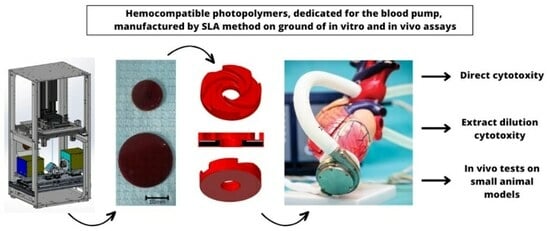
In Vitro and In Vivo Testing of Stereolithography (SLA)-Manufactured Haemocompatible Photopolymers for Blood Pump
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Development
- Omnirad 784 and Irgacure 784—photo-initiators for radical polymerization of unsaturated resins.
- CNT—carbon nanotubes.
- Dicumyl peroxide (DCP)—a radical initiator that can be thermally activated leading to an increase in conversion (hereinafter polymerization) in the post-treatment process at elevated temperatures.
- Quinoline Yellow—a component responsible for absorbing light, required for the SLA printing process.
2.2. Production of Printouts of Basic Test Materials and Cardiac Assist Pump Rotors
2.3. Optimisation of Biological Properties of Methacrylate-Based Materials
2.3.1. Cell Viability and Cytotoxicity Assessed Using Direct Tests
2.3.2. Cytotoxicity Evaluation Using Indirect Method
2.4. In Vivo Tests on Small Animal Models
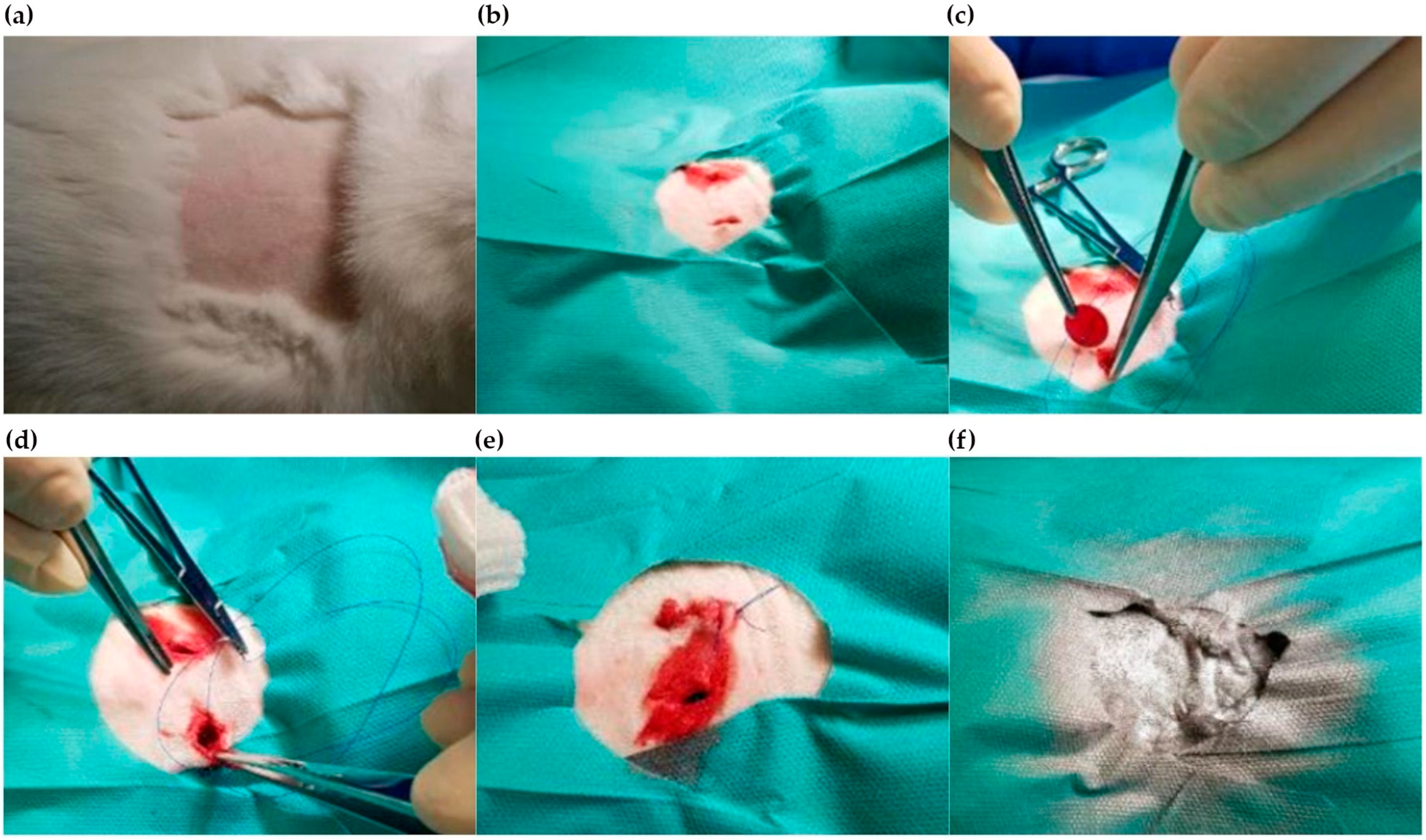
2.4.1. Implantation Process
2.4.2. Clinical Observations
- Breathing—dyspnoea (abdominal type, Kussmaul) apnoea, cyanosis, rapid breathing, nostril discharge.
- Physical activity—decreased/increased somnolence, loss of upright posture, anaesthesia, catalepsy, ataxia, unusual way of moving, extreme exhaustion, tremor, muscle bunching tremor.
- Convulsions—clonic, tonic, clonic–tonic, asphyxia, tetanic signs.
- Reflexes—corneal, upright, muscle contractility, reaction to light, startle reflex.
- Ocular symptoms—tearing, pupil constriction, pupil dilation, exophthalmos, opacity, iritis, conjunctivitis, red tears, drooping of the third eyelid.
- Cardiovascular symptoms—bradycardia, tachycardia, impotence, vasodilation, vasoconstriction.
- Salivation—salivation.
- Hairstyling—scraggly hair.
- Anaesthesia—decreased responsiveness.
- Muscle tension—hypotonia, hypertonia.
- Gastrointestinal symptoms—diarrhoea, vomiting.
- Skin—swelling, erythema.
2.4.3. Termination of the Experiment
2.4.4. Diagnostic Tests
2.4.5. Sectional Assessment
2.4.6. Histopathological Evaluation
3. Results
3.1. Printing of Methacrylate Materials for Optimization Tests Prior to In Vivo Studies
- OO’—≥550 mm
- Magnification—6.614 ± 1% (50 µm pixel size)
- Aperture DMD—12° (F/2.4)
- CTF at 10 Lp/mm—>75% as built
- Wavelengths [nm] and weights—439 (0.1) 449 (0.5) 459 (1) 469 (0.5) 479 (0.1)
- Distortion [%]—Need to have: <0.1, expected value < 0.05
- Optics—Projection optics designed to include ~45 mm glass or similar
3.2. Optimisation of Biological Properties of Methacrylate-Based Materials
3.2.1. Direct Cytotoxity Analysis
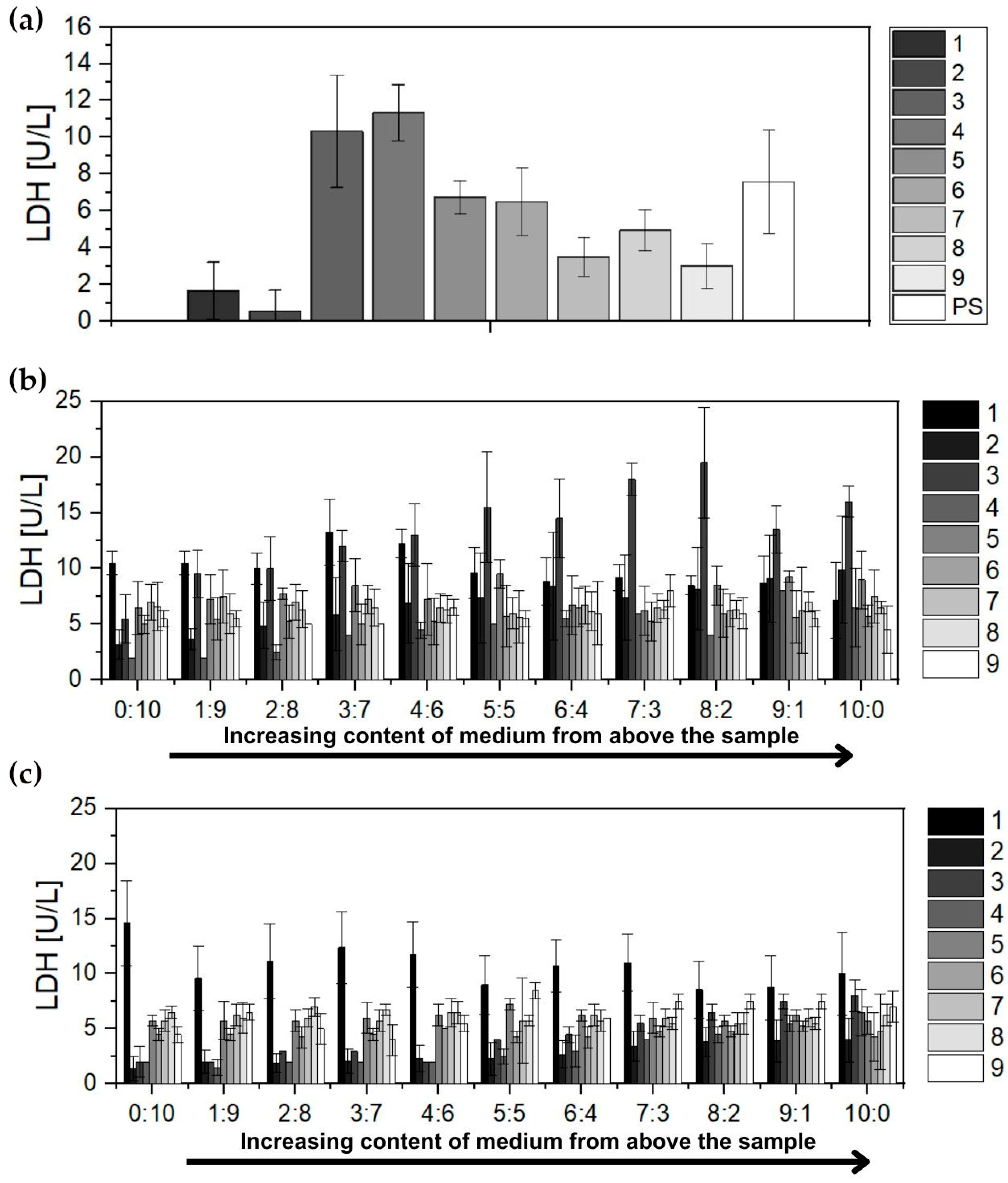
3.2.2. Lactate Dehydrogenase Analysis
3.3. In Vivo Tests on Small Animal Models
3.3.1. Clinical Observations
3.3.2. Diagnostic Tests
- White blood cell count (WBC)—the mean leukocyte count determined for all groups was within the reference range throughout the observation period.
- Erythrocyte count (RBC)—the mean erythrocyte count for all study groups determined throughout the observation period fluctuated in the lower reference range.
- Total haemoglobin concentration (HGB)—the mean total concentration determined for all groups throughout the observation period was within the lower reference range.
- Haematocrit (HCT)—the mean haematocrit value determined for all groups was within the lower reference range.
- Platelet count (PLT)—the mean platelet count determined for all groups throughout the observation period was within the lower reference range.
- Alanine aminotransferase (ALT)—the mean activity of the enzyme determined for all groups during the observation period was above the reference range.
- Aspartate aminotransferase (AST)—the mean activity of the enzyme in both groups fluctuated below the reference range.
- Lactate dehydrogenase (LDH)—the mean activity of the enzyme determined for all groups during follow-up was within the normal range.
- Gamma-glutamyltransferase (GGTP)—the mean activity of the enzyme in both groups before implantation of the test material was within the reference range. Before euthanasia, a slight increase in enzyme activity was observed in both groups above the reference range.
- Alkaline phosphatase (ALP)—the mean enzyme activity for all groups determined during the observation period was below the reference range.
- Other biochemical parameters were as follows:
- Albumin—mean albumin concentrations for all throughout the observation period were above the reference range.
- Creatinine—mean metabolite concentrations determined for both experimental groups before implantation and before euthanasia are within the reference range.
- Urea—mean metabolite concentrations determined for all groups before euthanasia and before implantation were within the reference range.
- Total protein—mean total protein concentrations fluctuated over the observation period for all groups within the lower limit of normal.
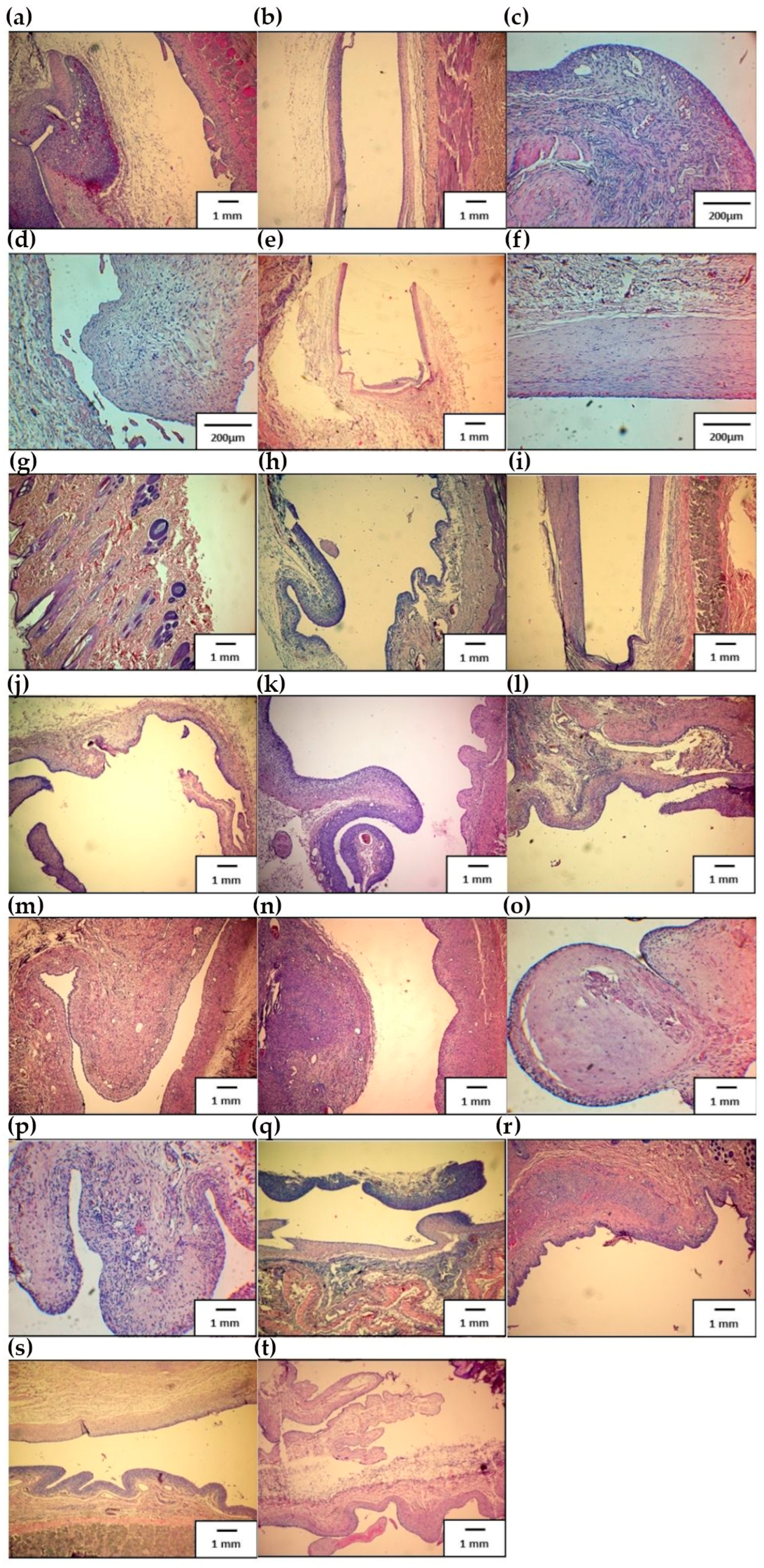
3.3.3. Post-Mortem Sectional Assessment
3.3.4. Histopathological Evaluation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balcioglu, O.; Kahraman, Ü.; Ergi, D.G.; Karaca, S.; Engin, C.; Yagdi, T. Association of Age, Sex, and Heart Failure Etiology in Continuous Flow Left Ventricular Assist Device Patients. Transplant. Proc. 2022, 55, 1294–1296. [Google Scholar] [CrossRef] [PubMed]
- Eurotransplant International Foundation, Annual Report 2011. Available online: https://www.eurotransplant.org/wp-content/uploads/2019/12/ar_2011.pdf (accessed on 17 November 2023).
- Khatana, S.A.M.; Grandin, E.W.; Rame, J.E.; Shen, C.; Yeh, R.W.; Groeneveld, P.W. Medicaid Expansion and Ventricular Assist Device Implantation: An Analysis of the INTERMACS Registry. J. Am. Coll. Cardiol. 2020, 76, 1501–1502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tansley, G.D.; Dargusch, M.S.; Fraser, J.F.; Pauls, J.P. Surface Coatings for Rotary Ventricular Assist Devices: A Systematic Review. ASAIO J. 2022, 68, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.E.; Desai, N.R. Caution with Conclusions and Context of Mechanical Circulatory Devices-Reply. JAMA Intern. Med. 2023, 183, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Consolo, F.; Pappalardo, F. Future Perspectives of Mechanical Circulatory Support with Left Ventricular Assist Devices: Lessons Learned from the HeartWare Ventricular Assist Device. ASAIO J. 2022, 68, 1–2. [Google Scholar] [CrossRef]
- Hullin, R.; Meyer, P.; Yerly, P.; Kirsch, M. Cardiac Surgery in Advanced Heart Failure. J. Clin. Med. 2022, 11, 773. [Google Scholar] [CrossRef]
- Milam, A.J.; Ramakrishna, H. Predicting Survival After HeartMate 3 Left Ventricular Assist Device Implantation-Progress Continues. J. Cardiothorac. Vasc. Anesth. 2023, 37, 1347–1348. [Google Scholar] [CrossRef] [PubMed]
- van der Heiden, C.W.; Zijderhand, C.F.; Veen, K.M.; Constantinescu, A.A.; Manintveld, O.C.; Brugts, J.J.; Bekkers, J.A.; Birim, O.; Bogers, A.J.J.C.; Caliskan, K. Mechanical Device Malfunction of the HeartMate II Versus the HeartMate 3 Left Ventricular Assist Device: The Rotterdam Experience. ASAIO J. 2023, 69, e80–e85. [Google Scholar] [CrossRef]
- Han, J.; Trumble, D.R. Cardiac Assist Devices: Early Concepts, Current Technologies, and Future Innovations. Bioengineering 2019, 6, 18. [Google Scholar] [CrossRef]
- Selzman, C.H.; Feller, E.D.; Walker, J.C.; Sheridan, B.C.; Silvestry, S.C.; Daly, R.C.; Anyanwu, A.C.; Madigan, D.; Liu, P.Y.; Frazier, O.H.; et al. The Jarvik 2000 Left Ventricular Assist Device: Results of the United States Bridge to Transplant Trial. ASAIO J. 2023, 69, 174–182. [Google Scholar] [CrossRef]
- Vieira, J.L.; Ventura, H.O.; Mehra, M.R. Mechanical circulatory support devices in advanced heart failure: 2020 and beyond. Prog. Cardiovasc. Dis. 2020, 63, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Gawlikowski, M.; Major, R.; Mika, B.; Komorowski, D.; Janiczak, K.; Tkacz, E.; Tamulewicz, A.; Piaseczna, N. Semi-Quantitative Method of Assessing the Thrombogenicity of Biomaterials Intended for Long-Term Blood Contact. Materials 2023, 16, 38. [Google Scholar] [CrossRef] [PubMed]
- Major, R.; Wilczek, G.; Więcek, J.; Gawlikowski, M.; Plutecka, H.; Kasperkiewicz, K.; Kot, M.; Pomorska, M.; Ostrowski, R.; Kopernik, M. Hemocompatibile Thin Films Assessed under Blood Flow Shear Forces. Molecules 2022, 27, 5696. [Google Scholar] [CrossRef] [PubMed]
- Dyner, A.; Major, R.; Major, Ł.; Szewczenko, J.; Lukaszkowicz, K.; Barabaszová, K.; Krzywiecki, M.; Basiaga, M. Biological properties of surface modified 316 LVM steel. Arch. Civ. Mech. Eng. 2023, 23, 237. [Google Scholar] [CrossRef]
- Kurtyka, P.; Kopernik, M.; Kaczmarek, M.; Surmiak, M.; Major, Ł.; Kustosz, R.; Więcek, J.; Kurtyka, K.; Bartkowiak, A.; Major, R. Biofunctional impact of textured coatings in the application of heart assist therapy. Arch. Civ. Mech. Eng. 2023, 23, 31. [Google Scholar] [CrossRef]
- Major, R.; Surmiak, M.; Gawlikowski, M.; Schwarz, R.; Kot, M.; Wiecek, J.; Lackner, J.M. Novel Hemocompatible Thiol-yne Based Photopolymers Obtained by the Advanced Stereolithography (SLA) Processing with Strongly Improved Surface Smoothness by a Novel Exposition Approach for Anti-aliasing. In Innovations in Biomedical Engineering. Lecture Notes in Networks and Systems; Gzik, M., Paszenda, Z., Piętka, E., Tkacz, E., Milewski, K., Jurkojć, J., Eds.; Springer: Cham, Switzerland, 2023; Volume 409, pp. 199–215. [Google Scholar] [CrossRef]
- Rab, T.; O’Neill, W. Mechanical circulatory support for patients with cardiogenic shock. Trends Cardiovasc. Med. 2019, 29, 410–417. [Google Scholar] [CrossRef]
- Urbanowicz, T.; Olasińska-Wiśniewska, A.; Michalak, M.; Bociański, M.; Krawczyk, D.; Straburzyńska-Migaj, E.; Wachowiak-Baszyńska, H.; Jemielity, M. Risk of Thrombus Formation in Patients on Mechanical Circulatory Support with POLVAD-MEV. Ann. Transplant. 2021, 26, e926555–e926563. [Google Scholar] [CrossRef]
- Kounis, N.G.; Soufras, G.D.; Davlouros, P.; Tsigkas, G.; Hahalis, G. Thrombus Formation Patterns in HeartMate II Continuous-Flow Left Ventricular Assist Devices: A Multifactorial Phenomenon Involving Kounis Syndrome? ASAIO J. 2014, 60, 369–371. [Google Scholar] [CrossRef]
- Dignat-George, F.; Boulanger, C.M. The many faces of endothelial microparticles. Arterioscler. Thromb. Vasc. Biol. 2022, 31, 27–33. [Google Scholar] [CrossRef]
- Roka-Moiia, Y.; Miller-Gutierrez, S.; Palomares, D.E.; Italiano, J.E.; Sheriff, J.; Bluestein, D.; Slepian, M.J. Platelet Dysfunction during Mechanical Circulatory Support: Elevated Shear Stress Promotes Downregulation of αIIbβ3 and GPIb via Microparticle Shedding Decreasing Platelet Aggregability. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1319–1336. [Google Scholar] [CrossRef]
- Lantada, A.D.; de Blas Romero, A.; Schwentenwein, M.; Jellinek, C.; Homa, J. Lithographybased ceramic manufacture (LCM) of auxetic structures: Present capabilities and challenges. Smart Mater. Struct. 2016, 25, 054015. [Google Scholar] [CrossRef]
- Zanchetta, E.; Cattaldo, M.; Franchin, G.; Schwentenwein, M.; Homa, J.; Colombo, P. Stereolithography of SiOC Ceramic Microcomponents. Adv. Mater. 2016, 28, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Schwentenwein, M.; Homa, J. Additive Manufacturing of Dense Alumina Ceramics. Int. J. Appl. Ceram. Technol. 2015, 12, 1–7. [Google Scholar] [CrossRef]
- Romero, A.B.; Pfaffinger, M.; Mitteramskogler, G.; Schwentenwein, M.; Jellinek, C.; Homa, J.; Stampfl, J. Lithography-based additive manufacture of ceramic biodevices with design controlled surface topographies. Int. J. Adv. Manuf. Technol. 2016, 88, 1547–1555. [Google Scholar] [CrossRef]
- Ligon-Auer, S.C.; Schwentenwein, M.; Gorsche, C.; Stampfl, J.; Liska, R. Toughening of photo-curable polymer networks: A review. Polym. Chem. 2016, 7, 257–286. [Google Scholar] [CrossRef]
- Slaughter, M.S.; Rogers, J.G.; Milano, C.A.; Russell, S.D.; Conte, J.V.; Feldman, D.; Sun, B.; Tatooles, A.J.; Delgado, R.M.; Long, J.W.; et al. Advanced Heart Failure Treated with Continuous-Flow Left Ventricular Assist Device. NEJM 2009, 361, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-6:2016; Biological Evaluation of Medical Devices; Part 6: Tests for Local Effects after Implantation. ISO: Geneva, Switzerland, 2016.
- ISO 10993-11:2017; Biological Evaluation of Medical Devices; Part 11: Tests for Systemic Toxicity. ISO: Geneva, Switzerland, 2017.
- Oesterreicher, A.; Ayalur-Karunakaran, S.; Moser, A.; Mostegel, F.H.; Edler, M.; Kaschnitz, P.; Pinter, G.; Trimmel, G.; Schlögl, S.; Griesser, T. Exploring thiol-yne based monomers as low cytotoxic building blocks for radical photopolymerization. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3484–3494. [Google Scholar] [CrossRef]
- Oesterreicher, A.; Gorsche, C.; Ayalur-Karunakaran, S.; Moser, A.; Edler, M.; Pinter, G.; Schlögl, S.; Liska, R.; Griesser, T. Exploring Network Formation of Tough and Biocompatible Thiol-yne Based Photopolymers. Macromol. Rapid Commun. 2016, 37, 1701–1706. [Google Scholar] [CrossRef]
- Oesterreicher, A.; Moser, A.; Edler, M.; Griesser, H.; Schlögl, S.; Pichelmayer, M.; Griesser, T. Investigating Photocurable Thiol-Yne Resins for Biomedical Materials. Macromol. Mater. Eng. 2017, 19, 1600450. [Google Scholar] [CrossRef]
- Oesterreicher, A.; Wiener, J.; Roth, M.; Moser, A.; Gmeiner, R.; Edler, M.; Pinter, G.; Griesser, T. Tough and degradable photopolymers derived from alkyne monomers for 3D printing of biomedical materials. Polym. Chem. 2016, 7, 5169–5180. [Google Scholar] [CrossRef]
- Quick, A.S.; Pereira, A.S.; Bruns, M.; Bückmann, T.; Rodriguez-Emmenegger, C.; Wegener, M.; Barner-Kowollik, C. Rapid Thiol-Yne-Mediated Fabrication and Dual Postfunctionalization of Micro-Resolved 3D Mesostructures. Adv. Funct. Mater. 2015, 25, 3735–3744. [Google Scholar] [CrossRef]
- Norbert, M.; Wolfgang, W.; Christoph, A.; Volker, R.; Robert, L.; Juergen, S.; Johannes, P. Light Hardening Dross for Stereolithographic Production of Dental Ceramics. EP2151214 (A1), 10 February 2010. Also Published as: US2010029801 (A1), JP2010031279 (A). Available online: https://patents.google.com/patent/EP2404590A1/en (accessed on 24 November 2023).
- Gottfried, R.; Wolfgang, W.; Christoph, A.; Johannes, P.; Juergen, S. Device and Method for Processing Light-Polymerizable Material for the Layered Assembly of Molds. WO2010045950 (A1), 29 April 2010. M-ERA.NET Call 2017; p. 17. Available online: https://patents.google.com/patent/US9067359 (accessed on 24 November 2023).
- Robert, L.; Johannes, P.; Juergen, S.; Wolfgang, W.; Christoph, A. Device and Method for Processing Light-Polymerizable Material for Building up Object in Layers. WO2010045951 (A1), 29 April 2010. Available online: https://eureka.patsnap.com/patent-US8741203B2 (accessed on 24 November 2023).
- Gottfried, R.; Wolfgang, W.; Juergen, S.; Johannes, P.; Johannes, H. Method and Device for Generative Production of a Mould with Non-Planar Layers. EP2251185 (A1), 17 November 2010. Also published as: US2010283188 (A1), JP2010259804 (A). Available online: https://patents.google.com/patent/US20100283188 (accessed on 24 November 2023).
- Lutzer, M.; Hummer, S.; Simsek, S.; Zimmermann, C.; Amsuessb, A.; Hutter, H.; Detz, H.; Stoeger-Pollach, M.; Bethge, O.; Bertagnolli, E. Rhodium Germanide Schottky Barrier Contacts. ECS J. Solid State Sci. Technol. 2015, 4, 387–392. [Google Scholar] [CrossRef]
- Lutzer, M.; Simsek, S.; Zimmermann, C.; Stoeger-Pollach, M.; Bethge, O.; Bertagnolli, E. Linearity optimization of ALD-grown ZrO2 MIM capacitors by inserting interfacial Zr-doped chromia layers. J. Appl. Phys. 2016, 119, 125304. [Google Scholar] [CrossRef]
- Zimmermann, C.; Bethge, O.; Winkler, K.; Lutzer, B.; Bertagnolli, E. Improving the ALD-grown Y2O3/Ge interface quality by surface and annealing treatments. Appl. Surf. Sci. 2016, 369, 377–383. [Google Scholar] [CrossRef]
- Zimmermann, C.; Bethge, O.; Lutzer, B.; Bertagnolli, E. Platinum-assisted Post Deposition annealing of the n-Ge/Y2O3 Interface. Semicond. Sci. Technol. 2016, 31, 075009. [Google Scholar] [CrossRef]
- Gross, B.C.; Erkal, J.L.; Lockwood, S.Y.; Chengpeng, C.; Spence, D.M. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef]
- Schuster, M.; Turecek, C.; Kaiser, B.; Stampfl, J.; Liska, R.; Varga, F. Evaluation of Biocompatible Photopolymers I: Photoreactivity and Mechanical Properties of Reactive Diluents. J. Polym. Sci. Part A Polym. Chem. 2007, 44, 547–557. [Google Scholar] [CrossRef]

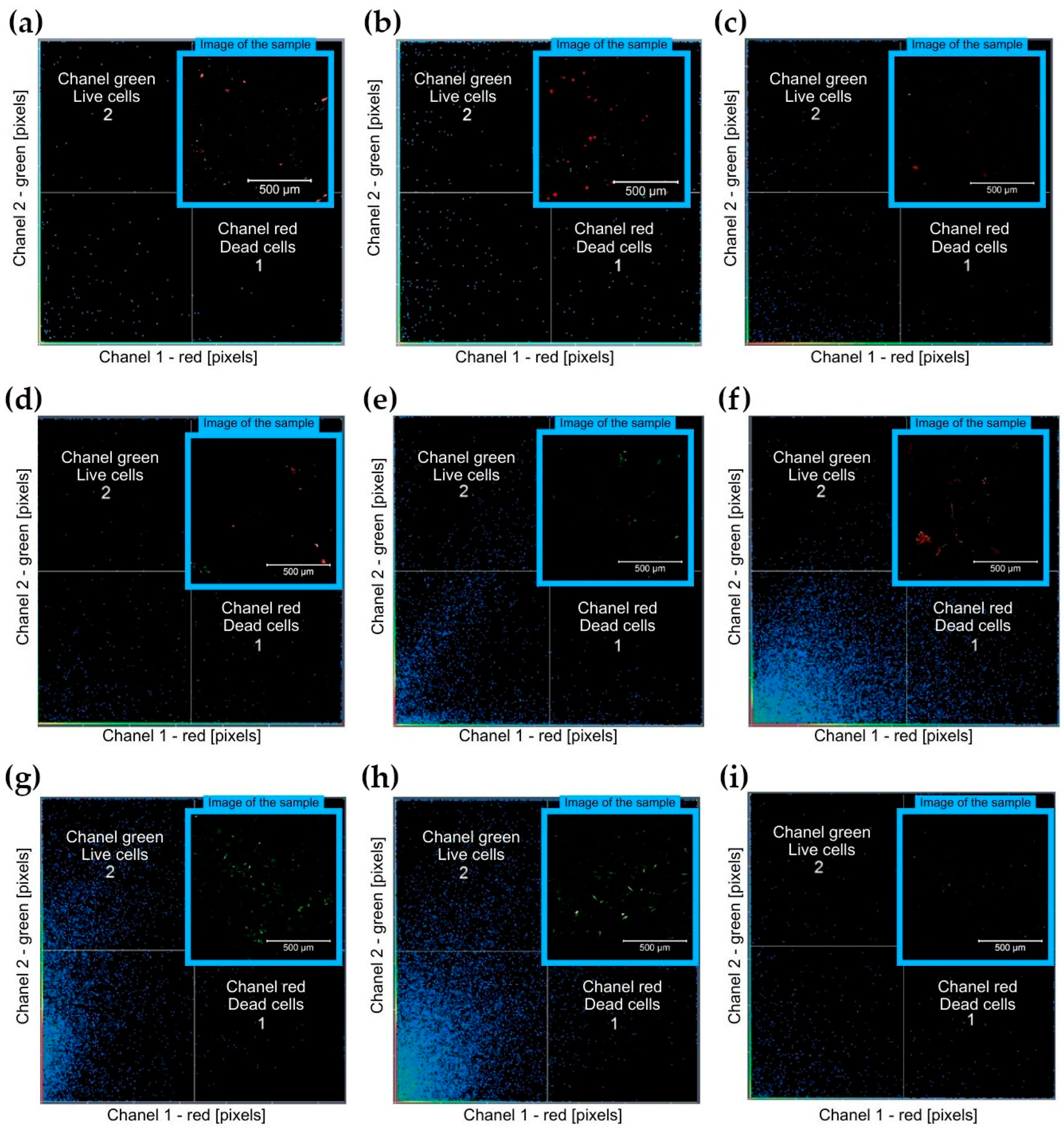


| No. | Material | Post-Processing | Surface Modification | Red Channel—Dead Cells [Pixels] | Green Channel—Live Cells [Pixels] |
|---|---|---|---|---|---|
| 1 | M10 + 0.8% Omnirad 784 | 80 °C | no coating | 1895.82 | 948.17 |
| 2 | M10 + 1 wt% CNT + 0.8 wt% Omnirad 784 | 80 °C | no coating | 2435.41 | 634.33 |
| 3 | M10 + 2 wt% DCP + 2.4 wt% Irgacure 784 + wt% 0.06 Quinoline Yellow | 70 °C | no coating | 1156.80 | 109.90 |
| 4 | M10 + 2 wt% DCP + 2.4 wt% Irgacure 784 + wt% 0.06 Quinoline Yellow | 100 °C | no coating | 706.90 | 117.10 |
| 5 | M10 + 2 wt% DCP + 2.4 wt% Irgacure 784 + wt% 0.06 Quinoline Yellow | 70 °C | PECVD a-CSiO:H | 228.15 | 351.76 |
| 6 | M10 + 2 wt% DCP + 2.4 wt% Irgacure 784 + wt% 0.06 Quinoline Yellow | 100 °C | PECVD a-CSiO:H | 142.71 | 139.00 |
| 7 | M10 + 2 wt% DCP + 2.4 wt% Irgacure 784 + wt% 0.06 Quinoline Yellow | 70 °C | ALD TiN | 57.21 | 4387.71 |
| 8 | M10 + 2 wt% DCP + 2.4 wt% Irgacure 784 + wt% 0.06 Quinoline Yellow | 100 °C | ALD TiN | 239.07 | 1665.33 |
| 9 | M10 + 2 wt% DCP + 2.4 wt% Irgacure 784 + wt% 0.06 Quinoline Yellow | 80 °C | no coating | 159.00 | 170.00 |
| Evaluated Group | Parameter | Normal Range for Animals | Control Group—Implantation | Study Group—Implantation | Conrol Group—Euthanasia | Study Group—Euthanasia | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unit | Mean | Std. Dev. | Mean | Std. Dev. | Mean | Std. Dev. | Mean | Std. Dev. | ||
| WBC | (×109/L) | 5.2–13.5 | 6.08 | 1.46 | 5.93 | 1.37 | 6.46 | 1.36 | 7.86 | 2.25 |
| RBC | (×1012/L) | 5–7.6 | 5.35 | 0.34 | 5.44 | 0.32 | 5.02 | 0.31 | 5.33 | 0.42 |
| HGB | (g/L) | 105–170 | 115.00 | 9.27 | 112.90 | 5.20 | 112.00 | 7.58 | 115.60 | 6.62 |
| HCT | (%) | 31–46.0 | 33.62 | 2.83 | 33.18 | 1.58 | 32.26 | 2.46 | 33.17 | 1.76 |
| PLT | (×109/L) | 100–512 | 184.60 | 33.76 | 126.20 | 52.65 | 172.40 | 79.58 | 194.30 | 32.32 |
| ALT | (U/L) | 31–53 | 110.02 | 38.73 | 81.29 | 37.11 | 107.92 | 50.44 | 95.49 | 59.78 |
| AST | (U/L) | 42–98 | 34.66 | 17.15 | 42.19 | 43.36 | 40.10 | 20.05 | 41.43 | 27.50 |
| Cr | (mg/dL) | 0.5–2.7 | 1.77 | 0.13 | 1.71 | 0.17 | 1.53 | 0.09 | 1.54 | 0.18 |
| LDH | (U/L) | 59–389 | 114.22 | 26.25 | 120.63 | 50.52 | 99.70 | 34.01 | 150.00 | 80.15 |
| TP | (g/L) | 60–83 | 59.50 | 4.37 | 56.69 | 4.02 | 60.42 | 1.67 | 59.15 | 2.91 |
| Urea | (mg/dL) | 26–84 | 51.00 | 6.58 | 44.69 | 3.94 | 47.88 | 5.65 | 43.93 | 5.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Major, R.; Gawlikowski, M.; Surmiak, M.; Janiczak, K.; Więcek, J.; Kurtyka, P.; Schwentenwein, M.; Jasek-Gajda, E.; Kopernik, M.; Lackner, J.M. In Vitro and In Vivo Testing of Stereolithography (SLA)-Manufactured Haemocompatible Photopolymers for Blood Pump. Appl. Sci. 2024, 14, 383. https://doi.org/10.3390/app14010383
Major R, Gawlikowski M, Surmiak M, Janiczak K, Więcek J, Kurtyka P, Schwentenwein M, Jasek-Gajda E, Kopernik M, Lackner JM. In Vitro and In Vivo Testing of Stereolithography (SLA)-Manufactured Haemocompatible Photopolymers for Blood Pump. Applied Sciences. 2024; 14(1):383. https://doi.org/10.3390/app14010383
Chicago/Turabian StyleMajor, Roman, Maciej Gawlikowski, Marcin Surmiak, Karolina Janiczak, Justyna Więcek, Przemysław Kurtyka, Martin Schwentenwein, Ewa Jasek-Gajda, Magdalena Kopernik, and Juergen M. Lackner. 2024. "In Vitro and In Vivo Testing of Stereolithography (SLA)-Manufactured Haemocompatible Photopolymers for Blood Pump" Applied Sciences 14, no. 1: 383. https://doi.org/10.3390/app14010383
APA StyleMajor, R., Gawlikowski, M., Surmiak, M., Janiczak, K., Więcek, J., Kurtyka, P., Schwentenwein, M., Jasek-Gajda, E., Kopernik, M., & Lackner, J. M. (2024). In Vitro and In Vivo Testing of Stereolithography (SLA)-Manufactured Haemocompatible Photopolymers for Blood Pump. Applied Sciences, 14(1), 383. https://doi.org/10.3390/app14010383