Aromatic Amines in Organic Synthesis Part III; p-Aminocinnamic Acids and Their Methyl Esters
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
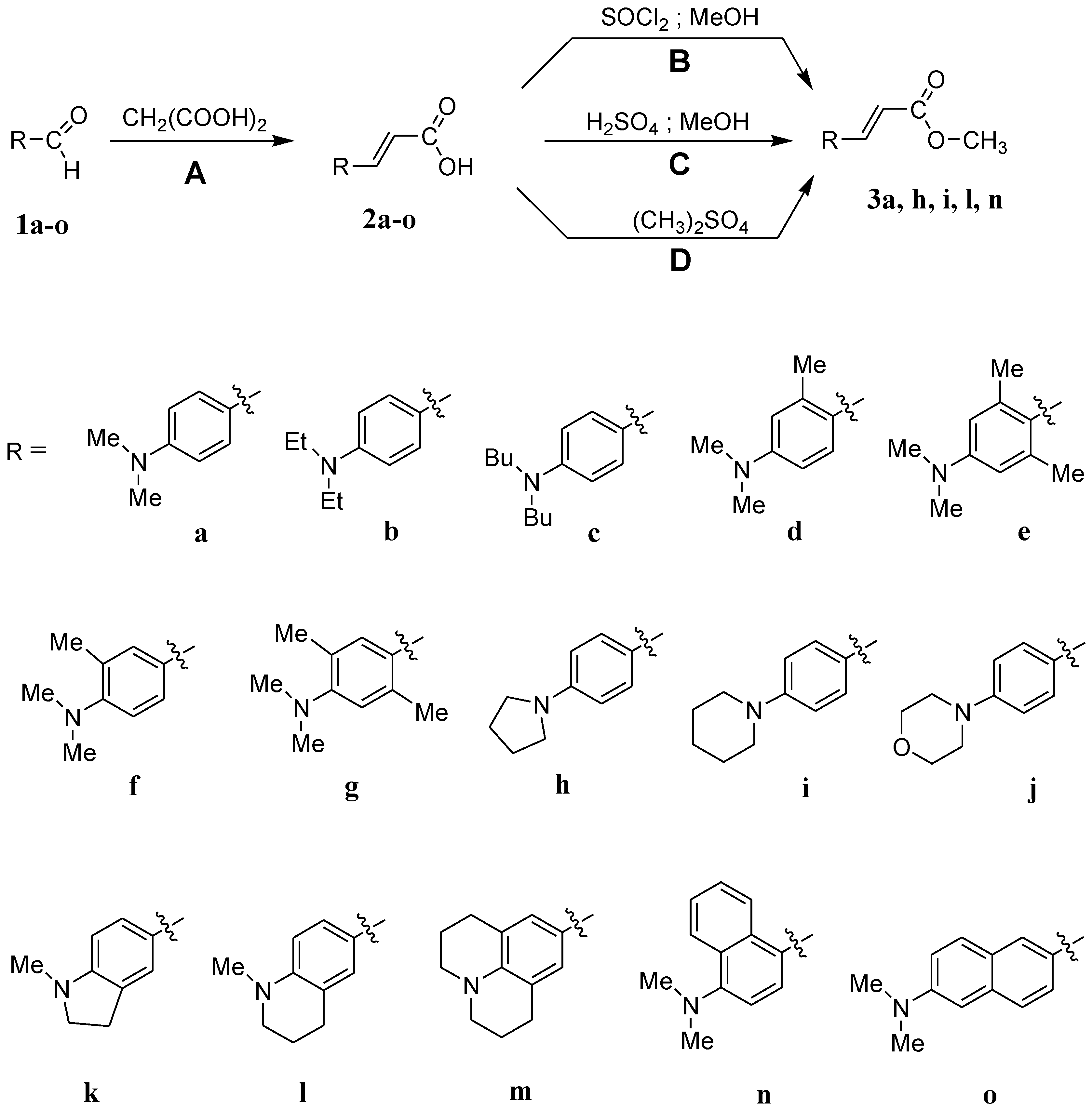
3.1. Synthesis
3.1.1. Synthesis of the Amine Derivatives of Cinnamic Acid
3.1.2. Synthesis of the Methyl p-(Dimethylamino)cinnamate
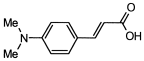
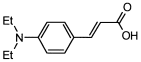
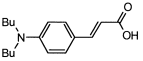
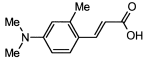

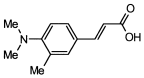


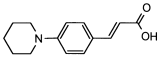
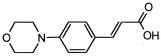
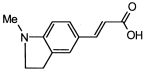
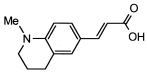
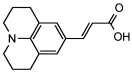
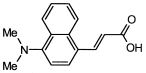
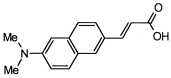

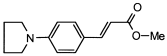
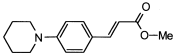
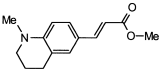
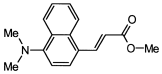
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malkin, J. Photophysical and Photochemical Properties of Aromatic Compounds; CRC: Boca Raton, FL, USA, 1992. [Google Scholar]
- Li, X.; Gao, X.; Shi, W.; Ma, H. Design Strategies for Water-Soluble Small Molecular Chromogenic and Fluorogenic Probes. Chem. Rev. 2014, 114, 590–659. [Google Scholar] [CrossRef] [PubMed]
- Lavis, L.D.; Raines, R.T. Bright Ideas for Chemical Biology. ACS Chem. Biol. 2008, 3, 142–155. [Google Scholar] [CrossRef]
- Costero, A.M.; Bañuls, M.J.; Aurell, M.J.; Ochando, L.E.; Doménech, A. Cation and anion fluorescent and electrochemical sensors derived from 4,4′-substituted biphenyl. Tetrahedron 2005, 61, 10309–10320. [Google Scholar] [CrossRef]
- Krawczyk, P.; Jędrzejewska, B.; Pietrzak, M.; Janek, T. Synthesis, spectroscopic, physicochemical properties and binding site analysis of 4-(1H-phenanthro [9,10-d]-imidazol-2-yl)-benzaldehyde fluorescent probe for imaging in cell biology: Experimental and theoretical study. J. Photochem. Photobiol. B Biol. 2016, 164, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-L.; Yang, L.-N.; Li, Z.-S. How to design more efficient organic dyes for dye-sensitized solar cells? Adding more sp2-hybridized nitrogen in the triphenylamine donor. J. Power Sources 2013, 223, 86–93. [Google Scholar] [CrossRef]
- Cook, W.D.; Chen, F. Enhanced photopolymerization of dimethacrylates with ketones, amines, and iodonium salts: The CQ system. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 5030–5041. [Google Scholar] [CrossRef]
- Chen, H.; Vahdati, M.; Xiao, P.; Dumur, F.; Lalevée, J. Water-Soluble Visible Light Sensitive Photoinitiating System Based on Charge Transfer Complexes for the 3D Printing of Hydrogels. Polymers 2021, 13, 3195. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Faeh, C.; Diederich, F. Fluorine in pharmaceuticals: Looking beyond intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, A.; Kostadinov, A.; Ivanov, D.; Dimov, D.; Stoyanov, S.; Nedelchev, L.; Nazarova, D.; Yancheva, D. Synthesis, spectroscopic and TD-DFT quantum mechanical study of azo-azomethine dyes. A laser induced trans-cis-trans photoisomerization cycle. Spectrochim. Acta Part A 2018, 192, 263–274. [Google Scholar] [CrossRef]
- Hubenova, Y.; Todorova, M.; Bakalska, R.; Mitov, M. Photophysical and Electrochemical Properties of Newly Synthesized Stilbazolium Dyes. ChemElectroChem 2022, 9, e202200918. [Google Scholar] [CrossRef]
- Luo, P.; Wang, M.; Liu, W.; Liu, L.; Xu, P. Activity-Based Fluorescent Probes Based on Hemicyanine for Biomedical Sensing. Molecules 2022, 27, 7750. [Google Scholar] [CrossRef]
- Mustroph, H. Hemicyanine dyes. Phys. Sci. Rev. 2023, 8, 1367–1379. [Google Scholar] [CrossRef]
- Mustroph, H. Merocyanine dyes. Phys. Sci. Rev. 2022, 7, 143–158. [Google Scholar] [CrossRef]
- Saady, A.; Varon, E.; Jacob, A.; Shav-Tal, Y.; Fischer, B. Applying styryl quinolinium fluorescent probes for imaging of ribosomal RNA in living cells. Dyes Pigment. 2020, 174, 107986. [Google Scholar] [CrossRef]
- Albelwi, F.F.; Al-anazi, M.; Naqvi, A.; Hritani, Z.M.; Okasha, R.M.; Afifi, T.H.; Hagar, M. Novel oxazolones incorporated azo dye: Design, synthesis photophysical-DFT aspects and antimicrobial assessments with In-silico and In-vitro surveys. J. Photochem. Photobiol. 2021, 7, 100032. [Google Scholar] [CrossRef]
- Rodrigues, C.A.B.; Mariz, I.F.A.; Maçôas, E.M.S.; Afonso, C.A.M.; Martinho, J.M.G. Two-photon absorption properties of push–pull oxazolones derivatives. Dyes Pigment. 2012, 95, 713–722. [Google Scholar] [CrossRef]
- Józefowicz, M.; Heldt, J.R.; Bajorek, A.; Pączkowski, J. Spectroscopic properties of ethyl 5-(4-dimethylaminophenyl)-3-amino-2,4-dicyanobenzoate. Chem. Phys. 2009, 363, 88–99. [Google Scholar] [CrossRef]
- Oliden-Sánchez, A.; Alvarado-Martínez, E.; Ramírez-Ornelas, D.E.; Vázquez, M.A.; Avellanal-Zaballa, E.; Bañuelos, J.; Peña-Cabrera, E. Extended BODIPYs as Red–NIR Laser Radiation Sources with Emission from 610 nm to 750 nm. Molecules 2023, 28, 4750. [Google Scholar] [CrossRef] [PubMed]
- Rybczyński, P.; Bousquet, M.H.E.; Kaczmarek-Kędziera, A.; Jędrzejewska, B.; Jacquemin, D.; Ośmiałowski, B. Controlling the fluorescence quantum yields of benzothiazole-difluoroborates by optimal substitution. Chem. Sci. 2022, 13, 13347–13360. [Google Scholar] [CrossRef]
- Kage, Y.; Kang, S.; Mori, S.; Mamada, M.; Adachi, C.; Kim, D.; Furuta, H.; Shimizu, S. An Electron-Accepting aza-BODIPY-Based Donor–Acceptor–Donor Architecture for Bright NIR Emission. Chem. Eur. J. 2021, 27, 5259–5267. [Google Scholar] [CrossRef]
- Shi, Z.; Han, X.; Hu, W.; Bai, H.; Peng, B.; Ji, L.; Fan, Q.; Li, L.; Huang, W. Bioapplications of small molecule Aza-BODIPY: From rational structural design to in vivo investigations. Chem. Soc. Rev. 2020, 49, 7533–7567. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K. Recent advances in the application of BODIPY in bioimaging and chemosensing. J. Mater. Chem. C 2019, 7, 11361–11405. [Google Scholar] [CrossRef]
- Donaire-Arias, A.; Poulsen, M.L.; Ramón-Costa, J.; Montagut, A.M.; Estrada-Tejedor, R.; Borrell, J.I. Synthesis of Chalcones: An Improved High-Yield and Substituent-Independent Protocol for an Old Structure. Molecules 2023, 28, 7576. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, M.; Józefowicz, M.; Bajorek, A.; Heldt, J.R. Experimental and Theoretical Studies of the Spectroscopic Properties of Chalcone Derivatives. J. Fluoresc. 2017, 27, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Khadieva, A.; Rayanov, M.; Shibaeva, K.; Piskunov, A.; Padnya, P.; Stoikov, I. Towards Asymmetrical Methylene Blue Analogues: Synthesis and Reactivity of 3-N′-Arylaminophenothiazines. Molecules 2022, 27, 3024. [Google Scholar] [CrossRef]
- Tiravia, M.; Sabuzi, F.; Valentini, F.; Conte, V.; Galloni, P. 3-Morpholino-7-[N-methyl-N-(4′-carboxyphenyl)amino]phenothiazinium Chloride. Molbank 2022, 2022, M1493. [Google Scholar] [CrossRef]
- Teng, C.; Yang, X.; Yang, C.; Li, S.; Cheng, M.; Hagfeldt, A.; Sun, L. Molecular Design of Anthracene-Bridged Metal-Free Organic Dyes for Efficient Dye-Sensitized Solar Cells. J. Phys. Chem. C 2010, 114, 9101–9110. [Google Scholar] [CrossRef]
- Nagy, M.; Fiser, B.; Szőri, M.; Vanyorek, L.; Viskolcz, B. Optical Study of Solvatochromic Isocyanoaminoanthracene Dyes and 1,5-Diaminoanthracene. Int. J. Mol. Sci. 2022, 23, 1315. [Google Scholar] [CrossRef] [PubMed]
- Demirçalı, A.; Karcı, F.; Sari, F. Synthesis and absorption properties of five new heterocyclic disazo dyes containing pyrazole and pyrazolone and their acute toxicities on the freshwater amphipod Gammarus roeseli. Color. Technol. 2021, 137, 280–291. [Google Scholar] [CrossRef]
- Szukalski, A.; Stottko, R.; Krawczyk, P.; Sahraoui, B.; Jędrzejewska, B. Application of the pyrazolone derivatives as effective modulators in the opto-electronic networks. J. Photochem. Photobiol. A Chem. 2023, 437, 114482. [Google Scholar] [CrossRef]
- Luo, Y.; Qiu, K.-M.; Lu, X.; Liu, K.; Fu, J.-Y.; Zhu, H.-L. Synthesis, biological evaluation, and molecular modeling of cinnamic acyl sulfonamide derivatives as novel antitubulin agents. Biorg. Med. Chem. 2011, 19, 4730–4738. [Google Scholar] [CrossRef]
- Abdel-Atty, M.M.; Farag, N.A.; Kassab, S.E.; Serya, R.A.T.; Abouzid, K.A.M. Design, synthesis, 3D pharmacophore, QSAR, and docking studies of carboxylic acid derivatives as Histone Deacetylase inhibitors and cytotoxic agents. Bioorg. Chem. 2014, 57, 65–82. [Google Scholar] [CrossRef] [PubMed]
- El-Batta, A.; Jiang, C.; Zhao, W.; Anness, R.; Cooksy, A.L.; Bergdahl, M. Wittig Reactions in Water Media Employing Stabilized Ylides with Aldehydes. Synthesis of α,β-Unsaturated Esters from Mixing Aldehydes, α-Bromoesters, and Ph3P in Aqueous NaHCO3. J. Org. Chem. 2007, 72, 5244–5259. [Google Scholar] [CrossRef] [PubMed]
- Baud, M.G.J.; Leiser, T.; Meyer-Almes, F.-J.; Fuchter, M.J. New synthetic strategies towards psammaplin A, access to natural product analogues for biological evaluation. Org. Biomol. Chem. 2011, 9, 659–662. [Google Scholar] [CrossRef]
- Handy, S.T. One-Pot Halogenation-Heck Coupling Reactions in Ionic Liquids. Synlett 2006, 2006, 3176–3178. [Google Scholar] [CrossRef]
- Gawinecki, R.; Andrzejak, S.; Puchala, A. Efficiency of the Vilsmeier-Haack method in the synthesis of p-aminobenzaldehydes. Org. Prep. Proced. Int. 1998, 30, 455–460. [Google Scholar] [CrossRef]
- van den Berg, O.; Sengers, W.G.F.; Jager, W.F.; Picken, S.J.; Wübbenhorst, M. Dielectric and Fluorescent Probes To Investigate Glass Transition, Melt, and Crystallization in Polyolefins. Macromolecules 2004, 37, 2460–2470. [Google Scholar] [CrossRef]
- Pietrzak, M.; Jędrzejewska, B.; Mądrzejewska, D.; Bajorek, A. Convenient Synthesis of p-Aminobenzoic Acids and their Methyl Esters. Org. Prep. Proced. Int. 2017, 49, 45–52. [Google Scholar] [CrossRef]
- Pietrzak, M.; Jędrzejewska, B. Aromatic Amines in Organic Synthesis. Part II. p-Aminocinnamaldehydes. Molecules 2021, 26, 4360. [Google Scholar] [CrossRef]
- Pereira, A.R.; Freitas, V.D.; Mateus, N.; Oliveira, J. Functionalization of 7-Hydroxy-pyranoflavylium: Synthesis of New Dyes with Extended Chromatic Stability. Molecules 2022, 27, 7351. [Google Scholar] [CrossRef]
- Divac, V.M.; Šakić, D.; Weitner, T.; Gabričević, M. Solvent effects on the absorption and fluorescence spectra of Zaleplon: Determination of ground and excited state dipole moments. Spectrochim. Acta Part A 2019, 212, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Tamulis, A.; Tamuliene, J.; Balevicius, M.L.; Rinkevicius, Z.; Tamulis, V. Quantum Mechanical Studies of Intensity in Electronic Spectra of Fluorescein Dianion and Monoanion Forms. Struct. Chem. 2003, 14, 643–648. [Google Scholar] [CrossRef]
- Batistela, V.R.; da Costa Cedran, J.; Moisés de Oliveira, H.P.; Scarminio, I.S.; Ueno, L.T.; Eduardo da Hora Machado, A.; Hioka, N. Protolytic fluorescein species evaluated using chemometry and DFT studies. Dyes Pigment. 2010, 86, 15–24. [Google Scholar] [CrossRef]
- Pilla, V.; Gonçalves, A.C.; Dos Santos, A.A.; Lodeiro, C. Lifetime and Fluorescence Quantum Yield of Two Fluorescein-Amino Acid-Based Compounds in Different Organic Solvents and Gold Colloidal Suspensions. Chemosensors 2018, 6, 26. [Google Scholar] [CrossRef]
- Koo, J.Y.; Heo, C.H.; Shin, Y.-H.; Kim, D.; Lim, C.S.; Cho, B.R.; Kim, H.M.; Park, S.B. Readily Accessible and Predictable Naphthalene-Based Two-Photon Fluorophore with Full Visible-Color Coverage. Chem. Eur. J. 2016, 22, 14166–14170. [Google Scholar] [CrossRef] [PubMed]
- Kazama, A.; Imai, Y.; Okayasu, Y.; Yamada, Y.; Yuasa, J.; Aoki, S. Design and Synthesis of Cyclometalated Iridium(III) Complexes—Chromophore Hybrids that Exhibit Long-Emission Lifetimes Based on a Reversible Electronic Energy Transfer Mechanism. Inorg. Chem. 2020, 59, 6905–6922. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Tanabe, H.; Tobita, S.; Shizuka, H. Solvent-Dependent Radiationless Transitions of Excited 1-Aminonaphthalene Derivatives. J. Phys. Chem. A 1997, 101, 4496–4503. [Google Scholar] [CrossRef]
- Lewis, F.D.; Hougland, J.L.; Markarian, S.A. Formation and Anomalous Behavior of Aminonaphthalene−Cinnamonitrile Exciplexes. J. Phys. Chem. A 2000, 104, 3261–3268. [Google Scholar] [CrossRef]
- Bajorek, A.; Trzebiatowska, K.; Jędrzejewska, B.; Pietrzak, M.; Gawinecki, R.; Pączkowski, J. Developing of Fluorescence Probes Based on Stilbazolium Salts for Monitoring Free Radical Polymerization Processes. II. J. Fluoresc. 2004, 14, 295–307. [Google Scholar] [CrossRef]
- Beach, S.F.; Hepworth, J.D.; Sawyer, J.; Hallas, G.; Marsden, R.; Mitchell, M.M.; Ibbitson, D.A.; Jones, A.M.; Neal, G.T. Dipole moments of some N-phenyl-substituted derivatives of pyrrolidine, piperidine, morpholine, and thiomorpholine. J. Chem. Soc. Perkin Trans. 2 1984, 2, 217–221. [Google Scholar] [CrossRef]
- Gawinecki, R.; Kolehmainen, E.; Kauppinen, R. 1H and 13C NMR studies of para-substituted benzaldoximes for evaluation of the electron donor properties of substituted amino groups. J. Chem. Soc. Perkin Trans. 2 1998, 1, 25–30. [Google Scholar] [CrossRef]
- Pelmus, M.; Ungureanu, E.-M.; Stanescu, M.D.; Tarko, L. Electrochemical and QSPR studies of several hydroxy- and amino-polysubstituted benzenes constituents of useful compounds. J. Appl. Electrochem. 2020, 50, 851–862. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Olmsted, J. Calorimetric determinations of absolute fluorescence quantum yields. J. Phys. Chem. 1979, 83, 2581–2584. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision B.05; Gaussian, Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
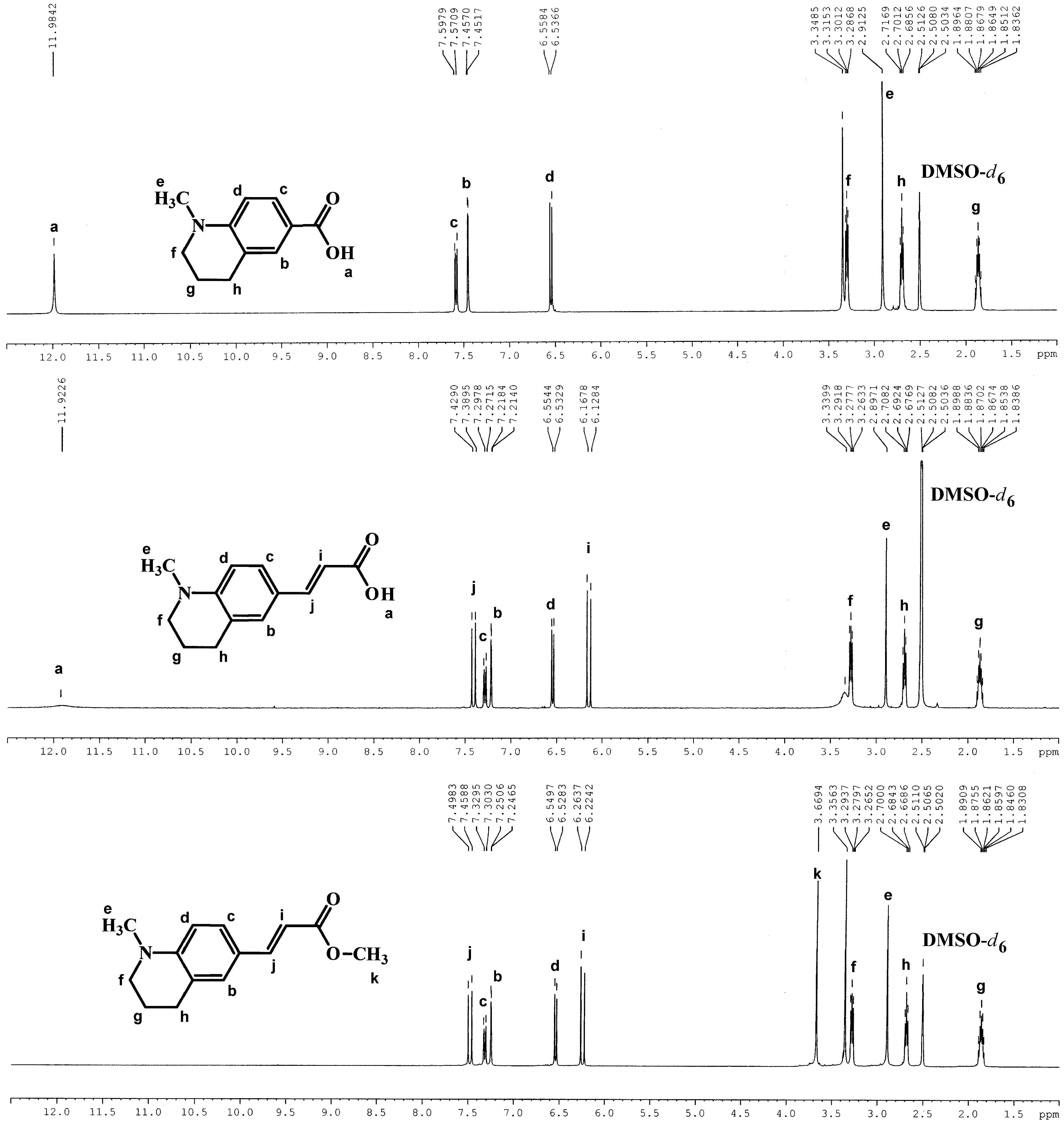

| Abbr. | 1,4-Dioxane | Ethyl Acetate | Methanol | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λmax abs | λmax fl | ϕfl | Δν | λmax abs | εmax | λmax fl | ϕfl | Δν | λmax abs | εmax | λmax fl | ϕfl | Δν | |
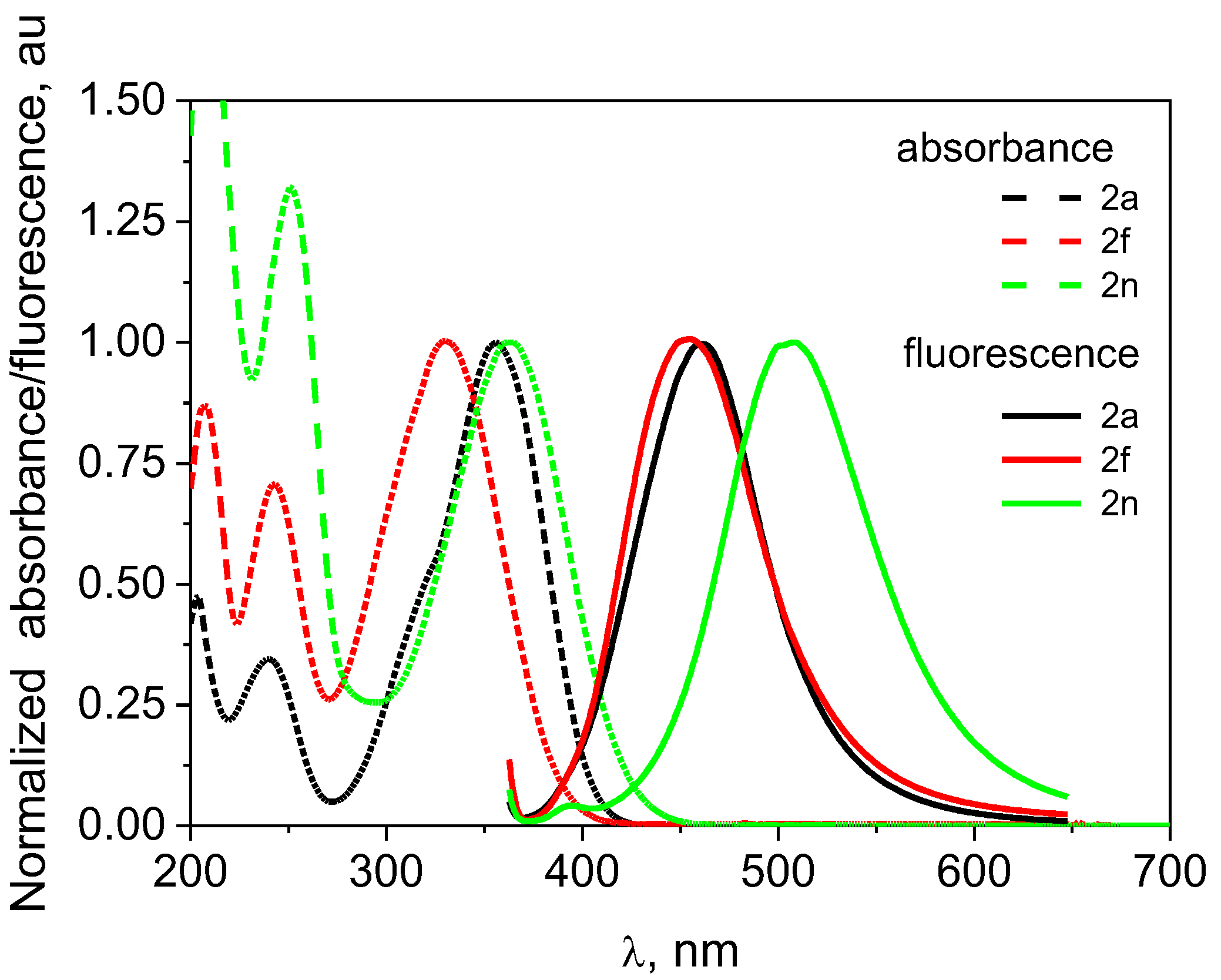
| 2a | 353 | 414 | 0.84 | 4174 | 355 | 28.9 | 426 | 0.61 | 4695 | 356 | 26.9 | 461 | 0.69 | 6398 |
| 2b | 363 | 418 | 0.96 | 3626 | 360 | 34.3 | 426 | 0.62 | 4304 | 366 | 31.2 | 462 | 1.29 | 5677 |
| 2c | 366 | 418 | 1.15 | 3399 | 364 | 36.5 | 427 | 0.93 | 4053 | 367 | 29.7 | 465 | 1.75 | 5743 |
| 2d | 359 | 438 | 0.52 | 5024 | 358 | 41.8 | 433 | 0.45 | 4838 | 365 | 33.9 | 463 | 0.67 | 5799 |
| 2e | 352 | 413 | 0.16 | 4196 | 352 | 23.7 | 432 | 0.30 | 5261 | 357 | 22.3 | 437 | 0.17 | 5128 |
| 2f | 332 | 438 | 3.48 | 7289 | 329 | 20.5 | 439 | 0.31 | 7616 | 330 | 18.4 | 454 | 0.64 | 8277 |
| 2g | 338 | 443 | 1.58 | 7012 | 336 | 19.3 | 445 | 1.80 | 7290 | 336 | 16.5 | 459 | 0.84 | 7975 |
| 2h | 362 | 420 | 0.89 | 3815 | 361 | 33.2 | 429 | 0.66 | 4391 | 361 | 32.4 | 463 | 1.07 | 6103 |
| 2i | 351 | 420 | 1.47 | 4681 | 348 | 25.5 | 430 | 1.28 | 5480 | 352 | 24.9 | 467 | 1.62 | 6996 |
| 2j | 341 | 416 | 1.21 | 5287 | 341 | 22.8 | 425 | 1.06 | 5796 | 341 | 27.5 | 466 | 1.67 | 7866 |
| 2k | 360 | 444 | 1.69 | 5255 | 357 | 27.2 | 443 | 1.63 | 5438 | 365 | 22.5 | 467 | 1.22 | 5984 |
| 2l | 365 | 439 | 1.44 | 4618 | 365 | 31.4 | 434 | 1.29 | 4356 | 371 | 25.6 | 465 | 1.36 | 5449 |
| 2m | 375 | 445 | 2.14 | 4195 | 374 | 27.2 | 444 | 2.38 | 4215 | 382 | 31.3 | 469 | 2.04 | 4856 |
| 2n | 365 | 464 | 0.57 | 5845 | 362 | 20.4 | 478 | 0.39 | 6704 | 365 | 16.8 | 508 | 0.67 | 7712 |
| 2o | 366 | 450 | 59.9 | 5100 | 370 | 17.2 | 471 | 73.8 | 5796 | 366 | 18.5 | 489 | 61.2 | 6873 |
| 3a | 357 | 417 | 0.81 | 4030 | 356 | 31.2 | 426 | 0.74 | 4616 | 364 | 29.9 | 467 | 1.27 | 6059 |
| 3h | 365 | 419 | 0.83 | 3531 | 364 | 31.4 | 429 | 0.81 | 4163 | 371 | 34.2 | 470 | 1.51 | 5678 |
| 3i | 354 | 424 | 1.56 | 4664 | 352 | 19.5 | 432 | 1.18 | 5261 | 358 | 32.2 | 473 | 2.06 | 6791 |
| 3l | 367 | 439 | 1.31 | 4469 | 367 | 32.2 | 434 | 1.29 | 4206 | 375 | 27.2 | 477 | 1.78 | 5702 |
| 3n | 366 | 465 | 0.61 | 5817 | 365 | 24.1 | 478 | 0.48 | 6477 | 366 | 15.6 | 511 | 1.00 | 7753 |
| No. | Solvent | λmax abs | λmax fl | Δν | ϕfl | χ2 | kr | knr | kr/knr | fos | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| εmax | α1 | α2 | |||||||||||
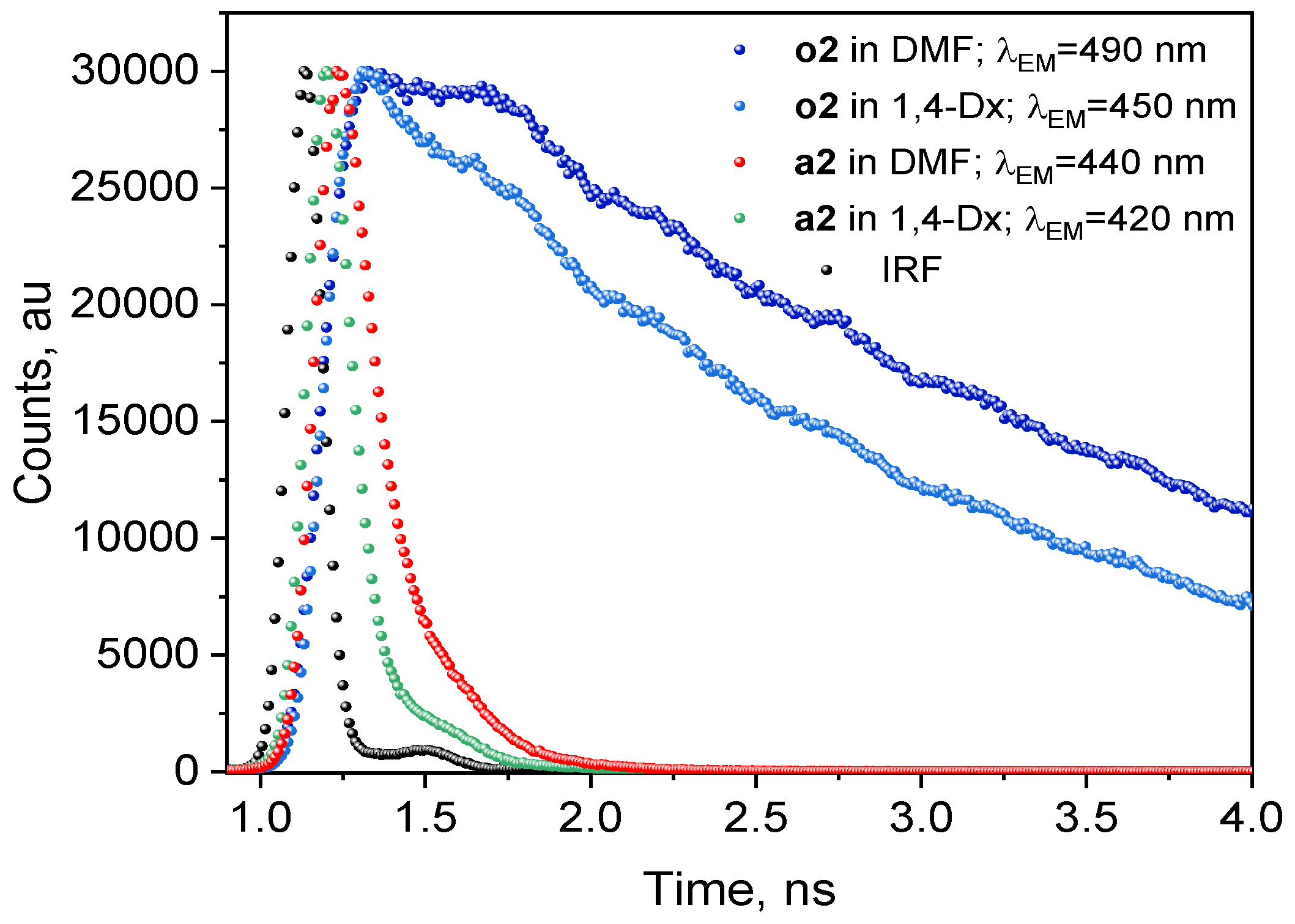
| 2a | 1,4-Dx | 353 | 414 | 4174 | 0.84 | 0.074 | 0.521 | 0.08 | 2.194 | 1.07 | 12.6 | 0.0085 | 0.619 |
| 27.4 | 98.95 | 1.05 | |||||||||||
| EtOAc | 355 | 426 | 4695 | 0.61 | 0.08 | 0.896 | 0.09 | 2.419 | 0.673 | 11.0 | 0.0061 | 0.625 | |
| 28.9 | 98.7 | 1.3 | |||||||||||
| DMF | 357 | 448 | 5690 | 2.06 | 0.127 | 2.455 | 0.14 | 1.700 | 1.48 | 7.04 | 0.021 | 0.646 | |
| 27.4 | 99.48 | 0.52 | |||||||||||
| 2o | 1,4-Dx | 366 | 450 | 5100 | 59.9 | 1.684 | 2.506 | 1.95 | 1.096 | 3.06 | 0.205 | 1.49 | 0.466 |
| 20.9 | 67.07 | 32.93 | |||||||||||
| EtOAc | 370 | 471 | 5796 | 73.8 | 1.777 | 2.454 | 2.09 | 1.002 | 3.52 | 0.125 | 2.82 | 0.401 | |
| 17.2 | 53.05 | 46.95 | |||||||||||
| DMF | 373 | 499 | 6842 | 70.8 | 1.836 | 2.673 | 2.53 | 1.110 | 2.80 | 0.115 | 2.42 | 0.539 | |
| 22.4 | 16.93 | 83.07 |
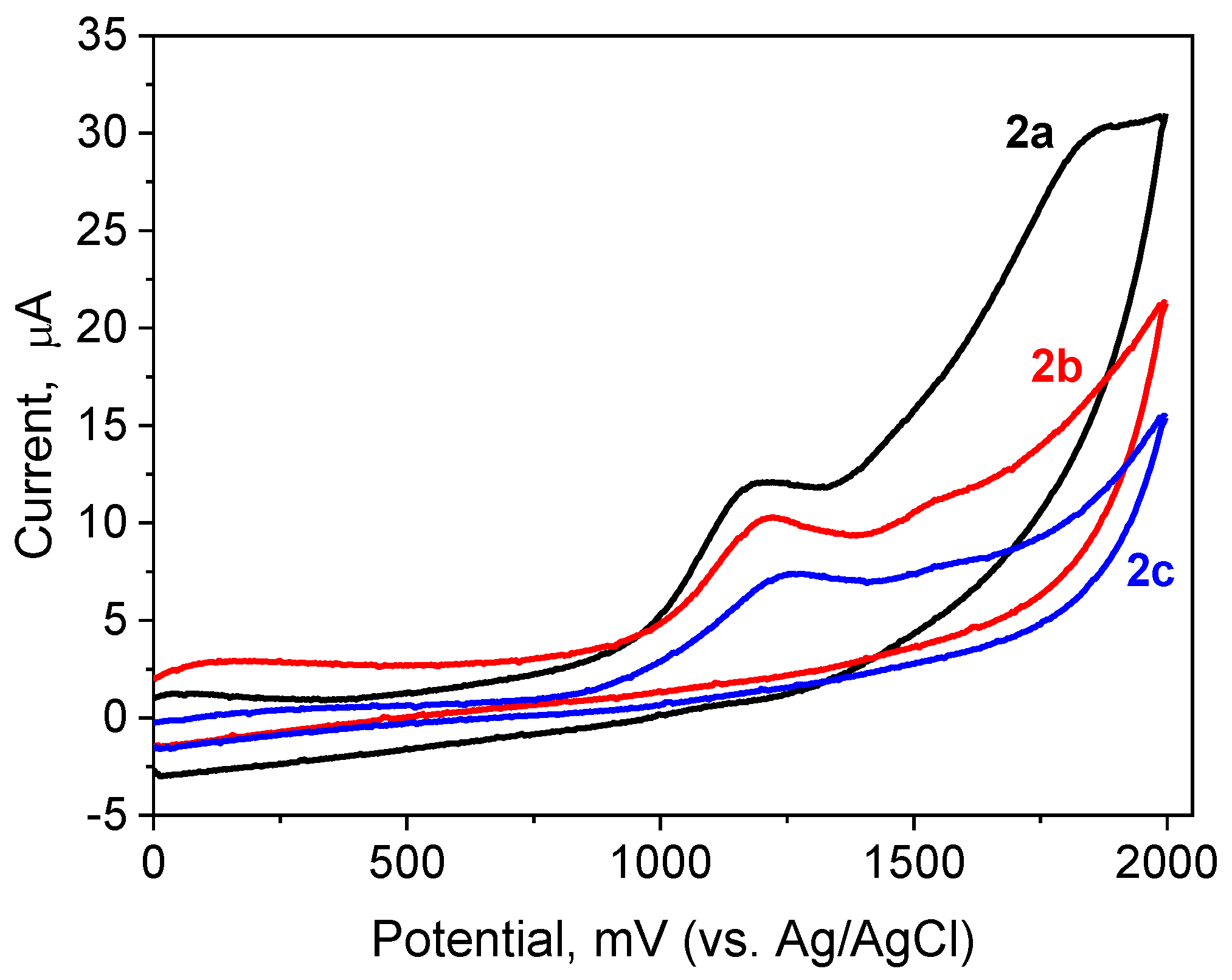
| Abbr. | Eox (mV) | Abbr. | Eox (mV) | Abbr. | Eox (mV) | Abbr. | Eox (mV) |
|---|---|---|---|---|---|---|---|
| 2a | 1132 | 2f | 1285 | 2k | 1072 | 3a | 1183 |
| 2b | 1213 | 2g | 1264 | 2l | 1150 | 3h | 1306 |
| 2c | 1261 | 2h | 993 | 2m | 1171 | 3i | 1210 |
| 2d | 1192 | 2i | 1084 | 2n | 1147 | 3l | 1240 |
| 2e | 1177 | 2j | 963 | 2o | 1066 | 3n | 1171 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrzak, M.; Jędrzejewska, B. Aromatic Amines in Organic Synthesis Part III; p-Aminocinnamic Acids and Their Methyl Esters. Appl. Sci. 2024, 14, 6032. https://doi.org/10.3390/app14146032
Pietrzak M, Jędrzejewska B. Aromatic Amines in Organic Synthesis Part III; p-Aminocinnamic Acids and Their Methyl Esters. Applied Sciences. 2024; 14(14):6032. https://doi.org/10.3390/app14146032
Chicago/Turabian StylePietrzak, Marek, and Beata Jędrzejewska. 2024. "Aromatic Amines in Organic Synthesis Part III; p-Aminocinnamic Acids and Their Methyl Esters" Applied Sciences 14, no. 14: 6032. https://doi.org/10.3390/app14146032
APA StylePietrzak, M., & Jędrzejewska, B. (2024). Aromatic Amines in Organic Synthesis Part III; p-Aminocinnamic Acids and Their Methyl Esters. Applied Sciences, 14(14), 6032. https://doi.org/10.3390/app14146032






