1. Introduction
To address the adverse impacts of climate change, clean energy such as solar energy, wind energy, and hydropower is gradually accelerating the pace of replacing traditional fossil energy [
1]. However, restrictions and technical barriers still exist, constraining the development of clean energy. Clean energy power generation is unstable, intermittent, unpredictable, etc., which requires that it be combined with thermal power units to improve the peak regulation ability. However, long-term operation under off-design conditions will cause great damage to thermal power units, and the peak periods of consumer electric power consumption and clean energy power are inconsistent. In order to handle these issues, developing energy storage system (ESS) technology is vital to guarantee the development of clean energy [
2].
At present, the pumped hydro energy storage system (PHS), the electrochemical energy storage system, the compressed air energy storage system (CAES), and the thermal energy storage system (TES) are the main mature energy storage technologies. Among them, the PHS is the most extensively applied [
3] for its superiority in its long lifetime, mature technologies, and low investment cost compared to battery and thermal energy storage systems [
4,
5]. However, it needs to equip upstream and downstream reservoirs, which requires suitable geographical conditions and long-period construction, making for an unignorable influence on regional ecology. Electrochemical energy storage realizes the energy conversion between chemical energy and electricity through chemical reactions. And it has received much attention from scholars due to the advantages of its short energy transition time, flexible site selection, and capacity configuration. However, it also has a great disadvantage in large capital investment [
6].
TES technology is often combined with other energy storage systems to improve the energy utilization and avoid the usage of fuel, which benefits both the environment and the construction cost. It stores the thermal energy in sensible, latent, and thermochemical forms [
7]. It has three mature classifications [
8]: sensible thermal energy storage (STES), latent thermal energy storage (LTES), and thermochemical energy storage (TCES). Specifically, STES often works at low–mid-range temperature conditions [
9], and the storage mediums typically used are water, oil, sand, molten salts, and some inorganic liquids [
10]. As to the medium of STES, molten salt is usually applied in the Solar Power Industry for its favorable heat transfer characteristics in the mid–high temperature range, and unsaturated water is also a preferred medium for its low cost [
8]. STES stores the thermal energy by increasing the temperature of the storage medium to store the sensible heat. Koçak et al. [
11] compared the thermal and economic performances of different kinds of TES technologies, considering that STES is the only cost-effective technology, especially for mid–high-temperature industrial applications. Gao et al. [
12] compared the thermal performances of the LTES system (where the storage medium is paraffin RT55) and STES system (where the storage medium is water), finding that water could make the thermal storage capacity more efficient than paraffin. Other scholars have also paid attention to the combination of TES and different forms of heat in coupling systems. Ortega-Fernández et al. [
13] utilized a TES unit to store the thermal energy during the compression stage and release it during the expansion stage, realizing the high cycle efficiency of an adiabatic-CAES plant. Chaychizadeh et al. [
14] employed a TES to store the released heat from the compression process and the heat transferred by electrical heaters from wind farm power. The dynamic simulation under steady wind power generation and real conditions were both conducted, and the results showed that the RTE reached 57.55% and the energy density reached 84.1 kWh/m
3.
Compared to other grand-scale energy storage systems, CAES has been studied widely [
15]. The high-pressure air pressurized by the compressor is reserved during the peak period, and it is released to generate power through the turbine in the off-peak period. The non-negligible advantages of CAES are its fast response, low construction cost, large capacity, and long lifetime [
16]. However, some technology restrictions still exist for the CAES system. In the current research, CAES can be widely classified into four types, including traditional CAES, AA-CAES (advanced adiabatic compressed air energy storage), LAES (compressed liquid air energy storage), and ICAES (isothermal compressed air energy storage) [
1]. For the traditional CAES, the efficiency is low because of the irreversible losses during internal flowing and energy conversion, as well as the waste gas emission after turbine expansion [
17]. And for the underground CAES, macro-cracks could appear in the lined underground caves because of high tensile stress and varying temperature during air injection [
15]. To improve system efficiency and reduce the waste gas pollution, AA-CAES is proposed, which would use stored heat from the compression process [
18]. The system efficiency of the traditional CAES is about 30% to 43%, while the AA-CAES can reach 50% to 75% [
17]. For the LAES, it compresses air at atmosphere pressure and then condenses it to the liquid state, improving the energy storage density and the system efficiency [
19,
20]. However, these three types of CAES technologies are restricted to fossil fuels and geographic conditions [
20,
21]. For the ICAES, it has no limitations of geographic conditions and it has low compression power and high efficiency. But the difficulties that it needs to overcome include finding a method to enhance the heat transfer efficiency to make the expansion and the compression close to the isothermal process [
1].
Compared to air, the critical temperature of CO
2 is close to normal temperature. CO
2 can be liquefied at room temperature (the critical point of CO
2 is at 7.4 MPa, 31.4 °C), and the density of CO
2 in the liquid phase and the supercritical phase is several times the density of air, making CO
2 more suitable to be the working medium of energy storage systems. The system structure of the compressed carbon dioxide energy storage (CCES) resembles the CAES. Morandin et al. [
22] first proposed a TC-CCES which used water to store heat energy and cool salt water to store cold energy, while the discharging time was 2 h and charging and discharging power was 50 MW. Ahmadi et al. [
23] proposed a TC-CCES which used cooling energy of liquefied natural gas (LNG) and thermal energy of a geothermal source. This paper systematically studied the influence of three key parameters (CO
2 turbine inlet temperature, inlet pressure, and outlet pressure) on the system performance. Liu et al. [
24] accomplished the exergetic analysis of a two-stage TC-CCES, determining the component with the greatest exergy loss. Zhang et al. [
25] integrated a CCES system with the organic Rankine cycle (ORC) to make full use of the stored compression intercooling energy during the storage period. The results demonstrated that the system net output energy, the system exergy efficiency, and the total product unit cost under typical conditions were 27.736 MW, 66.64%, and USD 20.34/GJ, respectively. Zhang et al. [
26] proposed a novel adsorption-type trans-critical compressed carbon dioxide ESS which achieved high-density and low-pressure CO
2 storage by using high-pressure absorbents to absorb CO
2 rather than CO
2 liquefaction. The thermodynamic simulation and parameter sensitivity analysis were conducted and the system round trip efficiency, the exergy efficiency, and the energy storage density under typical conditions were 66.68%, 67.79%, and 12.11 kWh/m
3, respectively. Zhang et al. [
27] proposed a new CCHP combined with the TC-CCES and wind energy (which includes wind power and wind energy waste heat). It improved the wind energy utilization by analyzing the influences of five main variables on the system performance. Hao et al. [
28] proposed a TC-CCES integrated with a heat pump subsystem, and the economic performance was analyzed by the life cycle cost method for CO
2 utilization. In the thermal energy storage (TES) subsystem, the heat pump cycle was applied to improve the quality of the heat stored in the thermal storage unit, and the working fluid of the heat pump was R245fa with its low boiling point, fairly high molecular mass, and high critical temperature (427 K).
Except for the above-mentioned TC-CCES systems, the SC-CCES has also aroused wide attention. Xu et al. [
29] proposed two SC-CCES systems, applying an underwater bag to reserve supercritical CO
2, which keeps the pressure constant during the energy conversion. Oh et al. [
30] constructed a SC-CCES system with two-stage compression. The system operation status was analyzed and the operational feasibility was discussed. Chen et al. [
31] came up with a SC-CCES coupled with the concentrating solar thermal subsystem, and a printed circuit heat exchanger was used to promote the system. This paper analyzed the influences of the mass flow and temperature of thermal oil, and calculated the economic indicators, identifying the viability of the system investment. Meanwhile, advanced exergy analysis was conducted on the SC-CCES system. Liu et al. [
24] performed the comprehensive analysis of the new two-stage SC-CCES by studying the conventional and advanced exergy analyses, finding that the result from the advanced exergetic analysis was more persuasive. He et al. [
32] found that the SC-CCES performs better than the CAES, and the recuperator has the biggest avoidable exergy loss. Zhang et al. [
33] established an SC-CCES coupled with a TES and compared it with the compressed liquid CO
2 energy storage (LCES) and the AA-CAES, finding that the energy storage efficiency of the AA-CAES was better than that of the CCES, while the system efficiency of the CCES was 4.05% higher than that of the LCES, and the energy density of the CCES was 2.8 times bigger than that of the AA-CAES. Moreover, the results showed that system minimum pressures have a major effect on the system performance compared to system maximum pressures. Huang et al. [
34] placed emphasis on the system layout by dividing super-critical heat exchangers into two sets so that different masses of thermal fluid were adjusted to fit the specific heat of supercritical CO
2. The exergy destructions of high-pressure heat exchangers could be significantly lessened, while the system round trip efficiency was improved by 2.84–5.26% compared to the baseline system. Chaychizadeh et al. [
14] put forward an SC-CCES system combined with a hierarchical water TES, which stored wind energy in the off-peak period and discharged it as the steady power in the peak period. Zhang et al. [
35] proposed the SC-CCES system with a low-temperature TES. The influences of main variables on the system performance were analyzed and the system optimization was completed by the genetic algorithm method.
In the ESS with trans-critical and supercritical CO
2 as the working fluids, each component needs to withstand high pressure. In case the operation pressure is higher than the CO
2 critical pressure, the difficulty and the cost for the system manufacturing increase and the safety for system operation are challenged. In response to these problems, Tang et al. [
36] and Sun et al. [
37] proposed the ESS based on the carbon dioxide phase transition process (PC-CCES). This system used a gas tank to reserve the gaseous working fluid at normal temperature and pressure. During the energy storage process, the working fluid was compressed first and then condensed, and finally reserved in the liquid tank. During the energy discharge period, the working medium evaporated and then expanded to generate power. The gas storage tank bore the highest pressure, close to the CO
2 critical pressure, while the pressure of other components was much lower than the critical value, which effectively solved the problem caused by high pressure in the supercritical or trans-critical systems. However, these systems also have problems, such as low system energy storage efficiency and the necessity of the additional heat source to evaporate the working fluid, because the storage pressure of the working fluid is lower than its critical pressure. Meanwhile, only one TES unit will raise the temperature difference of the heat exchanger for the severe changes of the specific heat capacity when CO
2 approaches the critical state.
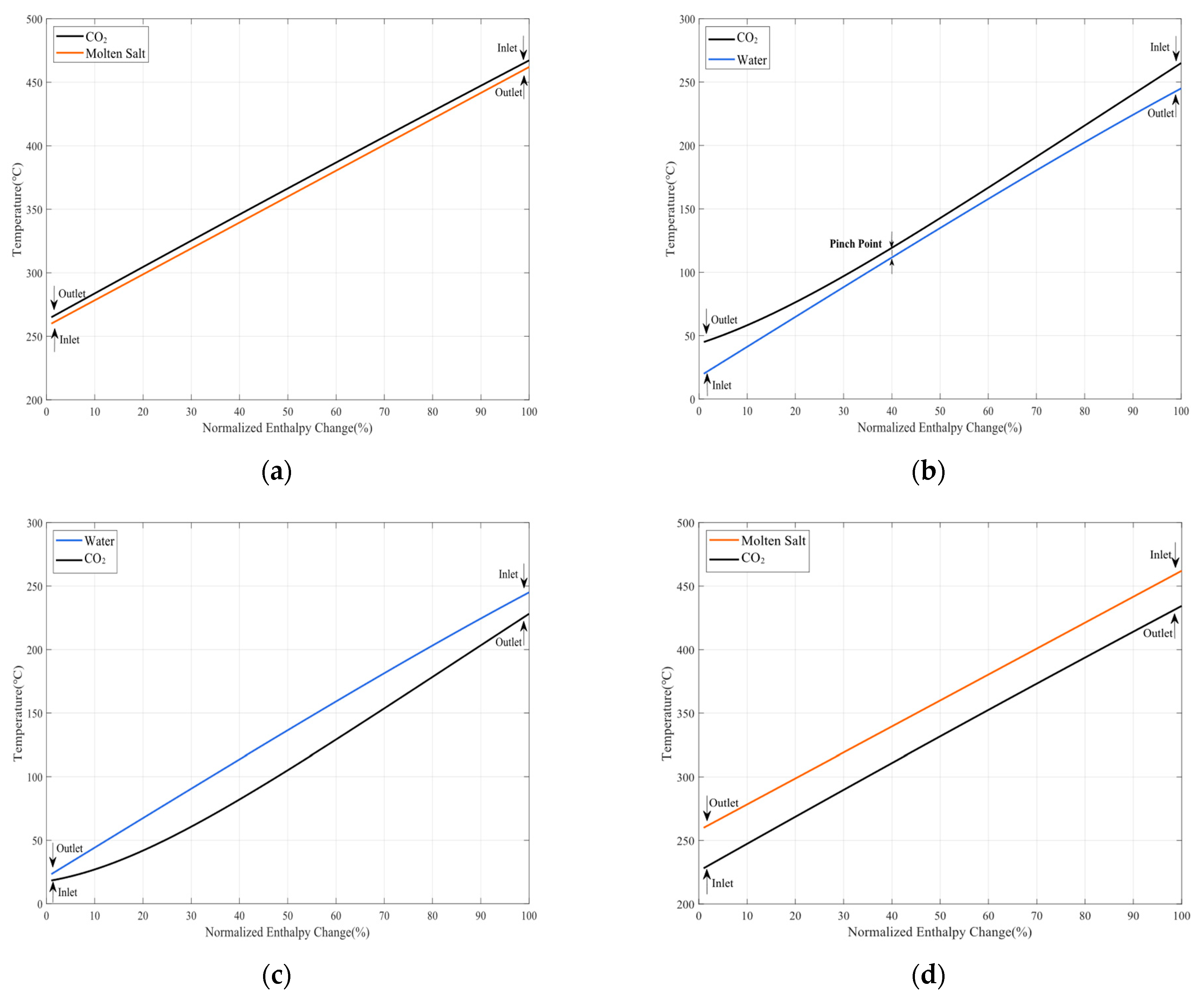
This paper puts forward an innovative compressed CO2 phase-change energy storage system in which gaseous carbon dioxide is reserved in a gas storage tank at normal temperature and pressure. Carbon dioxide is compressed and then condensed into the liquid state to store in a liquid tank during the energy storage period. The system maximum pressure is lower than carbon dioxide critical pressure, which can largely reduce the system manufacture investment and improve the operational security. During the energy discharge period, the STES is used to store the sensible heat from the compression and reheat CO2, so that the power capacity of the turbine is improved and the system energy storage efficiency is raised. To avoid the heat exchanger pinch points, this system proposes two-unit TES to complete heat exchange, respectively. Meanwhile, the throttle valve is applied to decrease both the pressure and the temperature of the evaporator, so that normal-temperature water can be used to evaporate CO2 in the evaporator.
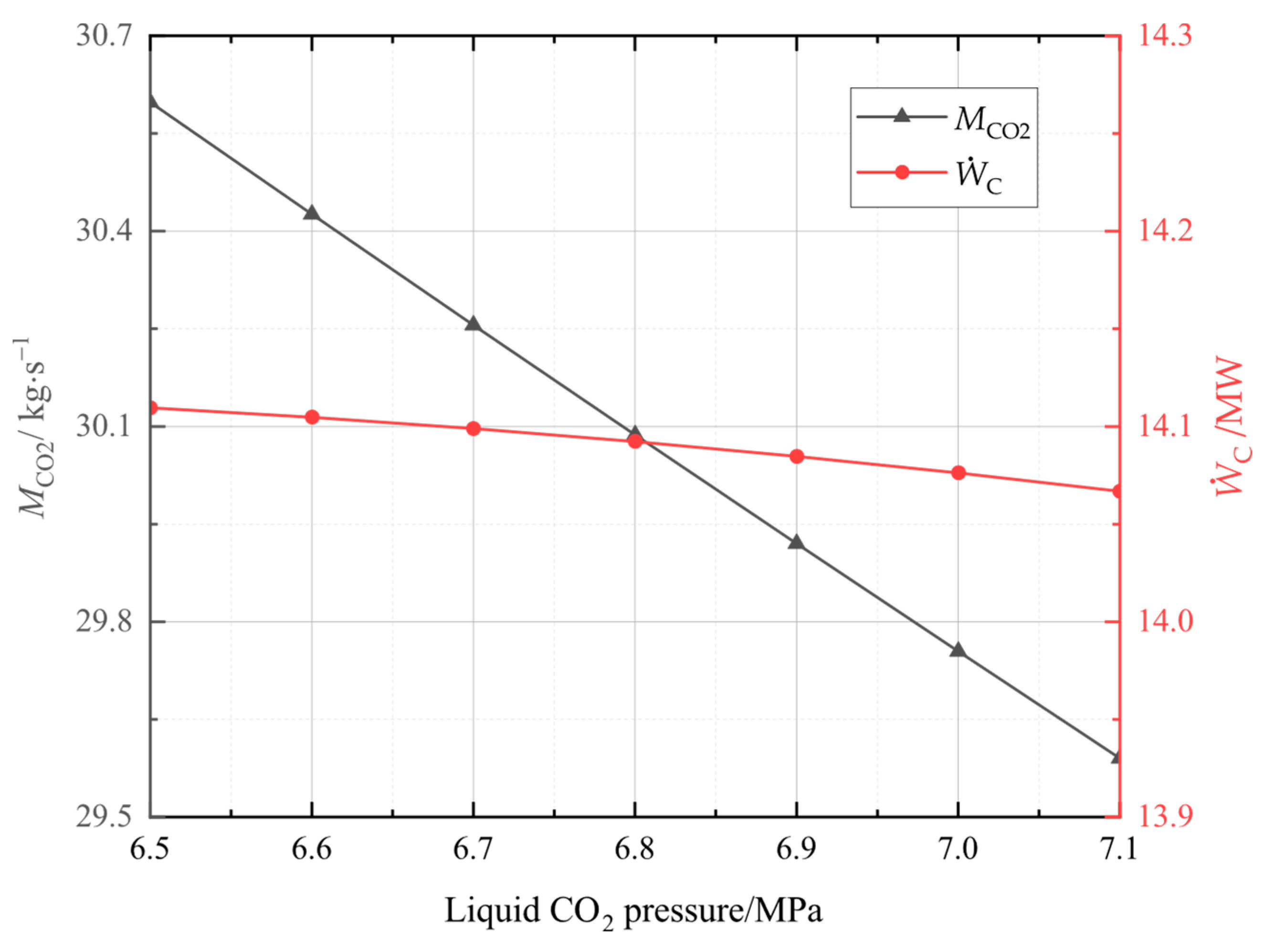
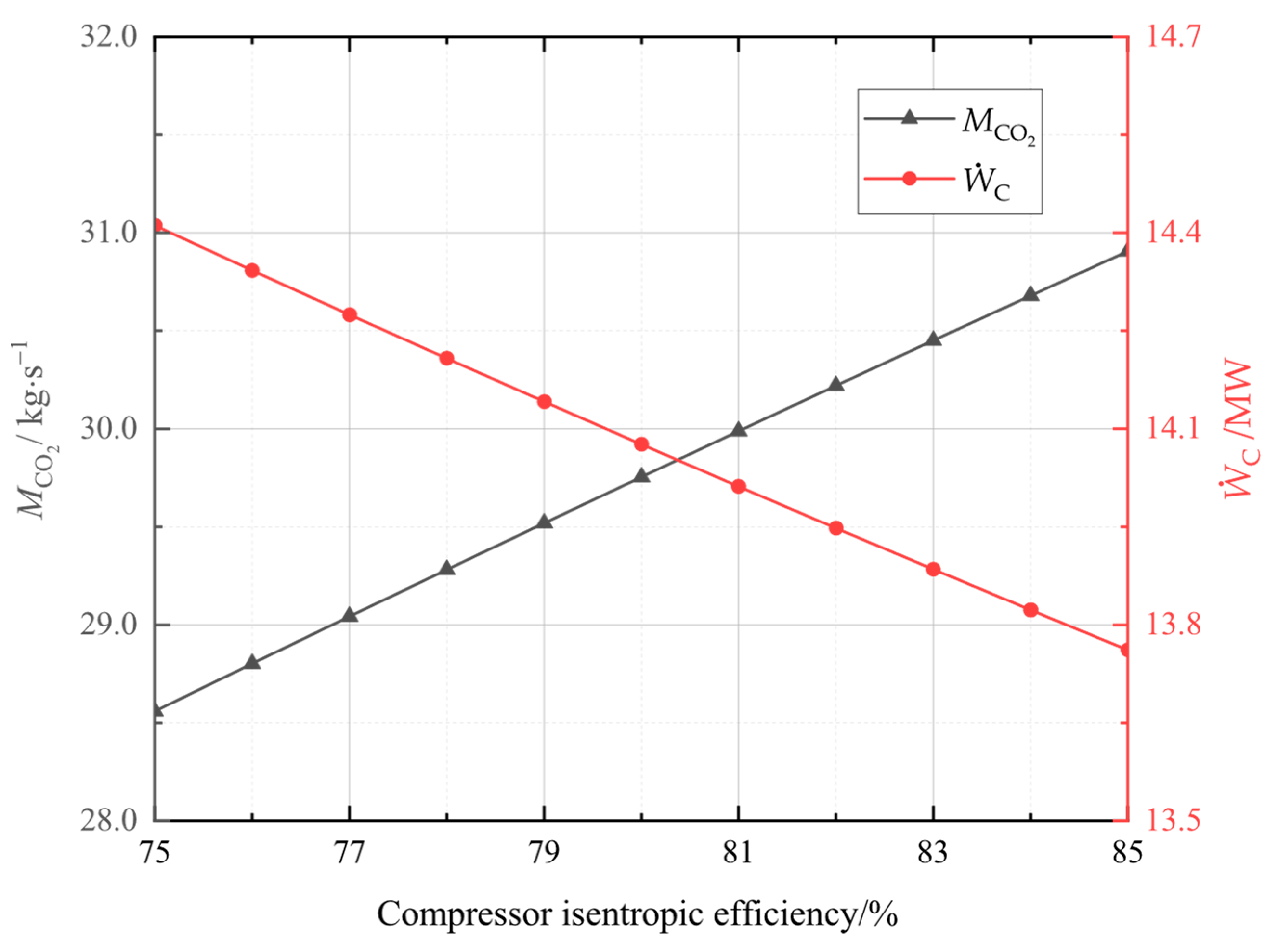
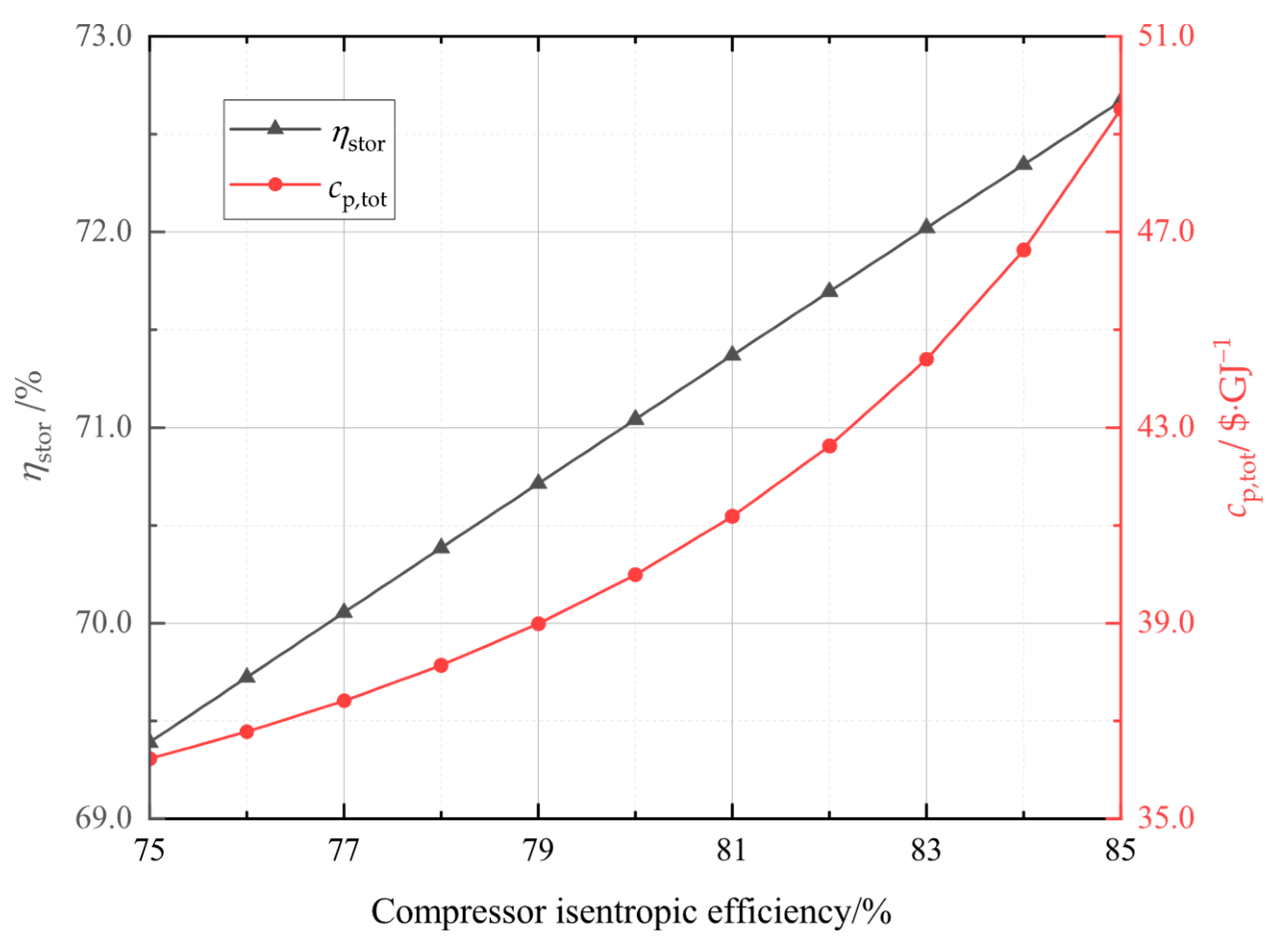
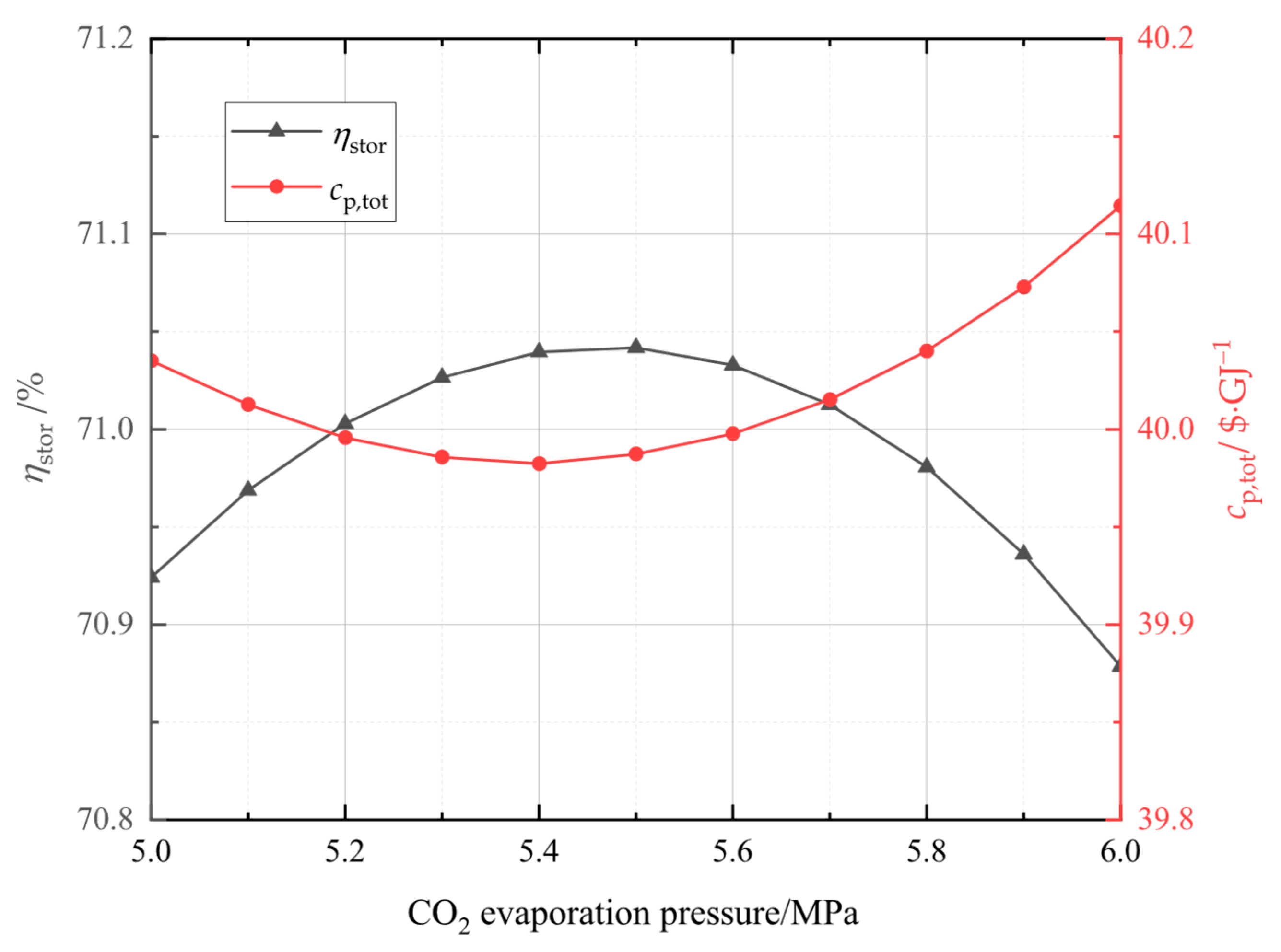
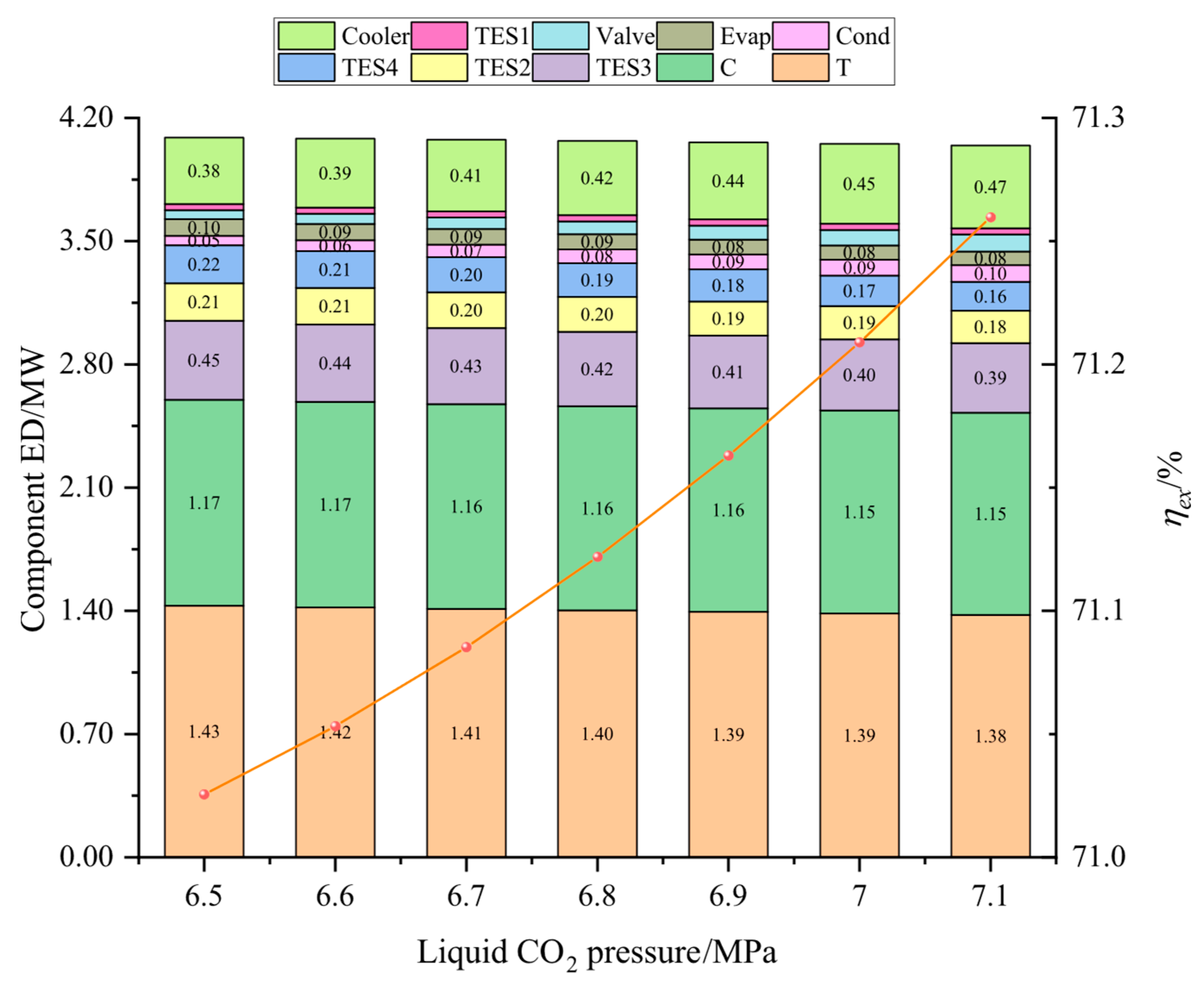
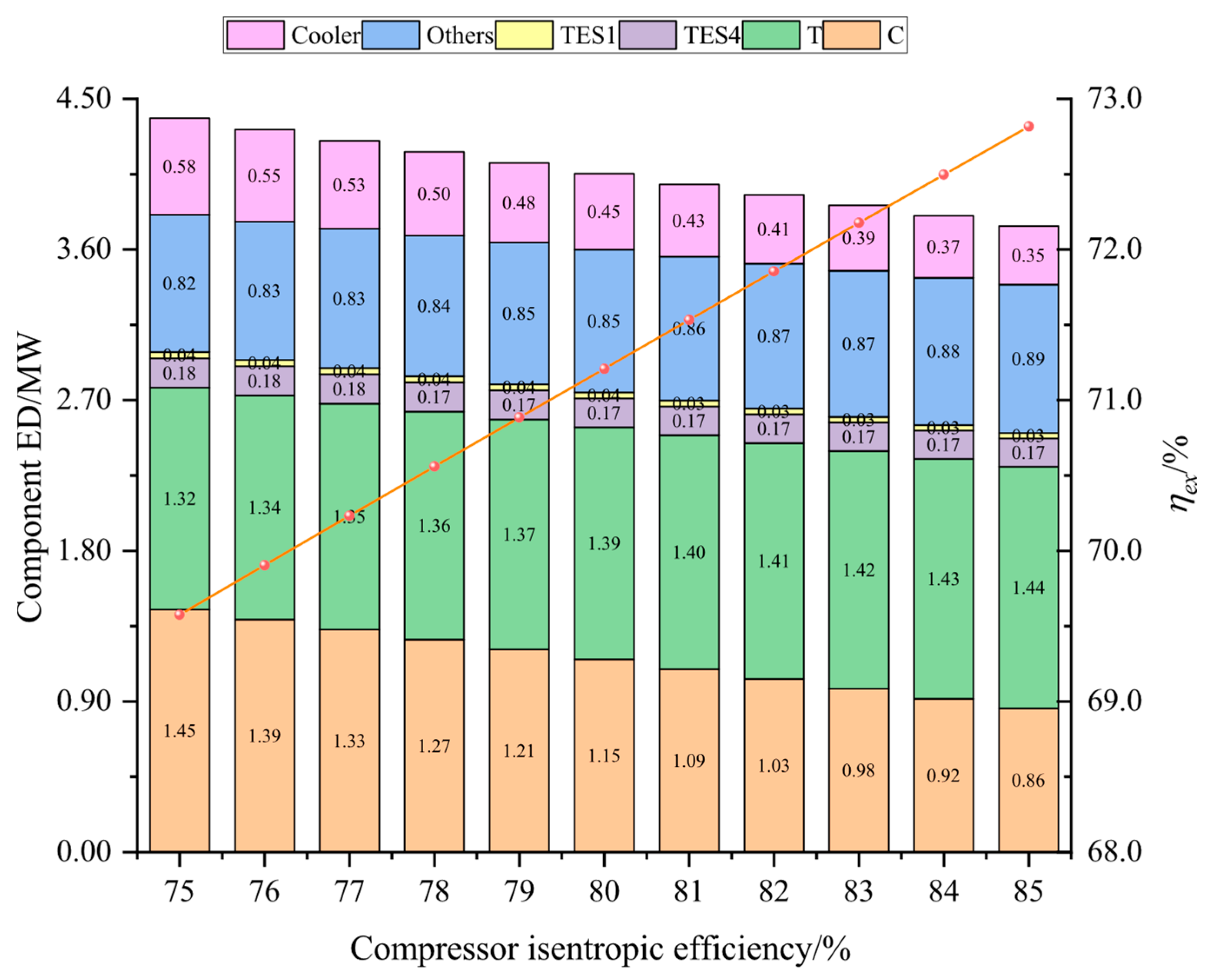
In this study, the system was established and the performance under typical operation conditions was calculated and analyzed. Then, the main variables, including liquid CO2 pressure, as well as compressor and turbine isentropic efficiencies, and CO2 evaporation pressure were studied for their effects on the system energy storage efficiency, the system unit output power cost, and the system exergetic efficiency. Finally, the multi-objective optimization was accomplished to acquire optimal operation parameters, then the system superiority was proved by comparing optimal energy storage efficiency with that of other systems.
2. System Process and Assumptions
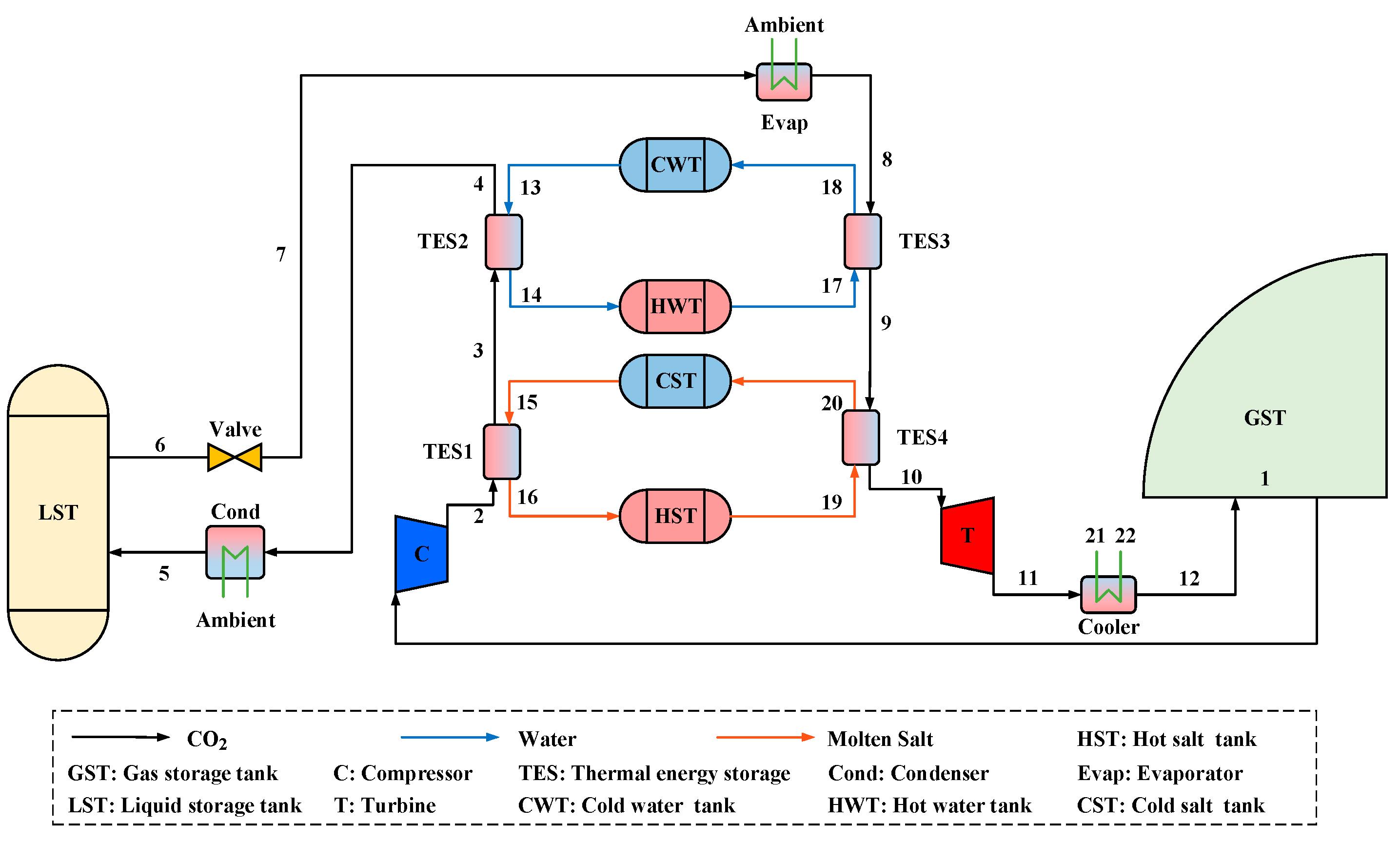
Figure 1 shows an innovative PC-CCES system: throttling expansion compressed CO
2 phase-change energy storage system. The system’s main components include a CO
2 gas storage tank, compressor, TES, condenser, liquid CO
2 storage tank, throttle valve, evaporator, turbine, cooler, cold water tank, hot water tank, cold salt tank, hot salt tank. The specific heat capacity of CO
2 varies dramatically when the physical property is close to the critical point, and it remains roughly unchanged when the physical property is far from the critical point. The temperature of CO
2 during the heat exchanging process has differing trends and it is necessary to assign two sets of TES units instead of only one set with a high terminal temperature difference. Based on a consideration of the temperature range, molten salt is selected in the high-temperature range for its sterling heat transfer performance, and water is selected in the middle-temperature range for its low cost.
The system energy charge process stores the pressure energy and the heat in different components. Carbon dioxide in the gas storage tank is of normal temperature and pressure, it is compressed to a high-temperature and high-pressure state during the energy storage period, and then it is liquefied and reserved in a liquid CO2 storage tank. Since the condensation temperature of CO2 is slightly higher than ambient temperature, the water with ambient temperature and pressure can be used to chill CO2. During the energy discharge period, liquid CO2 evaporates to gaseous CO2 by absorbing the heat from water with ambient temperature and pressure. Then, it enters the turbine to generate power, comes out in a normal state, and finally is reserved in the gas storage tank. The thermal energy produced during the energy charge period is reserved in the hot salt and the hot water tanks to increase the turbine power capability during the energy discharge period. Notably, the CO2 gas storage tank is flexible enough to keep pressure constant by changing its volume during the charging and discharging periods.
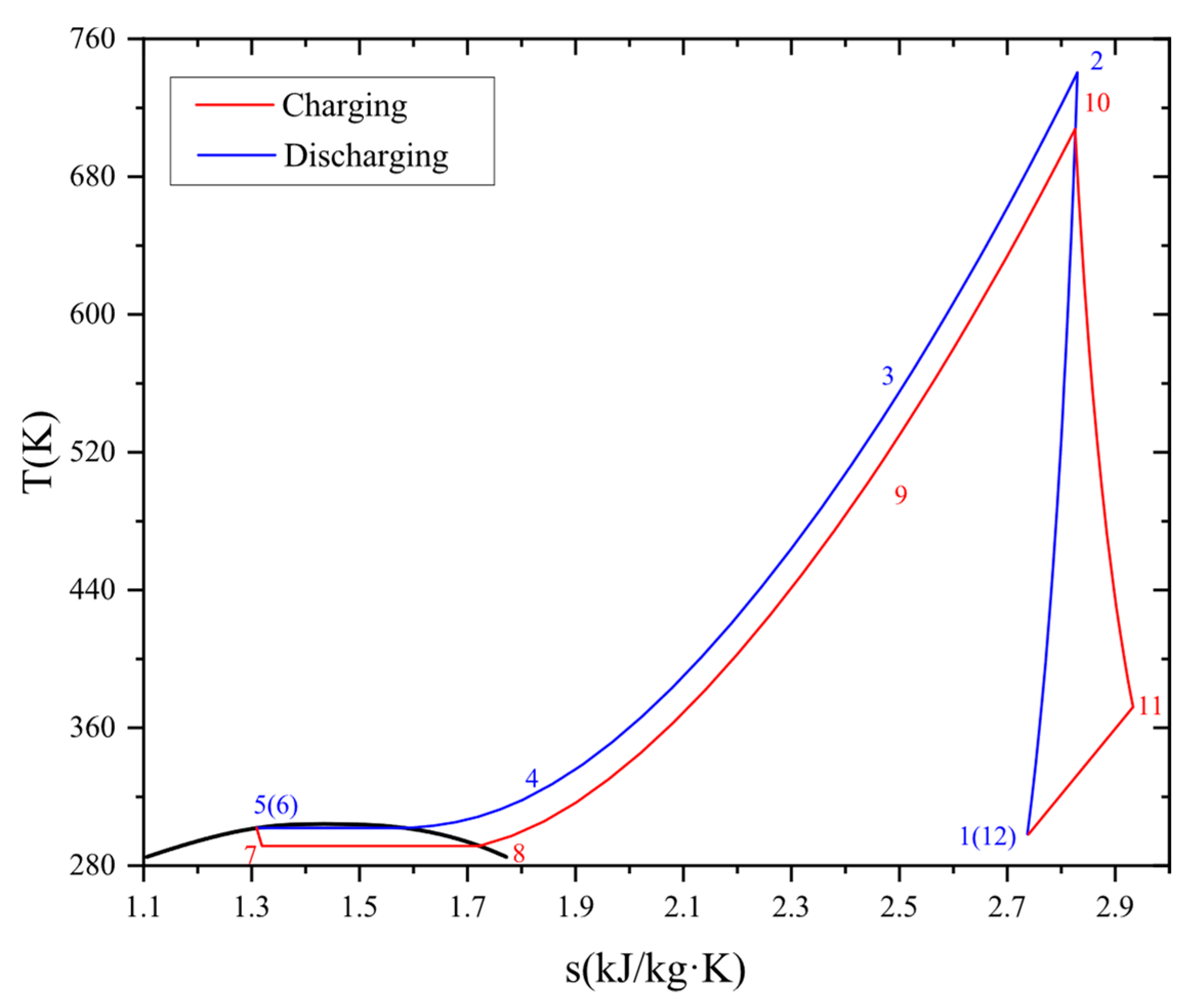
In this study, gaseous CO2 enters the compressor (1–2), the TES1, and the TES2 sequentially. CO2 with high temperature and pressure releases heat to the molten salt and cooling water (2–4) in the TES, then CO2 is further cooled to the liquid state during the condensation (4–5), and the liquid CO2 is reserved in the liquid storage tank, completing the energy charge process. In the energy discharge process, liquid CO2 expands into the gas–liquid two-phase state in the throttle (6–7), and then completely evaporates into a gaseous state through the evaporator (7–8). Gaseous CO2 absorbs heat from the TES3 and the TES4 (8–10), and then enters the turbine to output power (10–11). CO2 with low pressure is chilled by the environment (11–12) and finally reserved in the gas storage tank.
Figure 2 exhibits the temperature–entropy diagram of the system.