Abstract
Nanotechnology, a rapidly growing field, holds tremendous promise as it harnesses the unique properties and applications of nanoparticulate materials on a nanoscale. In parallel, the pressing global environmental concerns call for the development of sustainable chemical processes and the creation of new materials through eco-friendly synthesis methods. In this work, zero-valent iron nanoparticles (nZVI) were synthesized using an innovative and environmentally friendly approach as an alternative to conventional methods. This method leverages the antioxidant capacity of natural plant extracts to effectively reduce dissolved metals and produce nZVI. The chosen extract of green tea plays a pivotal role in this process. With the extract in focus, this study delves into the remarkable capability of nZVI in degrading two dyes commonly used in medicine, chrysoidine G and methylene blue, in aqueous solutions. Additionally, Fenton-type oxidation processes are explored by incorporating hydrogen peroxide into the nanoparticle mixture. By applying the statistical design of experiments and Response Surface Methodology, the influence of four key parameters—initial concentrations of Fe2+, Fe3+, H2O2, and polyphenols—on dye elimination efficiency in aqueous solutions is thoroughly analyzed. The obtained results demonstrate that advanced oxidation technologies, such as Fenton-type reactions in conjunction with nanoparticles, achieve an excellent efficiency of nearly 100% in eliminating the dyes. Moreover, this study reveals the synergistic effect achieved by simultaneously employing nZVI and the Fenton process, showcasing the potential for further advancements in the field.
1. Introduction
With a global market size valued at USD 29.9 billion in 2022, the dye and pigment industry constitutes a major economic sector. Furthermore, it is foreseeable that the market will grow to USD 39.7 billion by 2030, exhibiting an average annual increase of 3.6% throughout this period [1]. Historically, India, China, Germany, and the United States have led production. Due to their wide range of colors, versatility, and cost-effectiveness, synthetic dyes dominate the dye market. However, the dye industry has faced increasing scrutiny and regulatory measures related to environmental impact and sustainability [2], and efforts have been made to develop and promote eco-friendly or sustainable dyes with reduced environmental and health risks [3].
Textile dyes account for a significant portion of the overall dye market, although dyes find extensive use in manufacturing and industrial processes beyond textiles. They are employed in sectors such as plastics, paints, printing inks, cosmetics, and leather goods to add color, enhance appearance, and improve product quality. Dyes also play a part in environmental monitoring and assessment. They are used as tracers in studies related to water flow, hydrology, and pollution monitoring [4]. Dyes can help track the movement and dispersion of contaminants, identify pollution sources, and evaluate the effectiveness of environmental remediation efforts [5]. Finally, dyes have significant applications in scientific research, particularly in areas such as molecular biology, genetics, and cell biology [6]. Different dyes are used as fluorescent probes, indicators, or stains to visualize and study biological processes, cellular structures, and genetic material [6,7]. Dyes also play crucial roles in medical diagnostics and research, as they are used as contrast agents in medical imaging techniques such as X-rays, CT scans, and angiography to enhance visibility and highlight specific structures or functions in the body [8].
Chrysoidine G (4-Phenylazo-m-phenylenediamine monohydrochloride, C12H12N4·HCl, CAS Number 532-82-1) has been used in various medical applications, including wound healing, as it has shown antibacterial properties against certain types of bacteria commonly found in wound infections. In addition, chrysoidine has demonstrated antifungal activity against some species of fungi, particularly those causing superficial fungal infections such as dermatophytes [9].
Methylene blue (Methylthioninium chloride, 3,7-bis(Dimethylamino)phenothiazin-5-ium Chloride, C16H18ClN3S, CAS Number 61-73-4) has found use in different medical fields, such as diagnostic staining [10] and the treatment of urinary tract infections [11] or methemoglobinemia [12], a disease characterized by the presence of an abnormal form of hemoglobin in the blood. In addition, methylene blue has shown potential neuroprotective properties [13] and has been investigated as a therapeutic agent for neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease [14,15], as well as an adjunctive treatment for depression [16].
Despite their beneficial uses, chrysoidine and methylene blue can pose environmental risks including toxicity for aquatic or terrestrial organisms [17,18,19,20] and water pollution [21,22,23]. Furthermore, these adverse effects may be reinforced due to the potential of these molecules to bioaccumulate in organisms and their environmental persistence, which increase the potential for long-term exposure to organisms and ecosystems, amplifying the environmental risks.
To mitigate these hazards, it is necessary to use a range of methods that can remove or reduce the concentrations of these chemicals, preferably before discharging the effluents. The remediation of chrysoidine and methylene blue in water can be achieved through various treatment methods. For instance, activated carbon, zeolites, clay minerals, and other materials can be employed to adsorb chrysoidine and methylene blue from water [24,25,26,27,28,29,30,31,32]. Chemical coagulation and flocculation are also alternatives [33,34]. Membrane filtration techniques, such as reverse osmosis, nanofiltration, or ultrafiltration, can also effectively remove these dyes from water [35,36,37]. Nevertheless, all these methods involve the transference of the pollutant from one phase to another while leaving its molecular structure intact, retaining its potential risks. Certain microorganisms, however, possess the ability to biologically degrade dyes, and activated sludge processes or biofilters can be employed to remove chrysoidine and methylene blue from water [38,39,40,41]. Biological methods also have certain disadvantages, including specificity, slow degradation rates, sensitivity to environmental conditions, a need for nutrient supplementation, high operational costs, and the potential for the formation of harmful by-products [42]. Advanced oxidation processes (AOPs), such as ozonation, photocatalysis, or Fenton’s reagent, contribute to overcoming most of these inconveniences, as AOPs generate highly reactive hydroxyl radicals that break down the dye molecules into simpler and less toxic compounds. Ideally, complete mineralization of the pollutants into CO2 and water can be achieved.
Nanoscale Zero-Valent Iron (nZVI) is commonly used in advanced oxidation processes as a catalyst to facilitate the degradation of pollutants [43,44,45]. Due to its high surface area and ability to generate large quantities of reactive oxygen species (ROS) such as hydroxyl radicals (·OH), nZVI exhibits increased reactivity towards a wide variety of pollutants, including organic contaminants [43,44], heavy metals [46], and certain emerging contaminants [47]. Its oxidative potential enables the degradation or transformation of different pollutant classes. Interestingly, nZVI can be generated in situ, allowing it to be directly applied to contaminated sites [48,49,50,51]. This reduces the need for excavating and transporting contaminated soil or water, making it a cost-effective option in some scenarios. Finally, depending on the treatment process and conditions, nZVI particles can potentially be recovered and reused, reducing the overall cost and environmental impact of the treatment [52].
nZVI is typically produced through chemical or physical methods using iron precursors and a convenient reducing agent. In particular, green tea extract contains polyphenolic compounds with excellent reducing properties, meaning it can be used as an environmentally friendly reducing and stabilizing agent for nZVI [53,54]. This improves its dispersion and stability in aqueous solutions, preventing the oxidation of nZVI and enhancing its reactivity and longevity. All these properties increase the effectiveness of nZVI generated with the aid of green tea extract in pollutant removal [55].
However, the successful application of nZVI in AOPs requires careful optimization of operational parameters, such as pH, temperature, contact time, and dosage [56,57,58]. In this work, zero-valent iron nanoparticles have been synthesized using green tea leaf extract as an alternative to conventional reducing agents. Next, nZVI generated in situ was used to remove two dyes, namely chrysoidine G and methylene blue, from an aqueous solution through Fenton-type advanced oxidation processes. The statistical design of experiments and Response Surface Methodology have been applied to analyze the influence of four parameters (namely the initial concentrations of Fe2+, Fe3+, H2O2, and the reducing agent) on the efficiency of elimination of each dye.
2. Materials and Methods
2.1. Reagents
All the chemical reagents used were of the maximum grade of purity available. All the experiments were performed in Milli-Q®-grade deionized water. Tea bags from a local department store were used to extract the polyphenols, which were employed as a benign reducing agent. Iron (III) nitrate and iron (II) sulfate were purchased from Panreac (Barcelona, Spain), and chrysoidine, methylene blue, hydrogen peroxide, gallic acid, methanol, Folin–Ciocalteu’s phenol reagent, and sodium carbonate were purchased from Merck KGaA (Darmstadt, Germany).
2.2. Extraction of Polyphenols from Green Tea Leaves and Determination of the Polyphenol Content
The tea leaves were removed from their bags and pre-washed with Milli-Q water. They were then dried in an oven at 60 °C until constant weighing. To extract the polyphenols, 5 g of the dry tea sample was weighed and transferred to a three-necked flask. A total of 100 mL of Milli-Q water was added as a solvent and reflux extraction was carried out for 20 min, as previously described in the literature [59]. At the end of the process, the tea extract was separated from the solids by triple vacuum filtration. To avoid oxidative degradation of polyphenols, the resulting tea extract was stored in a freezer at −22 °C until use [60].
The composition of the natural extract was qualitatively analyzed using the following procedure. Initially, the samples were filtered through 0.45 μm nylon membrane filters. Subsequently, an HPLC system (Agilent Technologies HPLC 1260 Series, Santa Clara, CA, USA) equipped with a UV/Vis-Photo Diode Array Detector (DAD) and a Luna C18 column (3 μm, 150 mm × 2 mm, 100 Å, Phenomenex, Torrance, CA, USA) was utilized to separate and identify the polyphenols present in the sample. Two different mobile phases were employed. The first one (A) consisted of 0.1% formic acid in water (v/v), while the second one (B) was composed of 0.1% formic acid in methanol. The experiment involved the use of various gradients: 0–3 min, 10% B; 3–30 min, 50% B; 30–40 min, 60% B; 40–45 min, 60% B; 45–50 min, 100% B; 50–60 min, 10% B. A consistent flow rate of 0.25 mL·min−1 was maintained. The column temperature was held constant at 30 °C, and the injection volume was 20 μL. The DAD operated at λ = 280 nm.
For the sake of clarity, determination of the total polyphenol content in the leaf extract was preferred due to its composition as a mixture of various polyphenolic compounds. Quantification was conducted by UV–VIS spectrophotometry (Thermo Fisher Scientific, Waltham, MA, USA) using the Folin–Ciocalteu method [61]. This method relies on the reaction between phenolic compounds and Folin’s reagent under alkaline conditions, achieved by the addition of Na2CO3. Folin’s reagent in this assay consists of a solution of sodium tungstate and sodium molybdate in phosphoric acid. When this is reduced by phenolic groups, the yellow phosphomolybdotungstic acid (formed by both salts in an acidic medium) produces an intense blue complex, whose absorbance was determined spectrophotometrically using a Shimadzu UV-1800 Ultraviolet/Visible spectrophotometer (Cole-Parmer, Vernon Hills, IL, USA).
It is important to note that the quantification was performed based on a gallic acid standard line. Consequently, the concentration of polyphenols was determined as gallic acid equivalents. The procedure for establishing the standard line was as follows: Solutions with varying concentrations (ranging from 2.3 to 7.9 mg/L) of gallic acid standards were prepared in 25 mL flasks using a 196 mg/L stock solution. Subsequently, 0.5 mL of Folin’s reagent and 10 mL of 7.5% Na2CO3 were added to these solutions, followed by the addition of ultrapure water to reach the desired volume. The flasks were kept in the dark for one hour, after which the absorbance was measured at a fixed wavelength of 760 nm against a blank prepared in the same manner but without gallic acid.
Following the establishment of the calibration line, the quantification of polyphenols in the green tea extract was conducted. Various volumes of the extract (specifically, 50, 75, and 100 μL) were measured. To each volume, 0.5 mL of Folin’s reagent and 10 mL of Na2CO3 were added, and the solution was brought to a final volume of 25 mL by adding ultrapure water. After allowing the solution to stand for one hour in the absence of light, the absorbance was measured at 760 nm. This resulted in an average concentration of 3165 ± 180 equivalent mg of gallic acid per liter of extract. Consequently, the polyphenol content was determined to be 63,391 ± 3609 equivalent mg of gallic acid per kilogram of dry tea leaves.
2.3. Experimental Procedure
The experimental setup and procedure used are depicted in Figure 1. In brief, 25 mL of a tea extract solution (containing the polyphenols) is added to a beaker placed on a plate with continuous stirring. Then, 25 mL of Fe(II) and Fe(III) salt solution is added dropwise using a burette. The system is kept under continuous mechanical agitation for 5 min to ensure correct nanoparticle generation. The concentrations of polyphenols, Fe(II), and Fe(III) differ for each experiment depending on the experimental matrix, as described in the following section. Next, 64.3 mL of the dye solution (chrysoidine or methylene blue) is added to reach a final concentration of 150 mg·L−1, and a final volume of 150 mL is made up with Milli-Q water.
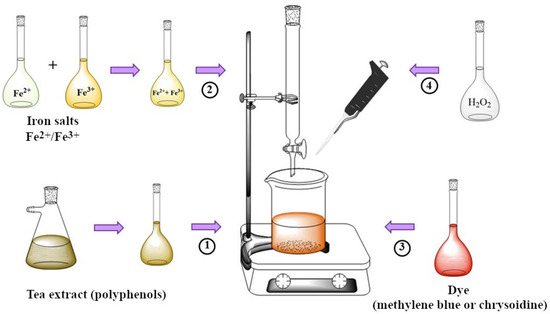
Figure 1.
Experimental setup for the in situ generation of nZVI and dye removal.
Subsequently, a quantity of hydrogen peroxide, determined for each experiment and so small that it can be neglected in the dilution (of the order of µL), is added and the solution is stirred for 8 min, which is sufficient to allow the Fenton process to be completed. Finally, an aliquot of 5 mL of the solution is taken and centrifuged at 5000× g rpm. To determine the final concentration of the dye, the supernatant is extracted after centrifugation and added to the spectrophotometric cell to acquire the corresponding spectrum.
2.4. Experimental Design
A statistical design of experiments (DOE) has been carried out with a double objective. On the one hand, the aim is to establish the influence of different operating variables on the dye removal efficiency of nZVI, which has been generated in situ; on the other hand, the goal is to analyze the possible interactions between these variables and to determine an optimum within the study limits. The selected operational variables, which are expected to have a significant effect on the target variables, are polyphenol concentration (PF), expressed as equivalent mg of gallic acid per liter; the concentrations of Fe(II) and Fe(III) in mol·L−1; and the concentration of hydrogen peroxide, also expressed in mol·L−1. The number of experiments that constitute the matrix is determined by considering the operational variables. For a factorial, central, composite, orthogonal, and rotatable design (FCCORD), the total numbers of experiments (NE) are:
where k is the number of operational variables (in this case, 4) and n is the number of replications of the central experiment. To ensure both rotatability and orthogonality, twelve replications of the central experiments are required. Thus, the experimental matrix consists of sixteen factorial experiments (i.e., 2k), eight axial experiments (i.e., 2k), and the sixteen replications of the central experiment, as mentioned earlier. Hence, NE equals 36.
NE = 2k + 2k + n
Table S1 (Supplementary Material) shows the operational variables that constitute the experimental design, the study region between the maximum and minimum values taken by these variables, the central value, and the steps. The experimental matrix shown in Table S2 (Supplementary Material) was designed from these values. It is worth noting that the experiments must be carried out in a precise order to ensure randomization in the experimental procedure.
To adequately apply this experimental methodology, it is of paramount importance to carefully determine the operating intervals. To properly select the working intervals of the experimental design, a series of preliminary experiments were run. The results of these preliminary tests and the design of the experimental matrix are discussed in detail in Section 3.
2.5. Characterization of n-ZVI
The samples prepared as described above were thoroughly characterized in terms of texture and morphology, chemical composition, and thermal behavior.
2.5.1. Texture and Morphology
Textural characterization was carried out using gas adsorption (N2 at 77 K). Semiautomatic Quantachrome Autosorb-1 equipment was used for the determination of the N2 adsorption isotherms. Briefly, 0.15 g of the sample was introduced into the glass vessel and degassed at 250 °C for 12 h at a pressure below 10−3 Torr. From the measured adsorption isotherms, the micropore volume (Vmi) was calculated by merely reading the volume of N2 adsorbed at p/p° equal to 0.10, whereas the mesopore volume (Vme) was also obtained from the N2 adsorption isotherm as Vme = V0.95 − Vmi, where V0.95 is the volume of nitrogen adsorbed at p/p° equal to 0.95. Both Vmi and Vme are expressed as liquid volumes (conversion factor, 1.4656 × 10−3).
The specific surface area (SBET) was estimated using the Brunauer, Emmet, and Teller (BET) method. The micropore volume (W0) was obtained by applying the Dubinin–Radushkevich equation.
The morphology of the samples was analyzed by Scanning Electron Microscopy (SEM). Images were acquired with the aid of HITACHI S-4800 equipment (MRL, Urbana, IL, USA), operating under high vacuum mode and with acceleration voltages between 0.5 and 30 kV. Secondary electrons (SE) and transmitted electrons (TE) detectors were used.
2.5.2. Chemical Characterization and Crystal Structure
The surface chemistry of the samples was analyzed by FT-IR spectroscopy using a Perkin Elmer 1720 spectrophotometer (Perkin Elmer, Shelton, CT, USA). Spectra were recorded in the range of wave numbers between 400 and 4000 cm−1 with a resolution of 2 cm−1. Fifty scans were registered for each record. Disks were prepared using KBr as a dispersing agent and binder. The sample-to-KBr proportion was 500:1, and the total mass of the disks was 200.5 mg.
The Energy Dispersive X-ray (EDX) spectrum was registered while acquiring the SEM images, with the aid of a Silicon Drift Detector (SDD) XFlash 5010 from Bruker (Singapore). Semi-quantitative chemical analysis was obtained from EDX spectra.
The crystal structure of the samples was studied by X-Ray Diffraction (XRD). Diffraction patterns were acquired with the aid of a Bruker D8 ADVANCE diffractometer equipped with a PSD Vantec detector within the 2θ range between 10° and 80° (step = 0.02°) using Cu Kα radiation (λ = 1.54184 Å).
2.5.3. Thermal Analysis
Thermal analyses were performed with the aid of an STA 449 F3 Jupiter-Netzsch (Netzsch Analyzing & Testing, Selb, Germany) thermobalance coupled with a QMS 403D Aëolos III-Netzsch mass spectrometer (Netzsch Analyzing & Testing). Thermogravimetric analysis (TGA) was performed within a temperature range between 40 and 800 °C with a heating rate of 5 °C/min. N2 was used as the inert gas, under a total flux of 100 mL min−1. Temperature Programmed Reduction (TPR) assays were conducted under the same experimental conditions, only varying the atmosphere (N2:H2, 90:10) and the total gas flux (200 mL min−1). The evolution of gases (namely H2O m/z = 18 and CO2 m/z = 44) was monitored throughout the experiment.
3. Results and Discussion
3.1. Characterization of Polyphenol Extracts
The determination of polyphenol content in the extract of green tea leaves was conducted following the procedure described in Section 2.2. The HPLC chromatogram of the extract is presented in Figure 2 (top).
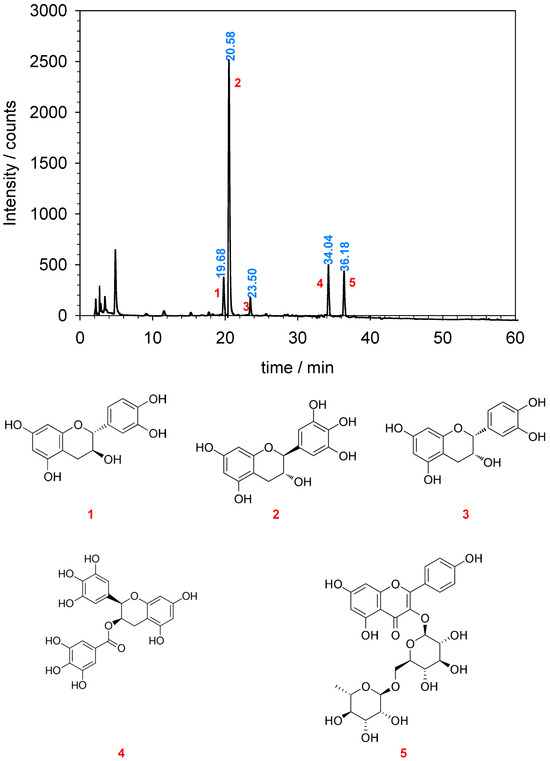
Figure 2.
HPLC-DAD profile of green tea leaf waste (top) and structures of the primary polyphenols identified (bottom). 1. Catechin; 2. Gallocatechin; 3. Epicatechin; 4. Epigallocatechin-3,5-digallate; 5. Kaempferol-rutinoside. Retention times are shown in blue.
Based on the available literature, the prominent peaks observed in the HPLC-DAD analysis have been assigned and identified [62,63,64,65]. The predominant polyphenols detected in the extract include (+)-catechin, (−)-gallocatechin, (−)-epicatechin, (−)-epigallocatechin-3,5-digallate, and kaempferol-rutinoside. These corresponding peaks have been labeled in the chromatogram, and their structures are illustrated in Figure 2 (bottom). This phenolic composition aligns with previously reported findings in the literature [66,67,68].
3.2. Texture and Morphology of the nZVI Samples
The N2 adsorption isotherm registered at 77 K, together with the pore size distribution plot of the nZVI sample, are depicted in Figure 3. From the N2 adsorption isotherm (Figure 3, top), it can be concluded that the isotherm belongs to Type IV of the BDDT classification. This type of isotherm is characterized by a pronounced hysteresis loop. This is typical for mesoporous solids. It has been established that the appearance of the hysteresis cycle is because the mesopore filling process is controlled by the capillary condensation phenomenon and percolation properties of the solid.
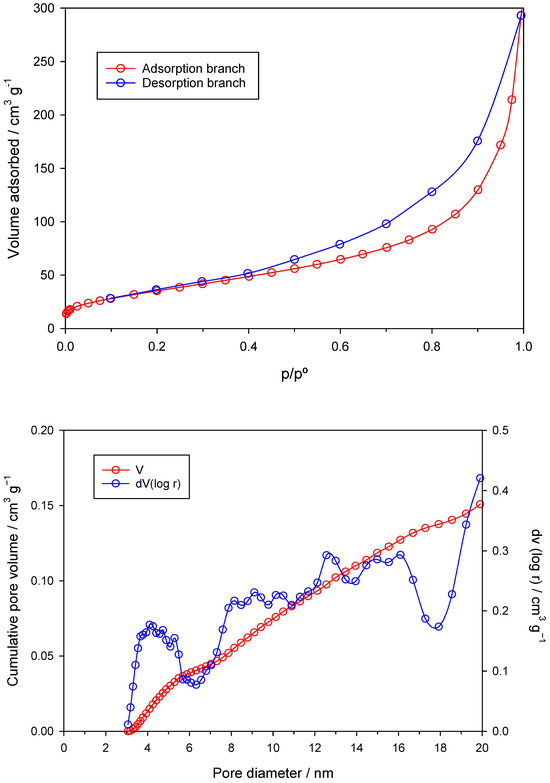
Figure 3.
N2 adsorption isotherm (top) and pore size distribution (bottom) of the nZVI sample.
In particular, the hysteresis loop that appears in the isotherm corresponds to the H3 type. This suggests that nZVI is a material with relatively high porosity development and with a significant presence of mesopores, as can be inferred from the absence of a pronounced plateau in the isotherm and the existence of a final section with a high slope. From the data of the adsorption isotherm, it is possible to calculate the volume of the micro- and mesopores (Vmi and Vme, respectively). Vmi = 0.027 cm3/g and Vme = 0.238 cm3/g were obtained as described in Section 2.5.1. It is therefore confirmed that the sample has a marked mesoporous character. By applying the Dubinin–Radushkevich (DR) equation, the micropore volume (W0) may also be calculated. For the nZVI studied here, W0 equals 0.031 cm3/g, a value that is in good agreement with the one calculated by directly reading the adsorption isotherm.
The specific surface area (SBET) of the sample was calculated by applying the well-known Brunauer, Emmett, and Teller (BET) equation. A value of 130 m2/g was obtained. The DR model also makes it possible to calculate the micropore surface area, which provides information on the contribution of micropores to the specific surface area of the sample. In this case, the micropore surface area was 102 m2/g. Considering the noticeable difference between this value and the one obtained by applying the BET equation, as well as the large value of Vme indicated above, it can be concluded that, for the nZVI sample under study, the contribution of mesopores to surface area and porosity is remarkable. Although most of the pristine nZVI previously reported in the literature exhibit values of SBET that range from 5 up to 61 m2/g [69], some authors have reported values of specific surface areas that reach 143 m2/g [70], i.e., similar or slightly larger than the ones reported here. Nevertheless, it is worth noting that these latter values were synthesized through the reduction of Fe3+ with a conventional reductant such as sodium borohydride, instead of using a greener approach as in this work.
Figure 3, bottom, shows the cumulative pore volume (red) and the pore size distribution (blue) in the region of the narrow and mid-size mesopores (3–32 nm), calculated by applying the DFT method. A wide variety of mesopore sizes can be observed in this plot. The total mesopore volume calculated as the area under the curve (AUC) of the cumulative pore volume (red line) equals 0.221 cm3/g, which is in very good agreement with that obtained by reading the adsorption isotherm (i.e., 0.238 cm3/g).
Scanning electron microscopy (SEM) was used to analyze the particle morphology of the nZVI sample. Figure 4 shows the SEM images of both samples. The presence of nanoparticle aggregates in the range of a few microns up to 100 microns can be appreciated. Images at larger magnification, as well as those acquired using the transmitted electron detector (STEM images), revealed that some of these aggregates were formed by nanoparticles, with a size around 100 nm or less. Aggregation of nZVI prepared by reduction of iron salts in the presence of polyphenols in aqueous medium has been repeatedly reported in the literature [55,71] and is regarded as a significant drawback of this kind of synthetic strategy [72].
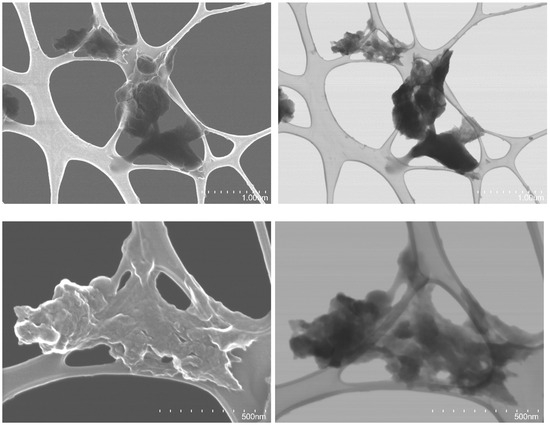
Figure 4.
SEM (left) and STEM (right) images of the nZVI under different magnifications.
In contrast to the conventional chemical method of synthesizing nZVI (e.g., using sodium borohydride as the reducing agent), which predominantly yields chain aggregates, green synthesis produces aggregates of various shapes such as cloud shapes and network structures [73]. The aggregation process is influenced by the synthesis conditions, primarily dictated by the chemical composition of the plant extract employed. Analysis using SEM revealed that the synthesized nZVI sample comprises a mixture of diverse aggregate forms due to the heterogeneous composition of the green tea extract utilized.
3.3. Chemical Characterization and Crystal Structure of the nZVI Samples
X-ray diffraction analysis was carried out to study the samples presented in this section. The XRD pattern is shown in Figure 5.
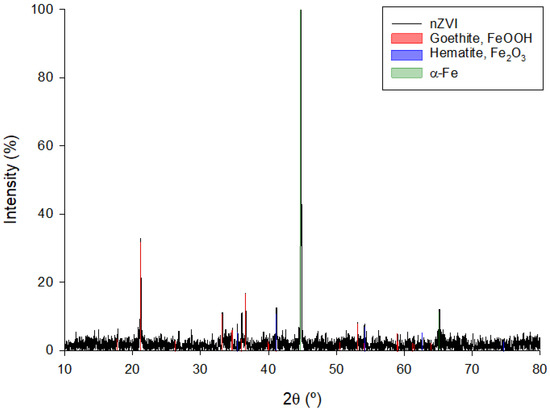
Figure 5.
XRD pattern of the nZVI sample.
The XRD patterns are coherent with the presence of crystalline Fe0. Thus, a strong diffraction peak at 2θ = 44.85° for the lattice plane of (1 1 0), and a second weaker peak at 2θ = 65.2° for the lattice plane of (2 0 0), can be easily appreciated. This confirms the generation of zero-valent iron nanoparticles because of the reduction of Fe2+ and Fe3+ by the polyphenols that are present in the green tea leaf extract. In addition, extra reflections corresponding to the XRD patterns of goethite (FeOOH) and hematite (Fe2O3) have been recorded. This is in good agreement with the fact that Fe0 can be at least partially re-oxidized by the aqueous media in which the synthesis was carried out. This is following other results previously published in the literature [55,74,75,76].
Other authors report similar XRD patterns but propose that the nanoparticles may consist of a Fe0 core that would be surrounded by iron oxide in a higher oxidation state (Fe2O3) or by iron oxyhydroxide (FeOOH) [71,77,78].
From the XRD pattern, a semi-quantitative analysis of the different crystalline phases in the sample can be obtained. The results of this analysis, together with the main characteristics of such phases, are summarized in Table 1.

Table 1.
Semi-quantitative analysis and crystal characteristics of the nZVI sample.
The Energy Dispersive X-ray (EDX) spectrum of the nZVI sample is shown in Figure 6. The semi-quantitative chemical analysis of the sample is shown as an insert in the figure. From the EDX spectrum, it can be concluded that nZVI is constituted by iron; however, the presence of relatively large amounts of other elements, mainly carbon and oxygen that are present in the carbonyl/carboxyl and phenolic moieties of polyphenols, reveals the presence of organic matter in the sample, corresponding to the dry residue of the polyphenol extract. The presence of small amounts of other oligo-elements such as phosphorus, chlorine, and sulfur, all of them essential for vegetal life, corroborates this hypothesis. Hence, it can be concluded that the surface of the nZVI sample under study appears to be coated with a layer of organic compounds initially present in the green tea leaf extract, mainly polyphenols. As a result, the elemental analysis reveals a relatively low iron content (6.12 wt% in mass, 1.67 in mol %) due to the presence of iron hidden beneath carbon and oxygen, which cannot be measured using EDS analysis [55,79,80,81]. The latter, however, does not hinder the ability of Fe to act as a catalyst in heterogeneous Fenton reactions, as the system is kept under constant stirring and the residual polyphenols that might deposit onto the nZVI particles are immediately removed. Only when the nanoparticles are filtered and dried does the remnant organic matter cover the nZVI.
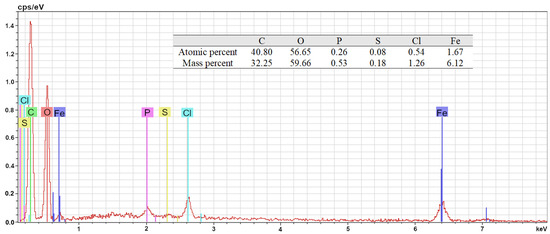
Figure 6.
Energy-Dispersive X-ray spectrum and semi-quantitative chemical analysis of the nZVI sample.
3.4. Thermal Analysis
The TG curve, together with the water (m/z = 18) and carbon dioxide (m/z = 44) release profiles, are shown in Figure 7, top. An initial weight loss starting from 40 °C and centered around 130 °C is attributable to the removal of humidity, with a significant mass decrease. As was foreseeable, this effect is coupled to an intense peak in the signal of water (blue line in Figure 7). A second weight loss effect centered around 225 °C and ending at approximately 320 °C can also be appreciated. Within this temperature range, another intense water release peak can be observed. According to the literature, this second effect can be due to the dehydration of goethite into hematite [82]. This water release overlaps with an increase in the signal corresponding to carbon dioxide (orange line in Figure 7). This signal reaches a maximum at approximately 365 °C and then decreases sharply. This behavior is coherent with an initial pyrolysis of the organic matter (residues of polyphenol extracts). At temperatures above 350 °C, no further water release is observed. Contrarily, the pyrolysis of the organic matter appears to take place in several stages, the last starting at around 650 °C and finishing at 780 °C, which is, approximately, only a few degrees before the thermal analysis ends.
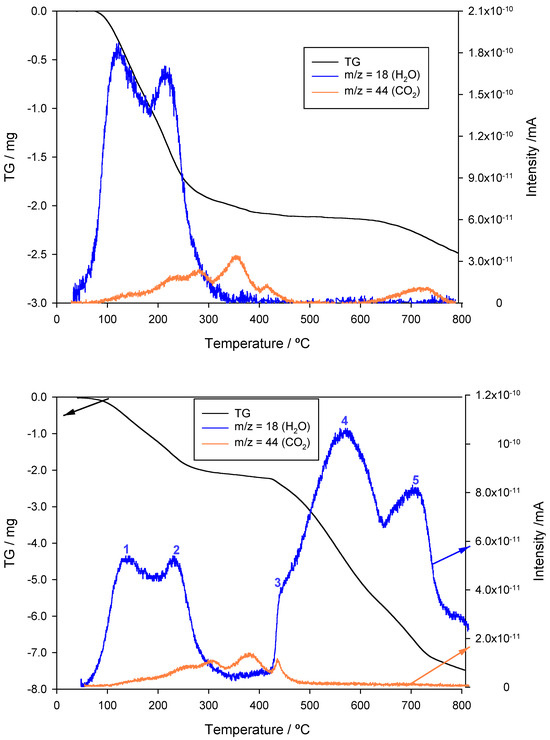
Figure 7.
TG (top) and TPR (bottom) plots showing water (m/z = 18) and carbon dioxide (m/z = 44) release profiles under inert (N2) and reducing (N2:H2 90:10) atmospheres.
The results of the TPR analysis are shown in Figure 7, bottom. Firstly, it can be seen that the mass loss plot is quite different from and more complex than the one registered under an inert atmosphere. The same applies to the gas release profiles, particularly for water. Although the two initial water release effects are very similar in both cases, up to three peaks can be seen in the TPR plot. For the sake of clarity, the five water release peaks have been marked in Figure 7, bottom.
As indicated above, peak number 1 (centered around 130 °C) corresponds in both cases to the loss of humidity. The same applies to peak number 2 (centered around 225 °C), which can be attributed to the dehydration of goethite, according to the following process:
From 350 °C and higher, the differences between the water release profiles in the TG and TPR experiments become quite evident. Firstly, there is a sharp increase in the signal of water between 410 and 425 °C, labeled number 3 in the TPR plot. This effect can be associated with a first reduction stage from hematite, Fe2O3, where all the iron is in oxidation state (III), to magnetite, Fe3O4, an oxide with an inverse spinel structure that consists of two Fe(III) cations and one Fe(II) cation, so that the neutrality of the crystal is maintained. Hence, Fe3O4 can be more accurately formulated as , where the t and o subscripts refer to the occupancy of tetrahedral and octahedral sites by FeII and/or FeIII cations within the cubic close packing (ccp) of oxide anions, thus giving rise to an occupancy factor, λ, equal to 0.5. This initial reduction reaction can be expressed as:
The water release continues in a slower but sustained manner, reaching a fourth peak at around 550 °C. This process is compatible with the subsequent reduction of magnetite into ferrous oxide, FeO, where all the iron has now been reduced into an oxidation state (II), according to the following reaction:
The reduction of iron finishes with the formation of the zero-valent metal from the ferrous oxide generated in the previous stage. Such a process begins at approximately 630 °C, reaches its maximum at around 690 °C (peak number 5), and is virtually completed at 750 °C. The chemical reaction associated with this latter effect is:
According to all of the above, the global reduction process, starting from goethite, can be summarized as:
3.5. Preliminary Tests and Determination of the Operational Variables and Working Intervals
In an experimental design such as the one used for the development of this work, it is of paramount importance to properly define the working range corresponding to each of the operating parameters (initial concentrations of H2O2, Fe2+, Fe3+, and polyphenols), whose effect on the response parameter (dye removal efficiency) is to be investigated. For this, it is necessary to perform a series of preliminary experiments to separately estimate the effect of each operating variable on the response variable.
Given the importance of the initial concentration of hydrogen peroxide for dye removal efficiency, a first series of experiments were performed in which the initial concentrations of Fe2+, Fe3+, and polyphenols were kept constant and H2O2 concentration varied between 0 and 1.5 × 10−2 M. It can be seen that in the absence of hydrogen peroxide, dye removal is barely above 60%, whereas with [H2O2]o = 2 × 10−3 M, dye removal can be as high as 85% or 82% for chrysoidine and methylene blue, respectively. Increasing this initial concentration to 3.75 × 10−3 M results in removing approximately 92% of the dye present in the solution. A further increase of [H2O2]o to 7.5 × 10−3 M or 1.5 × 10−2 M does not result in a higher removal efficiency of any of the dyes. Rather, the removal efficiency slightly decreases in both cases. This effect, repeatedly described in the literature, could be attributed to the generation of an excess of hydroxyl radicals at overly high hydrogen peroxide levels, which could recombine and reverse the iron-mediated catalytic degradation process [83]:
It is also possible that such radicals can be inactivated by reacting with excess hydrogen peroxide to form hydroperoxide radicals (HO2·), which have a lower oxidation potential than hydroxyl radicals, according to the reaction.
In light of the above, it was decided to use a hydrogen peroxide concentration of 2 × 10−3 M as the starting point for the experimental design, considering that an increase in [H2O2]o from 2 × 10−3 M to 3.75 × 10−3 M resulted in only a 6–7% increase in dye removal, as shown in Figure S1a.
The effect of varying the concentration of polyphenols in the solution on the removal of dye is also shown in Figure S1a. For the sake of brevity, only data corresponding to chrysoidine are depicted, as those corresponding to methylene blue are very similar. Solutions containing the amount of tea extract necessary to achieve a polyphenolic concentration between 0 and 240 mg·L−1 were used. Looking at the results shown in the graph, this variable in principle has a very limited influence on dye-removing efficiency. For this reason, and given the initial approach to the experimental design, it was decided to start from an intermediate concentration between the two values, that is, to use a polyphenol concentration of 100 mg·L−1 as the central point of the design.
The other two operating variables selected in this study were the initial concentrations of Fe(II) and Fe(III) cations. These cations have the dual function of catalyzing the decomposition of hydrogen peroxide into hydroxyl radicals (initial reaction of the Fenton and Fenton-like processes) and generating Fe nanoparticles through reduction by the polyphenols present in the green tea extract.
Figure S1b shows the influence of both variables on the removal efficiency of dye. The initial concentrations of H2O2 and polyphenols were kept constant at 2 × 10−3 M and 100 mg·L−1, respectively. Previous studies have shown that the optimal conditions for carrying out the advanced oxidation of organic pollutants through the integration of Fenton and Fenton-like processes correspond to a Fe (II):Fe (III) ratio between 1 and 2 [84]. Considering the results shown in Figure S1b, it can be concluded that the concentration of iron ions, regardless of their oxidation state, has little influence on the removal efficiency of dye. It remains constant at around 92–94% in all cases. Thus, it was decided to focus the experimental design on a Fe (II) concentration of 6 × 10−4 M and a Fe (III) concentration of 3 × 10−4 M, i.e., an Fe (II):Fe (III) ratio equal to 2. In the tests shown in Figure S1b, where the initial concentration of one of the two Fe cations was 0, the other was present at the concentration corresponding to the central point, since in the total absence of Fe ions, there would be neither the catalytic decomposition of H2O2 nor the formation of nanoparticles, and consequently, no pollutant removal would be achieved.
3.6. Analysis of the Statistical Design of Experiments
Table 2 lists the results of the removal efficiency of chrysoidine G and methylene blue for each of the 32 experiments that constitute the experimental matrix.

Table 2.
Experimental matrix and removal efficiency of chrysoidine G and methylene blue.
3.6.1. Numerical Analysis
The analysis of the results in an experimental design consists of two parts: numerical analysis and graphical analysis. The numerical analysis can be divided into three sections: (i) analysis of variance; (ii) derivation of a regression equation, with the corresponding analysis of its correlation coefficients; and (iii) determination of the experimental conditions that result in an optimum for the objective variable.
The analysis of variance, commonly known as ANOVA (from its initials in English), allows for testing the significance of each of the effects or variables in the experimental matrix. To do this, the parameter p is analyzed. If p is less than 0.05, then the operational variable in question has a statistically significant effect on the objective variable with a 95% probability. If it is greater than 0.05, then that effect is not statistically significant at that confidence level. Table S2 summarizes the result of the analysis of variance. From this Table, several highly relevant facts can be observed concerning the removal of chrysoidine. Firstly, it stands out that there are five effects with a p-value of less than 0.05, indicating that they have a statistically significant influence on the target variable with a 95% confidence level. Secondly, the R-squared statistic, obtained through a linear fit of the experimental values compared to those obtained from the experimental design, indicates that the model resulting from the analysis of the experimental design can explain 88.22% of the variability in the percentage of chrysoidine elimination.
Another determined parameter is the estimated standard error, which shows the standard deviation of the residuals. In this specific case, its value is 3.02, with the residual value being the difference between the observed value and the predicted value by the experimental design. Additionally, the calculation of the mean absolute error (1.59) was performed, which is defined as the average value of the residuals used to determine the previous parameter.
Lastly, the Durbin–Watson (DW) statistic is noteworthy, as it determines if there is a significant correlation among the data presented in Table 2, based on the order in which the data appear in the file. Since the p-value of this statistic is greater than 5.0%, it can be concluded that there is no autocorrelation in the residuals. In other words, the order in which the experiments comprising the matrix were carried out does not influence the obtained results in any way.
Regarding the removal of methylene blue, eight effects have a p-value below 0.05 and, hence, they have a statistically significant influence on the target variable. The R-squared statistic is as high as 93.21%, which suggests that the proposed model is suitable to fit the experimental data. The values of estimated standard error (6.16) and mean absolute error (3.66) corroborate this assertion. Again, according to the DW statistic and its p-value, no autocorrelation in the residuals is observed.
Once it has been determined through the ANOVA test that the model is suitable for effectively representing the influence of the study variables, given their p-values, it is possible to obtain the coefficients of the polynomial that fits the experimental values, according to a general equation:
y = βo + ∑i = 1nβi⋅Xi + ∑i = 1nβji⋅Xj⋅Xi + ∑i = 1nβii⋅X2i
In this particular case, the general equation is specialized into the following quadratic equations:
for the removal of chrysoidine, and
for the removal of methylene blue.
Removal %(Chrysoidine) = 88.8425 − 0.572917⋅[Fe2+] + 0.0670833⋅[Fe3+] + 6.41708⋅[H2O2] + 0.03625⋅[Polyphenols] + 1.38927⋅[Fe2+]2 − 0.118125⋅[Fe2+]⋅[Fe3+] − 0.231875⋅[Fe2+]⋅[H2O2] − 0.530625⋅[Fe2+]⋅[Polyphenols] + 1.52177⋅[Fe3+]2 − 0.154375⋅[Fe3+]⋅[H2O2] + 0.661875⋅[Fe3+]⋅[Polyphenols] − 2.14198⋅[H2O2]2 − 0.231875⋅[H2O2]⋅[Polyphenols] − 2.09948⋅[Polyphenols]2
Removal %(Methylene blue) = 85.1758 + 2.32172⋅[Fe2+] + 5.57271⋅[Fe3+] + 9.99818⋅[H2O2] − 14.5478⋅[Polyphenols] − 3.62988⋅[Fe2+]2 − 0.985287⋅[Fe2+]⋅[Fe3+] + 3.83218⋅[Fe2+]⋅[H2O2] − 1.92516⋅[Fe2+]⋅[Polyphenols] − 2.17238⋅[Fe3+]2 + 2.55629⋅[Fe3+]⋅[H2O2] + 2.71068⋅[Fe3+]⋅[Polyphenols] − 2.95788⋅[H2O2]2 + 7.66436 ⋅[H2O2]⋅[Polyphenols] − 2.79897⋅[Polyphenols]2
According to the coefficients shown in Equation (10), the concentration of hydrogen peroxide has the greatest positive influence on the target variable, with a value of +6.41708. This means that an appropriate concentration of hydrogen peroxide is crucial for achieving maximum elimination. However, it is worth noting that an excess of this concentration (reflected by the quadratic term of hydrogen peroxide concentration) is undoubtedly detrimental to the proper removal of the dye, with a value of −2.14198. This is in coherence with Equations (7) and (8). Another significant influence is worth highlighting. According to the obtained data, an excess of both iron solution concentrations (Fe2+ and Fe3+) has a favorable effect on achieving maximum removal of chrysoidine. This can be deduced from the values accompanying the quadratic terms of iron (II) and iron (III) solutions, which are +1.38927 and +1.52177, respectively. To conclude with the variables of major influence, a coefficient of -2.09948 can be observed in the quadratic term of polyphenol concentration, indicating that an excess of these compounds greatly hinders dye removal. A plausible explanation for this fact is related to the ease of oxidation of polyphenols by ·OH radicals. In other words, an excess of polyphenols may exert a scavenging role on ·OH radicals, thus decreasing the global oxidation of the pollutant.
Lastly, it can be observed that the other variables or combinations of variables also have both positive and negative coefficients in the regression equation. However, compared to the previously described coefficients, these values do not have a decisive influence on the target variable. All of the above perfectly aligns with the results obtained in the ANOVA test, as the variables with the greatest influence are those with a p-value less than 0.05 in the analysis, thereby determining a significant influence on the elimination of crystal violet in ultrapure water.
Regarding the removal of methylene blue, again, the concentration of hydrogen peroxide also plays a crucial role in the process. Interestingly, the concentration of polyphenols, as well as the quadratic terms of the concentrations of iron (II) and iron (III), exert a detrimental role in the removal of dye in this case. This suggests an important contribution of the Fenton and Fenton-like processes in the degradation of methylene blue, where Fe (II) and Fe (III) act as catalysts. In the presence of large amounts of polyphenols, these cationic species rapidly reduce into nZVI, with the subsequent loss of efficiency. However, an excess of iron salts, particularly Fe2+-, leads to a decrease in the removal percentage of dye. A plausible explanation for this could be the well-known scavenging ability of Fe2+ cations towards hydroxyl radicals:
Once the regression Equations (10) and (11) have been obtained, thanks to the capabilities of statistical experimental design, it is possible to theoretically predict the experimental conditions that would lead to the optimization (in this case, maximization) of the target variable. The optimal coded values of the operational variables that lead to this theoretical optimum are shown in Table 3.

Table 3.
Coded values corresponding to the maximum removal efficiency of dye.
For the removal of chrysoidine, the model has predicted three possible optima. Therefore, at least theoretically, by operating under those optimal conditions, complete elimination of the dye should be achieved. The experiments were carried out under the optimal conditions provided by the design, resulting in elimination percentages of 98.9% for optimum 1, 99.2% for optimum 2, and 97.3% for optimum 3. These data reflect that although the different optima do not yield the same elimination percentage, they do achieve a higher degree of elimination compared to any of the combinations in the experimental matrix (see Table 2), thus approaching the objective of the model, which is to achieve the highest possible elimination of chrysoidine.
One important aspect to consider in the obtained results from the different theoretical optima is the concentrations of certain variables, such as hydrogen peroxide. Due to the high cost of this reagent, it is advisable to use the lowest possible concentration while still ensuring the desired elimination efficiency. For this reason, considering the very similar elimination values obtained for optimum 1 and 2, from a practical point of view, the values obtained for optimum 1 are more convenient, achieving similar elimination values under more favorable conditions. Specifically, the conditions that result in maximum elimination of chrysoidine (98.9%) using the lowest concentration of hydrogen peroxide are obtained by decoding the values in Table 3 for optimum 1, which are as follows: [Fe2+] = 4.17 × 10−4 M; [Fe3+] = 5.89 × 10−4 M; [H2O2] = 3.01 × 10−3 M; and [Polyphenols] = 120 mg/L (gallic acid equivalents).
It is worth noting that these conditions lead to a double cost reduction in the process. Firstly, the molar ratio of Fe(II) to Fe(III) is decreased to values below even 1:1. Specifically, a Fe(II):Fe(III) ratio of 0.71:1 is used, while the literature suggests ratios between 1:1 and 2:1. Since Fe(III) salts have a lower price compared to Fe(II) salts, this results in lower reagent costs. Secondly, the molar ratio of total iron (Fe, including Fe2+ and Fe3+) to hydrogen peroxide is also reduced. The H2O2:(Fe2+ + Fe3+) ratio is around 3, while many sources in the literature use much higher ratios, reaching 9:1 or 10:1 (see [84] and references therein). Considering that hydrogen peroxide is the most expensive reagent in the process, a decrease in the required amount leads to a significant reduction in operating costs.
For the removal of methylene blue, the real values corresponding to a maximum removal efficiency are [Fe2+] = 5.39 × 10−4 M; [Fe3+] = 3.17 × 10−4 M; [H2O2] = 3.04 × 10−3 M; and [Polyphenols] = 77 mg/L (gallic acid equivalents). Again, the model predicts that operating under these conditions, total removal of the dye should be achieved. An experiment was conducted under those optimal conditions, resulting in a dye elimination percentage of 99.1%, which is very close to the total elimination predicted by the model.
In this case, the molar ratio of Fe(II) to Fe(III) equals 1.7, in line with the results previously reported in the literature [84]. Furthermore, as per the removal of chrysoidine, the H2O2:(Fe2+ + Fe3+) ratio is around 3, which also involves a significant reduction in operating costs.
3.6.2. Graphical Analysis
The Pareto chart of factors shows the greater or lesser influence of each study variable on the target variable, as well as the statistical significance of each variable. Therefore, it can be considered, to some extent, as a graphical representation of the ANOVA test. The horizontal bars represent the effect of each variable, namely the concentration of Fe2+, the concentration of Fe3+, the concentration of H2O2, and the concentration of polyphenols, as well as all their combinations. Figure 8a shows the Pareto chart for the elimination of chrysoidine.
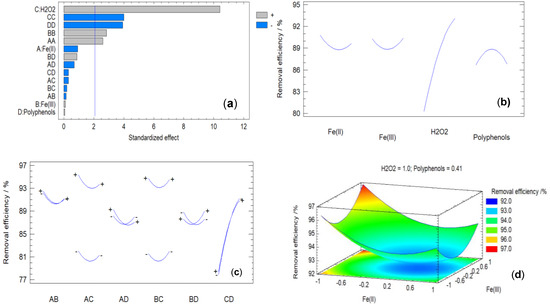
Figure 8.
Graphical analysis of the experimental design corresponding to the removal of chrysoidine: (a) Pareto factor plot; (b) Main effects plot; (c) Interactions plot; (d) Response surface plot.
The vertical line seen in Figure 8a corresponds to a p-value of 0.05. Factors whose bars exceed this line are considered to have a statistically significant effect on the dye elimination efficiency with a probability of 95%. As indicated earlier, these variables appear in the ANOVA test table (Table S2) with a value below 0.05. Additionally, the graph shows the type of influence exerted by each considered variable, i.e., whether its effect on the elimination of chrysoidine is positive or negative. Variables represented by gray bars have a positive effect on the target variable, while those represented by blue bars have a negative effect on the target variable.
Note that factors that have a positive influence on the dye elimination efficiency appear in Equation (10) preceded by a “+” sign, while factors that have a negative influence on the response variable are preceded by a “−” sign in the equation.
The Pareto chart allows for a more direct interpretation of the results discussed earlier in the ANOVA test. The five variables with the greatest influence and the degree of each variable demonstrate that an appropriate concentration of hydrogen peroxide has a very significant influence on the target variable. However, an excess of this concentration (CC bar) achieves the opposite effect. This is because the function of hydrogen peroxide in the elimination of dye lies in carrying out Fenton or Fenton-like radical processes. As indicated above, when the concentration of hydrogen peroxide is too high, a large amount of hydroxyl radicals can recombine with each other, reversing the catalytic decomposition process mediated by iron and regenerating the H2O2 molecule [84] according to reaction (7). It is also possible that these radicals deactivate by reacting with the excess of hydrogen peroxide, forming hydroperoxide radicals (HO2·), which have lower oxidation potential than hydroxyl radicals.
⋅OH+H2O2→ ⋅OOH+H2O
In this case, however, a sufficient concentration of hydrogen peroxide has not been reached for these scavenging reactions to play a predominant role compared to the catalytic decomposition of peroxide by cations such as Fe2+ or Fe3+, typical of Fenton or Fenton-like processes, respectively.
Another variable that has a negative influence is the excess of polyphenolic compounds. The explanation for this may lie in the antioxidant nature of these compounds. Additionally, the hydroxyl groups present in phenolic compounds confer chelating properties towards iron and other transition metals. Therefore, an excess of polyphenols could lead to the inhibition of Fenton reactions by reducing the amount of available iron to participate in the chain reactions that constitute the Fenton process. Furthermore, flavonoids present in tea extracts are effective scavengers of hydroxyl radicals (·OH) [85]. The scavenging capacity of these free radicals derives from the fact that their reduction potential is lower than that of the radicals generated in the Fenton process.
Another representation of great interest in the graphical analysis of the experimental design is the so-called main effects plot (Figure 8b). It allows us to observe the influence of the different operational variables individually (i.e., concentrations of Fe2+, Fe3+, hydrogen peroxide, and polyphenols) on the target variable. The plot represents the system’s final response when only one of these variables is modified while keeping the other three at their central values constant. This way, one can visually see which variable involved in the process has a stronger impact on the response. Based on Figure 8b, it can be concluded that the most influential variable is undoubtedly, as reflected in the previous data, the concentration of hydrogen peroxide. There is also an observed peak elimination at the concentration of polyphenols, as explained earlier.
Lastly, although they have a lesser influence, the concentrations of iron salts are of great importance as they both exhibit a minimum elimination and higher values as their concentrations increase or decrease. This is because both Fe3+ and Fe2+ can initiate Fenton-like reactions and lead to the formation of iron nanoparticles. These two processes generate a synergistic effect, as will be discussed later.
The reason for the need to increase or decrease the concentration of one of the iron salts is to maintain an excess of the other. One possible explanation for this observation lies in the fact that the formation of nanoparticles is somewhat favored for Fe3+ cations, but these cations give rise to “Fenton-like” reactions, which are less effective in dye elimination due to their lower oxidation power and slower kinetics. On the other hand, Fe2+ cations contribute to the development of the Fenton process, which is effective in the destruction of the dye [86].
Another graph of great interest in the analysis of experimental design data is the interaction plot (Figure 8c). Studying the presence (or absence) of interactions between the working variables is one of the key advantages of statistical experimental design and justifies, from a methodological point of view, its use over the traditional method of modifying “one variable at a time”. This method is valid if it is assumed that there are no interactions between variables, meaning that a change in one variable does not affect how the other variables influence the target variable.
In the interaction plot, each pair of curves represents the variation of the dye removal percentage by changing one variable at the extremes of the design, i.e., with its value equal to +1.0 (maximum value, marked with the “+” sign) and equal to −1.0 (minimum value, marked with the “−” sign), while the other variables are held constant at their central value. If the curves of a variable pair are parallel, modifying one variable does not affect the other. On the other hand, if two lines intersect, the interaction is evident. There can also be an intermediate situation where no clear behavior of the aforementioned types is observed. The data shown in Figure 8c reaffirm the hypotheses described earlier, demonstrating a significant interaction between the concentration of polyphenols and hydrogen peroxide. Additionally, a slight interaction between the iron (II) and iron (III) concentrations can also be observed, which, as previously mentioned, although not remarkable, does exist.
Among all the graphs analyzed in this section, perhaps the most important one is the response surface plot. From a mathematical standpoint, it is a graphical representation of Equation (10) (regression equation and correlation coefficients). From a results analysis perspective, it provides data along two different lines: the specific estimation of the optimum and the differential influence of each study factor. It allows for a qualitative understanding of the behavior of the entire system under study, the shape of the function defining the mathematical model, which factors are more influential than others, and in which regions of the studied space this influence is increased or decreased. To obtain the response surface plot, two out of the four variables must be fixed while modifying the other two, and then the efficiency of dye removal against both operational variables is graphically represented in the form of a surface plot. Furthermore, this graph allows us to observe the region where optimal elimination (above 95%) is achieved. In other words, it visualizes the value of the optimum 1 mentioned earlier (see Table 2). By keeping the values of initial concentrations of hydrogen peroxide and polyphenols fixed and equal to those corresponding to the optimum, Figure 8d has been plotted.
Figure S2 (Supplementary Material) shows the results of the graphical analysis corresponding to the removal of methylene blue. Most of the considerations made when dealing with the removal of chrysoidine are also applicable to the removal of methylene blue so these results will be discussed more succinctly.
One of the most relevant observations made by the Pareto diagram, Figure S2a, is that while the concentrations of Fe(II), Fe(III), and hydrogen peroxide have a positive influence on the elimination of methylene blue (i.e., increasing these concentrations increases the efficiency of the process), an increase in the initial concentration of polyphenols leads to a decrease in the percentage of dye removed. One possible explanation for this is that the use of polyphenols as reducers in the synthesis of iron nanoparticles removes Fe(II) and Fe(III) ions from the solution through their reduction and subsequent formation of nanoparticles. This, in turn, implies a decrease in the efficiency of the Fenton process since part of the catalyst necessary for the decomposition of hydrogen peroxide and the generation of hydroxyl radicals is being removed. However, it is worth mentioning that experimenting in the complete absence of hydrogen peroxide (where all dye removal from the medium would be attributable to its adsorption on the iron nanoparticles) achieves an elimination efficiency of 53% (experiment 21 in Table 1), which is much higher than other cases previously reported in the literature [87,88,89]. Additionally, polyphenols play an important role in the elimination of methylene blue, not so much in terms of the efficiency of elimination but rather in terms of the kinetics of the process.
Indeed, based on Figure S2b, it can be concluded that an increase in the concentration of polyphenols leads to a lower efficiency of the dye removal process throughout the entire working range, as indicated by the steep negative slope of the corresponding line. On the other hand, the concentrations of Fe(II), Fe(III), and hydrogen peroxide appear to have a positive effect on the elimination of methylene blue. It is noteworthy that in the case of Fe(II) concentration, a maximum is reached beyond which a further increase in concentration has a negative impact on dye removal. For the concentrations of Fe(III) and H2O2, although the positive influence gradually diminishes, no maximum is observed.
This is particularly important considering the well-known regeneration reaction of H2O2 from ·OH radicals, as shown in Equation (7), or the deactivation of such radicals through reactions with excess hydrogen peroxide, as shown in Equation (13). However, similar to the case of chrysoidine, a sufficient concentration of hydrogen peroxide has not been achieved for these scavenging reactions to play a predominant role.
The interaction plot, Figure S2c, suggests that the only pair of variables that clearly do not exhibit an interaction with one another are the concentration of Fe(II), denoted as A, and the concentration of Fe(III), denoted as B. In this case, the lines are parallel, indicating no interaction between these variables. In the remaining cases, although the lines do not intersect (or at least not within the graphically represented interval), marked differences in the slopes of the “+” and “−” lines can be observed. This is indicative of the presence of interactions between the variables. This interaction is particularly evident in the case of polyphenols (D) with Fe(III) (B), and especially, as expected based on previous discussions, with hydrogen peroxide (C). Indeed, since the addition of polyphenols removes the catalyst from the system (Fe2+ and Fe3+ ions), the role of H2O2 as a generator of hydroxyl radicals is noticeably affected.
Figure S2d shows the response surface plot corresponding to the removal of methylene blue. In this case, both iron ion concentrations have been fixed at their optimal values (see Table 2), while the concentrations of hydrogen peroxide and polyphenols were varied, and the change in the efficiency of methylene blue elimination was represented accordingly. It is striking how strongly the concentration of hydrogen peroxide influences the elimination efficiency, especially at low (−1 and close to −1) polyphenol concentrations. Similarly, in the absence of hydrogen peroxide, polyphenols have a significant effect on the response variable. This is evident from the steep slope of the response surface curve at both extremes. In contrast, at coded values below −0.5 for polyphenol concentration and above 0.5 for H2O2 concentration, the response surface is nearly flat, and elimination efficiencies close to 100% are achieved (intense red color zone).
From all of this, it can be inferred that by carefully selecting a working interval or optimal experimental conditions, the proposed method combining iron nanoparticle adsorption and radical oxidation through the Fenton (and/or “Fenton-like”) process is capable of effectively removing methylene blue from aqueous solutions, achieving elimination efficiencies close to 100%.
3.7. Contribution of Fe0 Nanoparticles to the Elimination Process of Dyes
As indicated in the previous section, it can be observed that experiment number 21 in Table 1, which is carried out in the total absence of hydrogen peroxide (coded value equal to −2), results in a removal efficiency equal to 61.5% and 53.0% for chrysoidine and methylene blue, respectively. Therefore, in this experiment, dye removal is solely attributed to the effect of iron nanoparticles.
In the literature, numerous references can be found regarding the elimination of dyes in general, and azo dyes in particular, through treatment with iron nanoparticles. However, at the time of writing this work, to the best of the authors’ knowledge, there is no existing literature on the use of iron nanoparticles for the elimination of chrysoidine in solution. Several of the consulted studies suggest that the removal of the dye in solution is mediated by adsorption processes. For example, García et al. [90] investigated the removal of the azo dye Reactive Black 5 through adsorption by iron nanoparticles and biomass of the seaweed Macrocystis pyrifera. According to these authors, elimination efficiencies between 69% and 80% can be achieved by the combined use of biomass and nanoparticles, and they attribute this high removal capacity to the immobilizing effect of the biomass on the nanoparticles. Additionally, Lin et al. [91] also attribute the high removal rates of another azo dye, Acid Black 24, to adsorption phenomena by micro- and nano-sized Fe0 particles. According to these authors, an increase in particle size leads to lower efficiency in removing the contaminant due to the subsequent decrease in specific surface area. However, many more authors justify the high removal efficiency of azo dyes by iron nanoparticles based on their high reactivity, specifically their ability to break the azo group’s double bond (N=N). This bond cleavage, according to some authors [92,93], would be conditioned by a two-stage process. The first stage, which is reversible, involves the oxidation of Fe0 within the nanoparticle to Fe2+ and/or Fe3+ and the addition of hydrogen to the azo group’s double bond (N=N), resulting in the formation of an unstable reaction intermediate. The second stage involves the cleavage of the bond and the formation of two aminated compounds. Schematically, the process can be represented as in Scheme 1:
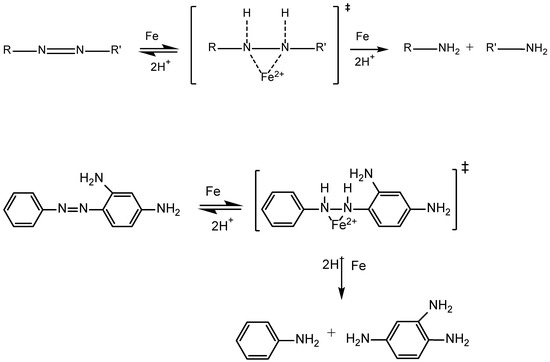
Scheme 1.
Possible mechanism of azo group cleavage by Fe0 nanoparticles (above) and particularization in the case of chrysoidine G. ‡ denotes a unstable reaction intermediate.
Other authors propose a combined mechanism in which the dye removal occurs through both adsorption phenomena and the cleavage of the azo group’s double bond mediated by iron nanoparticles [94,95,96]. Thus, the process can be described in five stages: (i) transfer of a dye molecule from the bulk solution to the vicinity of the iron nanoparticle surface; (ii) adsorption of the dye onto the nanoparticle surface; (iii) cleavage of the azo group’s double bond (N=N) through the chemical reaction described above; (iv) desorption of the reaction products from the nanoparticle surface; and (v) transfer of the reaction products from the vicinity of the nanoparticle back into the bulk solution. This overall process is schematically represented in Figure 9.
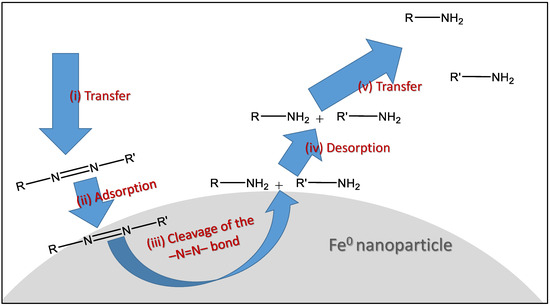
Figure 9.
Stages in the degradation of an azo dye by Fe0 nanoparticle-catalyzed double bond cleavage.
To verify whether the elimination process of chrysoidine by Fe0 nanoparticles was solely due to adsorption or if there was also a contribution from the catalytic cleavage of the azo group, a sample of nanoparticles was prepared and then brought into contact with the dye solution without the addition of hydrogen peroxide, thus excluding the contribution of the Fenton process to chrysoidine degradation. The prepared nanoparticles were filtered and dried. Subsequently, the infrared spectrum of a portion of these nanoparticles alone and in contact with the dye was recorded. For comparative purposes, the spectrum of the dye itself was also recorded, along with the spectrum of a sample of nanoparticles that were not in contact with chrysoidine.
Figure S3 (Supplementary Material) represents the FT-IR spectrum of the dyes. Various bands can be observed in the spectrum, and their assignment is provided below. Some of these bands are common to both dyes. For instance, the bands centered around 1600 and 1390 cm−1 correspond to the stretching vibration of the –C=C– bonds in the aromatic ring. Additionally, the characteristic bands of primary amino groups can be seen, centered around 3400 and 3300 cm−1, which can be attributed to the symmetric and asymmetric stretching vibrations of the N–H bonds. Finally, the bands centered around 1240–1250 cm−1 correspond to the vibrations of C–N bonds in aryl amines.
Regarding chrysoidine G in particular, the most characteristic spectral features of azo dyes are the bands located at 1509 and 1158 cm−1, which are typical of the azo (–N=N–) chromophore.
In the case of methylene blue, the band centered at 1600 cm−1 can also be due to CH=N stretching. –C–N and N–N stretching is the cause of the peaks that appear at 1252 cm−1 and 1224 cm−1, respectively. A typical spectral feature of methylene blue is the band centered at 1064 cm−1, which is attributable to the C–S–C bonds. Finally, the out-of-plane bending vibrations of C–H bonds give rise to the bands located around 890 cm−1 and 840 cm−1.
On the other hand, Figure 10 displays the FT-IR spectra corresponding to the nanoparticles both before and after their contact with the dyes. The spectra of the nZVI sample in contact with chrysoidine G show a remarkable similarity. The spectrum of the nanoparticles without the dye displays distinct bands at 3305, 2330, 1613, and 1018 cm−1, which are known to be characteristic of iron nanoparticles according to sources in the literature [97,98]. Notably, the two spectra are almost identical, and there are no noticeable peaks related to the dye. If the dye had been adsorbed onto the iron nanoparticles, the main bands seen in the spectrum of the dye (Figure S3) would have been observed in the nanoparticles’ spectrum after contact. However, this is not the case, which unequivocally rules out the existence of adsorption processes between the iron nanoparticles and chrysoidine. These findings align with previously published studies on the removal of other azoic dyes using Fe0 nanoparticles [96,99,100].
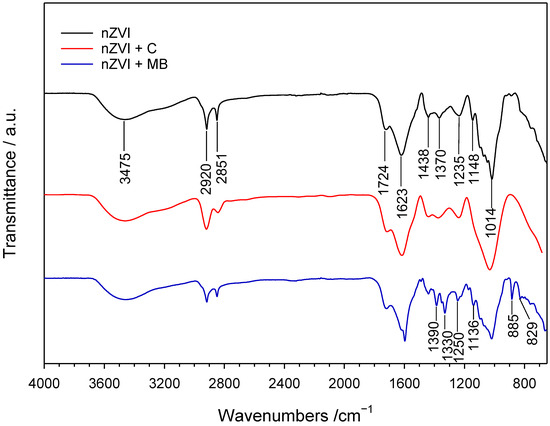
Figure 10.
FT-IR of nZVI before and after contact with chrysoidine-G (C) and methylene blue (MB).
In contrast, the FTIR spectrum of the nZVI sample exposed to methylene blue indicates that adsorption plays a significant role in the dye removal process. The spectrum of nZVI after contact with the dye solution exhibits several spectral features corresponding to methylene blue. Specifically, the bands originally observed in the methylene blue spectrum at 1392, 1335, 1252, 1174, 1139, 889, and 837 cm−1 are also evident in the spectrum of nZVI used for treating the methylene blue solution. However, in this latter case, these bands appear at slightly lower wavenumbers, ranging between 2 and 8 cm−1 lower. This indicates a weakening of the bonds in the respective organic moieties, as a consequence of the presence of interactions between the adsorbed dye molecules and the active sites of nZVI, which behave as a sorbent.
3.8. Contribution of the Fenton/Fenton-like Processes to the Removal of Dyes
In the previous section, the contribution of Fe0 nanoparticles to the elimination process of the dye in aqueous solution was analyzed. As mentioned before, the results of the experimental design are presented in Table 1. Experiment number 10 was conducted in the absence of tea extract, and therefore, the total elimination of chrysoidine G or methylene blue (74.4% and 85.5%, respectively) can be attributed to the oxidation of the molecule through Fenton and Fenton-like processes, considering that the reducing action of the polyphenols present in the extract is essential for nanoparticle formation.
In the case of chrysoidine G, there are numerous studies on the possible degradation mechanisms of azoic dyes in general using Fenton-like processes (see, for example, [101], and the references cited therein). However, for chrysoidine G in particular, information is much more limited.
Firstly, it is worth noting that in the case of the “Fenton-like” process, the initial reactions that occur are:
Fe2+ can then react with hydrogen peroxide to generate hydroxyl radicals:
This latter reaction constitutes the first stage of the conventional Fenton process. Therefore, according to the literature, the Fenton-like process can be considered analogous to Fenton but with a certain delay in its initial stages.
Schematically, the main reactions leading to the generation of radicals from the catalytic decomposition of hydrogen peroxide in the presence of Fe2+ are shown in Figure S4 (Supplementary Material).
However, proposing a general mechanism for the degradation of azoic dyes through attack by hydroxyl radicals is far from a simple task. In any case, it seems that there is a commonly accepted agreement that these radicals attack the azo group’s double bond (–N=N–), either through hydrogenation or hydroxylation of the double bond. The first stage of both processes is illustrated in Scheme 2.
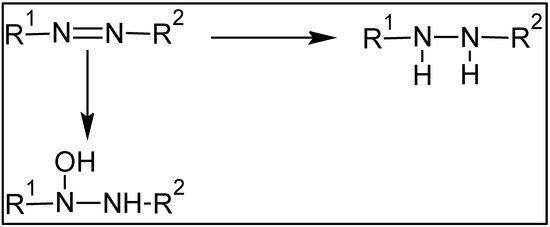
Scheme 2.
Processes of breaking the double bond of the azo group by radical attack. Hydrogenation (top) and hydroxylation (bottom).
Next, the hydroxylated or hydrogenated molecule undergoes successive bond cleavages, leading to the formation of various reaction intermediates that, in the case of chrysoidine, contain oxygenated benzene rings. These intermediate compounds can undergo further oxidation with ring opening, ideally leading to complete mineralization into CO2 and H2O [101].
For methylene blue, the earliest stage of degradation by ·OH radicals can proceed through ring- or sulfur-hydroxylation, methyl-oxidation, or N-demethylation (see Scheme 3), with probably concurrent participation of the three processes [102].
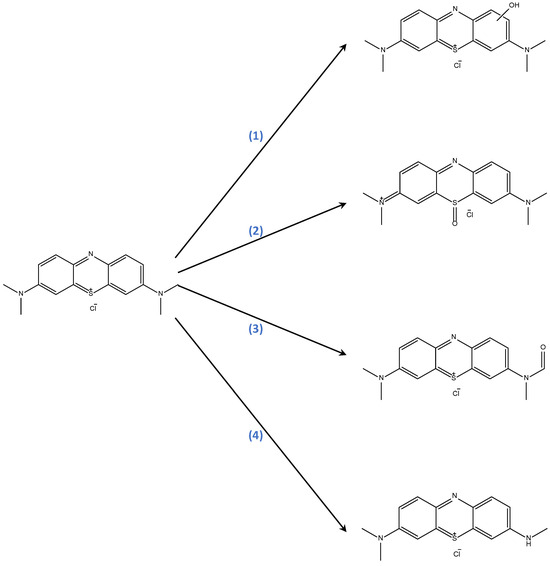
Scheme 3.
Plausible initial stage of the ·OH-mediated degradation of methylene blue in the Fenton process. (1) ring-hydroxylation; (2) sulfur-hydroxylation; (3) methyl-oxidation; (4) N-demethylation.
Elucidating the mechanism and determining the reaction intermediates is a laborious and not always feasible task. In this particular case, where iron nanoparticles have been generated in situ, that is, in the presence of an extremely complex mixture of polyphenols and other molecules initially present in the green tea leaf extract, amd can also undergo bond cleavage, trying to propose a degradation pathway would involve a remarkably speculative procedure.
3.9. Synergistic Effect of Nanoparticle Formation and Advanced Oxidation Processes on the Removal of Dyes
The experimental results presented in Table 2 demonstrate that the elimination of dyes using only Fe0 nanoparticle formation (Experiment 21) reaches 61.5% for chrysoidine and 53.0% for methylene blue. In contrast, when exclusively applying radical oxidation through Fenton and/or Fenton-like processes (Experiment 10), the elimination achieves results of 74.4% for chrysoidine and 85.5% for methylene blue. However, when both procedures are combined, the contaminant can be eliminated by up to 96.4% (Experiment 12 for chrysoidine) or 97.5% (Experiment 9 for methylene blue), which represents almost complete removal. Therefore, it can be affirmed that under appropriate conditions, a synergy can be created between radical oxidation processes and dye degradation mediated by the presence of iron nanoparticles. For methylene blue, as indicated above, adsorption of the dye onto the surface of nZVI also contributes to the removal efficiency [54,103].
The use of Fe0 nanoparticles as heterogeneous catalysts in Fenton-like processes for contaminant degradation significantly reduces the problems encountered in homogeneous Fenton processes (where the iron catalyzing hydrogen peroxide decomposition is in the form of Fe2+ and/or Fe3+ salts in solution). These drawbacks include generation of large amounts of iron sludge after treatment that needs to be disposed of; consumption of significant quantities of Fe2+ and hydrogen peroxide, with the molar ratio of H2O2 to Fe2+ reaching 9:1; the requirement to operate under acidic conditions (pH < 3) to prevent the precipitation of iron salts as hydroxides or oxohydroxides; and the need for the neutralization of initially acidified solutions before disposal as waste [104,105].
In contrast to these drawbacks, the use of Fe0 nanoparticles as heterogeneous catalysts offers significant advantages, such as virtually no precipitation of iron hydroxides or oxyhydroxides since the concentration of Fe2+ or Fe3+ ions in solution is much lower; easy separation of the catalyst after application; possibility of catalyst reuse in certain cases; and an extended pH range for operation [106].
However, the use of nanoparticulate Fe0 as a heterogeneous catalyst in Fenton and Fenton-like processes does have some disadvantages. One of the most important is that the contaminant molecules need to transfer from the solution to the surface of the material to reach the active sites for degradation. Additionally, the reactions involved in these heterogeneous processes are usually slower compared to conventional homogeneous processes at the same concentrations. On the other hand, heterogeneous reactions are generally more efficient in terms of H2O2 consumption.
Regarding the oxidation mechanism, according to the literature [106], it can be proposed that oxidation occurs through two different mechanisms: (i) a process catalyzed homogeneously by iron ions released into the solution; and (ii) heterogeneous reactions between the solutes and the surface groups of iron nanoparticles.
The reactions involved in the heterogeneous catalytic Fenton process by Fe0 nanoparticles are schematically represented in Figure 11.
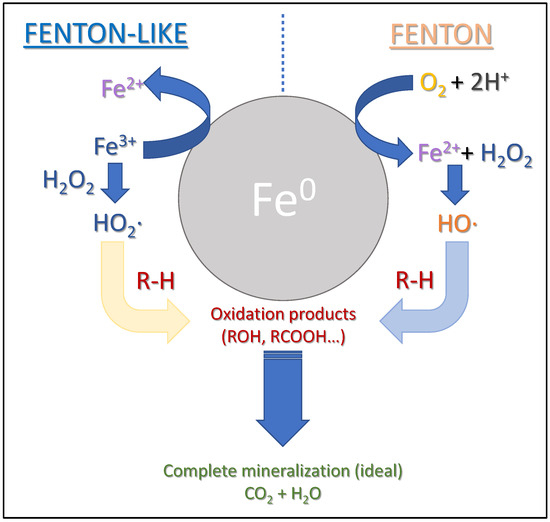
Figure 11.
Schematic representation of the reactions involved in the Fenton process that is heterogeneously catalyzed by Fe0 nanoparticles.
On the right side of Figure 11, the oxidation of Fe0 by O2 (under aerobic conditions) in the presence of an acidic medium is schematically depicted, resulting in the generation of Fe2+ and H2O2. On the left side of the Figure, the reduction of Fe3+ to Fe2+ mediated by Fe0 is illustrated by the reaction:
Once the Fe2+ ion is formed, either through one pathway or the other, the cascade of radical reactions known as the Fenton process begins, with its first stage being the catalytic decomposition of hydrogen peroxide, as mentioned earlier:
The hydroxyl radicals (·OH) contribute to the oxidation of the dye molecule. In other words, the Fe0/H2O/O2 system is capable of generating oxidizing species such as H2O2 and ·OH. Subsequently, the reactions would follow the typical mechanisms of the Fenton process to produce intermediate oxidized products or, ideally, complete mineralization of the organic compound. This explains the synergistic effect observed experimentally in the degradation of chrysoidine by the combined use of Fe0 nanoparticles and the reagents involved in the Fenton process.
4. Conclusions
By adding a solution of Fe (II) and Fe (III) salts to another solution containing tea extract, the in situ green synthesis of zero-valent iron nanoparticles (nZVI) has been successfully performed. Operating in this manner, stable iron nanoparticles were obtained without unwanted flocculation phenomena. The influence of four working variables, specifically the initial concentrations of hydrogen peroxide, polyphenols, Fe(II), and Fe(III) in solution, on the efficiency of removing two commonly used dyes in medicine, chrysoidine G and methylene blue, was analyzed. Preliminary tests indicated a significant influence of the hydrogen peroxide parameter on the overall process. A marked synergistic effect on dye removal was observed when combining the treatment involving the formation of Fe0 nanoparticles with the Fenton/Fenton-like process. For chrysoidine G, the degradation of the dye begins with the cleavage of the azo group’s double bond (–N=N–), followed by hydroxylation and/or hydrogenation. In a subsequent stage, the hydroxylated or hydrogenated molecule undergoes successive bond cleavages, resulting in various reaction intermediates containing oxygenated benzene rings. These intermediate compounds can undergo further oxidation with ring opening, ideally leading to complete mineralization into CO2 and H2O. The use of FT-IR spectroscopy has revealed that the removal of chrysoidine G by iron nanoparticles does not involve adsorption processes, where the dye remains retained on the surface of the nanoparticles. However, the adsorption of chrysoidine before the azo group’s cleavage mentioned in the previous paragraph cannot be completely ruled out. For methylene blue, adsorption onto the active sites of nZVI remarkably contributed to the removal of dye. Statistical analysis of the experimental results has confirmed that, as suggested by preliminary tests, the initial concentration of hydrogen peroxide is the factor that most significantly influences the efficiency of chrysoidine removal. Through the use of the statistical design of experiments, optimization of the dye removal process has been achieved. Under suitable conditions, it is possible to eliminate 98.9% of the initially present chrysoidine in solution, and 99.1% of methylene blue. Moreover, these conditions result in cost reduction by reducing the required amount of the two more expensive reagents, hydrogen peroxide and Fe(II) salts, by half.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app14156558/s1, Table S1. Coded and real values of the operational variables in the experimental design; Table S2. Results of the analysis of variance (ANOVA); Figure S1. Preliminary runs. Influence of H2O2 and polyphenol concentration (a) and Fe2+ and Fe3+ (b); Figure S2. Graphical analysis of the experimental design corresponding to the removal of methylene blue: (a) Pareto factor plot; (b) Main effects plot; (c) Interactions plot; (d) Response surface plot; Figure S3. FT-IR spectra of chrysoidine-G and methylene blue; Figure S4. Schematic representation of radical generation in Fenton and/or Fenton-like processes.
Author Contributions
Conceptualization, E.M.C.-C.; methodology, E.M.C.-C.; validation, E.M.C.-C., M.F.A.-F. and C.F.-G.; formal analysis, M.F.A.-F., C.R.-R. and C.F.-G.; investigation, C.R.-R., M.C.-P. and A.G.-T.; writing—original draft preparation, M.F.A.-F., C.F.-G. and E.M.C.-C.; visualization, C.R.-R., A.G.-T. and M.C.-P.; supervision, E.M.C.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Global Industry Analysts Inc. Pigments and Dyes—A Global Strategic Business Report; Global Industry Analysts Inc.: San Jose, CA, USA, 2023. [Google Scholar]
- Hossain, M.Y.; Liang, Y.; Pervez, M.N.; Ye, X.; Dong, X.; Hassan, M.M.; Cai, Y. Effluent-Free Deep Dyeing of Cotton Fabric with Cacao Husk Extracts Using the Taguchi Optimization Method. Cellulose 2021, 28, 517–532. [Google Scholar] [CrossRef]
- Masek, A.; Plota, A.; Chrzastowska, J.; Piotrowska, M. Novel Hybrid Polymer Composites Based on Anthraquinone and Eco-Friendly Dyes with Potential for Use in Intelligent Packaging Materials. Int. J. Mol. Sci. 2021, 22, 12524. [Google Scholar] [CrossRef]
- Swarnkar, K.; Nikam, V.; Gupta, K.; Pearson, J.M. Review of the State-of-the-Art for Monitoring Urban Drainage Water Quality Using Rhodamine WT Dye as a Tracer. ISH J. Hydraul. Eng. 2023, 29, 531–539. [Google Scholar] [CrossRef]
- Cascarano, R.N.; Reeves, D.M.; Henry, M.A. A Dye Tracer Approach for Quantifying Fluid and Solute Flux Across the Sediment–Water Interface. Groundwater 2021, 59, 428–437. [Google Scholar] [CrossRef]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium Dyes as Tools in Cell Biology: New Insights into Their Cellular Reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, R.; Chen, Y.; Liu, X.; Wang, K.; Mao, L.; Yuan, J.; Zhang, X.; Tao, L.; Wei, Y. A Polymerizable Aggrega-tion-Induced Emission Dye for Fluorescent Nanoparticles: Synthesis, Molecular Structure and Application in Cell Imaging. Polym. Chem. 2019, 10, 2162–2169. [Google Scholar] [CrossRef]
- Biyase, N.G. Contrast and Dyes. South. Afr. J. Anaesth. Analg. 2020, 26, S12–S16. [Google Scholar] [CrossRef]
- Wright, P.M.; Seiple, I.B.; Myers, A.G. The Evolving Role of Chemical Synthesis in Antibacterial Drug Discovery. Angew. Chem. Int. Ed. 2014, 53, 8840–8869. [Google Scholar] [CrossRef]
- Lian, C.; Zhang, J.Z.; Li, Y.R.; Liu, H.L.; Liu, X.J.; Li, X.L. Methylene Blue Staining: A Novel Application to Identify the Damaged Tissues on the Surface of Pressure Ulcers. Int. Wound J. 2019, 16, 570–571. [Google Scholar] [CrossRef]
- Gama, C.R.B.; Pombo, M.A.G.; Nunes, C.P.; Gama, G.F.; Mezitis, S.G.E.; Neto, M.S.; Guimarães, O.R.; Geller, M.; Oliveira, L.; da Fonseca, A.d.S.; et al. Treatment of Recurrent Urinary Tract Infection Symptoms with Urinary Antiseptics Containing Me-thenamine and Methylene Blue: Analysis of Etiology and Treatment Outcomes. Res. Rep. Urol. 2020, 12, 639–649. [Google Scholar] [CrossRef]
- Mak, R.S.P.; Liebelt, E.L. Methylene Blue: An Antidote for Methemoglobinemia and Beyond. Pediatr. Emerg. Care 2021, 37, 478–479. [Google Scholar] [CrossRef]
- Stelmashook, E.V.; Genrikhs, E.E.; Mukhaleva, E.V.; Kapkaeva, M.R.; Kondratenko, R.V.; Skrebitsky, V.G.; Isaev, N.K. Neuro-protective Effects of Methylene Blue In Vivo and In Vitro. Bull. Exp. Biol. Med. 2019, 167, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Luger, P.; Dittrich, B.; Benecke, L.; Sterzel, H. Charge Density Studies on Methylene Blue—A Potential Anti-Alzheimer Agent. Z. Naturforschung Sect. B J. Chem. Sci. 2018, 73, 99–108. [Google Scholar] [CrossRef]
- Smith, E.S.; Clark, M.E.; Hardy, G.A.; Kraan, D.J.; Biondo, E.; Gonzalez-Lima, F.; Cormack, L.K.; Monfils, M.; Lee, H.J. Daily Consumption of Methylene Blue Reduces Attentional Deficits and Dopamine Reduction in a 6-OHDA Model of Parkinson’s Disease. Neuroscience 2017, 359, 8–16. [Google Scholar] [CrossRef]
- Delport, A.; Harvey, B.H.; Petzer, A.; Petzer, J.P. Methylene Blue and Its Analogues as Antidepressant Compounds. Metab. Brain Dis. 2017, 32, 1357–1382. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Iqbal, J.; Zhu, Y.; Wang, F.; Zhang, F.; Chen, W.; Wu, T.; Du, Y. Chitosan/Al2O3-HA Nanocomposite Beads for Efficient Removal of Estradiol and Chrysoidin from Aqueous Solution. Int. J. Biol. Macromol. 2020, 145, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, Y.; Li, M.; Han, S.; Yang, X.; Liu, R. Toxic Effects of Chrysoidine on Human Serum Albumin: Isothermal Titration Calorimetry and Spectroscopic Investigations. Luminescence 2016, 31, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Z.; Feng, Y.X.; Yue, D.M. Phytotoxicity of Methylene Blue to Rice Seedlings. Glob. J. Environ. Sci. Manag. 2015, 1, 199–204. [Google Scholar] [CrossRef]
- Tkaczyk-Wlizło, A.; Mitrowska, K. Occurrence and Ecotoxicological Risk Assessment of Pharmacologically Active Dyes in the Environmental Water of Poland. Chemosphere 2023, 313, 137432. [Google Scholar] [CrossRef]
- Liu, S.; Ge, H.; Wang, C.; Zou, Y.; Liu, J. Agricultural Waste/Graphene Oxide 3D Bio-Adsorbent for Highly Efficient Removal of Methylene Blue from Water Pollution. Sci. Total Environ. 2018, 628–629, 959–968. [Google Scholar] [CrossRef]
- Azil, K.; Ferria, K.; Bouzid, S. Cladless Optical Fiber Sensor Based on Evanescent Wave Absorption for Monitoring Methylene Blue Induced Water Pollution. J. Opt. Soc. Am. B 2020, 37, A253. [Google Scholar] [CrossRef]
- Rahimi Aqdam, S.; Otzen, D.E.; Mahmoodi, N.M.; Morshedi, D. Adsorption of Azo Dyes by a Novel Bio-Nanocomposite Based on Whey Protein Nanofibrils and Nano-Clay: Equilibrium Isotherm and Kinetic Modeling. J. Colloid Interface Sci. 2021, 602, 490–503. [Google Scholar] [CrossRef]
- Raghav, L.; Patanjali, P.; Patanjali, N.; Singh, R. Adsorption of Malachite Green and Chrysoidine-Y by Sn-Pillared Clay. Environ. Qual. Manag. 2023, 32, 181–193. [Google Scholar] [CrossRef]
- Mariyam, A.; Mittal, J.; Sakina, F.; Baker, R.T.; Sharma, A.K. Adsorption Behaviour of Chrysoidine r Dye on a Metal/Halide-Free Variant of Ordered Mesoporous Carbon. Desalination Water Treat. 2021, 223, 425–433. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Z.; Gou, J.; Dong, S. Highly Efficient Adsorption and Removal of Chrysoidine Y from Aqueous Solution by Magnetic Graphene Oxide Nanocomposite. Arab. J. Chem. 2019, 12, 3064–3074. [Google Scholar] [CrossRef]
- Matheswaran, M.; Karunanithi, T. Adsorption of Chrysoidine R by Using Fly Ash in Batch Process. J. Hazard. Mater. 2007, 145, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Bentahar, S.; Dbik, A.; Khomri, M.E.; El Messaoudi, N.; Lacherai, A. Adsorption of Methylene Blue, Crystal Violet and Congo Red from Binary and Ternary Systems with Natural Clay: Kinetic, Isotherm, and Thermodynamic. J. Environ. Chem. Eng. 2017, 5, 5921–5932. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Q.; Zhang, L.; Pei, L.; Xue, H.; Li, Z. Adsorption of Methylene Blue and Congo Red from Aqueous Solution on 3D MXene/Carbon Foam Hybrid Aerogels: A Study by Experimental and Statistical Physics Modeling. J. Environ. Chem. Eng. 2023, 11, 109206. [Google Scholar] [CrossRef]
- Tran, T.H.; Le, H.H.; Pham, T.H.; Nguyen, D.T.; La, D.D.; Chang, S.W.; Lee, S.M.; Chung, W.J.; Nguyen, D.D. Comparative Study on Methylene Blue Adsorption Behavior of Coffee Husk-Derived Activated Carbon Materials Prepared Using Hydrothermal and Soaking Methods. J. Environ. Chem. Eng. 2021, 9, 105362. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Ghaedi, M.; Hajati, S.; Ghaedi, A.M.; Goudarzi, A.; Asfaram, A. Random Forest Model for the Ultrasonic-Assisted Removal of Chrysoidine G by Copper Sulfide Nanoparticles Loaded on Activated Carbon; Response Surface Methodology Approach. RSC Adv. 2015, 5, 59335–59343. [Google Scholar] [CrossRef]
- Raghav, L.; Patanjali, P.; Chopra, I.; Singh, R. Adsorption and Kinetic Studies for Removal of Basic Dyes Using Pillared Bentonites. Indian J. Chem. Technol. 2022, 29, 47–53. [Google Scholar]
- Saitoh, T.; Saitoh, M.; Hattori, C.; Hiraide, M. Rapid Removal of Cationic Dyes from Water by Coprecipitation with Aluminum Hydroxide and Sodium Dodecyl Sulfate. J. Environ. Chem. Eng. 2014, 2, 752–758. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, C.; Liu, X.; Sui, M.; Meng, Y. Selective Flocculation of Pollutants in Wastewater Using PH Responsive HM-Alginate/Chitosan Complexes. J. Environ. Chem. Eng. 2017, 5, 5406–5410. [Google Scholar] [CrossRef]
- Jaafar, A.; El-Husseini, S.; Platas-Iglesias, C.; Bilbeisi, R.A. Zeolitic Imidazolate Framework (AMCD-ZIF) Functionalised Mem-brane for the Removal of Dyes from Water. J. Environ. Chem. Eng. 2022, 10, 108019. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Cai, X.; Zhou, H.; Kong, Z.; Ma, X.; Gao, L.; Tan, H.; Cai, J. Tunable Reduced Graphene Oxide Nanofiltration Membrane for Efficient Retention and Separation of Dye/Salt Based on Molecular Interactions. J. Environ. Chem. Eng. 2023, 11, 109735. [Google Scholar] [CrossRef]
- Torabian, A.; Bihdendi, G.R.N.; Ranjbar, P.Z.; Razmkhah, N. Efficiency Evaluation of Textile Basic Dye Removal from Water by Nanofiltration. Iran. J. Environ. Health Sci. Eng. 2007, 4, 177–180. [Google Scholar]
- Kakuta, T.; Aoki, F.; Okada, T.; Shindo, H.; Yoshizawa, K.; Koizumi, T.; Nojiro, K. Biodegradation of Azo-Dye by a Yeast Candida Curvata AN723. J. Fiber Sci. Technol. 1998, 54, 54–57. [Google Scholar] [CrossRef]
- Kanekar, P.; Sarnaik, S. Microbial Process for Treatment of Phenol Bearing Dye—Industry Effluent in a Fixed Film Bioreactor. J. Environ. Sci. Health Part A Environ. Sci. Eng. Toxicol. 1995, 30, 1817–1826. [Google Scholar] [CrossRef]
- Ramakrishnan, R.K.; Padil, V.V.T.; Waclawek, S.; Cerník, M.; Tiwari, D. Sustainable, Biodegradable, and Recyclable Bio-Sponge for Rapid and Practical Bioremediation of Dye from Water. J. Environ. Chem. Eng. 2022, 10, 108285. [Google Scholar] [CrossRef]
- Tabasum, B.; Dhagale, P.R.; Nitnaware, K.M.; Nikule, H.A.; Nikam, T.D. New Chemical Products Formation from Textile Dye Degradation, Chitinolytic and Antioxidant Activity in New Strain Nbpc5-18 of Cellulosimicrobium Sp. TH-20. J. Environ. Chem. Eng. 2019, 7, 103114. [Google Scholar] [CrossRef]
- Solayman, H.M.; Hossen, M.A.; Abd Aziz, A.; Yahya, N.Y.; Leong, K.H.; Sim, L.C.; Monir, M.U.; Zoh, K.-D. Performance Eval-uation of Dye Wastewater Treatment Technologies: A Review. J. Environ. Chem. Eng. 2023, 11, 109610. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; He, D.; Pan, X. Application of Iron-Based Materials in Heterogeneous Advanced Oxidation Processes for Wastewater Treatment: A Review. Chem. Eng. J. 2021, 407, 127191. [Google Scholar] [CrossRef]
- Liu, J.; Peng, C.; Shi, X. Preparation, Characterization, and Applications of Fe-Based Catalysts in Advanced Oxidation Processes for Organics Removal: A Review. Environ. Pollut. 2022, 293, 118565. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wang, S.; Yu, Z.; Cao, X. Characteristics and Application of Iron-Based Materials in Heterogeneous Fenton Oxidation for Wastewater Treatment: A Review. Environ. Sci. 2023, 9, 1266–1289. [Google Scholar] [CrossRef]
- Guo, S.; Yang, H.; Sun, Q.; Zhang, G.; Zhao, T.; Zhou, Y.; Li, X.; Gao, P. Evaluation of a Novel Carbon-Based Micro-Nano Ze-ro-Valent Iron Composite for Immobilization of Heavy Metals in Soil. J. Environ. Chem. Eng. 2023, 11, 109740. [Google Scholar] [CrossRef]
- Barka, E.; Noutsopoulos, C.; Galani, A.; Panagou, I.; Kalli, M.; Koumaki, E.; Malamis, S.; Mamais, D. Removal of Contaminants of Emerging Concern from Wastewater Using an Integrated Column System Containing Zero Valent Iron Nanoparticles. Water 2023, 15, 598. [Google Scholar] [CrossRef]
- Yang, W.; Xi, D.; Li, C.; Yang, Z.; Lin, Z.; Si, M. “In-Situ Synthesized” Iron-Based Bimetal Promotes Efficient Removal of Cr(VI) in by Zero-Valent Iron-Loaded Hydroxyapatite. J. Hazard. Mater. 2021, 420, 126540. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Zhu, J.-J.; Li, P.-H.; Sun, Y.-F.; Yang, M.; Huang, X.-J. In-Situ Growth of Zero-Valent Iron in FeOx/Mn3O4 to Improve the Surficial Redox for High-Efficient Electrocatalysis of Pb(II). Chem. Eng. J. 2022, 430, 132959. [Google Scholar] [CrossRef]
- Samaraweera, H.; Nawalage, S.; Nayanathara, R.M.O.; Peiris, C.; Karunaratne, T.N.; Gunatilake, S.R.; Thirumalai, R.V.K.G.; Zhang, J.; Zhang, X.; Mlsna, T. In Situ Synthesis of Zero-Valent Iron-Decorated Lignite Carbon for Aqueous Heavy Metal Remediation. Processes 2022, 10, 1659. [Google Scholar] [CrossRef]
- Wu, X.; Wang, X.; Xie, Y.; Ren, N.; Ma, J.; Ning, P. Facile In-Situ Construction of Highly Dispersed Nano Zero-Valent Iron Modified Black TiO2 Z-Scheme Recyclable Heterojunction with Highly Efficient Visible-Light-Driven Photocatalytic Activity. Appl. Catal. B 2022, 310, 121325. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Tian, S.; Zhu, S.; Chen, Z.; Si, H. The Effect of Zero-Valent Iron/Fe3+ Coupling and Reuse on the Properties of Anoxic Sludge. J. Clean. Prod. 2022, 344, 131031. [Google Scholar] [CrossRef]
- Soliemanzadeh, A.; Fekri, M. Synthesis of Green and Ecofriendly Iron Nanoparticles Using Plant Part Extracts: Application on the Removal of Phosphorus from Aqueous Media. Inorg. Nano-Met. Chem. 2021, 51, 340–351. [Google Scholar] [CrossRef]
- Han, X.; Zhao, Y.; Zhao, F.; Wang, F.; Tian, G.; Liang, J. Novel Synthesis of Nanoscale Zero-Valent Iron from Iron Ore Tailings and Green Tea for the Removal of Methylene Blue. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130412. [Google Scholar] [CrossRef]
- Panić, S.; Petronijević, M.; Vukmirović, J.; Grba, N.; Savić, S. Green Synthesis of Nanoscale Zero-Valent Iron Aggregates for Catalytic Degradation of Textile Dyes. Catal. Lett. 2023, 153, 3605–3619. [Google Scholar] [CrossRef]
- Shojaei, S.; Shojaei, S. Optimization of Process Variables by the Application of Response Surface Methodology for Dye Removal Using Nanoscale Zero-Valent Iron. Int. J. Environ. Sci. Technol. 2019, 16, 4601–4610. [Google Scholar] [CrossRef]
- Khoshro, S.; Mirbagheri, N.S.; Sabbaghi, S. Removal of Nitrate from Aqueous Solution Using Nano Zerovalent Iron-Reduced Graphene Oxide Composite: Optimization of Parameters. Water Environ. J. 2020, 34, 608–621. [Google Scholar] [CrossRef]
- Amiri, M.J. Synthesis and Optimization of Spherical NZVI (20–60 Nm) Immobilized in Bio-Apatite-Based Material for Efficient Removal of Phosphate: Box-Behnken Design in a Fixed-Bed Column. Environ. Sci. Pollut. Res. 2022, 29, 67751–67764. [Google Scholar] [CrossRef] [PubMed]
- Scherer, R.; Godoy, H.T. Effects of Extraction Methods of Phenolic Compounds from Xanthium strumarium L. and Their An-tioxidant Activity|Efeito de Métodos de Extração de Compostos Fenólicos de Xanthium strumarium L. e Suas Atividades Antioxidantes. Rev. Bras. Plantas Med. 2014, 16, 41–46. [Google Scholar] [CrossRef]
- Jorgensen, E.M.; Marin, A.B.; Kennedy, J.A. Analysis of the Oxidative Degradation of Proanthocyanidins under Basic Conditions. J. Agric. Food Chem. 2004, 52, 2292–2296. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Gil, M.I.; Cremin, P.; Waterhouse, A.L.; Hess-Pierce, B.; Kader, A.A. HPLC-DAD-ESIMS Analysis of Phenolic Compounds in Nectarines, Peaches, and Plums. J. Agric. Food Chem. 2001, 49, 4748–4760. [Google Scholar] [CrossRef]
- Tao, W.; Zhou, Z.; Zhao, B.; Wei, T. Simultaneous Determination of Eight Catechins and Four Theaflavins in Green, Black and Oolong Tea Using New HPLC–MS–MS Method. J. Pharm. Biomed. Anal. 2016, 131, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Nian, B.; Chen, L.; Yi, C.; Shi, X.; Jiang, B.; Jiao, W.; Liu, Q.; Lv, C.; Ma, Y.; Zhao, M. A High Performance Liquid Chromatography Method for Simultaneous Detection of 20 Bioactive Components in Tea Extracts. Electrophoresis 2019, 40, 2837–2844. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.; Rego, C.; Lima, E.; Marcone, M.; Baptista, J. Comparative Analysis of the Polyphenols, Caffeine, and Antioxidant Activities of Green Tea, White Tea, and Flowers from Azorean Camellia Sinensis Varieties Affected by Different Harvested and Processing Conditions. Antioxidants 2021, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, D.; Cobbs, M.; Rahhal, S.; Bakarr Kanu, A. The Use of Liquid Chromatography and Mass Spectrometry to Identify and Quantify Chemical Components in Tea Extracts. Anal. Chem. Lett. 2022, 12, 292–301. [Google Scholar] [CrossRef]
- Yang, X.-R.; Wang, Y.-Y.; Li, K.-K.; Li, J.; Li, C.-R.; Shi, X.-G.; Ko, C.-H.; Leung, P.-C.; Ye, C.-X.; Song, X.-H. Cocoa Tea (Camellia Ptilophylla Chang), a Natural Decaffeinated Species of Tea—Recommendations on the Proper Way of Preparation for Con-sumption. J. Funct. Foods 2011, 3, 305–312. [Google Scholar] [CrossRef]
- Zhang, C.; Suen, C.L.-C.; Yang, C.; Quek, S.Y. Antioxidant Capacity and Major Polyphenol Composition of Teas as Affected by Geographical Location, Plantation Elevation and Leaf Grade. Food Chem. 2018, 244, 109–119. [Google Scholar] [CrossRef]
- Kim, Y.; Goodner, K.L.; Park, J.-D.; Choi, J.; Talcott, S.T. Changes in Antioxidant Phytochemicals and Volatile Composition of Camellia Sinensis by Oxidation during Tea Fermentation. Food Chem. 2011, 129, 1331–1342. [Google Scholar] [CrossRef]
- Suazo-Hernández, J.; Sepúlveda, P.; Cáceres-Jensen, L.; Castro-Rojas, J.; Poblete-Grant, P.; Bolan, N.; Mora, M.D.L.L. NZVI-Based Nanomaterials Used for Phosphate Removal from Aquatic Systems. Nanomaterials 2023, 13, 399. [Google Scholar] [CrossRef]
- Som, I.; Suman, S.; Roy, M.; Gupta, S.; Saha, R. Effective Phosphate Removal from Sewage Water Using Zerovalent Iron Na-nomaterial as an Adsorbent. Water Qual. Res. J. 2022, 57, 177–199. [Google Scholar] [CrossRef]
- Badmus, K.O.; Coetsee-Hugo, E.; Swart, H.; Petrik, L. Synthesis and Characterisation of Stable and Efficient Nano Zero Valent Iron. Environ. Sci. Pollut. Res. 2018, 25, 23667–23684. [Google Scholar] [CrossRef]
- He, Y.; Gao, J.F.; Feng, F.Q.; Liu, C.; Peng, Y.Z.; Wang, S.Y. The Comparative Study on the Rapid Decolorization of Azo, An-thraquinone and Triphenylmethane Dyes by Zero-Valent Iron. Chem. Eng. J. 2012, 179, 8–18. [Google Scholar] [CrossRef]
- Machado, S.; Pacheco, J.G.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Characterization of Green Zero-Valent Iron Nanoparticles Produced with Tree Leaf Extracts. Sci. Total Environ. 2015, 533, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lin, J.; Chen, Z.; Megharaj, M.; Naidu, R. Green Synthesized Iron Nanoparticles by Green Tea and Eucalyptus Leaves Extracts Used for Removal of Nitrate in Aqueous Solution. J. Clean. Prod. 2014, 83, 413–419. [Google Scholar] [CrossRef]
- Fang, S.; Zhang, J.; Niu, Y.; Ju, S.; Gu, Y.; Han, K.; Wan, X.; Li, N.; Zhou, Y. Removal of Nitrate Nitrogen from Wastewater by Green Synthetic Hydrophilic Activated Carbon Supported Sulfide Modified Nanoscale Zerovalent Iron: Characterization, Performance and Mechanism. Chem. Eng. J. 2023, 461, 141990. [Google Scholar] [CrossRef]
- Akhgar, B.N.; Kavanlouei, M.; Kazemi, H.; Ghavidel, S.; Dardman, S. Effect of Synthesis Routes on the Microstructural Properties of Nano Zero-Valent Metals of Fe and Cu Synthesized from the Industrial Copper Solvent Extraction Process. Mater. Chem. Phys. 2023, 302, 127704. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, W.-X. Fine Structural Features of Nanoscale Zero-Valent Iron Characterized by Spherical Aberration Corrected Scanning Transmission Electron Microscopy (Cs-STEM). Analyst 2014, 139, 4512–4518. [Google Scholar] [CrossRef] [PubMed]
- Kozma, G.; Rónavári, A.; Kónya, Z.; Kukovecz, Á. Environmentally Benign Synthesis Methods of Zero-Valent Iron Nanopar-ticles. ACS Sustain. Chem. Eng. 2016, 4, 291–297. [Google Scholar] [CrossRef]
- Mareedu, T.; Poiba, V.; Vangalapati, M. Green Synthesis of Iron Nanoparticles by Green Tea and Black Tea Leaves Extract. Mater. Today Proc. 2021, 42, 1498–1501. [Google Scholar] [CrossRef]
- Huang, L.; Weng, X.; Chen, Z.; Megharaj, M.; Naidu, R. Green Synthesis of Iron Nanoparticles by Various Tea Extracts: Com-parative Study of the Reactivity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 130, 295–301. [Google Scholar] [CrossRef]
- Kuang, Y.; Wang, Q.; Chen, Z.; Megharaj, M.; Naidu, R. Heterogeneous Fenton-like Oxidation of Monochlorobenzene Using Green Synthesis of Iron Nanoparticles. J. Colloid Interface Sci. 2013, 410, 67–73. [Google Scholar] [CrossRef]
- Iwamoto, I.; Kurniawan, A.; Hasegawa, H.; Kashiwaya, Y.; Nomura, T.; Akiyama, T. Reduction Behaviors and Generated Phases of Iron Ores Using Ammonia as Reducing Agent. ISIJ Int. 2022, 62, 2483–2490. [Google Scholar] [CrossRef]
- Rivero, M.J.; Alonso, E.; Dominguez, S.; Ribao, P.; Ibañez, R.; Ortiz, I.; Irabien, A. Kinetic Analysis and Biodegradability of the Fenton Mineralization of Bisphenol A. J. Chem. Technol. Biotechnol. 2014, 89, 1228–1234. [Google Scholar] [CrossRef]
- Domínguez, J.R.; González, T.; Palo, P.; Cuerda-Correa, E.M. Fenton + Fenton-like Integrated Process for Carbamazepine Degradation: Optimizing the System. Ind. Eng. Chem. Res. 2012, 51, 2531–2538. [Google Scholar] [CrossRef]
- Cadenas, E.; Packer, L. Handbook of Antioxidants, 2nd ed.; Marcel Dekker: New City, NY, USA, 2001; ISBN 9780824705473. [Google Scholar]
- Neyens, E.; Baeyens, J. A Review of Classic Fenton’s Peroxidation as an Advanced Oxidation Technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Raman, C.D.; Kanmani, S. Textile Dye Degradation Using Nano Zero Valent Iron: A Review. J. Environ. Manag. 2016, 177, 341–355. [Google Scholar] [CrossRef]
- Moon, B.-H.; Park, Y.-B.; Park, K.-H. Fenton Oxidation of Orange II by Pre-Reduction Using Nanoscale Zero-Valent Iron. Desalination 2011, 268, 249–252. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, C.; Megharaj, M. Characterization of Iron-Polyphenol Nanoparticles Synthesized by Three Plant Extracts and Their Fenton Oxidation of Azo Dye. ACS Sustain. Chem. Eng. 2014, 2, 1022–1025. [Google Scholar] [CrossRef]
- García, F.E.; Plaza-Cazón, J.; Montesinos, V.N.; Donati, E.R.; Litter, M.I. Combined Strategy for Removal of Reactive Black 5 by Biomass Sorption on Macrocystis Pyrifera and Zerovalent Iron Nanoparticles. J. Environ. Manag. 2018, 207, 70–79. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Weng, C.-H.; Chen, F.-Y. Effective Removal of AB24 Dye by Nano/Micro-Size Zero-Valent Iron. Sep. Purif. Technol. 2008, 64, 26–30. [Google Scholar] [CrossRef]
- Freyria, F.S.; Bonelli, B.; Sethi, R.; Armandi, M.; Belluso, E.; Garrone, E. Reactions of Acid Orange 7 with Iron Nanoparticles in Aqueous Solutions. J. Phys. Chem. C 2011, 115, 24143–24152. [Google Scholar] [CrossRef]
- Cao, J.; Wei, L.; Huang, Q.; Wang, L.; Han, S. Reducing Degradation of Azo Dye by Zero-Valent Iron in Aqueous Solution. Chemosphere 1999, 38, 565–571. [Google Scholar] [CrossRef]
- Valsaraj, K.T. Elements of Environmental Engineering: Thermodynamics and Kinetics, 3rd ed.; CRC Press-Taylor & Francis Group: Boca Raton, FL, USA, 2000. [Google Scholar]
- Simsek, U.B.; Turabik, M. Decolorization Mechanisms of an Anionic Dye by Using Zero-Valent Iron Nanoparticles: Application of Response Surface Modeling for the Optimization Process. Desalination Water Treat. 2017, 81, 303–314. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Xi, B.; Meng, X.; Gong, B.; Li, R.; Peng, X.; Liu, H. Decolorization of Methyl Orange by a New Clay-Supported Nanoscale Zero-Valent Iron: Synergetic Effect, Efficiency Optimization and Mechanism. J. Environ. Sci. 2017, 52, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Weng, X.; Dharmarajan, R.; Chen, Z. Characterization and Reactivity of Iron Based Nanoparticles Synthesized by Tea Extracts under Various Atmospheres. Chemosphere 2017, 169, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Wang, D.; Li, Y. Green Chemistry for Nanoparticle Synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-X.; Jin, X.-Y.; Chen, Z.; Megharaj, M.; Naidu, R. Removal of Methyl Orange from Aqueous Solution Using Benton-ite-Supported Nanoscale Zero-Valent Iron. J. Colloid Interface Sci. 2011, 363, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, F.; Chen, Z.; Megharaj, M.; Naidu, R. Heterogeneous Fenton Oxidation of Direct Black G in Dye Effluent Using Functional Kaolin-Supported Nanoscale Zero Iron. Environ. Sci. Pollut. Res. 2014, 21, 1936–1943. [Google Scholar] [CrossRef]
- Shen, J.-H.; Horng, J.-J.; Wang, Y.-S.; Zeng, Y.-R. The Use of Reactive Index of Hydroxyl Radicals to Investigate the Degradation of Acid Orange 7 by Fenton Process. Chemosphere 2017, 182, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Ferroudj, N.; Combes, A.; Pichon, V.; Abramson, S. Tracking the Degradation Pathway of Three Model Aqueous Pollutants in a Heterogeneous Fenton Process. J. Environ. Chem. Eng. 2019, 7, 102987. [Google Scholar] [CrossRef]
- Hamdy, A.; Mostafa, M.K.; Nasr, M. Zero-Valent Iron Nanoparticles for Methylene Blue Removal from Aqueous Solutions and Textile Wastewater Treatment, with Cost Estimation. Water Sci. Technol. 2018, 78, 367–378. [Google Scholar] [CrossRef]
- Mukherjee, R.; Kumar, R.; Sinha, A.; Lama, Y.; Saha, A.K. A Review on Synthesis, Characterization, and Applications of Nano Zero Valent Iron (NZVI) for Environmental Remediation. Crit. Rev. Environ. Sci. Technol. 2016, 46, 443–466. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A Review on Fenton and Improvements to the Fenton Process for Wastewater Treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Litter, M.I.; Slodowicz, M. An Overview on Heterogeneous Fenton and PhotoFenton Reactions Using Zerovalent Iron Materials. J. Adv. Oxid. Technol. 2017, 20, 20160164. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).