Abstract
Upon degranulation, basophils and mast cells secrete an array of inflammatory mediators, including histamine, which leads to not only allergic inflammation but also other inflammatory diseases. We previously reported that an aqueous extract from enzyme-treated, dried sardine inhibits the degranulation of RBL-2H3 cells and attenuates the symptoms of Japanese cedar pollinosis in mice. This study evaluated an antiallergic effect of dipeptides containing acidic amino acid residue in an antigen-induced degranulation assay using RBL-2H3 cells. The result showed that acidic amino acid residue-containing dipeptides inhibit the degranulation of RBL-2H3 cells without cytotoxicity. Additionally, L-histidyl-L-glutamic acid (His-Glu), one of the acidic amino acid residue-containing dipeptides tested in this study, inhibited calcium ionophore-induced degranulation. We also found that His-Glu suppressed microtubule reorganization in RBL-2H3 cells after antigen stimulation. His-Glu slightly, but not significantly, suppressed the elevation of cytosolic calcium ion concentration leading to degranulation. Immunoblot analysis revealed that His-Glu significantly suppressed the phosphorylation of phosphoinositide 3-kinase and Akt, but not that of Syk or phospholipase Cγ. Overall results suggest that acidic amino acid residue-containing dipeptides can be used as food ingredients with an antiallergic effect.
1. Introduction
An allergy is defined as a systemic or localized abnormal response of the immune system to a normally harmless substance, which is called an allergen. In most allergic reactions, immunoglobulin E (IgE) specific to the allergen is produced in the body and binds to high-affinity IgE receptor (FcεRI) on the surface of basophils in the bloodstream and of mast cells in tissues. Upon exposure to the allergen, it binds to these cells via IgE-binding FcεRI, and then the cells release histamine and other chemical mediators [1], causing allergic symptoms such as smooth muscle contraction, increased vascular permeability, and increased glandular secretion. Antihistamines and other drugs are on the market as antiallergic agents; however, they sometimes exhibit harmful side effects [2]. In recent years, there has been an increase in the number of papers on the antiallergic effects of nutrients and dietary components [3]. Although the antiallergic effects of food ingredients are not as strong as those of drugs, they are attracting attention because they have fewer harmful side effects. Therefore, developing functional foods containing antiallergic ingredients to prevent and attenuate allergic symptoms is an effective countermeasure.
Food-derived peptides have recently drawn attention as functional food ingredients. Food-derived peptides are generally found in fermented or enzymatically hydrolyzed food products [4]. Some bioactive peptides derived from foods are thought to be effective in preventing the onset of several diseases including hypertension, cancers, obesity, and cardiovascular diseases [5]. For example, soy bioactive peptides alter the composition and functions of gut microorganisms [6], and seaweed bioactive peptides can potentially prevent cardiovascular diseases and diabetes [7]. Recently, type 2 diabetes management using food protein-derived bioactive peptides has received much attention [8,9,10]. The effective use of bioactive peptides derived from by-products of food production has also attracted attention [11].
We previously reported that an aqueous extract from enzyme-treated, dried sardine inhibits the degranulation of RBL-2H3 cells and attenuates the symptoms of Japanese cedar pollinosis in mice [12]. As a result of the component analysis, we found that the enzyme-treated, dried sardine extract was rich in glutamic acid. Therefore, this study aimed to evaluate the inhibitory effect of acidic amino acids and their dipeptides on the degranulation of RBL-2H3 cells. Our findings demonstrate a degranulation-inhibiting impact of glutamyl and aspartyl dipeptides, and we have identified part of the mechanism by which they exert their antiallergic effects.
2. Materials and Methods
2.1. Reagents
Sodium L-glutamate monohydrate and 4-nitrophenyl 2-acetamido-2-deoxy-β-D-glucopyranoside were purchased from Tokyo Chemical Industry (Tokyo, Japan). L-aspartic acid was purchased from Fujifilm Wako Pure Chemical (Osaka, Japan). Dipeptides containing L-glutamic acid and/or L-aspartic acid were obtained from GL Biochem (Shanghai, China), except for L-glutamyl-L-glutamic acid (Glu-Glu), which was obtained from Peptide Institute (Osaka, Japan). Dipeptides and amino acids were dissolved in distilled deionized water at 4.0 mM. Dulbecco’s modified Eagle’s medium (DMEM), penicillin, streptomycin, fetal bovine serum (FBS), bovine serum albumin (BSA), mouse anti-dinitrophenyl (DNP) monoclonal IgE, DNP-human serum albumin (HSA) conjugate, a calcium ionophore A23187, and Triton X-100 were purchased from Sigma-Aldrich (St. Louis, MO, USA). All antibodies used in this study were obtained from Cell Signaling Technology (Danvers, MA, USA). All other chemicals were purchased from Nacalai Tesque (Kyoto, Japan) or Fujifilm Wako Pure Chemical unless otherwise noted.
2.2. Cell Culture
The rat basophilic leukemia RBL-2H3 cells were obtained from the American Type Culture Collection (Rockville, MD, USA). The cells were cultured in DMEM supplemented with 100 U/mL of penicillin, 100 µg/mL of streptomycin, and 5% FBS at 37 °C under humidified 5% CO2.
2.3. Antigen-Induced Degranulation Assay
RBL-2H3 cells were seeded at 4.0 × 104 cells/well in a 96-well culture plate (Violamo, Osaka, Japan). After preculturing at 37 °C overnight, the cells were sensitized with 25 ng/mL of anti-DNP IgE for 2 h at 37 °C. The cells were subsequently washed with modified Tyrode’s (MT) buffer (20 mM HEPES, 135 mM NaCl, 5.0 mM KCl, 1.8 mM CaCl2, 1.0 mM MgCl2, 5.6 mM glucose, and 0.05% BSA, pH 7.4) to remove excess anti-DNP IgE. Dipeptides and amino acids were equally mixed with 2 × MT buffer and then added at 120 μL/well. After incubation for 10 min, DNP-HSA, the antigen of anti-DNP IgE, was added to each well (final concentration: 50 ng/mL) to induce degranulation. After incubation for 30 min, the supernatant was transferred to a new 96-well microplate. The cells were then lysed in MT buffer containing 0.1% Triton X-100 for 30 min, and the cell lysate was transferred to a new 96-well microplate. The colorimetric substrate solution (3.3 mM 4-nitrophenyl 2-acetamido-2-deoxy-β-D-glucopyranoside dissolved in 0.1 M citrate buffer, pH 4.5) was added to both the culture supernatant and cell lysate. After incubation for 25 min at 37 °C, the enzymatic reaction was terminated by adding 2.0 M glycine buffer (pH 10.4). The absorbance was measured at 415 nm using an iMark microplate reader (Bio-Rad Laboratories, Hercules, CA, USA). The release rate of β-hexosaminidase from cells was calculated as described previously [13].
2.4. Calcium Ionophore-Induced Degranulation Assay
RBL-2H3 cells were seeded at 4.0 × 104 cells/well in a 96-well culture plate (Violamo) and precultured at 37 °C overnight. After removing the culture medium, a mixture of 2 × MT buffer and a dipeptide/amino acid sample was added at 120 μL/well. After incubation for 10 min, A23187 was added to each well (final concentration: 1.0 µM) to induce degranulation. After incubation for 30 min, subsequent operations were carried out as described in Section 2.3.
2.5. Cell Viability Assay
RBL-2H3 cells were seeded and sensitized in the same manner as the antigen-induced degranulation assay. After treating cells with samples and inducing IgE-mediated degranulation as shown above, the culture medium was removed, and the cells were washed with phosphate-buffered saline (PBS, pH 7.4). Fresh DMEM containing WST-8 reagent (Nacalai Tesque) was then added to each well. The absorbance was measured at 450 nm using the iMark microplate reader.
2.6. Monitoring of Cytosolic Calcium Ion Concentration
The cytosolic calcium ion concentration in RBL-2H3 cells was monitored using Fluo-3 acetoxymethyl ester (Dojindo Laboratories, Mashiki, Japan) according to the manufacturer’s instructions. RBL-2H3 cells were seeded at 4.0 × 104 cells/well in a black 96-well culture plate (Nunc, Copenhagen, Denmark) and precultured at 37 °C overnight. In the case of antigen-induced degranulation, the cells were sensitized with 25 ng/mL of anti-DNP IgE for 2 h at 37 °C. The cells were then pretreated with Fluo-3 acetoxymethyl ester for 1 h at 37 °C. After removing the reagent, cells were washed with PBS twice. The cells were subsequently treated with 2.0 mM L-histidyl-L-glutamic acid (His-Glu) for 10 min at 37 °C. After removing the reagent, cells were washed with PBS once. DNP-HSA was then added to each well (final concentration: 50 ng/mL). In the case of calcium ionophore-induced degranulation, the cells pretreated with Fluo-3 acetoxymethyl ester were washed with PBS twice and treated with 2.0 mM His-Glu for 10 min at 37 °C. After removing the reagent, cells were washed with PBS once. A23187 was then added to each well (final concentration: 1.0 µM). Fluorescence was monitored immediately after inducing degranulation using an Infinite 200 Pro microplate reader (Tecan, Männedorf, Switzerland) with an excitation wavelength of 480 nm and an emission wavelength of 530 nm.
2.7. Morphological Analysis of RBL-2H3 Cells
RBL-2H3 cells were seeded into a 35 mm culture dish (Violamo) containing a coverslip at 6.0 × 105 cells/dish. After sensitizing with 50 ng/mL of anti-DNP IgE, the cells were treated with 2.0 mM His-Glu at 37 °C for 10 min. After removing the reagent, the cells were stimulated with 50 ng/mL of DNP-HSA at 37 °C for 10 min. After washing with PBS twice, the cells were fixed with 4% paraformaldehyde for 15 min. Then, after washing with PBS thrice more, the cells were blocked with 5% BSA and 0.3% Triton X-100 in PBS for 1 h. After removing the reagents, the cells were stained with an anti-α-tubulin antibody labeled with Alexa Fluor 488 at 4 °C overnight. After washing with PBS thrice, images were acquired on a fluorescence microscope (BX53, Olympus, Tokyo, Japan).
2.8. Immunoblot Analysis
RBL-2H3 cells were seeded into a 35 mm culture dish (Violamo) at 5.0 × 105 cells/dish. After sensitizing with 50 ng/mL of anti-DNP IgE, the cells were washed with MT buffer once and then treated with 2.0 mM His-Glu at 37 °C for 10 min. The cells were then stimulated with 50 ng/mL of DNP-HSA for 5 min. Cell lysates were subsequently prepared and used for immunoblotting as described previously [14].
2.9. Statistical Analysis
Data were expressed as mean ± SEM. Statistical analysis was performed using GraphPad Prism version 7.02 (GraphPad Software, San Diego, CA, USA). Statistical significance was determined via Dunnett’s multiple comparison test. Values with p < 0.05 were considered statistically significant.
3. Results and Discussion
3.1. Inhibitory Effect of Acidic Amino Acid Residue-Containing Dipeptides on IgE-Mediated Degranulation of RBL-2H3 Cells
This study evaluated the antiallergic effect of acidic amino acids and dipeptides containing an L-glutamic or L-aspartic acid residue in an antigen-induced degranulation assay using RBL-2H3 cells. The result showed that L-glutamic acid is not effective in inhibiting the degranulation of RBL-2H3 cells (Table 1). L-aspartic acid, an anionic amino acid structurally similar to L-glutamic acid, was also investigated; however, no inhibitory effect was observed (Table 1).

Table 1.
Inhibition of antigen-induced degranulation of RBL-2H3 cells by an acidic amino acid or dipeptide.
We then evaluated various dipeptides containing an L-glutamic or L-aspartic acid residue because some dipeptides are known to benefit humans [15]. As a result, L-glutamic acid-containing dipeptides tested in this study inhibited the degranulation of RBL-2H3 cells at 1 mM (Table 1). These peptides suppressed the degranulation in a concentration-dependent manner. The concentration-dependent curve of His-Glu is shown in Figure 1A. The position (carboxy or amino terminus) of L-glutamic acid residue seems to have little effect on the degranulation-inhibitory potency in all samples tested (Table 1). We also evaluated dipeptides containing an L-aspartic acid residue. All of the L-aspartic acid-containing dipeptides tested showed slightly weaker inhibitory effects on the degranulation of RBL-2H3 cells than L-glutamic acid-containing dipeptides (Table 1). Therefore, a shorter side chain of L-aspartic acid residue might be less favorable to the degranulation-inhibitory effect. A strategy to predict and discover novel bioactive peptides using artificial intelligence is becoming more prevalent [16]. Utilizing such a strategy may help explain the correlation between dipeptide structure and degranulation-inhibitory activity. No cytotoxicity was observed in any of the tested dipeptides at 1 mM (Table 1). Thus, the suppressed extracellular release of β-hexosaminidases by L-glutamic or L-aspartic acid-containing dipeptides is based on degranulation inhibition rather than on cytotoxicity.
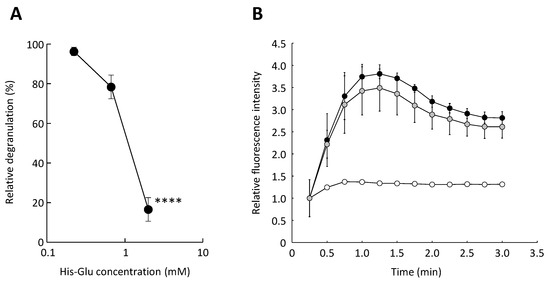
Figure 1.
Effect of an acidic amino acid residue-containing dipeptide on antigen-induced degranulation. Anti-DNP IgE-sensitized RBL-2H3 cells were treated with His-Glu and subsequently stimulated with antigen to induce degranulation. (A) Relative degranulation was calculated based on the enzymatic activity of β-hexosaminidase, used as a marker of degranulation. (B) The elevation in cytosolic calcium ion concentration in RBL-2H3 cells was monitored using Fluo-3. The relative fluorescence intensity of Fluo-3 reflects the cytosolic calcium ion concentration. Closed circle, RBL-2H3 cells untreated with His-Glu and stimulated with antigen; gray circle, the cells treated with 2.0 mM His-Glu and stimulated with antigen; open circle, the cells not treated with His-Glu and not stimulated with antigen. Data are shown as mean ± SEM (n = 3). **** p < 0.0001 against the control by Dunnett’s test.
3.2. Effect of an L-Glutamic Acid-Containing Dipeptide His-Glu on the Antigen-Induced Elevation of Cytosolic Calcium Ion Concentration in RBL-2H3 Cells
Next, we attempted to elucidate the mechanism by which acidic amino acid residue-containing dipeptides suppress degranulation. Antigen-induced degranulation firstly causes an elevation in cytosolic calcium ion concentration accompanied by a calcium ion influx from outside of the cell [17]. We thus investigated the effect of acidic amino acid residue-containing dipeptides on the elevation of cytosolic calcium ion concentration during degranulation. Two millimolar His-Glu was used in the following experiments as a representative of acidic amino acid residue-containing dipeptides, at which concentration there is no cytotoxicity. As can be seen in Figure 1B, the relative fluorescence intensity representing cytosolic calcium ion concentration rapidly increased upon stimulation with an antigen. Additionally, in cells exposed to His-Glu, cytosolic calcium ion concentration quickly increased, as well as in the control cells. The calcium ion influx tended to decrease when treating the cells with His-Glu; however, no statistically significant difference was observed between the His-Glu-treated and control cells. Since previous studies have shown that some dipeptides possess a chelating property [18,19], the inhibitory effect of acidic amino acid residue-containing dipeptides on the degranulation of RBL-2H3 cells was suspected due to the chelating effect, which blocks the influx of calcium ion into cells. However, it was suggested that the degranulation-inhibitory effect of acidic amino acid residue-containing dipeptides is not mainly caused by the chelating effect, because the increase in cytosolic calcium ion concentration accompanied by an influx from outside the cell tended to be downregulated, but not statistically significant, in cells treated with His-Glu. Thus, we consider that the degranulation-inhibitory effect of His-Glu was not due to inhibition of the increase in cytosolic calcium ion concentration, but to another mechanism, although a weak chelating effect of His-Glu may have occurred.
3.3. Inhibitory Effect of His-Glu on Calcium Ionophore-Mediated Degranulation of RBL-2H3 Cells
Since His-Glu did not significantly suppress the elevation in cytosolic calcium ion concentration in RBL-2H3 cells, we investigated whether acidic amino acid residue-containing dipeptides inhibit degranulation when calcium ions are artificially transported into the cell. A23187 is a divalent cation ionophore and induces mast cell degranulation [20]. We confirmed that A23187-induced degranulation was significantly inhibited when RBL-2H3 cells were treated with His-Glu (Figure 2A). We also confirmed that His-Glu tended to downregulate the elevation in cytosolic calcium ion concentration in RBL-2H3 cells (Figure 2B), but not to a statistically significant degree. This fact suggests that L-glutamic acid-containing dipeptides possess a weak chelating effect and inhibit degranulation mainly by affecting intracellular processes after the increase in the cytosolic calcium ion concentration, such as the formation of the SNARE complex. There is a calcium ion-independent pathway inducing mast cell degranulation, which mainly consists of Fyn, Gab2, phosphoinositide 3-kinase (PI3K), and Akt [21,22]. The pathway is activated upon antigen binding to IgE and does not require an increase in calcium ion concentration in the cytosol. Acidic amino acid residue-containing dipeptides might affect this pathway to inhibit the degranulation of RBL-2H3 despite rising the calcium ion concentration in the cytosol.
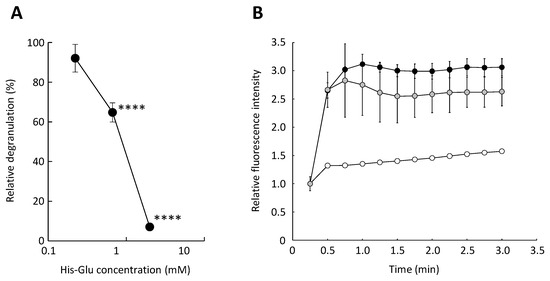
Figure 2.
Effect of an acidic amino acid residue-containing dipeptide on A23187-induced degranulation. RBL-2H3 cells were treated with His-Glu and subsequently stimulated with A23187 to induce degranulation. (A) Relative degranulation was calculated based on the enzymatic activity of β-hexosaminidase used as a marker of degranulation. (B) The elevation in cytosolic calcium ion concentration in RBL-2H3 cells was monitored using Fluo-3. The relative fluorescence intensity of Fluo-3 reflects the cytosolic calcium ion concentration. Closed circle, RBL-2H3 cells untreated with His-Glu and stimulated with A23187 (control); gray circle, the cells treated with 2.0 mM His-Glu and stimulated with A23187; open circle, the cells not treated with His-Glu and not stimulated with A23187. Data are shown as mean ± SEM (n = 3). **** p < 0.0001 against the control by Dunnett’s test.
3.4. Effect of His-Glu on the Antigen-Induced Morphological Change of RBL-2H3 Cells
The morphological change of RBL-2H3 cells was next observed after antigen stimulation. When basophils and mast cells are stimulated with an antigen, microtubules are reorganized and transport intracellular granules to the plasma membrane for degranulation [23]. An anti-tubulin antibody labeled with an Alexa Fluor 488 fluorescent dye was used for immunofluorescence staining of RBL-2H3 cells. As shown in Figure 3, we confirmed that microtubules in the cell are reorganized after antigen stimulation. Before degranulation, RBL-2H3 cells show a round shape. After antigen stimulation, RBL-2H3 cells show an elongated shape. When RBL-2H3 cells treated with His-Glu were stimulated with an antigen, microtubule reorganization was inhibited, and the shape of cells looked round. This result suggests that L-glutamic acid-containing dipeptides inhibit degranulation by blocking microtubule reorganization. Microtubule remodeling occurs with increased calcium ion concentration in the cytosol. However, it has also been reported that microtubule reorganization occurs independently of an increase in cytosolic calcium ion concentration [21]. Thus, inhibiting the calcium ion-independent signaling pathway by L-glutamic acid-containing dipeptides might be a possible mechanism to suppress the degranulation of RBL-2H3 cells.
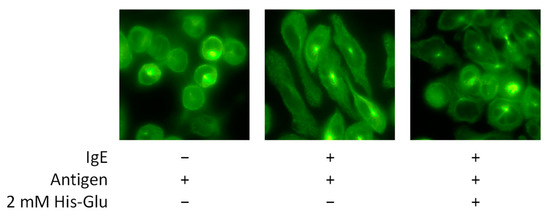
Figure 3.
Effect of an acidic amino acid residue-containing dipeptide on microtubule reorganization in RBL-2H3 cells. Degranulation was induced with antigen after IgE-sensitized RBL-2H3 cells were treated with 2.0 mM His-Glu. After staining with an anti-α-tubulin antibody labeled with Alexa Fluor 488, the cells were observed under a fluorescence microscope with ×60 magnification of an objective lens.
3.5. Effect of His-Glu on Intracellular Signaling Pathways in RBL-2H3 Cells
Since it was suggested that His-Glu inhibits degranulation by affecting the calcium ion-independent pathway, we performed an immunoblot analysis. Figure 4 shows that Syk and phospholipase Cγ (PLCγ) were phosphorylated after antigen stimulation of RBL-2H3 cells. We confirmed that His-Glu does not affect the phosphorylation of PLCγ1 or PLCγ2. The phosphorylation of Syk tended to decrease by treating the cells with His-Glu, but not significantly. These signaling proteins play an important role in signaling that increases calcium ion concentrations in the cytosol [24]. Thus, this result was as expected because His-Glu did not significantly inhibit the increase in cytosolic calcium ion concentrations (Figure 1B). Figure 4 also shows that PI3K and Akt were phosphorylated after antigen stimulation in RBL-2H3 cells. We found that His-Glu significantly downregulates the phosphorylation of PI3K and Akt. PI3K and Akt play a crucial role in the reorganization of microtubules in basophils and mast cells to carry intracellular granules for degranulation [25]. These results indicated that L-glutamic acid-containing dipeptides downregulate the phosphorylation of PI3K and Akt, leading to suppressed degranulation by inhibiting the reorganization of microtubules.
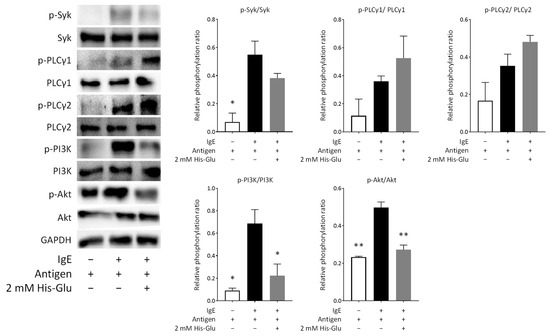
Figure 4.
Effect of an acidic amino acid residue-containing dipeptide on signaling pathways leading to degranulation. IgE-sensitized RBL-2H3 cells were treated with 2.0 mM His-Glu and subsequently stimulated with antigen for 5 min to induce degranulation. Cell lysates were then prepared and used for immunoblotting. The phosphorylation levels of Syk, PLCγ1, PLCγ2, PI3K, and Akt were evaluated. The p-Syk, p-PLCγ1, p-PLCγ2, p-PI3K, and p-Akt represent phosphorylated Syk, phosphorylated PLCγ1, phosphorylated PLCγ2, phosphorylated PI3K, and phosphorylated Akt, respectively. The relative phosphorylation ratio indicates the ratio of the amount of phosphorylated protein to the amount of whole protein. A representative blot from two independent experiments is shown. Data are expressed as the mean ± SEM. * p < 0.05 and ** p < 0.01 against control (antigen positive/His-Glu negative) by Dunnett’s test.
4. Conclusions
We found that glutamic acid, aspartic acid, and poly L-glutamic acid do not inhibit antigen-induced degranulation of RBL-2H3 cells, but acidic amino acid-containing dipeptides do demonstrate inhibitive activity without cytotoxicity. L-glutamic acid-containing dipeptides suppressed the degranulation of RBL-2H3 cells induced with a calcium ionophore A23187. We also found that His-Glu tends to suppress, but not significantly, the elevation in cytosolic calcium ion concentration upon antigen and A23187 stimulation, and inhibits microtubule reorganization in RBL-2H3 cells after antigen stimulation. The results of immunoblot analysis showed that His-Glu does not significantly affect the phosphorylation of Syk or PLCγ, but impacts that of PI3K and Akt. Overall results suggest that there are three mechanisms underlying the inhibitory effect of acidic amino acid residue-containing dipeptides on the degranulation of RBL-2H3 cells: a chelating effect, inhibition of intracellular processes after calcium flux, and blocking microtubule reorganization. Figure 1B and Figure 2B indicate that the chelating effect of acidic amino acid residue-containing dipeptides seems weak. Thus, we consider that acidic amino acid residue-containing dipeptides mainly inhibit intracellular processes after the increase in the cytosolic calcium ion concentration, such as the formation of the SNARE complex, and/or block the microtubule reorganization through downregulated phosphorylation of PI3K and Akt as shown in Figure 5. The exact mechanism of the degranulation-inhibitory activity of acidic amino acid residue-containing dipeptides has not yet been elucidated, and further investigation is needed. Based on our data, L-glutamic acid-containing dipeptides can be used as food ingredients to attenuate allergic symptoms. Further studies are required to examine whether allergic symptoms can be ameliorated with L-glutamic acid-containing dipeptides in vivo.
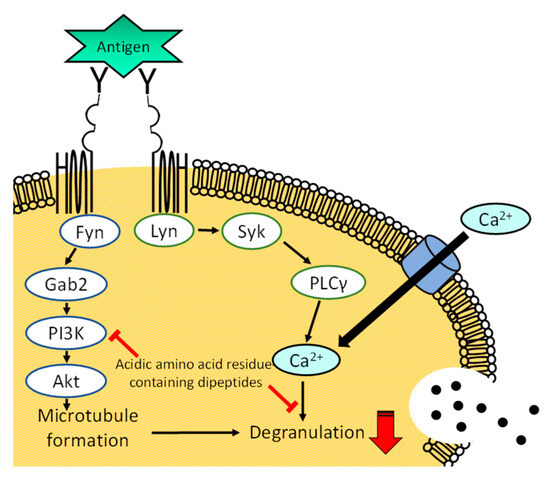
Figure 5.
A scheme describing the proposed mechanism of degranulation inhibition by acidic amino acid residue-containing peptides. The Lyn/Syk/PLCγ pathway is calcium ion-dependent, while the Fyn/Gab2/PI3K pathway is calcium ion-independent.
Author Contributions
Conceptualization, T.S.; formal analysis, K.N., M.I. (Momoko Ishida) and T.S.; investigation, T.H., M.I. (Mitsumasa Izumi), N.K., S.Y., A.O. and Y.T.; writing—original draft preparation, K.N.; writing—review and editing, K.N. and T.S.; supervision, K.N. and T.S.; funding acquisition, T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Grants-in-Aid for Scientific Research (KAKENHI Grant Number JP22K05512) from the Japan Society for the Promotion of Science.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the reported results are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stone, K.D.; Prussin, C.; Metcalfe, D.D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010, 125, S73–S80. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, R.; Peng, C.; Chen, X.; Li, J. Pharmacogenomics for the efficacy and side effects of antihistamines. Exp. Dermatol. 2022, 31, 993–1004. [Google Scholar] [CrossRef]
- Zhang, P. The Role of Diet and Nutrition in Allergic Diseases. Nutrients 2023, 15, 3683. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Wu, J. Impact of food-derived bioactive peptides on gut function and health. Food Res. Int. 2021, 147, 110485. [Google Scholar] [CrossRef] [PubMed]
- Sreelekshmi, P.J.; Devika, V.; Aiswarya, L.S.; Jeevan, S.R.; Ramanunni, K.; Nair, P.B.; Sadanandan, S. Recent Advances in Bioactive Peptides as Functional Food for Health Promotions and Medicinal Applications. Protein Pept. Lett. 2023, 30, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J. Soy bioactive peptides and the gut microbiota modulation. Appl. Microbiol. Biotechnol. 2020, 104, 9009–9017. [Google Scholar] [CrossRef] [PubMed]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive Peptides Derived from Seaweed Protein and Their Health Benefits: Antihypertensive, Antioxidant, and Antidiabetic Properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Mandal, S.; Tomar, S.K.; Anand, S. Food protein-derived bioactive peptides in management of type 2 diabetes. Eur. J. Nutr. 2015, 54, 863–880. [Google Scholar] [CrossRef] [PubMed]
- Antony, P.; Vijayan, R. Bioactive Peptides as Potential Nutraceuticals for Diabetes Therapy: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 9059. [Google Scholar] [CrossRef]
- Mojica, L.; Luna-Vital, D.A.; González de Mejía, E. Characterization of peptides from common bean protein isolates and their potential to inhibit markers of type-2 diabetes, hypertension and oxidative stress. J. Sci. Food Agric. 2017, 97, 2401–2410. [Google Scholar] [CrossRef]
- Görgüç, A.; Gençdağ, E.; Yılmaz, F.M. Bioactive peptides derived from plant origin by-products: Biological activities and techno-functional utilizations in food developments—A review. Food Res. Int. 2020, 136, 109504. [Google Scholar] [CrossRef]
- Awane, S.; Nishi, K.; Nakamoto, M.; Osajima, K.; Suemitsu, T.; Sugahara, T. Inhibitory effects of enzyme-treated dried sardine extract on IgE-mediated degranulation of RBL-2H3 cells and a murine model of Japanese cedar pollinosis. RSC Adv. 2016, 6, 85718–85726. [Google Scholar] [CrossRef]
- Nishi, K.; Kanayama, Y.; Kim, I.H.; Nakata, A.; Nishiwaki, H.; Sugahara, T. Docosahexaenoyl ethanolamide mitigates IgE-mediated allergic reactions by inhibiting mast cell degranulation and regulating allergy-related immune cells. Sci. Rep. 2019, 9, 16213. [Google Scholar] [CrossRef] [PubMed]
- Kanda, K.; Nishi, K.; Kadota, A.; Nishimoto, S.; Liu, M.C.; Sugahara, T. Nobiletin suppresses adipocyte differentiation of 3T3-L1 cells by an insulin and IBMX mixture induction. Biochim. Biophys. Acta 2012, 1820, 461–468. [Google Scholar] [CrossRef]
- Yuan, H.; Luo, Z.; Ban, Z.; Reiter, R.J.; Ma, Q.; Liang, Z.; Yang, M.; Li, X.; Li, L. Bioactive peptides of plant origin: Distribution, functionality, and evidence of benefits in food and health. Food Funct. 2022, 13, 3133–3158. [Google Scholar] [CrossRef] [PubMed]
- Kussmann, M. Prediction, Discovery, and Characterization of Plant- and Food-Derived Health-Beneficial Bioactive Peptides. Nutrients 2022, 14, 4810. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.T.; Beaven, M.A. Regulators of Ca2+ signaling in mast cells: Potential targets for treatment of mast cell-related diseases? Adv. Exp. Med. Biol. 2011, 716, 62–90. [Google Scholar]
- Zhao, L.; Huang, S.; Cai, X.; Hong, J.; Wang, S. A specific peptide with calcium chelating capacity isolated from whey protein hydrolysate. J. Funct. Foods 2010, 125, S73–S80. [Google Scholar] [CrossRef]
- Tang, N.; Skibsted, L.H. Calcium Binding to Amino Acids and Small Glycine Peptides in Aqueous Solution: Toward Peptide Design for Better Calcium Bioavailability. J. Agric. Food Chem. 2016, 64, 4376–4389. [Google Scholar] [CrossRef]
- Johansen, T. Mechanism of histamine release from rat mast cells induced by the ionophore A23187: Effects of calcium and temperature. Br. J. Pharmacol. 1978, 63, 643–649. [Google Scholar] [CrossRef]
- Nishida, K.; Yamasaki, S.; Ito, Y.; Kabu, K.; Hattori, K.; Tezuka, T.; Nishizumi, H.; Kitamura, D.; Goitsuka, R.; Geha, R.S.; et al. FcεRI-mediated mast cell degranulation requires calcium-independent microtubule-dependent translocation of granules to the plasma membrane. J. Cell Biol. 2005, 170, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Kalesnikoff, J.; Galli, S. New developments in mast cell biology. Nat. Immunol. 2008, 9, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Lazki-Hagenbach, P.; Klein, O.; Sagi-Eisenberg, R. The actin cytoskeleton and mast cell function. Curr. Opin. Immunol. 2021, 72, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Siraganian, R.P.; de Castro, R.O.; Barbu, E.A.; Zhang, J. Mast cell signaling: The role of protein tyrosine kinase Syk, its activation and screening methods for new pathway participants. FEBS Lett. 2010, 584, 4933–4940. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Kempuraj, D.; Tagen, M.; Conti, P.; Kalogeromitros, D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol. Rev. 2007, 217, 65–78. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).