Improving the Phosphatase-Catalyzed Synthesis of 5′-Nucleotides: A Reaction Engineering Approach
Abstract
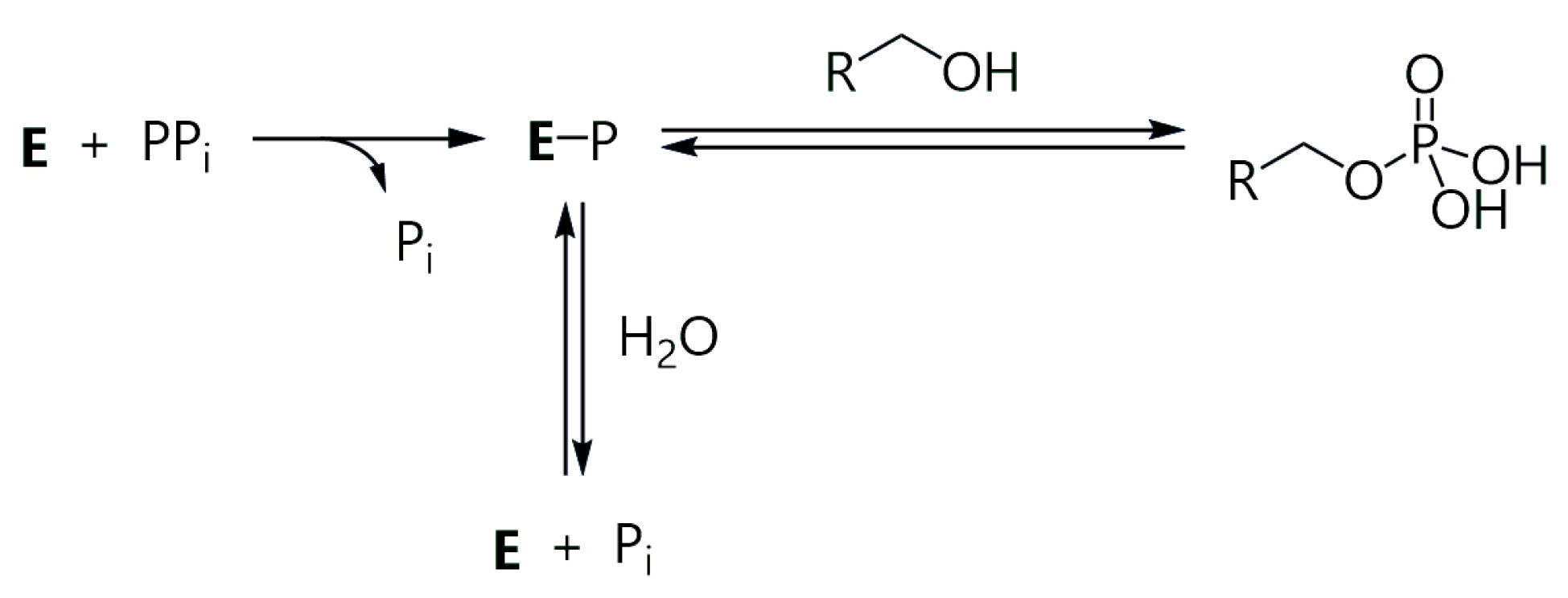
1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Expression and Purification of Recombinant PhoC-Mm
2.3. PhoC-Mm Activity Assay
2.4. Phosphorylation of Inosine Catalyzed by Phoc-Mm (Analytical Scale)
2.5. Phosphorylation of Inosine Catalyzed by Phoc-Mm (Semi-Preparative Scale)
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bowler, M.W.; Cliff, M.J.; Waltho, J.P.; Blackburn, G.M. Why did nature select phosphate for its dominant roles in biology? New J. Chem. 2010, 34, 784–794. [Google Scholar] [CrossRef]
- Sullenger, B.A.; Nair, S. From the RNA world to the clinic. Science 2016, 352, 1417–1420. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Fu, S.; Ying, J.; Zhao, Y. Prebiotic Chemistry: A review of nucleoside phosphorylation and polymerization. Open Biol. 2023, 13, 220234. [Google Scholar] [CrossRef] [PubMed]
- Jordheim, L.P.; Durantel, D.; Zoulim, F.; Dumontet, C. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat. Rev. Drug Discov. 2013, 12, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Pradere, U.; Garnier-Amblard, E.C.; Coats, S.J.; Amblard, F.; Schinazi, R.F. Synthesis of nucleoside phosphate and phosphonate prodrugs. Chem. Rev. 2014, 114, 9154–9218. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Meyerhof, W.; Hellfritsch, C.; Hofmann, T. Sweet and umami taste: Natural products, their chemosensory targets, and beyond. Angew. Chem. Int. Ed. 2011, 50, 2220–2242. [Google Scholar] [CrossRef] [PubMed]
- Morelli, C.F.; Pappalardo, V.; Brockhoff, A.; Pieraccini, S.; Sironi, M.; Sangiorgio, S.; Scarabattoli, L.; Speranza, G.; Rabuffetti, M. Purine 5′-ribonucleotide-l-glutamate hybrids as potential tools to investigate the mechanism of Umami taste reception. ChemistrySelect 2022, 7, e202204123. [Google Scholar] [CrossRef]
- Roy, B.; Depaix, A.; Périgaud, C.; Peyrottes, S. Recent trends in nucleotide synthesis. Chem. Rev. 2016, 116, 7854–7897. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Kato, T.; Takenishi, T. A novel method for phosphorylation of nucleosides to 5′-nucleotides. Tetrahedron Lett. 1967, 8, 5065–5068. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Kato, T.; Takenishi, T. Studies of phosphorylation. III. Selective phosphorylation of unprotected nucleosides. Bull. Chem. Soc. Jpn. 1969, 42, 3505–3508. [Google Scholar] [CrossRef]
- Domon, K.; Puripat, M.; Fujiyoshi, K.; Hatanaka, M.; Kawashima, S.A.; Yamatsugu, K.; Kanai, M. Catalytic chemoselective O-phosphorylation of alcohols. ACS Cent. Sci. 2020, 6, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Benner, S.A. Abiotic synthesis of nucleoside 5′-triphosphates with nickel borate and cyclic trimetaphosphate (CTMP). Astrobiology 2021, 21, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Shepard, S.M.; Jessen, H.J.; Cummins, C.C. Beyond triphosphates: Reagents and methods for chemical oligophosphorylation. J. Am. Chem. Soc. 2022, 144, 7517–7530. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, A.; Barry, A.; Burke, A.J.; Hutton, A.E.; Bell, E.L.; Green, A.P.; O’Reilly, E. Biocatalysis: Landmark discoveries and applications in chemical synthesis. Chem. Soc. Rev. 2024, 53, 2828–2850. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, R. The power of biocatalysts for highly selective and efficient phosphorylation reactions. Catalysts 2022, 12, 1436. [Google Scholar] [CrossRef]
- Ubiali, D.; Speranza, G. Enzymatic phosphorylation of nucleosides. In Enzymatic and Chemical Synthesis of Nucleic Acid Derivatives, 1st ed.; Fernández-Lucas, J., Ed.; Wiley-VCH: Weinheim, Germany, 2019; pp. 29–42. [Google Scholar]
- Serra, I.; Conti, S.; Piškur, J.; Clausen, A.R.; Munch-Petersen, B.; Terreni, M.; Ubiali, D. Immobilized Drosophila melanogaster deoxyribonucleoside kinase (DmdNK) as a high performing biocatalyst for the synthesis of purine arabinonucleotides. Adv. Synth. Catal. 2014, 356, 563–570. [Google Scholar] [CrossRef]
- Serra, I.; Ubiali, D.; Piškur, J.; Munch-Petersen, B.; Bavaro, T.; Terreni, M. Immobilization of deoxyadenosine kinase from Dictyostelium discoideum (DddAK) and its application in the 5′-phosphorylation of arabinosyladenine and arabinosyl-2-fluoroadenine. ChemistrySelect 2017, 2, 5403–5408. [Google Scholar] [CrossRef]
- Robescu, M.S.; Serra, I.; Terreni, M.; Ubiali, D.; Bavaro, T. A multi-enzymatic cascade reaction for the synthesis of vidarabine 5′-monophosphate. Catalysts 2020, 10, 60. [Google Scholar] [CrossRef]
- Fehlau, M.; Kaspar, F.; Hellendahl, K.F.; Schollmeyer, J.; Neubauer, P.; Wagner, A. Modular enzymatic cascade synthesis of nucleotides using a (d)ATP regeneration system. Front. Bioeng. Biotechnol. 2020, 8, 854. [Google Scholar]
- Widlanski, T.S.; Taylor, W. Chemistry and enzymology of phosphatases. In Comprehensive Natural Products Chemistry, 1st ed.; Barton, D., Nakanishi, K., Meth-Cohn, O., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 139–162. [Google Scholar]
- Médici, R.; Garaycoechea, J.I.; Valino, A.L.; Pereira, C.A.; Lewkowicz, E.S.; Iribarren, A.M. A comparative study on phosphotransferase activity of acid phosphatases from Raoultella planticola and Enterobacter aerogenes on nucleosides, sugars, and related compounds. Appl. Microbiol. Biotechnol. 2014, 98, 3013–3022. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Mihara, Y.; Yamada, H. A New Enzymatic method of selective phosphorylation of nucleosides. J. Mol. Catal. B Enzym. 1999, 6, 271–277. [Google Scholar] [CrossRef]
- Asano, Y.; Mihara, Y.; Yamada, H. A novel selective nucleoside phosphorylating enzyme from Morganella morganii. J. Biosci. Bioeng. 1999, 87, 732–738. [Google Scholar] [CrossRef]
- Tasnádi, G.; Zechner, M.; Hall, M.; Baldenius, K.; Ditrich, K.; Faber, K. Investigation of acid phosphatase variants for the synthesis of phosphate monoesters. Biotechnol. Bioeng. 2017, 114, 2187–2195. [Google Scholar] [CrossRef]
- Mihara, Y.; Utagawa, T.; Yamada, H.; Asano, Y. Phosphorylation of nucleosides by the mutated acid phosphatase from Morganella morganii. Appl. Environ. Microbiol. 2000, 66, 2811–2816. [Google Scholar] [CrossRef] [PubMed]
- Mihara, Y.; Utagawa, T.; Yamada, H.; Asano, Y. Acid phosphatase/phosphotransferases from enteric bacteria. J. Biosci. Bioeng. 2001, 92, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Hasan, Z.; Hartog, A.F.; van Herk, T.; Wever, R. Phosphorylation and dephosphorylation of polyhydroxy compounds by class a bacterial acid phosphatases. Org. Biomol. Chem. 2003, 1, 2833–2839. [Google Scholar] [CrossRef]
- Mihara, Y.; Ishikawa, K.; Suzuki, E.; Asano, Y. Improving the pyrophosphate-inosine phosphotransferase activity of Escherichia blattae acid phosphatase by sequential site-directed mutagenesis. Biosci. Biotechnol. Biochem. 2004, 68, 1046–1050. [Google Scholar] [CrossRef]
- Kragl, U. The role of reaction engineering in bioprocess development. Chimia 2020, 74, 378. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- VanEtten, R.L.; Waymack, P.P.; Rehkop, D.M. Transition metal ion inhibition of enzyme-catalyzed phosphate ester displacement reactions. J. Am. Chem. Soc. 1974, 96, 6782–6785. [Google Scholar] [CrossRef] [PubMed]
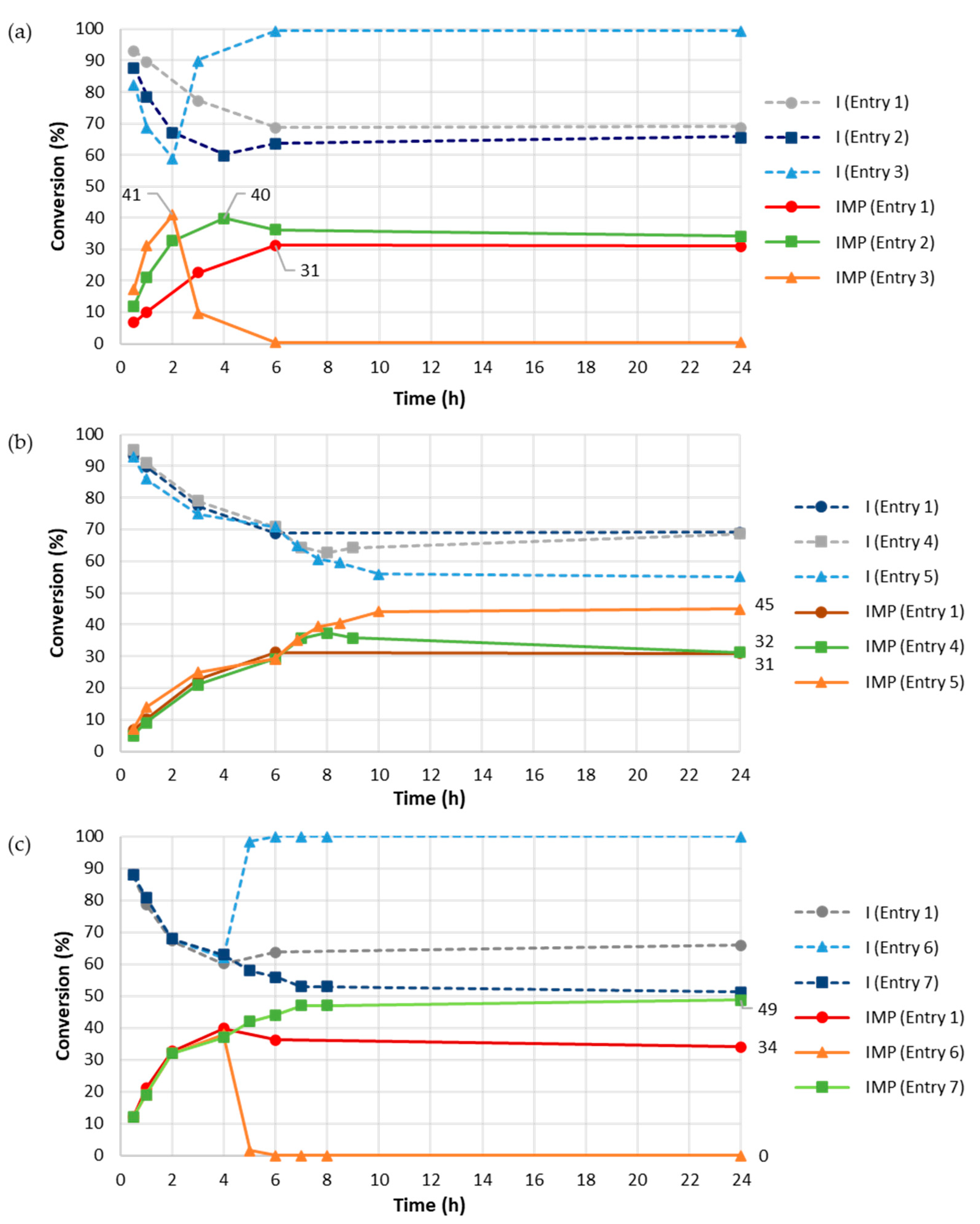
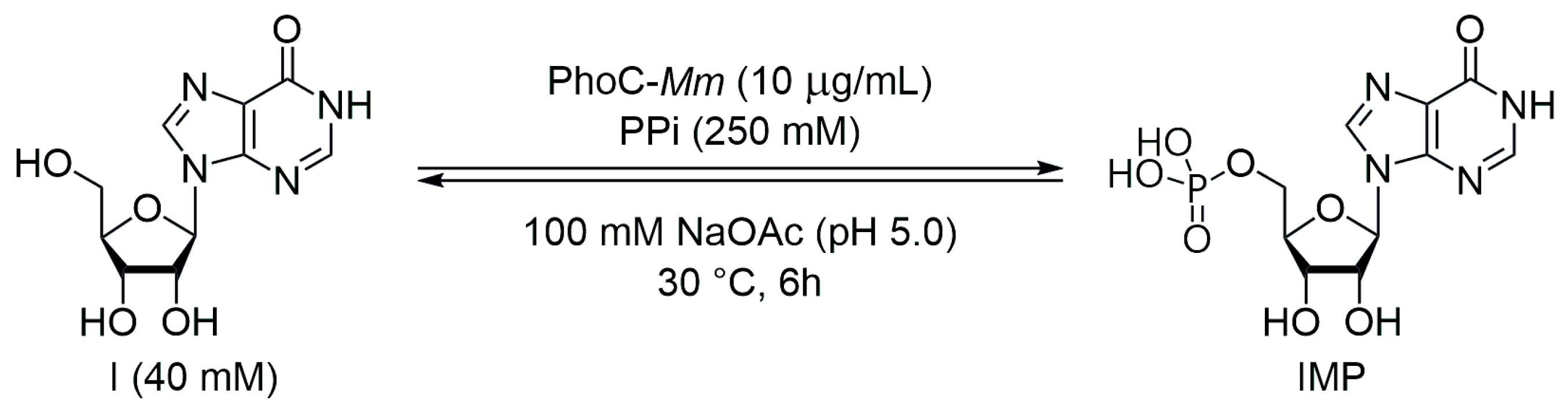
| Entry 1 | Assessed Variable | I (mM) | PPi (mM) | Buffer | Time (h) 2 | IMP (%) 3 |
|---|---|---|---|---|---|---|
| 1 | reference reaction | 40 | 250 | NaOAc 100 mM pH 5.0 | 6 | 31 |
| 2 | buffer | 40 | 250 | MES 100 mM pH 5.5 | 24 | 29 |
| 3 | buffer | 40 | 250 | Sodium citrate 100 mM pH 5.0 | 6 | 14 |
| Entry 1 | Assessed Variable | I (mM) | PPi (mM) | Buffer | Time (h) 2 | IMP (%) 3 |
|---|---|---|---|---|---|---|
| 1 | reference reaction | 40 | 250 | NaOAc 100 mM pH 5.0 | 6 | 31 |
| 4 | [PPi] | 40 | 200 | NaOAc 100 mM pH 5.0 | 6 | 21 |
| 5 | [PPi] | 40 | 400 | NaOAc 100 mM pH 5.0 | 6 | 19 |
| 6 | fed-batch PPi addition 4 | 40 | 250 | NaOAc 100 mM pH 5.0 | 6 | 9 |
| 7 | fed-batch PPi addition 5 | 40 | 500 | NaOAc 100 mM pH 5.0 | 8 | 34 |
| 8 | fed-batch PPi addition 6 | 80 | 750 | NaOAc 100 mM pH 5.0 | 9 | 44 |
| Entry 1 | Assessed Variable | I (mM) | PPi (mM) | Buffer | Time (h) 2 | IMP (%) 3 |
|---|---|---|---|---|---|---|
| 1 | reference reaction | 40 | 250 | NaOAc 100 mM pH 5.0 | 6 | 31 |
| 9 | [I] 4 | 100 | 250 | NaOAc 100 mM pH 5.0 | 6 | 22 |
| 10 | [I] 5 | 200 | 250 | NaOAc 100 mM pH 5.0 | 6 | 18 |
| 11 | [I] 6 | 100 | 250 | NaOAc 100 mM pH 5.0 | 6 | 29 |
| 12 | [I] | 80 | 250 | NaOAc 100 mM pH 5.0 | 6 | 26 |
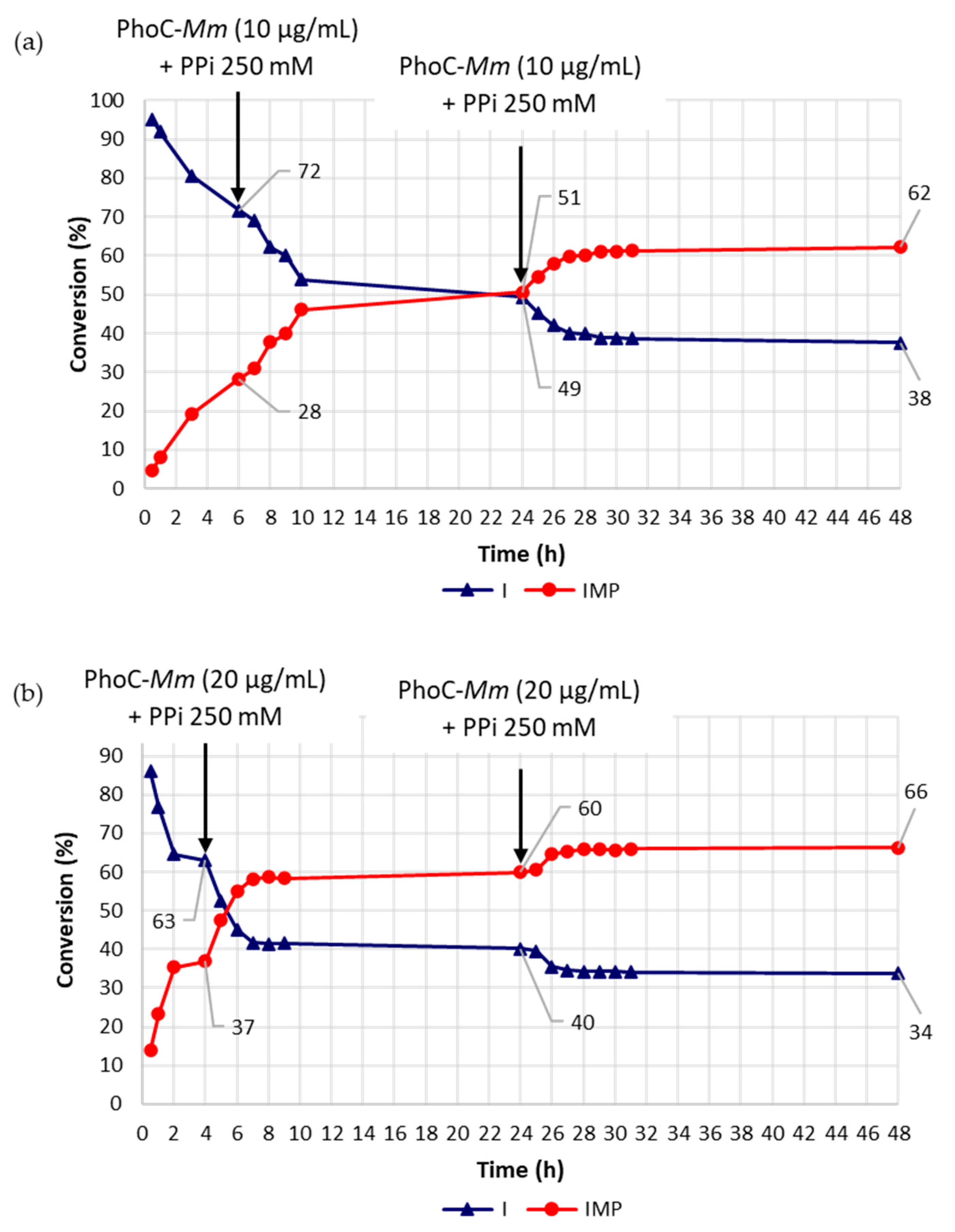
| Entry 1 | Assessed Variable(s) | PhoC-Mm (μg/mL (Addition Time)) | PPi (mM (Addition Time)) | Time (h) 2 | IMP (%) 3 |
|---|---|---|---|---|---|
| 1 | reference reaction | 10 (t0) | 250 (t0) | 6 | 31 |
| 2 | [Phoc-Mm] | 20 (t0) | 250 (t0) | 4 | 40 |
| 3 | [Phoc-Mm] | 30 (t0) | 250 (t0) | 2 | 41 |
| 4 | multiple additions of Phoc-Mm and PPi | 10 (t0), 10 (6 h) | 250 (t0) | 8 | 37 |
| 5 | multiple additions of Phoc-Mm and PPi | 10 (t0), 10 (6 h) | 250 (t0), 250 (6 h) | 10 | 44 |
| 6 | multiple additions of Phoc-Mm and PPi | 20 (t0), 20 (4 h) | 250 (t0) | 4 | 37 |
| 7 | multiple additions of Phoc-Mm and PPi | 20 (t0), 20 (4 h) | 250 (t0); 250 (4 h) | 24 | 49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robescu, M.S.; Bavaro, T.; Medici, F.; Speranza, G.; Ubiali, D.; Rabuffetti, M. Improving the Phosphatase-Catalyzed Synthesis of 5′-Nucleotides: A Reaction Engineering Approach. Appl. Sci. 2024, 14, 6227. https://doi.org/10.3390/app14146227
Robescu MS, Bavaro T, Medici F, Speranza G, Ubiali D, Rabuffetti M. Improving the Phosphatase-Catalyzed Synthesis of 5′-Nucleotides: A Reaction Engineering Approach. Applied Sciences. 2024; 14(14):6227. https://doi.org/10.3390/app14146227
Chicago/Turabian StyleRobescu, Marina S., Teodora Bavaro, Fabrizio Medici, Giovanna Speranza, Daniela Ubiali, and Marco Rabuffetti. 2024. "Improving the Phosphatase-Catalyzed Synthesis of 5′-Nucleotides: A Reaction Engineering Approach" Applied Sciences 14, no. 14: 6227. https://doi.org/10.3390/app14146227
APA StyleRobescu, M. S., Bavaro, T., Medici, F., Speranza, G., Ubiali, D., & Rabuffetti, M. (2024). Improving the Phosphatase-Catalyzed Synthesis of 5′-Nucleotides: A Reaction Engineering Approach. Applied Sciences, 14(14), 6227. https://doi.org/10.3390/app14146227








