Abstract
Vitamin D plays a crucial role in the human body, influencing a wide range of physiological processes from bone health to immune function. The complex biochemical pathways involved in the synthesis, metabolism, and action of Vitamin D are explored, emphasizing its importance in nutrition and food technology. This review also investigates the regulatory mechanisms that control Vitamin D metabolism and its systemic effects on calcium homeostasis, cell proliferation, differentiation, and immune modulation. The role of Vitamin D3 in regulating blood pressure and atherosclerosis in the onset of cardiovascular disorders is discussed. Given the importance of Vitamin D in food science and technology, the regulatory mechanisms that control Vitamin D metabolism and its systemic effects on calcium homeostasis are also investigated, integrating innovative approaches and advanced technologies to improve human health through nutrition. Additionally, the review assesses the influence of food processing on Vitamin D levels and discusses cutting-edge technologies as innovative strategies to mitigate Vitamin D loss during food processing. This comprehensive exploration aims to improve our understanding of the biochemical pathways of Vitamin D and its relevance to food science, contributing to the development of new strategies for food fortification and the promotion of optimal health through diet.
1. Introduction
The importance of Vitamin D for human health is widely recognized. It plays a crucial role in a number of physiological processes such as bone health (by promoting calcium absorption in the gut), immune function (by modulating the immune response), and the regulation of calcium metabolism, which is crucial for bone formation and maintenance. Vitamin D deficiency can lead to a range of health problems, including osteoporosis, increased risk of infections, and potentially chronic diseases [1]. Recent research has indeed highlighted a close correlation between Vitamin D deficiency and the onset of cardiovascular disorders [2].
This review aims to explore the complex biochemical pathways involved in the synthesis, metabolism, and action of Vitamin D within the human body, with a particular interest in the prevention of cardiovascular disorders through innovative dietary and technological strategies. The implications of food fortification and food processing on Vitamin D availability and efficacy will also be discussed, integrating advanced approaches and cutting-edge technologies to improve human health through nutrition.
2. Vitamin D Metabolism, Sources and Supplementation
Research on Vitamin D metabolism has focused on calcium homeostasis and bone metabolism [3]. Subsequently, when 25-hydroxyVitamin D (25OHD) and then 1,25-hydroxyVitamin D [1,25(OH)2D] were identified, the research was also extended to other sectors including infections, immunity, cancer, and cardiovascular diseases. Vitamin D plays a role in regulating the immune system. It works by controlling the activity of suppressor T lymphocytes, cytokine synthesis, and the cell death process [4]. In particular, Vitamin D also stimulates the absorption of phosphate in the intestine and prevents its excretion through the kidneys [5]. Therefore, Vitamin D represents a molecule with a large pleiotropic functional profile.
The term Vitamin D refers to a significant group of fat-soluble substances with the main chain made up of cholesterol rings. 25(OH)D (calcifediol or calcidiol) is the main circulating compound, while 1,25(OH)2D (calcitriol) represents the active compound and interacts with the Vitamin D/hormone D receptor (VDR) [6]. 1,25(OH)2D binds to the VDR to initiate or inhibit gene transcription, causing the heterodimerization of the VDR and the retinoid X receptor [7]. When the heterodimer translocates into the nucleus, the complex binds to the Vitamin D consensus sequences, and gene transcription is modulated. At the circulatory level, Vitamin D (2 and 3) attaches to Vitamin D binding protein (DBP) and is then accumulated in adipose tissue [8]. Subsequently, metabolization into 25(OH)D occurs in the liver by hydroxylase (cytochrome P450(CYP)2R1 and CYP27A1). This process can also occur in other tissues in an autocrine and/or paracrine manner [9]. Hydroxylation then occurs in the renal tubule to produce the active molecule 1,25(OH)2D. During this process, cubilin and megalin, two proteins that allow the DBP-25(OH)D complex to enter through the cellular receptors of the renal tubule [10], intervene. A decrease in cubilin and megalin determines a greater urinary loss of 25(OH)D. Furthermore, the cells of the renal tubule present two hydroxylases belonging to the cytochrome P450 family, i.e., 1-alpha-hydroxylase (CYP27B1) and 24-alpha-hydroxylase (CYP24A1), which, by hydroxylating 25(OH)D, produce the active form of Vitamin D (1,25(OH)2D-calcitriol) or the inactive metabolite 24,25(OH) 2D [10].
The main production site of Vitamin D is the skin [6]. Vitamin D3 (cholecalciferol) is produced in the skin when exposed to UVB rays with a wavelength of 290–315 nm. These radiations convert 7-dehydrocholesterol (7-DHC) into pre-Vitamin D, which in turn is converted into Vitamin D3 in a thermosensitive but non-catalytic process, which involves the formation of three double bonds [11]. Both UVB intensity and skin pigment levels influence the rate of D3 formation. Vitamin D skin production will therefore be influenced by different factors such as latitude, seasonality, clothing, pollution and the use of UV filters [12,13]. In summer, 15 min of full-body sunlight at midday is equivalent to 250 μg of cholecalciferol [14]. However, many factors influence the effectiveness of this combination (Figure 1).
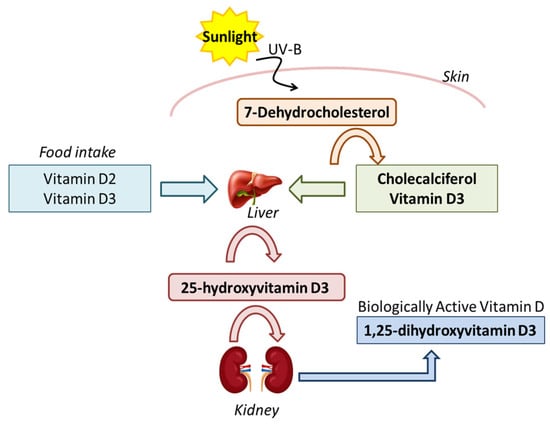
Figure 1.
Pathway of Vitamin Production. The vitamin D production pathway involves the conversion of 7-dehydrocholesterol in the skin to vitamin D3 via UV-B. Vitamin D3 (and D2 from food) is hydroxylated in the liver to 25-hydroxyvitamin D3 and then in the kidneys to 1,25-dihydroxyvitamin D3, the biologically active form.
Compared to synthesis at the skin level, the intake of Vitamin D from foods is lower. The main food source of Vitamin D is foods of animal origin (cholecalciferol), but mushrooms (ergocalciferol) are also an important resource [15]. Vitamin D is a fat-soluble molecule, and therefore for its absorption it requires bile salts, micelles, and chylomicrons for its transport. Oily fish, eggs, and liver also have a high content of Vitamin D. Over the years, many populations have included foods fortified with Vitamin D (ergocalciferol or cholecalciferol) in their diet, mainly milk (cow or vegetable), butter, margarine, and breakfast cereals. Therefore, for those who suffer from Vitamin D deficiency due to insufficient sun exposure or if skin synthesis is reduced (e.g., in the elderly), a supplement should be prescribed [16]. To date, Vitamin D supplementation is considered an effective treatment to produce better health outcomes. Many clinical studies regarding Vitamin D supplementation show beneficial effects on health, but some of them have not shown any positive effects on different types of pathologies [4]. These differences also depend on the methods and times of administration. In fact, many researchers underline the importance of a very long follow-up especially for chronic diseases; the method of administration is also important: some authors prefer intermittent bolus administration and others daily administration (Vitamin D oral intermittent treatment (DO IT) study, a randomized clinical trial with individual loading regimen). Recent data shows that intermittent bolus administration (which is the highest) should be avoided as it can cause harmful health effects [17]. Finally, the doses of administration also play a decisive role: high doses (i.e., ~1000–2000 IU/day) should be used compared to too low doses (i.e., ~400–800 IU/day) in order to observe significant results. Despite these considerations, Vitamin D supplementation has been shown to reduce the risk of lung disease, cancer, type 2 diabetes, and autoimmune diseases [18,19,20,21]. Additionally, individuals with cardiovascular disease may benefit from Vitamin D supplementation, a finding that warrants further investigation [22,23].
3. Methodology
In this comprehensive narrative review, we aimed to collect and summarize the literature concerning the effects of Vitamin D on cardiovascular diseases and the implications of different fortification and processing methods. To achieve this goal, we performed an extensive literature search using Pubmed databases (https://pubmed.ncbi.nlm.nih.gov; accessed 1 April 2024). The literature review explored using the search string: targeting publications from January 2008 to December 2024. The search strategy was meticulously crafted to include a wide range of keywords and phrases such as ‘Vitamin D fortification’, ‘Vitamin D metabolism’, ‘Vitamin D and cardiovascular health’, ‘impact of food processing on Vitamin D levels’, and ‘mitigation strategies for Vitamin D content in processed foods’. We also included studies that specifically discussed Vitamin D biochemical pathways and its role in human health, expanding our search to include terms such as ‘Vitamin D biochemical pathways’, ‘Vitamin D in clinical nutrition’, and ‘Vitamin D in food science’. Each article was selected based on its relevance to the central themes of the review, the quality of the evidence presented, and the clarity of the results reported. Once the articles were retrieved, we used a structured approach to data extraction. Key information was catalogued, including study design, sample size, main outcomes, and conclusions regarding the impact of Vitamin D on health and food processing techniques. These data were then critically analyzed to assess the consistency of findings across studies and to evaluate the strength of evidence supporting the role of Vitamin D in improving health and reducing disease. Our analysis aimed not only to summarize the results but also to identify gaps in the current research landscape. The review process was designed iteratively, allowing for the inclusion of new studies that met our inclusion criteria as they became available. The final data synthesis provides a detailed overview of the current understanding of the role of Vitamin D and the efficacy of various fortification strategies, offering valuable insights into potential areas for future research.
4. Vitamin D Deficiency and Cardiovascular Risk
Vitamin D deficiency is associated with many health consequences, including skeletal problems (i.e., rickets, fractures, osteomalacia, osteopenia) and non-skeletal problems [24,25]. Non-skeletal complications include heart disease and complications such as systolic and diastolic dysfunction, abdominal aortic aneurysm, and hypertension [26,27,28,29,30,31]. Vitamin D deficiency is associated with several cardiovascular risk factors. By increasing the synthesis of renin and angiotensin II, Vitamin D deficiency can induce the production of reactive oxygen species (ROS). Furthermore, being implicated in the processes of calcification and proliferation of smooth muscles, Vitamin D levels directly influence cardiovascular health [32]. A study from the Intermountain Integrated Health System prospective database of 41,504 patients showed an association between Vitamin D deficiency and an increased risk of peripheral vascular disease (PVD), heart failure and coronary heart disease/myocardial infarction, and stroke [33]. Furthermore, following the Hill criteria, low serum 25(OH)D levels have been associated with the risk of cardiovascular disease [34]. Results from a meta-analysis involving 65,994 individuals also show a linear and inverse relationship between Vitamin D levels and the risk of cardiovascular disease [35]. Recent studies demonstrate that Vitamin D can regulate the expression of specific metalloproteinases and their inhibitors, which promote the development of heart failure [36]. Finally, growing evidence reveals the anti-inflammatory activity of Vitamin D in inhibiting the activation of tumor necrosis factor-alpha (TNF-α), nuclear factor kappa B (NF-kB), and interleukin-10, all known important contributors of cardiovascular disease [37].
4.1. Vitamin D Deficiency and Coronary Artery Disease
The relationship between Vitamin D deficiency and coronary heart disease (CAD) has been demonstrated in various studies. The risk of coronary heart disease seems to be associated with Vitamin D deficiency. The main indicator of this possible relationship is the presence of VDR in the myocardium, vascular endothelial cells, and fibroblasts. In detail, epidemiological studies highlight that Vitamin D deficiency, causing the activation of the renin–angiotensin–aldosterone system (RAAS), causes an increase in CAD by increasing anti-inflammatory and decreasing proinflammatory mediators [38]. Preliminary studies show a relationship between Vitamin D deficiency and cases of acute myocardial infarction (AMI) in the short and long term [38]. The study included 18,225 male patients aged between 40 and 75 years. Over 10 years, results showed an association between low Vitamin D levels and an increased risk of AMI [27]. In particular, men deficient (≤15 ng/mL) in 25(OH)D were at increased risk of AMI compared to those considered to be sufficient (≥30 ng/mL) in 25(OH)D. It has also been shown that hospitalized patients with AMI had a high incidence of Vitamin D deficiency. Lee et al. found low levels of Vitamin D in 96% of 239 patients with acute coronary syndrome (ACS) [39]. Dziedzic et al. found low levels of Vitamin D in patients who had episodes of myocardial infarction during their lifetime [40]. Increased risks of ischemic heart disease, myocardial infarction, and premature death were also associated with low Vitamin D levels during a large prospective study that included 10,170 individuals [41]. Furthermore, a meta-analysis of 18 studies found an increased risk of ischemic heart disease and premature death in subjects with low Vitamin D levels [41].
Despite these observations, there is still no certain protocol to support the benefit of Vitamin D supplementation in the treatment/prevention of coronary heart disease. This is partly due to the few ongoing or completed clinical studies examining the effects of Vitamin D on patients with AMI or ACS.
4.2. Vitamin D Deficiency and Hypertension
Hypertension is the most common cardiovascular disease in adults (29%), and it is estimated that in 2025 there will be approximately 1.6 billion cases of hypertension in the global population [42]. As mentioned previously, Vitamin D deficiency is assumed to increase blood pressure via the renin–angiotensin system.
An increase in renin expression levels and plasma angiotensin II production are observed in mice lacking the VDR receptor. This condition leads to hypertension, cardiac hypertrophy, and increased water intake. Administering 1,25(OH)2D3 suppresses renin expression in VDR-null mice [43]. Vitamin D regulates the proliferation of endothelial and vascular smooth muscle cells and VDR is present in these cells [44]. Endothelial dysfunction plays an important role in cardiovascular diseases as well as hypertension. Vitamin D deficiency affecting endothelial cells can induce hypertension [45]. In endothelium-specific VDR knockout mice, aortic relaxation is impaired by acetylcholine, as are increased sensitivity to the hypertensive effects of angiotensin II and the increased expression of myocardial genes sensitive to hypertrophy induced by angiotensin II infusion [46]. These observations indicate that the endothelial VDR may have an important role in blood pressure control.
Similar results highlight an increase in the production of superoxide anion, angiotensin II, systolic blood pressure, and atrial natriuretic peptide production in rats with Vitamin D deficiency. The same authors also identify 51 genes involved in the regulation of oxidative stress and hypertrophy myocardial [47]. Another study shows how a finger rich in Vitamin D is able to reverse the increase in systolic and diastolic blood pressure, high plasma renin–angiotensin activity, and reduced urinary sodium excretion in Vitamin D-deficient mice after 6 weeks [48]. It therefore seems that hypertension and an acceleration of atherosclerosis are linked to Vitamin D deficiency. A study of 3316 patients showed consistent increases in plasma renin concentrations and angiotensin 2 with a parallel decrease in 25(OH)D and 1,25(OH)2D [49]. Obese hypertensive individuals also present a significant increase in the activity of the renin–angiotensin system together with a decrease in 25(OH)D levels [50]. Vaidya et al. demonstrate that genetic variability in the Fok1 gene encoding VDR, in association with 25(OH)D levels, is related to hypertension, suggesting that the Vitamin D–VDR complex could be a regulator of renin in humans [51].
4.3. Vitamin D Deficiency and Acute Myocardial Infarction
Referring back to the previous paragraph, regarding coronary heart disease, the association between Vitamin D and acute myocardial infarction (AMI) deserves further investigation. Indeed, many studies present conflicting results. Some authors demonstrate an increase in Vitamin D deficiency in patients who have suffered an AMI both in Italy and in the United States [39,52]. Another study carried out on 314 subjects hospitalized for AMI highlights that 85% of patients had 25(OH)D deficiency regardless of sex or age, but it is associated with some risk factors such as diabetes mellitus and calcium deficiency [53]. This trend has also been confirmed in other prospective studies which state a high incidence of Vitamin D deficiency in patients admitted to hospitals for AMI [54,55]. Further data have been provided on the association between low Vitamin D levels and AMI. Ng et al. in a large study show the link between Vitamin D deficiency and AMI or acute coronary syndrome in 1259 hospitalized patients [56]. As mentioned above there are also conflicting results regarding the association between Vitamin D deficiency and AMI. Ford et al. show how VDR KO mice subjected to experimental AMI had normal cardiac function compared to wild type mice [57]. These results highlight the importance of the contribution of Vitamin D to AMI and other cardiac complications such as thrombosis, arrhythmias, and heart failure, which should still be studied in depth.
4.4. Vitamin D Deficiency and Heart Failure
Many studies also correlate myocardial infarction with Vitamin D deficiency. In particular, a significant increase in biomarkers linked to heart failure, apoptosis, and inflammation was observed in VDR KO mice. The administration of paricalcitol (PC), an activated Vitamin D analog, promotes cardioprotection through the activation of anti-apoptotic and anti-inflammatory mechanisms [58]. In fact, it has been shown that Vitamin D deficiency correlates with an increase in inflammatory cytokines (TNF-α, IL-6, and IL-1β), which decrease when Vitamin D is supplemented. Alongside this role it seems that Vitamin D also has an anti-fibrotic and anti-hypertrophic role at the cardiac level. In fact, mesenchymal multipotent cells (MMCs) exposed to 5′-azacytidine to induce a fibrotic phenotype, and then treated with active Vitamin D (1,25D), presented a decreased expression of fibrotic genes [59]. Epidemiological studies also confirm the importance of Vitamin D in subjects suffering from heart failure. Increased mortality in patients with heart failure (due to left ventricular dysfunction) appears to be associated with low levels of 25-hydroxyVitamin D [60] A recent study published in 2023 shows how Vitamin D can be used today as a prognostic biomarker in heart failure. In particular, the authors demonstrate that in 70 patients with left ventricular heart failure there was also a significant drop in 25(OH)D levels [61]. All these results clarify the importance of Vitamin D in correct cardiovascular function and underline the need to study new types of Vitamin D supplementation and dietary models in relation to heart failure. Table 1 summarizes the possible role of Vitamin D for various cardiovascular disease (CVD) risk factors.

Table 1.
The role of Vitamin D3 on CVD risk factors.
4.5. Vitamin D Supplementation and CVD
Recent investigations into the potential benefits of Vitamin D supplementation in the prevention of CVD have led to conclusions that question the efficacy of such interventions in a large population. Vitamin D supplementation did not significantly reduce the incidence of CVD events [68]. This finding is supported by Virtanen et al. [69], who demonstrated that Vitamin D3 supplementation did not reduce the incidence of major CVD events among older adults, potentially due to the sufficiency of baseline Vitamin D levels in participants. The efficacy of high-dose Vitamin D (200,000 IU, followed a month later by monthly doses of 100,000 IU, or placebo for a median of 3.3 years) supplementation in preventing CVD was questioned in a study by Scragg et al. [70] that revealed no preventive benefit, suggesting the need for further research into the effects of different dosing regimens. Complementing this, Neale et al. [71] reported that the monthly administration of Vitamin D3 (60,000 IU per month) did not reduce all-cause mortality in unscreened elderly subjects. A randomized controlled trial [72] showed that high-dose Vitamin D supplementation did not improve markers of blood glucose, inflammation, neurohormonal activation, or lipids. Similarly, Desouza [73] observed a small favorable effect on atherosclerotic CVD risk score in individuals with prediabetes not selected for Vitamin D insufficiency, but no decrease in major adverse cardiovascular events. Further studies [74,75] found no reduction in major CVD events or improvement in post-stroke outcomes with Vitamin D or omega-3 fatty acid supplementation. Gepner et al. [76] and Pilz et al. [77] also reported no significant improvements in cardiovascular risk factors, with the latter study noting an increase in triglycerides with Vitamin D supplementation in hypertensive patients with low 25-hydroxyVitamin D levels. Finally, Wood et al. [78] concluded that improving Vitamin D status through dietary supplementation is unlikely to reduce conventional CVD risk factors, highlighting the complexity of Vitamin D’s role in cardiovascular health and disease prevention. In summary, although Vitamin D supplementation has been widely promoted for its potential health benefits, evidence from RCTs suggests that it does not significantly reduce the incidence of major cardiovascular events or cancer or improve certain cardiovascular risk factors in the general population.
5. Impact of Dietary Vitamin D Fortification on Blood Vitamin D Levels and Cardiovascular Outcomes
Fortification strategies, while jurisdictional in nature, demonstrate variable impacts on cardiovascular health across different states. Dairy products, bread, and breakfast cereals are the foods most commonly fortified with Vitamin D [79]. In countries where there is no mandatory fortification policy, data show that the fortification of foods with Vitamin D could counteract Vitamin D deficiency [80]. In Finland, for example, data modeling studies led to a policy of mandatory Vitamin D food fortification that increased 25(OH)D concentrations in the population by 18 nmol/L [81]. In vitro studies suggest that altering the lipid composition of fortified foods increases the absorption of Vitamin D3 [78]. Proposed fortification strategies may not be as effective because the matrix and composition of foods may alter Vitamin D absorption and bioavailability [82]. Supplements and fortified foods serve as additional sources of Vitamin D. Both Vitamin D2 and D3 can be synthetically produced for supplementation and fortification purposes [83]. The debate on the bioequivalence of Vitamin D2 and D3 in fortification and supplementation suggests that D3 may be superior, especially when administered in the form of supplements or injections. Despite numerous researches that have investigated Vitamin D2 and D3 additionally, there is a lack of research directly comparing the effect of Vitamin D2 and D3 from biofortified foods on 25(OH)D concentrations. The fortification of dairy products with Vitamin D is considered cost-effective and bioavailable, so much so that some countries have mandated fortification [84]. Studies show a significant increase in 25(OH)D levels following the consumption of fortified dairy products, although most research has focused on female participants [85]. The efficacy of fortified foods for males and various dietary preferences remains important [86]. In addition, non-dairy fortification strategies, including bars and juices, meet the needs of those who avoid dairy products. These studies emphasize the importance of selecting appropriate foods for fortification to accommodate different dietary habits and ensure widespread improvement in Vitamin D status [87,88].
Although both D2 and D3 can be effective in increasing Vitamin D levels when added to foods, D3 generally shows superior bioavailability and efficacy in improving Vitamin D status [89]. The choice of foods to be fortified is critical in maximizing the benefits of Vitamin D fortification policies, particularly in meeting the nutritional needs of different population groups [90]. Fortifying bread with 11.3 μg of Vitamin D/100 g could result in a daily Vitamin D intake of 23.7 μg, raising the serum 25(OH)D concentration in the German population from 45 nmol/L to 75 nmol/L during winter as demonstrated by Brawn et al. [80]. Moreover, a 12-month study involving nursing home residents who consumed bread fortified with 125 μg (5000 IU) Vitamin D3 daily observed an increase in serum 25(OH)D levels from 28.5 nmol/L to 125.6 nmol/L [91].
While these strategies have successfully elevated Vitamin D levels, the direct correlation between these increases, and a significant reduction in major cardiovascular events is not clearly established. Despite effectively increasing serum vitamin D levels, supplementation did not yield clear cardiovascular benefits in several studies, suggesting that the role of vitamin D in cardiovascular health might be more complex and not solely dependent on improving Vitamin D status. This underscores the potential limitations of fortification in addressing the multifactorial nature of cardiovascular diseases [75]. For instance, in Finland, despite the rise in 25(OH)D levels due to mandatory fortification, the expected decline in cardiovascular incidents has not been conclusively observed, suggesting that other factors like dietary patterns, genetic predispositions, and lifestyle choices play significant roles [81]. Studies often show that micronutrient enhancements improve markers of general health but fail to demonstrate efficacy in preventing complex conditions such as heart disease, which involve multifactorial etiologies beyond simple nutritional deficiencies. This highlights the need for integrated health strategies that go beyond nutritional fortification, incorporating public health initiatives aimed at overall lifestyle and environmental changes to effectively combat cardiovascular diseases [92]. Table 2 synthesizes in vivo studies on the impact of Vitamin D-fortified foods, drawn from systematic reviews and meta-analysis of randomized trials. Based on the variability of results observed in current studies, policy recommendations should consider more localized fortification strategies that take into account regional dietary patterns, sunlight exposure, and genetic factors that may influence Vitamin D metabolism. Adapting policies to regional health profiles could improve the effectiveness of fortification in preventing cardiovascular disease and other complex health conditions [93]. Continued research is essential to better understand the differential impacts of Vitamin D2 and D3, as well as the optimal levels of fortification needed to influence public health outcomes positively. This should include long-term studies that not only assess biochemical markers but also directly correlate these with reductions in disease incidence, particularly in diverse population groups [94].

Table 2.
In vivo studies on the impact of Vitamin D-fortified food.
6. The Impact of Processing on Vitamin D Levels in Foods and Mitigation Strategies
6.1. Impact of Processing on Vitamin D Levels
The effectiveness of dietary Vitamin D fortification in improving blood Vitamin D levels and cardiovascular outcomes is intricately linked to how Vitamin D is processed and maintained in fortified foods. Fortification strategies are crucial for enhancing Vitamin D levels in the population, especially in regions with limited sunlight exposure. However, the actual benefits of such fortification on cardiovascular health are still under debate, as studies show variable impacts on cardiovascular outcomes. This is compounded by the fact that the processing of Vitamin D-fortified foods can significantly affect the nutrient’s stability and bioavailability. Without proper handling during processing, the potential cardiovascular benefits of Vitamin D fortification might not be fully realized, underscoring the need for optimized processing methods to maintain the efficacy of fortified foods in boosting Vitamin D status and potentially improving cardiovascular health. Both Vitamin D3 and D2 are susceptible to degradation from heat, light, oxidation, and other processing factors, which may compromise its stability and bioavailability in food products. Food scientists must account for these considerations during food processing, storage, and distribution to minimize nutrient loss and ensure product quality and safety. Moreover, innovative processing technologies and packaging materials are continuously explored to enhance the stability and retention of Vitamin D in processed foods. Figure 2 illustrates the impact of various processing methods on Vitamin D3 levels in foods and highlights the key mitigation strategies employed to counteract these effects.
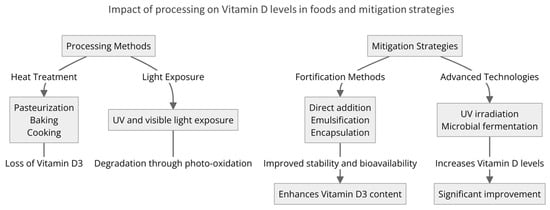
Figure 2.
The impact of various processing methods on Vitamin D3 levels in foods and fortification approaches, including direct addition, emulsification, encapsulation, UV irradiation, and microbial fermentation, which are crucial in enhancing the stability and bioavailability of Vitamin D3, thus improving overall nutritional quality.
6.1.1. Pasteurization
Several studies have demonstrated that food processing, especially at high temperatures, alters the Vitamin D content of foods. Pasteurization, a common heat treatment used to extend the shelf life of dairy products, has been shown to cause a significant reduction in Vitamin D levels. Gomes et al. investigated the effects of pasteurization on Vitamin D compounds in donor breast milk. They found significant reductions in Vitamin D2 and D3 concentration, with losses ranging from 10% to 20% after pasteurization. This study highlights the sensitivity of Vitamin D compounds to the pasteurization process [103]. Similarly, another study by Capozzo et al. observed a significant reduction in Vitamin D (56%) following high-temperature, short-time (HTST) pasteurization and ultra-high-temperature (UHT) processing [104]. As reported by Tsai et al. (2017), Vitamin D3 has poor thermal stability, which results in a significant reduction (p ≤ 0.05) of about 50% in Vitamin D3 in pasteurized milk compared with raw milk [105]. The heat sensitivity of Vitamin D is a key factor contributing to its degradation during pasteurization. Moreover, Vitamin D is susceptible to thermal degradation, especially at elevated temperatures and prolonged heating times. Research by Jakobsen et al. [106] elucidated the kinetics of Vitamin D degradation during heat treatment, showing that the degradation rate increases with rising temperatures. Jakobsen et al. have highlighted that the stability of Vitamin D in food matrices such as oil and mushrooms significantly decreases when subjected to higher cooking temperatures. Their research found that lower temperatures tend to preserve Vitamin D levels better than high temperatures, which can significantly accelerate the degradation process.
6.1.2. Baking and Cooking
Processing methods such as baking, cooking, frying, and water boiling induce a significant reduction in Vitamin D, although the extent of this loss varies depending on the type of food being cooked and the specific methods of heat application. Jakobsen et al. report that during heat treatment in an oven for 40 min at typical cooking temperatures, eggs and margarine retained about 39–45% of their Vitamin D content while frying preserved 82–84% of the vitamin, whereas boiling eggs achieved a retention rate of 86–88%. In the case of baked bread, rye bread retained 69% of its Vitamin D3, which was less than the 85% retention observed in wheat bread. Vitamin D2 retention was, instead, somewhat better, at 73% in rye bread and 89% in wheat bread [107]. Another study by Hill et al. [108] demonstrated that both storage and cooking practices influence Vitamin D metabolites, with certain cooking methods showing true retention rates for Vitamin D3 ranging from 50–152%. The findings suggest that 25-hydroxyVitamin D3-enriched eggs can serve as a potent dietary source of Vitamin D, with their total Vitamin D activity being significantly higher in comparison to non-enriched eggs depending on the storage and cooking method used.
6.1.3. Light Exposure
Vitamin D is sensitive to UV and visible light, leading to degradation through photo-oxidation. Foods exposed to light during processing or storage, especially those in transparent packaging, are at risk of losing their Vitamin D content. Saffert et al. have demonstrated the effects of package light transmittance on the vitamin content of milk, showing that transparent packaging led to more pronounced losses in Vitamin D3 compared to pigmented or less transparent packaging. This aligns with the general observation that light exposure can degrade sensitive vitamins like Vitamin D3 [109]. Furthermore, Vitamin D3-enriched bread exposed to light for 2 h showed a reduction of 15–20% in Vitamin D content, underscoring the sensitivity of both forms of Vitamin D to light during post-baking storage [110]. In detail, D2 and D3 vitamins exhibit different susceptibilities to oxidative degradation, with Vitamin D3 generally considered more stable, due to its structure, under similar conditions [110]. The impact of processing on Vitamin D underscores the importance of considering strategies to mitigate nutrient losses in processed foods.
6.2. Mitigation Strategies to Improve Vitamin D Content in Processed Foods
Recent advancements in the field of nutrition and food technology have provided innovative solutions to improve the bioavailability and stability of Vitamin D in processed foods, addressing the challenge of Vitamin D deficiency on a global scale. Stability during processing, storage, and in vitro bioaccessibility are a key point to address for setting the best technological strategies aiming to enhance or maintain nutritional value in terms of Vitamin D in processed foods.
6.2.1. Fortification
The most widely used approach to restore or enhance the Vitamin D content of processed products is fortification. Vitamin D fortification, particularly in dairy products, cereals, juices, and margarine, has been instrumental in enhancing population-wide intake, especially in regions with limited sunlight exposure. In many countries, including the UK, the exposure of the skin to UVB radiation or supplementation is the primary means of obtaining Vitamin D. According to the latest UK National Diet and Nutrition Survey data, collected in 2012/13 and 2013/14, mean dietary intakes of Vitamin D were reported as 3.1 µg/d for adult men and 2.5 µg/d for adult women. However, lower dietary intakes ranging from 1.25–1.6 µg/d have been observed among South Asian women in the UK [111].
To achieve sustainable fortification, direct addition, emulsification, or encapsulation are widely employed. Direct addition is predominantly used for enriching milk and dairy products [112]. Typically, Vitamin D is dissolved in a food-safe organic solvent, such as ethanol and butter oil, before being evenly distributed through homogenization within the food matrix. However, challenges arise with Vitamin D’s stability within aqueous food matrices and its tendency to adhere to packaging materials, like polypacks or tetrapacks, which can lead to degradation. In the emulsification technique, an oil phase containing Vitamin D is dispersed into fine droplets within water, and these droplets are then incorporated into target foods like cheese, milk, and bread. Achieving a stable emulsion faces hurdles such as the homogenization of Vitamin D in the food matrix and the limited availability of food-grade emulsifiers. Manufacturers may utilize fortification techniques to add Vitamin D to dairy products post-pasteurization, offsetting the losses incurred during processing. Adding Vitamin D at stages of processing that expose it to the least amount of heat, light, and oxygen can help in retaining its levels. However, fortification levels may vary by region and regulations but are typically designed to provide a significant portion of the recommended daily intake of Vitamin D per serving. To reduce the loss of Vitamin D upon food processing, complementary approaches include the optimization of processing as the pasteurization parameters, such as temperature and duration, to minimize Vitamin D degradation while ensuring food safety. Modifying processing conditions, such as lowering temperatures and reducing exposure to oxygen and light during manufacturing, can help preserve Vitamin D content.
6.2.2. UV Irradiation and Biofortification
Besides post-processing fortification, alternative fortification methods can be explored to enhance the Vitamin D content of processed foods. Among them, the ultraviolet (UV) irradiation of dairy products post-pasteurization can stimulate the synthesis of Vitamin D, effectively increasing its levels without compromising food safety. The EU Novel Food Regulation oversees the approval and safety evaluation of UV-treated foods, considering them as novel foods. EFSA, under EC regulation no. 258/97, endorsed UV radiation for post-pasteurization milk processing to enhance shelf life and Vitamin D levels [113]. The European Commission specifies allowed UV wavelengths for treating different foods to increase Vitamin D. Ultraviolet (UV) irradiation, predominantly UVB and more recently UVC radiation, stimulates the synthesis of Vitamin D in certain foods, particularly those containing precursor molecules such as ergosterol or proVitamin D2. After irradiation with UVB light (290–315 nm), 7-dehydrocholesterol undergoes a photoisomerization reaction, followed by a thermal isomerization reaction, resulting in the formation of Vitamin D3. In this context, the European Commission (EC) allowed specific wavelength ranges for different foods (200–800 nm for mushrooms, 240–315 nm for bread, 200–310 nm for milk, not specified for baker’s yeast) [112]. Within the realm of dairy products, UV irradiation is strategically employed post-pasteurization to bolster their Vitamin D content. By subjecting pasteurized dairy items to UV irradiation, manufacturers can catalyze the conversion of precursor molecules inherent in the product, such as ergosterol in milk, into active Vitamin D2 (ergocalciferol) [112,114]. Studies have shown that Vitamin D2 levels in fungi can be significantly enhanced by exposing them to UVB light [114]. As reported by Lavelli et al., UV irradiation significantly increased Vitamin D2 content in mushrooms, reaching up to 57 µg/g dry weight in A. bisporus, and up to 37 µg/g dry weight in P. ostreatus [115]. Additionally, UV irradiation circumvents the need for heat or chemical additives, thereby preserving the sensory and nutritional integrity of the food product, rendering it an appealing option for Vitamin D enhancement without compromising food safety or consumer acceptance.
6.2.3. Microbial Fermentation
Among the innovative approaches to fortifying foods with Vitamin D, microbial fermentation represents a novel strategy. Certain microorganisms such as Amycolata sp., Rhodococcus erythropolis can convert Vitamin D3 to 1α,25-dihydroxyVitamin D3 via 25-hydroxyVitamin D3 during fermentation processes [116]. By selecting appropriate strains and optimizing fermentation conditions, manufacturers can enhance the Vitamin D content of fermented foods such as yogurt and cheese, offering consumers an additional source of this essential nutrient. Recently, EFSA evaluated the safety of Vitamin D-enriched UV-treated baker’s yeast, Saccharomyces cerevisiae, as a novel food ingredient, by revealing that the UV-treated baker’s yeast is capable of converting ergosterol into Vitamin D2, significantly enhancing the Vitamin D2 content of food products without compromising safety [117]. Amycolatopsis autotrophica has been shown to convert Vitamin D3 to its more potent forms, 25-hydroxyVitamin D3, and 1α,25-dihydroxyVitamin D3, via microbial hydroxylation. The process was further optimized by using cyclodextrin to increase the hydroxylation efficiency significantly [118]. Since Vitamin D3 has inadequate absorption compared to its corresponding hydroxylated derivatives, an efficient biotransformation of Vitamin D3 to 25-hydroxyVitamin D3 by a newly isolated Bacillus cereus strain was proposed by Tang et al., 2020 [119]. Moreover, Sasaki et al. reported the bioconversion of D3 derivatives for the first time by screening approximately 300 Streptomyces strains. Among them, S. sclerotialus FERM BP-1370 and S. roseosporus FERM BP-1574 were found to be able to introduce hydroxy group at C-1α position of calcifediol and C-25 position of 1α(OH)VD3, respectively. Microbial fermentation presents a viable strategy for enhancing the levels of Vitamin D3, with specific microbial species offering promising routes for the bioconversion and improved bioavailability of this essential vitamin. This approach has implications not only for dietary supplements but also for the fortification of foods, potentially contributing to better health outcomes by addressing Vitamin D3 deficiency.
6.2.4. Encapsulation
In the field of food technology, fortifying foods with Vitamin D presents numerous challenges, such as ensuring compatibility with various food matrices, achieving even dispersion and uniformity, maintaining stability, and delivering bioavailable doses capable of addressing Vitamin D deficiencies. These obstacles have catalyzed the development of innovative fortification methods across different food products. The increasing consumer interest in functional foods has also propelled the creation and application of novel nanomaterials tailored for food fortification, with numerous nanomaterials proving to be effective vehicles for Vitamin D enhancement. Furthermore, cutting-edge processing methods, including microencapsulation and nanoemulsions, are being explored to encapsulate Vitamin D efficiently, thereby protecting it from thermal degradation during food processing. This technique facilitates controlled release, boosts the physicochemical stability and bioavailability of Vitamin D, and enhances its dispersibility and uniformity within complex food matrices. Microencapsulation involves the encapsulation of active ingredients, such as vitamins, within microscopic particles or capsules made of various materials, including proteins, carbohydrates, lipids, and polymers. The encapsulation process creates a protective barrier around the active ingredient, preventing its degradation or interaction with external factors such as heat, light, moisture, and oxygen. Abbasi et al. (2014) investigated the stability of Vitamin D3 encapsulated in nanoparticles of whey protein isolate against degradation parameters such as UV light or oxygen [120]. This study highlights the potential of encapsulation techniques in protecting Vitamin D3 from environmental factors. In addition, Diarrassouba et al. (2014) explored the increased stability and protease resistance of the β-lactoglobulin/Vitamin D3 complex, demonstrating that this complexation can significantly protect Vitamin D3 from the damaging effects of UV light [121]. Similar results has been achieved by Loewen, Chan, and Li-Chan (2018), who aimed to optimize Vitamin D3 loading in re-assembled casein micelles and assess the effect of loading on the stability of Vitamin D3 during storage with light exposure [122]. When a composite gel made from whey protein isolate and lotus root amylopectin was used as an encapsulation method, Liu et al. (2020) markedly highlighted an enhancement in the bioavailability of Vitamin D3 offering substantial protection against degradation from UV light exposure [123]. Moreover, microcapsules can be designed to release the encapsulated Vitamin D slowly over time, ensuring sustained availability and absorption in the body. Encapsulating Vitamin D in microcapsules can enhance its solubility in aqueous solutions, facilitating its incorporation into dairy products without affecting their texture or sensory properties. Moreover, recent advances suggest that nanotechnology can offer substantial stability and uniformity by protecting many vitamins against a wide range of conditions and substances that could otherwise affect their integrity during food processing [124]. The nanoencapsulation of Vitamin D also offers protection against degradation during processing and storage, ensuring the retention of its nutritional benefits in the final product. As an example, David and Livney used potato proteins as a natural material to protect and deliver Vitamin D3 in beverages [125]. Nanoemulsions can serve as efficient carriers for encapsulating the vitamin within the dispersed oil phase. The nanoemulsion droplets (typically less than 100 nanometers), act as protective compartments, preventing the direct exposure of Vitamin D to heat and other deleterious factors during processing and providing a protective shell that shields it from thermal degradation during pasteurization [126]. For example, research by Gupta et al. demonstrated that Vitamin D3 encapsulated in nanoemulsions showed a 50% improvement in bioavailability compared to non-encapsulated forms. This improvement is attributed to the enhanced solubility and protection against degradation mechanisms provided by the nanoemulsion [127].
Furthermore, the protective capability of nanoemulsions against oxidative degradation is remarkable. A study by Silva et al. [128] found that Vitamin D3 stability increased by up to 70% when encapsulated in nanoemulsions compared to its stability in conventional emulsions under similar conditions. Enhancing the stability of Vitamin D3 is vital for prolonging the shelf life of fortified processed foods, guaranteeing its effectiveness from the moment of production through to the point of consumption. In addition, Lin et al. (2016) focused on the enhancement of Vitamin D3’s physicochemical stability and in vitro bioaccessibility using corn protein hydrolysate as a novel nano-vehicle, showcasing innovative approaches to maintaining Vitamin D3’s stability against UV light [129].
Controlled release is another significant benefit offered by nanoemulsion technology. According to Jaiswal et al. [130], nanoemulsions can be engineered to release encapsulated nutrients in response to specific triggers, such as pH changes or enzymatic action in the gastrointestinal tract. This targeted release mechanism can lead to a more efficient absorption and utilization of Vitamin D3, potentially enhancing its health benefits. Moreover, the development of ovalbumin–pectin nanocomplexes for Vitamin D3 encapsulation showed enhanced storage stability and sustained release in simulated gastrointestinal digestion. The encapsulation efficiency reached up to 96.37%, with a significant improvement in storage stability and a controlled release profile in simulated intestinal fluid, demonstrating that protein–polysaccharide complexes can effectively encapsulate Vitamin D3 for improved bioavailability [131]. Among the innovative strategies for improving Vitamin D3 stability, the nanostructured Lipid Carriers (NLCs) has been shown to provide a controlled release mechanism in simulated gastrointestinal fluids, increasing oral bioavailability and providing a promising strategy for delivering lipophilic vitamins like Vitamin D3. Park et al. [132] examined the release profile of Vitamin D3 from NLCs in simulated gastrointestinal fluids, representing the stomach and intestinal environments. The results showed that NLCs could remain stable and protect Vitamin D3 in simulated stomach fluid, releasing more than 90% of the encapsulated Vitamin D3 upon reaching simulated intestinal fluid. Additionally, Rabelo et al., 2018 focused on chitosan-coated NLCs for Vitamin D loading and studied their physical stability, indicating that coating NLCs with chitosan could effectively stabilize Vitamin D3, preventing its degradation in simulated gastric fluids [133].
Despite these promising outcomes, challenges remain. Concerns about the long-term stability of nanoemulsions and potential impacts on the sensory attributes of fortified foods need to be addressed. Furthermore, the safety and regulatory aspects of nanotechnology in food products warrant careful consideration. For instance, comprehensive toxicological assessments are essential to ensure consumer safety. The European Food Safety Authority (EFSA) and the Food and Drug Administration (FDA) are actively working on establishing guidelines for the use of nanotechnology in food products [134,135]. In conclusion, the incorporation of nanoemulsion technology in food fortification strategies presents a promising approach to improving the stability and bioavailability of Vitamin D3 in processed foods. However, overcoming the technological and regulatory challenges will be crucial for the successful application of this technology. Ongoing research and innovation in this field are essential to unlock the full potential of nanoemulsions for Vitamin D3 fortification and to contribute significantly to addressing nutritional deficiencies.
7. Conclusions
The main findings of this review emphasize the crucial role of Vitamin D not only in calcium metabolism and bone health but also in blood pressure regulation, inflammation, and endothelial function, all of which are relevant to cardiovascular health. Research has shown that Vitamin D deficiency is associated with an increased risk of cardiovascular disease, suggesting the importance of food fortification and the use of supplements as effective strategies to improve Vitamin D status in the general population. However, current evidence indicates that Vitamin D fortification or supplementation neither significantly reduces the incidence of major cardiovascular events nor improves certain cardiovascular risk factors in the general population. These findings raise questions about the efficacy of Vitamin D fortification strategies in the prevention of cardiovascular disease, suggesting, furthermore, the need for further research to explore the effects of different dosing regimens and the use of Vitamin D in specific population groups with proven deficiency. There is a clear need for further research to optimize intervention strategies, especially for individuals with specific nutritional needs or at risk of Vitamin D deficiency. Moreover, the stability, bioaccessibility, and bioavailability of vitamin-D in the food system are important parameters to pay attention to. Innovative mitigation strategies such as UV irradiation, microbial fermentation, and advanced encapsulation techniques like nanoemulsions have been developed to counteract its losses. Employing these strategies effectively requires a careful consideration of processing conditions and food matrices to optimize Vitamin D retention and functionality. As research progresses, these methods are likely to evolve further, offering more efficient and widely applicable solutions to maintain and enhance Vitamin D levels in fortified foods.
Author Contributions
Conceptualization, G.A., M.L. and S.B.; writing—original draft preparation, G.A., M.L. and S.B.; writing—review and editing, G.A., M.L. and S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Janousek, J.; Pilarová, V.; Macáková, K.; Nomura, A.; Veiga-Matos, J.; da Silva, D.D.; Remiao, F.; Saso, L.; Malá-Ládová, K.; Maly, J.; et al. Vitamin D: Sources, physiological role, biokinetics, deficiency, therapeutic use, toxicity, and overview of analytical methods for detection of vitamin D and its metabolites. Crit. Rev. Clin. Lab. Sci. 2022, 59, 517–554. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.H. Vitamin D Deficiency and Cardiovascular Diseases: The Causality or Association? Int. J. Gerontol. 2018, 12, 75. [Google Scholar] [CrossRef]
- McCollum, E.V.; Simmonds, N.; Becker, J.E.; Shipley, P.G. Studies on experimental rickets: XXI. an experimental demonstration of the existence of a vitamin which promotes calcium deposition. J. Biol. Chem. 1922, 53, 293–312. [Google Scholar] [CrossRef]
- Rebelos, E.; Tentolouris, N.; Jude, E. The Role of Vitamin D in Health and Disease: A Narrative Review on the Mechanisms Linking Vitamin D with Disease and the Effects of Supplementation. Drugs 2023, 83, 665–685. [Google Scholar] [CrossRef]
- Akimbekov, N.S.; Digel, I.; Sherelkhan, D.K.; Razzaque, M.S. Vitamin D and Phosphate Interactions in Health and Disease. Phosphate Metab. Physiol. Toxic. 2022, 1362, 37–46. [Google Scholar] [CrossRef]
- Baker, A.R.; McDonnell, D.P.; Hughes, M.; Crisp, T.M.; Mangelsdorf, D.J.; Haussler, M.R.; Pike, J.W.; Shine, J.; Omalley, B.W. Cloning and expression of full-length cdna-encoding human vitamin-D receptor. Proc. Natl. Acad. Sci. USA 1988, 85, 3294–3298. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, D.P.; Mangelsdorf, D.J.; Pike, J.W.; Haussler, M.R.; Omalley, B.W. Molecular-cloning of complementary-DNA encoding the avian receptor for vitamin-D. Science 1987, 235, 1214–1217. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Han, S.N. The Role of Vitamin D in Adipose Tissue Biology: Adipocyte Differentiation, Energy Metabolism, and Inflammation. J. Lipid Atheroscler. 2021, 10, 130–144. [Google Scholar] [CrossRef]
- Jones, G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 2008, 88, 582S–586S. [Google Scholar] [CrossRef]
- Kaseda, R.; Hosojima, M.; Sato, H.; Saito, A. Role of Megalin and Cubilin in the Metabolism of Vitamin D3. Ther. Apher. Dial. 2011, 15, 14–17. [Google Scholar] [CrossRef]
- Holick, M.F.; Maclaughlin, J.A.; Clark, M.B.; Holick, S.A.; Potts, J.T.; Anderson, R.R.; Blank, I.H.; Parrish, J.A. Photosynthesis of previtamin-D3 in human-skin and the physiologic consequences. Science 1980, 210, 203–205. [Google Scholar] [CrossRef]
- Webb, A.R.; Decosta, B.R.; Holick, M.F. Sunlight regulates the cutaneous production of vitamin-D3 by causing its photodegradation. J. Clin. Endocrinol. Metab. 1989, 68, 882–887. [Google Scholar] [CrossRef]
- Neale, R.E.; Khan, S.R.; Lucas, R.M.; Waterhouse, M.; Whiteman, D.C.; Olsen, C.M. The effect of sunscreen on Vitamin D: A review. Br. J. Dermatol. 2019, 181, 907–915. [Google Scholar] [CrossRef]
- Neville, J.J.; Palmieri, T.; Young, A.R. Physical Determinants of Vitamin D Photosynthesis: A Review. J. Bone Miner. Res. Plus 2021, 5, e10460. [Google Scholar] [CrossRef]
- Schmid, A.; Walther, B. Natural Vitamin D Content in Animal Products. Adv. Nutr. 2013, 4, 453–462. [Google Scholar] [CrossRef]
- Lamberg-Allardt, C. Vitamin D in foods and as supplements. Prog. Biophys. Mol. Biol. 2006, 92, 33–38. [Google Scholar] [CrossRef]
- Trivedi, D.P.; Doll, R.; Khaw, K.T. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: Randomised double blind controlled trial. BMJ-Br. Med. J. 2003, 326, 469–472. [Google Scholar] [CrossRef]
- van Etten, E.; Mathieu, C. Immunoregulation by 1,25-dihydroxyvitamin D3:: Basic concepts. J. Steroid Biochem. Mol. Biol. 2005, 97, 93–101. [Google Scholar] [CrossRef]
- Murdaca, G.; Tonacci, A.; Negrini, S.; Greco, M.; Borro, M.; Puppo, F.; Gangemi, S. Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun. Rev. 2019, 18, 102350. [Google Scholar] [CrossRef]
- El-Sharkawy, A.; Malki, A. Vitamin D Signaling in Inflammation and Cancer: Molecular Mechanisms and Therapeutic Implications. Molecules 2020, 25, 3219. [Google Scholar] [CrossRef]
- Blasiak, J.; Chojnacki, J.; Pawlowska, E.; Jablkowska, A.; Chojnacki, C. Vitamin D May Protect against Breast Cancer through the Regulation of Long Noncoding RNAs by VDR Signaling. Int. J. Mol. Sci. 2022, 23, 3189. [Google Scholar] [CrossRef]
- Thompson, B.; Waterhouse, M.; English, D.R.; McLeod, D.S.; Armstrong, B.K.; Baxter, C.; Romero, B.D.; Ebeling, P.R.; Hartel, G.; Kimlin, M.G.; et al. Vitamin D supplementation and major cardiovascular events: D-Health randomised controlled trial. BMJ-Br. Med. J. 2023, 381, e075230. [Google Scholar] [CrossRef]
- Ye, H.W.; Li, Y.X.; Liu, S.M.; Zhang, X.F.; Liang, H.Z.; Wang, Y.; Wang, R.X.; Liu, H.; Wen, Y.; Jing, C.X.; et al. Association between serum 25-hydroxyvitamin D and vitamin D dietary supplementation and risk of all-cause and cardiovascular mortality among adults with hypertension. Nutr. J. 2024, 23, 33. [Google Scholar] [CrossRef]
- Judd, S.E.; Tangpricha, V. Vitamin D Deficiency and Risk for Cardiovascular Disease. Am. J. Med. Sci. 2009, 338, 40–44. [Google Scholar] [CrossRef]
- Basit, S. Vitamin D in health and disease: A literature review. Br. J. Biomed. Sci. 2013, 70, 161–172. [Google Scholar] [CrossRef]
- Zittermann, A.; Schleithoff, S.S.; Koerfer, R. Vitamin D insufficiency in congestive heart failure: Why and what to do about it? Heart Fail. Rev. 2006, 11, 25–33. [Google Scholar] [CrossRef]
- Giovannucci, E.; Liu, Y.; Hollis, B.W.; Rimm, E.B. 25-hydroxyvitamin D and risk of myocardial infarction in—A prospective study. Arch. Intern. Med. 2008, 168, 1174–1180. [Google Scholar] [CrossRef]
- Kim, D.H.; Sabour, S.; Sagar, U.N.; Adams, S.; Whellan, D.J. Prevalence of Hypovitaminosis D in Cardiovascular Diseases (from the National Health and Nutrition Examination Survey 2001 to 2004). Am. J. Cardiol. 2008, 102, 1540–1544. [Google Scholar] [CrossRef]
- Kendrick, J.; Targher, G.; Smits, G.; Chonchol, M. 25-Hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis 2009, 205, 255–260. [Google Scholar] [CrossRef]
- Wong, Y.Y.E.; Flicker, L.; Yeap, B.B.; McCaul, K.A.; Hankey, G.J.; Norman, P.E. Is Hypovitaminosis D Associated with Abdominal Aortic Aneurysm, and is There a Dose-response Relationship? Eur. J. Vasc. Endovasc. Surg. 2013, 45, 657–664. [Google Scholar] [CrossRef]
- Hiemstra, T.F.; Lim, K.; Thadhani, R.; Manson, J.E. Vitamin D and Atherosclerotic Cardiovascular Disease. J. Clin. Endocrinol. Metab. 2019, 104, 4033–4050. [Google Scholar] [CrossRef]
- Heston, T.F. Hypovitaminosis D in Hypertension. South. Med. J. 2010, 103, 723–724. [Google Scholar] [CrossRef]
- Anderson, J.L.; May, H.T.; Horne, B.D.; Bair, T.L.; Hall, N.L.; Carlquist, J.F.; Lappé, D.L.; Muhlestein, J.B.; Grp, I.H.C.S. Relation of Vitamin D Deficiency to Cardiovascular Risk Factors, Disease Status, and Incident Events in a General Healthcare Population. Am. J. Cardiol. 2010, 106, 963–968. [Google Scholar] [CrossRef]
- Weyland, P.G.; Grant, W.B.; Howie-Esquivel, J. Does Sufficient Evidence Exist to Support a Causal Association between Vitamin D Status and Cardiovascular Disease Risk? An Assessment Using Hill’s Criteria for Causality. Nutrients 2014, 6, 3403–3430. [Google Scholar] [CrossRef]
- Pilz, S.; Tomaschitz, A.; Drechsler, C.; Zittermann, A.; Dekker, J.M.; März, W. Vitamin D Supplementation: A Promising Approach for the Prevention and Treatment of Strokes. Curr. Drug Targets 2011, 12, 88–96. [Google Scholar] [CrossRef]
- Polly, P.; Tan, T.C. The role of vitamin D in skeletal and cardiac muscle function. Front. Physiol. 2014, 5, 86755. [Google Scholar] [CrossRef]
- Roffe-Vazquez, D.N.; Huerta-Delgado, A.S.; Castillo, E.C.; Villarreal-Calderón, J.R.; Gonzalez-Gil, A.M.; Enriquez, C.; Garcia-Rivas, G.; Elizondo-Montemayor, L. Correlation of Vitamin D with Inflammatory Cytokines, Atherosclerotic Parameters, and Lifestyle Factors in the Setting of Heart Failure: A 12-Month Follow-Up Study. Int. J. Mol. Sci. 2019, 20, 5811. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 Dietary Reference Intakes for Calcium and Vitamin D: What Dietetics Practitioners Need to Know. J. Am. Diet. Assoc. 2011, 111, 524–527. [Google Scholar] [CrossRef]
- Lee, J.H.; Gadi, R.; Spertus, J.A.; Tang, F.M.; O’Keefe, J.H. Prevalence of Vitamin D Deficiency in Patients With Acute Myocardial Infarction. Am. J. Cardiol. 2011, 107, 1636–1638. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, E.A.; Gasior, J.S.; Pawlowski, M.; Dabrowski, M. Association of Vitamin D Deficiency and Degree of Coronary Artery Disease in Cardiac Patients with Type 2 Diabetes. J. Diabetes Res. 2017, 2017, 3929075. [Google Scholar] [CrossRef] [PubMed]
- Brondum-Jacobsen, P.; Benn, M.; Jensen, G.B.; Nordestgaard, B.G. 25-Hydroxyvitamin D Levels and Risk of Ischemic Heart Disease, Myocardial Infarction, and Early Death Population-Based Study and Meta-Analyses of 18 and 17 Studies. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2794–2802. [Google Scholar] [CrossRef]
- Martini, L.A.; Wood, R.J. Vitamin D and blood pressure connection: Update on epidemiologic, clinical, and mechanistic evidence. Nutr. Rev. 2008, 66, 291–297. [Google Scholar] [CrossRef]
- Li, Y.C.; Kong, J.; Wei, M.J.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Investig. 2002, 110, 229–238. [Google Scholar] [CrossRef]
- Kassi, E.; Adamopoulos, C.; Basdra, E.K.; Papavassiliou, A.G. Role of Vitamin D in Atherosclerosis. Circulation 2013, 128, 2517–2531. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency—Reply. N. Engl. J. Med. 2007, 357, 1981–1982. [Google Scholar] [CrossRef]
- Ni, W.; Watts, S.W.; Ng, M.; Chen, S.; Glenn, D.J.; Gardner, D.G. Elimination of Vitamin D Receptor in Vascular Endothelial Cells Alters Vascular Function. Hypertension 2014, 64, 1290–1298. [Google Scholar] [CrossRef]
- Argacha, J.F.; Egrise, D.; Pochet, S.; Fontaine, D.; Lefort, A.; Libert, F.; Goldman, S.; van de Borne, P.; Berkenboom, G.; Moreno-Reyes, R. Vitamin D Deficiency-induced Hypertension Is Associated With Vascular Oxidative Stress and Altered Heart Gene Expression. J. Cardiovasc. Pharmacol. 2011, 58, 65–71. [Google Scholar] [CrossRef]
- Weng, S.; Sprague, J.E.; Oh, J.; Riek, A.E.; Chin, K.; Garcia, M.; Bernal-Mizrachi, C. Vitamin D Deficiency Induces High Blood Pressure and Accelerates Atherosclerosis in Mice. PLoS ONE 2013, 8, e54625. [Google Scholar] [CrossRef]
- Tomaschitz, A.; Pilz, S.; Ritz, E.; Grammer, T.; Drechsler, C.; Boehm, B.O.; März, W. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin. Chim. Acta 2010, 411, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Forman, J.P.; Williams, J.S. Vitamin D and the vascular sensitivity to angiotensin II in obese Caucasians with hypertension. J. Hum. Hypertens. 2011, 25, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Sun, B.; Forman, J.P.; Hopkins, P.N.; Brown, N.J.; Kolatkar, N.S.; Williams, G.H.; Williams, J.S. The Fok1 vitamin D receptor gene polymorphism is associated with plasma renin activity in Caucasians. Clin. Endocrinol. 2011, 74, 783–790. [Google Scholar] [CrossRef]
- Aleksova, A.; Janjusevic, M.; Zhou, X.N.O.; Zandonà, L.; Chicco, A.; Stenner, E.; Beltrami, A.P.; D’Errico, S.; Sinagra, G.; Marketou, M.; et al. Persistence of vitamin D deficiency among Italian patients with acute myocardial infarction. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1283–1294. [Google Scholar] [CrossRef]
- Karur, S.; Veerappa, V.; Nanjappa, M.C. Study of vitamin D deficiency prevalence in acute myocardial infarction. IJC Heart Vessel. 2014, 3, 57–59. [Google Scholar] [CrossRef]
- Correia, L.C.L.; Sodré, F.; Garcia, G.; Sabino, M.; Brito, M.; Kalil, F.; Barreto, B.; Lima, J.C.; Noya-Rabelo, M.M. Relation of Severe Deficiency of Vitamin D to Cardiovascular Mortality During Acute Coronary Syndromes. Am. J. Cardiol. 2013, 111, 324–327. [Google Scholar] [CrossRef]
- De Metrio, M.; Milazzo, V.; Rubino, M.; Cabiati, A.; Moltrasio, M.; Marana, I.; Campodonico, J.; Cosentino, N.; Veglia, F.; Bonomi, A.; et al. Vitamin D Plasma Levels and In-Hospital and 1-Year Outcomes in Acute Coronary Syndromes A Prospective Study. Medicine 2015, 94, e857. [Google Scholar] [CrossRef]
- Ng, L.L.; Sandhu, J.K.; Squire, I.B.; Davies, J.E.; Jones, D.J.L. Vitamin D and prognosis in acute myocardial infarction. Int. J. Cardiol. 2013, 168, 2341–2346. [Google Scholar] [CrossRef]
- Ford, K.; Latic, N.; Slavic, S.; Zeitz, U.; Dolezal, M.; Andrukhov, O.; Erben, R.G.; Andrukhova, O. Lack of vitamin D signalling per se does not aggravate cardiac functional impairment induced by myocardial infarction in mice. PLoS ONE 2018, 13, e0204803. [Google Scholar] [CrossRef]
- Rai, V.; Agrawal, D.K. Role of Vitamin D in Cardiovascular Diseases. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1039–1059. [Google Scholar] [CrossRef]
- Artaza, J.N.; Norris, K.C. Vitamin D reduces the expression of collagen and key profibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. J. Endocrinol. 2009, 200, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Cubbon, R.M.; Lowry, J.E.; Drozd, M.; Hall, M.; Gierula, J.; Paton, M.F.; Byrom, R.; Kearney, L.C.; Barth, J.H.; Kearney, M.T.; et al. Vitamin D deficiency is an independent predictor of mortality in patients with chronic heart failure. Eur. J. Nutr. 2019, 58, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- Szabo, T.M.; Nagy, E.E.; Kirchmaier, A.; Heidenhoffer, E.; Gabor-Kelemen, H.L.; Frasineanu, M.; Cseke, J.; German-Sallo, M.; Frigy, A.; Sun, J. Total 25-Hydroxyvitamin D Is an Independent Marker of Left Ventricular Ejection Fraction in Heart Failure with Reduced and Mildly Reduced Ejection Fraction. Biomolecules 2023, 13, 1578. [Google Scholar] [CrossRef] [PubMed]
- McMullan, C.J.; Borgi, L.; Curhan, G.C.; Fisher, N.; Forman, J.P. The effect of vitamin D on renin-angiotensin system activation and blood pressure: A randomized control trial. J. Hypertens. 2017, 35, 822–829. [Google Scholar] [CrossRef]
- Amirkhizi, F.; Pishdadian, A.; Asghari, S.; Hamedi-Shahraki, S. Vitamin D status is favorably associated with the cardiovascular risk factors in adults with obesity. Clin. Nutr. Espen 2021, 46, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Khanolkar, S.; Hirani, S.; Mishra, A.; Vardhan, S.; Prasad, R.; Wanjari, M. Exploring the Role of Vitamin D in Atherosclerosis and Its Impact on Cardiovascular Events: A Comprehensive Review. Cureus J. Med. Sci. 2023, 15, e42470. [Google Scholar] [CrossRef] [PubMed]
- Argano, C.; Mirarchi, L.; Amodeo, S.; Orlando, V.; Torres, A.; Corrao, S. The Role of Vitamin D and Its Molecular Bases in Insulin Resistance, Diabetes, Metabolic Syndrome, and Cardiovascular Disease: State of the Art. Int. J. Mol. Sci. 2023, 24, 5485. [Google Scholar] [CrossRef] [PubMed]
- Al Mheid, I.; Patel, R.; Murrow, J.; Morris, A.; Rahman, A.; Fike, L.; Kavtaradze, N.; Uphoff, I.; Hooper, C.; Tangpricha, V.; et al. Vitamin D Status Is Associated With Arterial Stiffness and Vascular Dysfunction in Healthy Humans. J. Am. Coll. Cardiol. 2011, 58, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Mozos, I.; Marginean, O. Links between Vitamin D Deficiency and Cardiovascular Diseases. BioMed Res. Int. 2015, 2015, 109275. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, J.K.; Nurmi, T.; Aro, A.; Bertone-Johnson, E.R.; Hypponen, E.; Kroger, H.; Lamberg-Allardt, C.; Manson, J.E.; Mursu, J.; Mantyselka, P.; et al. Vitamin D supplementation and prevention of cardiovascular disease and cancer in the Finnish Vitamin D Trial: A randomized controlled trial. Am. J. Clin. Nutr. 2022, 115, 1300–1310. [Google Scholar] [CrossRef]
- Scragg, R.; Stewart, A.W.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Sluyter, J.; Murphy, J.; Khaw, K.T.; Camargo, C.A. Effect of Monthly High-Dose Vitamin D Supplementation on Cardiovascular Disease in the Vitamin D Assessment Study A Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 608–616. [Google Scholar] [CrossRef]
- Neale, R.E.; Baxter, C.; Romero, B.D. The D-Health Trial: A randomised controlled trial of the effect of vitamin D on mortality. Lancet Diabetes Endocrinol. 2022, 10, 120–128. [Google Scholar] [CrossRef]
- Miao, J.; Bachmann, K.N.; Huang, S.; Su, Y.R.; Dusek, J.; Newton-Cheh, C.; Arora, P.; Wang, T.J. Effects of Vitamin D Supplementation on Cardiovascular and Glycemic Biomarkers. J. Am. Heart Assoc. 2021, 10, e017727. [Google Scholar] [CrossRef]
- Desouza, C.; Chatterjee, R.; Vickery, E.M.; Nelson, J.; Johnson, K.C.; Kashyap, S.R.; Lewis, M.R.; Margolis, K.; Pratley, R.; Rasouli, N.; et al. The effect of vitamin D supplementation on cardiovascular risk in patients with prediabetes: A secondary analysis of the D2d study. J. Diabetes Its Complicat. 2022, 36, 108230. [Google Scholar] [CrossRef]
- Manson, J.E.; Bassuk, S.S.; Cook, N.R.; Lee, I.M.; Mora, S.; Albert, C.M.; Buring, J.E.; Grp, V.R. Vitamin D, Marine n-3 Fatty Acids, and Primary Prevention of Cardiovascular Disease Current Evidence. Circ. Res. 2020, 126, 112–128. [Google Scholar] [CrossRef]
- Rist, P.M.; Buring, J.E.; Cook, N.R.; Manson, J.E.; Rexrode, K.M. Effect of vitamin D and/or omega-3 fatty acid supplementation on stroke outcomes: A randomized trial. Eur. J. Neurol. 2021, 28, 809–815. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gepner, A.D.; Ramamurthy, R.; Krueger, D.C.; Korcarz, C.E.; Binkley, N.; Stein, J.H. A Prospective Randomized Controlled Trial of the Effects of Vitamin D Supplementation on Cardiovascular Disease Risk. PLoS ONE 2012, 7, e36617. [Google Scholar] [CrossRef]
- Pilz, S.; Gaksch, M.; Kienreich, K.; Grübler, M.; Verheyen, N.; Fahrleitner-Pammer, A.; Treiber, G.; Drechsler, C.; Hartaigh, B.O.; Obermayer-Pietsch, B.; et al. Effects of Vitamin D on Blood Pressure and Cardiovascular Risk Factors A Randomized Controlled Trial. Hypertension 2015, 65, 1195–1201. [Google Scholar] [CrossRef]
- Wood, A.D.; Secombes, K.R.; Thies, F.; Aucott, L.; Black, A.J.; Mavroeidi, A.; Simpson, W.G.; Fraser, W.D.; Reid, D.M.; Macdonald, H.M. Vitamin D3 Supplementation Has No Effect on Conventional Cardiovascular Risk Factors: A Parallel-Group, Double-Blind, Placebo-Controlled RCT. J. Clin. Endocrinol. Metab. 2012, 97, 3557–3568. [Google Scholar] [CrossRef]
- McCourt, A.F.; O’Sullivan, A.M. Using food fortification to improve vitamin D bioaccessibility and intakes. Proc. Nutr. Soc. 2022, 81, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Sandmann, A.; Ignatius, A.; Amling, M.; Barvencik, F. New perspectives on vitamin D food fortification based on a modeling of 25(OH)D concentrations. Nutr. J. 2013, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, T.; Itkonen, S.T.; Lundqvist, A.; Erkkola, M.; Koskela, T.; Lakkala, K.; Dowling, K.G.; Hull, G.L.J.; Kröger, H.; Karppinen, J.; et al. The positive impact of general vitamin D food fortification policy on vitamin D status in a representative adult Finnish population: Evidence from an 11-y follow-up based on standardized 25-hydroxyvitamin D data. Am. J. Clin. Nutr. 2017, 105, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Lavelli, V.; D’Incecco, P.; Pellegrino, L. Vitamin D Incorporation in Foods: Formulation Strategies, Stability, and Bioaccessibility as Affected by the Food Matrix. Foods 2021, 10, 1989. [Google Scholar] [CrossRef] [PubMed]
- Maurya, V.K.; Aggarwal, M. Factors influencing the absorption of vitamin D in GIT: An overview. J. Food Sci. Technol.-Mysore 2017, 54, 3753–3765. [Google Scholar] [CrossRef] [PubMed]
- Buttriss, J.L.; Lanham-New, S.A. Is a vitamin D fortification strategy needed? Nutr. Bull. 2020, 45, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Black, L.J.; Seamans, K.M.; Cashman, K.D.; Kiely, M. An Updated Systematic Review and Meta-Analysis of the Efficacy of Vitamin D Food Fortification. J. Nutr. 2012, 142, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- McCourt, A.; McNulty, B.A.; Walton, J.; O’Sullivan, A. Efficacy and safety of food fortification to improve Vitamin D intakes of older adults. Nutrition 2020, 75–76, 110837. [Google Scholar] [CrossRef]
- Madsen, K.H.; Rasmussen, L.B.; Andersen, R.; Molgaard, C.; Jakobsen, T.; Bjerrum, P.J.; Andersen, E.W.; Mejborn, H.; Tetens, I. Randomized controlled trial of the effects of vitamin D-fortified milk and bread on serum 25-hydroxyvitamin D concentrations in families in Denmark during winter: The VitmaD study. Am. J. Clin. Nutr. 2013, 98, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Tangpricha, V.; Koutkia, P.; Rieke, S.M.; Chen, T.C.; Perez, A.A.; Holick, M.F. Fortification of orange juice with vitamin D: A novel approach for enhancing vitamin D nutritional health. Am. J. Clin. Nutr. 2003, 77, 1478–1483. [Google Scholar] [CrossRef]
- Guo, J.; Jackson, K.G.; Taha, C.; Li, Y.; Givens, D.I.; Lovegrove, J.A. A 25-Hydroxycholecalciferol-Fortified Dairy Drink Is More Effective at Raising a Marker of Postprandial Vitamin D Status than Cholecalciferol in Men with Suboptimal Vitamin D Status. J. Nutr. 2017, 147, 2076–2082. [Google Scholar] [CrossRef]
- Reyes-Garcia, R.; Garcia-Martin, A.; Palacios, S.; Salas, N.; Mendoza, N.; Quesada-Charneco, M.; Fonolla, J.; Lara-Villoslada, F.; Mañoz-Torres, M. Factors Predicting the Response to a Vitamin D-Fortified Milk in Healthy Postmenopausal Women. Nutrients 2019, 11, 2641. [Google Scholar] [CrossRef]
- Costan, A.R.; Vulpoi, C.; Mocanu, V. Vitamin D Fortified Bread Improves Pain and Physical Function Domains of Quality of Life in Nursing Home Residents. J. Med. Food 2014, 17, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Witte, K.K.; Clark, A.L. Chronic heart failure and multiple micronutrient supplementation: Realistic hope or idealistic conjecture? Heart Fail. Monit. 2005, 4, 123–129. [Google Scholar] [PubMed]
- Pilz, S.; März, W.; Cashman, K.D.; Kiely, M.E.; Whiting, S.J.; Holick, M.F.; Grant, W.B.; Pludowski, P.; Hiligsmann, M.; Trummer, C.; et al. Rationale and Plan for Vitamin D Food Fortification: A Review and Guidance Paper. Front. Endocrinol. 2018, 9, 373. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bollerslev, J.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Formenti, A.M.; Lazaretti-Castro, M.; et al. Consensus statement from 2nd International Conference on Controversies in Vitamin D. Rev. Endocr. Metab. Disord. 2020, 21, 89–116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gasparri, C.; Perna, S.; Spadaccini, D.; Alalwan, T.; Girometta, C.; Infantino, V.; Rondanelli, M. Is vitamin D-fortified yogurt a value-added strategy for improving human health? A systematic review and meta-analysis of randomized trials. J. Dairy Sci. 2019, 102, 8587–8603. [Google Scholar] [CrossRef]
- Al Khalifah, R.; Alsheikh, R.; Alnasser, Y.; Alhelali, N.; Naji, A.; Al Backer, N. The impact of vitamin D food fortification and health outcomes in children: A systematic review and meta-regression. Syst. Rev. 2020, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- Emadzadeh, M.; Rashidmayvan, M.; Sahebi, R.; Sadeghi, R.; Ferns, G.A.; Ghayour-Mobarhan, M. The effect of vitamin D fortified products on anthropometric indices: A systematic review and meta-analysis. Complement. Ther. Clin. Pract. 2020, 41, 101242. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, E.; Kiely, M.E.; James, A.P.; Singh, T.; Pham, N.M.; Black, L.J. Vitamin D Food Fortification and Biofortification Increases Serum 25-Hydroxyvitamin D Concentrations in Adults and Children: An Updated and Extended Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Nutr. 2021, 151, 2622–2635. [Google Scholar] [CrossRef] [PubMed]
- Emadzadeh, M.; Mehdizadeh, A.; Sharifan, P.; Khoshakhlagh, M.; Sahebi, R.; Sadeghi, R.; Ferns, G.A.; Ghayour-Mobarhan, M. The Effects of Vitamin D Fortified Products on Bone Biomarkers: A Systematic Review and Meta-Analysis. Iran. J. Public Health 2022, 51, 278. [Google Scholar]
- Nikooyeh, B.; Neyestani, T.R. The effects of vitamin D-fortified foods on circulating 25(OH)D concentrations in adults: A systematic review and meta-analysis. Br. J. Nutr. 2022, 127, 1821–1838. [Google Scholar] [CrossRef]
- Szabó, É.; Csölle, I.; Felso, R.; de Gaudry, D.K.; Nyakundi, P.N.; Ibrahim, K.; Metzendorf, M.I.; Ferenci, T.; Lohner, S. Benefits and Harms of Edible Vegetable Oils and Fats Fortified with Vitamins A and D as a Public Health Intervention in the General Population: A Systematic Review of Interventions. Nutrients 2023, 15, 5135. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; O’Neill, C.M. Strategic food vehicles for vitamin D fortification and effects on vitamin D status: A systematic review and meta-analysis of randomised controlled trials. J. Steroid Biochem. Mol. Biol. 2024, 238, 106448. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.P.; Shaw, P.N.; Whitfield, K.; Koorts, P.; McConachy, H.; Hewavitharana, A.K. Effect of pasteurisation on the concentrations of vitamin D compounds in donor breastmilk. Int. J. Food Sci. Nutr. 2016, 67, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Cappozzo, J.C.; Koutchma, T.; Barnes, G. Chemical characterization of milk after treatment with thermal (HTST and UHT) and nonthermal (turbulent flow ultraviolet) processing technologies. J. Dairy Sci. 2015, 98, 5068–5079. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Lin, H.Y.; Hong, W.P.; Lin, C.P. Evaluation of preliminary causes for vitamin D series degradation via DSC and HPLC analyses. J. Therm. Anal. Calorim. 2017, 130, 1357–1369. [Google Scholar] [CrossRef]
- Loznjak, P.; Jakobsen, J. Stability of vitamin D3 and vitamin D2 in oil, fish and mushrooms after household cooking. Food Chem. 2018, 254, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Maurya, V.K.; Bashir, K.; Aggarwal, M. Vitamin D microencapsulation and fortification: Trends and technologies. J. Steroid Biochem. Mol. Biol. 2020, 196, 105489. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Kuznesof, S.; Waller, A.; Davies, S.; Wilson, S.; Ritchie, A.; Duesterloh, A.; Harbord, L.; Hill, T.R. The Influence of Storage and Cooking on the Vitamin D Content of 25-Hydroxyvitamin D3-Enriched Eggs. Foods 2023, 12, 2522. [Google Scholar] [CrossRef] [PubMed]
- Saftert, A.; Pieper, G.; Jetten, J. Effect of package light transmittance on vitamin content of milk. Part 2: UHT whole milk. Packag. Technol. Sci. 2008, 21, 47–55. [Google Scholar] [CrossRef]
- Jakobsen, J.; Knuthsen, P. Stability of vitamin D in foodstuffs during cooking. Food Chem. 2014, 148, 170–175. [Google Scholar] [CrossRef]
- Wilson, L.R.; Tripkovic, L.; Hart, K.H.; Lanham-New, S.A.; Team, D.D.S. Vitamin D deficiency as a public health issue: Using vitamin D2 or vitamin D3 in future fortification strategies. Proc. Nutr. Soc. 2017, 76, 392–399. [Google Scholar] [CrossRef]
- Cashman, K.D.; Kiely, M. Tackling inadequate vitamin D intakes within the population: Fortification of dairy products with vitamin D may not be enough. Endocrine 2016, 51, 38–46. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, N.a.A.N. Safety of UV-treated milk as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2016, 14, 4370. [Google Scholar] [CrossRef]
- Ko, J.A.; Lee, B.H.; Lee, J.S.; Park, H.J. Effect of UV-B exposure on the concentration of vitamin D2 in sliced shiitake mushroom (Lentinus edodes) and white button mushroom (Agaricus bisporus). J. Agric. Food Chem. 2008, 56, 3671–3674. [Google Scholar] [CrossRef] [PubMed]
- Gallotti, F.; Lavelli, V. The Effect of UV Irradiation on Vitamin D(2) Content and Antioxidant and Antiglycation Activities of Mushrooms. Foods 2020, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, J.; Miyazaki, A.; Saito, M.; Adachi, T.; Mizoue, K.; Hanada, K.; Omura, S. Transformation of vitamin-D3 to 1-alpha, 25-dihydroxyvitamin-D3 via 25-hydroxyvitamin-D3 using amycolata sp strains. Appl. Microbiol. Biotechnol. 1992, 38, 152–157. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Safety of Vitamin D-Enriched UV-Treated Baker’s Yeast. EFSA J. 2014, 12, 2014. [Google Scholar]
- Takeda, K.; Asou, T.; Matsuda, A.; Kimura, K.; Okamura, K.; Okamoto, R.; Sasaki, J.; Adachi, T.; Omura, S. Application of cyclodextrin to microbial transformation of vitamin-D3 to 25-hydroxyvitamin D3 and 1-alpha,25-dihydroxyvitamin D3. J. Ferment. Bioeng. 1994, 78, 380–382. [Google Scholar] [CrossRef]
- Tang, D.D.; Liu, W.; Huang, L.; Cheng, L.M.; Xu, Z.N. Efficient biotransformation of vitamin D3 to 25-hydroxyvitamin D3 by a newly isolated Bacillus cereus strain. Appl. Microbiol. Biotechnol. 2020, 104, 765–774. [Google Scholar] [CrossRef]
- Abbasi, A.; Emam-Djomeh, Z.; Mousavi, M.A.E.; Davoodi, D. Stability of vitamin D3 encapsulated in nanoparticles of whey protein isolate. Food Chem. 2014, 143, 379–383. [Google Scholar] [CrossRef]
- Diarrassouba, F.; Garrait, G.; Remondetto, G.; Alvarez, P.; Beyssac, E.; Subirade, M. Increased stability and protease resistance of the β-lactoglobulin/vitamin D3 complex. Food Chem. 2014, 145, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Loewen, A.; Chan, B.; Li-Chan, E.C.Y. Optimization of vitamins A and D3 loading in re-assembled casein micelles and effect of loading on stability of vitamin D3 during storage. Food Chem. 2018, 240, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Kong, X.L.; Li, Q.M.; Zhang, H.L.; Zha, X.Q.; Luo, J.P. Stability and bioavailability of vitamin D3 encapsulated in composite gels of whey protein isolate and lotus root amylopectin. Carbohydr. Polym. 2020, 227, 115337. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, A.; Schneider, Y.J. Engineered Nanomaterials in Food: Implications for Food Safety and Consumer Health. Int. J. Environ. Res. Public Health 2014, 11, 5720–5750. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Livney, Y.D. Potato protein based nanovehicles for health promoting hydrophobic bioactives in clear beverages. Food Hydrocoll. 2016, 57, 229–235. [Google Scholar] [CrossRef]
- Pateiro, M.; Gómez, B.; Munekata, P.E.S.; Barba, F.J.; Putnik, P.; Kovacevic, D.B.; Lorenzo, J.M. Nanoencapsulation of Promising Bioactive Compounds to Improve Their Absorption, Stability, Functionality and the Appearance of the Final Food Products. Molecules 2021, 26, 1547. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.D.; Cerqueira, M.A.; Vicente, A.A. Nanoemulsions for Food Applications: Development and Characterization. Food Bioprocess Technol. 2012, 5, 854–867. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Y.H.; Yang, X.Q.; Guo, J.; Wang, J.M. Corn protein hydrolysate as a novel nano-vehicle: Enhanced physicochemical stability and in vitro bioaccessibility of vitamin D3. LWT—Food Sci. Technol. 2016, 72, 510–517. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Xiang, C.Y.; Gao, J.; Ye, H.X.; Ren, G.R.; Ma, X.J.; Xie, H.J.; Fang, S.; Lei, Q.F.; Fang, W.J. Development of ovalbumin-pectin nanocomplexes for vitamin D3 encapsulation: Enhanced storage stability and sustained release in simulated gastrointestinal digestion. Food Hydrocoll. 2020, 106, 105926. [Google Scholar] [CrossRef]
- Park, S.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Development of nanostructured lipid carriers for the encapsulation and controlled release of vitamin D3. Food Chem. 2017, 225, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, R.S.; Oliveira, I.F.; da Silva, V.M.; Prata, A.S.; Hubinger, M.D. Chitosan coated nanostructured lipid carriers (NLCs) for loading Vitamin D: A physical stability study. Int. J. Biol. Macromol. 2018, 119, 902–912. [Google Scholar] [CrossRef]
- Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; Ricci, A.; et al. Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health. EFSA J. 2018, 16, e05327. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Guidance for Industry: Safety of Nanomaterials in Cosmetic Products. Final, June 2014. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-safety-nanomaterials-cosmetic-products (accessed on 1 April 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).