Characterization of Mesenchymal Stromal Cells after Serum Starvation for Extracellular Vesicle Production
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Mesenchymal Stromal Cell Isolation, Expansion, and Starvation
2.2. Characterization of MSCs
2.2.1. Flow Cytometry
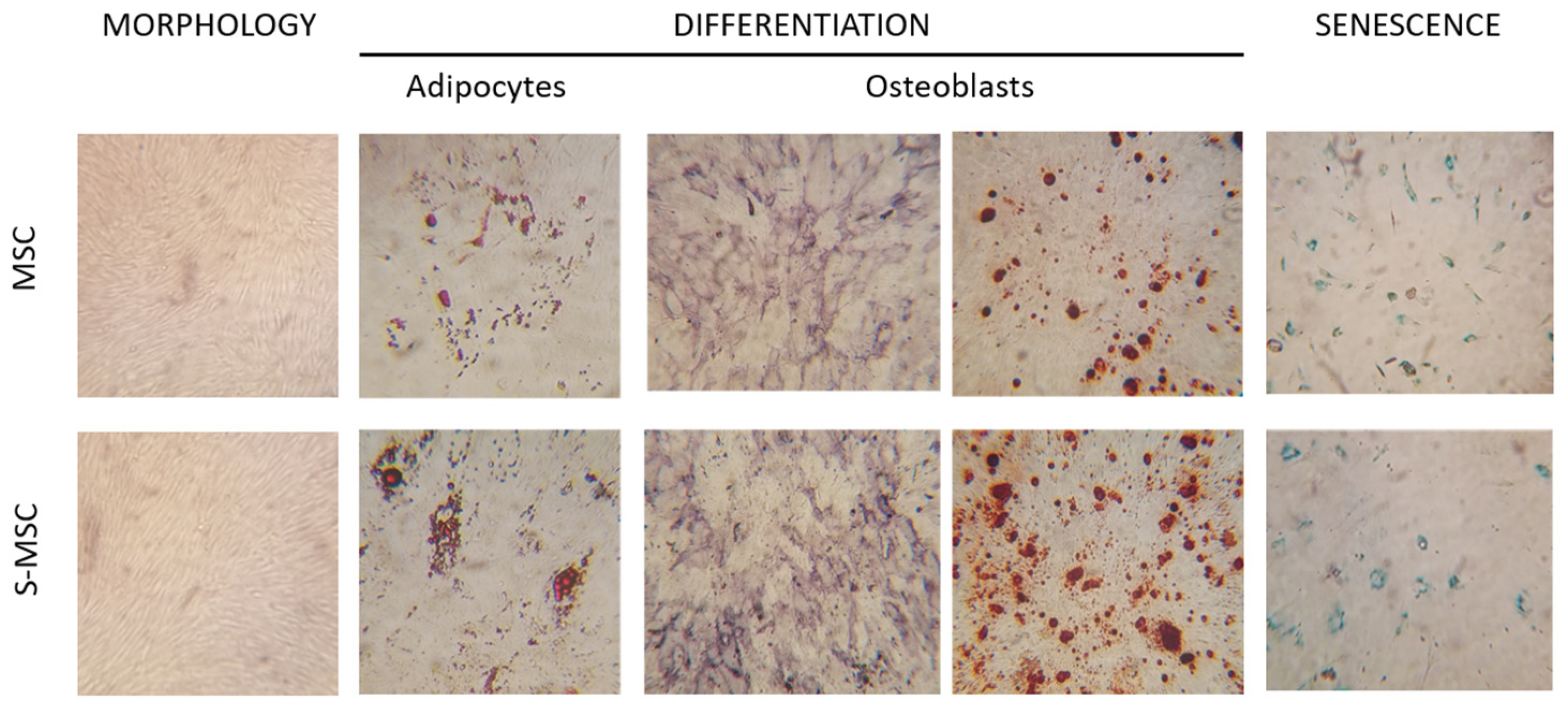
2.2.2. Differentiation Assay
2.2.3. Senescence
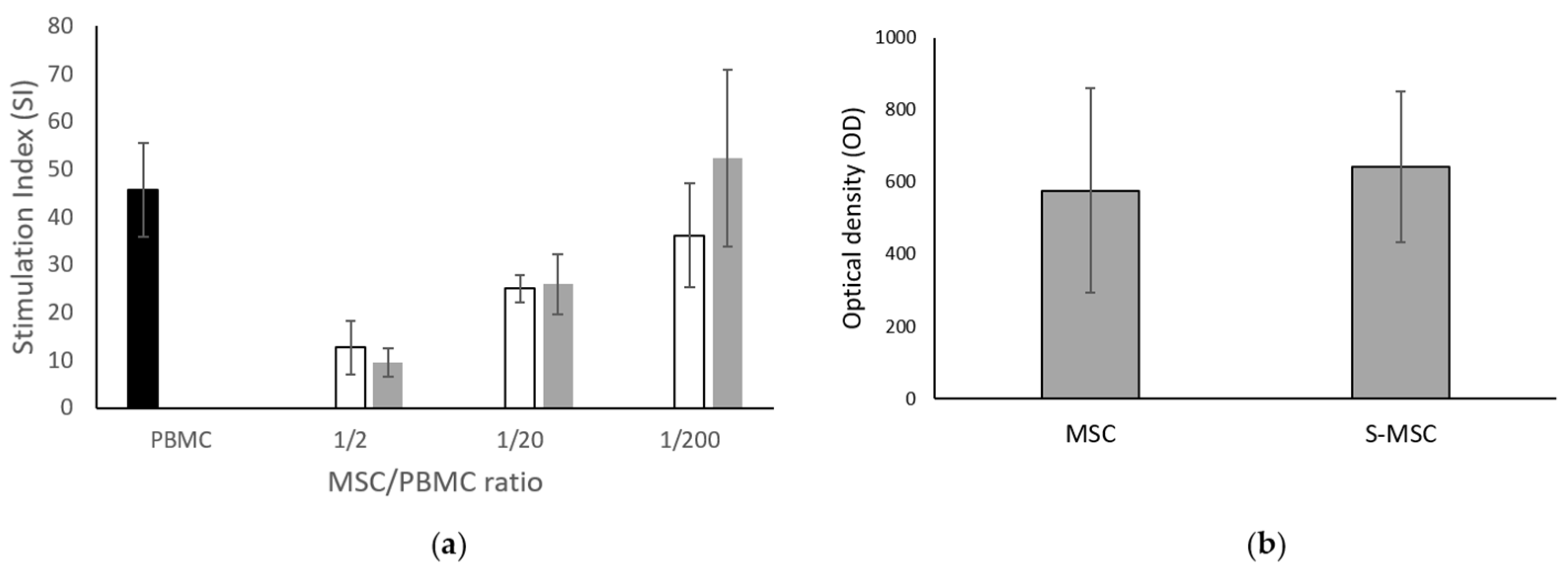
2.2.4. MSC Immunomodulation Capacity
2.2.5. MSC Metabolic Activity
2.2.6. Cytogenetic Analysis by Conventional Karyotype
2.3. Transmission Electron Microscopy (TEM)
2.4. Statistical Analysis
3. Results
3.1. MSC and S-MSC Characterization
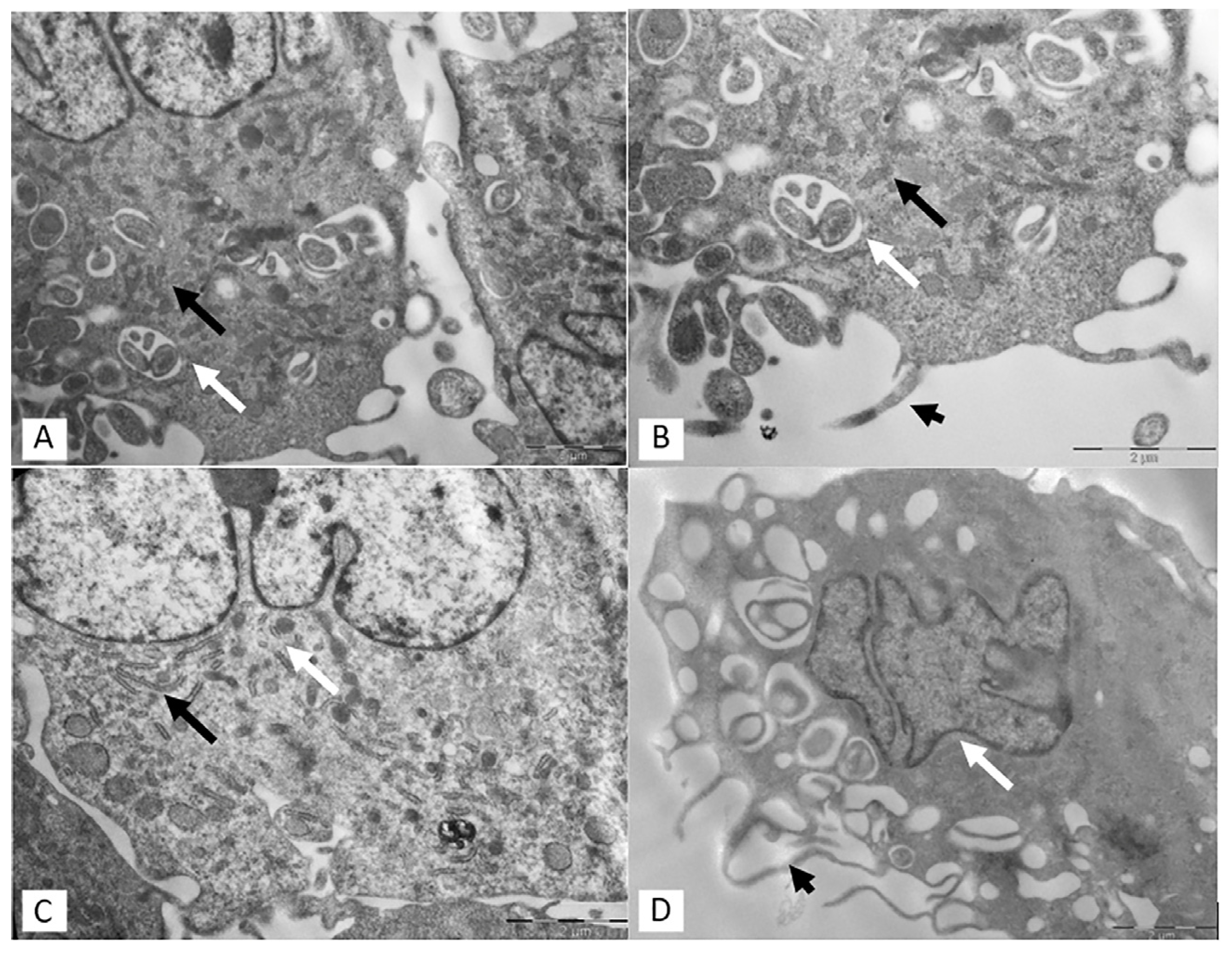
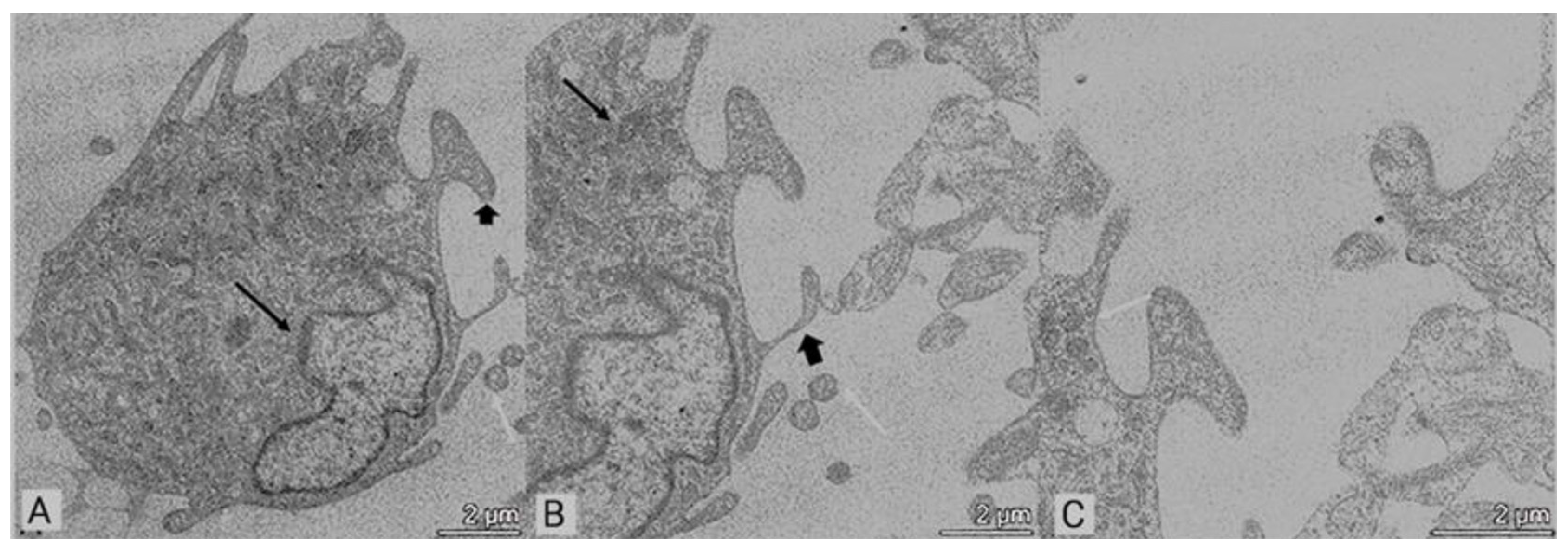
3.2. Transmission Electron Microscopy (TEM)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, A.; AboQuella, N.M.; Wang, H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res. Ther. 2023, 14, 66. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, Y.; Li, H.J. Advances in mesenchymal stem cell exosomes: A review. Stem Cell Res. Ther. 2021, 12, 71. [Google Scholar] [CrossRef]
- Manzoor, T.; Saleem, A.; Farooq, N.; Dar, L.A.; Nazir, J.; Saleem, S.; Ismail, S.; Gugjoo, M.B.; Shiekh, P.A.; Ahmad, S.M. Extracellular vesicles derived from mesenchymal stem cells—A novel therapeutic tool in infectious diseases. Inflamm. Regen. 2023, 43, 17. [Google Scholar] [CrossRef]
- Patel, D.B.; Gray, K.M.; Santharam, Y.; Lamichhane, T.N.; Stroka, K.M.; Jay, S.M. Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles. Bioeng. Trans. Med. 2017, 2, 170–179. [Google Scholar] [CrossRef]
- Vu, B.T.; Le, H.T.; Nguyen, K.N.; Van Pham, P. Hypoxia, Serum starvation, and TNF-a can modify the immunomodulation potency of human adipose-derived stem cells. In Advances in Mesenchymal Stem Cells and Tissue Engineering; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Jung, P.Y.; Ryu, H.; Rhee, K.J.; Hwang, S.; Lee, C.G.; Gwon, S.Y.; Kim, J.; Kim, J.; Yoo, B.S.; Baik, S.K.; et al. Adipose tissue-derived mesenchymal stem cells cultured at high density express IFN-b and TRAIL and suppress the growth of H460 human lung cancer cells. Cancer Lett. 2019, 202, 440–441. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Miller, R.; Dubuke, M.L.; Rockwell, H.E.; Coles, A.H.; Sapp, E.; Didiot, M.C.; Echeverria, D.; Stoppato, M.; Sere, Y.Y.; et al. Serum deprivation of mesenchymal stem cells improves exosome activity and alters lipid and protein composition. iScience 2019, 16, 230–241. [Google Scholar] [CrossRef]
- Oskowitz, A.; McFerrin, H.; Gutschow, M.; Carter, M.L.; Pochampally, R. Serum-deprived human multipotent mesenchymal stromal cells (MSCs) are highly angiogenic. Stem Cell Res. 2011, 6, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Bakopoulou, A.; Kritis, A.; Andreadis, D.; Papachristou, E.; Leyhausen, G.; Koidis, P.; Geurtsen, W.; Tsiftsoglou, A. Angiogenic potential and secretome of human apical papilla mesenchymal stem cells in various stress microenvironments. Stem Cells Dev. 2015, 24, 2496–2512. [Google Scholar] [CrossRef] [PubMed]
- Hahm, J.; Kim, J.; Park, J. Strategies to enhance extracellular vesicle production. Tissue Eng. Regen. Med. 2021, 18, 513–524. [Google Scholar] [CrossRef]
- Binder, B.Y.; Sagun, J.E.; Leach, J.K. Reduced serum and hypoxic culture conditions enhance the osteogenic potential of human mesenchymal stem cells. Stem Cell Rev. Rep. 2015, 11, 387–393. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, S.H.; Li, T.Z.; Suh, H. Influence of in vitro biomimicked stem cell ‘niche’ for regulation of proliferation and differentiation of human bone marrow-derived mesenchymal stem cells to myocardial phenotypes: Serum starvation without aid of chemical agents and prevention of spontaneous stem cell transformation enhanced by the matrix environment. J. Tissue Eng. Regen. Med. 2016, 10, E1–E13. [Google Scholar]
- Avanzini, M.A.; Bernardo, M.E.; Cometa, A.M.; Perotti, C.; Zaffaroni, N.; Novara, F.; Visai, L.; Moretta, A.; Del Fante, C.; Villa, R.; et al. Generation of mesenchymal stromal cells in the presence of platelet lysate: A phenotypic and functional comparison of umbilical cord blood- and bone marrow-derived progenitors. Haematologica 2009, 94, 1649–1660. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Lo Sicco, C.; Reverberi, D.; Balbi, C.; Ulivi, V.; Principi, E.; Pascucci, L.; Becherini, P.; Bosco, M.C.; Varesio, L.; Franzin, C.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem Cells Transl. Med. 2017, 6, 1018–1028. [Google Scholar] [CrossRef]
- Magarotto, F.; Sgrò, A.; Dorigo Hochuli, A.H.; Andreetta, M.; Grassi, M.; Saggioro, M.; Nogara, L.; Tolomeo, A.M.; Francescato, R.; Collino, F.; et al. Muscle functional recovery is driven by extracellular vesicles combined with muscle extracellular matrix in a volumetric muscle loss murine model. Biomaterials 2021, 269, 120653. [Google Scholar] [CrossRef]
- Almeria, C.; Weiss, R.; Roy, M.; Tripisciano, C.; Kasper, C.; Weber, V.; Egger, D. Hypoxia Conditioned Mesenchymal Stem Cell-Derived Extracellular Vesicles Induce Increased Vascular Tube Formation in vitro. Front. Bioeng. Biotechnol. 2019, 7, 292. [Google Scholar] [CrossRef]
- Gregorius, J.; Wang, C.; Stambouli, O.; Hussner, T.; Qi, Y.; Tertel, T.; Börger, V.; Mohamud Yusuf, A.; Hagemann, N.; Yin, D.; et al. Small extracellular vesicles obtained from hypoxic mesenchymal stromal cells have unique characteristics that promote cerebral angiogenesis, brain remodeling and neurological recovery after focal cerebral ischemia in mice. Basic. Res. Cardiol. 2021, 116, 40. [Google Scholar] [CrossRef]
- Gorgun, C.; Africano, C.; Ciferri, M.C.; Bertola, N.; Reverberi, D.; Quarto, R.; Ravera, S.; Tasso, R. Preconditioned Mesenchymal Stromal Cell-Derived Extracellular Vesicles (EVs) Counteract Inflammaging. Cells 2022, 11, 3695. [Google Scholar] [CrossRef]
- Kim, J.Y.; Rhim, W.K.; Seo, H.J.; Lee, J.Y.; Park, C.G.; Han, D.K. Comparative Analysis of MSC-Derived Exosomes Depending on Cell Culture Media for Regenerative Bioactivity. Tissue Eng. Regen. Med. 2021, 18, 355–367. [Google Scholar] [CrossRef]
- Guo, S.; Debbi, L.; Zohar, B.; Samuel, R.; Arzi, R.S.; Fried, A.I.; Carmon, T.; Shevach, D.; Redenski, I.; Schlachet, I.; et al. Stimulating Extracellular Vesicles Production from Engineered Tissues by Mechanical Forces. Nano Lett. 2021, 21, 2497–2504. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sun, L.; Zhang, J.; Chiang, C.; Pan, J.; Wang, X.; Kwak, K.J.; Li, H.; Zhao, R.; Rima, X.Y.; et al. Exosomal mRNAs for Angiogenic–Osteogenic Coupled Bone Repair. Adv. Sci. 2023, 10, 2302622. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.H.A.; Antunes, M.A.; Dos Santos, C.C.; Weiss, D.J.; Cruz, F.F.; Rocco, P.R.M. Strategies to improve the therapeutic effects of mesenchymal stromal cells in respiratory diseases. Stem Cell Res. Ther. 2018, 9, 45. [Google Scholar] [CrossRef]
- Moya, A.; Larochette, N.; Paquet, J.; Deschepper, M.; Bensidhoum, M.; Izzo, V.; Kroemer, G.; Petite, H.; Logeart-Avramoglou, D. Quiescence Preconditioned Human Multipotent Stromal Cells Adopt a Metabolic Profile Favorable for Enhanced Survival under Ischemia. Stem Cells 2017, 35, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Mylotte, L.A.; Duffy, A.M.; Murphy, M.; O’Brien, T.; Samali, A.; Barry, F.; Szegezdi, E. Metabolic flexibility permits mesenchymal stem cell survival in an ischemic environment. Stem Cells 2008, 26, 1325–1336. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asti, A.L.; Croce, S.; Valsecchi, C.; Lenta, E.; Grignano, M.A.; Gregorini, M.; Carolei, A.; Comoli, P.; Zecca, M.; Avanzini, M.A.; et al. Characterization of Mesenchymal Stromal Cells after Serum Starvation for Extracellular Vesicle Production. Appl. Sci. 2024, 14, 5821. https://doi.org/10.3390/app14135821
Asti AL, Croce S, Valsecchi C, Lenta E, Grignano MA, Gregorini M, Carolei A, Comoli P, Zecca M, Avanzini MA, et al. Characterization of Mesenchymal Stromal Cells after Serum Starvation for Extracellular Vesicle Production. Applied Sciences. 2024; 14(13):5821. https://doi.org/10.3390/app14135821
Chicago/Turabian StyleAsti, Anna Lia, Stefania Croce, Chiara Valsecchi, Elisa Lenta, Maria Antonietta Grignano, Marilena Gregorini, Adriana Carolei, Patrizia Comoli, Marco Zecca, Maria Antonietta Avanzini, and et al. 2024. "Characterization of Mesenchymal Stromal Cells after Serum Starvation for Extracellular Vesicle Production" Applied Sciences 14, no. 13: 5821. https://doi.org/10.3390/app14135821
APA StyleAsti, A. L., Croce, S., Valsecchi, C., Lenta, E., Grignano, M. A., Gregorini, M., Carolei, A., Comoli, P., Zecca, M., Avanzini, M. A., & Rampino, T. (2024). Characterization of Mesenchymal Stromal Cells after Serum Starvation for Extracellular Vesicle Production. Applied Sciences, 14(13), 5821. https://doi.org/10.3390/app14135821






