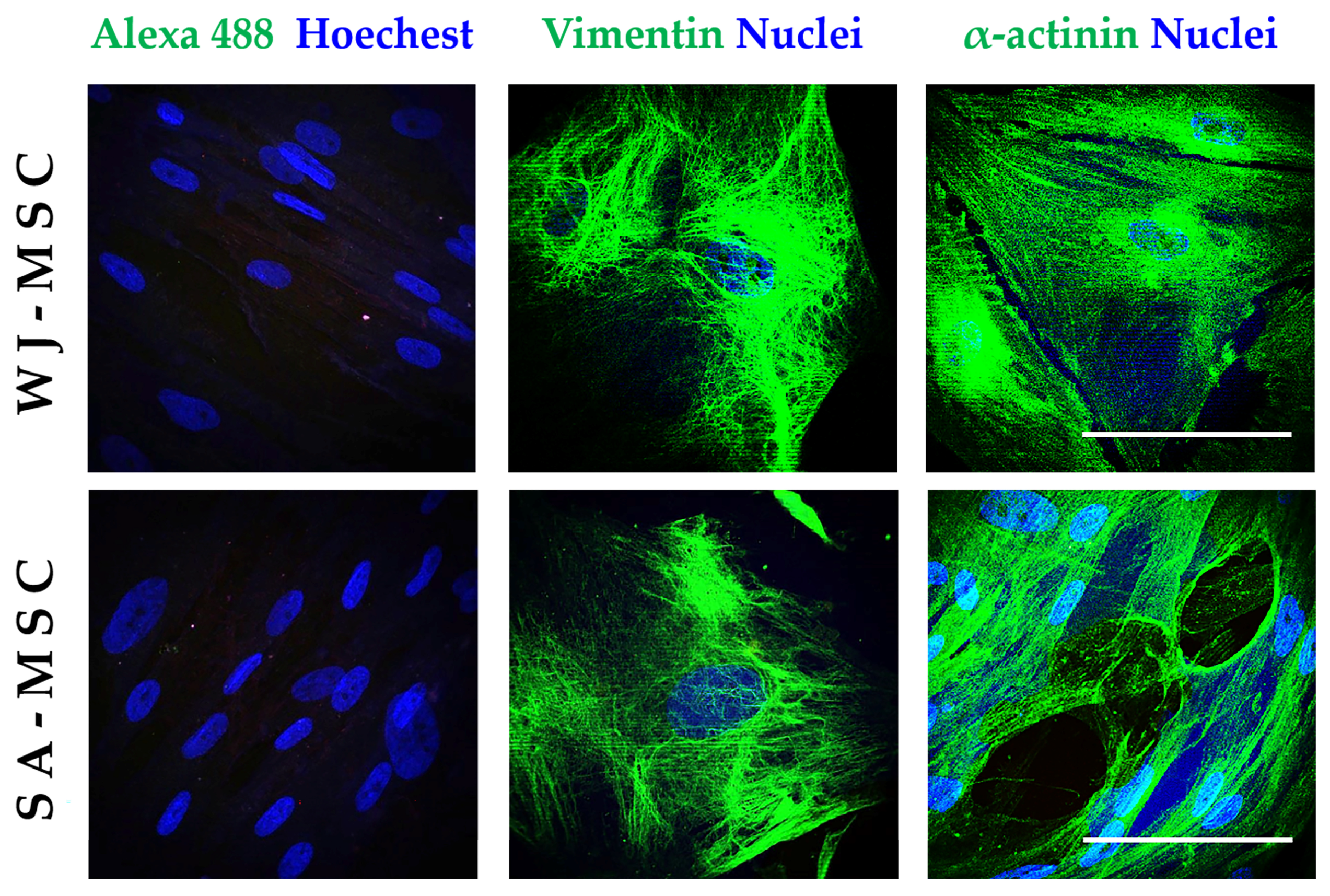
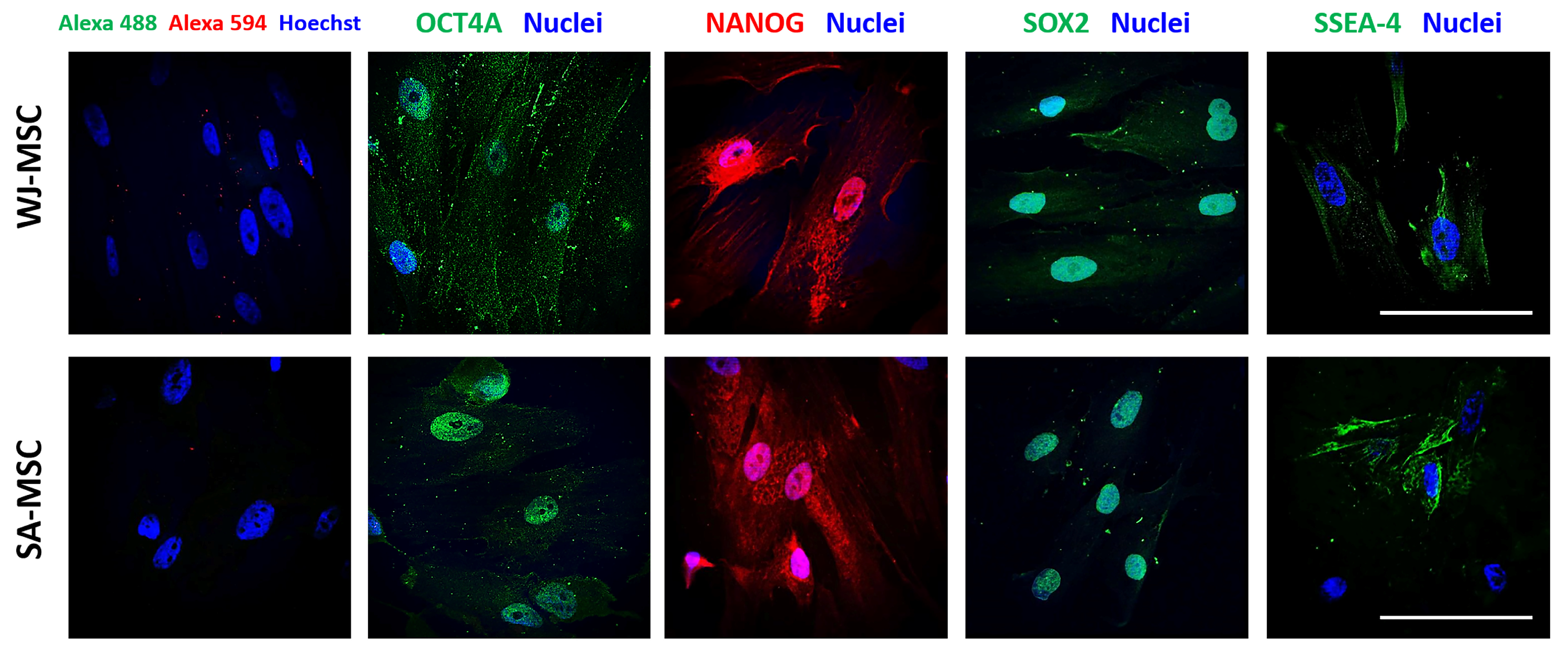
2.5. Immunofluorescence Assays
2.6. RNA Extraction and RT-PCR Analyses
2.7. Statistics
3. Results
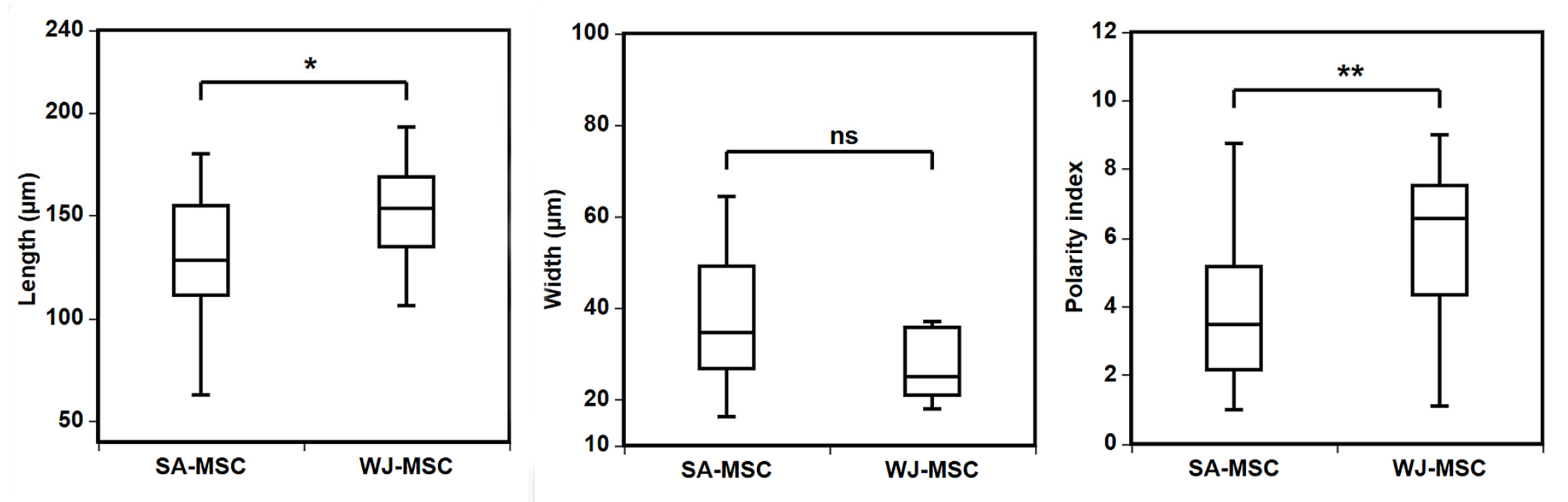
3.1. Identification of Umbilical Cord-Derived Cells
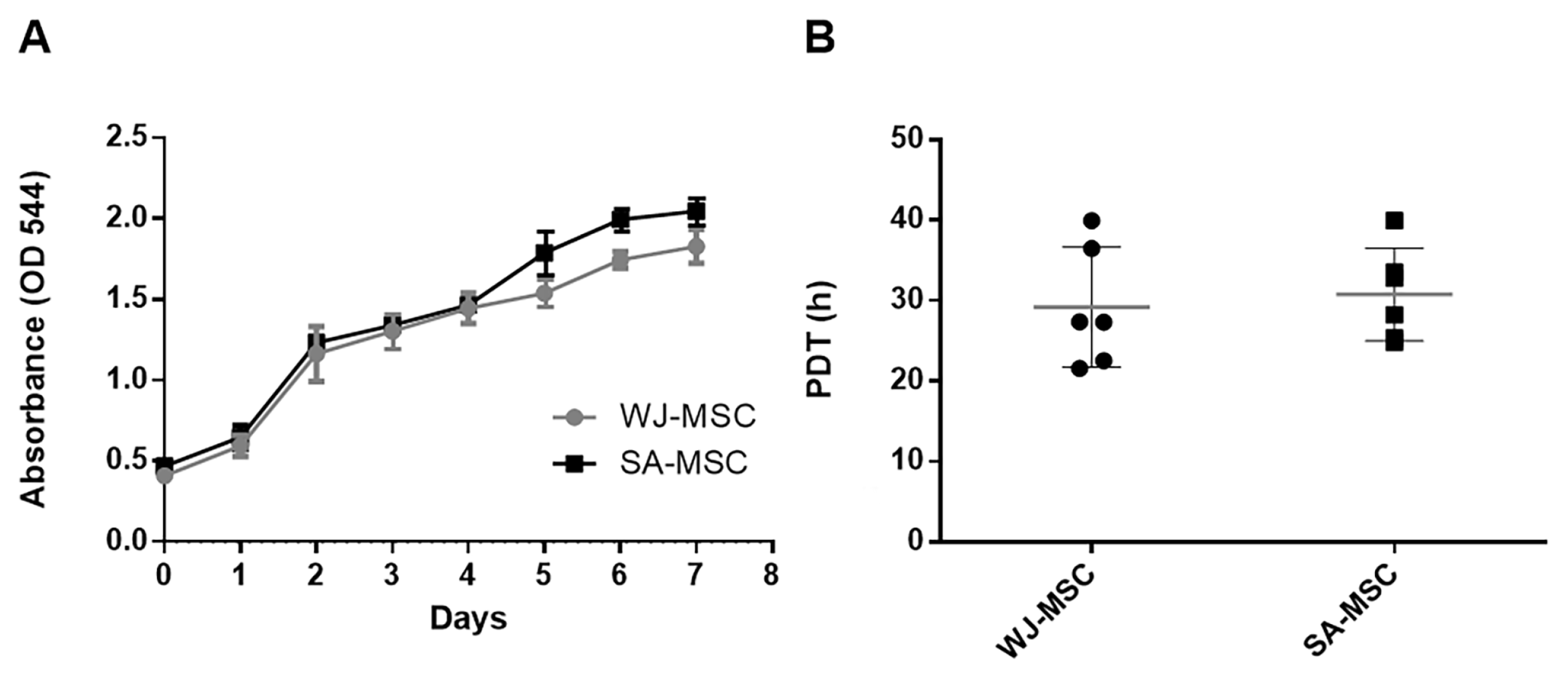
3.2. Growth Characteristics
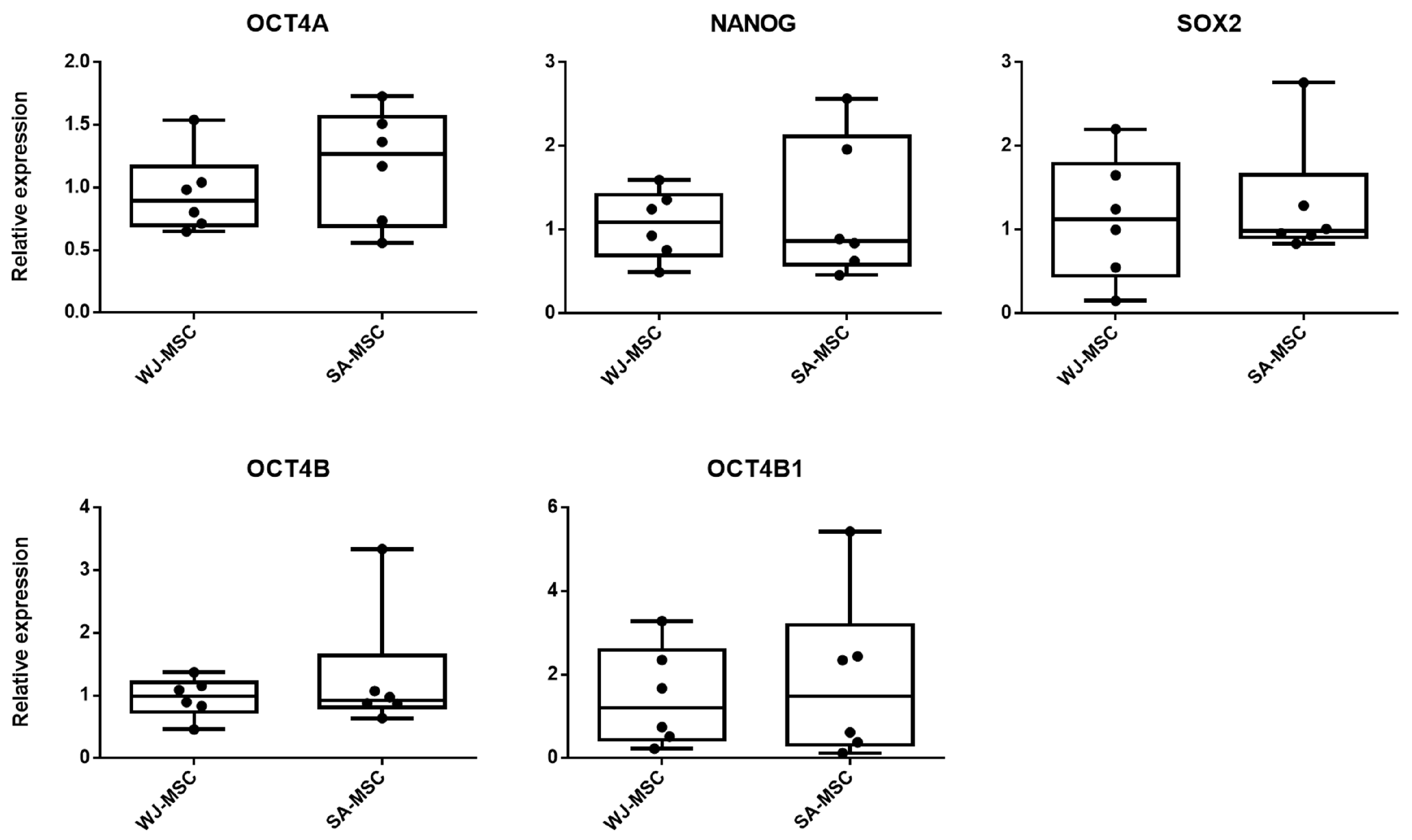
3.3. Expression of Pluripotency-Associated Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayala-Cuellar, A.P.; Kang, J.H.; Jeung, E.B.; Choi, K.C. Roles of Mesenchymal Stem Cells in Tissue Regeneration and Immunomodulation. Biomol. Ther. 2019, 27, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Karahuseyinoglu, S. Concise review: Human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells 2007, 25, 2886–2895. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.; Zhang, Y. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed]
- Hass, R.; Kasper, C.; Bohm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. CCS 2011, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Choudhery, M.S.; Badowski, M.; Muise, A.; Harris, D.T. Comparison of human mesenchymal stem cells derived from adipose and cord tissue. Cytotherapy 2013, 15, 330–343. [Google Scholar] [CrossRef]
- Drela, K.; Lech, W.; Figiel-Dabrowska, A.; Zychowicz, M.; Mikula, M.; Sarnowska, A.; Domanska-Janik, K. Enhanced neuro-therapeutic potential of Wharton’s Jelly-derived mesenchymal stem cells in comparison with bone marrow mesenchymal stem cells culture. Cytotherapy 2016, 18, 497–509. [Google Scholar] [CrossRef]
- Baksh, D.; Yao, R.; Tuan, R.S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 2007, 25, 1384–1392. [Google Scholar] [CrossRef]
- Hamidian Jahromi, S.; Li, Y.; Davies, J.E. Effect of Tumor Necrosis Factor Alpha Dose and Exposure Time on Tumor Necrosis Factor-Induced Gene-6 Activation by Neonatal and Adult Mesenchymal Stromal Cells. Stem Cells Dev. 2018, 27, 44–54. [Google Scholar] [CrossRef]
- Nagamura-Inoue, T.; Kato, S.; Najima, Y.; Isobe, M.; Doki, N.; Yamamoto, H.; Uchida, N.; Takahashi, A.; Hori, A.; Nojima, M.; et al. Immunological influence of serum-free manufactured umbilical cord-derived mesenchymal stromal cells for steroid-resistant acute graft-versus-host disease. Int. J. Hematol. 2022, 116, 754–769. [Google Scholar] [CrossRef]
- Umer, A.; Khan, N.; Greene, D.L.; Habiba, U.E.; Shamim, S.; Khayam, A.U. The Therapeutic Potential of Human Umbilical Cord Derived Mesenchymal Stem Cells for the Treatment of Premature Ovarian Failure. Stem Cell Rev. Rep. 2023, 19, 651–666. [Google Scholar] [CrossRef]
- Gherghel, R.; Macovei, L.A.; Burlui, M.-A.; Cardoneanu, A.; Rezus, I.-I.; Mihai, I.R.; Rezus, E. Osteoarthritis—The Role of Mesenchymal Stem Cells in Cartilage Regeneration. Appl. Sci. 2023, 13, 10617. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, H.; Ren, X.; Jiang, F.; Zhou, P. Therapeutic effects of different intervention forms of human umbilical cord mesenchymal stem cells in the treatment of osteoarthritis. Front. Cell Dev. Biol. 2023, 11, 1246504. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, S.; Peng, C. Human umbilical cord mesenchymal stem cells for psoriasis: A phase 1/2a, single-arm study. Signal Transduct. Target. Ther. 2022, 7, 263. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Guan, H.; Oishi, H.; Takahashi, S.; Zhang, C. Human umbilical cord mesenchymal stem cells in diabetes mellitus and its complications: Applications and research advances. Int. J. Med. Sci. 2023, 20, 1492–1507. [Google Scholar] [CrossRef]
- Patel, A.A.; Mohamed, A.H.; Rizaev, J.; Mallick, A.K.; Qasim, M.T.; Abdulmonem, W.A.; Jamal, A.; Hattiwale, H.M.; Kamal, M.A.; Ahmad, F. Application of mesenchymal stem cells derived from the umbilical cord or Wharton’s jelly and their extracellular vesicles in the treatment of various diseases. Tissue Cell 2024, 89, 102415. [Google Scholar] [CrossRef]
- Yudintceva, N.; Mikhailova, N. Mesenchymal Stem Cells and MSCs-Derived Extracellular Vesicles in Infectious Diseases: From Basic Research to Clinical Practice. Bioengineering 2022, 9, 662. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ma, J.; Yi, P.; Wu, J.; Zhao, F.; Tu, W.; Liu, W.; Li, T.; Deng, Y.; Hao, J.; et al. Human umbilical cord mesenchymal stem cells restore the ovarian metabolome and rescue premature ovarian insufficiency in mice. Stem Cell Res. Ther. 2020, 11, 466. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Z.; Wen, J.; Jiang, F.; Xia, Y. Human Amniotic Mesenchymal Stem Cells Promote Endogenous Bone Regeneration. Front. Endocrinol. 2020, 11, 543623. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mao, X.; Zhong, Y.; Zhao, X.; Li, C.; Jiang, J.; Hong, Z.; Wang, N.; Wang, F. Human amniotic mesenchymal stem cells inhibit immune rejection injury from allogeneic mouse heart transplantation: A preliminary study on the microRNA expression. Transpl. Immunol. 2024, 84, 102022. [Google Scholar] [CrossRef]
- Stefańska, K.; Ożegowska, K.; Hutchings, G.; Popis, M.; Moncrieff, L.; Dompe, C.; Janowicz, K.; Pieńkowski, W.; Gutaj, P.; Shibli, J.A. Human Wharton’s Jelly-Cellular Specificity, Stemness Potency, Animal Models, and Current Application in Human Clinical Trials. J. Clin. Med. 2020, 9, 1102. [Google Scholar] [CrossRef]
- Huang, P.Y.; Shih, Y.H.; Tseng, Y.J.; Ko, T.L.; Fu, Y.S.; Lin, Y.Y. Xenograft of human umbilical mesenchymal stem cells from Wharton’s jelly as a potential therapy for rat pilocarpine-induced epilepsy. Brain Behav. Immun. 2016, 54, 45–58. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M. Umbilical Cord Lining Membrane and Wharton’s Jelly-Derived Mesenchymal Stem Cells: The Similarities and Differences. Open Tissue Eng. Regen. Med. J. 2011, 4, 21–27. [Google Scholar] [CrossRef]
- Vieira Paladino, F.; de Moraes Rodrigues, J. The Immunomodulatory Potential of Wharton’s Jelly Mesenchymal Stem/Stromal Cells. Stem Cells Int. 2019, 2019, 3548917. [Google Scholar] [CrossRef]
- Kita, K.; Gauglitz, G.G.; Phan, T.T.; Herndon, D.N.; Jeschke, M.G. Isolation and characterization of mesenchymal stem cells from the sub-amniotic human umbilical cord lining membrane. Stem Cells Dev. 2010, 19, 491–502. [Google Scholar] [CrossRef]
- Subramanian, A.; Fong, C.Y.; Biswas, A.; Bongso, A. Comparative Characterization of Cells from the Various Compartments of the Human Umbilical Cord Shows that the Wharton’s Jelly Compartment Provides the Best Source of Clinically Utilizable Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0127992. [Google Scholar] [CrossRef]
- Alatyyat, S.M.; Alasmari, H.M.; Aleid, O.A.; Abdel-Maksoud, M.S.; Elsherbiny, N. Umbilical cord stem cells: Background, processing and applications. Tissue Cell 2020, 65, 101351. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.K.; Vellasamy, S.; Tan, B.C.; Abdullah, M.; Vidyadaran, S.; Seow, H.F.; Ramasamy, R. Generation of mesenchymal stem cell from human umbilical cord tissue using a combination enzymatic and mechanical disassociation method. Cell Biol. Int. 2011, 35, 221–226. [Google Scholar] [CrossRef]
- Hua, J.; Gong, J.; Meng, H.; Xu, B.; Yao, L.; Qian, M.; He, Z.; Zou, S.; Zhou, B.; Song, Z. Comparison of different methods for the isolation of mesenchymal stem cells from umbilical cord matrix: Proliferation and multilineage differentiation as compared to mesenchymal stem cells from umbilical cord blood and bone marrow. Cell Biol. Int. 2013, 38, 198–210. [Google Scholar] [CrossRef]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry. Part A J. Int. Soc. Anal. Cytol. 2018, 93, 19–31. [Google Scholar] [CrossRef]
- Teoh, P.L.; Mohd Akhir, H.; Abdul Ajak, W.; Hiew, V.V. Human Mesenchymal Stromal Cells Derived from Perinatal Tissues: Sources, Characteristics and Isolation Methods. Malays. J. Med. Sci. MJMS 2023, 30, 55–68. [Google Scholar] [CrossRef]
- Zheng, S.; Gao, Y.; Chen, K.; Liu, Y.; Xia, N.; Fang, F. A Robust and Highly Efficient Approach for Isolation of Mesenchymal Stem Cells From Wharton’s Jelly for Tissue Repair. Cell Transpl. 2022, 31, 9636897221084354. [Google Scholar] [CrossRef]
- Salehinejad, P.; Alitheen, N.B.; Ali, A.M.; Omar, A.R.; Mohit, M.; Janzamin, E.; Samani, F.S.; Torshizi, Z.; Nematollahi-Mahani, S.N. Comparison of different methods for the isolation of mesenchymal stem cells from human umbilical cord Wharton’s jelly. Vitr. Cell. Dev. Biol. Anim. 2012, 48, 75–83. [Google Scholar] [CrossRef]
- Freshney, R. Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010; Volume 346, p. 796. [Google Scholar]
- Hendijani, F. Explant culture: An advantageous method for isolation of mesenchymal stem cells from human tissues. Cell Prolif. 2017, 50, e12334. [Google Scholar] [CrossRef]
- Skiles, M.L.; Marzan, A.J.; Brown, K.S.; Shamonki, J.M. Comparison of umbilical cord tissue-derived mesenchymal stromal cells isolated from cryopreserved material and extracted by explantation and digestion methods utilizing a split manufacturing model. Cytotherapy 2020, 22, 581–591. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Layton, C. The Hematoxylin and Eosin. In Theory & Practice of Histological Techniques, 7th ed.; Suvarna, S.K., Layton, C., Bancroft, J.D., Eds.; Churchill Livingstone of El Sevier: Philadelphia, PA, USA, 2013; Volume 10–11, pp. 172–214. [Google Scholar] [CrossRef]
- Kestendjieva, S.; Kyurkchiev, D.; Tsvetkova, G.; Mehandjiev, T.; Dimitrov, A.; Nikolov, A.; Kyurkchiev, S. Characterization of mesenchymal stem cells isolated from the human umbilical cord. Cell Biol. Int. 2008, 32, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Jez, M.; Ambady, S.; Kashpur, O.; Grella, A.; Malcuit, C.; Vilner, L.; Rozman, P.; Dominko, T. Expression and differentiation between OCT4A and its Pseudogenes in human ESCs and differentiated adult somatic cells. PLoS ONE 2014, 9, e89546. [Google Scholar] [CrossRef]
- Todtenhaupt, P.; Franken, L.A.; Groene, S.G.; van Hoolwerff, M.; van der Meeren, L.E.; van Klink, J.M.M.; Roest, A.A.W.; de Bruin, C.; Ramos, Y.F.M.; Haak, M.C.; et al. A robust and standardized method to isolate and expand mesenchymal stromal cells from human umbilical cord. Cytotherapy 2023, 25, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Hendijani, F.; Sadeghi-Aliabadi, H.; Haghjooy Javanmard, S. Comparison of human mesenchymal stem cells isolated by explant culture method from entire umbilical cord and Wharton’s jelly matrix. Cell Tissue Bank. 2014, 15, 555–565. [Google Scholar] [CrossRef]
- Doan, C.C.; Le, T.L.; Hoang, N.S.; Doan, N.T.; Le, V.D.; Do, M.S. Differentiation of umbilical cord lining membrane-derived mesenchymal stem cells into endothelial-like cells. Iran. Biomed. J. 2014, 18, 67–75. [Google Scholar] [CrossRef]
- Semenova, E.; Grudniak, M.P.; Machaj, E.K.; Bocian, K.; Chroscinska-Krawczyk, M.; Trochonowicz, M.; Stepaniec, I.M.; Murzyn, M.; Zagorska, K.E.; Boruczkowski, D.; et al. Mesenchymal Stromal Cells from Different Parts of Umbilical Cord: Approach to Comparison & Characteristics. Stem Cell Rev. Rep. 2021, 17, 1780–1795. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Nagamura-Inoue, T.; Tsunoda, H.; Yuzawa, M.; Yamamoto, Y.; Yorozu, P.; Agata, H.; Tojo, A. Stage-specific embryonic antigen 4 in Wharton’s jelly-derived mesenchymal stem cells is not a marker for proliferation and multipotency. Tissue Eng. Part A 2014, 20, 1314–1324. [Google Scholar] [CrossRef]
- Mori, Y.; Ohshimo, J.; Shimazu, T.; He, H.; Takahashi, A.; Yamamoto, Y.; Tsunoda, H.; Tojo, A.; Nagamura-Inoue, T. Improved explant method to isolate umbilical cord-derived mesenchymal stem cells and their immunosuppressive properties. Tissue Eng. Part C Methods 2015, 21, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Hassan, G.; Kasem, I.; Soukkarieh, C.; Aljamali, M. A Simple Method to Isolate and Expand Human Umbilical Cord Derived Mesenchymal Stem Cells: Using Explant Method and Umbilical Cord Blood Serum. Int. J. Stem Cells 2017, 10, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Bharti, D.; Shivakumar, S.B.; Park, J.K.; Ullah, I.; Subbarao, R.B.; Park, J.S.; Lee, S.L.; Park, B.W.; Rho, G.J. Comparative analysis of human Wharton’s jelly mesenchymal stem cells derived from different parts of the same umbilical cord. Cell Tissue Res. 2018, 372, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Jang, K.H.; Jo, C.H. Minimal Cube Explant Provides Optimal Isolation Condition of Mesenchymal Stem Cells from Umbilical Cord. Tissue Eng. Regen. Med. 2022, 19, 793–807. [Google Scholar] [CrossRef]
- Otte, A.; Bucan, V.; Reimers, K.; Hass, R. Mesenchymal stem cells maintain long-term in vitro stemness during explant culture. Tissue Eng. Part C Methods 2013, 19, 937–948. [Google Scholar] [CrossRef]
- Yoon, J.H.; Roh, E.Y.; Shin, S.; Jung, N.H.; Song, E.Y.; Chang, J.Y.; Kim, B.J.; Jeon, H.W. Comparison of explant-derived and enzymatic digestion-derived MSCs and the growth factors from Wharton’s jelly. BioMed Res. Int. 2013, 2013, 428726. [Google Scholar] [CrossRef]
- Araújo, A.B.; Salton, G.D.; Furlan, J.M.; Schneider, N.; Angeli, M.H.; Laureano, Á.M.; Silla, L.; Passos, E.P.; Paz, A.H. Comparison of human mesenchymal stromal cells from four neonatal tissues: Amniotic membrane, chorionic membrane, placental decidua and umbilical cord. Cytotherapy 2017, 19, 577–585. [Google Scholar] [CrossRef]
- Wang, S.; Qu, X.; Zhao, R.C. Clinical applications of mesenchymal stem cells. J. Hematol. Oncol. 2012, 5, 19. [Google Scholar] [CrossRef]
- Jovic, D.; Yu, Y.; Wang, D.; Wang, K.; Li, H.; Xu, F.; Liu, C.; Liu, J.; Luo, Y. A Brief Overview of Global Trends in MSC-Based Cell Therapy. Stem Cell Rev. Rep. 2022, 18, 1525–1545. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, R.; Zou, Q.; Chen, Y.; Zhou, M.; Li, X.; Ran, R.; Chen, Q. Comparison of the Biological Characteristics of Mesenchymal Stem Cells Derived from the Human Placenta and Umbilical Cord. Sci. Rep. 2018, 8, 5014. [Google Scholar] [CrossRef] [PubMed]
- Dulugiac, M.; Moldovan, L.; Zarnescu, O. Comparative studies of mesenchymal stem cells derived from different cord tissue compartments-The influence of cryopreservation and growth media. Placenta 2015, 36, 1192–1203. [Google Scholar] [CrossRef]
- Boyer, L.A.; Lee, T.I.; Cole, M.F.; Johnstone, S.E.; Levine, S.S.; Zucker, J.P.; Guenther, M.G.; Kumar, R.M.; Murray, H.L.; Jenner, R.G.; et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005, 122, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Stoyanova, E.; Oreshkova, T.; Mourdjeva, M. Generation of human induced pluripotent stem cells from adipose-derived stromal/stem cells isolated from a 75-year-old patient. Arch. Biol. Sci. 2017, 69, 715–721. [Google Scholar] [CrossRef]
- Kellner, S.; Kikyo, N. Transcriptional regulation of the Oct4 gene, a master gene for pluripotency. Histol. Histopathol. 2010, 25, 405–412. [Google Scholar] [CrossRef]
- Mehravar, M.; Ghaemimanesh, F.; Poursani, E.M. An Overview on the Complexity of OCT4: At the Level of DNA, RNA and Protein. Stem Cell Rev. Rep. 2021, 17, 1121–1136. [Google Scholar] [CrossRef]
- He, H.; Nagamura-Inoue, T.; Yuzawa, M.; Yamamoto, Y.; Tsunoda, H.; Tojo, A. Comparative Analysis of SSEA4+ and SSEA4− Mesenchymal Stem Cells Derived From Wharton’s Jelly of Umbilical Cord. Blood 2012, 120, 4735. [Google Scholar] [CrossRef]
- Weiss, M.L.; Medicetty, S.; Bledsoe, A.R.; Rachakatla, R.S.; Choi, M.; Merchav, S.; Luo, Y.; Rao, M.S.; Velagaleti, G.; Troyer, D. Human umbilical cord matrix stem cells: Preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells 2006, 24, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Qin, X.; Fan, C.; Wang, M.; Chen, F.; Liao, M.; Zhong, H.; Wang, H.; Ma, L. Comparison of Biological Characteristics of Human Umbilical Cord Wharton’s Jelly-Derived Mesenchymal Stem Cells from Extremely Preterm and Term Infants. Tissue Eng. Regen. Med. 2023, 20, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Deuse, T.; Stubbendorff, M.; Tang-Quan, K.; Phillips, N.; Kay, M.A.; Eiermann, T.; Phan, T.T.; Volk, H.D.; Reichenspurner, H.; Robbins, R.C.; et al. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transplant. 2011, 20, 655–667. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) | Ta (°C) |
|---|---|---|---|
| oct4a | AGTGAGAGGCAACCTGGAGA | GTGAAGTGAGGGCTCCCATA | 60 |
| oct4b | TATGGGAGCCCTCACTTCAC | CAAAAACCCTGGCACAACT | 60 |
| oct4b1 | AGACTATTCCTTGGGGCCACAC | GGCTGAATACCTTCCCAAATAGA | 63 |
| sox2 | ACACCAATCCCATCCACACT | GCAAACTTCCTGCAAAGCTC | 60 |
| nanog | GTCCCGGTCAAGAACAGAA | TGCGTCACACCATTGCTATT | 60 |
| β-actin | TGACGGGGTCACCCACACACTGTGCCCATCTA | CTAGAAGCATTTGCGGACGATGGAGGG | 62 |
| Author | Origin of the Explant | Size of the Explants | Discarding of the Explants (Days) | Initial Cell Number Cells/cm (cm2) | Time for Reaching Confluence of 80–90% (Days) | Population Doubling Time (on Average)/Passage Number |
|---|---|---|---|---|---|---|
| Our results | WJ | 10 mm2 | 10 with transferring of the explant to new culture dish for another 10 days | 5–6 × 104 cells/cm2 or 18 × 104 cells/cm | 16–18 | ~29.2 (P3) |
| SA | 10 mm2 | 3–4 × 104 cells/cm2 or 12 × 104 cells/cm | ~31.9 (P3) | |||
| Hua et al., 2013 [29] | WJ | 10 mm of UC 5 mm of UC 1 mm of UC | 10 | 6.7 × 104 cells/cm 1.9 × 104 cells/cm 2.6 × 104 cells/cm | 21 8–10 8–10 | ND |
| Todtenhaupt et al., 2023 [41] | WJ | ~1 cm2 | 10 | 2.15 × 104 cells/cm2 | 10–12 | ~ 26.6 h (P1) |
| Hendijani et al., 2014 [42] | UC WJ | 1–3 mm2 1–3 mm2 | ND | ND | 22 | ~23 h (P6) ~24 h(P6) |
| Doan et al., 2014 [43] | Amnion | 2–3 mm3 | 10–12 | ND | 21 | ND |
| Kita et al., 2010 [25] | Amnion | ~1 inch long/2.5 cm of UC | ND | ND | ND | ND |
| Semenova et al., 2021 [44] | Amnion WJ Perivascular region | 2–3 mm3 2–3 mm3 2–3 mm3 | 14 | ND | 14 | ~35.25 h up to P12 ~32.4 ± 3.6 up to P12 ~27.8 ± 4.8 up to P12 |
| He et al., 2014 [45] | WJ | 1– 2 mm3 | 4–21 | NA | 14–21 | ND |
| Mori et al., 2015 [46] | WJ | 1–2 mm3 | 9–12 | NA | 20.1 ± 4.4 | ND |
| Hassan et al., 2017 [47] | UC | 5 cm2 | 14 | ND | ND | 38–95 h (P3) |
| Bharti et al., 2018 [48] | WJ | 1 to 2 mm | 10–12 | ND | 18–22 | ~49.30 h |
| Lee et al., 2020 [49] | WJ | 1–2 mm 2–4 mm 10 mm | ND | NA | 12 | ~31.6 up to P3 ~28.5 ± 1.6 h up to P3 ~33.4 ± 1.2 h up to P3 |
| Zheng et al., 2022 [32] | WJ | 1–2 mm | 8 | NA | 8 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kestendjieva, S.; Chervenkov, M.; Oreshkova, T.; Mourdjeva, M.; Stoyanova, E. Mesenchymal Stromal/Stem Cells Isolated by Explant Culture Method from Wharton’s Jelly and Subamnion Possess Similar Biological Characteristics. Appl. Sci. 2024, 14, 8036. https://doi.org/10.3390/app14178036
Kestendjieva S, Chervenkov M, Oreshkova T, Mourdjeva M, Stoyanova E. Mesenchymal Stromal/Stem Cells Isolated by Explant Culture Method from Wharton’s Jelly and Subamnion Possess Similar Biological Characteristics. Applied Sciences. 2024; 14(17):8036. https://doi.org/10.3390/app14178036
Chicago/Turabian StyleKestendjieva, Snejana, Mihail Chervenkov, Tsvetelina Oreshkova, Milena Mourdjeva, and Elena Stoyanova. 2024. "Mesenchymal Stromal/Stem Cells Isolated by Explant Culture Method from Wharton’s Jelly and Subamnion Possess Similar Biological Characteristics" Applied Sciences 14, no. 17: 8036. https://doi.org/10.3390/app14178036
APA StyleKestendjieva, S., Chervenkov, M., Oreshkova, T., Mourdjeva, M., & Stoyanova, E. (2024). Mesenchymal Stromal/Stem Cells Isolated by Explant Culture Method from Wharton’s Jelly and Subamnion Possess Similar Biological Characteristics. Applied Sciences, 14(17), 8036. https://doi.org/10.3390/app14178036







