Abstract
The vascular system maintains cellular homeostasis by transporting oxygen, nutrients, and metabolic waste products. The vascular system is involved in a variety of fundamental physiological phenomena and is closely associated with human vascular diseases. Additionally, the stability of drugs in the vasculature affects their efficacy. Therefore, researchers have used vascular models to study vascular diseases, assess drug stability, and screen drugs. However, there are many shortcomings in the animal models and in vitro two-dimensional vascular models that have been extensively developed. In this paper, we specifically review the construction methods of in vitro vascular models and classify the specific methods into photolithography, soft lithography, self-assembly, template, 3D bioprinting, and laser degradation/cavitation. The first two are microfluidics-based methods and the last three are non-microfluidics-based methods. The vascular model construction methods reviewed in this paper overcome the shortcomings of traditional models—which cannot accurately reproduce the human vascular microenvironment—and can assist in the construction of in vitro 3D vascular models and tissue engineering vascularization. These models can be reused by perfusion devices, and the cells within the channels reside on biocompatible materials that are used to simulate the microenvironment and 3D cellular organization of the vasculature in vivo. In addition, these models are reproducible in shape and length, allowing experiments to be repeated, which is difficult to do with natural vessels. In vitro vascular models are widely used in research and drug screening for diseases associated with endothelial dysfunction, cancer, and other vascular abnormalities.
1. Introduction
The vascular system plays a vital role in the human body as it exists in various tissues and organs, serving as the foundation for human life activities [1]. Not only does it deliver nutrients and oxygen to the body’s tissues but it also conducts immune surveillance and metabolic waste removal. Capillaries are located within 100 to 150 μm of every cell in the body, and cells that exceed the oxygen diffusion limit will die due to insufficient oxygen and nutrients [2]. Vascular disease can have a significant impact on the human body, leading to various conditions such as cancer, tumor cell metastasis, atherosclerosis, hypertension, sickle cell anemia, diabetes, sepsis, and ischemic stroke [3]. Among these, atherosclerosis is the primary cause of cardiovascular disease, which has the highest mortality rate compared to other diseases [4,5]. Moreover, drug stability in blood vessels can affect its efficacy. As a result, researchers have developed numerous physiologically relevant animal and in vitro models for preclinical studies including pharmacodynamic, pharmacokinetic, pharmaceutical, and toxicological safety evaluations of blood vessels and related diseases (Table 1).

Table 1.
Comparison of traditional in vitro models [6,7,8,9,10,11,12,13].
Drug development spending has increased over the past 20 years but the number of drugs approved annually has decreased due to the limitations of current animal and in vitro models in predicting efficacy. While animal models have contributed significantly to drug development and safety, they fall short in simulating the human body’s physiological or pathological environment [6]. Animal models tend to overestimate the effectiveness of drug treatments, resulting in only 10–15% of drugs approved for human use, even when effective in animal models [7]. This is likely due to differences in species’ physiological environments, bias in animal model research, and differences in research methods between animal models and human clinical trials [8]. Additionally, some human tissues or systems are too complex or costly to model accurately using animal models, such as atherosclerosis and the human blood–brain barrier tissue [9]. The use of animal models is also time-consuming, laborious, and raises ethical concerns [10].
In vitro vascular models can be broadly classified into two-dimensional (2D) and three-dimensional (3D) models. Two-dimensional models are typically constructed using culture dishes, which offer the advantages of low cost, ease of operation, and compatibility with high-resolution microscopes [11]. However, despite being widely used as high-throughput tools for drug discovery and screening, the success rate of drugs from phase I clinical trials to market approval is only 9.6% using 2D models. Moreover, 2D models are typically static and do not accurately reproduce the microenvironment of fluid flow. Therefore, conventional 2D models are often inadequate for studying the mechanisms of human vascular disease and developing related drugs [12,13].
Tissue engineering is an interdisciplinary technique that combines cell biology and materials science to develop new structures with specific morphological shapes and functions to replace damaged tissues and organs. However, the production of vascular structures remains a major challenge [14]. Vascularizing tissues and organs in vitro is crucial for tissue-engineered constructs to mimic the in vivo environment, since cells within these constructs may die after crossing the oxygen diffusion limit distance due to insufficient oxygen and nutrient uptake. Therefore, researchers are increasingly interested in constructing 3D vascular models in vitro, given the rapid development of tissue engineering, biomaterials science, cell biology, 3D bioprinting technology, and microfabrication technology. An in vitro 3D vascular model can reproduce the microenvironment of the vascular system in vitro by manipulating gas, nutrient diffusion, and shear stress [11]. Due to its unique advantages, 3D vascular models have the potential to replace animal models and 2D vascular models as research platforms for studying vascular diseases and related drugs.
The human body has a network of blood vessels that control the flow of blood into and out of tissues. The vascular system can be divided into veins, arteries, and capillaries based on their internal diameter. Veins and arteries have diameters of 1–20 mm, while capillaries can be as small as 10 µm [15]. The walls of arteries and veins consist of three layers—the tunica intima, tunica media, and tunica adventitia—from the inside to the outside. The tunica intima comprises a layer of endothelial cells that are in direct contact with the blood flow in the lumen. Endothelial cells are arranged along the direction of blood flow and act as a selective permeability barrier between the vessel wall and circulating blood [11,16]. They maintain vascular function, integrity, and the internal environment balance. Endothelial dysfunction is a sign of the onset of atherosclerosis [8]. Between the endothelial cells and media lies the basement membrane protein, mainly composed of laminin and type IV collagen, while elastin forms the outer layer. The media is mainly composed of smooth muscle cells that regulate blood flow and pressure by causing vasoconstriction and vasodilation. Therefore, most mechanical properties of arteries are regulated by this layer [17]. Finally, fibroblasts and loose connective tissue form the outermost layer of blood vessels, the tunica adventitia, which repairs vascular damage and anchors blood vessels [18].
In contrast to arterial and venous walls, capillary walls are thinner and consist of a single layer of endothelial cells supported on the outside by pericytes. This thin monolayer facilitates efficient transport of oxygen and nutrients, and the timely removal of metabolic waste [19]. These characteristics make capillaries crucial for drug extravasation and regulating substance exchange. The endothelial cells of arteries and veins are continuous monolayers, while capillaries can be classified into three types based on the different tissue functions of their endothelial cells: continuous, fenestrated, and sinusoidal capillaries [20].
To construct vascular models in vitro, it is crucial to understand the main processes of vessel formation in vivo: vasculogenesis and angiogenesis. Vasculogenesis is the initial formation of blood vessels, which primarily occurs during embryogenesis [21]. Endothelial progenitor cells and hematopoietic stem cells first form blood islands, and then the edge of the island differentiates into multiple small endothelial cell clusters that invade the surrounding tissue. These expanded cell clusters eventually connect to form a basic vascular network [19,22]. Stable vascular endothelial cells are tightly interconnected by transmembrane adhesive proteins such as VE-cadherin, N-cadherin, claudins, and tight junction proteins, which enhance the permeability barrier function of endothelial cells to better control substance exchange and cell migration [23].
In contrast to vasculogenesis, angiogenesis refers to the formation of new blood vessels from existing vascular networks, which typically occurs during key points in morphogenesis, injury, and pathogenesis [24]. Therefore, angiogenesis is more common than vasculogenesis in adults. Sprouting angiogenesis and intussusceptive angiogenesis (non-sprouting angiogenesis) are the two primary ways to form new blood vessels during angiogenesis, and both occur in physiological or pathological tissues and organs [25,26].
The human body has an oxygen-sensing mechanism that allows for timely physiological adaptations to fluctuations in oxygen concentration. Therefore, sprouting angiogenesis is typically caused by hypoxia-inducible factors (HIFs) or inflammation. HIFs are heterodimeric transcription factors consisting of α and β subunits. HIF-1β is not regulated by oxygen concentration, while HIF-1α is produced in large quantities under hypoxic conditions and forms heterodimers with HIF-1β. The gradual accumulation of HIF activates the expression of many angiogenic genes and initiates the angiogenic process [27]. Angiogenic factors mainly include vascular endothelial growth factor, fibroblast growth factor, angiopoietin, and chemokines [28]. Among these, vascular endothelial growth factor is the most important factor in angiogenesis, occurring in the early stage of the angiogenic cascade response, and is associated with the initial activation of endothelial cells [29]. Additionally, potential genetic factors and hemodynamic stimuli can also promote angiogenesis to some extent [30].
In addition, intussusceptive angiogenesis refers to the process by which existing blood vessels invaginate through the vessel wall to form tissue columns in the lumen, eventually splitting the original vessel into two parallel vessels. This process is therefore also known as split angiogenesis [31,32]. The specific process is as follows: first, the endothelial cells on both sides of the blood vessels touch each other to form a contact zone, and then, the endothelial cell membranes gradually become thinner and fuse to form tiny holes. Supporting cells such as mesenchymal stem cells, fibroblasts, and pericytes then invade and form columnar or rod-like structures to fill the gap between the two new blood vessels. Eventually, new vascular channels are formed in the original blood vessels as the hole structures gradually enlarge and merge [33,34]. Although shear stress and blood flow velocity have been shown to play a critical role in this process, we currently know little about the cellular and molecular mechanisms of intussusception angiogenesis and need to establish experimental models for research [26,35]. Compared to sprouting angiogenesis, intussusceptive angiogenesis occurs at a faster rate. During intussusceptive angiogenesis, endothelial cells appear to become larger and thinner, and the process does not involve endothelial cell proliferation, requiring less energy. As a result, most tumors form vessels rapidly through this process [31,36].
Traditional animal models and two-dimensional in vitro models are insufficient in replicating the human pathological and physiological environment accurately. Additionally, the introduction of vascular networks allows for continuous supply of nutrients and oxygen to cells in tissue engineering constructs. As a result, a wide range of in vitro three-dimensional vascular models have been developed. An ideal in vitro vascular model can simulate a three-dimensional tissue structure with appropriate cell composition and disease characteristics. In this paper, we classify in vitro vascular model construction methods into microfluidic-based and non-microfluidic-based methods based on the fabrication method. We consider five in vitro vascular model construction methods: photolithography, soft lithography, self-assembly, template, 3D bioprinting, and laser degradation/cavitation. These models vary in complexity and physiological correlation but provide a variety of tools for studying vascular disease and tissue-engineering vascularization methods. We discuss and summarize the latest in vitro vascular model fabrication methods, highlighting their advantages and limitations. Finally, we present the applications of different methods in related diseases and provide opinions and insights on future research and fabrication methods.
2. Construction of In Vitro Vascular Models
In recent years, researchers have utilized various fabrication techniques to construct in vitro vascular models. However, traditional two-dimensional in vitro models and animal models have several drawbacks. With the advancement of microfabrication technology, 3D bioprinting technology, and laser technology, 3D in vitro vascular models can reproduce the microenvironment of complex geometric vascular systems in vitro by manipulating gas, nutrient diffusion, and shear stress. The advantages and disadvantages of different construction methods for in vitro 3D vascular models are described in detail in Table 2.

Table 2.
Overview of strategies for constructing in vitro 3D vascular models [37,38,39,40,41,42,43,44,45,46,47,48,49,50].
2.1. Construction of In Vitro Vascular Model Based on Microfluidic Technology
Microfluidics is a technique used for manipulating liquids and gases in channels with cross-sectional dimensions of 10–100 μm, and microfluidic chips are the primary platform for utilizing microfluidics. In recent decades, the advancement of microfluidics technology has accelerated the development of extracorporeal vascular models due to their light weight, small size, portability, precise fluid control, and maneuverability [51]. Additionally, this technology can accurately control the physiological structure of blood vessels, which can be designed as straight lines, bifurcations, narrow structures, and other physiological structures that are difficult to achieve with traditional methods [52]. The small size of the microfluidic chips also reduces wastage of reagents and biological samples. These advantages over traditional in vitro models make microfluidic chips an ideal platform for constructing in vitro vascular models. This platform can be used not only to elucidate the mechanism of vascular formation and related factors but also as a platform for vascular disease and drug screening. We divide the construction methods of in vitro vascular models based on microfluidic technology into photolithography, soft lithography, and self-assembly. The principles, steps, advantages, and disadvantages of each method are presented below.
2.1.1. Photolithography and Soft Lithography
Photolithography and soft lithography are two common methods for constructing vascular models on microfluidic chips using microfluidic techniques [53]. Photolithography starts with a uniform coating of photoresist on a silicon wafer, then, a patterned photolithographic mask is placed on top of the photoresist. The photoresist is then exposed or unexposed to UV light and removed with developer after UV cross-linking to produce a master with a clear microstructure on the substrate. The photolithography process requires a high degree of cleanliness of the environment to prevent contamination of the silicon wafer with particles [53].
Microfluidic chips are generally fabricated using lithography and replication molding technology following these steps (Figure 1): (i) fabrication of a silicon wafer mold by photolithography; (ii) mixing polydimethylsiloxane (PDMS) prepolymer and the curing agent in the silicon wafer mold for subsequent curing; (iii) removal of PDMS from the mold and irreversibly bonding it to a rigid or flexible substrate (e.g., glass, silicon, or polymer) to form a closed microfluidic chip [54,55]. This sealing method is known as bonding technology and relies on the formation of irreversible chemical bonds between the activated PDMS and the substrate surface. Silicon-based materials (e.g., glass or silicon) are more widely used in the field of microchannel sealing because they can be bonded to PDMS by a simple surface activation treatment [56].
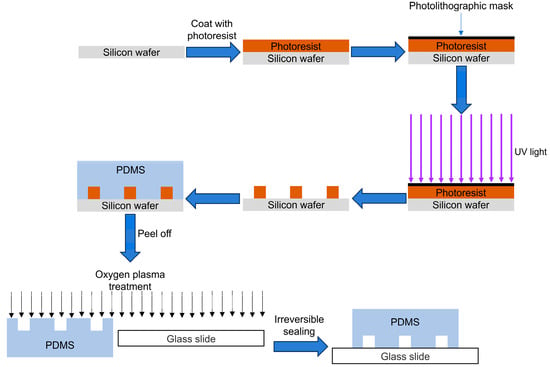
Figure 1.
Schematic diagram of microfluidic chip preparation.
Although conventional photolithography is well-established and has been extensively used for the fabrication of microfluidic chips, it is limited in the construction of in vitro vascular models due to its high cost, time, and labor-intensive nature, limited control over surface chemical properties, and limitations of photosensitive materials such as photoresists [37,38].
With the advancement of microfabrication technology, we can now utilize soft lithography to construct intricate vascular models in vitro. Soft lithography, a non-photolithographic method first proposed by Whitesides at Harvard University in the late 1990s, employs elastic materials such as PDMS as molds to replace the hard molds used in traditional photolithography technology. This technology is ideal for microstructure replication molding and offers a low-cost, convenient, effective, and time-efficient method for forming and fabricating micro- and nano-vascular structure models, overcoming the limitations of photolithography [39].
Early photolithography was the conventional method for fabricating microfluidic chips, using materials such as silicon wafers and glass. However, the process was time-consuming and expensive, making mass production of microfluidic chips even more uneconomical [57]. Nowadays, microfluidic chip fabrication primarily involves photolithography of organic polymers like photoresists that are cheaper and easier to fabricate into microfluidic chips than silicon wafers and glass; moreover, these polymers possess better mechanical properties than silicon wafers and glass [58]. PDMS is the most commonly used material for soft lithography, followed by thermoplastics like polymethyl methacrylate, polyvinyl chloride, polycarbonate, thermoplastic elastomer, and polyurethane [57,59,60]. Although thermoplastics can be reused and are transparent, they have poor permeability, high cost, high stiffness, and are not easy to bend. Hence, most researchers prefer PDMS as a photolithography and soft lithography material.
PDMS is an elastomer that possesses excellent optical, mechanical, and electrical properties, which make it the primary material for fabricating micro- and nanovascular structures using photolithography and soft lithography techniques. It is particularly suitable for simulating blood vessels [38]. PDMS is a macromolecular polymer consisting of repeated silicon–oxygen bonds, offering several advantages: PDMS material has lower cost, is easy to manufacture microfluidic chips, and exhibits good biocompatibility and mechanical performance. The transmittance of PDMS in the range of 390–780 nm wavelength can reach 90%, making it suitable for various optical detections such as UV/visible absorption and fluorescence [61]. Its excellent resistance to bio-degradation, non-toxicity, and good permeability to oxygen and carbon dioxide make PDMS channels suitable for cell studies. The material can also be used as insulation for pacemakers and as a coating for biomedical implant materials, with only minimal foreign body reactions [62,63]. The Young’s modulus of PDMS is approximately 1–3 MPa, which is four orders of magnitude lower than glass (approximately 50 GPa), making it highly flexible. Therefore, it can recover its original shape after external forces are removed. It is suitable for curved surfaces and can be demolded from molds with intricate structures without damaging itself [64]. It has the ability to replicate microscale features, making it ideal for accurately constructing channel structures on microfluidic chips. Another key advantage of PDMS is that it can irreversibly bond with glass or itself without the need for adhesive [65]. Therefore, PDMS material has become the main material for constructing vascular models due to these advantages.
Although PDMS has numerous advantages, it may also have some limitations in certain applications. For instance, it tends to swell in many common solvents such as toluene [57]. Additionally, shrinkage during the curing of the PDMS prepolymer can affect the fabrication and processing of microfluidic chips. Among these limitations, hydrophobicity is the most significant drawback of PDMS. The intrinsic hydrophobicity of PDMS restricts its application in specific fields, as most experiments in microchannels require the use of water-soluble fluids (e.g., blood, buffers) or cells [66]. For example, the unmodified highly hydrophobic PDMS surface is not conducive to long-term cell culture, which can lead to cell detachment from the wall, agglomeration, and death [38]. Additionally, the high hydrophobicity of PDMS makes it easy to adsorb other hydrophobic biological small molecules and biological solvents, which can result in reagent loss, local concentration changes, bubble formation, and even inaccurate quantitative analysis [67]. To overcome this drawback, several surface modification strategies to improve the hydrophilicity of PDMS surfaces are described below, which can be classified into surface activation modification, physical adsorption, and chemical modification methods according to different principles (Table 3) [68]. Increasing the hydrophilicity of the PDMS surface is conducive to the attachment of biomaterials and cells, which can make microfluidic technology more widely used in the construction of vascular models.

Table 3.
Surface modification strategies for PDMS [69,70,71,72,73,74,75,76,77,78,79,80,81,82].
2.1.2. Self-Assembly
Self-assembly is a technique where endothelial cells naturally form blood vessels in a hydrogel after fulfilling the conditions of vasculogenesis or angiogenesis in vitro, which are also the two primary ways blood vessels form in vivo. The main advantage of self-assembly is that the process of vessel formation is similar to that of the vasculature in vivo. As a result, the vessel model created using this technique closely resembles an in vivo blood vessel in terms of function and morphology [40].
Microfluidic chips are a common platform for studying self-assembly, and they can be inoculated with different hydrogels containing endothelial cells. This method differs from other vascular construction methods because endothelial cells are mixed directly with the hydrogel and placed in a microfluidic device. The cells then spontaneously reconstruct the three-dimensional vasculature in a few days, using the mechanisms of vasculogenesis and angiogenesis, resulting in vessels with more physiological properties than those constructed by other methods [12]. Kim et al. [83] developed a microfluidic three-dimensional in vitro vascular model by co-culturing human umbilical vein endothelial cells and fibroblasts to study the effects of interstitial flow and angiogenic factors on vascular growth. Interstitial flow was found to promote the sprouting and growth of blood vessels under the synergistic effect of angiogenic factors. Pauty et al. [84] established an in vitro model of VEGF-induced sprouting angiogenesis in a microfluidic chip, which can be used to screen the anti-angiogenic properties of candidate compounds and study the mechanism of angiogenesis in vitro and the endothelial barrier’s function. The self-assembly method is preferred for studying vasculogenesis or angiogenesis and can also be used to study the effects of mechanical stress and chemical factors on vessel formation, as well as the morphological characteristics of blood vessels in abnormal locations such as near tumors.
However, this method has some limitations. Although it can adjust the morphological characteristics of vessels (e.g., average vessel diameter, average branch length, number of branches, proportion of vascularized area, etc.) by controlling key parameters—such as mechanical properties of the hydrogel, cell seeding density, and paracrine signaling factors—it cannot control microchannels well and ensure easy perfusion. Additionally, constructing a vascular network using this method requires considerable time and effort, which limits its widespread application and research.
There are two methods of constructing in vitro vascular models based on microfluidics: photolithography—including soft lithography—and self-assembly. Microfluidic chips constructed by photolithography and soft lithography have several advantages, including reduced reagent waste, precise fluid control, and high operability, which allow for the construction of vascular models with complex geometries. However, the most commonly used material in microfluidics, PDMS, has some limitations due to its hydrophobicity, which can limit its application. Therefore, several surface modification strategies have been developed to increase the hydrophilicity of the PDMS surface and promote the wider application of microfluidic technology in the construction of vascular models. In addition, the self-assembly method using microfluidics constructs in vitro vascular models that are closer to the human physiological environment. Although this method can control changes in vascular morphology by adjusting parameters, it cannot easily control the formation of the vascular network. Overall, these advantages over traditional in vitro models make microfluidic chips an ideal platform for the construction of in vitro vascular models.
2.2. Construction of In Vitro Vascular Model Based on Non-Microfluidics-Based Methods
2.2.1. Construction of Vascular Models In Vitro Using Templates
The template method involves casting materials around a template and then removing it to form a hollow tube, onto which endothelial cells are seeded to form a vascular model [19]. One of the most commonly used methods is to cast materials around small diameter needles or fiber rods, which are then removed after solidification [85]. In 2006, Chrobak et al. [86] were pioneers of the needle model method and used it to construct a perfused vascular model with an internal diameter of 120 μm in vitro. The model maintained cell morphological stability for 2–3 weeks, indicating good stability and potential for drug screening and inflammation treatment. In addition to needles, fiber rods can also be used as templates. In a separate study, Mori et al. [87] used nylon wire as a template to construct a skin model with perfusion vessels by removing the template and inoculating endothelial cells on the inner wall. The model showed endothelial function similar to in vivo and permeability similar to human skin and traditional models. Drugs for skin diseases can be developed by measuring the amount of drug that passes through the skin into the bloodstream. The researchers used oxygen plasma treatment to improve the perfusion capacity of the model, which maintained stable perfusion for 1–2 weeks and showed endothelial function and permeability similar to in vivo and traditional models, providing sufficient time for subsequent skin drug and cosmetic development. While the template method is simple and can form a circular channel after extraction of the template, this step is critical and may encounter issues such as difficult template extraction, biomaterial remaining on the template after extraction, or pipeline collapse. These issues may be related to the viscosity of the hydrogel material, the chemical properties of the template surface, temperature, and other parameters. By optimizing these parameters, the success rate of circular pipeline construction can be improved.
In addition to the one-step method for constructing circular microchannels, the template method can also be used to construct vascular models by synthesizing two semicircular microchannels and splicing them together to form a complete circular microchannel (Figure 2). In a study by Choi et al. [88], two semicircular microchannels were aligned and cross-linked to form a seamless closed circular microchannel, onto which smooth muscle cells and endothelial cells were co-cultured. Co-culturing the two types of cells promoted the rearrangement of the endothelial cells and made their arrangement direction closer to the actual vascular structure. However, optimizing the concentration of the inoculated cells is necessary to ensure that the cells of the lower layer are completely covered before proceeding with the inoculation of the upper layer. This method also places high demands on the sealing of the channel during splicing, as misalignment or incomplete cross-linking can lead to leakage or even disintegration of the tubing. In another study, He et al. [41] used a similar method to prepare a closed circular channel and inoculated endothelial cells into it to form a vascular model. The cells were evenly distributed in a single layer and maintained a normal phenotype and high viability. The researchers tested the stability of the model and found that it did not leak when the perfusion velocity was higher than the average velocity in the natural artery, indicating good stability. The template method allows for the direct formation or splicing of models through a simple approach, offering advantages of low cost and easy operation. The cross-section of the channel made by this method is similar to the cross-section of natural blood vessels in a circular structure, which is closer to the physiological structure of real blood vessels and difficult to achieve by microfluidic technology. However, this method requires high mechanical properties of the hydrogel filled around the model to avoid tube collapse and structural damage to the hydrogel. Additionally, due to limitations in fabrication methods, template methods can only be used to construct simple branched, narrow, or single straight vessel structures, and cannot be used to construct complex vascular networks.
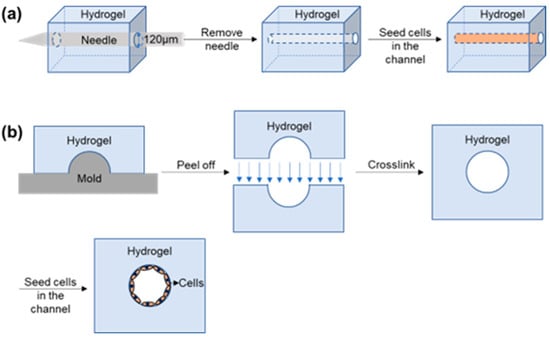
Figure 2.
Schematic diagrams of in vitro vascular models prepared by template methods. (a) The schematic diagram of an in vitro vascular model prepared using the stainless-steel needle one-step method. (b) Schematic diagram of an in vitro vascular model prepared using the semi-synthetic method.
2.2.2. Construction of Vascular Model In Vitro by 3D Bioprinting
The use of 3D bioprinting technology has been on the rise because of its potential to create vascularized tissue structures and replicate intricate vascular structures in vitro [2,89]. This technology offers the advantage of not only precisely printing cells, biomaterials, and cell-loaded biomaterials separately but also combining them to build 3D tissue structures directly [90]. These combined biomaterials are referred to as “bioinks”.
To date, in the field of bioprinting, various techniques are used for 3D bioprinting of perfusable vascular channels in vitro, including extrusion bioprinting, inkjet bioprinting, and light-assisted bioprinting [48]. Extrusion and inkjet bioprinting can be categorized into direct and indirect bioprinting methods [91]. Direct printing involves depositing cell-laden bioinks layer by layer onto hollow structures and requires rapid cross-linking to maintain stable structures [92]. On the other hand, indirect printing, also known as sacrificial bioprinting, involves embedding the printed sacrificial material within a larger cell-laden material. The sacrificial template is then removed to create a hollow channel, which allows for the inoculation of cells and is particularly effective in constructing complex structures [93]. It is important to note that each bioprinting technology has its own advantages and limitations. Furthermore, these technologies differ in resolution, manufacturing time, and design requirements [94].
Extrusion bioprinting is a cost-effective and simple technology that is suitable for most high-viscosity bioinks. It is also the most widely used bioprinting technology for constructing blood vessels [2,42]. This method uses a pneumatic system, mechanical (piston or screw), or electromagnetic drive system to continuously drip bioink from a needle, accurately depositing cell-laden biomaterials to form a stable vascular structure. As a result, very high cell densities can be achieved using this technology [43]. However, extrusion bioprinting has low resolution and can easily cause bioink blockage at the nozzle, as well as shear-stress-induced reduction in cell viability [44].
For instance, Zhang et al. [95] used a coaxial nozzle system to bioprint sodium alginate loaded with human-umbilical-vein smooth muscle cells to form a perfusable vascular channel in vitro. Although shear force had a negative effect on cell viability during printing, the viability of human umbilical vein smooth muscle cells in the channel increased with increasing culture time, even exceeding the viability of the original cells. Therefore, to improve cell viability, bioink materials should closely mimic the natural extracellular matrix. In other words, the mechanical properties and biodegradability of the bioink will affect cell expansion, which limits the use of this printing technology for biological materials [96].
Inkjet bioprinting uses thermal, piezoelectric, or electromagnetic actuation mechanisms to deposit cell suspension droplets through nozzles in a high-throughput manner [46]. This method ensures high cell viability (typically 80–90%) and has the advantages of fast printing speed, high resolution, low cost, and compatibility with various bioinks [2,45]. However, high-viscosity bioink can cause nozzle blockage, so this method requires bioink with lower viscosity and lower cell density [46,47]. Moreover, inkjet bioprinting is not commonly used for large-scale vascularized tissue construction due to the limited space for bioink in the printhead and ink cartridge [45].
On the other hand, light-assisted bioprinting uses laser pulse energy to deposit cell-laden biomaterials on the substrate, which can cross-link and solidify the biological materials without requiring nozzles [94]. This method can print bioinks with higher viscosity and also has the advantages of high cell viability, high resolution, and high printing speed. However, it needs to be operated in a sterile environment, which makes it complicated to operate. Additionally, this method requires the use of photosensitive bioink, which results in high cost [48]. Therefore, this printing method has not been widely used.
In summary, although inkjet and light-assisted bioprinting also have many advantages, the strict requirements for cell density and low cost make extrusion bioprinting the most commonly used bioprinting technology for in vitro printing of vascular structures.
In addition to technical properties, bioprinting can also use one or more materials simultaneously [97]. Various hydrogel materials such as alginate, collagen, hyaluronic acid, gelatin, and poly(ethylene glycol) (PEG) can be used as bioinks due to their biocompatibility and high viscosity [98]. However, these biomaterials cannot be used alone for bioprinting hollow microchannels because of their low mechanical strength. Biomaterials with low mechanical strength will not be able to support the weight of the hollow microchannels, leading to the collapse of the microchannels.
For instance, Khademhosseini et al. [99] designed a cell-responsive bioink using three biological materials: gelatin methacryloyl, sodium alginate, and 4-arm poly(ethylene glycol)-tetra-acrylate. The alginate component is first cross-linked with calcium ions, and then the photo-cross-linking of gelatin methacryloyl and 4-arm poly(ethylene glycol)-tetra-acrylate can further fix the morphology of the microchannel. The bioink has good mechanical strength and biological properties, which are conducive to constructing a complex 3D perfusable vascular network and promoting the proliferation and expansion of encapsulated cells. The use of 3D bioprinting technology can construct biologically relevant, perfusable, and highly organized 3D vascular models in vitro, which are challenging to achieve using traditional microfabrication techniques. Therefore, the emergence of advanced 3D bioprinting technology and bioink formulations may represent a significant milestone in the construction of large-scale vascularized tissues.
Bioprinting and pre-manufactured microchannels followed by endothelial cell seeding are two distinct methods for constructing vascular models. Bioprinting utilizes layer-by-layer deposition of biomaterials and various cell types to precisely control the shape, size, and complex structure of blood vessels [89]. In contrast, the method of pre-manufactured microchannels involves creating predefined microchannel structures and then seeding cells on their inner surfaces, typically achieved through injection or coating of cell suspensions, ensuring cell attachment to form single-layer or multi-layer cell sheets [19]. Bioprinting enables more precise spatial control and integrated construction of multiple cell types (such as endothelial cells, smooth muscle cells, and pericytes), allowing for the construction of complex vascular network structures including hierarchical branching and the precise arrangement of different cell types. Bioprinting offers significant advantages in building complex tissue models and personalized medicine but it often comes with higher costs and risks of compromising cell viability [90]. In contrast, the method of pre-manufactured microchannels followed by endothelial cell seeding is limited by the precision and complexity of microchannel manufacturing technology, typically suited for simple single-layer or basic channel structures, suitable for constructing vascular models with simpler structures and for functional assessments [100]. This method may be more cost-effective and applicable in certain basic research and specific application scenarios. In conclusion, the choice of method depends on the specific requirements of the research or application, as well as the available technologies and resources.
In the process of constructing vascular models, there are significant differences in cell types between bioprinting and pre-manufactured microchannels followed by endothelial cell seeding, particularly in simulating complex structures and functions involving multiple cell types. Bioprinting utilizes single or multiple cell types to build vascular models. For single-cell types such as endothelial cells, bioprinting allows precise control over cell distribution and density, thereby constructing vascular structures with consistency and standardized morphology. Additionally, bioprinting can simultaneously print multiple cell types within the same structure, such as endothelial cells, smooth muscle cells, pericytes, and astrocytes, thus enabling the simulation of complex multi-cellular vascular structures and functions [97]. The method of pre-manufactured microchannels followed by endothelial cell seeding typically employs one or two cell types to construct vascular models. For single-cell type vascular models, this method involves precise manufacturing of the predefined shapes and sizes of the microchannels, followed by seeding endothelial cells on their inner surfaces to form a single-layer cell. When constructing dual-cell type vascular models, this method allows sequential seeding of different types of cells on the inner surfaces of microchannels. For example, smooth muscle cells may be seeded before endothelial cells to simulate complex cellular tissue structures [88]. In summary, bioprinting offers significant advantages in simulating complex multi-cellular vascular structures but it may involve higher costs and technological complexity. In contrast, the method of pre-manufactured microchannels followed by endothelial cell seeding is more cost-effective and straightforward, suitable for certain specific application needs.
2.2.3. Construction of Vascular Model In Vitro by Laser Degradation or Laser Cavitation Molding
Laser degradation is a method that selectively degrades or ablates the cell-filled hydrogel using laser energy to form a network structure. Endothelial cells are then arranged and grown within the hydrogel to form a complex three-dimensional vascular network, as shown in Figure 3. This method has high resolution, is easy to use, and is not limited by complex three-dimensional shapes. Additionally, sterility is ensured as this method uses light to create three-dimensional networks [49,50]. Hyaluronic acid, collagen, PEG, agarose, and other materials can be used to construct three-dimensional networks by laser degradation. This method allows spatiotemporal control of fluid channel formation and fluid flow.
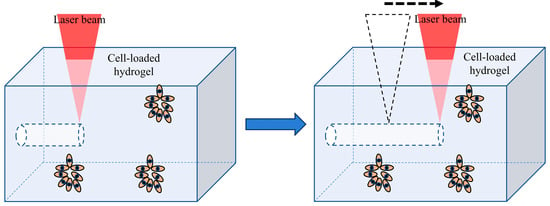
Figure 3.
Fabrication of vascular networks in hydrogels by laser degradation.
However, this thermal ablation process can cause denaturation of proteins in the hydrogel and has some impact on cell compatibility [101]. Moreover, this method requires the use of expensive multi-photon systems for high resolution. For example, Rayner et al. [102] successfully constructed an endothelialized glomerular structure in vitro using multiphoton and laser-guided angiogenesis techniques. They first used lithography to create two parallel vessels into which human umbilical vein endothelial cells were seeded. A 10 µm channel was then created in the hydrogel by laser ablation to connect the pre-formed parallel vessels, resulting in the formation of a glomerular vessel model.
Researchers have combined laser degradation technology with image-guided laser control to form highly complex, tortuous, and high-resolution three-dimensional structures in hydrogels. These networks accurately reproduce the structure, size, and density of microvessels in the body and can form endothelial networks together with endothelial cells [103]. This method can accurately simulate the vascular network in vivo by controlling parameters such as the total length of the network, the number of branching points, and the average vessel diameter. Moreover, this image-guidance method relies on the use of virtual masks so that the masks can be easily designed and modified by simple digital operations, which is different from the need to redesign and modify the master using lithography technology. In summary, this method can be used to build capillary-sized vascular networks and replicate the dense and tortuous structures of vascular networks in vivo [103].
Although the above method has many advantages, it can have some impact on the proteins in cells and hydrogels. Therefore, a more ideal pipeline construction method has recently been developed by a research team. Enrico et al. [15] proposed a new method to construct a three-dimensional pipeline structure by laser cavitation. The researchers first used a low-energy femtosecond infrared laser pulse to perform non-ablative recombination of collagen hydrogels. The expansion of the cavitation bubbles rearranged the hydrogel structure to create a circular hollow tube with a diameter of 20–60 μm, which was stable for at least 8 days under physiological conditions. The researchers then inoculated the tube with endothelial cells to create a vascular model.
Unlike the laser degradation method, this method can minimize the thermal diffusion volume and mechanical stress on the cells to improve cell viability. This method has no effect on cell viability whether the cells are outside the tube or inside the hydrogel. However, this method is only applicable to collagen hydrogels [50]. In summary, laser-induced cavitation in collagen hydrogels allows the formation of microchannels of any shape and size, which are then filled with endothelial cells to create a simulated vascular network in vivo. This method forms a 3D microvascular tissue model by irradiating collagen hydrogel with a femtosecond laser, making it possible to use a 3D tissue model to study complex tissues such as tumors and neural tissue.
There are several methods for building in vitro vessel models that are not based on microfluidic technology, including the template method, 3D bioprinting method, laser degradation method, and laser cavitation molding method. The template method is inexpensive, easy to use, and produces a blood vessel model with a cross-section similar to the physiological structure. However, it is limited to constructing a single blood vessel structure and is prone to pipeline collapse, requiring high mechanical strength biomaterials. Three-dimensional bioprinting, on the other hand, allows for the direct production of more accurate vessel models. Extrusion bioprinting, in particular, stands out as a cost-effective and user-friendly technique that is compatible with various high-viscosity bioprinting technologies. It is the most widely used bioprinting technology for constructing blood vessels. However, there is a risk of the bioprinting ink clogging and reduced cell viability. Selecting biomaterials with high biocompatibility can help improve cell viability. Lastly, the laser degradation cavitation method offers higher resolution, simple operation, and is not limited by complex three-dimensional shapes. It also maintains a sterile environment and can create a more physiological capillary network by controlling the basic parameters of blood vessels while preserving cell vitality. However, this method can be expensive and has limitations in terms of biomaterial selection.
3. Application
Table 4 summarizes the various methods for constructing blood vessels and their specific applications. Key technical details of soft lithography in building vascular models include using PDMS as the primary mold material, accurately replicating microscale structures through UV lithography, and curing PDMS prepolymer mixtures after mold preparation. These molds are irreversibly bonded with glass or PDMS itself to design and manufacture PDMS microfluidic chips containing microchannels and microcavities, simulating the complex structures and flow conditions of vascular systems [54]. Soft lithography enables the creation of minute structural features such as channels and branches to mimic the intricate geometries of blood vessels. Additionally, soft lithography allows precise control over material surface properties and structural morphology, ensuring that the constructed vascular models possess excellent biocompatibility and biomimetic characteristics [104]. By introducing biomolecules, cell cultures, or simulating blood flow within the vessels, vascular models fabricated through soft lithography can be used to study hemodynamics, drug delivery, and disease modeling, providing crucial support for medical research and treatment.

Table 4.
Research progress on constructing in vitro vascular models [105,106,107,108].
Self-assembly technology utilizes endothelial cells to naturally form blood vessels within hydrogels under specific conditions. This method mimics the process of blood vessel formation in vivo by seeding hydrogels containing endothelial cells onto microfluidic chips. Under the influence of growth factors and other factors, endothelial cells utilize their own mechanisms of vascular generation to spontaneously reconstruct three-dimensional vascular systems in a short period of time [40]. Unlike other methods of constructing blood vessels, self-assembly technology directly mixes endothelial cells with hydrogels and places them in microfluidic devices. The significance of this method lies in the fact that the generated vascular models closely resemble natural blood vessels in both function and morphology, possessing superior physiological characteristics [12]. Therefore, this technology holds significant value in research and clinical applications, enabling the development of personalized vascular models and serving as a preferred method for studying vascular development or angiogenesis. It can also be used to investigate the effects of mechanical stress and chemical factors on vascular formation, as well as the morphological characteristics of vessels near abnormal sites such as tumors.
The template method involves casting material (such as hydrogel) around appropriately sized stainless-steel needles or fiber rods, allowing it to solidify before removing the template to form hollow channels [85]. This approach can also use molds to prepare two semicircular microchannels, which, when aligned and cross-linked, form a seamless closed circular microchannel [88]. By optimizing the cell seeding concentration and sequence, ensuring complete coverage and normal arrangement of underlying cells, the physiological similarity and functionality of the model are enhanced. The template method allows for direct formation or assembly of models, with channel cross-sections resembling the circular structure of natural blood vessels—closer to the physiological structure of real vessels—which is challenging to achieve with microfluidic technology. However, due to manufacturing limitations, the template method is only suitable for constructing simple branch, narrow, or straight blood vessel structures and cannot be used for constructing complex vascular networks.
The importance of 3D bioprinting in specific vascular applications lies in its ability to precisely control and customize vascular structures to meet medical and research needs. This technology can print complex vascular structures with micrometer-level accuracy, including various components such as endothelial cells, matrix scaffolds, and vessel walls. This precision is crucial for mimicking the morphology and function of natural blood vessels. Bioprinting materials must possess compatibility and biocompatibility with the body’s environment to support cell adhesion, proliferation, and differentiation [89]. Choosing appropriate bioinks and scaffold materials is a key technological challenge. Designing various types of vessels, including those with different diameters, branches, bends, and diverse biological and physical characteristics according to specific needs, demonstrates the flexibility of this technology, making it suitable for various clinical applications and research scenarios. By integrating multiple cell types (such as endothelial cells, smooth muscle cells, and fibroblasts) during printing, it is possible to better simulate the complex structure and function of natural blood vessels, thereby enhancing the success rate and functional performance of vascular construction [91]. Controlling the printing microenvironment and the release of growth factors can promote vascular generation and regeneration, which is crucial for repairing damaged vessels or improving blood supply.
Laser degradation and laser cavitation technologies have significant application value in constructing vascular models. These technologies utilize precise energy control and local action characteristics of lasers to simulate and reconstruct complex vascular structures. By using high-energy laser pulses, the focus and energy of the laser can be precisely controlled to construct fine structures and channels on different material surfaces or within tissues [49]. Therefore, in constructing vascular models, this technology can be used to create highly refined microstructures, such as the intricate internal structures of vessel walls or various complex forms mimicking vessel walls. Lasers can achieve local evaporation or chemical changes on different material surfaces or tissues to form specific shapes or structures. When constructing vascular models, different types of biocompatible materials or simulated tissues can be used, and their shapes and microstructures can be precisely controlled using laser technology to simulate the characteristics of real blood vessels. This technology allows high-precision processing at the microscopic scale, enabling constructed vascular models to possess cellular-level structural features [50]. Such precision and complexity enable researchers to more accurately study the physiological and pathological processes of blood vessels, such as hemodynamics and mechanisms of thrombosis, thereby advancing vascular model development.
3.1. Endothelial Dysfunction
Endothelial dysfunction is associated with the development of thrombosis, atherosclerosis, and inflammatory diseases [109]. The mechanisms underlying thrombosis and atherosclerosis are complex and involve numerous factors. Therefore, in vitro models play a crucial role in predicting disease progression, risk levels, and determining treatment options. Tsai et al. [110] constructed an in vitro thrombus model to evaluate the efficacy of antithrombotic drugs. The researchers first inoculated endothelial cells into the microfluidic channel. Studies have shown that endothelial cells assemble into a single layer within 24–48 h under perfusion conditions and begin to function appropriately. Tumor necrosis factor alpha was then used to activate the endothelial cells, causing tube occlusion to form a thrombus model. The researchers used this model to test the effectiveness of hydroxyurea drugs on thrombosis. The study showed that the fluid blockage in the tube improved after adding the drugs. Thus, the thrombus model can be used to evaluate antithrombotic drugs and serve as a screening platform for drug discovery. However, due to the microfabrication technology of the microfluidic chip, the cross-section of the above model is rectangular and does not match the physiological structure of the blood vessel. Therefore, it cannot fully simulate the vascular microenvironment. Nonetheless, the endothelial cells inoculated into the channel can form an approximately cylindrical vascular cavity, making the system more physiological. Additionally, cell-loaded hydrogel constructs composed of growth factors, hydrogels, and cells provide a favorable in vitro environment for studying endothelial dysfunction. Thus, cell-loaded hydrogels with ideal geometry, size, and composition can be developed to bring the model closer to the physiological environment.
Chen et al. [111] (Figure 4) used collagen gel to co-culture endothelial cells and smooth muscle cells. Studies have shown that co-culture can affect cell gene expression and lead to the production of a variety of adhesion molecules and chemokines. This model provides new insights into the mechanism of interaction between leukocytes and the vessel wall in patients with atherosclerosis.
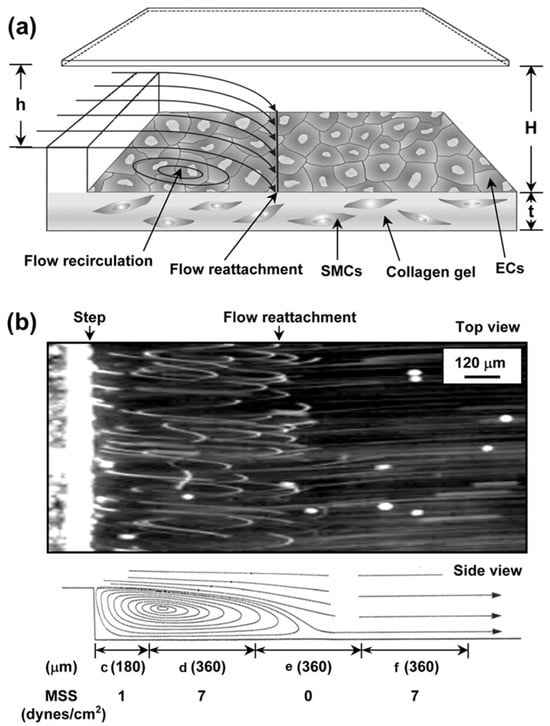
Figure 4.
Schematic diagram and flow patterns of the vertical-step flow (VSF) channel structure. (a) Schematic diagram of the channel. ((b), (top)) Microphotographs showing experimental flow patterns in the VSF channel (top view). ((b), (bottom)) Side-view schematic diagram of VSF streamlines inferred from top-view microphotographs. Flow separation occurs in the region farthest from the step, forming four distinct flow regions: (c) stagnation zone, (d) recirculation vortex zone, (e) reattachment flow zone, and (f) flow redeveloping into laminar flow region as shown at the bottom of the figure. Reprinted/adapted with permission from Ref. [111]. 2006, Amer Soc Hematology.
In another study, Dorweiler et al. [112] used fibrin gel to construct an atherosclerosis model. The researchers first inoculated smooth muscle cells into the fibrin gel, and after 14 days, the smooth muscle cells formed a mature network structure. Then, the endothelial cells were inoculated on the surface of the gel, and the two cell types were continuously cultured for 4–6 weeks. The study confirmed that the model had high viability and functional integrity. After 28 days, low-density lipoprotein was used to induce atherosclerosis. The model had good long-term stability and could be used to study atherosclerosis for up to 6 weeks. However, established in vitro models cannot fully simulate human endothelial dysfunction. Additionally, these models still have shortcomings such as poor reproducibility, lack of homogeneity, and inadequate mechanical properties. Therefore, cell-loaded collagen can be combined with microfluidic chips to create more advanced models.
3.2. Blood Vessels Associated with Cancer
Cancer research utilizing in vitro vascular models is another important area of study. The blood vessels surrounding tumors play a critical role in tumor growth by supplying oxygen and nutrients and also contribute to metastasis [11]. As a result, various in vitro vascular models have been developed to investigate the impact of vasculature on cancer. In one study, Dereli et al. [113] created a three-dimensional microfluidic cell array composed of three PDMS layers. This model maintained nutrient supply and waste metabolism of the encapsulated cells through continuous perfusion in the microchannel. The research demonstrated that this model sustained high cell viability for up to two weeks. It was successfully employed to establish human breast cancer and lung cancer models using the respective cancer cells, enabling high-throughput screening of potential anti-cancer drugs. In another study, Gospodinova et al. [114] utilized a hydroxyethyl cellulose-based bioink mixed with sodium alginate to develop a cervical cancer model via 3D bioprinting technology using HeLa cells. Cell viability was minimally affected during the process, and after one week of cultivation, the cell count increased significantly. The research indicated that low concentrations of sodium alginate in the bioink had little impact on cell viability. However, higher concentrations of sodium alginate (5%) and longer printing times have a negative effect on initial cell viability. The researchers used this model to evaluate the effectiveness of paclitaxel and observed its cytotoxic effect on the hydrogel. This model can be utilized for screening other anti-cancer drugs.
In addition to screening anti-cancer drugs, cancer-related models can also be utilized to investigate the mechanism of angiogenesis at cancer sites and the phenomenon of cancer metastasis. Carvalho et al. [115] (Figure 5) replicated the budding process of endothelial cells in the tumor environment using a tumor chip, and by day 5, the model formed a vascular-like structure. This result indicates that the chip successfully simulated the angiogenesis process in the rectal cancer microenvironment. Compared to traditional in vitro models, this platform is closer to the physiological environment and shows promise as a drug screening platform. However, the lack of pericyte support in this model can hinder the stability of blood vessels. Additionally, this model lacks important features of natural blood vessels, such as fluid flow. Chen et al. [116] developed a triangular multi-cavity tumor chip platform. Research shows that the chip can ensure dynamic culture and maintain high vitality in human breast cancer cells for at least one week. Starting on day 8, the hydrogel began to gradually degrade, and by day 15, most of the hydrogel had degraded. Furthermore, research indicates that the large-pore hydrogel—formed by adjusting the proportion of hydrogel—promotes cell growth, providing a good platform for constructing in vitro tumor models, investigating the mechanism of tumor metastasis, and screening antitumor metastasis drugs.
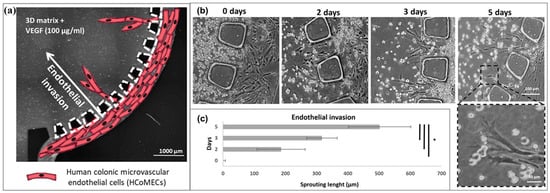
Figure 5.
Schematic diagram of endothelial cell invasion: (a) schematic diagram depicting the invasion of human colonic microvascular endothelial cells (HCoMECs) from the side channel into the central chamber in response to VEGF in the matrix; (b) formation of endothelial sprouts in a microfluidic device; (c) quantification of endothelial invasion. The asterisk (*) denotes statistical differences (p < 0.01). Reprinted/adapted with permission from Ref. [115]. 2019, Amer Assoc Advancement Science.
Buchanan et al. [117] developed an in vitro tumor vessel model using the template method. Tumor cells had a survival rate of more than 90% in the hydrogel and maintained proliferative activity during the three-day experiment. By evaluating shear-induced endothelial morphological changes, it was found that endothelial cells maintained their integrity under the physiological shear-stress environment. The study also investigated the impact of shear stress on microvessels and the paracrine signaling of breast cancer cells. However, current in vitro cancer models have limitations. For instance, three or more cell cultures in the blood–brain barrier model make it difficult to observe cell growth and take a long time to measure transmembrane resistance. Furthermore, current cancer models lack the immune system, which plays a crucial role in the immune clearance of tumor cells. To understand tumor and immune-mediated processes in vitro, the constructed cancer model must include key immune components. Finally, the current cancer model only integrates the vascular system and ignores the role of the lymphatic system in cancer metastasis. Therefore, an integrated cancer model that incorporates both blood vessels and lymphatic channels is expected to provide a more physiological model for drug delivery screening or tumor metastasis research.
3.3. Blood–Brain Barrier
The blood–brain barrier is a critical physiological barrier that protects the brain from external influences. It regulates the selective transport system between the brain and blood and metabolizes substances in the brain [118]. However, the blood–brain barrier also limits the effective treatment of brain-related diseases by preventing drugs from reaching the brain [119]. Therefore, the development of in vitro blood–brain barrier models is crucial.
In vitro blood–brain barrier models are generally achieved by co-culturing multiple cell types, and cell co-culture is particularly critical to the maturation and stability of the blood–brain barrier model. Yeon et al. [120] (Figure 6) used soft lithography technology to fabricate microfluidic chips and incubated endothelial cells and primary astrocytes to form a blood–brain barrier model. The study demonstrated that the permeability of the blood–brain barrier model was consistent with in vivo data, allowing for continuous monitoring of drug permeability across the blood–brain barrier and reducing culture time. However, research indicates that reactive oxygen species can cause connexin dispersion and affect the stability of the model. Therefore, antioxidants can be added to increase the model’s stability. However, as this model only contains two cell types and lacks pericytes, it still falls short of simulating the real blood–brain barrier. In another study, Delsing et al. [121] found that co-culturing endothelial cells, primary astrocytes, pericytes, and neurons in the blood–brain barrier model significantly enhanced the expression of trans-endothelial resistance and tight junction proteins, promoting blood–brain barrier formation by measuring transmembrane resistance. Co-culture also increased the expression of specific transporters, endothelial cell maturity, and model stability, allowing for distinguishing which new drug candidates are substrates for efflux transporters.
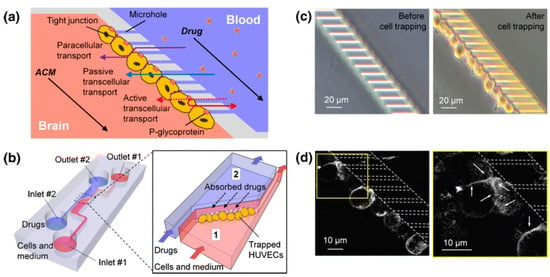
Figure 6.
Schematic diagram of the blood–brain barrier (BBB) model. (a) Design of microfluidic devices simulating the BBB model. (b) Schematic diagram of the microfluidic device. Human-umbilical-vein endothelial cells (HUVECs) suspended in culture medium are injected and trapped within the microchannel array. Number 1 is the cell injection port, and Number 2 is the drug injection port. (c) Tight contact between HUVECs trapped on microchannels. (d) Staining of HUVECs using monoclonal antibodies showing tight junctions between endothelial cells. Reprinted/adapted with permission from Ref. [120]. 2012, Springer.
However, the blood–brain barrier model requires co-culturing multiple cells simultaneously, resulting in a long culture cycle, complex procedures, and high costs. Furthermore, primary cells are preferred over biological immortal cell lines in this model as they better replicate the biological properties of cells in vivo.
3.4. Vascularized In Vitro Organs-on-Chips
Organ-on-a-chip is an innovative technology that combines tissue engineering and microfluidics to recreate human tissues and organs on a small chip. This technology aims to accurately simulate the complex structure, microenvironment, and physiological functions of human tissues and organs [122]. One crucial aspect of this technology is the development of perfusable vascular structures to ensure a constant supply of nutrients and removal of metabolic waste for the engineered tissues and organs. In this context, the focus will be on the vascularized organ chip model, which involves the integration of functional blood vessel networks within the chip.
Zhu et al. [123] developed an alveolar chip that can monitor the expansion and contraction of the lung organ in real-time by changing the color of the chip. The top and bottom layers of the chip simulate respiration or blood flow, respectively, while the sustained stretchability of the chip film mimics the physiological changes in the alveoli. This method extends the application of organ chips for real-time monitoring, which has potential applications in biological research, disease monitoring, and drug discovery. The study showed that the chip maintains high cell viability even under cyclic stretching and helps to maintain tight connections between cells.
Apart from single organ chips like lung, heart, liver, kidney, and small intestine, multiple tissues and organs can be constructed simultaneously and connected through the chip pipeline to form multi-organ chips. For instance, Oleaga et al. [124] (Figure 7) developed a four-organ microchip for toxicity testing that included liver, heart, skeletal muscle, and neurons. The results of all drug treatments during a 14-day incubation period with continuous media circulation in the device were generally consistent with toxicity results published in human and animal data. The model can study cardiotoxicity through the study of liver metabolites and serve as a new platform for preclinical studies.
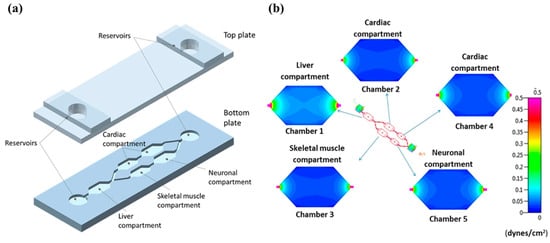
Figure 7.
(a) Schematic diagram of the microfluidic platform showing different cellular compartments. (b) Distribution of shear stress across the compartments of the system. Reprinted/adapted with permission from Ref. [124]. 2016, Nature Portfolio.
Lee et al. [125] developed a heart and breast cancer microarray platform for disease modeling and monitoring of cancer-chemotherapy-induced cardiotoxicity. They encapsulated breast cancer cells in hydrogel beads to create a tissue model and controlled the average diameter of the beads to avoid necrosis in the core region of the cell beads. Additionally, researchers encapsulated heart spheroids in a water gel to form a cardiac tissue model, which has a similar Young’s modulus to that of natural human heart tissue. The sensing platform has excellent accuracy and high sensitivity with very low detection limits, which can be used to detect and predict cardiotoxicity caused by early chemotherapy in patients.
Applying biofabrication methods to organs-on-chips involves a series of challenges that affect their effectiveness and reliability in biomedical research and applications [13,126,127]. First, biofabrication methods need to achieve highly precise control at the microscale to simulate complex biological structures and functions. In particular, in the construction of organ chips, precise control over cell arrangement, tissue structure, and the simulation of microenvironments pose challenges to the complexity and accuracy of manufacturing techniques. Secondly, the biological materials used in organ chips must possess excellent biocompatibility and mechanical properties, while being compatible with the materials used in the manufacturing process. This encompasses various aspects such as bioinks, scaffold materials, cell culture substrates, etc., all of which must meet engineering requirements while maintaining cellular functionality and growth capabilities. Additionally, organ chips need to accurately simulate cell behavior and microenvironments under physiological conditions, including the effects of fluid dynamics, cell–cell interactions, and signal transduction. Furthermore, achieving efficient operation and controllability of organ chips requires integrating advanced manufacturing technologies and automation systems, such as 3D printing, microfluidics, sensors, and control systems working synergistically. Finally, standardization and reproducibility are essential factors in the manufacturing process. Organ chips must demonstrate high levels of standardization and reproducibility to ensure the reliability of experimental results and comparability of data. This involves standard operating procedures for manufacturing processes, quality control, and validation, as well as consistency and credibility in data analysis.
Although organ chips are advanced, they cannot fully replicate the functions of living organs since organs cannot exist in isolation from the organism. Therefore, accurately analyzing the cell and gene expression levels of normal human tissues and organs is necessary to more accurately simulate the physiological environment of real organs. Additionally, the cost of using this tool in the medical field is still high, despite research funding support. Finally, the biggest challenge for organs-on-chips is the source of cells. Human-derived cells are most commonly used due to interspecies differences in non-human-derived cells but the sourcing of human terminal cells has been a limitation for various related research applications.
4. Summary and Prospects
The vasculature plays a crucial role in the body, and its dysfunction is closely linked to major diseases. Researchers have utilized various animal models and two-dimensional static in vitro platforms to study vascular diseases and develop related drugs. However, these models often fail to accurately replicate the vascular pathology and physiological environment in vitro. Furthermore, although tissue engineering has made significant advancements in recent decades, creating functional vascular constructs remains a major challenge. The vasculature is responsible for supplying nutrients to tissue engineering constructs, facilitating the removal of metabolic waste, and regulating signaling molecules involved in tissue growth. Traditional microfabrication techniques primarily focus on the overall structure while neglecting the intricate internal vascular network. This limitation makes it difficult to fabricate complex vascular networks. Therefore, this review examines the construction methods and applications of three-dimensional in vitro vascular models, aiming to address these challenges and improve our understanding of vascular biology and pathology.
This article began by discussing the limitations of traditional models, the functional structure of blood vessels, and the process of blood vessel formation. Currently, most perfusion extracorporeal vascular models are developed using microfluidic technology. To complement these methods, this review categorized the construction of extracorporeal vascular models into microfluidics-based and non-microfluidics-based methods based on different fabrication techniques. Specifically, we explored the principles, advantages, and disadvantages of various vascular fabrication methods, including lithography and soft lithography, self-assembly, template, 3D bioprinting, and laser degradation/cavitation. These methods aim to create in vitro vascular models that accurately simulate the complexity of blood vessels. While the complexity and physiological context of these models may differ, they offer valuable approaches for studying the physiological/pathological environment of blood vessels and the vascularization of tissue engineering constructs. The use of microfluidics technology, self-assembly, and 3D bioprinting to construct in vitro vascular and disease models remains a prominent research focus. These methods can be combined to create more physiologically relevant models. However, it is important to note that these three methods can only generate vascular networks with large diameters and simple geometries. Although laser degradation technology provides higher resolution and complexity, it may compromise cell viability and hydrogel integrity. With the advancement of laser cavitation molding technology, it is now possible to produce smaller and more intricate tubes without compromising cell viability. It is anticipated that laser cavitation molding technology will find widespread use in studying complex tissues in the near future.
During the process of in vitro vascular construction, the diameter and curvature of microchannels are influenced by the preparation methods, which significantly affect the formation of endothelial monolayers, barrier function, and cell viability [16,128]. A smaller diameter promotes tighter cell–cell interactions and closer connections between endothelial cells, facilitating the formation of a denser and more intact endothelial monolayer, crucial for maintaining continuous integrity [2]. Curvature also plays a pivotal role. Microchannels with smaller curvature typically support more uniform cell alignment and monolayer formation compared to those with larger curvature, which may disrupt cell alignment and monolayer integrity. In terms of barrier function, the diameter of the microchannels affects the permeability of endothelial monolayers. Smaller diameters enhance permeability, indicating that dense packing of endothelial cells contributes to better barrier function. Similarly, microchannels with smaller curvature often maintain better barrier integrity by promoting stable cell–cell connections and reducing the likelihood of cell detachment or disruption. However, while smaller diameters enhance connections and barrier function, spatial and nutrient constraints may limit cell migration and affect cellular vitality. In contrast, larger diameters facilitate cell migration within microchannels but may compromise barrier integrity by allowing looser cell–cell connections. In conclusion, the diameter and curvature of microchannels formed by fabrication methods play a crucial role in the formation of endothelial monolayers, barrier function, cellular vitality, and migration within vascular models. These parameters are indispensable considerations in the design of microfluidic systems for studying vascular biology, drug delivery, and disease modeling.
Shear stress and other mechanical factors strongly influence endothelial cells [100]. Fluid flow and shear stress play crucial roles in the construction of vascular models and their biological impacts. Fluid flow not only affects the structural formation of models but also directly influences the function and behavior of cells. Adjusting the speed and direction of fluid can affect the distribution and aggregation of biological materials (such as cells and growth factors) within the model, thereby enhancing the physiological realism and stability of vascular models. Furthermore, shear stress significantly influences the biological behavior of cells; simulating and adjusting shear stress can better mimic the biological environment and study its biological effects [16]. Flow rate is also crucial for biological models; appropriate flow rates can affect cell distribution, growth rates, and interactions between cells [129]. Therefore, when designing and operating organ chips, considering the relevance of flow rate to the physiological aspects of the studied biological model is a key factor in ensuring the biological significance and repeatability of experimental results. In summary, understanding the impact of fluid flow and shear stress on vascular models, and appropriately controlling flow rates, is essential for enhancing the biological relevance and physiological fidelity of the models. Overcoming these challenges requires deep collaboration and innovation across the fields of engineering, biology, and materials science. Further advancements in high-precision fluid control technology, biomaterials engineering, and novel chip designs will help enhance the complexity and physiological fidelity of vascular models.
The growth factors guide the formation of new blood vessels by activating signaling pathways [40,100]. When designing vascular models, leveraging factors such as the extracellular matrix (ECM) and growth factors, especially in 3D models, offers significant advantages. Firstly, the ECM in a 3D space provides a more complex and physiologically realistic support structure, mimicking the three-dimensional structure and microenvironment of real vascular tissues. This intricate structure supports cell growth, differentiation, and functional expression, better reflecting the realities of tissue engineering and biological research compared to 2D models. Secondly, growth factors like vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) exhibit more precise release and gradient control in a 3D environment, mimicking dynamic stimuli encountered by cells in vivo, thereby promoting proliferation and vascular formation of endothelial cells [129]. Therefore, integrating ECM and growth factors to construct 3D vascular models not only enhances their biological relevance and physiological fidelity but also provides a more accurate and reliable experimental platform for studying vascular-related disease mechanisms and drug screening.
However, the in vitro model suffers from poor homogeneity and is susceptible to factors such as cell source, cell type, and cell state. Additionally, the type of culture medium, serum concentration, and external environment can also influence the model. Furthermore, there is a lack of standardized characterization and stability evaluation methods for in vitro models, leading to a lack of reproducibility, which hinders their widespread use. When it comes to cell types, primary cells are closer to the human physiological environment compared to immortal cell lines. However, the cost of culturing primary cells is high, and the availability of materials is limited. Moreover, as primary cells undergo more passages, their tight junctions weaken, resulting in increased permeability that affects model stability. Therefore, it is crucial to improve experimental techniques, select high-quality serum and culture media, and optimize cell cryopreservation and resuscitation procedures.
In the process of constructing vascular models, there are two additional challenges: cellular spatial arrangement and maintaining uniform distribution [130,131]. Firstly, cellular spatial arrangement involves effectively organizing and arranging various cell types such as endothelial cells, smooth muscle cells, and other supporting cells to simulate the complex structure and functions of real blood vessels. This requires precise control over cell positions and relationships to ensure the model accurately reflects the physiological characteristics of the vascular system. Secondly, maintaining a uniform distribution of cells is another key issue. In three-dimensional space, the uniformity of cell distribution directly affects the stability and reproducibility of the model. Uneven distribution can lead to localized metabolic and signaling abnormalities, thereby impacting the interpretation of experimental results and the application value of models. To address these challenges, advanced cell culture techniques and microfluidic systems are typically relied upon to precisely control cell positioning and environmental conditions. These technologies not only facilitate uniform cell distribution but also enhance model complexity and physiological relevance, providing a reliable platform and tool for the development of vascular models used in drug screening and disease research.
Although in vitro vascular models are generally more physiologically relevant than two-dimensional models, they still struggle to replicate the complex metabolic conditions of the human body. As a result, their physiological relevance and clinical applicability have not been fully investigated and proven. Current complex in vitro models consist of single or multiple tissues and organs, but integrating all these components to simulate specific physiological functions remains a major challenge due to difficulties and high costs. Additionally, it is challenging to replicate the differences in blood pressure, flow rate, and dynamic components across different parts of the complex in vitro model, making it difficult for vascular models to fully replicate the in vivo environment.
The vascular models discussed in this article are reusable through perfusion devices, and the endothelial cells in the pipeline are on materials with good biocompatibility, which can simulate the microenvironment of blood vessels and three-dimensional cellular tissues in vivo. Additionally, these models are reproducible in shape and length, enabling repeated experiments that are difficult to conduct with natural blood vessels. However, due to technical limitations, current engineered vascular models can only last for a few weeks, limiting their use in the early stages of acute and chronic diseases. Therefore, it is important to focus on improving the long-term stability of in vitro vascular models, which can be reflected in endothelial homeostasis, perfusion capacity, permeability, and contractility of blood vessels. Furthermore, current models primarily use cell lines that can be used alone or in combination with different cells. Advances in stem cell culture technology and the use of patient-derived cells will improve the physiological relevance of the model.
In conclusion, this review is valuable in expediting the development of new multi-scale, multi-material, and multi-dimensional strategies that are crucial for designing and developing complex in vitro vascular models. For instance, the use of hydrogel constructs made from other biological materials such as hyaluronic acid, alginic acid, gelatin, chitosan, or their combinations can overcome the limitations of current materials. Additionally, advancements in the in vitro vascular models summarized in this article will promote basic scientific research and facilitate the translation of vascular engineering into clinical applications. Many physiological and pathological vascular models have yielded significant results in drug development. We should adopt a dialectical approach towards existing in vitro vascular models, and vascular design and research should always be guided by practical issues. Moreover, we should have a more comprehensive understanding of basic vascular biology and the advantages and limitations of existing models. I believe that with the continuous development and innovation of biomaterial formulations, manufacturing technologies, and cell biology, in vitro vascular models will introduce new cells and novel materials to achieve higher resolution, structural complexity, and durability. Therefore, future vascular models will more realistically simulate the in vivo vascular environment to study complex vascular pathological problems, making their applications in biomedical fields more widespread.
Author Contributions
Writing—original draft preparation, Z.H. and P.C.; writing—review and editing, G.Y. and Z.O.; supervision, G.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kim, S.; Kim, W.; Lim, S.; Jeon, J.S. Vasculature-On-A-Chip for In Vitro Disease Models. Bioengineering 2017, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Wang, Y.T.; Zhong, L.N.; Pan, F.W.; Wang, J. Advances in Tissue Engineering of Vasculature through Three-Dimensional Bioprinting. Dev. Dyn. 2021, 250, 1717–1738. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Bornfeldt, K.E.; Tall, A.R. Atherosclerosis Successes, Surprises, and Future Challenges. Circ. Res. 2016, 118, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Herrington, W.; Lacey, B.; Sherliker, P.; Armitage, J.; Lewington, S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ. Res. 2016, 118, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Whelan, I.T.; Moeendarbary, E.; Hoey, D.A.; Kelly, D.J. Biofabrication of Vasculature in Microphysiological Models of Bone. Biofabrication 2021, 13, 032004. [Google Scholar] [CrossRef] [PubMed]
- Puryear, J.R.; Yoon, J.K.; Kim, Y. Advanced Fabrication Techniques of Microengineered Physiological Systems. Micromachines 2020, 11, 730. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.X.; Millican, R.; Lynd, T.; Gangasani, M.; Malhotra, S.; Sherwood, J.; Hwang, P.T.; Cho, Y.; Brott, B.C.; et al. Recent Progress in In Vitro Models for Atherosclerosis Studies. Front. Cardiovasc. Med. 2022, 8, 790529. [Google Scholar] [CrossRef] [PubMed]
- Grifno, G.N.; Farrell, A.M.; Linville, R.M.; Arevalo, D.; Kim, J.H.; Gu, L.; Searson, P.C. Tissue-Engineered Blood-Brain Barrier Models via Directed Differentiation of Human Induced Pluripotent Stem Cells. Sci. Rep. 2019, 9, 13957. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kankala, R.K.; Zhang, J.T.; Hao, L.Z.; Zhu, K.; Wang, S.B.; Zhang, Y.S.; Chen, A.Z. Modeling Endothelialized Hepatic Tumor Microtissues for Drug Screening. Adv. Sci. 2020, 7, 2002002. [Google Scholar] [CrossRef] [PubMed]
- Luque-González, M.A.; Reis, R.L.; Kundu, S.C.; Caballero, D. Human Microcirculation-On-Chip Models in Cancer Research: Key Integration of Lymphatic and Blood Vasculatures. Adv. Biosyst. 2020, 4, 2000045. [Google Scholar] [CrossRef] [PubMed]
- Shirure, V.S.; Hughes, C.C.W.; George, S.C. Engineering Vascularized Organoid-On-A-Chip Models. Annu. Rev. Biomed. Eng. 2021, 23, 141–167. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.S.Y.; Guo, F.; Zhang, B.Y. Modeling Organ-Specific Vasculature with Organ-On-A-Chip Devices. Nanotechnology 2018, 30, 024002. [Google Scholar] [CrossRef] [PubMed]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological Strategies for Engineering Complex Tissues. Nat. Nanotechnol. 2011, 6, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Enrico, A.; Voulgaris, D.; Ostmans, R.; Sundaravadivel, N.; Moutaux, L.; Cordier, A.; Niklaus, F.; Herland, A.; Stemme, G. 3D Microvascularized Tissue Models by Laser-Based Cavitation Molding of Collagen. Adv. Mater. 2022, 34, 2109823. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.; Vogt, F.; Schmitz-Rode, T.; Jockenhoevel, S.; Mela, P. Bioengineered Vascular Constructs as Living Models for in Vitro Cardiovascular Research. Drug Discov. Today 2016, 21, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.H.; Balestrini, J.L.; Udelsman, B.V.; Zhou, K.C.; Zhao, L.P.; Ferruzzi, J.; Starcher, B.C.; Levene, M.J.; Humphrey, J.D.; Niklason, L.E. Biaxial Stretch Improves Elastic Fiber Maturation, Collagen Arrangement, and Mechanical Properties in Engineered Arteries. Tissue Eng. Part C Methods 2016, 22, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Schmitz-Rixen, T.; Hamilton, G.; Seifalian, A.M. Achieving the Ideal Properties for Vascular Bypass Grafts Using a Tissue Engineered Approach: A Review. Med. Biol. Eng. Comput. 2007, 45, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Pollet, A.M.A.O.; den Toonder, J.M.J. Recapitulating the Vasculature Using Organ-On-Chip Technology. Bioengineering 2020, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Augustin, H.G.; Koh, G.Y. Organotypic Vasculature: From Descriptive Heterogeneity to Functional Pathophysiology. Science 2017, 357, eaal2379. [Google Scholar] [CrossRef] [PubMed]
- Kant, R.J.; Coulombe, K.L.K. Integrated Approaches to Spatiotemporally Directing Angiogenesis in Host and Engineered Tissues. Acta Biomater. 2018, 69, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Hou, L.Q.; Huang, N.F. Vascularization of Three-Dimensional Engineered Tissues for Regenerative Medicine Applications. Acta Biomater. 2016, 41, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Rouwkema, J.; Khademhosseini, A. Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends Biotechnol. 2016, 34, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.M.; Gerecht, S. Microfluidics and Biomaterials to Study Angiogenesis. Curr. Opin. Chem. Eng. 2016, 11, 114–122. [Google Scholar] [CrossRef]
- Rodriguez, D.; Watts, D.; Gaete, D.; Sormendi, S.; Wielockx, B. Hypoxia Pathway Proteins and Their Impact on the Blood Vasculature. Int. J. Mol. Sci. 2021, 22, 9191. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Vimalraj, S.; Pavani, K.; Nikarika, R.; Sumantran, V.N. Intussusceptive Angiogenesis as a Key Therapeutic Target for Cancer Therapy. Life Sci. 2020, 252, 117670. [Google Scholar] [CrossRef] [PubMed]
- Fallah, J.; Rini, B.I. HIF Inhibitors: Status of Current Clinical Development. Curr. Oncol. Rep. 2019, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Nejad, A.E.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Javanmard, S.H.; Taherian, M.; Ahmadlou, M. The Role of Hypoxia in the Tumor Microenvironment and Development of Cancer Stem Cell: A Novel Approach to Developing Treatment. Cancer Cell Int. 2021, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.; Sadeghinia, A.; Kahroba, H.; Samadi, A.; Heidari, H.R.; Bradaran, B.; Zeinali, S.; Molavi, O. Therapeutic Targeting of Angiogenesis Molecular Pathways in Angiogenesis-Dependent Diseases. Biomed. Pharmacother. 2019, 110, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Teleanu, D.M. Tumor Angiogenesis and Anti-Angiogenic Strategies for Cancer Treatment. J. Clin. Med. 2020, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S.; Saravanan, S.; Anuradha, D.; Chatterjee, S. Models to Investigate Intussusceptive Angiogenesis: A Special Note on CRISPR/Cas9 Based System in Zebrafish. Int. J. Biol. Macromol. 2018, 123, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Nitzsche, B.; Rong, W.W.; Goede, A.; Hoffmann, B.; Scarpa, F.; Kuebler, W.M.; Secomb, T.W.; Pries, A.R. Coalescent Angiogenesis-Evidence for a Novel Concept of Vascular Network Maturation. Angiogenesis 2022, 25, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Pezzella, F. Sprouting and Nonsprouting Angiogenesis in Tumors. Tumor Vasc. 2020, 25, 2927. [Google Scholar]
- Pasut, A.; Becker, L.M.; Cuypers, A.; Carmeliet, P. Endothelial Cell Plasticity at the Single-Cell Level. Angiogenesis 2021, 24, 311–326. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, G.; Muñoz-Félix, J.M.; Pedrosa, A.R.; Hodivala-Dilke, K.M. “Splitting the Matrix”: Intussusceptive Angiogenesis Meets MT1-MMP. EMBO Mol. Med. 2019, 12, e11663. [Google Scholar] [CrossRef] [PubMed]
- Zuazo-Gaztelu, I.; Casanovas, O. Unraveling the Role of Angiogenesis in Cancer Ecosystems. Front. Oncol. 2018, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.Y.; Ingber, D.E. Soft Lithography in Biology and Biochemistry. Annu. Rev. Biomed. Eng. 2001, 3, 335–373. [Google Scholar] [CrossRef] [PubMed]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2022, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Pasman, T.; Grijpma, D.; Stamatialis, D.; Poot, A. Flat and Microstructured Polymeric Membranes in Organs-On-Chips. J. R. Soc. Interface 2018, 15, 20180351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wan, Z.P.; Kamm, R.D. Vascularized Organoids on a Chip: Strategies for Engineering Organoids with Functional Vasculature. Lab Chip 2021, 21, 473–488. [Google Scholar] [CrossRef] [PubMed]
- He, J.K.; Chen, R.M.; Lu, Y.J.; Zhan, L.; Liu, Y.X.; Li, D.C.; Jin, Z.M. Fabrication of Circular Microfluidic Network in Enzymatically-Crosslinked Gelatin Hydrogel. Mater. Sci. Eng. C 2016, 59, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I.T.; Hospodiuk, M. Current Advances and Future Perspectives in Extrusion-Based Bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed]
- Rider, P.; Kacarevic, Z.P.; Alkildani, S.; Retnasingh, S.; Barbeck, M. Bioprinting of Tissue Engineering Scaffolds. J. Tissue Eng. 2018, 9, 2041731418802090. [Google Scholar] [CrossRef] [PubMed]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.C.; Reid, R.R. 3-D Bioprinting Technologies in Tissue Engineering and Regenerative Medicine: Current and Future Trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ma, X.Y.; Gou, M.L.; Mei, D.Q.; Zhang, K.; Chen, S.C. 3D Printing of Functional Biomaterials for Tissue Engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I.T.; Moncal, K.K.; Gudapati, H. Evaluation of Bioprinter Technologies. Addit. Manuf. 2017, 13, 179–200. [Google Scholar] [CrossRef]
- Holzl, K.; Lin, S.M.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.X.; Ovsianikov, A. Bioink Properties before, during and after 3D Bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef] [PubMed]
- Betz, J.F.; Ho, V.B.; Gaston, J.D. 3D Bioprinting and Its Application to Military Medicine. Mil. Med. 2020, 185, E1510–E1519. [Google Scholar] [CrossRef] [PubMed]
- Applegate, M.B.; Coburn, J.; Partlow, B.P.; Moreau, J.E.; Mondia, J.P.; Marelli, B.; Kaplan, D.L.; Omenetto, F.G. Laser-Based Three-Dimensional Multiscale Micropatterning of Biocompatible Hydrogels for Customized Tissue Engineering Scaffolds. Proc. Natl. Acad. Sci. USA 2015, 112, 12052–12057. [Google Scholar] [CrossRef] [PubMed]
- Brandenberg, N.; Lutolf, M.P. In Situ Patterning of Microfluidic Networks in 3D Cell-Laden Hydrogels. Adv. Mater. 2016, 28, 7450–7456. [Google Scholar] [CrossRef] [PubMed]
- Morbioli, G.G.; Speller, N.C.; Stockton, A.M. A Practical Guide to Rapid-Prototyping of PDMS-Based Microfluidic Devices: A Tutorial. Anal. Chim. Acta 2020, 1135, 150–174. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, J.M.; Park, K.Y.; Virumbrales-Munoz, M.; Beebe, D.J. Toward Improved In Vitro Models of Human Cancer. APL Bioeng. 2021, 5, 010902. [Google Scholar] [CrossRef] [PubMed]
- Christoffersson, J.; Mandenius, C.F. Fabrication of a Microfluidic Cell Culture Device Using Photolithographic and Soft Lithographic Techniques. Methods Mol. Biol. 2019, 1994, 227–233. [Google Scholar] [PubMed]
- Wang, Z.; Samanipour, R.; Kim, K. Organ-On-A-Chip Platforms for Drug Screening and Tissue Engineering. In Biomedical Engineering: Frontier Research and Converging Technologies; Springer International Publishing: New York, NY, USA, 2015; pp. 209–233. [Google Scholar]
- Jiang, B.; White, A.; Ou, W.Q.; Belleghem, S.V.; Stewart, S.; Shamul, J.H.; Rahaman, S.O.; Fisher, J.P.; He, X. Noncovalent Reversible Binding-Enabled Facile Fabrication of Leak-Free PDMS Microfluidic Devices without Plasma Treatment for Convenient Cell Loading and Retrieval. Bioact. Mater. 2022, 16, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Borok, A.; Laboda, K.; Bonyar, A. PDMS Bonding Technologies for Microfluidic Applications: A Review. Biosensors 2021, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Raj, M.K.; Chakraborty, S. PDMS Microfluidics: A Mini Review. J. Appl. Polym. Sci. 2020, 137, 48958. [Google Scholar] [CrossRef]
- Fan, Y.Q.; Liu, S.C.; Zhang, Y.J. Direct Bonding of Polymer/Glass-Based Microfluidic Chips with Dry Film Photoresist. Microsyst. Technol. 2018, 24, 1659–1665. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Scott, S.; Ali, Z. Fabrication Methods for Microfluidic Devices: An Overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.L.; Liu, H.H.; Zhang, B.J.; Han, Y.; Shen, C.; Lin, Q.; Chen, H. Development of Antibacterial and High Light Transmittance Bulk Materials: Incorporation and Sustained Release of Hydrophobic or Hydrophilic Antibiotics. Colloids Surf. B Biointerfaces 2016, 141, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Liu, J. Implantable Liquid Metal-Based Flexible Neural Microelectrode Array and Its Application in Recovering Animal Locomotion Functions. J. Micromech. Microeng. 2017, 27, 104002. [Google Scholar] [CrossRef]
- Tavakoli, S.; Nemati, S.; Kharaziha, M.; Akbari-Alavijeh, S. Embedding CuO Nanoparticles in PDMS-SiO2 Coating to Improve Antibacterial Characteristic and Corrosion Resistance. Colloid Interface Sci. Commun. 2019, 28, 20–28. [Google Scholar] [CrossRef]
- Sala, F.; Ficorella, C.; Osellame, R.; Käs, J.A.; Vázquez, R.M. Microfluidic Lab-On-A-Chip for Studies of Cell Migration under Spatial Confinement. Biosensors 2022, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with Designer Functionalities-Properties, Modifications Strategies, and Applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Tanyeri, M.; Tay, S. Viable Cell Culture in PDMS-Based Microfluidic Devices. Methods Cell Biol. 2018, 148, 3–33. [Google Scholar] [PubMed]
- Kuddannaya, S.; Bao, J.N.; Zhang, Y.L. Enhanced in Vitro Biocompatibility of Chemically Modified Poly(dimethylsiloxane) Surfaces for Stable Adhesion and Long-Term Investigation of Brain Cerebral Cortex Cells. ACS Appl. Mater. Interfaces 2015, 7, 25529–25538. [Google Scholar] [CrossRef] [PubMed]
- Gokaltun, A.; Yarmush, M.L.; Asatekin, A.; Usta, O.B. Recent Advances in Nonbiofouling PDMS Surface Modification Strategies Applicable to Microfluidic Technology. Technology 2017, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Natyanun, S.; Pussadee, N. Hydrophobicity Recovery of Polydimethylsiloxane Treated with Oxygen Plasma and Ion Implantation. J. Phys. Conf. Ser. 2018, 1144, 012110. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Baassiri, K.; Mahmoudi, Z.; Perumal, A.S.; Rajendran, K.; Rubies, G.M.; Nicolau, D.V. Hydrophobic Recovery of PDMS Surfaces in Contact with Hydrophilic Entities: Relevance to Biomedical Devices. Materials 2022, 15, 2313. [Google Scholar] [CrossRef] [PubMed]
- Deagen, M.E.; Chan, E.P.; Schadler, L.S.; Ullal, C.K. Corona Treatment for Nanotransfer Molding Adhesion. ACS Appl. Polym. Mater. 2019, 1, 997–1005. [Google Scholar]
- Akther, F.; Yakob, S.B.; Nguyen, N.T.; Ta, H.T. Surface Modification Techniques for Endothelial Cell Seeding in PDMS Microfluidic Devices. Biosensors 2020, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Shahriar, M.; Liu, J.C.; Xu, H.Q.; Zhang, Z.Y.; Xu, C.X. Effects of Corona Treatment on Cellular Attachment and Morphology on Polydimethylsiloxane Micropillar Substrates. JOM 2022, 74, 3408–3418. [Google Scholar] [CrossRef]
- Ozcam, A.E.; Efimenko, K.; Genzer, J. Effect of Ultraviolet/Ozone Treatment on the Surface and Bulk Properties of Poly(dimethyl Siloxane) and Poly(vinylmethyl Siloxane) Networks. Polymer 2014, 55, 3107–3119. [Google Scholar] [CrossRef]
- Raveendran, R.; Namboothiry, M.A.G. Surface-Treated Poly(dimethylsiloxane) as a Gate Dielectric in Solution-Processed Organic Field-Effect Transistors. ACS Omega 2018, 3, 11278–11285. [Google Scholar] [CrossRef] [PubMed]
- Siddique, A.; Meckel, T.; Stark, R.W.; Narayan, S. Improved Cell Adhesion under Shear Stress in PDMS Microfluidic Devices. Colloids Surf. B Biointerfaces 2017, 150, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Sivarapatna, A.; Ghaedi, M.; Xiao, Y.; Han, E.; Aryal, B.; Zhou, J.; Fernandez-Hernando, C.; Qyang, Y.B.; Hirschi, K.K.; Niklason, L.E. Engineered Microvasculature in PDMS Networks Using Endothelial Cells Derived from Human Induced Pluripotent Stem Cells. Cell Transp. 2017, 26, 1365–1379. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, R.; Lee, N.Y. Microfluidic Device Fabrication Mediated by Surface Chemical Bonding. Analyst 2020, 145, 4096–4110. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.Y.; Chuah, Y.J.; Ang, W.T.; Zheng, N.; Wang, D.A. Optimization of a Polydopamine (PD)-Based Coating Method and Polydimethylsiloxane (PDMS) Substrates for Improved Mouse Embryonic Stem Cell (ESC) Pluripotency Maintenance and Cardiac Differentiation. Biomater. Sci. 2017, 5, 1156–1173. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.J.; Xu, J.J.; Chen, H.Y. In-Situ Grafting Hydrophilic Polymer on Chitosan Modified Poly(dimethylsiloxane) Microchip for Separation of Biomolecules. J. Chromatogr. A 2007, 1147, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.W.; Ren, X.Q.; Bachman, M.; Sims, C.E.; Li, G.P.; Allbritton, N. Surface Modification of Poly(dimethylsiloxane) Microfluidic Devices by Ultraviolet Polymer Grafting. Anal. Chem. 2002, 74, 4117–4123. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Georgescu, A.; Oh, J.M.; Kwon, K.W.; Huh, D. Polydopamine-Based Interfacial Engineering of Extracellular Matrix Hydrogels for the Construction and Long-Term Maintenance of Living Three-Dimensional Tissues. ACS Appl. Mater. Interfaces 2019, 11, 23919–23925. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chung, M.; Ahn, J.; Lee, S.; Jeon, N.L. Interstitial Flow Regulates the Angiogenic Response and Phenotype of Endothelial Cells in a 3D Culture Model. Lab Chip 2016, 16, 4189–4199. [Google Scholar] [CrossRef] [PubMed]
- Pauty, J.; Usuba, R.; Cheng, I.G.; Hespel, L.; Takahashi, H.; Kato, K.; Kobayashi, M.; Nakajima, H.; Lee, E.; Yger, F. A Vascular Endothelial Growth Factor-Dependent Sprouting Angiogenesis Assay Based on an In Vitro Human Blood Vessel Model for the Study of Anti-Angiogenic Drugs. EBioMedicine 2018, 27, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kato, K.; Ueyama, K.; Kobayashi, M.; Baik, G.; Yukawa, Y.; Suehiro, J.I.; Matsunaga, Y.Y. Visualizing Dynamics of Angiogenic Sprouting from a Three-Dimensional Microvasculature Model Using Stage-Top Optical Coherence Tomography. Sci. Rep. 2017, 7, 42426. [Google Scholar] [CrossRef] [PubMed]
- Chrobak, K.M.; Potter, D.R.; Tien, J. Formation of Perfused, Functional Microvascular Tubes In Vitro. Microvasc. Res. 2006, 71, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Morimoto, Y.; Takeuchi, S. Skin Integrated with Perfusable Vascular Channels on a Chip. Biomaterials 2017, 116, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Seo, T.S. Orthogonal Co-Cultivation of Smooth Muscle Cell and Endothelial Cell Layers to Construct in Vivo-Like Vasculature. Biomicrofluidics 2019, 13, 014115. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Chen, M.J.; Fan, X.Q.; Zhou, H.F. Recent Advances in Bioprinting Techniques: Approaches, Applications and Future Prospects. J. Transl. Med. 2016, 14, 271. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; Burdick, J.A.; Highley, C.; Lee, S.J.; Morimoto, Y.; Takeuchi, S.; Yoo, J.J. Biofabrication Strategies for 3D in Vitro Models and Regenerative Medicine. Nat. Rev. Mater. 2018, 3, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Sasmal, P.; Datta, P.; Wu, Y.; Ozbolat, I.T. 3D Bioprinting for Modelling Vasculature. Microphysiol. Syst. 2018, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D Bioprinting System to Produce Human-Scale Tissue Constructs with Structural Integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-Dimensional Bioprinting of Thick Vascularized Tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; Boland, T.; Burdick, J.A.; De Maria, C.; Derby, B.; Forgacs, G.; Groll, J.; Li, Q.; Malda, J.; Mironov, V.A.; et al. Biofabrication: A guide to technology and terminology. Trends Biotechnol. 2018, 36, 384–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Yu, Y.; Akkouch, A.; Dababneh, A.; Dolati, F.; Ozbolat, I.T. In Vitro Study of Directly Bioprinted Perfusable Vasculature Conduits. Biomater. Sci. 2015, 3, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.J.; Kim, K.; Kim, D.H. 3D Bioprinting for Engineering Complex Tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Tomasina, C.; Bodet, T.; Mota, C.; Moroni, L.; Camarero-Espinosa, S. Bioprinting Vasculature: Materials, Cells and Emergent Techniques. Materials 2019, 12, 2701. [Google Scholar] [CrossRef] [PubMed]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D Bioprinting: An Overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.T.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.J.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D Bioprinting of Perfusable Vascular Constructs Using a Blend Bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, S.; Tavakol, D.N.; Vunjak-Novakovic, G. From Arteries to Capillaries: Approaches to Engineering Human Vasculature. Adv. Funct. Mater. 2020, 30, 1910811. [Google Scholar] [CrossRef] [PubMed]
- Sima, F.; Sugioka, K.; Vazquez, R.M.; Osellame, R.; Kelemen, L.; Ormos, P. Three-Dimensional Femtosecond Laser Processing for Lab-On-A-Chip Applications. Nanophotonics 2018, 7, 613–634. [Google Scholar] [CrossRef]
- Rayner, S.G.; Howard, C.C.; Mandrycky, C.J.; Stamenkovic, S.; Himmelfarb, J.; Shih, A.Y.; Zheng, Y. Multiphoton-Guided Creation of Complex Organ-Specific Microvasculature. Adv. Healthc. Mater. 2021, 10, 2100031. [Google Scholar] [CrossRef] [PubMed]
- Heintz, K.A.; Bregenzer, M.E.; Mantle, J.L.; Lee, K.H.; West, J.L.; Slater, J.H. Fabrication of 3D Biomimetic Microfluidic Networks in Hydrogels. Adv. Healthc. Mater. 2016, 5, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Kwon, K.W.; Park, M.C.; Lee, S.H.; Kim, S.M.; Suh, K.Y. Soft Lithography for Microfluidics: A Review. BioChip J. 2008, 2, 1–11. [Google Scholar]
- Nguyen, D.H.T.; Stapleton, S.C.; Yang, M.T.; Cha, S.S.; Choi, C.K.; Galie, P.A.; Chen, C.S. Biomimetic Model to Reconstitute Angiogenic Sprouting Morphogenesis In Vitro. Proc. Natl. Acad. Sci. USA 2013, 110, 6712–6717. [Google Scholar] [CrossRef] [PubMed]
- Moya, M.L.; Hsu, Y.H.; Lee, A.P.; Hughes, C.C.W.; George, S.C. In Vitro Perfused Human Capillary Networks. Tissue Eng. Part C Methods 2013, 19, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Heintz, K.A.; Mayerich, D.; Slater, J.H. Image-Guided, Laser-Based Fabrication of Vascular-Derived Microfluidic Networks. J. Vis. Exp. 2017, 119, e55101. [Google Scholar] [PubMed]
- Gao, G.; Park, J.Y.; Kim, B.S.; Jang, J.; Cho, D.W. Coaxial Cell Printing of Freestanding, Perfusable, and Functional In Vitro Vascular Models for Recapitulation of Native Vascular Endothelium Pathophysiology. Adv. Healthc. Mater. 2018, 7, 1801102. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.I. Blood Flow and Arterial Endothelial Dysfunction: Mechanisms and Implications. Comptes Rendus Phys. 2013, 14, 479–496. [Google Scholar] [CrossRef]
- Tsai, M.; Kita, A.; Leach, J.; Rounsevell, R.; Huang, J.N.; Moake, J.; Ware, R.E.; Fletcher, D.A.; Lam, W.A. In Vitro Modeling of the Microvascular Occlusion and Thrombosis That Occur in Hematologic Diseases Using Microfluidic Technology. J. Clin. Investig. 2012, 122, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.N.; Chang, S.F.; Lee, P.L.; Chang, K.; Chen, L.J.; Usami, S.; Chien, S.; Chiu, J.J. Neutrophils, Lymphocytes, and Monocytes Exhibit Diverse Behaviors in Transendothelial and Subendothelial Migrations under Coculture with Smooth Muscle Cells in Disturbed Flow. Blood 2006, 107, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Dorweiler, B.; Torzewski, M.; Dahm, M.; Ochsenhirt, V.; Lehr, H.A.; Lackner, K.J.; Vahl, C.F. A Novel in Vitro Model for the Study of Plaque Development in Atherosclerosis. Thromb. Haemost. 2006, 95, 182–189. [Google Scholar] [PubMed]
- Dereli-Korkut, Z.; Akaydin, H.D.; Ahmed, A.H.R.; Jiang, X.J.; Wang, S.H. Three Dimensional Microfluidic Cell Arrays for ex Vivo Drug Screening with Mimicked Vascular Flow. Anal. Chem. 2014, 86, 2997–3004. [Google Scholar] [CrossRef] [PubMed]
- Gospodinova, A.; Nankov, V.; Tomov, S.; Redzheb, M.; Petrov, P.D. Extrusion Bioprinting of Hydroxyethylcellulose-Based Bioink for Cervical Tumor Model. Carbohydr. Polym. 2021, 260, 117793. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.R.; Barata, D.; Teixeira, L.M.; Giselbrecht, S.; Reis, R.L.; Oliveira, J.M.; Truckenmüller, R.; Habibovic, P. Colorectal Tumor-On-A-Chip System: A 3D Tool for Precision Onco-Nanomedicine. Sci. Adv. 2019, 5, eaaw1317. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xue, Y.F.; Xu, L.T.; Li, W.L.; Chen, Y.L.; Zheng, S.N.; Dai, R.; Liu, J. Recapitulation of Dynamic Nanoparticle Transport around Tumors Using a Triangular Multi-Chamber Tumor-On-A-Chip. Lab Chip 2022, 22, 4191–4204. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, C.F.; Voigt, E.E.; Szot, C.S.; Freeman, J.W.; Vlachos, P.P.; Rylander, M.N. Three-Dimensional Microfluidic Collagen Hydrogels for Investigating Flow-Mediated Tumor-Endothelial Signaling and Vascular Organization. Tissue Eng. Part C Methods 2014, 20, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Prabhu, A. In Vitro Blood Brain Barrier Models: Molecular Aspects and Therapeutic Strategies in Glioma Management. Curr. Res. Transl. Med. 2022, 71, 103376. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.R.; de Menezes, L.R.; do Nascimento, D.F.; Souza, D.H.S.; Reynaud, F.; Marques, M.F.V.; Tavares, M.I.B. Polymeric Nanoparticles Assembled with Microfluidics for Drug Delivery across the Blood-Brain Barrier. Eur. Phys. J. Spec. Top. 2016, 225, 779–795. [Google Scholar] [CrossRef]
- Yeon, J.H.; Na, D.; Choi, K.; Ryu, S.W.; Choi, C.; Park, J.K. Reliable Permeability Assay System in a Microfluidic Device Mimicking Cerebral Vasculatures. Biomed. Microdevices 2012, 14, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Delsing, L.; Donnes, P.; Sanchez, J.; Clausen, M.; Voulgaris, D.; Falk, A.; Herland, A.; Brolén, G.; Zetterberg, H.; Hicks, R.; et al. Barrier Properties and Transcriptome Expression in Human iPSC-Derived Models of the Blood-Brain Barrier. Stem Cells 2018, 36, 1816–1827. [Google Scholar] [CrossRef] [PubMed]
- Esch, E.W.; Bahinski, A.; Huh, D. Organs-On-Chips at the Frontiers of Drug Discovery. Nat. Rev. Drug Discov. 2015, 14, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Sun, L.Y.; Wang, Y.; Cai, L.J.; Zhang, Z.H.; Shang, Y.X.; Zhao, Y.J. A Biomimetic Human Lung-On-A-Chip with Colorful Display of Microphysiological Breath. Adv. Mater. 2022, 34, 2108972. [Google Scholar] [CrossRef] [PubMed]
- Oleaga, C.; Bernabini, C.; Smith, A.S.T.; Srinivasan, B.; Jackson, M.; McLamb, W.; Platt, V.; Bridges, R.; Cai, Y.Q.; Santhanam, N.; et al. Multi-Organ Toxicity Demonstration in a Functional Human in Vitro System Composed of Four Organs. Sci. Rep. 2016, 6, 20030. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Mehrotra, S.; Zare-Eelanjegh, E.; Rodrigues, R.O.; Akbarinejad, A.; Ge, D.; Amato, L.; Kiaee, K.; Fang, Y.C.; Rosenkranz, A.; et al. A Heart-Breast Cancer-On-A-Chip Platform for Disease Modeling and Monitoring of Cardiotoxicity Induced by Cancer Chemotherapy. Small 2021, 17, e2004258. [Google Scholar] [CrossRef] [PubMed]
- Fritschen, A.; Blaeser, A. Biosynthetic, Biomimetic, and Self-Assembled Vascularized Organ-On-A-Chip Systems. Biomaterials 2021, 268, 120556. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Ingber, D.E. Microfluidic Organs-On-Chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [PubMed]
- Bogorad, M.I.; Destefano, J.; Wong, A.D.; Searson, P.C. Tissue-Engineered 3D Microvessel and Capillary Network Models for the Study of Vascular Phenomena. Microcirculation 2017, 24, e12360. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, A.; Albers, H.J.; Passier, R.; Mummery, C.L.; van den Berg, A.; Orlova, V.V.; van der Meer, A.D. Advanced in Vitro Models of Vascular Biology: Human Induced Pluripotent Stem Cells and Organ-On-Chip Technology. Adv. Drug Deliv. Rev. 2019, 140, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Naderi-Meshkin, H.; Cornelius, V.A.; Eleftheriadou, M.; Potel, K.N.; Setyaningsih, W.A.W.; Margariti, A. Vascular Organoids: Unveiling Advantages, Applications, Challenges, and Disease Modelling Strategies. Stem Cell Res. Ther. 2023, 14, 292. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kankala, R.K.; Ou, C.W.; Chen, A.Z.; Yang, Z.L. Advances in Hydrogel-Based Vascularized Tissues for Tissue Repair and Drug Screening. Bioact. Mater. 2022, 9, 198–220. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).