Abstract
Applications of the Drosophila melanogaster (D.m.) research model have an important means both for genetic investigations and for the study of metal toxicity, because D.m. has physiological mechanisms comparable to those in human organisms. In this research, the toxic effect of lead (Pb2+) and copper (Cu2+) on four D.m. genotypes—the wild genotype (Oregon-R, used as control) and three mutant genotypes (white, brown, and white-vestigial)—was compared. Five replicates were made to observe the development progress of monitorized genotypes exposed to five different concentrations 0, 0.50, 0.75, 1.00, and 2.00 mM of copper (CuSO4) and lead Pb(C2H3O2)2. Proliferation rates of larvae, pupae, and adults depend on genetic factors, metals used (copper or lead), and their concentrations. The white-vestigial mutant genotype showed the greatest sensitivity at IC50 concentration (inhibition of proliferation of more than 50% compared with the control sample) at doses of 1.00 mM for Cu2+ and 2.00 mM for Pb2+. In contrast, the control genotype (Oregon-R) showed only an inhibition IC50 concentration of 2.00 mM for Cu2+. The white-vestigial mutant genotype showed the greatest sensitivity at IC50 concentration (inhibition of proliferation of more than 50% compared with the control sample) at doses of 1.00 mM for Cu2+ and 2.00 mM for Pb2+. In contrast, the control genotype (Oregon-R) showed an inhibition at the IC50 concentration of 2.00 mM for Cu2+. The results conclude that (i) the dose influences the prolificacy rate in a directly proportional way, (ii) the comparative analyses between Cu2+ and Pb2+ revealed a more acute effect of Cu2+, and (iii) differentiated prolificacy values according to genotypes were recorded. Those reflect the importance of using D.m. as a research model in the comparative studies of the interactions between genetic factors and metal toxicity. Also, this study provides significant information on non-toxic maximum doses for organisms.
1. Introduction
Global industrialization and modernization have brought rapid progress, yet they have been accompanied by the issue of pollution, which poses serious threats to human health. The excessive presence of metals because of agricultural practices, industrial processes, and natural sources poses a significant threat due to the challenge of removing them from the ecosystem and their adverse effects on human health [1,2].
Before the 2015 ban on ocean dumping, 60 thousand tons of heavy metals were introduced into the environment, with copper significantly present. Land-based waste had a major impact on copper pollution in marine environments, with anthropogenic copper input being up to 141 times higher than atmospheric deposition [3]. Mining activities and the use of fungicides have resulted in copper contamination of soil, water, and sediment exceeding safe levels [4]. In a study conducted by McFarland et al. in 2022, out of 318 million Americans, only 131 million had blood lead levels below 5 μg/dL [5].
In addition, recent studies show a dramatic accumulation of heavy metals in plants. Cu values varied between 13.278 and 42.586, and Pb levels ranged between 1.311 and 16.238, as well as for other metals [6]. However, various studies showed differences in the pollution regulations, reflecting the difficulty of identifying a maximum allowed limit applicable on a global scale, due to contextual divergences including social, geographical, and genetic factors [7,8].
Therefore, the question arises of how to establish improved international standards for assessing ecosystem quality and food safety, considering genetic influence [9,10]. This approach would facilitate the implementation of more effective preventive and corrective measures on a global scale regarding metal limits [11].
Drosophila melanogaster (D.m.) is a standard model in research and can be an important bioindicator for understanding toxicity limits [12]. The genetic similarities between D.m. and humans, coupled with its short life cycle, highlight its potential for successful application in diagnosing and understanding the impact of metals on organisms [13]. This contribution can aid in the timely prevention of toxic effects by establishing intervention limits and assessing risks for various ecosystems [14].
Copper, a metal with metabolic implications, present in all the cells of the body, is essential for the growth and development of organisms and participating in enzymatic reactions and maintaining homeostasis [15]. Also, in high concentrations, it becomes toxic [16]. The body’s copper intake comes from copper-rich foods, such as vegetables, fruits, meat, cereals, and water [17]. The amount of copper in the blood of healthy people is 1.23 ± 0.16 mg/L [18]. High concentrations of copper act as an enzyme inhibitor and limit the metabolic activity [19,20,21].
Lead, a heavy metal that affects a range of physiological and biochemical processes, such as metabolism and internal organ function, contributes to the development of diseases [22]. When there is approximately 0.23 mg/L (10 μg/dL) of lead in the blood, medical interventions are necessary [23]. Lead accumulation in organisms [24] is caused by the presence of lead in various foods, with acceptable levels regulated by the Centers for Disease Control and Prevention [25].
Studies have shown that exposure to high concentrations of lead and copper stimulates the production of reactive oxygen species (ROS) [26,27,28,29,30].
From a genotypic point of view, D.m. has a wide range of mutant genotypes [31,32], which gives it a significant advantage in comparative ecotoxicological studies [33]. Studies on D.m. have shown a different sensitivity to lead and copper depending on the sex of the adults (X chromosome) [21]. Similarly, D.m. mutants showed influences on ABC transporters [34]. ABC transport is a system made up of a family of transmembrane proteins and proteins that bind nucleosides to adenosine triphosphate (ATP) [35], playing a role in transporting a variety of molecules and ions into and out of the cell. This transport mechanism is essential for normal cell function and plays an important role in physiological processes such as nutrient uptake and cell detoxification [36].
Both mutant genotypes, white eye color (gene present in the X chromosome [37,38]) and brown eye color (gene present in the II chromosome [39]), influence heterodynamic guanine transport [40]. Guanine is one of the four nitrogenous bases that make up nucleic acids [16], contributing to the stability and replication of DNA and RNA and has an influence on all physiological systems, including detoxification processes [41,42,43].
Studies revealed that the vestigial genotype of D.m. interacts with the translation elongation factor (TEF-1) [44,45], indicating the possibility of a protein similar to the “vg” gene in human cells. Thus, the use of the D.m. study model and by extension, the white, brown, and vestigial mutants, may provide a better understanding of the mechanisms of detoxification and limitation of metal concentrations.
In this study, (i) the impacts of lead and copper on the developmental stages (larval stage III, pupal, and adult) of D.m. were monitored; (ii) comparations between toxicity effects stimulated by the two metals were made; and (iii) the four genotypes responses when exposed to similar toxicity levels were compared.
2. Materials and Methods
2.1. Chemical Products
Copper sulphate pentahydrate (CuSO4-5H2O) was purchased from SilverChemicals (București, Romania) and lead acetate Pb(C2H3O2)2-3H2O was purchased from SticlarieLaborator (Iasi, Romania), both with the highest purity available.
2.2. Stocks
Four genotypes of Drosophila melanogaster (D.m.): the wild Oregon-R genotype, and three mutant genotypes: the white, brown, and white-vestigial were studied in the Genetic Laboratory of the University of Life Sciences “King Michael I” of Timișoara.
Studies have shown that sex influences tolerance to toxic levels of Cu2+ and Pb2+, showing greater sensitivity in females than in males [46]. In this respect, we selected mutant genotypes linked to the sex chromosomes (X chromosome).
The wild genotype (Oregon-R) was used as a control variant, with an open body, red eyes, and normal wings (Figure 1a) [47,48].

Figure 1.
Drosophila melanogaster (D.m) genotypes studied: (a)—wild genotype (Oregon-R), (b)—brown mutant genotype, (c)—white mutant genotype, (d)—white-vestigial mutant genotype.
The white mutant genotype is different from the wild type due to the presence of the recessive gene responsible for the white eye color. This gene is located on the X chromosome [49,50] at a distance of 1.5 centimorgans (cMo) from the 0 end and is designated “w” (Figure 1c). Studies highlight the involvement of the “w” gene in the regulation of copulation success in D.m. [37].
The brown mutant genotype is characterized by the presence of the recessive gene “bw”, located on chromosome II at 104 cMo from the 0 end [51]. This gene blocks the formation of normal red pigment, giving the eyes a brown color (Figure 1d). Studies showed that there is a link between the genes for white and brown eye color, as they are involved in the guanine transport [52].
The white-vestigial mutant genotype is characterized by the white color of the eyes, generated by the presence of the recessive gene “w”, located on chromosome I, and by the presence of reduced and rudimentary wings caused by the presence of the recessive gene “vg”, located on chromosome II (Figure 1d). Studies showed the influence of the “vg” gene in the translation elongation process (TEF) [53], a process necessary for the generation of messenger RNA (mRNA) proteins, thus reducing the capacity of detoxification mechanisms [54].
2.3. Preparing the Cultural Environment
The recipe, according to Animesh Kumar Mohapatra and Priyamvada Pandey (2018) [55], based on wheat flour (250 mL distilled water with 40 g sugar and 100 g wheat flour, 4 g yeast, 2 mL propionic acid, and 7 g agar-agar) was used to prepare the culture medium. Propionic acid was added after the medium had reached room temperature. The experimental variants were maintained at a constant temperature (20 °C, [51]) under 12-h light/dark cycle conditions.
2.4. Growth Medium and Experimental Variants of the Metals Cu2+ (CuSO4) and Pb2+ (C2H3O2)2
In this study, four concentrations of Cu2+ and Pb2+ were used to determine the effect on the life cycle of D.m. using the following experimental variants:
- -
- wheat flour-based control medium [55].
- -
- control medium based on wheat flour supplemented with a solution of copper sulphate CuSO4-5H2O (0.50 mM, 0.75 mM, 1.00 mM, and 2.00 mM);
- -
- control medium based on wheat flour supplemented with lead acetate solution (Pb(C2H3O2)2-3H2O (0.50 mM, 0.75 mM, 1.00 mM, and 2.00 mM).
For the preparation of the experimental media, the concentrations were chosen on the basis of the LC50 for Cu2+ (lethal concentration for 50% of the population) of 3 mM [56].
A stock solution with an initial concentration of 400 mM for CuSO4 and (Pb(C2H3O2)2 was used to prepare the experimental variants. This solution was diluted with distilled water and reduced to concentrations of 0.50 mM, 0.75 mM, 1.00 mM, and 2.00 mM in control medium. The diluted solutions were mixed with the medium after cooling and poured into culture dishes (125 mL) at a rate of 20 g/dish.
2.5. Determination of the Inhibition Index (IC50) of Proliferation after Exposure to Cu2+ (CuSO4) and Pb2+ Pb(C2H3O2)2
The research evaluated and quantified the larva (III)-pupal-adult transition, the number of larval stage III (Figure 2(a.3)), the number of pupae (including newly hatched ones, Figure 2(a.4)), and the number of emerged adults (Figure 2(b.2,b.4)). Recently hatched (10–12 h post-hatch [57]) D.m. adults (Figure 2) were transferred in a 5:5 ratio (♀-virgin: ♂) for fertilization and oviposition for 24 h in dishes (125 mL) with culture medium (20 g), after which they were removed from the medium.
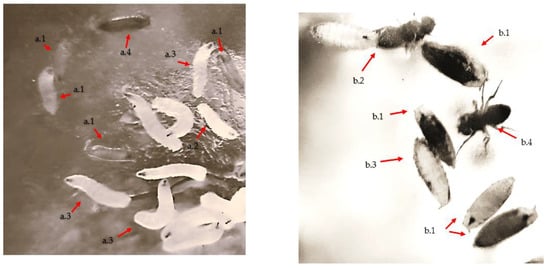
Figure 2.
Development stages of Drosophila melanogaster (D.m.) larvae, (a.1)—larval stage I, (a.2)—larval stage II, (a.3)—larval stage III, (a.4)—larval pupation; evolution of the formation of the adult pupal: (b.1)—pupa, (b.2)—hatching, (b.3)—hatched pupal, (b.4)—adult.
The experiment was carried out in five replicates, following the same conditions for all the experimental variants. Observations were made at the same time interval for all genotypes each day for a period of 15 days, in order to follow the evolution of the life cycle stages. After adult elimination, fertilization, and oviposition (after 24 h), the following observations were made for the five replicates/experimental variants: between 3 and 8 days, the number of stage III larvae (Figure 2(a.3)) was monitored, between 6 and 11, the number of pupae was monitored, and between 10 and 15, the number of adults was monitored. In the experiment, the impact of Cu2+ and Pb2+ was monitored during the developmental stages of D.m. (larva III, pupal, and adult) (Figure 2) in order to determine the toxicity of the two metals and to compare the effect of inhibition of proliferation between them (1):
2.6. Statistical Calculation Methods Used to Determine the Levels of Proliferation and Toxicity between Cu2+ and Pb2+
A statistical analysis was carried out using a multivariate ANOVA test. That revealed how genotypes responded to various toxicity levels and concentrations of Cu2+ and Pb2+ in different stages of developmental period. Significance was determined at a confidence level of p < 0.05. The results were subjected to a comparative analysis using the Tukey test. Statistical interpretation was performed using Rstudio 4.3.2. software.
3. Results
During our Drosophila melanogaster (D.m) study, our research revealed the number of larvae (stage III), pupae, and adults depends on the following: genotypes (Oregon-R, white, brown, and white-vestigial), used metals (Cu2+ or Pb2+), and their concentrations (0.50 mM, 0.75 mM, 1.00 mM, and 2.00 mM). During the experiments, the IC50 (inhibition of proliferation of more than 50% compared with the control sample) levels of D.m. genotypes were identified based on their exposure to various concentrations of metals.
3.1. Influence of Genotype, Concentration, and Type of Metal Cu2+ and Pb2+ on Proliferation
Results showed that there are significant differences between wild-type genotypes (Oregon-R, control) and mutant genotypes, white, brown, and white-vestigial, in the numbers of larvae formed at stage III under different experimental conditions.
A directly proportional correlation between the metals used (Cu2+ and Pb2+), and their concentration, as well as the genotypes, was identified in terms of the number of larvae formed (p < 0.001) (Table 1).

Table 1.
Analysis of the factors involved in the proliferation inhibition process at different concentrations of copper (CuSO4) and lead Pb Pb(C2H3O2)2 at larval stage III.
The studied genotypes have a significant influence on the number of individuals resulting from exposure to Cu2+ and Pb2+, thus confirming the significant role of genetic predisposition to toxicity (Table 1).
At the larval stage, the influence of the genotype and the concentrations of metals used was found to present significant variations in prolificacy levels throughout the life cycle (p < 0.001) (Table 1).
Analysis of the interaction between the genetic factor and metal concentration levels (Cu2+ and Pb2+) significantly influenced pupal and adult formation in D.m. (Table 2 and Table 3) (p < 0.001). The results indicate that as the concentration increases, the ability to proliferate specifically affects certain genotypes, some being more tolerant (showing a lower inhibition concentration) and others are more sensitive.

Table 2.
Analysis of factors involved in the inhibition of proliferation at different concentrations of copper (CuSO4) and lead Pb(C2H3O2)2 in pupal formation.

Table 3.
Analysis of the factors involved in inhibiting proliferation at different concentrations of copper (CuSO4) and lead (Pb(C2H3O2)2 in adult development.
Analysis of the interaction between concentration and metals used (Cu2+ or Pb2+) had a significant impact on the number of individuals during the development cycle (p < 0.001). This reflects differences in the response of proliferation rates according to the type of metal to which D.m. genotypes are exposed.
3.2. Influence of Copper (CuSO4) on Proliferation during the Life Cycle (Larva III-Pupal-Adult)
The results obtained showed the same impact of the reduction in prolificacy for Cu2+ and Pb2+, with the effect of reducing individuals in the larval, pupal, and adult stages.
The inhibition degree during the life cycle of D.m. resulting from exposure to metals was influenced by genotype, concentration, and used metal (Cu2+ or Pb2+) (p < 0.001) (Table 1, Table 2 and Table 3).
The proliferation rate of D.m. genotypes is inversely proportional to the increasing concentration of copper during larval stage III, pupal, and adult development (Table 4).

Table 4.
Results on the proliferation of Drosophila melanogaster (D.m) genotypes exposed to different concentrations of copper.
Significant differences in response to copper concentrations were observed between the genotypes used (p < 0.05). The white-vestigial genotype showed the greatest sensitivity to the highest concentrations of Cu2+ compared with the other genotypes.
The Oregon-R genotype showed proliferation rate values of less than 50% (IC50) at copper concentrations of 2.00 mM at larval stage III. The same toxicity effect was observed in pupal and adult formation, with IC50 present at a concentration of 2.00 mM (Table 4).
At a concentration of 2.00 mM Cu2+, the brown genotype recorded an increased toxicity level (IC50), which resulted in the number of larval stages III, pupae, and adults being significantly reduced compared to the control.
In the case of the white genotype, the copper concentration of 2.00 mM (IC50) had the same toxic effect, leading to a reduction in the proliferation of this genotype during the life cycle. The highest sensitivity was recorded in the white-vestigial genotype, showing the lowest number of larvae, formed pupae, or adults (p < 0.05).
Additionally, the white-vestigial, pupal, and adult formation showed an IC50 value at the concentration of 1 mM of Cu2+. This aspect could have occurred due to the prolonged exposure to copper at this concentration and the cumulative effect of the “w’’ (white-gene) and “vg’’ (vestigial-gene) genes. These genes are implicated in the replication of DNA and RNA, indirectly causing delays or deficiencies in the detoxification responses (Table 4).
The development of adults in the studied genotypes was influenced in an inversely proportional manner to increasing concentrations for all the monitored stages (Table 4 and Figure 2). At a 2.00 mM concentration of Cu2+, the IC50 value was observed in all studied genotypes (4/4) (Table 4 and Figure 3). Similarly, at a concentration of 1.00 mM of Cu2+, the IC50 value was observed only for the white-vestigial genotype in the pupal and adult formation (1/4). The results highlight an inhibition of D.m. studied genotypes proliferation caused by increasing copper (CuSO4) concentration.
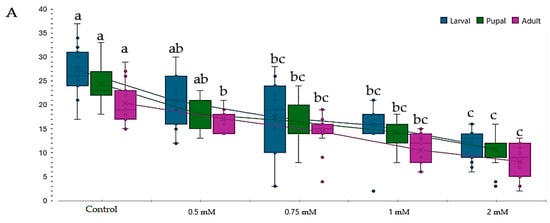
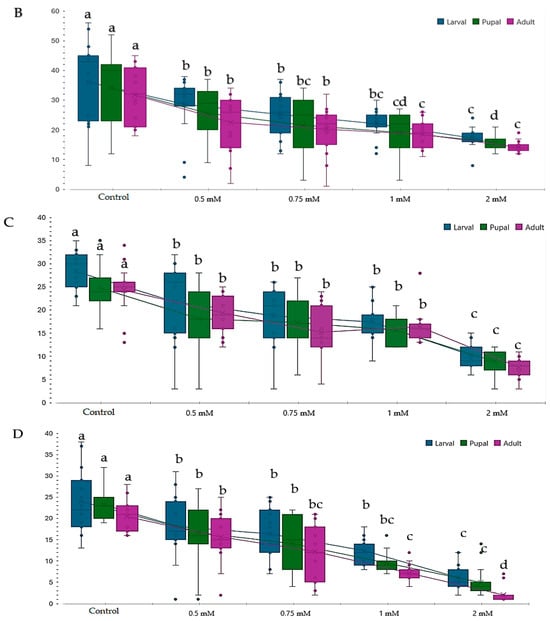
Figure 3.
Effect of copper on different genotypes of Drosophila melanogaste (D.m.): (A)—wild-type genotype (Oregon-R), (B)—brown mutant genotype, (C)—white mutant genotype, (D)—white-vestigial mutant genotype. Notes: The different superscript letters (a–d) indicate significant differences (p < 0.05), with “a” representing the best.
3.3. Influence of Lead Pb(C2H3O2)2 on Proliferation during the Life Cycle (Larva III-Pupa-Adult)
Analysis of the number of stage III larvae of D.m. genotypes exposed to different concentrations of Pb2+ revealed significant differences in the rate of proliferation as an effect of toxicity.
The results revealed significant variations in the number of individuals developed under different experimental conditions. Investigations indicated that genotype exerted a significant influence on the development of D.m. post-exposure, confirming a higher genetic predisposition to toxicity for certain genotypes. Prolificacy values peaked for the Oregon-R genotypes, averaged for the brown and white genotypes, and reached their lowest for the white-vestigial genotype (Table 5).

Table 5.
Results on the proliferation of Drosophila melanogaster (D.m.) genotypes exposed to different concentrations of lead Pb(C2H3O2)2.
During the early stages of the development cycle, larvae exposed to lead concentrations between 0.50 and 1.00 mM exhibited a modest reduction in proliferation. However, this reduction lacked statistical significance concerning genotype proliferation (p > 0.05). The wild type (Oregon-R) displayed a notable reduction in the number of stage III larvae at higher concentrations, particularly at 2.00 mM. A similar trend was observed for the white and brown genotypes, implying a shared sensitivity to lead (Table 5 and Figure 4).
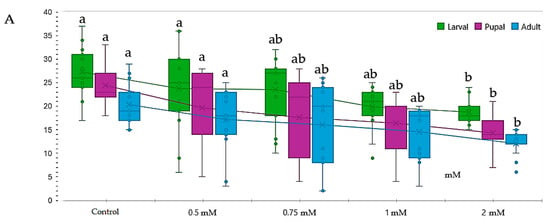
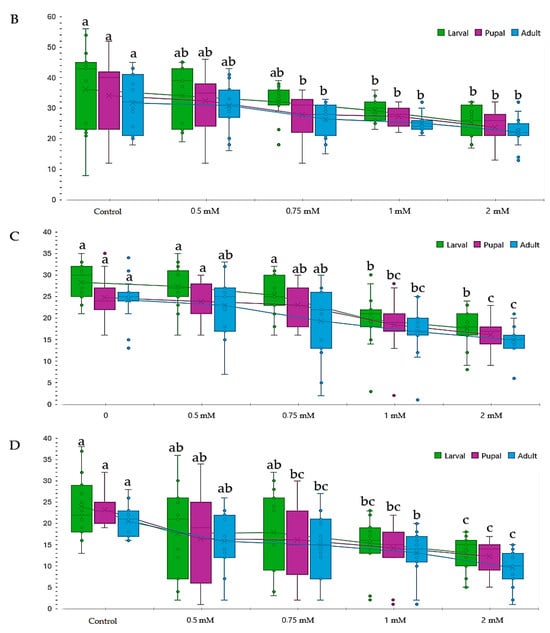
Figure 4.
Effect of lead on different genotypes of Drosophila melanogaster (D.m.): (A)—wild genotype (Oregon-R), (B)—brown mutant genotype, (C)—white mutant genotype, (D)—vestigial white mutant genotype. Notes: The different superscript letters (a–c) indicate significant differences (p < 0.05), with “a” representing the best.
In contrast, the white-vestigial genotype exhibited a heightened sensitivity to lead, with a significant reduction in the number of III larvae observed at concentrations of 1.00 mM and above (Table 5 and Figure 4).
The IC50 value for lead at a concentration of 2.00 mM was only present in the white-vestigial genotype (1/4), unlike other genotypes, which displayed greater tolerance at this concentration (Figure 4).
These results showed that genotypes exhibit varying responses to lead concentrations (p < 0.05).
Studies on mutant genotypes demonstrated that the white-vestigial genotype formed significantly fewer pupae in most of the studied variants compared to the Oregon-R genotype, which exhibited the highest tolerance during the pupal and adult stages.
Overall, the analysis of lead’s effect on D.m. underscores the metal’s significant impact on organism development and health. This emphasizes the necessity for ongoing investigations in this field and the development of effective strategies to safeguard the environment and public health.
3.4. Comparative Study of Different Concentrations of Lead and Copper Toxic Effects on Drosophila melanogaster
Comparative analysis of copper and lead toxicity unveiled significant variations in pupal formation and adult emergence. Examination of the toxicity disparities revealed that, in comparison to lead, copper displayed a more acute toxicity at both low and high concentrations (Figure 5).
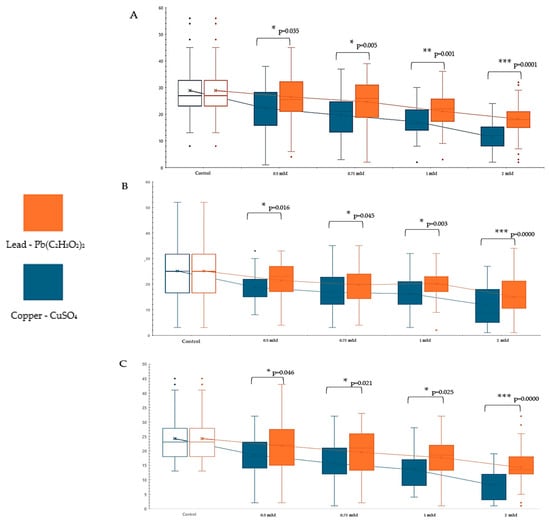
Figure 5.
Comparative study of the toxic effects on Drosophila melanogaster (D.m.) at different concentrations of lead and copper: (A)—larvae stage III, (B)—pupae, (C)—adult. “*”—p < 0.05, “**”—p < 0.01, “***”—Significant at p < 0.001.
These discrepancies persisted across other concentrations, indicating the prevailing dominance of copper toxicity over lead toxicity. Consequently, the level of significance escalated with increasing concentration. Thus, at a concentration of 1.00 mM, copper’s toxicity was significantly more pronounced (p < 0.05) than lead. At 2.00 mM, the significance level escalated to p < 0.0001. These intervals were markedly different from each other (Figure 5) and also from the control. These findings underscore a more acute toxic effect of copper compared to lead.
4. Discussion
Drosophila melanogaster (D.m.) possesses numerous genes and detoxification mechanisms similar to those found in humans [58]. Hence, it should be used to understand the physiological aspects of metals, as an essential indicator for determining toxicity levels of Cu2+ and Pb2+, and to assess the toxic impact of heavy metals.
Although it has beneficial effects on organisms, high concentrations of Cu2+ indicate negative effects, manifested by an inhibition of proliferation resulting in the reduction in larvae, pupae, and adult number. The Cu2+ toxicity phenomenon was proved using the Oregon-R genotype at concentrations of 0.50–2 mM range [59,60]. Other studies reveled an inhibition of proliferation in Cu2+ depending on the sex (0.40 mM for females and 0.75 mM for males [46]).
The application of the D.m. research model revealed inhibitory effects for Pb2+ concentrations, similar to results previously recorded by other researchers [61].
The onset of oxidative stress at concentrations of 0.20 mM Pb2+ along with a mutagenic effect (manifesting as wing spots of 0.40 mM) was demonstrated [62].
As our study and others suggest, genetic factors play an important and decisive role in assessing heavy metal toxicity levels. Research indicates the possibility of developing genotypes with enhanced tolerance to Cu2+ at concentrations of up to 50 mM, with survival rates exceeding 75% [63]. This stands in contrast to other genotypes, which exhibit survival rates of less than 18%.
The application of the D.m. model provided valuable insights into both acute and chronic toxicity, owing to the rapidity of its life cycle and the ease with which multiple generations can be generated and observed [64].
Comparatively, our results suggest that Cu2+ effects impact more then Pb2+, with a greater reduction in adult number. This pronounced effect of Cu2+ over Pb2+ was also observed in plants [65]. This result may be understandable in itself, as copper (CuSO4) is often used as an antimicrobial and antibacterial agent, playing a homeostatic role, whereas lead accumulation presents a markedly different toxicological profile.
Both Cu2+ and Pb2+ can induce the production of reactive oxygen species (ROS), causing oxidative stress [66,67]. Consequently, ROS induces programmed cell death (PCD) [68,69].
Also, the levels of glutathione (GSH), an antioxidant and essential compound for cellular detoxification, can be significantly affected by exposure to copper and lead. GSH acts as a cytoplasmic buffer to counteract high concentrations of copper ions, facilitating their utilization in metabolic processes, maintaining copper homeostasis, and aiding in its detoxification [70,71].
On the other hand, in the case of Pb2+, GSH transforms lead ions into a less toxic form. However, prolonged or high concentrations of exposure to lead can overcome the capacity of the GSH detoxification system. Other research revealed that individuals chronically exposed to high levels of lead (50 μg/dL) had lower GSH levels compared to the control group (1.5 μg/dL) [72].
Utilizing the D.m. research model, we discovered that the white vestigial genotype exhibited increased susceptibility, possibly due to the presence of “vg” genes involved in the translation elongation process (TEF), which could impair detoxification mechanisms. The acute effects of copper were evident across all genotypes, indicating differing detoxification mechanisms compared to lead.
5. Conclusions
Genetic factors significantly influence the proliferation capacity of Drosophila melanogaster (D.m.). The white-vestigial genotype, identified as the most sensitive, exhibited lower proliferation capacity compared to the Oregon-R genotype and other mutants.
Comparative analysis between Cu2 and Pb2 unveiled that the toxic effects of copper are more acute compared to lead. The disparities between the toxical effects of copper and lead escalate with increasing metal concentrations, proving the acute effect of copper. The significant reduction in larvae, pupae, and adults was observed at 0.5 mM for Cu2+ and at 1.00 mM fo Pb2+ The acute impact of copper, compared to the chronic effects of lead, was observed not only in the Oregon-R genotype but also in the mutant genotypes. This observation suggests the existence of distinct detoxification mechanisms between the two metals.
Copper exhibited IC50 toxicity for all genotypes within the 2.00 mM concentration (four out of four genotypes).
Lead demonstrated an IC50 toxicity at 2.00 mM only for the white-vestigial genotype (one out of four genotypes).
These findings underscore the importance of ongoing research in ecotoxicology providing valuable support in the decision-making process for safeguarding the environment and public health against heavy metal pollution.
Author Contributions
Conceptualization: P.I.; Data curation: O.E., T.-C.A.-M. and G.M.; Formal analysis: O.E. and L.D.; Fund acquisition: M.A.A., C.D. and C.A.; Investigation: S.I., P.I., O.E., L.D., M.A.A., P.S., V.L.-G. and G.M.; Methodology: S.I., P.I. and G.M.; Project administration: C.D.; Resources: S.I., P.S., P.C., C.A. and G.M.; Validation: S.I., P.I., T.-C.A.-M., P.S., L.D., V.L.-G. and S.C.; Visualization: T.-C.A.-M., C.D., P.C. and G.M.; Writing—original version: S.I., O.E. and S.C.; Writing—revision and editing: S.I., O.E., T.-C.A.-M. and S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was supported by the project “Increasing the impact of excellence research on innovation and technology transfer capacity at USV Timisoara”. code 6PFE. submitted in the competition Programme 1-Development of the national R&D system. Sub-programme 1.2-Institutional performance. Sub-programme 1.2-Institutional performance. Institutional development projects—Development projects of excellence in R&D&I.
Institutional Review Board Statement
The research carried out in this article did not involve animals or human beings.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors would like to thank the funding institutions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Ali, M.M.; Hossain, D.; Al-Imran, A.; Khan, M.S.; Begum, M.; Osman, M.H. Environmental pollution with heavy metals: A public health concern. In Heavy Metals—Their Environmental Impacts and Mitigation; IntechOpen: London, UK, 2021; pp. 771–783. [Google Scholar]
- Jung, J.-M.; Kim, C.-J.; Chung, C.-S.; Kim, T.; Choi, K.-Y. Heavy metal characterization of land-based waste dumped at three ocean dumping sites in the Republic of Korea. Mar. Pollut. Bull. 2023, 193, 115205. [Google Scholar] [CrossRef]
- Alkhanjaf, A.A.M.; Sharma, S.; Sharma, M.; Kumar, R.; Arora, N.K.; Kumar, B.; Umar, A.; Baskoutas, S.; Mukherjee, T.K. Microbial strategies for copper pollution remediation: Mechanistic insights and recent advances. Environ. Pollut. 2024, 346, 123588. [Google Scholar] [CrossRef]
- McFarland, M.J.; Hauer, M.E.; Reuben, A. Half of US population exposed to adverse lead levels in early childhood. Proc. Natl. Acad. Sci. USA 2022, 119, e2118631119. [Google Scholar] [CrossRef]
- Karahan, F. Evaluation of Trace Element and Heavy Metal Levels of Some Ethnobotanically Important Medicinal Plants Used as Remedies in Southern Turkey in Terms of Human Health Risk. Biol. Trace Element Res. 2022, 201, 493–513. [Google Scholar] [CrossRef]
- Harmonisation, P.T. Derivation Methods of Soil Screening Values in Europe. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=1315ea61e2c6e168e151175735cb6253ddef33d7 (accessed on 2 April 2024).
- Sexton, J.P.; Hangartner, S.B.; Hoffmann, A.A. Genetic Isolation by Environment or Distance: Which Pattern of Gene Flow is Most Common? Evolution 2013, 68, 1–15. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, M.; Ji, M.; Fan, J.; Xie, J.; Wei, X.; Jiang, X.; Xu, J.; Chen, L.; Yin, R.; et al. Air Pollution, Genetic Factors, and the Risk of Lung Cancer A Prospective Study in the UK Biobank. Am. J. Respir. Crit. Care Med. 2021, 204, 817–825. [Google Scholar] [CrossRef]
- Brumberg, H.L.; Karr, C.J.; Bole, A.; Ahdoot, S.; Balk, S.J.; Bernstein, A.S.; Byron, L.G.; Landrigan, P.J.; Marcus, S.M.; Nerlinger, A.L.; et al. Ambient Air Pollution: Health Hazards to Children. Pediatrics 2021, 147, e2021051484. [Google Scholar] [CrossRef]
- Rajendran, S.; Priya, T.; Khoo, K.S.; Hoang, T.K.; Ng, H.-S.; Munawaroh, H.S.H.; Karaman, C.; Orooji, Y.; Show, P.L. A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere 2021, 287, 132369. [Google Scholar] [CrossRef] [PubMed]
- Bhat, V.S.; Kudva, A.K.; Naik, H.V.; Raghu, S.V.; De Padova, P.; Hegde, G. Toxicological Profiling of Onion-Peel-Derived Mesoporous Carbon Nanospheres Using In Vivo Drosophila melanogaster Model. Appl. Sci. 2022, 12, 1528. [Google Scholar] [CrossRef]
- Curcio, R.; Lunetti, P.; Zara, V.; Ferramosca, A.; Marra, F.; Fiermonte, G.; Cappello, A.R.; De Leonardis, F.; Capobianco, L.; Dolce, V. Drosophila melanogaster Mitochondrial Carriers: Similarities and Differences with the Human Carriers. Int. J. Mol. Sci. 2020, 21, 6052. [Google Scholar] [CrossRef]
- Everman, E.R.; Macdonald, S.J.; Kelly, J.K. The genetic basis of adaptation to copper pollution in Drosophila melanogaster. Front. Genet. 2023, 14, 1144221. [Google Scholar] [CrossRef] [PubMed]
- Alaraby, M.; Hernández, A.; Marcos, R. Copper oxide nanoparticles and copper sulphate act as antigenotoxic agents in drosophila melanogaster. Environ. Mol. Mutagen. 2017, 58, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Balinski, M.A.; Woodruff, R.C. Differential sexual survival of Drosophila melanogaster on copper sulfate. Genetica 2017, 145, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef]
- Soylak, M.; Kirnap, M. Serum Copper and Zinc Concentrations of Patients with Rheumatoid Arthritis from Kayseri_Turkey. Fresenius Environ. Bull. 2001, 10, 409–410. [Google Scholar]
- Swain, J.; Gutteridge, J.M. Prooxidant iron and copper, with ferroxidase and xanthine oxidase activities in human atherosclerotic material. FEBS Lett. 1995, 368, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Lavrent`yeva, S.I.; Ivachenko, L.Y.; Golokhvast, K.S.; Nawaz, M.A. Ribonuclease activity of Glycine max and Glycine soja sprouts as a marker adaptation to copper sulphate and zinc sulphate toxicity. Biochem. Syst. Ecol. 2019, 83, 66–70. [Google Scholar] [CrossRef]
- Fiskin, K.; Kandemir, S.; Hamamci, D.; Yesilada, E.; Bozcuk, A. Age-related changes in catalase, glutathione reductase activities, the amount of glutathione in total body of oregon and vestigial Drosophila melanogaster. Arch. Gerontol. Geriatr. 1994, 19, 85–90. [Google Scholar] [CrossRef]
- Nwaka, S.; Hudson, A. Innovative lead discovery strategies for tropical diseases. Nat. Rev. Drug Discov. 2006, 5, 941–955. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Nester, M.; Devol, E.; Shinwari, N.; Al-Shahria, S. Determinants of Blood Lead Levels in Saudi Arabian Schoolgirls. Int. J. Occup. Environ. Health 1999, 5, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Sekara, A.; Poniedzialeek, M.; Ciura, J.; Jedrszczyk, E. Cadmium and Lead Accumulation and Distribution in the Organs of Nine Crops: Implications for Phytoremediation. Pol. J. Environ. Stud. 2005, 14, 509–516. [Google Scholar]
- Geng, Y.; Faber, K.N.; de Meijer, V.E.; Blokzijl, H.; Moshage, H. How does hepatic lipid accumulation lead to lipotoxicity in non-alcoholic fatty liver disease? Hepatol. Int. 2021, 15, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Segin, T.B.; Hnatush, S.O.; Gorishniy, M.B. The processes of lipid peroxidation in the cells of Chlorobium limicola IMV K-8 under the influence of copper (II) sulphate. Biosyst. Divers. 2016, 24, 72–77. [Google Scholar] [CrossRef]
- Siddique, H.R.; Gupta, S.C.; Mitra, K.; Bajpai, V.K.; Mathur, N.; Murthy, R.C.; Saxena, D.K.; Chowdhuri, D.K. Adverse effect of tannery waste leachates in transgenic Drosophila melanogaster: Role of ROS in modulation of Hsp70, oxidative stress and apoptosis. J. Appl. Toxicol. 2008, 28, 734–748. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Himalian, R.; Shabir, S.; Obaid, A.A.; Alamri, A.S.; Galanakis, C.M.; Singh, S.K.; Vamanu, E. Protection of Phytoextracts against Rotenone-Induced Organismal Toxicities in Drosophila melanogaster via the Attenuation of ROS Generation. Appl. Sci. 2022, 12, 9822. [Google Scholar] [CrossRef]
- Mackay, T.F.C.; Huang, W. Charting the genotype-phenotype map: Lessons from the Drosophila melanogaster Genetic Reference Panel. Wiley Interdiscip Rev. Dev. Biol. 2018, 7, e289. [Google Scholar] [CrossRef]
- Kondrashov, A.S.; Houle, D. Genotype—Environment interactions and the estimation of the genomic mutation rate in Drosophila melanogaster. Proc. R. Soc. B Biol. Sci. 1994, 258, 221–227. [Google Scholar] [CrossRef]
- Anderson, S.; Sadinski, W.; Shugart, L.; Brussard, P.; Depledge, M.; Ford, T.; Hose, J.; Stegeman, J.; Suk, W.; Wirgin, I.; et al. Genetic and Molecular Ecotoxicology: A Research Framework. Environ. Health Perspect. 1994, 102, 3–8. [Google Scholar] [CrossRef]
- Wipf, I.J. Investigating the Role of ATP-Binding Cassette Transporters in Drosophila melanogaster Testis Stem Cells. Master’s Thesis, University of Northern Colorado, Greeley, CO, USA, 2021. [Google Scholar]
- Licht, A.; Schneider, E. ATP binding cassette systems: Structures, mechanisms, and functions. Cent. Eur. J. Biol. 2011, 6, 785–801. [Google Scholar] [CrossRef]
- Kang, J.; Park, J.; Choi, H.; Burla, B.; Kretzschmar, T.; Lee, Y.; Martinoia, E. Plant ABC Transporters. Arab. Book 2011, 9, e0153. [Google Scholar] [CrossRef]
- Xiao, C.; Qiu, S.; Robertson, R.M. The white gene controls copulation success in Drosophila melanogaster. Sci. Rep. 2017, 7, 17712. [Google Scholar] [CrossRef]
- Qian, S.; Pirrotta, V. Dosage compensation of the Drosophila white gene requires both the X chromosome environment and multiple intragenic elements. Genetics 1995, 139, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Slatis, H.M. Position Effects at the Brown Locus in Drosophila Melanogaster. Genetics 1955, 40, 5–23. [Google Scholar] [CrossRef]
- Grubbs, N.; Haas, S.; Beeman, R.W.; Lorenzen, M.D. The ABCs of Eye Color in Tribolium castaneum: Orthologs of the Drosophila white, scarlet, and brown Genes. Genetics 2015, 199, 749–759. [Google Scholar] [CrossRef]
- Babazadeh, R.; Schneider, K.L.; Fischbach, A.; Hao, X.; Liu, B.; Nystrom, T. The yeast guanine nucleotide exchange factor Sec7 is a bottleneck in spatial protein quality control and detoxifies neurological disease proteins. Sci. Rep. 2023, 13, 14068. [Google Scholar] [CrossRef] [PubMed]
- Vetter, I.R.; Wittinghofer, A. The Guanine Nucleotide-Binding Switch in Three Dimensions. Science 2001, 294, 1299–1304. [Google Scholar] [CrossRef]
- Mandel, M.; Marmur, J. [109] Use of ultraviolet absorbance-temperature profile for determining the guanine plus cytosine content of DNA. Methods Enzymol. 1968, 12, 195–206. [Google Scholar] [CrossRef]
- Maeda, T.; Chapman, D.L.; Stewart, A.F.R. Mammalian Vestigial-like 2, a Cofactor of TEF-1 and MEF2 Transcription Factors That Promotes Skeletal Muscle Differentiation. J. Biol. Chem. 2002, 277, 48889–48898. [Google Scholar] [CrossRef]
- Vaudin, P.; Delanoue, R.; Davidson, I.; Silber, J.; Zider, A. TONDU (TDU), a novel human protein related to the product of vestigial (vg) gene of Drosophila melanogaster interacts with vertebrate TEF factors and substitutes for Vg function in wing formation. Development 1999, 126, 4807–4816. [Google Scholar] [CrossRef]
- Halmenschelager, P.T.; da Rocha, J.B.T. Biochemical CuSO4 Toxicity in Drosophila melanogaster depends on sex and developmental stage of exposure. Biol. Trace Elem. Res. 2019, 189, 574–585. [Google Scholar] [CrossRef]
- Lints, F.; Lints, C.; Bullens, P.; Bourgois, M.; Delincé, J. Unexplained variations in life span of the Oregon-R strain of Drosophila melanogaster over a four-year period. Exp. Gerontol. 1989, 24, 265–271. [Google Scholar] [CrossRef]
- Siddique, Y.H.; Haidari, M.; Khan, W.; Fatima, A.; Jyoti, S.; Khanam, S.; Naz, F.; Rahul; Ali, F.; Singh, B.R.; et al. Toxic potential of copper-doped ZnO nanoparticles in Drosophila melanogaster (Oregon R). Toxicol. Mech. Methods 2015, 25, 425–432. [Google Scholar] [CrossRef]
- Lefevre, G.; Green, M.M. Genetic duplication in the white-split interval of the X chromosome in Drosophila melanogaster. Chromosoma 1972, 36, 391–412. [Google Scholar] [CrossRef]
- Green, M.M. 2010: A Century of Drosophila Genetics Through the Prism of the white Gene. Genetics 2010, 184, 3–7. [Google Scholar] [CrossRef]
- Rabinow, L.; Nguyen-Huynh, A.T.; Birchler, J.A. A trans-acting regulatory gene that inversely affects the expression of the white, brown and scarlet loci in Drosophila. Genetics 1991, 129, 463–480. [Google Scholar] [CrossRef]
- Campbell, J.L.; Nash, H.A. Volatile general anesthetics reveal a neurobiological role for the white and brown genes of Drosophila melanogaster. J. Neurobiol. 2001, 49, 339–349. [Google Scholar] [CrossRef]
- Aarts, J.M.M.J.G.; Alink, G.M.; Franssen, H.J.; Roebroeks, W. Evolution of Hominin Detoxification: Neanderthal and Modern Human Ah Receptor Respond Similarly to TCDD. Mol. Biol. Evol. 2020, 38, 1292–1305. [Google Scholar] [CrossRef]
- Gachon, F.; Olela, F.F.; Schaad, O.; Descombes, P.; Schibler, U. The circadian PAR-domain basic leucine zipper transcription factors DBP, TEF, and HLF modulate basal and inducible xenobiotic detoxification. Cell Metab. 2006, 4, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.K.; Pandey, P. Fecundity of inbred fruit fly Drosophila melanogaster on different solid culture media: An analysis. J. Appl. Nat. Sci. 2018, 10, 1109–1114. [Google Scholar] [CrossRef]
- Klimaczewski, C.V.; Ecker, A.; Piccoli, B.; Aschner, M.; Barbosa, N.V.; Rocha, J.B.T. Peumus boldus attenuates copper-induced toxicity in Drosophila melanogaster. Biomed. Pharmacother. 2018, 97, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Va, D.P.; Sa, A.A.; Paul, S.F. wonder animal model for genetic studies-Drosophila melanogaster-its life cycle and breeding methods-a review. Sri Ramachandra J. Med. 2009, 2, 33–38. [Google Scholar]
- Wang, Y.; Misto, M.; Yang, J.; Gehring, N.; Yu, X.; Moussian, B. Toxicity of Dithiothreitol (DTT) to Drosophila melanogaster. Toxicol. Rep. 2021, 8, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Burke, R. Molecular physiology of copper in Drosophila melanogaster. Curr. Opin. Insect Sci. 2022, 51, 100892. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.A.; Schneuwly, S. Copper and Zinc Homeostasis: Lessons from Drosophila melanogaster. Front. Genet. 2017, 8, 223. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Men, L.; Wang, J.; Zhang, Y.; Chickenyen, S.; Wang, Y.; Zhou, F. Redox Reactions of Copper Complexes Formed with Different β-Amyloid Peptides and Their Neuropathalogical Relevance. Biochemistry 2007, 46, 9270–9282. [Google Scholar] [CrossRef] [PubMed]
- Southon, A.; Burke, R.; Norgate, M.; Batterham, P.; Camakaris, J. Copper homoeostasis in Drosophila melanogaster S2 cells. Biochem. J. 2004, 383, 303–309. [Google Scholar] [CrossRef]
- Avram, O.R.; Caragea, G.; Varzaru, C.A. Copper and its role in the human body-the importance of establishing copper concentrations in the body. Rom. J. 2021, 124, 2. [Google Scholar]
- Demir, E.; Demir, F.T. Drosophila: A promising model for evaluating the toxicity of environmental pollutants. Karaelmas Mühendislik Derg. 2022, 12, 101–118. [Google Scholar]
- An, Y.-J. Assessment of comparative toxicities of lead and copper using plant assay. Chemosphere 2006, 62, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Komarnicka, U.K.; Lesiów, M.K.; Witwicki, M.; Bieńko, A. The Bright and Dark Sides of Reactive Oxygen Species Generated by Copper–Peptide Complexes. Separations 2022, 9, 73. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Guan, X.; Zhou, L.; Asad, M.A.U.; Xu, Y.; Pan, G.; Cheng, F. ABA-triggered ROS burst in rice developing anthers is critical for tapetal programmed cell death induction and heat stress-induced pollen abortion. Plant Cell Environ. 2023, 46, 1453–1471. [Google Scholar] [CrossRef] [PubMed]
- Emilian, O.; Ioan, S.; Irina, P.; Raul, P.; Adriana, C.; Dorin, C.; Ciprian, S. Cytological Applications of the Vacuolization Phenomenon as a Means of Determining Saline Cytotoxicity. Appl. Sci. 2023, 13, 8461. [Google Scholar] [CrossRef]
- Ho, T.; Ahmadi, S.; Kerman, K. Do glutathione and copper interact to modify Alzheimer’s disease pathogenesis? Free. Radic. Biol. Med. 2022, 181, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Babak, M.V.; Ahn, D. Modulation of intracellular copper levels as the mechanism of action of anticancer copper complexes: Clinical relevance. Biomedicines 2021, 9, 852. [Google Scholar] [CrossRef]
- Devóz, P.P.; Dos Reis, M.B.; Gomes, W.R.; Maraslis, F.T.; Ribeiro, D.L.; Antunes, L.M.G.; Batista, B.L.; Grotto, D.; Reis, R.M.; Barbosa, F., Jr. Adaptive epigenetic response of glutathione (GSH)-related genes against lead (Pb)-induced toxicity, in individuals chronically exposed to the metal. Chemosphere 2021, 269, 128758. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).