Advanced and Potential Methods for Extraction of Bioactive Compounds from Avocado Peel—A Review
Abstract
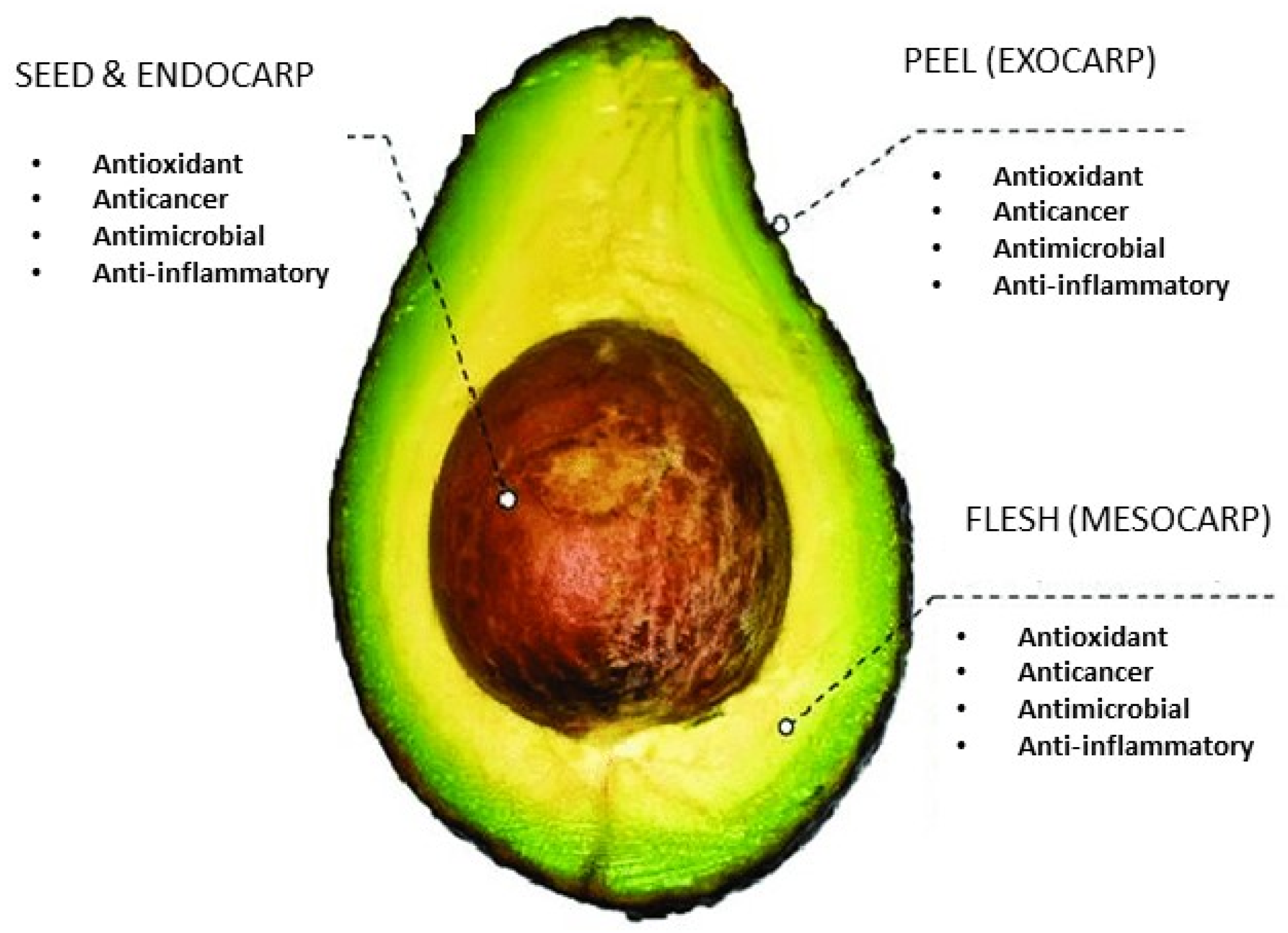
1. Introduction
2. Method
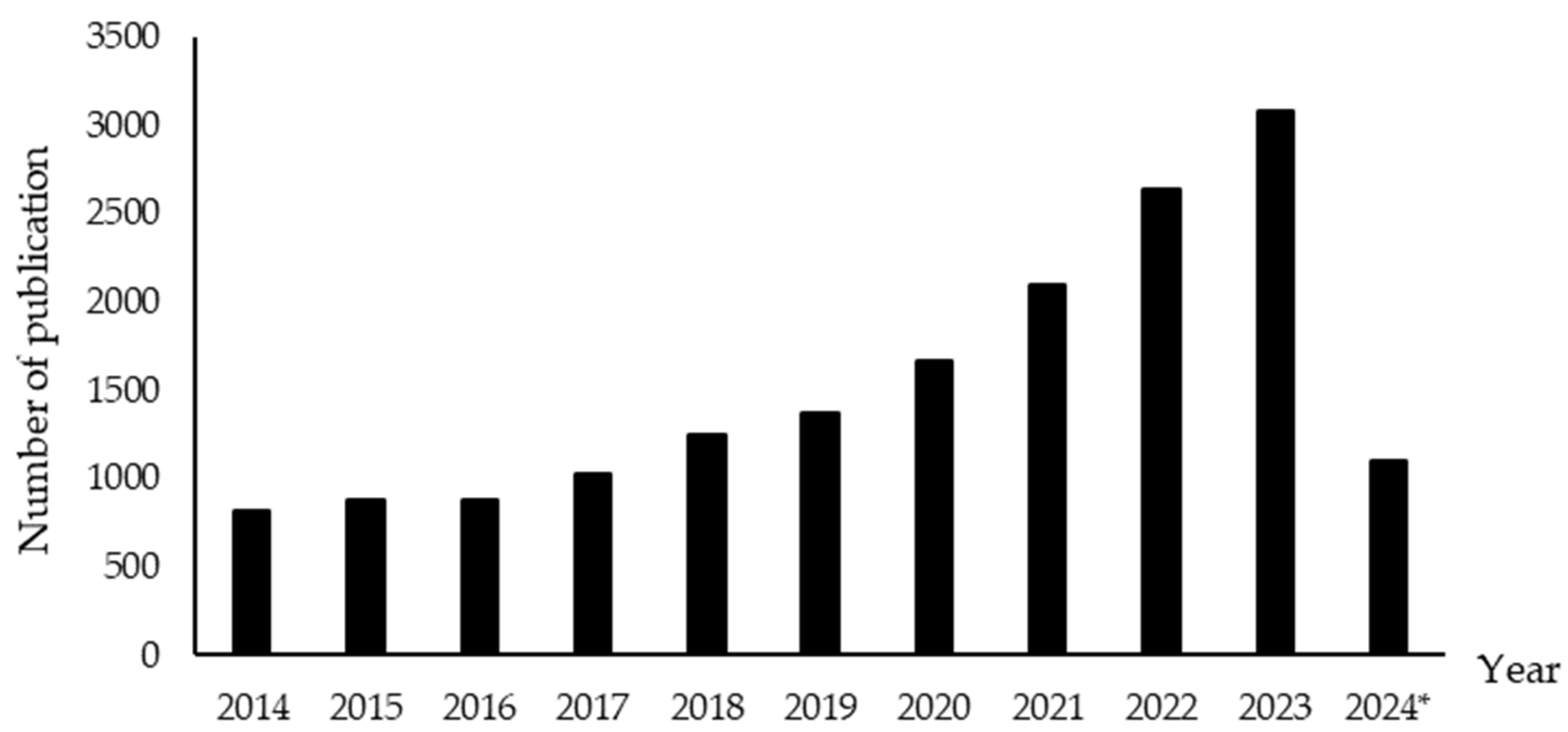
3. Research Trends in Extraction of Bioactive Compounds from Avocado Peel
4. Advanced Methods for Extraction of Bioactive Compounds from Avocado Peel
4.1. Microwave-Assisted Extraction (MAE)
4.2. Ultrasound-Assisted Extraction (UAE)
4.3. Enzyme-Assisted Extraction (EAE)
4.4. Pressurized Liquid Extraction (PLE)
4.5. Supercritical Fluid Extraction (SFE)
4.6. Natural Deep Eutectic Solvents (NaDESs) Extraction
4.7. Three-Phase Partitioning (TPP)
5. Potential Techniques for Extraction of Bioactive Compounds from Avocado Peel
5.1. Pulsed-Electric Field Extraction (PEF)
5.2. High Voltage Electric Discharge Plasma (HVED)
5.3. Centrifugal Partition Extraction (CPE)
5.4. Surfactant-Mediated Extraction (SME)
6. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muhammad, D.R.A.; Zulfa, F.; Purnomo, D.; Widiatmoko, C.; Fibri, D.L.N. Consumer acceptance of chocolate formulated with functional ingredient. IOP Conf. Ser. Earth Environ. Sci. 2021, 637, 012081. [Google Scholar] [CrossRef]
- Muhammad, D.R.A. Nanoencapsulation: A new way of using herbs and spices in food and its related products. Rev. Agric. Sci. 2022, 10, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Nkhili, E.; Tomao, V.; El Hajji, H.; El Boustani, E.S.; Chemat, F.; Dangles, O. Microwave-assisted water extraction of green tea polyphenols. Phytochem. Anal. 2009, 20, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, D.R.A.; Wikandari, R. Extraction and stability assessment of the bioactive compounds from berries. In Berry Bioactive Compound By-Products, 1st ed.; Khalifa, I., Nawaz, A., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 1–44. [Google Scholar]
- Sirohi, R.; Gaur, V.K.; Pandey, A.K.; Sim, S.J.; Kumar, S. Harnessing fruit waste for poly-3-hydroxybutyrate production: A review. Bioresour. Technol. 2021, 326, 124734. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carpena, J.G.; Morcuende, D.; Andrade, M.J.; Kylli, P.; Estévez, M. Avocado (Persea americana Mill.) phenolics, in vitro antioxidant and antimicrobial activities, and inhibition of lipid and protein oxidation in porcine patties. J. Agric. Food Chem. 2011, 59, 5625–5635. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Del Pino-García, R.; Curiel, J.A.; Lozano-Sánchez, J.; Segura-Carretero, A. Functional ingredient from avocado peel: Microwave-assisted extraction, characterization and potential applications for the food industry. Food Chem. 2021, 352, 129300. [Google Scholar] [CrossRef] [PubMed]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Marathe, S.J.; Jadhav, S.B.; Bankar, S.B.; Dubey, K.K.; Singhal, R.S. Improvements in the extraction of bioactive compounds by enzymes. Curr. Opin. Food Sci. 2019, 25, 62–72. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Kamal, S.M.M. Subcritical water extraction of bioactive compounds from plants and algae: Applications in pharmaceutical and food ingredients. Food Eng. Rev. 2016, 8, 23–34. [Google Scholar] [CrossRef]
- Ko, M.J.; Nam, H.H.; Chung, M.S. Conversion of 6-gingerol to 6-shogaol in ginger (Zingiber officinale) pulp and peel during subcritical water extraction. Food Chem. 2019, 270, 149–155. [Google Scholar] [CrossRef]
- Alkhalaf, M.I.; Alansari, W.S.; Ibrahim, E.A.; ELhalwagy, M.E. Anti-oxidant, anti-inflammatory and anti-cancer activities of avocado (Persea americana) fruit and seed extract. J. King Saud Univ.-Sci. 2019, 31, 1358–1362. [Google Scholar] [CrossRef]
- Jimenez, P.; Garcia, P.; Quitral, V.; Vasquez, K.; Parra-Ruiz, C.; Reyes-Farias, M.; Garcia-Diaz, D.F.; Robert, P.; Encina, C.; Soto-Covasich, J. Pulp, leaf, peel and seed of avocado fruit: A review of bioactive compounds and healthy benefits. Food Rev. Int. 2021, 37, 619–655. [Google Scholar] [CrossRef]
- Araújo, R.G.; Rodriguez-Jasso, R.M.; Ruiz, H.A.; Pintado, M.M.E.; Aguilar, C.N. Avocado by-products: Nutritional and functional properties. Trends Food Sci. Technol. 2018, 80, 51–60. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Ummat, V.; Tiwari, B.; Rajauria, G. Exploring ultrasound, microwave and ultrasound–microwave assisted extraction technologies to increase the extraction of bioactive compounds and antioxidants from brown macroalgae. Mar. Drugs 2020, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Araujo, R.G.; Rodríguez-Jasso, R.M.; Ruíz, H.A.; Govea-Salas, M.; Pintado, M.; Aguilar, C.N. Recovery of bioactive components from avocado peels using microwave-assisted extraction. Food Bioprod. Process. 2021, 127, 152–161. [Google Scholar] [CrossRef]
- Bilgin, M.; Sahin, S.; Dramur, M.U.; Sevgili, L.M. Obtaining scarlet sage (Salvia coccinea) extract through homogenizer-and ultrasound-assisted extraction methods. Chem. Eng. Commun. 2013, 200, 1197–1209. [Google Scholar] [CrossRef]
- Mittal, R.; Tavanandi, H.A.; Mantri, V.A.; Raghavarao, K.S.M.S. Ultrasound assisted methods for enhanced extraction of phycobiliproteins from marine macro-algae, Gelidium pusillum (Rhodophyta). Ultrason. Sonochem. 2017, 38, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Thakur, P.; Sonawane, S.; Potoroko, I.; Sonawane, S.H. Recent advances in ultrasound-assisted synthesis of nano-emulsions and their industrial applications. Curr. Pharm. Biotechnol. 2021, 22, 1748–1758. [Google Scholar]
- Hefzalrahman, T.; Morsi, M.K.; Morsy, N.F.; Hammad, K.S. Application of enzyme and ultrasound assisted extraction of polyphenols from avocado (Persea americana Mill.) peel as natural antioxidants. Acta Sci. Pol. Technol. Aliment. 2022, 21, 129–138. [Google Scholar]
- Rodríguez-Martínez, B.; Ferreira-Santos, P.; Gullón, B.; Teixeira, J.A.; Botelho, C.M.; Yáñez, R. Exploiting the potential of bioactive molecules extracted by ultrasounds from avocado peels—Food and nutraceutical applications. Antioxidants 2021, 10, 1475. [Google Scholar] [CrossRef] [PubMed]
- Cláudio, A.F.M.; Ferreira, A.M.; Freire, C.S.; Silvestre, A.J.; Freire, M.G.; Coutinho, J.A. Optimization of the gallic acid extraction using ionic-liquid-based aqueous two-phase systems. Sep. Purif. Technol. 2012, 97, 142–149. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Chen, S.; Zhang, M.; Wang, Z. Ionic liquids based simultaneous ultrasonic and microwave assisted extraction of phenolic compounds from burdock leaves. Anal. Chim. Acta 2012, 716, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Qiu, H.; Li, J.; Liu, X.; Jiang, S. 1-Hexadecyl-3-methylimidazolium ionic liquid as a new cationic surfactant for separation of phenolic compounds by MEKC. Chromatographia 2009, 69, 1093–1096. [Google Scholar] [CrossRef]
- Strati, I.F.; Gogou, E.; Oreopoulou, V. Enzyme and high pressure assisted extraction of carotenoids from tomato waste. Food Bioprod. Process. 2015, 94, 668–674. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Getachew, A.T.; Jacobsen, C.; Holdt, S.L. Emerging technologies for the extraction of marine phenolics: Opportunities and challenges. Mar. Drugs 2020, 18, 389. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Quirantes-Piné, R.; Segura-Carretero, A. Optimization of drying process and pressurized liquid extraction for recovery of bioactive compounds from avocado peel by-product. Electrophoresis 2018, 39, 1908–1916. [Google Scholar] [CrossRef]
- Yeh, H.-Y.; Chuang, C.-H.; Chen, H.-C.; Wan, C.-J.; Chen, T.-L.; Lin, L.-Y. Bioactive components analysis of two various gingers (Zingiber officinale Roscoe) and antioxidant effect of ginger extracts. Food Sci. Technol. 2014, 55, 329–334. [Google Scholar] [CrossRef]
- Mazyan, W.I.; O’connor, E.; Martin, E.; Vogt, A.; Charter, E.; Ahmadi, A. Effects of temperature and extraction time on avocado flesh (Persea americana) total phenolic yields using subcritical water extraction. Processes 2021, 9, 159. [Google Scholar] [CrossRef]
- Pinto, M.I.; Micaelo, C.; Vale, C.; Sontag, G.; Noronha, J.P. Screening of priority pesticides in Ulva sp. seaweeds by selective pressurized solvent extraction before gas chromatography with electron capture detector analysis. Arch. Environ. Contam. Toxicol. 2014, 67, 547–556. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.D.P.; Parada-Alfonso, F.; Ibáñez, E.; Cifuentes, A. On-line coupling of supercritical fluid extraction and chromatographic techniques. J. Sep. Sci. 2017, 40, 213–227. [Google Scholar] [CrossRef]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective extraction of bioactive compounds from plants using recent extraction techniques: A review. J. Chromatogr. A 2021, 1635, 461770. [Google Scholar] [CrossRef]
- Kraujalienė, V.; Pukalskas, A.; Venskutonis, P.R. Multi-stage recovery of phytochemicals from buckwheat (Fagopyrum esculentum Moench) flowers by supercritical fluid and pressurized liquid extraction methods. Ind. Crops Prod. 2017, 107, 271–280. [Google Scholar] [CrossRef]
- Restrepo-Serna, D.L.; Solarte-Toro, J.C.; Cardona-Alzate, C.A. A Biorefinery Approach for an Integral Valorisation of Avocado Peel and Seeds Through Supercritical Fluids. Waste Biomass Valorization 2022, 13, 3973–3988. [Google Scholar] [CrossRef]
- Herrero, M.; del Pilar Sánchez-Camargo, A.; Cifuentes, A.; Ibáñez, E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef]
- King, J.W. Modern supercritical fluid technology for food applications. Annu. Rev. Food Sci. Technol. 2014, 5, 215–238. [Google Scholar] [CrossRef]
- Kua, Y.L.; Gan, S.; Morris, A.; Ng, H.K. Ethyl lactate as a potential green solvent to extract hydrophilic (polar) and lipophilic (non-polar) phytonutrients simultaneously from fruit and vegetable by-products. Sustain. Chem. Pharm. 2016, 4, 21–31. [Google Scholar] [CrossRef]
- Herrero, M.; Castro-Puyana, M.; Mendiola, J.A.; Ibañez, E. Compressed fluids for the extraction of bioactive compounds. TrAC Trends Anal. Chem. 2013, 43, 67–83. [Google Scholar] [CrossRef]
- Gilbert-López, B.; Mendiola, J.A.; Ibáñez, E. Green foodomics. Towards a cleaner scientific discipline. TrAC Trends Anal. Chem. 2017, 96, 31–41. [Google Scholar] [CrossRef]
- Zoccali, M.; Donato, P.; Mondello, L. Recent advances in the coupling of carbon dioxide-based extraction and separation techniques. TrAC Trends Anal. Chem. 2019, 116, 158–165. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. TrAC Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Mišan, A.; Nađpal, J.; Stupar, A.; Pojić, M.; Mandić, A.; Verpoorte, R.; Choi, Y.H. The perspectives of natural deep eutectic solvents in agri-food sector. Crit. Rev. Food Sci. Nutr. 2020, 60, 2564–2592. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Gómez, A.V.; Tadini, C.C.; Biswas, A.; Buttrum, M.; Kim, S.; Boddu, V.M.; Cheng, H.N. Microwave-assisted extraction of soluble sugars from banana puree with natural deep eutectic solvents (NADES). LWT 2019, 107, 79–88. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, B.; Ferreira-Santos, P.; Alfonso, I.M.; Martínez, S.; Genisheva, Z.; Gullón, B. Deep eutectic solvents as a green tool for the extraction of bioactive phenolic compounds from avocado peels. Molecules 2022, 27, 6646. [Google Scholar] [CrossRef]
- Marathe, S.J.; Shah, N.N.; Singhal, R.S. Three phase partitioning (TPP) as an extraction technique for oleaginous materials. In Three Phase Partitioning, 1st ed.; Gupta, M., Roy, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 267–284. [Google Scholar]
- Sonar, M.P.; Rathod, V.K. Extraction of type ii antidiabetic compound corosolic acid from Lagerstroemia speciosa by batch extraction and three phase partitioning. Biocatal. Agric. Biotechnol. 2020, 27, 101694. [Google Scholar] [CrossRef]
- Panadare, D.C.; Rathod, V.K. Three phase partitioning for extraction of oil: A review. Trends Food Sci. Technol. 2017, 68, 145–151. [Google Scholar] [CrossRef]
- Jiménez-Velázquez, P.; Valle-Guadarrama, S.; Alia-Tejacal, I.; Salinas-Moreno, Y.; García-Cruz, L.; Pérez-López, A.; Guerra Ramírez, D. Separation of Bioactive Compounds from Epicarp of ‘Hass’ Avocado Fruit through Aqueous Two-Phase Systems. Food Bioprod. Process. 2020, 123, 238–250. [Google Scholar] [CrossRef]
- Rojas-García, A.; Fuentes, E.; Cádiz-Gurrea, M.D.L.L.; Rodriguez, L.; Villegas-Aguilar, M.D.C.; Palomo, I.; Arráez-Román, D.; Segura-Carretero, A. Biological Evaluation of Avocado Residues as a Potential Source of Bioactive Compounds. Antioxidants 2022, 11, 1049. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; Mulyani, S.; Abdullah, R.; Bohari, B. Antioxidant Properties of the Methanolic Extract of Avocado Fruit Peel (Persea americana Mill.) from Indonesia. J. Adv. Pharm. Technol. Res. 2022, 13, 166–170. [Google Scholar] [PubMed]
- Ferreira, S.M.; Falé, Z.; Santos, L. Sustainability in Skin Care: Incorporation of Avocado Peel Extracts in Topical Formulations. Molecules 2022, 27, 1782. [Google Scholar] [CrossRef]
- García-Ramón, F.; Malnati-Ramos, M.; Rios-Mendoza, J.; Vivar-Méndez, J.; Nieva-Villegas, L.M.; Cornelio-Santiago, H.P.; Sotelo-Méndez, A. Avocado Hass Peel from Industrial By-Product: Effect of Extraction Process Variables on Yield, Phenolic Compounds and Antioxidant Capacity. Front. Sustain. Food Syst. 2023, 7, 1255941. [Google Scholar] [CrossRef]
- Juma, I.; Englund, J.-E.; Ortiz, R.; Geleta, M.; Tibuhwa, D.D.; Carlsson, A.S.; Nyomora, A.; Fatih, M.; Hovmalm, H.P. Polyphenolic Content and Radical Scavenging Activities of the Peel, Pulp and Seed of Avocado (Persea americana Mill.) Grown in Tanzania. Tanzan. J. Sci. 2023, 49, 230–239. [Google Scholar] [CrossRef]
- Lister, I.N.E.; Amiruddin, H.L.; Fachrial, E.; Girsang, E. Anti-Aging Effectiveness of Avocado Peel Extract Ointment (Persea americana Mill.) against Hydration, Collagen, and Elasticity Levels in Wistar Rat. J. Pharm. Res. Int. 2021, 33, 173–184. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Quero, J.; Osada, J.; Martín-Belloso, O.; Rodríguez-Yoldi, M.J. Phenolic-Rich Extracts from Avocado Fruit Residues as Functional Food Ingredients with Antioxidant and Antiproliferative Properties. Biomolecules 2021, 11, 977. [Google Scholar] [CrossRef]
- Da Silva, G.G.; Pimenta, L.P.S.; Melo, J.O.F.; Mendonça, H.D.O.P.; Augusti, R.; Takahashi, J.A. Phytochemicals of Avocado Residues as Potential Acetylcholinesterase Inhibitors, Antioxidants, and Neuroprotective Agents. Molecules 2022, 27, 1892. [Google Scholar] [CrossRef]
- Martínez-Gutiérrez, E. Study of Influence of Extraction Method on the Recovery Bioactive Compounds from Peel Avocado. Molecules 2023, 28, 2557. [Google Scholar] [CrossRef]
- Skenderidis, P.; Leontopoulos, S.; Petrotos, K.; Giavasis, I. Vacuum Microwave-Assisted Aqueous Extraction of Polyphenolic Compounds from Avocado (Persea americana) Solid Waste. Sustainability 2021, 13, 2166. [Google Scholar] [CrossRef]
- Skenderidis, P.; Leontopoulos, S.; Petrotos, K.; Mitsagga, C.; Giavasis, I. The In Vitro and In Vivo Synergistic Antimicrobial Activity Assessment of Vacuum Microwave Assisted Aqueous Extracts from Pomegranate and Avocado Fruit Peels and Avocado Seeds Based on a Mixtures Design Model. Plants 2021, 10, 1757. [Google Scholar] [CrossRef]
- Razola-Díaz, M.D.C.; Verardo, V.; Guerra-Hernández, E.J.; García-Villanova Ruiz, B.; Gómez-Caravaca, A.M. Response Surface Methodology for the Optimization of Flavan-3-Ols Extraction from Avocado By-Products via Sonotrode Ultrasound-Assisted Extraction. Antioxidants 2023, 12, 1409. [Google Scholar] [CrossRef] [PubMed]
- Grisales-Mejía, J.F.; Torres-Castañeda, H.; Andrade-Mahecha, M.M.; Martínez-Correa, H.A. Green Extraction Methods for Recovery of Antioxidant Compounds from Epicarp, Seed, and Seed Tegument of Avocado Var. Hass (Persea americana Mill.). Int. J. Food Sci. 2022, 2022, 1965757. [Google Scholar] [CrossRef]
- Monzón, L.; Becerra, G.; Aguirre, E.; Rodríguez, G.; Villanueva, E. Ultrasound-Assisted Extraction of Polyphenols from Avocado Residues: Modeling and Optimization Using Response Surface Methodology and Artificial Neural Networks. Sci. Agropecu. 2021, 12, 33–40. [Google Scholar] [CrossRef]
- Aguilar-Méndez, M.A.; Campos-Arias, M.P.; Quiroz-Reyes, C.N.; Jesús, E.R.; Cruz-Hernández, M.A. Fruit Peels as Sources of Bioactive Compounds with Antioxidant and Antimicrobial Properties. Rev. Fac. Cienc. Agrar. 2020, 52, 360–372. [Google Scholar]
- Trujillo-Mayol, I.; Casas-Forero, N.; Pastene-Navarrete, E.; Lima Silva, F.; Alarcón-Enos, J. Fractionation and Hydrolyzation of Avocado Peel Extract: Improvement of Antibacterial Activity. Antibiotics 2020, 10, 23. [Google Scholar] [CrossRef]
- Della Posta, S.; Gallo, V.; Ascrizzi, A.M.; Gentili, A.; De Gara, L.; Dugo, L.; Fanali, C. Development of a Green Ultrasound-Assisted Procedure for the Extraction of Phenolic Compounds from Avocado Peel with Deep Eutectic Solvents. Green Anal. Chem. 2023, 7, 100083. [Google Scholar] [CrossRef]
- Oliveira, C.S.D.; Andrade, J.K.S.; Rajan, M.; Narain, N. Influence of the Phytochemical Profile on the Peel, Seed and Pulp of Margarida, Breda and Geada Varieties of Avocado (Persea americana Mill) Associated with Their Antioxidant Potential. Food Sci. Technol. 2022, 42, e25822. [Google Scholar] [CrossRef]
- King-Loeza, Y.; Ciprián-Macías, D.A.; Cardador-Martínez, A.; Martín-del-Campo, S.T.; Castañeda-Saucedo, M.C.; Ramírez-Anaya, J.D.P. Functional Composition of Avocado (Persea americana Mill. Var Hass) Pulp, Extra Virgin Oil, and Residues Is Affected by Fruit Commercial Classification. J. Agric. Food Res. 2023, 12, 100573. [Google Scholar] [CrossRef]
- Grisales-Mejía, J.F.; Álvarez-Rivera, G.; Torres-Castañeda, H.G.; Andrade-Mahecha, M.M.; Martínez-Correa, H.A.; Mendiola, J.A.; Cifuentes, A.; Ibañez, E. Hass Avocado (Persea americana Mill.) Residues as a New Potential Source of Neuroprotective Compounds Using Pressurized Liquid Extraction. J. Supercrit. Fluids 2024, 204, 106117. [Google Scholar] [CrossRef]
- Restrepo-Serna, D.L.; Cardona-Alzate, C.A. The Avocado Peel as a Source of Catechins: A Comparison between Extraction Technologies and the Influence of Fruit Variety. Sustain. Chem. Pharm. 2024, 39, 101556. [Google Scholar] [CrossRef]
- Del Castillo-Llamosas, A.; Rodríguez-Martínez, B.; Del Río, P.G.; Eibes, G.; Garrote, G.; Gullón, B. Hydrothermal Treatment of Avocado Peel Waste for the Simultaneous Recovery of Oligosaccharides and Antioxidant Phenolics. Bioresour. Technol. 2021, 342, 125981. [Google Scholar] [CrossRef] [PubMed]
- Avalos-Viveros, M.; Santolalla-Vargas, C.-E.; Santes-Hernández, V.-F.; Martínez-Flores, H.-E.; Torres-García, E.; López-Meza, J.-E.; Virgen-Ortiz, J.-J.; Pérez-Calix, E.; García-Pérez, M.-E. Valorization of Avocado Peels by Conventional Extraction and Hydrothermal Carbonization for Cosmeceutical Applications. Sustain. Chem. Pharm. 2023, 36, 101335. [Google Scholar] [CrossRef]
- Toepfl, S.; Mathys, A.; Heinz, V.; Knorr, D. Potential of high hydrostatic pressure and pulsed electric fields for energy efficient and environmentally friendly food processing. Food Rev. Int. 2006, 22, 405–423. [Google Scholar] [CrossRef]
- Buckow, R.; Ng, S.; Toepfl, S. Pulsed electric field processing of orange juice: A review on microbial, enzymatic, nutritional, and sensory quality and stability. Compr. Rev. Food Sci. Food Saf. 2013, 12, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Evrendilek, G.A. Impacts of pulsed electric field and heat treatment on quality and sensory properties and microbial inactivation of pomegranate juice. Food Sci. Technol. Int. 2017, 23, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Fincan, M.; Dejmek, P. In situ visualization of the effect of a pulsed electric field on plant tissue. J. Food Eng. 2002, 55, 223–230. [Google Scholar] [CrossRef]
- Puertolas, E.; Koubaa, M.; Barba, F.J. An overview of the impact of electrotechnologies for the recovery of oil and high-value compounds from vegetable oil industry: Energy and economic cost implications. Food Res. Int. 2016, 80, 19–26. [Google Scholar] [CrossRef]
- Yan, L.G.; He, L.; Xi, J. High intensity pulsed electric field as an innovative technique for extraction of bioactive compounds—A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2877–2888. [Google Scholar] [CrossRef]
- Bansal, V.; Sharma, A.; Ghanshyam, C.; Singla, M.L. Optimization and characterization of pulsed electric field parameters for extraction of quercetin and ellagic acid in emblica officinalis juice. J. Food Meas. Charact. 2014, 8, 225–233. [Google Scholar] [CrossRef]
- Fincan, M. Extractability of phenolics from spearmint treated with pulsed electric field. J. Food Eng. 2015, 162, 31–37. [Google Scholar] [CrossRef]
- Kwao, S.; Al-Hamimi, S.; Damas, M.E.V.; Rasmusson, A.G.; Galindo, F.G. Effect of guard cells electroporation on drying kinetics and aroma compounds of Genovese basil (Ocimum basilicum L.) leaves. Innov. Food Sci. Emerg. Technol. 2016, 38, 15–23. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- López, N.; Puértolas, E.; Condón, S.; Raso, J.; Alvarez, I. Enhancement of the extraction of betanine from red beetroot by pulsed electric fields. J. Food Eng. 2009, 90, 60–66. [Google Scholar] [CrossRef]
- López, N.; Puértolas, E.; Condón, S.; Álvarez, I.; Raso, J. Effects of pulsed electric fields on the extraction of phenolic compounds during the fermentation of must of Tempranillo grapes. Innov. Food Sci. Emerg. Technol. 2008, 9, 477–482. [Google Scholar] [CrossRef]
- Delsart, C.; Ghidossi, R.; Poupot, C.; Cholet, C.; Grimi, N.; Vorobiev, E.; Milisic, V.; Peuchot, M.M. Enhanced extraction of phenolic compounds from Merlot grapes by pulsed electric field treatment. Am. J. Enol. Vitic. 2012, 63, 205–211. [Google Scholar] [CrossRef]
- Boussetta, N.; Vorobiev, E. Extraction of valuable biocompounds assisted by high voltage electrical discharges: A review. Comptes Rendus Chim. 2014, 17, 197–203. [Google Scholar] [CrossRef]
- El Kantar, S.; Boussetta, N.; Rajha, H.N.; Maroun, R.G.; Louka, N.; Vorobiev, E. High voltage electrical discharges combined with enzymatic hydrolysis for extraction of polyphenols and fermentable sugars from orange peels. Food Res. Int. 2018, 107, 755–762. [Google Scholar] [CrossRef]
- Misra, N.N.; Martynenko, A.; Chemat, F.; Paniwnyk, L.; Barba, F.J.; Jambrak, A.R. Thermodynamics, transport phenomena, and electrochemistry of external field-assisted nonthermal food technologies. Crit. Rev. Food Sci. Nutr. 2018, 58, 1832–1863. [Google Scholar] [CrossRef]
- Xi, J.; He, L.; Yan, L.G. Continuous extraction of phenolic compounds from pomegranate peel using high voltage electrical discharge. Food Chem. 2017, 230, 354–361. [Google Scholar] [CrossRef]
- Almohammed, F.; Koubaa, M.; Khelfa, A.; Nakaya, M.; Mhemdi, H.; Vorobiev, E. Pectin recovery from sugar beet pulp enhanced by high-voltage electrical discharges. Food Bioprod. Process. 2017, 103, 95–103. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Barba, F.J.; Parniakov, O.; Galanakis, C.M.; Lebovka, N.; Grimi, N.; Vorobiev, E. High voltage electrical discharges, pulsed electric field, and ultrasound assisted extraction of protein and phenolic compounds from olive kernel. Food Bioproc. Technol. 2015, 8, 885–894. [Google Scholar] [CrossRef]
- Sarkis, J.R.; Boussetta, N.; Tessaro, I.C.; Marczak, L.D.F.; Vorobiev, E. Application of pulsed electric fields and high voltage electrical discharges for oil extraction from sesame seeds. J. Food Eng. 2015, 153, 20–27. [Google Scholar] [CrossRef]
- Barba, F.J.; Brianceau, S.; Turk, M.; Boussetta, N.; Vorobiev, E. Effect of alternative physical treatments (ultrasounds, pulsed electric fields, and high-voltage electrical discharges) on selective recovery of bio-compounds from fermented grape pomace. Food Bioproc. Technol. 2015, 8, 1139–1148. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Lebovka, N.; Vorobiev, E. Extraction assisted by pulsed electric energy as a potential tool for green and sustainable recovery of nutritionally valuable compounds from mango peels. Food Chem. 2016, 192, 842–848. [Google Scholar] [CrossRef]
- Nagaosa, Y.; Wang, T. High performance centrifugal partition chromatographic separation of alkaline earth metal ions with bis-2-ethylhexylphosphinic acid. J. Sep. Sci. 2003, 26, 953–956. [Google Scholar] [CrossRef]
- Lee, J.H.; Ko, J.Y.; Kim, H.H.; Kim, C.Y.; Jang, J.H.; Nah, J.W.; Jeon, Y.J. Efficient approach to purification of octaphlorethol A from brown seaweed, Ishige foliacea by centrifugal partition chromatography. Algal Res. 2017, 22, 87–92. [Google Scholar] [CrossRef]
- Lee, J.H.; Ko, J.Y.; Oh, J.Y.; Kim, C.Y.; Lee, H.J.; Kim, J.; Jeon, Y.J. Preparative isolation and purification of phlorotannins from Ecklonia cava using centrifugal partition chromatography by one-step. Food Chem. 2014, 158, 433–437. [Google Scholar] [CrossRef]
- Anaëlle, T.; Leon, E.S.; Laurent, V.; Elena, I.; Mendiola, J.A.; Stéphane, C.; Nelly, K.; Luc, M.; Valérie, S.P. Green improved processes to extract bioactive phenolic compounds from brown macroalgae using Sargassum muticum as model. Talanta 2013, 104, 44–52. [Google Scholar] [CrossRef]
- Bojczuk, M.; Żyżelewicz, D.; Hodurek, P. Centrifugal partition chromatography–A review of recent applications and some classic references. J. Sep. Sci. 2017, 40, 1597–1609. [Google Scholar] [CrossRef]
- Sharma, S.; Kori, S.; Parmar, A. Surfactant mediated extraction of total phenolic contents (TPC) and antioxidants from fruits juices. Food Chem. 2015, 185, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Gümüş Yılmaz, G.; Gómez Pinchetti, J.L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Comparison of extraction techniques and surfactants for the isolation of total polyphenols and phlorotannins from the brown algae lobophora variegata. Anal. Lett. 2019, 52, 2724–2740. [Google Scholar] [CrossRef]
| Extraction Method | Important Finding | Ref. |
|---|---|---|
| Maceration | The extract contained 48 compounds, where the major components were flavonoids and procyanidins. The extract inhibited platelet aggregation (at 1, 0.75, and 0.5 mg/mL) and reduced enzymatic inhibition, especially inhibition of xanthine oxidase, hyaluronidase, and acetylcholinesterase. | [53] |
| Avocado peel methanolic extract contained phenolic compounds (21.833 ± 0.118 mg/100 g extract), flavonoids (2.607 ± 0.111 mg/100 g extract), tannins (38.357 ± 0.202 mg/100 g extract), saponins (8.874% ± 0.031%), and alkaloids (9.95 ± 0.035 mg CE/g extract) that contributed to its antioxidant activities. Its IC50 was 185.891 ± 1.598 ppm. | [54] | |
| The ethanolic extract inhibited the growth of Staphylococcus. A longer extraction time (1.5, 3, and 4 h) showed a higher antioxidant and antibacterial activity. | [55] | |
| The optimal maceration of avocado peel was obtained with 40% ethanol, 49.3 °C, solvent/feed ratio of 14.3 mL/g, and 60 min process. The optimal extract showed the highest total phenolic content (44.24 mg GAE/g peel dw), total flavonoid content (786.08 mg QE/g peel dw), antioxidant capacity against DPPH (564.82 μmTE/g peel dw), FRAP (1006.21 82 μmTE/g peel dw), and ABTS (804.40 82 μmTE/g peel dw). | [56] | |
| The total polyphenolic content of avocado peel, pulp, and seed ethanolic extract was 200, 245, and 424 mg GAE/100 g DW, respectively. The total flavonoid contents of avocado peel, pulp, and seed ethanolic extract were 36.06, 36.98, and 32.54 mg RE/100 g DW, respectively. The radical scavenging activity of avocado peel, pulp, and seed extract was 4.90, 3.24, and 3.63 μg/mL, respectively. | [57] | |
| The extract contained the phenolic compound (59.55 GAE mg/gram extract), flavonoid (2.96 QE mg/gram extract), and tannin (22.63 TAE mg/gram extract). The extract significantly changed hydration levels, collagen, and skin elasticity with 2 times application per day for 4 weeks in male rats (in vivo). | [58] | |
| The avocado peel extract contained phenolics compounds (309.95 ± 25.33 mmol GA/100 g of extract), flavonoids (12.54 ± 0.52 mmol Cat.eq/100 g of extract), and anthocyanins (622.37 ± 17.26 mmol Cyanidin-3-glucoside eq./100 g of extract). The extract showed an antiproliferative effect mediated by apoptosis, oxidative stress reduction, and antiproliferative effect. | [59] | |
| The extracts showed a high content of Ca, Mg, Fe, Zn, ω-6 linoleic acid, and flavonoids. The extract showed acetylcholinesterase inhibition with no significant difference with eserine control. | [60] | |
| The wet grinding plus maceration showed the highest value of total phenols (2143.1 mg GAE/100 g dry weight), chlorogenic acid (244.3 mg/100 g dry matter), and epicatechin (181.7 mg/100 g dry matter). The wet grinding plus maceration method used accessible technology and more environment-friendly solvent than others. | [61] | |
| Microwave-assisted extraction | The extract showed high matrix metalloproteinases inhibitory capacity and antioxidant activity. The total phenolic content of the extract was 18.1–68.8 mg GAE/g peel DE, which was affected by time, temperature, and solvents during extraction. | [7] |
| The highest antioxidant capacity was obtained by 74.48 °C–4.13 min and 66.37 °C–0.97 min extractions with 42.58% ethanol. The extract showed a high polyphenolic content (3.79.28 ± 19.35 mg GAE/g dry extract) and high antioxidant activity measured by DPPH, ABTS, and ORAC assay. | [16] | |
| Vacuum microwave-assisted aqueous extraction | The optimal extractions were temperature of 79.64 and 78.11 °C, time of 11.89 and 11.75 min, ratio of water and avocado peel 16.45 and 10.02%, and microwave power of 5708.04 and 5699.10 W. The conditions showed the highest TPC (0.352 gallic acid equivalent-GAE/g fresh avocado peel/min) and DPPH radical scavenging activity (0.104 L/min). | [62] |
| Vacuum microwave-assisted aqueous extraction | - The avocado peel extract contained flavonoids that exhibited antioxidant, antimicrobial, and antifungal activity. | [63] |
| Ultrasound-assisted extraction | The extract by Sonotrode extraction had higher flavan-3-ols recovery (54%) and antioxidant activity (62–76%) than ultrasound bath extraction. Sonotrode extraction was an alternative to non-thermal, low-time-consuming, and scalable methods for extracting the bioactive compounds as functional ingredients. | [64] |
| The extract with acetone–water solvent showed higher total phenolic content (208.5 ± 19.8 mg GAE/g DE) than the extract with ethanol (183.4 ± 6.0 mg GAE/g DE) and ethanol–water solvent (192.6 ± 11.1 mg GAE/g DE). | [65] | |
| The optimal extraction obtained by response surface methodology was 50.9 °C of temperature, 49.5% of ethanol/water, and 61.8 min. The extract contained 124.050–125.187 mg GAE/g of phenolic content. | [66] | |
| The ethanol concentration higher than 40% decreased phenolic content. The optimized extract was obtained with 38.46% of ethanol, 44.06 min, and 50 °C. The extract contained high phenolic and flavonoid compounds. It influenced the metabolic activity of normal and cancer cells, but the extract had positive effects on metabolic activity and inhibited cancer (Caco-2, A549, and HeLa) cells. The extract showed low cellular toxicity in normal cells but negatively affected cancer cells, particularly HeLa cells. | [22] | |
| The avocado peel extract had higher phenolic content and antioxidant activity but lower IC50 value (59 ppm) than cocoa bean, coconut, and cactus pear extract. The avocado peel extract showed inhibitory activity against Staphylococcus aureus, Shigella dysenteriae, and Candida albicans. | [67] | |
| Ultrasound–microwave combined extraction | The avocado extract had higher phenolic compounds and antioxidant activity than the aqueous fraction and the acid-microwave hydrolyzed fraction. The extract inhibited growth of Pseudomonas aeruginosa and Bacillus cereus, Staphylococcus aureus, Salmonella spp., and Salmonella spp. | [68] |
| Ultrasound-assisted deep eutectic solvent extraction | The optimal extraction conditions were a matrix/solvent ratio of 1:30 (w/v), an extraction time of 15 min, and a temperature of 25 °C. The phenolic compounds of 8.29 ± 0.07 g GAE/100 g of dry avocado peel were extracted by the optimal extraction above. | [69] |
| Ultrasound-assisted extraction and enzyme-assisted extraction | The major polyphenolic compounds of the extract were benzoic acid, vanillic acid, syringic acid, and resveratrol. The ultrasound-assisted extraction yielded phenolic extraction equal to that of enzyme-assisted extraction. The extract of ultrasound-assisted extraction showed a solid-to-solvent ratio of 1:20 (w/v), 20% ultrasonic intensity, 30 min showed the highest polyphenols (35.4 mg GAE/g of dried peel). | [21] |
| Enzymatic-assisted extraction | The avocado peel extract treated by peptidase showed higher total phenolics (45.46 mg GAE/g DW) and antioxidant activities (FRAP: 1547.00 µmolTE/g, DPPH: 243.93 µmolTE/g, ABTS assay: 211.96 µmolTE/g) than the methanolic extract. | [70] |
| Total phenolic content (TPC) of avocado peel (296.5–515.1 mg/100 g DM) between TPC of avocado pulp (38.0–41.0 mg/100 g DM) and TPC of avocado seed (395.3–663.93 mg/100 g DM). The antioxidant capacity of avocado peel extract (ABTS: 6.407–20.96 mmol TE/g, DPPH: 9.341–22.85 μmol TE/g) was lower than avocado seed extract (ABTS: 14.70–24.54 mmol TE/g, DPPH: 24.78–52.08 μmol TE/g), but higher than avocado pulp (ABTS: 3.587–5.748 mmol TE/g, DPPH: 0.635–0.962 μmol TE/g) and avocado oil (ABTS: 0.320–0.561 mmol TE/g, DPPH: 0.040–0.351 μmol TE/g). | [71] | |
| Natural deep eutectic solvent (NaDES) extraction | The best solvents used were choline chloride-acetic acid and -lactic acid., The deep eutectic solvents were more efficient than ethanol. The extract contained higher phenolics (92.03 ± 2.11 mg GAE/g DAP) and flavonoid content (186.01 ± 3.27 mg RE/g DAP) than conventional extract with ethanol. | [48] |
| Pressurized liquid extraction | The extraction with pure water (without ethanol) at 100 bar and 40 °C obtained a high extraction yield (26.8 ± 0.9%), antioxidant capacity (ABTS: 3350 ± 179 µmol TE/g dry extract, ORAC: 0.14 ± 0.01 µg/mL), total phenol content (505 ± 25 mg GAE/g dry extract), and acetylcholinesterase inhibition (33.6 ± 2.9 µg/mL). | [72] |
| The total phenolic content of avocado peel extract was 158.8 ± 25.9 mg GAE/g DE—higher than the phenolic content of avocado seed tegument extract (9.5 ± 0.16 mg GAE/g DE) but lower than avocado seed extract (11.9 ± 0.05 mg GAE/g DE). The antioxidant capacity of avocado peel extract was 1329.4 ± 492.1 μmol TE/g DE of DPPH, 829.8 ± 445.4 μmol TE/g DE of ABTS, and 3215.1 ± 668.4 μmol Fe2+/g DE of FRAP. | [65] | |
| Supercritical fluid extraction | Avocado peel extract contained phenolic acid, flavonoid, quercetin, and catechin. The production cost is 5.52 USD/kg for stand-alone extraction processes, with profit margins of 21.14%. | [37] |
| The supercritical fluids extraction increased 14.20–17.14% extraction yield but decreased catechins concentration on Lorena variety. The optimal conditions during supercritical fluids extraction were 60 C, 0.2 mg avocado peel every liter of solvent, 30 kHz, and 60 min. The minimum extraction cost was 8.21 USD/kg of avocado peel extract. | [73] | |
| Aqueous two-phase extraction | The extraction based on polyethylene glycol with 24.9–14.5% sodium nitrate and 12.2–15.5% magnesium sulfate recovered more than 82% flavonoids, phenols, and condensable tannin from the avocado peel. | [52] |
| Hydrothermal treatment | This extraction was due to increased oligosaccharides and polyphenolics recovery. The optimal extraction obtained by 150 °C with the highest oligosaccharide recovery (14.3 g oligosaccharides/100 g avocado peel) and antioxidant phenolics recovery (3.48 g gallic acid equivalents/100 g AP and 10.80 g Trolox equivalents/100 g avocado peel measured with ABTS●+ assay). | [74] |
| Combination of maceration and hydrothermal carbonization | The extract was divided into ethanolic extract, liquid phases, and heavy bio-oils. Ethanolic extract had the highest proanthocyanidins content. The liquid phases were high in total phenols, flavonoids, and hydroxynamic acids. Heavy bio-oils inhibited tyrosinase and elastase activities significantly. | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, D.R.A.; Ayouaz, S.; Rachmawati, A.N.; Madani, K.; Fibri, D.L.N.; Rafi, M.; Julianti, E.; Fahmy, K. Advanced and Potential Methods for Extraction of Bioactive Compounds from Avocado Peel—A Review. Appl. Sci. 2024, 14, 6018. https://doi.org/10.3390/app14146018
Muhammad DRA, Ayouaz S, Rachmawati AN, Madani K, Fibri DLN, Rafi M, Julianti E, Fahmy K. Advanced and Potential Methods for Extraction of Bioactive Compounds from Avocado Peel—A Review. Applied Sciences. 2024; 14(14):6018. https://doi.org/10.3390/app14146018
Chicago/Turabian StyleMuhammad, Dimas Rahadian Aji, Siham Ayouaz, Annisa Noor Rachmawati, Khodir Madani, Dwi Larasatie Nur Fibri, Mohamad Rafi, Elisa Julianti, and Khandra Fahmy. 2024. "Advanced and Potential Methods for Extraction of Bioactive Compounds from Avocado Peel—A Review" Applied Sciences 14, no. 14: 6018. https://doi.org/10.3390/app14146018
APA StyleMuhammad, D. R. A., Ayouaz, S., Rachmawati, A. N., Madani, K., Fibri, D. L. N., Rafi, M., Julianti, E., & Fahmy, K. (2024). Advanced and Potential Methods for Extraction of Bioactive Compounds from Avocado Peel—A Review. Applied Sciences, 14(14), 6018. https://doi.org/10.3390/app14146018








