The Use of Beech Bark (Latin: Fagus sylvatica) and Birch Bark (Latin: Betula pendula Roth) for the Removal of Cationic Dyes from Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Sorbents
2.2. Sorbates (Dyes)
2.3. Preparation of Sorbents
- −
- Beech bark—fine fraction (BBeF);
- −
- Beech bark—coarse fraction (BBeC);
- −
- Birch bark—fine fraction (BBeF);
- −
- Birch bark—coarse fraction (BBeC).
2.4. Preparation of Dyes
2.5. Effect of the pH Value on the Efficiency of Dye Sorption
2.6. Research to Determine the Equilibrium Time
2.7. Determination of the Maximum Sorption Capacity
2.8. Calculation Methods
- Qe—Mass of the sorbed dye [mg/g DM];
- Co—Initial dye concentration [mg/L];
- Cs—Concentration of dye after sorption [mg/L];
- V—Volume of solution [L];
- M—Sorbent mass [g DM].
- q—Instantaneous value of sorbed dye [mg/g];
- qe—The amount of dye sorbed at the equilibrium state [mg/g];
- t—Time of sorption [min];
- k1—Pseudo-first-order adsorption rate constant [1/min];
- k2—Pseudo-second-order adsorption rate constant [g/(mg × min)];
- kid—Intraparticular diffusion model adsorption rate constant [mg/(g × min0.5)].
- Langmuir’s isotherm (5):
- Qe—Equilibrium amount of sorbed dye [mg/g DM];
- Qmax—Maximum sorption capacity [mg/g DM];
- KC—Constant used in the Langmuir’s equation [L/mg];
- Ce—Concentration of dye remaining in the solution [mg/L].
- Freundlich‘s isotherm (6):
- Qe—Actual sorption of sorbate on the sorbent [mg/g DM];
- K—Sorption equilibrium constant used in Freundlich’s model;
- Ce—Concentration of dye remaining in the solution [mg/L];
- n—Heterogeneity parameter.
3. Results and Discussion
3.1. FTIR Analysis
3.2. Effect of pH on the Efficiency of Dye Sorption
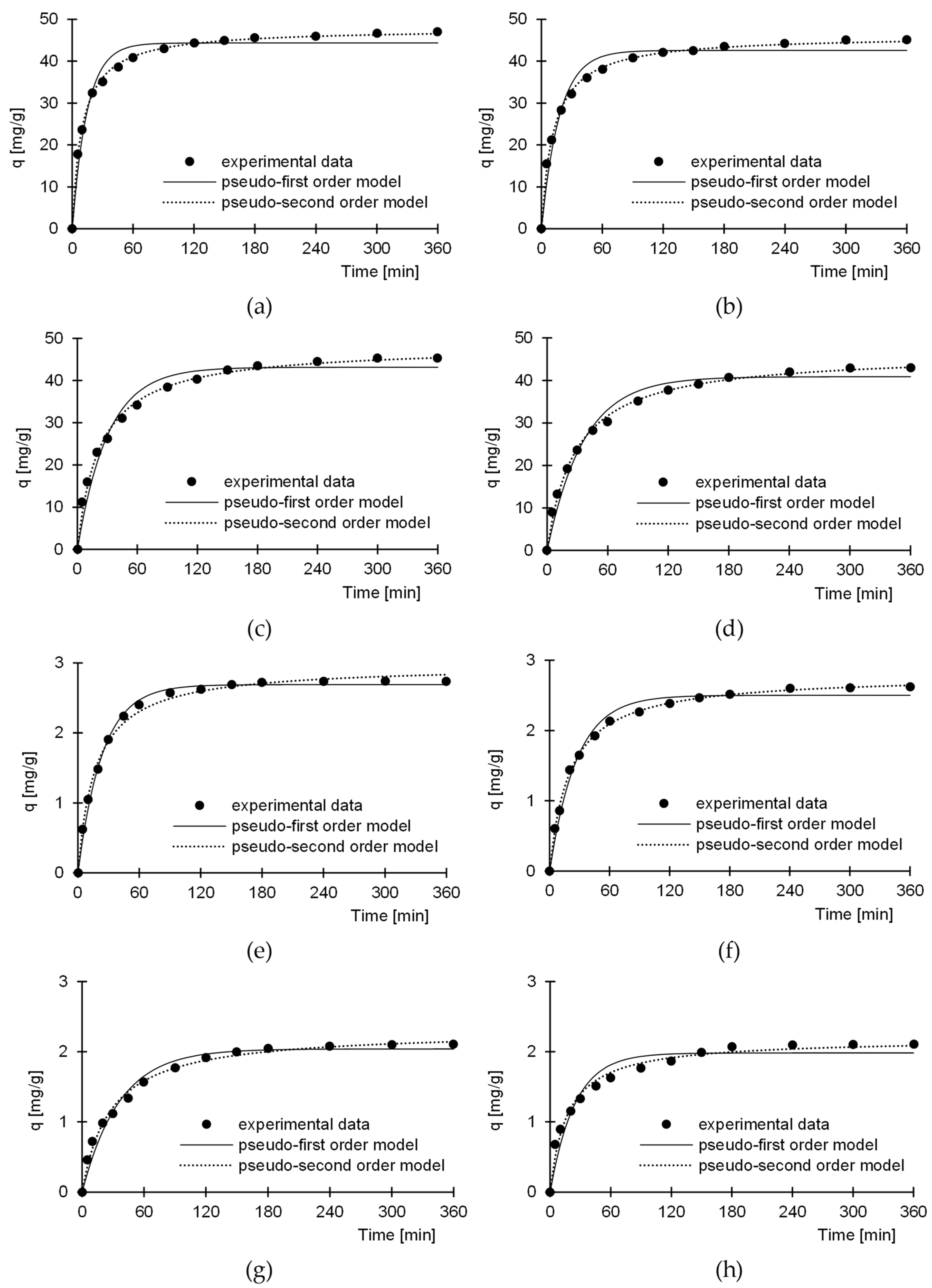
3.3. Kinetics of Dye Sorption
3.4. Sorption Capacity of Beech and Birch Barks
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiśniewska, M.; Chibowski, S.; Wawrzkiewicz, M.; Onyszko, M.; Bogatyrov, V. CI Basic Red 46 Removal from Sewage by Carbon and Silica Based Composite: Equilibrium, Kinetic and Electrokinetic Studies. Molecules 2022, 27, 1043. [Google Scholar] [CrossRef] [PubMed]
- Nigam, P.; Armour, G.; Banat, I.M.; Singh, D.; Marchant, R. Physical Removal of Textile Dyes from Effluents and Solid-State Fermentation of Dye-Adsorbed Agricultural Residues. Bioresour. Technol. 2000, 72, 219–226. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of Dyes in Textile Effluent: A Critical Review on Current Treatment Technologies with a Proposed Alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Ihaddaden, S.; Aberkane, D.; Boukerroui, A.; Robert, D. Removal of Methylene Blue (Basic Dye) by Coagulation-Flocculation with Biomaterials Bentonite and Opuntia Ficus Indica. J. Water Process Eng. 2022, 49, 102952. [Google Scholar] [CrossRef]
- Klimiuk, E.; Filipkowska, U.; Korzeniowska, A. Effects of PH and Coagulant Dosage on Effectiveness of Coagulation of Reactive Dyes from Model Wastewater by Polyaluminium Chloride (PAC). Pol. J. Environ. Stud. 1999, 8, 73–80. [Google Scholar]
- Ghalwa, N.M.; Saqer, A.M.; Farhat, N.B. Removal of Reactive Red 24 Dye by Clean Electrocoagulation Process Using Iron and Aluminum Electrodes. J. Chem. Eng. Process Technol. 2015, 7, 269. [Google Scholar] [CrossRef]
- Nandi, B.K.; Patel, S. Effects of Operational Parameters on the Removal of Brilliant Green Dye from Aqueous Solutions by Electrocoagulation. Arab. J. Chem. 2017, 10, S2961–S2968. [Google Scholar] [CrossRef]
- Ahangarnokolaei, M.A.; Ganjidoust, H.; Ayati, B. Optimization of Parameters of Electrocoagulation/Flotation Process for Removal of Acid Red 14 with Mesh Stainless Steel Electrodes. J. Water Reuse Desalination 2018, 8, 278–292. [Google Scholar] [CrossRef]
- Janoš, P.; Buchtová, H.; Rýznarová, M. Sorption of Dyes from Aqueous Solutions onto Fly Ash. Water Res. 2003, 37, 4938–4944. [Google Scholar] [CrossRef]
- Yang, Z.; Asoh, T.A.; Uyama, H. Removal of Cationic or Anionic Dyes from Water Using Ion Exchange Cellulose Monoliths as Adsorbents. Bull. Chem. Soc. Jpn. 2019, 92, 1453–1461. [Google Scholar] [CrossRef]
- Hassan, M.M.; Carr, C.M. A Critical Review on Recent Advancements of the Removal of Reactive Dyes from Dyehouse Effluent by Ion-Exchange Adsorbents. Chemosphere 2018, 209, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Raut, P.; Pal, D.; Singh, V.K. Dye Removal Using Activated Sludge. Biol. Approaches Dye. Contain. Wastewater 2022, 2, 1–16. [Google Scholar] [CrossRef]
- Widajatno, R.L.; Kardena, E.; Arifianingsih, N.N.; Helmy, Q. Activated sludge: Conventional dye treatment technique. In Biological Approaches in Dye-Containing Wastewater; Springer: Singapore, 2022; Volume 1, pp. 119–153. [Google Scholar] [CrossRef]
- Rane, A.; Joshi, S.J. Biodecolorization and Biodegradation of Dyes: A Review. Open Biotechnol. J. 2021, 15, 97–108. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Estrella-Nuñez, J.; Pardo, S.; Bucio, E. Interaction of Dye Molecules with Fungi: Operational Parameters and Mechanisms. In Dye Biodegradation, Mechanisms and Techniques. Sustainable Textiles: Production, Processing, Manufacturing & Chemistry; Muthu, S.S., Khadir, A., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’rabet, S.; Lgaz, H.; El Bachiri, A.; El Harfi, A. Dyes: Classification, Pollution, and Environmental Effects. Muthu, S.S., Khadir, A., Eds.; In Dye Biodegradation, Mechanisms and Techniques. Sustainable Textiles: Production, Processing, Manufacturing & Chemistry; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Kamal, I.M.; Abdeltawab, N.F.; Ragab, Y.M.; Farag, M.A.; Ramadan, M.A. Biodegradation, Decolorization, and Detoxification of Di-Azo Dye Direct Red 81 by Halotolerant, Alkali-Thermo-Tolerant Bacterial Mixed Cultures. Microorganisms 2022, 10, 994. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic Degradation of Dyes Using Semiconductor Photocatalysts to Clean Industrial Water Pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Panchal, D.; Sharma, A.; Pal, S. Novel Photocatalytic Techniques for Organic Dye Degradation in Water. In Photocatalytic Degradation of Dyes: Current Trends and Future Perspectives; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–22. [Google Scholar] [CrossRef]
- Nazri, M.K.H.M.; Sapawe, N. A Short Review on Photocatalytic toward Dye Degradation. Mater. Today Proc. 2020, 31, A42–A47. [Google Scholar] [CrossRef]
- Negarestani, M.; Etemadifar, P.; Kheradmand, A. Advanced Oxidation Processes for Dye Removal; Springer: Berlin/Heidelberg, Germany, 2021; pp. 71–128. [Google Scholar] [CrossRef]
- Thanavel, M.; Bankole, P.O.; Selvam, R.; Govindwar, S.P.; Sadasivam, S.K. Synergistic Effect of Biological and Advanced Oxidation Process Treatment in the Biodegradation of Remazol Yellow RR Dye. Sci. Rep. 2020, 10, 20234. [Google Scholar] [CrossRef]
- Buthiyappan, A.; Abdul Aziz, A.R.; Wan Daud, W.M.A. Recent Advances and Prospects of Catalytic Advanced Oxidation Process in Treating Textile Effluents. Rev. Chem. Eng. 2016, 32, 1–47. [Google Scholar] [CrossRef]
- Cuiping, B.; Xianfeng, X.; Wenqi, G.; Dexin, F.; Mo, X.; Zhongxue, G.; Nian, X. Removal of Rhodamine B by Ozone-Based Advanced Oxidation Process. Desalination 2011, 278, 84–90. [Google Scholar] [CrossRef]
- Basturk, E.; Karatas, M. Decolorization of antraquinone dye Reactive Blue 181 solution by UV/H2O2 process. J. Photochem. Photobiol. A Chem. 2015, 299, 67–72. [Google Scholar] [CrossRef]
- Hidalgo, A.M.; León, G.; Gómez, M.; Murcia, M.D.; Gómez, E.; Macario, J.A. Removal of Different Dye Solutions: A Comparison Study Using a Polyamide NF Membrane. Membranes 2020, 10, 408. [Google Scholar] [CrossRef] [PubMed]
- Moradihamedani, P. Recent Advances in Dye Removal from Wastewater by Membrane Technology: A Review. Polym. Bull. 2022, 79, 2603–2631. [Google Scholar] [CrossRef]
- Dasgupta, J.; Sikder, J.; Chakraborty, S.; Curcio, S.; Drioli, E. Remediation of Textile Effluents by Membrane Based Treatment Techniques: A State of the Art Review. J. Environ. Manag. 2015, 147, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Cevallos-Mendoza, J.; Amorim, C.G.; Rodríguez-Díaz, J.M.; Montenegro, M.d.C.B.S.M. Removal of Contaminants from Water by Membrane Filtration: A Review. Membranes 2022, 12, 570. [Google Scholar] [CrossRef] [PubMed]
- Gubari, M.Q.; Zwain, H.M.; Hassan, W.H.; Vakili, M.; Majdi, A. Desalination of Pigment Industry Wastewater by Reverse Osmosis Using OPM-K Membrane. Case Stud. Chem. Environ. Eng. 2023, 8, 100401. [Google Scholar] [CrossRef]
- Mulyanti, R.; Susanto, H. Wastewater Treatment by Nanofiltration Membranes. IOP Conf. Ser. Earth Environ. Sci. 2018, 142, 012017. [Google Scholar] [CrossRef]
- Desiriani, R.; Susanto, H.; Aryanti, N. Performance Evaluation of Nanofiltration Membranes for Dye Removal of Synthetic Hand-Drawn Batik Industry Wastewater. Environ. Prot. Eng. 2022, 48, 51–68. [Google Scholar] [CrossRef]
- Abdi, G.; Alizadeh, A.; Zinadini, S.; Moradi, G. Removal of Dye and Heavy Metal Ion Using a Novel Synthetic Polyethersulfone Nanofiltration Membrane Modified by Magnetic Graphene Oxide/Metformin Hybrid. J. Memb. Sci. 2018, 552, 326–335. [Google Scholar] [CrossRef]
- Aouni, A.; Fersi, C.; Cuartas-Uribe, B.; Bes-Pía, A.; Alcaina-Miranda, M.I.; Dhahbi, M. Reactive Dyes Rejection and Textile Effluent Treatment Study Using Ultrafiltration and Nanofiltration Processes. Desalination 2012, 297, 87–96. [Google Scholar] [CrossRef]
- Meez, E.; Rahdar, A.; Kyzas, G.Z. Sawdust for the Removal of Heavy Metals from Water: A Review. Molecules 2021, 26, 4318. [Google Scholar] [CrossRef]
- Gupta, V.K. Suhas Application of Low-Cost Adsorbents for Dye Removal—A Review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent Advances on the Removal of Dyes from Wastewater Using Various Adsorbents: A Critical Review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Shukla, A.; Zhang, Y.H.; Dubey, P.; Margrave, J.L.; Shukla, S.S. The Role of Sawdust in the Removal of Unwanted Materials from Water. J. Hazard. Mater. 2002, 95, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.R.; Pai, R.S. Adsorption of Cu(II), Ni(II) and Zn(II) on Modified Jute Fibres. Bioresour. Technol. 2005, 96, 1430–1438. [Google Scholar] [CrossRef]
- Rubio, A.J.; Silva, I.Z.; Gasparotto, F.; Paccola, E.A.S.; Silva, C.N.; Emanuelli, I.P.; Bergamasco, R.; Yamaguchi, N.U. Removal of Methylene Blue Using Cassava Bark Residue. Chem. Eng. Trans. 2018, 65, 751–756. [Google Scholar] [CrossRef]
- Shamsheer, H.B.; Mughal, T.A.; Ishaq, A.; Zaheer, S.; Zahid, K. Extraction of Ecofriendly Leather Dyes from Plants Bark. Pak. J. Sci. Ind. Res. Ser. A Phys. Sci. 2017, 60, 96–100. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K. Removal of Anionic Dye Congo Red from Aqueous Solution by Raw Pine and Acid-Treated Pine Cone Powder as Adsorbent: Equilibrium, Thermodynamic, Kinetics, Mechanism and Process Design. Water Res. 2012, 46, 1933–1946. [Google Scholar] [CrossRef] [PubMed]
- Litefti, K.; Freire, M.S.; Stitou, M.; González-Álvarez, J. Adsorption of an Anionic Dye (Congo Red) from Aqueous Solutions by Pine Bark. Sci. Rep. 2019, 9, 16530. [Google Scholar] [CrossRef]
- Annadurai, G.; Juang, R.S.; Lee, D.J. Use of Cellulose-Based Wastes for Adsorption of Dyes from Aqueous Solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef]
- Gul, S.; Kanwal, M.; Qazi, R.A.; Gul, H.; Khattak, R.; Khan, M.S.; Khitab, F.; Krauklis, A.E. Efficient Removal of Methyl Red Dye by Using Bark of Hopbush. Water 2022, 14, 2831. [Google Scholar] [CrossRef]
- Al-Zawahreh, K.; Barral, M.T.; Al-Degs, Y.; Paradelo, R. Competitive Removal of Textile Dyes from Solution by Pine Bark-Compost in Batch and Fixed Bed Column Experiments. Environ. Technol. Innov. 2022, 27, 102421. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.N.W.; Jaafar, J.; Asri, S.E.A.M.; Ismail, A.F. Physicochemical Properties of “Green” Nanocrystalline Cellulose Isolated from Recycled Newspaper. RSC Adv. 2015, 5, 29842–29849. [Google Scholar] [CrossRef]
- Pavithra, R.; Gunasekaran, S.; Sailatha, E.S.; Kamatchi, S. Investigations on Paper Making Raw Materials and Determination of Paper Quality by FTIR-UATR and UV-Vis DRS Spectroscopy. Int. J. Curr. Res. Acad. Rev. 2015, 3, 42–59. [Google Scholar]
- Woźniak, M.; Ratajczak, I.; Szentner, K.; Kwaśniewska, P.; Mazela, B. Propolis and Organosilanes in Wood Protection. Part I: FTIR Analysis and Biological Tests. Ann. Wars. Univ. Life Sci. SGGW 2015, 91, 218–224. [Google Scholar]
- Kaya, M.; Sargin, I.; Aylanc, V.; Tomruk, M.N.; Gevrek, S.; Karatoprak, I.; Colak, N.; Sak, Y.G.; Bulut, E. Comparison of Bovine Serum Albumin Adsorption Capacities of α-Chitin Isolated from an Insect and β-Chitin from Cuttlebone. J. Ind. Eng. Chem. 2016, 38, 146–156. [Google Scholar] [CrossRef]
- Bhavsar, P.S.; Dalla Fontana, G.; Zoccola, M. Sustainable Superheated Water Hydrolysis of Black Soldier Fly Exuviae for Chitin Extraction and Use of the Obtained Chitosan in the Textile Field. ACS Omega 2021, 6, 8884–8896. [Google Scholar] [CrossRef] [PubMed]
- Halib, N.; Amin, M.C.I.M.; Ahmad, I. Physicochemical Properties and Characterization of Nata de Coco from Local Food Industries as a Source of Cellulose. Sains Malays. 2012, 41, 205–211. [Google Scholar]
- Vârban, R.; Cris, I.; Vârban, D.; Ona, A.; Olar, L.; Stoie, A.; Tefan, R.S.; Cavaco, A.M.; Marques Da Silva, J.; Guerra, R.; et al. Comparative FT-IR Prospecting for Cellulose in Stems of Some Fiber Plants: Flax, Velvet Leaf, Hemp and Jute. Appl. Sci. 2021, 11, 8570. [Google Scholar] [CrossRef]
- Popescu, M.C.; Popescu, C.M.; Lisa, G.; Sakata, Y. Evaluation of Morphological and Chemical Aspects of Different Wood Species by Spectroscopy and Thermal Methods. J. Mol. Struct. 2011, 988, 65–72. [Google Scholar] [CrossRef]
- Md Salim, R.; Asik, J.; Sani Sarjadi, M. Chemical Functional Groups of Extractives, Cellulose and Lignin Extracted from Native Leucaena Leucocephala Bark. Wood Sci. Technol. 2021, 55, 295–313. [Google Scholar] [CrossRef]
- Rao, H.; Yang, Y.; Hu, X.; Yu, J.; Jiang, H. Identification of an Ancient Birch Bark Quiver from a Tang Dynasty (A.D. 618-907) Tomb in Xinjiang, Northwest China. Econ. Bot. 2017, 71, 32–44. [Google Scholar] [CrossRef]
- Bouatay, F.; Dridi-Dhaouadi, S.M.F.; Mhenni, M.F. Valorization of Tunisian Pottery Clay onto Basic Dyes Adsorption. Int. J. Environ. Res. 2014, 8, 1053–1066. [Google Scholar]
- Mahmoodi, N.M.; Arami, M.; Bahrami, H.; Khorramfar, S. Novel Biosorbent (Canola Hull): Surface Characterization and Dye Removal Ability at Different Cationic Dye Concentrations. Desalination 2010, 264, 134–142. [Google Scholar] [CrossRef]
- El Haddad, M.; Mamouni, R.; Saffaj, N.; Lazar, S.; Saïd Lazar, S. Removal of a Cationic Dye-Basic Red 12-from Aqueous Solution by Adsorption onto Animal Bone Meal Removal of a Cationic Dye-Basic Red 12-from Aqueous Solution by Adsorption onto Animal Bone Meal. J. Assoc. Arab. Univ. Basic. Appl. Sci. 2018, 12, 48–54. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, W.; Song, Y.; Zhuang, H.; Tang, H. Removal of Cationic Dye BR46 by Biochar Prepared from Chrysanthemum Morifolium Ramat Straw: A Study on Adsorption Equilibrium, Kinetics and Isotherm. J. Mol. Liq. 2021, 340, 116617. [Google Scholar] [CrossRef]
- Yeddou, N.; Bensmaili, A. Kinetic Models for the Sorption of Dye from Aqueous Solution by Clay-Wood Sawdust Mixture. Desalination 2005, 185, 499–508. [Google Scholar] [CrossRef]
- Sahnoun, A.Y.; Selatnia, A.; Mitu, L.; Ayeche, R.; Daoud, N.; Dahoun-Tchoulak, Y. Basic Red 46 Adsorption Studies onto Pyrolyzed By-Product Biomass. Appl. Water Sci. 2024, 14, 111. [Google Scholar] [CrossRef]
- Karim, A.B.; Mounir, B.; Hachkar, M.; Bakasse, M.; Rais, Z.; Yaacoubi, A. Adsorption of BR46 Dye Using Raw and Purified Clay. J. Water Sci. Environ. Technol. 2017, 2, 233–240. [Google Scholar]
- Zamouche, M.; Hamdaoui, O. Sorption of Rhodamine B by Cedar Cone: Effect of Ph and Ionic Strength. Energy Procedia 2012, 18, 1228–1239. [Google Scholar] [CrossRef]
- Shen, K.; Gondal, M.A. Removal of Hazardous Rhodamine Dye from Water by Adsorption onto Exhausted Coffee Ground. J. Saudi Chem. Soc. 2017, 21, S120–S127. [Google Scholar] [CrossRef]
- Pengthamkeerati, P.; Satapanajaru, T.; Chatsatapattayakul, N.; Chairattanamanokorn, P.; Sananwai, N. Alkaline Treatment of Biomass Fly Ash for Reactive Dye Removal from Aqueous Solution. Desalination 2010, 261, 34–40. [Google Scholar] [CrossRef]
- Alivio, R.K.O.; Go, A.W.; Angkawijaya, A.E.; Santoso, S.P.; Gunarto, C.; Soetaredjo, F.E. Extractives-Free Sugarcane Bagasse as Adsorbent for the Removal of Rhodamine B (Basic Violet 10) with High Capacity and Reusability. J. Ind. Eng. Chem. 2023, 124, 175–200. [Google Scholar] [CrossRef]
- Al-Zawahreh, K.; Barral, M.T.; Al-Degs, Y.; Paradelo, R. Comparison of the Sorption Capacity of Basic, Acid, Direct and Reactive Dyes by Compost in Batch Conditions. J. Environ. Manag. 2021, 294, 113005. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.; Paradelo, R.; García, P.; González, A.; Al-Zawahreh, K.; Barral, M.T. Influence of Zinc and Humic Acids on Dye Adsorption from Water by Two Composts. Int. J. Environ. Res. Public Health 2023, 20, 5353. [Google Scholar] [CrossRef]
- Oyekanmi, A.A.; Ahmad, A.; Hossain, K.; Rafatullah, M. Adsorption of Rhodamine B Dye from Aqueous Solution onto Acid Treated Banana Peel: Response Surface Methodology, Kinetics and Isotherm Studies. PLoS ONE 2019, 14, e0216878. [Google Scholar] [CrossRef] [PubMed]







| Component | Beech Bark | Birch Bark |
|---|---|---|
| Lignin [%] | 32.87 | 40.30 |
| Cellulose [%] | 30.49 | 25.20 |
| Hemicellulose [%] | 32.34 | 30.50 |
| Ash; mineral substances; other ingredients [%] | 4.30 | 4.00 |
| Dye | Basic Violet 10—(BV10) | Basic Red 46—(BR46) |
|---|---|---|
| Structural formula |  |  |
| Molar mass | 479 g/mol | 401 g/mol |
| Summaric formula | C28H31ClN2O3 | C18H21BrN6 |
| λmax | 554 [nm] | 530 [nm] |
| Type of dye | cationic | cationic |
| Application | dyeing paper, leather, cotton; paint production | dyeing of fibers and synthetic fibers; printing |
| Dye | Conc. of Dye [mg/L] | pH of Dye Solutions | Sorbent Dose [g DM/L] | Mixing Speed [r.p.m] | Sorpt. Time [min] | Temp. [°C] |
|---|---|---|---|---|---|---|
| BV10 | 5 | 2/3/4/5/6/7/8/9/10/11 | 1 | 120 | 120 | 22 |
| BR46 | 50 | 2/3/4/5/6/7/8 | 1 | 120 | 120 | 22 |
| Sorbent | Dye | Conc. of Dye [mg/L] | pH of Dye Solutions | Sorbent Dose (for All Series) [g DM/L] | Sampling Time (for All Series) [min] | Mixing Speed [r.p.m.] | Temp. [°C] |
|---|---|---|---|---|---|---|---|
| BBeF | BV10 | 5 | Optimal, determined for each dye in point 2.5 | 1 | 0, 5, 10, 20, 30, 45, 60, 90, 120, 150, 180, 240, 300, 360 | 120 | 22 |
| BBeC | BR46 | 50 | |||||
| BBiF | BV10 | 5 | |||||
| BBiC | BR46 | 50 |
| Sorbent | Sorbent Dose (for All Series) [g/L] | Dye | Dye Conc. [mg/L] | pH of the Solutions | Sampling Time [min] | Mixing Speed [r.p.m.] | Temp. [°C] |
|---|---|---|---|---|---|---|---|
| BBeF BBeC BBiF BBiC | 1 | BV10 BR46 | 10, 50, 75, 100, 200, 300, 400, 500 | Optimal, determined for each dye in point 2.5 | Equilibrium time determined for each dye in Section 2.6 | 120 | 22 |
| Dye (Initial Conc.) | Sorbent | Pseudo-First-Order Model | Pseudo-Second-Order Model | Exp. Data | Equil. Time | ||||
|---|---|---|---|---|---|---|---|---|---|
| k1 | qe,(cal.) | R2 | k2 | qe,(cal.) | R2 | ||||
| [1/min] | [mg/g] | - | [g/mg × min] | [mg/g] | - | [mg/g] | [min] | ||
| BR46 (50 mg/L) | BBeC | 0.0564 | 42.55 | 0.9665 | 0.0018 | 46.19 | 0.9975 | 45.10 | 300 |
| BBeF | 0.0678 | 44.33 | 0.9669 | 0.0021 | 47.80 | 0.9980 | 46.99 | 300 | |
| BBiC | 0.0277 | 40.89 | 0.9773 | 0.0008 | 46.45 | 0.9969 | 43.01 | 300 | |
| BBiF | 0.0328 | 43.12 | 0.9712 | 0.0009 | 48.24 | 0.9955 | 45.34 | 300 | |
| BV10 (5 mg/L) | BBeC | 0.0373 | 2.50 | 0.9842 | 0.0899 | 2.79 | 0.9986 | 2.62 | 180 |
| BBeF | 0.0418 | 2.69 | 0.9951 | 0.0195 | 2.97 | 0.9946 | 2.74 | 180 | |
| BBiC | 0.0409 | 1.98 | 0.9425 | 0.0271 | 2.18 | 0.9858 | 2.10 | 180 | |
| BBiF | 0.0277 | 2.04 | 0.9764 | 0.0159 | 2.30 | 0.9926 | 2.11 | 180 | |
| Dye (Initial Conc.) | Sorbent | PHASE 1 | PHASE 2 | PHASE 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| kd1 | Time | R2 | kd2 | Time | R2 | kd3 | Time | R2 | ||
| * | [min] | - | * | [min] | - | * | [min] | - | ||
| BR46 (50 mg/L) | BBeC | 5.940 | 30 | 0.9895 | 1.758 | 60 | 0.9501 | 0.472 | 210 | 0.9804 |
| BBeF | 6.553 | 30 | 0.9811 | 1.638 | 90 | 0.9449 | 0.353 | 180 | 0.9724 | |
| BBiC | 4.278 | 45 | 0.9990 | 2.308 | 75 | 0.9923 | 0.810 | 180 | 0.9634 | |
| BBiF | 4.461 | 60 | 0.9908 | 1.621 | 120 | 0.9721 | 0.467 | 120 | 0.9973 | |
| BV10 (5 mg/L) | BBeC | 0.314 | 20 | 0.9823 | 0.213 | 40 | 0.9997 | 0.069 | 120 | 0.9901 |
| BBeF | 0.344 | 45 | 0.9949 | 0.118 | 45 | 0.9824 | 0.029 | 90 | 0.9044 | |
| BBiC | 0.287 | 10 | 0.997 | 0.174 | 35 | 0.9955 | 0.082 | 135 | 0.9964 | |
| BBiF | 0.222 | 20 | 0.9967 | 0.164 | 70 | 0.9889 | 0.071 | 90 | 0.9652 | |
| Isotherm | Basic Red 46 | Basic Violet 10 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BBeF | BBeC | BBiF | BBiC | BBeF | BBeC | BBiF | BBiC | ||
| Langmuir | Qmax [mg/g DM] | 131.5 | 123.0 | 111.7 | 111.2 | 18.0 | 17.3 | 16.5 | 15.9 |
| KC [L/g DM] | 0.088 | 0.066 | 0.091 | 0.056 | 0.286 | 0.229 | 0.145 | 0.130 | |
| R2 | 0.998 | 0.995 | 0.994 | 0.999 | 0.994 | 0.998 | 0.994 | 0.991 | |
| Freundlich | n [-] | 0.265 | 0.267 | 0.230 | 0.282 | 0.144 | 0.154 | 0.170 | 0.174 |
| K [(mg. g DM−1).(L/g DM)n] | 30.07 | 26.9 | 29.3 | 22.1 | 8.27 | 7.48 | 6.36 | 5.99 | |
| R2 | 0.879 | 0.894 | 0.855 | 0.896 | 0.761 | 0.800 | 0.786 | 0.768 | |
| Dye | Sorbent | Sorption Capacity [mg/g DM] | Time Sorption [min] | pH | Source |
|---|---|---|---|---|---|
| BR46 | beech tree bark | 131.5 | 300 | 5 | own research |
| birch tree bark | 111.7 | 300 | 5 | own research | |
| ceramic clay | 28.05 | 120 | 6 | [57] | |
| rapeseed husks | 49.00 | 60 | 8 | [58] | |
| bone meal | 24.56 | 90 | 6 | [59] | |
| biochar prepared from Chrysanthemum morifolium Ramat straw | 32.26 (180 °C) 49.5 (200 °C) 53.19 (220 °C) | 60 | 10 | [60] | |
| clay–wood sawdust mixture | 30.12 | 30 | 7 | [61] | |
| pyrolyzed by-product biomass | 135.0 | 5 | 7.5 | [62] | |
| carbon and silica-based composite | 41.3 (293 K) 87.31 (313 K) 176.1 (333 K) | 120 | 4.7 | [1] | |
| raw and purified clay | 54.0 (raw) 72.0 (purified) | 10 (raw) 20 (purified) | 6 | [63] | |
| BV10 | beech tree bark | 18.0 | 240 | 3 | own research |
| birch tree bark | 17.3 | 240 | 3 | own research | |
| cedar cone | 17.2 | 480 | 5 | [64] | |
| exhausted coffee ground | 2.5 | 180 | 2 | [65] | |
| fly ash | 1.9 | 4320 | [66] | ||
| sugarcane bagasse | 88.7 | - | 4 | [67] | |
| pine bark–compost | 126 | - | [68] | ||
| compost | 41.7 | 1440 | 5.3 | [69] | |
| banana peels | 9.5 | 60 | 2 | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipkowska, U.; Jóźwiak, T.; Filipkowska, M.; Deptuła, M. The Use of Beech Bark (Latin: Fagus sylvatica) and Birch Bark (Latin: Betula pendula Roth) for the Removal of Cationic Dyes from Aqueous Solutions. Appl. Sci. 2024, 14, 6128. https://doi.org/10.3390/app14146128
Filipkowska U, Jóźwiak T, Filipkowska M, Deptuła M. The Use of Beech Bark (Latin: Fagus sylvatica) and Birch Bark (Latin: Betula pendula Roth) for the Removal of Cationic Dyes from Aqueous Solutions. Applied Sciences. 2024; 14(14):6128. https://doi.org/10.3390/app14146128
Chicago/Turabian StyleFilipkowska, Urszula, Tomasz Jóźwiak, Magdalena Filipkowska, and Magdalena Deptuła. 2024. "The Use of Beech Bark (Latin: Fagus sylvatica) and Birch Bark (Latin: Betula pendula Roth) for the Removal of Cationic Dyes from Aqueous Solutions" Applied Sciences 14, no. 14: 6128. https://doi.org/10.3390/app14146128
APA StyleFilipkowska, U., Jóźwiak, T., Filipkowska, M., & Deptuła, M. (2024). The Use of Beech Bark (Latin: Fagus sylvatica) and Birch Bark (Latin: Betula pendula Roth) for the Removal of Cationic Dyes from Aqueous Solutions. Applied Sciences, 14(14), 6128. https://doi.org/10.3390/app14146128






