Mitochondrial Dysfunction in Aristolochic Acid I-Induced Kidney Diseases: What We Know and What We Do Not Know
Abstract
1. Introduction
2. Mitochondria and Kidney Diseases
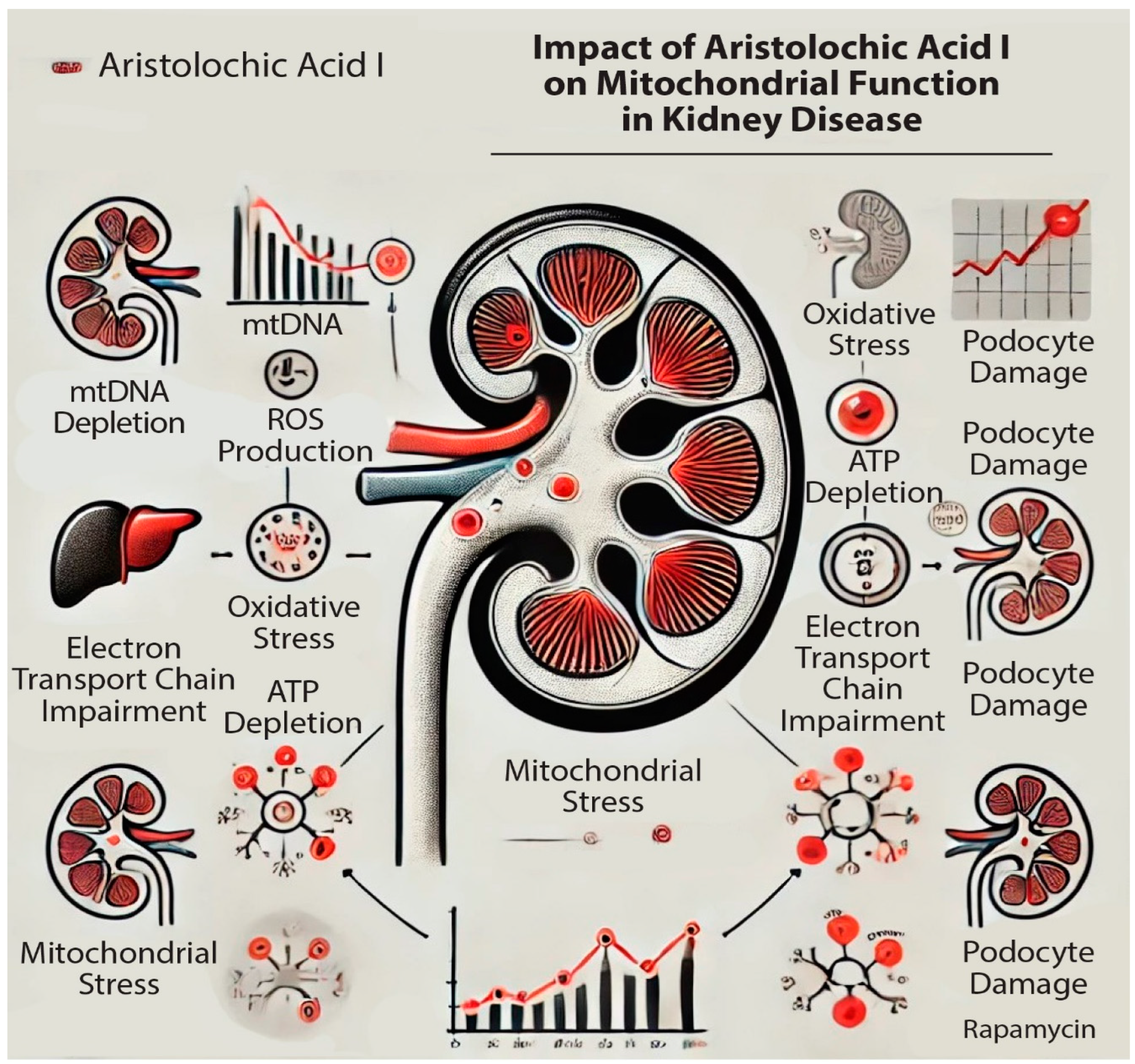
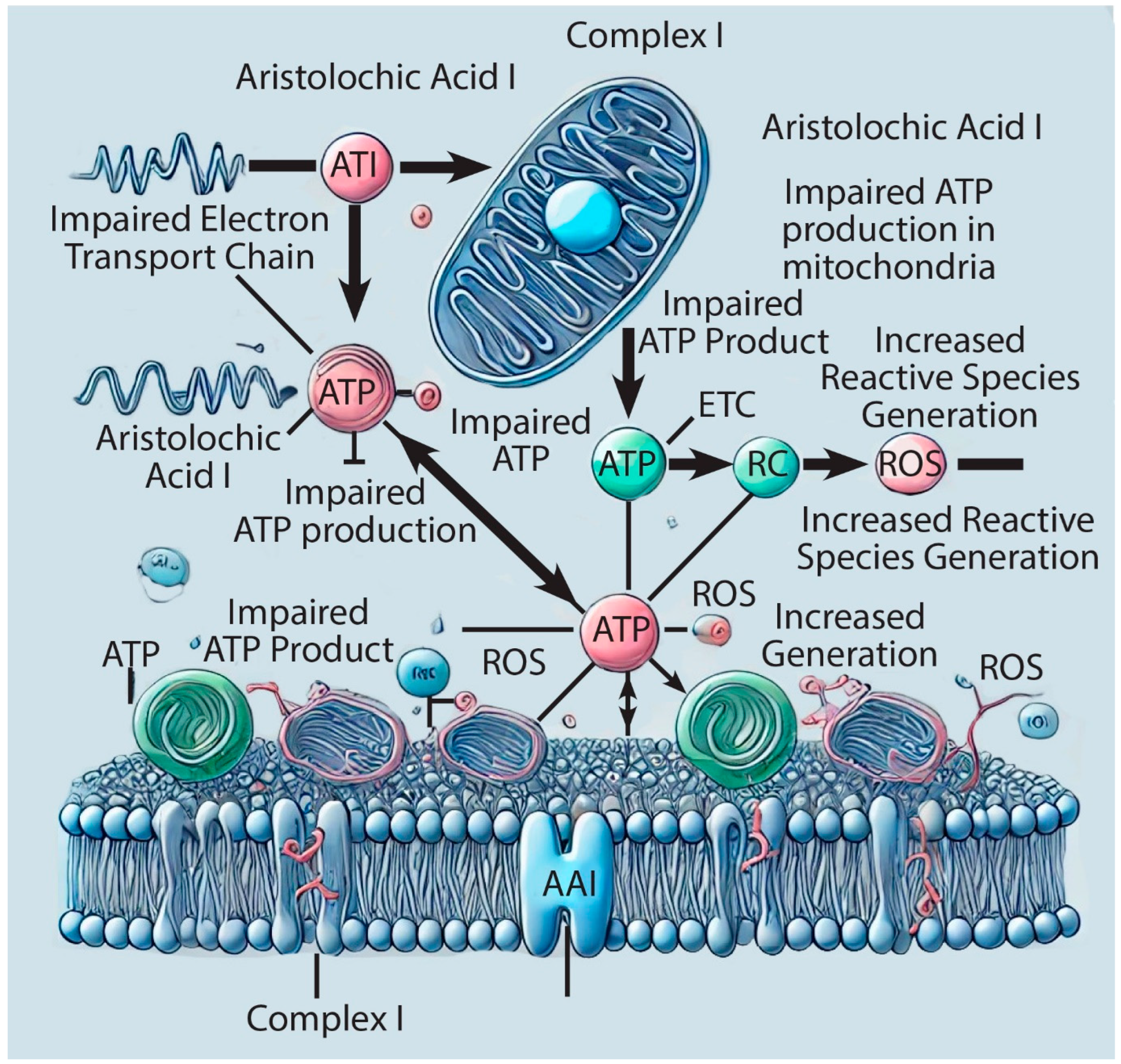
3. Mitochondrial Dysfunction in AAI-Induced Kidney Diseases
3.1. Methods of Investigations and Biomarker Detection in AAI-Induce Mitochondrial Dysfunction
3.2. Investigation Methods of Mitochondrial Function
3.2.1. Assay of Oxygen Consumption Measurement
3.2.2. Respiratory Chain Complexes
3.2.3. Mitochondrial Membrane Potential (MMP)
3.2.4. Assay of Adenine Nucleotide Translocator (ANT) Activity
3.2.5. Measurement of Calcium
3.2.6. Mitochondrial DNA (mtDNA)
3.3. Other Types of Investigation Methods and Markers of Mitochondrial Dysfunction
4. Discussions
4.1. Diagnosis and Prognosis of Mitochondrial Dysfunction in AAI-Induced Kidney Diseases
4.2. Treatment of Mitochondrial Dysfunction in AAI-Induced Kidney Diseases
4.3. AAI-Induced Mitochondrial Dysfunction in Other Organs
4.4. The Cytoprotective Effects of AAI
5. Conclusions and Future Perspectives
5.1. Future Directions
5.2. Potential Therapeutic Approaches
5.3. Importance and Potential Impact
5.4. Effects of AAI on Mitochondrial Function
5.5. Mutational Signature and Carcinogenesis
5.6. Clinical Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gifford, F.J.; Gifford, R.M.; Eddleston, M.; Dhaun, N. Endemic Nephropathy Around the World. Kidney Int. Rep. 2017, 2, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, J.; Wang, J.; Feng, X.; Wu, H.; Huang, R.; Fan, J.; Yu, X.; Yang, X. Mitochondrial Dysfunction Is Involved in Aristolochic Acid I-Induced Apoptosis in Renal Proximal Tubular Epithelial Cells. Hum. Exp. Toxicol. 2020, 39, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, P.; Wei, F.; Lin, R.-C.; Khan, I.A.; Pasco, D.S. Structure Activity Relationships of Aristolochic Acid Analogues: Toxicity in Cultured Renal Epithelial Cells. Kidney Int. 2005, 67, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Arlt, V.M. Aristolochic Acid as a Probable Human Cancer Hazard in Herbal Remedies: A Review. Mutagenesis 2002, 17, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Debelle, F.D.; Vanherweghem, J.-L.; Nortier, J.L. Aristolochic Acid Nephropathy: A Worldwide Problem. Kidney Int. 2008, 74, 158–169. [Google Scholar] [CrossRef]
- Tatu, C.A.; Orem, W.H.; Finkelman, R.B.; Feder, G.L. The Etiology of Balkan Endemic Nephropathy: Still More Questions than Answers. Environ. Health Perspect. 1998, 106, 689. [Google Scholar] [CrossRef]
- Han, J.; Xian, Z.; Zhang, Y.; Liu, J.; Liang, A. Systematic Overview of Aristolochic Acids: Nephrotoxicity, Carcinogenicity, and Underlying Mechanisms. Front. Pharmacol. 2019, 10, 648. [Google Scholar] [CrossRef]
- Chan, C.-K.; Liu, Y.; Pavlović, N.M.; Chan, W. Etiology of Balkan Endemic Nephropathy: An Update on Aristolochic Acids Exposure Mechanisms. Chem. Res. Toxicol. 2018, 31, 1109–1110. [Google Scholar] [CrossRef]
- Jadot, I.; Declèves, A.-E.; Nortier, J.; Caron, N. An Integrated View of Aristolochic Acid Nephropathy: Update of the Literature. IJMS 2017, 18, 297. [Google Scholar] [CrossRef]
- Yang, L.; Su, T.; Li, X.-M.; Wang, X.; Cai, S.-Q.; Meng, L.-Q.; Zou, W.-Z.; Wang, H.-Y. Aristolochic Acid Nephropathy: Variation in Presentation and Prognosis. Nephrol. Dial. Transplant. 2012, 27, 292–298. [Google Scholar] [CrossRef]
- Braga, P.C.; Alves, M.G.; Rodrigues, A.S.; Oliveira, P.F. Mitochondrial Pathophysiology on Chronic Kidney Disease. IJMS 2022, 23, 1776. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Bao, Q.; Sun, L.; Huang, X.; Wang, T.; Zhang, S.; Li, H.; Zhang, L. Possible Role of mtDNA Depletion and Respiratory Chain Defects in Aristolochic Acid I-Induced Acute Nephrotoxicity. Toxicol. Appl. Pharmacol. 2013, 266, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.; Ham, Y.-H. Probing the Hidden Role of Mitochondrial DNA Damage and Dysfunction in the Etiology of Aristolochic Acid Nephropathy. Chem. Res. Toxicol. 2021, 34, 1903–1909. [Google Scholar] [CrossRef]
- Acin-Perez, R.; Benador, I.Y.; Petcherski, A.; Veliova, M.; Benavides, G.A.; Lagarrigue, S.; Caudal, A.; Vergnes, L.; Murphy, A.N.; Karamanlidis, G.; et al. A Novel Approach to Measure Mitochondrial Respiration in Frozen Biological Samples. EMBO J. 2020, 39, e104073. [Google Scholar] [CrossRef]
- Murphy, M.P.; Smith, R.A.J. Drug Delivery to Mitochondria: The Key to Mitochondrial Medicine. Adv. Drug Deliv. Rev. 2000, 41, 235–250. [Google Scholar] [CrossRef]
- Zhang, X.; Agborbesong, E.; Li, X. The Role of Mitochondria in Acute Kidney Injury and Chronic Kidney Disease and Its Therapeutic Potential. IJMS 2021, 22, 11253. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.A.; Schnellmann, R.G. Persistent Disruption of Mitochondrial Homeostasis after Acute Kidney Injury. Am. J. Physiol.-Ren. Physiol. 2012, 302, F853–F864. [Google Scholar] [CrossRef] [PubMed]
- Fontecha-Barriuso, M.; Lopez-Diaz, A.M.; Guerrero-Mauvecin, J.; Miguel, V.; Ramos, A.M.; Sanchez-Niño, M.D.; Ruiz-Ortega, M.; Ortiz, A.; Sanz, A.B. Tubular Mitochondrial Dysfunction, Oxidative Stress, and Progression of Chronic Kidney Disease. Antioxidants 2022, 11, 1356. [Google Scholar] [CrossRef]
- Zhou, Y.; Bian, X.; Fang, L.; He, W.; Dai, C.; Yang, J. Aristolochic Acid Causes Albuminuria by Promoting Mitochondrial DNA Damage and Dysfunction in Podocyte. PLoS ONE 2013, 8, e83408. [Google Scholar] [CrossRef]
- Qi, X.; Cai, Y.; Gong, L.; Liu, L.; Chen, F.; Xiao, Y.; Wu, X.; Li, Y.; Xue, X.; Ren, J. Role of Mitochondrial Permeability Transition in Human Renal Tubular Epithelial Cell Death Induced by Aristolochic Acid. Toxicol. Appl. Pharmacol. 2007, 222, 105–110. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, P.; Chen, J.; Yang, C.; Xia, F.; Zhang, J.; Tang, H.; Liu, D.; Gu, L.; Shi, Q.; et al. Dissection of Targeting Molecular Mechanisms of Aristolochic Acid-Induced Nephrotoxicity via a Combined Deconvolution Strategy of Chemoproteomics and Metabolomics. Int. J. Biol. Sci. 2022, 18, 2003–2017. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.; Batuman, V. Aristolochic Acid I Induces Proximal Tubule Injury through ROS/HMGB1/Mt DNA Mediated Activation of TLRs. J. Cell. Mol. Med. 2022, 26, 4277–4291. [Google Scholar] [CrossRef] [PubMed]
- Hsin, Y.-H.; Cheng, C.-H.; Tzen, J.T.C.; Wu, M.-J.; Shu, K.-H.; Chen, H.-C. Effect of Aristolochic Acid on Intracellular Calcium Concentration and Its Links with Apoptosis in Renal Tubular Cells. Apoptosis 2006, 11, 2167–2177. [Google Scholar] [CrossRef]
- Tafani, M.; Schneider, T.G.; Pastorino, J.G.; Farber, J.L. Cytochrome C-Dependent Activation of Caspase-3 by Tumor Necrosis Factor Requires Induction of the Mitochondrial Permeability Transition. Am. J. Pathol. 2000, 156, 2111–2121. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-K.; Wei, C.-W.; Pan, Y.-R.; Cherng, S.-H.; Chang, W.-J.; Wang, H.-F.; Yu, Y.-L. Vitamin C Attenuates the Toxic Effect of Aristolochic Acid on Renal Tubular Cells via Decreasing Oxidative Stress-Mediated Cell Death Pathways. Mol. Med. Rep. 2015, 12, 6086–6092. [Google Scholar] [CrossRef]
- Pastorino, J.G.; Chen, S.-T.; Tafani, M.; Snyder, J.W.; Farber, J.L. The Overexpression of Bax Produces Cell Death upon Induction of the Mitochondrial Permeability Transition. J. Biol. Chem. 1998, 273, 7770–7775. [Google Scholar] [CrossRef]
- Jiménez-Uribe, A.P.; Pedraza-Chaverri, J. Promising Therapeutic Strategies Targeting Mitochondria in Kidney Diseases: From Small Molecules to Whole Mitochondria. Future Pharmacol. 2022, 2, 256–275. [Google Scholar] [CrossRef]
- Ho, H.-J.; Shirakawa, H. Oxidative Stress and Mitochondrial Dysfunction in Chronic Kidney Disease. Cells 2022, 12, 88. [Google Scholar] [CrossRef]
- Galvan, D.L.; Mise, K.; Danesh, F.R. Mitochondrial Regulation of Diabetic Kidney Disease. Front. Med. 2021, 8, 745279. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Wang, J.; Fan, J.; Feng, X.; Yu, X.; Yang, X. Possible Role of Mitochondrial Injury in Caulis Aristolochia Manshuriensis-Induced Chronic Aristolochic Acid Nephropathy. Drug Chem. Toxicol. 2017, 40, 115–124. [Google Scholar] [CrossRef]
- Cleveland, K.H.; Schnellmann, R.G. Pharmacological Targeting of Mitochondria in Diabetic Kidney Disease. Pharmacol. Rev. 2023, 75, 250–262. [Google Scholar] [CrossRef]
- Penzo, D.; Petronilli, V.; Angelin, A.; Cusan, C.; Colonna, R.; Scorrano, L.; Pagano, F.; Prato, M.; Di Lisa, F.; Bernardi, P. Arachidonic Acid Released by Phospholipase A2 Activation Triggers Ca2+-Dependent Apoptosis through the Mitochondrial Pathway. J. Biol. Chem. 2004, 279, 25219–25225. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Anger, E.E.; Zhang, X.; Su, S.; Su, C.; Zhao, S.; Yu, F.; Li, J. Protective Effects of Mitochondrial Uncoupling Protein 2 against Aristolochic Acid I-Induced Toxicity in HK-2 Cells. IJMS 2022, 23, 3674. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Zhou, C.; Xu, Q.; Gao, H.; Huo, M.; Jiang, X.; Yu, W. Catalpol Attenuates Renal Injury by Regulating Oxidative Stress and Inflammation Response. Res. Sq. 2022. preprint. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, S.; Wu, J.; Chen, W.; Sun, H.; Peng, W.; Yu, X.; Yang, X. Autophagy Inhibitors Promoted Aristolochic Acid I Induced Renal Tubular Epithelial Cell Apoptosis via Mitochondrial Pathway but Alleviated Nonapoptotic Cell Death in Mouse Acute Aritolochic Acid Nephropathy Model. Apoptosis 2014, 19, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Baudoux, T.E.R.; Pozdzik, A.A.; Arlt, V.M.; De Prez, E.G.; Antoine, M.-H.; Quellard, N.; Goujon, J.-M.; Nortier, J.L. Probenecid Prevents Acute Tubular Necrosis in a Mouse Model of Aristolochic Acid Nephropathy. Kidney Int. 2012, 82, 1105–1113. [Google Scholar] [CrossRef]
- Yang, C.-C.; Wu, C.-T.; Chen, L.-P.; Hung, K.-Y.; Liu, S.-H.; Chiang, C.-K. Autophagy Induction Promotes Aristolochic Acid-I-Induced Renal Injury in Vivo and in Vitro. Toxicology 2013, 312, 63–73. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiang, L.; Su, T.; Liu, G.; Yang, L. Overview of Aristolochic Acid Nephropathy: An Update. Kidney Res. Clin. Pract. 2023, 42, 579–590. [Google Scholar] [CrossRef]
- Zhang, J.; Chan, C.-K.; Pavlović, N.M.; Chan, W. Effects of Diet on Aristolochic Acid-DNA Adduct Formation: Implications for Balkan Endemic Nephropathy Etiology. Chem. Res. Toxicol. 2023, 36, 438–445. [Google Scholar] [CrossRef]
- Zhang, Y.; ShiYang, X.; Zhang, Y.; Li, Y.; Shi, X.; Xiong, B. Exposure to Aristolochic Acid I Compromises the Maturational Competency of Porcine Oocytes via Oxidative Stress-Induced DNA Damage. Aging 2019, 11, 2241–2252. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X.; Zhou, C.; Jia, Y.; Liu, S.; Xiong, Z.; Guo, X.; Fei, X.; Jiang, X.; Yu, W. Aristolochic Acid Induces Mitochondrial Apoptosis through Oxidative Stress in Rats, Leading to Liver Damage. Toxicol. Mech. Methods 2021, 31, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Au, C.-K.; Ham, Y.-H.; Chan, W. Bioaccumulation and DNA Adduct Formation of Aristolactam I: Unmasking a Toxicological Mechanism in the Pathophysiology of Aristolochic Acid Nephropathy. Chem. Res. Toxicol. 2023, 36, 322–329. [Google Scholar] [CrossRef]
- Fernandes, C.A.H.; Cardoso, F.F.; Cavalcante, W.G.L.; Soares, A.M.; Dal-Pai, M.; Gallacci, M.; Fontes, M.R.M. Structural Basis for the Inhibition of a Phospholipase A2-Like Toxin by Caffeic and Aristolochic Acids. PLoS ONE 2015, 10, e0133370. [Google Scholar] [CrossRef]
- Okada, H.; Watanabe, Y.; Inoue, T.; Kobayashi, T.; Kanno, Y.; Shiota, G.; Nakamura, T.; Sugaya, T.; Fukamizu, A.; Suzuki, H. Transgene-Derived Hepatocyte Growth Factor Attenuates Reactive Renal Fibrosis in Aristolochic Acid Nephrotoxicity. Nephrol. Dial. Transplant. 2003, 18, 2515–2523. [Google Scholar] [CrossRef]
- Gao, R.; Zheng, F.; Liu, Y.; Zheng, D.; Li, X.; Bo, Y.; Liu, Y. Aristolochic Acid I-Induced Apoptosis in LLC-PK1 Cells and Amelioration of the Apoptotic Damage by Calcium Antagonist. Chin. Med. J. 2000, 113, 418–424. [Google Scholar] [PubMed]
- Rosenthal, M.D.; Vishwanath, B.S.; Franson, R.C. Effects of Aristolochic Acid on Phospholipase A2 Activity and Arachidonate Metabolism of Human Neutrophils. Biochim. Biophys. Acta 1989, 1001, 1–8. [Google Scholar] [CrossRef]
- Chandra, V.; Jasti, J.; Kaur, P.; Srinivasan, A.; Betzel, C.; Singh, T.P. Structural Basis of Phospholipase A2 Inhibition for the Synthesis of Prostaglandins by the Plant Alkaloid Aristolochic Acid from a 1.7 Å Crystal Structure. Biochemistry 2002, 41, 10914–10919. [Google Scholar] [CrossRef]
- Vishwanath, B.S.; Fawzy, A.A.; Franson, R.C. Edema-Inducing Activity of Phospholipase A2 Purified from Human Synovial Fluid and Inhibition by Aristolochic Acid. Inflammation 1988, 12, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Gijón, M.A.; Leslie, C.C. Regulation of Arachidonic Acid Release and Cytosolic Phospholipase A2 Activation. J. Leukoc. Biol. 1999, 65, 330–336. [Google Scholar] [CrossRef]
- Liu, X.; Shi, J.; Jiao, Y.; An, J.; Tian, J.; Yang, Y.; Zhuo, L. Integrated Multi-Omics with Machine Learning to Uncover the Intricacies of Kidney Disease. Brief. Bioinform. 2024, 25, bbae364. [Google Scholar] [CrossRef]
- Li, J.J.; Liu, J.; Lupino, K.; Liu, X.; Zhang, L.; Pei, L. Growth Differentiation Factor 15 Maturation Requires Proteolytic Cleavage by PCSK3, -5, and -6. Mol. Cell. Biol. 2018, 38, e00249-18. [Google Scholar] [CrossRef] [PubMed]
- Verissimo, T.; de Seigneux, S. New Evidence of the Impact of Mitochondria on Kidney Health and Disease. Nat. Rev. Nephrol. 2024, 20, 81–82. [Google Scholar] [CrossRef] [PubMed]
| Types of Samples | Imaging Investigations | Biochemical/ELISA | Western Blot | Molecular Biology Assays | Immunology Assays | Ref |
|---|---|---|---|---|---|---|
| L929–TNF-α cells | TEM | Caspase 3, 8 MDH assay | PARP; Cytochrome c | cleavage of DNA | - | [24] |
| MH1C1–A23187 treated cells | Fluorescence microscopy | - | Caspase 8, 9, and 3 cleavage Cytochrome c | - | - | [32] |
| MDKC cells LLC-PK1 cells | Fluorescence microscopy | - | Mitochondrial/cytosolic fractions-cytochrome c | Cellular DNA-EtBr-UV | Flow cytometry: PI | [23] |
| CD-1 mouse Mice podocyte cells | HE and PAS TEM Immunohistochemical staining–WT1 Immunofluorescent staining | Urine albumin and creatinine Albumin influx assay | WT1 Mitobiogenesis: SDHA, COXI, actin Cytochrome C Tubulin Actin | Comet assay mtDNA copy number assay: cytochrome B COX III | DCF fluorescence Flow cytometer | [19] |
| Rats HK-2 cell | - | caspase 3 ATP | Cytochrome c | - | - | [20] |
| HEK293 cells L02 cells | - | ATP | - | DNA: 8-oxo-dG dA-ALI (LC-MS/MS) | - | [13] |
| NRK-52E cells | - | ATP | Cleaved-Caspase 3 | q-PCR mtDNA copy number | Flow-cytometry-Annexin V/PI DCF-ROS | [2] |
| SD rats | HE; TEM | BUN; Cr; ATP | - | mtDNA | - | [12] |
| HK-2 cells | Immunofluorescence microscopy-BrdU assay | IL6 KIM1 NO assay | TLR2 TLR4 TLR6 TLR9 | q-PCR | Apoptosis assay (PI; Annexin V) Cell cycle | [22] |
| NRK-52E cells | Inverted phase contrast and fluorescence microscopy | Caspase H2O2 and O2− ratios | Cleaved-caspase-3 Tubulin | - | - | [25] |
| Jurkat cells | - | Caspase 3 | Cytochrome c PARP | DNA fragmentation | - | [26] |
| HK-2 cells | Inverted phase contrast and fluorescence microscopy: apoptosis; ROS | Caspase 3 LDH assay MDA assay GSH-Px assay | UCP2 | - | - | [33] |
| SD rats | HE; PAS; TEM Confocal microscopy | BUN and Cr | Cleaved-Caspase-3 COX-I, NDUFβ8, PGC-1α | q-PCR mtDNA copy number | - | [30] |
| NRK-2E cells C57BL/6NJ mice | Optical and fluorescence microscopy; TEM HE; PAS; IHC | BUN and Cr MDA, GSH-Px, SOD, T-AOC IL-1, IL-6, IL-12 NGAL, KIM-1 | + | qRT-PCR | Flow-cytometry: apoptosis-Annexin V/PI | [34] |
| BALB/c mice | HE, PAS; IHC; TUNEL; TEM | BUN and Scr | LC3-I; LC3-II; Beclin-1 | - | - | [35] |
| C57BL/6 mice | HE; IHC; TEM | PCr | - | AA-DNA | - | [36] |
| Wistar rats NRK52E cells | Immunofluorescence and fluorescence microscopy TEM | - | Beclin-1, Atg5, PARP, LC3 | - | Flow-cytometry | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukinich-Gruia, A.T.; Calma, C.L.; Szekely, F.A.E.; Cristea, I.-M.; Pricop, M.-A.; Simina, A.-G.; Ordodi, V.L.; Pavlović, N.M.; Tatu, C.A.; Paunescu, V. Mitochondrial Dysfunction in Aristolochic Acid I-Induced Kidney Diseases: What We Know and What We Do Not Know. Appl. Sci. 2024, 14, 7961. https://doi.org/10.3390/app14177961
Lukinich-Gruia AT, Calma CL, Szekely FAE, Cristea I-M, Pricop M-A, Simina A-G, Ordodi VL, Pavlović NM, Tatu CA, Paunescu V. Mitochondrial Dysfunction in Aristolochic Acid I-Induced Kidney Diseases: What We Know and What We Do Not Know. Applied Sciences. 2024; 14(17):7961. https://doi.org/10.3390/app14177961
Chicago/Turabian StyleLukinich-Gruia, Alexandra T., Crenguta L. Calma, Flavia A. E. Szekely, Iustina-Mirabela Cristea, Maria-Alexandra Pricop, Alina-Georgiana Simina, Valentin L. Ordodi, Nikola M. Pavlović, Calin A. Tatu, and Virgil Paunescu. 2024. "Mitochondrial Dysfunction in Aristolochic Acid I-Induced Kidney Diseases: What We Know and What We Do Not Know" Applied Sciences 14, no. 17: 7961. https://doi.org/10.3390/app14177961
APA StyleLukinich-Gruia, A. T., Calma, C. L., Szekely, F. A. E., Cristea, I.-M., Pricop, M.-A., Simina, A.-G., Ordodi, V. L., Pavlović, N. M., Tatu, C. A., & Paunescu, V. (2024). Mitochondrial Dysfunction in Aristolochic Acid I-Induced Kidney Diseases: What We Know and What We Do Not Know. Applied Sciences, 14(17), 7961. https://doi.org/10.3390/app14177961






