Abstract
The sit-to-stand (STS) movement is important in improving satisfaction after total knee arthroplasty (TKA). Reports on motion analysis using a combination of motion capture systems, force plates, and surface electromyography after TKA are limited. We aimed to compare the STS movement of patients aged over 60 who underwent modern and conventional TKA with more than 6 months of postoperative follow-up. Ten patients underwent surgery with a modern implant (Group I: Smith and Nephew JOURNEY II, Memphis, TN, USA), and ten with a conventional implant (Group II: Smith and Nephew LEGION, Memphis, TN, USA). STS movement kinematics and kinetic data were measured by synchronising a motion capture system with a force plate. Surface electromyography was used to measure the muscle activity. STS time was shorter in Group I than in Group II. Maximum knee-extension angular velocity and maximum knee-extension moment were greater in Group I than in Group II. Electromyography revealed that Group I tended to have less activity in the quadriceps femoris than Group II. Group II had a greater hip-extension moment and vertical ground reaction force, and the hip joint seemed to compensate for knee function. Group I possibly used the quadriceps muscle more effectively, due to the implant shape.
1. Introduction
Total knee arthroplasty (TKA) is recommended for patients with advanced to end-stage osteoarthritis (OA) who have decreased activities of daily living (ADL). In Japan, it is estimated that the proportion of patients with knee OA is increasing year by year; the number of people over the age of 40 with knee OA is 25.3 million, and the number of patients with knee OA with symptoms is 8 million [1]. Knee OA results in progressive wear and degeneration of joint cartilage, causing pain and impaired ADL. In addition, progressive injury and loss of articular cartilage cause deformation of the femur and tibia and contracture of the surrounding soft tissues, such as muscles and ligaments, resulting in limited range of motion and muscle weakness in the knee joint. Such pain, limited range of motion, and muscle weakness lead to ADL impairment [2,3]. If the knee OA is resistant to conservative treatments, surgical treatment is performed. There are several surgical treatments, and in cases of severe joint deformity or limited range of motion, TKA is the optimal surgical method. TKA is a surgical procedure that reduces pain and improves range of motion and stability by removing articular surfaces with cartilage damage and meniscal degeneration and correcting deformities. Generally, TKA has two designs: the Cruciate-Retaining type, which preserves the posterior cruciate ligament, and the Posterior-Stabilised type, which compensates for the posterior cruciate ligament. As the number of patients with knee OA increases, TKA also increases. Several favourable clinical results have been reported to date [4,5,6], and various designs and concepts of TKA have been improved. Various implants have been developed to help regain normal knee-joint function, including a unicompartmental arthroplasty and a bilateral cruciate ligament-sparing type that preserves both the posterior cruciate ligament (PCL) and anterior cruciate ligament (ACL). In recent years, implants have also been developed in which the anteroposterior stability of both cruciate ligaments is substituted by the shape of the implant. An insert with an asymmetrical, anatomical articular-surface shape is used, and the mediolateral and lateral thicknesses of the femoral implant are varied, to create a physiological articular-surface inclination in the coronal plane. This reproduces the longitudinal stability of the ACL and PCL with the characteristic insert that is not found in other models (Figure 1). Currently, it has been demonstrated that knee-joint kinematics evaluation using the 2D–3D registration method shows joint dynamics closer to those of a normal knee [7,8].
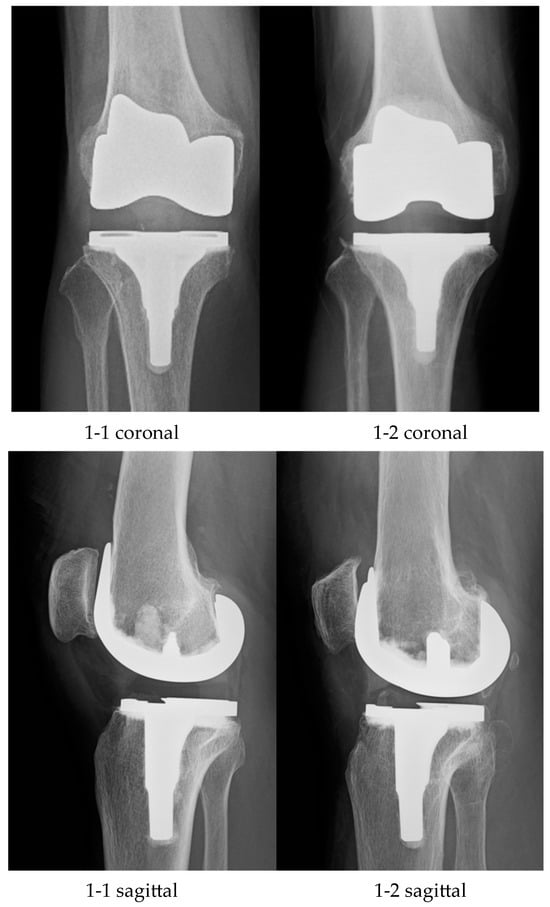
Figure 1.
1-1: Modern TKA; 1-2: Conventional TKA; TKA, total knee arthroplasty.
Patient satisfaction after TKA has been previously reported. In recent years, good long-term results have been reported, with survival rates of 94.2% at 25 years and 92.4% at 30 years, with reoperation, including revision surgery, as the end-point [6]. In addition, stable clinical results have been reported in clinical evaluations such as pain improvement effect, knee-joint range of motion, walking ability, and plain radiographic evaluation [5,9,10]. It has been suggested that, although the pain-improving effect is similar to that of total hip arthroplasty, TKA is less effective in improving joint function and quality of life [11]. In addition, the favourable postoperative clinical results that have been reported so far are the results of the physician’s evaluation, which is different from the patient’s own evaluation. Therefore, in recent years, in addition to objective evaluations by physicians, patient-based evaluations of pain, symptoms, and daily life have been developed, such as J-KOOS (Japanese Knee Injury and Osteoarthritis Outcome Score) [12] and WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) [13]. It is becoming increasingly important to evaluate knee joint function through patient-based evaluation, in which patients are asked to choose a graded evaluation based on questions regarding several items, such as daily activities. In post-TKA patient-based evaluations, there are reports of patient dissatisfaction regarding walking, standing, kneeling, and squatting [14,15]. Various factors have been reported to reduce satisfaction, including pain, limited range of motion, alignment on plain radiographs, and instability in soft tissue balance [16,17,18]. Abnormal kinematics is also thought to be a contributing factor [16,19]. In addition, there have been reports of poor outcomes that cannot be diagnosed with plain radiographic images alone [20] and of decreased quadriceps muscle strength being a factor in poor outcomes after TKA surgery [21].
Motion analysis of the knee joint has been studied using several methods: the 2D–3D registration method, using continuous fluoroscopic X-ray imaging [22,23], accelerometers [24], motion capture systems [25,26], model-based image-matching methods [21], navigation analysis [27], and many others. In vivo kinetic analysis after TKA surgery has mainly been evaluated using 2D–3D registration methods. The computer-aided design (CAD) model for implants has been superimposed from 2D to 3D on its 2D-projection image taken using X-ray fluoroscopic images, and the 3D relative movement between the femoral and tibia sides can be precisely measured. This allows for the six degrees of freedom of the knee joint to be flexed and extended and the varus and valgus to be internally and externally rotated, as well as the relative position of the femur and tibia and the state of contact between the femoral component and the insert. In addition to normal knees, kinematics evaluation of TKA patients’ knees is widely performed. Many studies have revealed that, after TKA surgery, patients exhibit joint movements different from those of normal knees, called paradoxical motions, which are abnormal forward movements of the femur during knee flexion. It has also been reported that the medial pivot motion, which is the rotational movement of the knee joint around the medial femoral condyle that is observed in normal knees, is attenuated following TKA [28,29]. While this 2D–3D registration method can evaluate precise kinematics of the knee joint, it has drawbacks, such as limitations in the imaging range, effects of X-ray exposure, and mechanical evaluation difficulty.
There are three main types of motion capture systems: optical, magnetic, and inertial sensors, but optical motion capture is the most commonly used. This is a dynamic analysis method that involves attaching infrared reflective markers to the body surface, photographing them with an optical camera, and analysing their trajectories. Although equipment is required for measurement, any movement can be evaluated within the measurement space, and kinematics evaluation is possible without invasiveness or X-ray exposure. Although it is widely known for its use in creating computer graphics for movies, it is often applied in the field of orthopaedics as a kinematic analysis method for sports movements and ADL [30,31]. A ground-reaction-force sensor is a device that can measure the ground reaction force that counteracts gravity from the soles of the feet. By synchronising the motion capture system and the ground-reaction-force plates, kinematics such as joint positions and joint-motion angles, angular velocities, and angular accelerations can be calculated, and joint moments and kinetics can be calculated from the relationship with the ground-reaction-force vector. Although this method is inferior to the 2D–3D registration method in terms of accuracy, it is useful in that there are no restrictions on movement, and, in addition to kinematics evaluation, it is possible to estimate mechanical loads on the body and joints. ADLs such as walking, standing up, and climbing stairs have been analysed for healthy subjects, patients with knee OA, and patients after TKA surgery. Although patients after TKA have improved walking and standing movements compared to those of patients with knee OA, their walking ability, such as walking speed, decreases compared to that of healthy individuals, and they exhibit abnormalities in knee-joint-extension moment and varus moment [32,33,34,35,36,37].
One motion analysis method is electromyography. An electromyogram records and displays the myoelectric potential that causes muscles to contract. Surface electromyography evaluates muscle activity by pasting electrodes on the skin, making it possible to estimate muscle tension and muscle fatigue in a minimally invasive manner. It allows for static evaluation and the examination of changes in biological functions during movement, and is used in fields such as sports, rehabilitation, and ergonomics. With the development of multi-channel and wearable measurement equipment, as well as advances in signal processing methods and analysis tools, evaluations are becoming easier and more precise. Previously, surface electromyography analysis of ADL, such as walking and standing up, was performed on patients after TKA surgery [38,39]. It has been reported that in patients after TKA, the quadriceps muscle becomes abnormally overactive during walking or standing up, and co-contraction with the hamstrings occurs [39,40].
Therefore, we focused on motion analysis using motion capture, force plates, and surface electromyography. Our institution previously conducted a motion analysis of patients who underwent ACL reconstruction surgery using our unique system, which synchronises surface electromyography with a motion capture system and force plates [41]. However, there are limited reports on motion analysis using a combination of motion capture systems, force plates, and surface electromyography in patients who have undergone TKA [42]. To further improve postoperative function after TKA, a modern implant with a shape similar to a biological knee has been developed. Although kinematic analyses of modern implants using 2D–3D registration have been performed [7,8], the kinetics and muscle activity remain unclear. Therefore, we aimed to compare the kinematics, kinetics, and muscle activity of patients who underwent TKA during sit-to-stand (STS) movement using a modern implant that mimics the normal knee function and a conventional implant, by analysing data from a motion capture system synchronised with force plates and surface electromyography.
We hypothesised that patients who underwent TKA using modern implants would show kinematics, kinetics, and muscle activity similar to those of healthy individuals.
2. Materials and Methods
Twenty patients participated in this study and were divided into two groups. One group had 10 patients who had undergone surgery using a modern implant (Journey II; Smith and Nephew, Memphis, TN, USA) that mimicked a normal knee function (Group I) (Figure 1(1-1)), and the other group had 10 patients who had undergone surgery using a conventional implant (Legion; Smith and Nephew, Memphis, TN, USA) (Group II) (Figure 1(1-2)). All patients were aged 60 years or older, had undergone TKA surgery at our hospital or a related facility after 2016, and had more than 6 months of postoperative experience. The implant selection criteria were not specifically set, but were determined according to the surgeon’s intentions. Patients with a history of lower limb surgery or fracture, neuromuscular disorders, collagen diseases, skin disorders preventing marker attachment, inability to perform STS movement, or severe knee-joint range-of-motion restriction were excluded. Table 1 lists the patients’ demographic data. This study was approved by the Ethics Committee of the University of Tsukuba Hospital (approval number: H29-243). Informed consent was obtained from each participant, prior to the motion analysis.

Table 1.
Patients’ demographic data.
2.1. Surgery and Postoperative Rehabilitation
All TKA surgeries were performed by the same surgeon or first assistant (A.K.). A midline longitudinal skin incision was made, and the medial parapatellar approach was used. The distal femoral cut was set at a 6° valgus angle with an intramedullary alignment rod, and the tibia was cut 90° from the mechanical axis, using an intramedullary rod. The ACL was removed in all cases, and the PCL was removed in highly degenerative cases. The flexion and extension gaps were determined using the measured resection technique. In all cases, the knee could bend from 0° of extension to 110° of flexion during surgery. Depending on the degree of cartilage damage and deformation, the patella was either resected or replaced. In all cases, patellar tracking was good, and lateral release was not required. Rehabilitation was initiated immediately after surgery using the same protocol, including range-of-motion training and full-weight-bearing walking training, with pain control.
The STS movement was analysed by synchronising a three-dimensional motion analysis device (Vicon MX; Vicon Motion System Inc., Oxford, UK), a floor force plate (AccuGait; AMTI Inc., Watertown, NY, USA), and wireless surface electromyography (Trigno Lab; Delsys, Inc., Boston, MA, USA). The STS movement was performed by adjusting the height of the chair such that the knee joint was flexed to 90° and aligning the rotation of the knee and ankle joints to ground, with both feet on the floor force plate. Both hands were fixed in a position adjacent to the iliac crest, and the patients performed three STS movements at their comfortable speed (Figure 2).
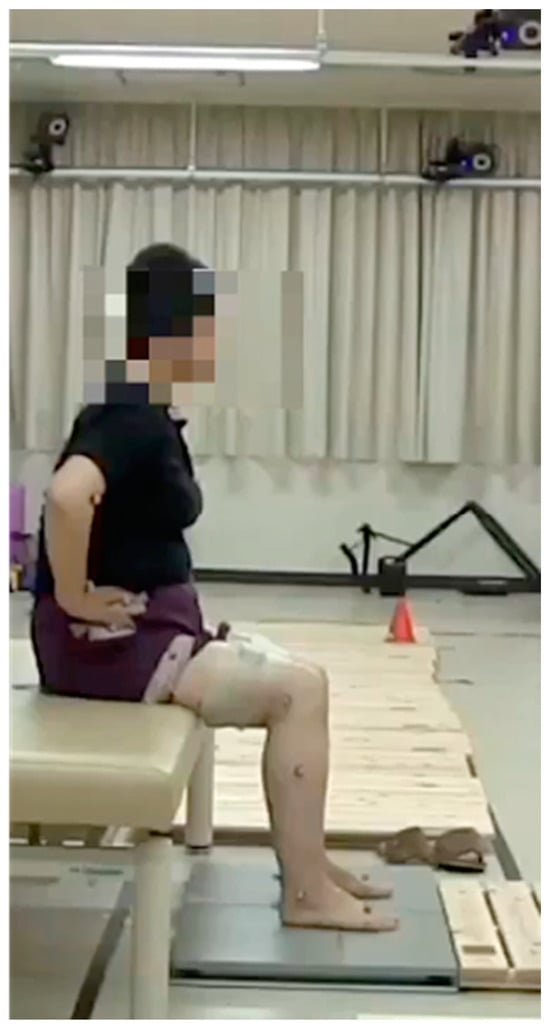
Figure 2.
The sit-to-stand movement setup.
Sixteen infrared cameras (MX-T20; Vicon Motion System Inc., Oxford, UK) were used, and the sampling frequency was set at 100 Hz. Fourteen-millimetre infrared reflection markers were affixed at 40 points, in accordance with the Plug-in Gait model [25]. The force plate measured the ground reaction force at a sampling frequency of 1000 Hz when in contact with the ground. Surface electromyography was used to measure the muscle activity at six locations on each lower limb (vastus medialis, rectus femoris, vastus lateralis, semitendinosus, biceps femoris, and gluteus medius) during the STS movement, at a sampling frequency of 2000 Hz. Electrode placement for the surface electromyography was performed as previously described [43]. Before measuring the motion, the maximum voluntary isometric contraction (MVC) during knee flexion, extension, and hip abduction was measured.
2.2. Evaluation Items and Statistical Analysis
We set the standing-up interval from the point at which the movement started and the hip joint began to flex, to the point at which the hip joint finished extending. We measured the STS time, knee-extension angular velocity, and joint angles in the sagittal plane of the hip, knee, and ankle, as kinematics. The moments of the knee and hip joints and the vertical ground reaction force (vGRF) were calculated as kinetics. Additionally, we measured the integrated electromyography of each muscle during the STS interval, using surface electromyography. We calculated the mean of the three STS movements for each item, compared them between Groups I and II, and analysed them using an unpaired t-test. Statistical significance was set at p < 0.05. All statistical analyses were performed using SPSS Statistics (version 21.0; International Business Machines Co., New York, NY, USA).
3. Results
Group I consisted of ten patients with a mean age of 69.4 years. Group II comprised ten patients with a mean age of 70.3 years. No significant difference was observed between the two groups. The details of the two groups are presented in Table 1.
3.1. Kinematics
The STS time was 1.1 ± 0.3 s in Group I and 1.2 ± 0.2 s in Group II (p = 0.32). The maximum knee-extension angular velocity was 126.5 ± 31.7°/s in Group I and 116.4 ± 29.5°/s in Group II (p = 0.49). The maximum range of motion of the hip joint in the sagittal plane was 80.2 ± 11.1° in Group I and 81.7 ± 7.5° in Group II (p = 0.74), with no significant differences between Groups I and II (Table 2).

Table 2.
Comparison of kinematic data.
3.2. Kinetics
The maximum knee-extension moment was 0.37 ± 0.11 Nm/kg in Group I and 0.34 ± 0.12 Nm/kg in Group II (p = 0.59), and the maximum knee-varus moment was 0.03 ± 0.06 Nm/kg and 0.04 ± 0.03 Nm/kg, respectively (p = 0.54). The maximum hip-extension moment was 0.47 ± 0.18 Nm/kg in Group I and 0.67 ± 0.16 Nm/kg in Group II (p = 0.02). The mean vertical ground reaction force was 4.06 ± 0.5 N/kg and 4.68 ± 0.4 N/kg, respectively (p = 0.01) (Table 3).

Table 3.
Average peak moment and vGRF.
3.3. Surface Electromyography
The integrated electromyography during the standing phase revealed that the vastus medialis muscle activity in Group I was 56.8 ± 17.9 (%MVC), compared to 71.9 ± 26.0 in Group II (p = 0.17). The rectus femoris muscle activity was 27.2 ± 14.9 and 31.9 ± 9.8 in Groups I and II, respectively (p = 0.44). The vastus lateralis muscle activity was 70.0 ± 25.3 and 77.4 ± 21.8, respectively (p = 0.52). The semitendinosus muscle activity was 29.3 ± 18.0 and 24.2 ± 13.4 (p = 0.51), and the bicep femoris muscle activity was 39.0 ± 2 1.7 and 32.9 ± 13.0, respectively (p = 0.48) (Table 4).

Table 4.
Average maximum EMG value (% MVC) during the phase of standing.
4. Discussion
This study is the first to evaluate the postoperative in vivo kinematics, kinetics, and muscle activity of a modern implant that mimics normal knee function. The most important finding of this study was that patients with modern implants had smoother knee functions. This study revealed the following three points. First, regarding kinematics, Group I, using the modern implant, tended to show a shorter standing-up time and faster knee-extension angular velocity compared to those of Group II, who used the conventional implant. Second, regarding kinetics, Group I showed a lower hip-extension moment and vGRF, and a significant difference was observed for the two items. Third, Group I tended to exhibit lower activity in the quadriceps femoris muscle.
The STS movement is a repetitive action performed in daily life, and the quality of life decreases when this motion is impaired. Reports have shown that there is still room for improvement in rising and squatting motions in patients after TKA [14,15]. Various factors, such as pain, muscle strength, and original disease, affect the STS movement; however, knee extension muscle strength is known to be a vital factor [39].
Previous studies on patients with conventional TKA have shown a decrease in knee-extension range of motion and knee-extension angular velocity, compared with that of healthy individuals [44,46]. In the present study, there was no significant difference in the kinematic data between the two groups; however, in Group I, the STS time was shorter and the knee-extension angular velocity was higher, indicating the possibility that the STS movement was smoother in Group I.
Regarding the kinetic data, studies have shown that the maximum knee-extension moment decreases and hip-extension moment increases in patients with TKA compared to those in healthy individuals [45,47]. Furthermore, it was found that the vGRF increases in the non-operated limb compared to the operated limb. Although no difference was observed in the knee-joint-extension moment in this study, the hip-extension moment was greater in Group II than in Group I. It has been found that patients who underwent TKA compensate by extending the hip joint during STS [45]. The STS movement was achieved by effectively using the knee joint, as the vGRF was smaller in Group I (similar to that observed in healthy individuals) compared to that in Group II.
Studies have shown that the activity of the quadriceps muscle, which is involved in the knee extension mechanism, is lower on the operated side than on the non-operated side in patients with conventional TKA [45,47,48]. This is believed to result from STS without a load on the operating side. In contrast, a study comparing the muscle activity with different implant types showed that single-radius TKA, which uses a larger knee-extension lever arm, resulted in less quadricep muscle activity than the multi-radius type [49]. In Group I, there was a tendency for decreased quadricep muscle activity, which may be attributed to load issues similar to those reported in the study comparing different types of implants. This study demonstrated that Journey II reproduces the normal anteroposterior position in terms of implant shape compared to conventional implants, implying that the lever arm in the extension mechanism is enlarged and the extensor muscle is activated effectively [50].
Overall, considering the kinematics, kinetics, and muscle-activity results, it can be suggested that Group I patients were able to perform the STS movement more smoothly and effectively using the quadriceps muscles compared to those with conventional implants.
This study has several limitations. First, although there was no significant difference between the two groups, there is a possibility of selection bias, owing to the lack of uniformity in the preoperative patient’s condition and the status of the opposite knee. Second, the number of cases in this retrospective study was small, but the power of the evaluation item with a significant difference was more than 0.83 in the power analysis; thus, we consider the results valid. Third, the average postoperative measurement period was 14 months in Group I and 16 months in Group II. Evaluation is also necessary for mid- and long-term follow-ups.
By analysing and comparing the kinematics, kinetics, and muscle activity in patients with different models of TKA after surgery, the impact of the implant can be evaluated in more detail. Furthermore, this research analysis can be used as an objective evaluation method for other knee joint surgeries, such as unicompartmental knee arthroplasty (UKA) or osteotomy, to improve symptoms.
5. Conclusions
Motion analysis was performed on patients after TKA, using a motion capture system synchronised with a force plate and surface electromyography. In a comparison between a modern artificial knee joint that mimics normal knee function and a conventional artificial knee joint, the modern artificial knee joint allowed smooth STS movement in a patient. Patients with modern artificial joints can use the quadriceps muscle more effectively in performing STS movements, because of the implant shape.
Author Contributions
Investigation, K.H., A.K., H.K., M.K., K.O., N.K. and M.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the University of Tsukuba Hospital (protocol code H29-243 and date of approval 26 December 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yoshimura, N.; Muraki, S.; Oka, H.; Mabuchi, A.; En-Yo, Y.; Yoshida, M.; Saika, A.; Yoshida, H.; Suzuki, T.; Yamamoto, S.; et al. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: The research on osteoarthritis/osteoporosis against disability study. J. Bone Min. Metab. 2009, 27, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Gherghel, R.; Iordan, D.A.; Mocanu, M.D.; Onu, A.; Onu, I. Osteoarthritis is not a disease, but rather an accumulation of predisposing factors. A systematic review. Balneo PRM Res. J. 2021, 12, 218–226. [Google Scholar] [CrossRef]
- Onu, I.; Matei, D.; Sardaru, D.-P.; Cascaval, D.; Onu, A.; Gherghel, R.; Serban, I.L.; Mocanu, G.D.; Iordan, D.A.; Murariu, G.; et al. Rehabilitation of Patients with Moderate Knee Osteoarthritis Using Hyaluronic Acid Viscosupplementation and Physiotherapy. Appl. Sci. 2022, 12, 3165. [Google Scholar] [CrossRef]
- Diduch, D.R.; Insall, J.N.; Scott, W.N.; Scuderi, G.R.; Font-Rodriguez, D. Total knee replacement in young, active patients. Long-term follow-up and functional outcome. J. Bone Jt. Surg. Am. 1997, 79, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Tan, Y.; Deng, Y.; Chen, L. Posterior cruciate-retaining versus posterior stabilized total knee arthroplasty: A meta-analysis of randomized controlled trials. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Ritter, M.A.; Keating, E.M.; Sueyoshi, T.; Davis, K.E.; Barrington, J.W.; Emerson, R.H. Twenty-Five-Years and Greater, Results After Nonmodular Cemented Total Knee Arthroplasty. J. Arthroplast. 2016, 31, 2199–2202. [Google Scholar] [CrossRef] [PubMed]
- Grieco, T.F.; Sharma, A.; Dessinger, G.M.; Cates, H.E.; Komistek, R.D. In Vivo Kinematic Comparison of a Bicruciate Stabilized Total Knee Arthroplasty and the Normal Knee Using Fluoroscopy. J. Arthroplast. 2018, 33, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Hamai, S.; Okazaki, K.; Gondo, H.; Wang, Y.; Ikebe, S.; Higaki, H.; Shimoto, T.; Mizu-Uchi, H.; Akasaki, Y.; et al. Knee kinematics in bi-cruciate stabilized total knee arthroplasty during squatting and stair-climbing activities. J. Orthop. 2018, 15, 650–654. [Google Scholar] [CrossRef]
- Bercik, M.J.; Joshi, A.; Parvizi, J. Posterior cruciate-retaining versus posterior-stabilized total knee arthroplasty: A meta-analysis. J. Arthroplast. 2013, 28, 439–444. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, Y.; Kwon, O.R.; Kim, J.S. Functional outcome and range of motion of high-flexion posterior cruciate-retaining and high-flexion posterior cruciate-substituting total knee prostheses: A prospective, randomized study. J. Bone Jt. Surg. Am. 2009, 91, 753–760. [Google Scholar] [CrossRef]
- de Beer, J.; Petruccelli, D.; Adili, A.; Piccirillo, L.; Wismer, D.; Winemaker, M. Patient perspective survey of total hip vs total knee arthroplasty surgery. J. Arthroplast. 2012, 27, 865–869.e5. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Takeuchi, R.; Sawaguchi, T.; Ishikawa, H.; Saito, T.; Goldhahn, S. Cross-cultural adaptation and validation of the Japanese Knee Injury and Osteoarthritis Outcome Score (KOOS). J. Orthop. Sci. 2011, 16, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Hanyu, T.; Sledge, C.B.; Lingard, E.A. Validation of a Japanese patient-derived outcome scale for assessing total knee arthroplasty: Comparison with Western Ontario and McMaster Universities osteoarthritis index (WOMAC). J. Orthop. Sci. 2003, 8, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Noble, P.C.; Conditt, M.A.; Cook, K.F.; Mathis, K.B. The John Insall Award: Patient expectations affect satisfaction with total knee arthroplasty. Clin. Orthop. Relat. Res. 2006, 452, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, H.; Okazaki, K.; Mizu-Uchi, H.; Hamai, S.; Tashiro, Y.; Matsuda, S.; Iwamoto, Y. Correlations between patient satisfaction and ability to perform daily activities after total knee arthroplasty: Why aren’t patients satisfied? J. Orthop. Sci. 2015, 20, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Nishio, Y.; Onodera, T.; Kasahara, Y.; Takahashi, D.; Iwasaki, N.; Majima, T. Intraoperative medial pivot affects deep knee flexion angle and patient-reported outcomes after total knee arthroplasty. J. Arthroplast. 2014, 29, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Kawahara, S.; Okazaki, K.; Tashiro, Y.; Iwamoto, Y. Postoperative alignment and ROM affect patient satisfaction after TKA. Clin. Orthop. Relat. Res. 2013, 471, 127–133. [Google Scholar] [CrossRef]
- Azukizawa, M.; Kuriyama, S.; Nakamura, S.; Nishitani, K.; Lyman, S.; Morita, Y.; Furu, M.; Ito, H.; Matsuda, S. Intraoperative medial joint laxity in flexion decreases patient satisfaction after total knee arthroplasty. Arch. Orthop. Trauma. Surg. 2018, 138, 1143–1150. [Google Scholar] [CrossRef]
- Van Onsem, S.; Verstraete, M.; Van Eenoo, W.; Van Der Straeten, C.; Victor, J. Are TKA Kinematics During Closed Kinetic Chain Exercises Associated with Patient-reported Outcomes? A Preliminary Analysis. Clin. Orthop. Relat. Res. 2020, 478, 255–263. [Google Scholar] [CrossRef]
- Brander, V.A.; Stulberg, S.D.; Adams, A.D.; Harden, R.N.; Bruehl, S.; Stanos, S.P.; Houle, T. Predicting total knee replacement pain: A prospective, observational study. Clin. Orthop. Relat. Res. 2003, 416, 27–36. [Google Scholar] [CrossRef]
- Koga, H.; Nakamae, A.; Shima, Y.; Iwasa, J.; Myklebust, G.; Engebretsen, L.; Bahr, R.; Krosshaug, T. Mechanisms for noncontact anterior cruciate ligament injuries: Knee joint kinematics in 10 injury situations from female team handball and basketball. Am. J. Sports Med. 2010, 38, 2218–2225. [Google Scholar] [CrossRef] [PubMed]
- Komistek, R.D.; Mahfouz, M.R.; Bertin, K.C.; Rosenberg, A.; Kennedy, W. In vivo determination of total knee arthroplasty kinematics: A multicenter analysis of an asymmetrical posterior cruciate retaining total knee arthroplasty. J. Arthroplast. 2008, 23, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Hoff, W.A.; Komistek, R.D.; Dennis, D.A.; Gabriel, S.M.; Walker, S.A. Three-dimensional determination of femoral-tibial contact positions under in vivo conditions using fluoroscopy. Clin. Biomech. 1998, 13, 455–472. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Walker, P.S.; Zuckerman, J.D.; Slover, J.; Jaffe, F.; Karia, R.J.; Kim, J.H. The potential of accelerometers in the evaluation of stability of total knee arthroplasty. J. Arthroplast. 2013, 28, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Kadaba, M.P.; Ramakrishnan, H.K.; Wootten, M.E. Measurement of lower extremity kinematics during level walking. J. Orthop. Res. 1990, 8, 383–392. [Google Scholar] [CrossRef]
- Andriacchi, T.P.; Dyrby, C.O.; Johnson, T.S. The use of functional analysis in evaluating knee kinematics. Clin. Orthop. Relat. Res. 2003, 410, 44–53. [Google Scholar] [CrossRef]
- Hamada, D.; Wada, K.; Takasago, T.; Goto, T.; Nitta, A.; Higashino, K.; Fukui, Y.; Sairyo, K. Native rotational knee kinematics are lost in bicruciate-retaining total knee arthroplasty when the tibial component is replaced. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3249–3256. [Google Scholar] [CrossRef]
- Bellemans, J.; Banks, S.; Victor, J.; Vandenneucker, H.; Moemans, A. Fluoroscopic analysis of the kinematics of deep flexion in total knee arthroplasty: Influence of posterior condylar offset. J. Bone Jt. Surg. Br. 2002, 84, 50–53. [Google Scholar] [CrossRef]
- Dennis, D.A.; Komistek, R.D.; Mahfouz, M.R.; Haas, B.D.; Stiehl, J.B. Multicenter determination of in vivo kinematics after total knee arthroplasty. Clin. Orthop. Relat. Res. 2003, 416, 37–57. [Google Scholar] [CrossRef]
- Schache, A.G.; Blanch, P.D.; Rath, D.A.; Wrigley, T.V.; Starr, R.; Bennell, K.L. A comparison of overground and treadmill running for measuring the three-dimensional kinematics of the lumbo-pelvic-hip complex. Clin. Biomech. 2001, 16, 667–680. [Google Scholar] [CrossRef]
- Holden, J.P.; Selbie, W.S.; Stanhope, S.J. A proposed test to support the clinical movement analysis laboratory accreditation process. Gait Posture 2003, 17, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Zeni, J.A., Jr.; Higginson, J.S. Differences in gait parameters between healthy subjects and persons with moderate and severe knee osteoarthritis: A result of altered walking speed? Clin. Biomech. 2009, 24, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Kim, E.Y. Comparing self-selected speed walking of the elderly with self-selected slow, moderate, and fast speed walking of young adults. Ann. Rehabil. Med. 2014, 38, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Bolink, S.A.; Naisas, H.; Senden, R.; Essers, H.; Heyligers, I.C.; Meijer, K.; Grimm, B. Validity of an inertial measurement unit to assess pelvic orientation angles during gait, sit-stand transfers and step-up transfers: Comparison with an optoelectronic motion capture system. Med. Eng. Phys. 2016, 38, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Nagura, T.; Otani, T.; Suda, Y.; Matsumoto, H.; Toyama, Y. Is high flexion following total knee arthroplasty safe?: Evaluation of knee joint loads in the patients during maximal flexion. J. Arthroplast. 2005, 20, 647–651. [Google Scholar] [CrossRef] [PubMed]
- McClelland, J.A.; Webster, K.E.; Feller, J.A. Gait analysis of patients following total knee replacement: A systematic review. Knee 2007, 14, 253–263. [Google Scholar] [CrossRef]
- Naili, J.E.; Brostrom, E.W.; Gutierrez-Farewik, E.M.; Schwartz, M.H. The centre of mass trajectory is a sensitive and responsive measure of functional compensations in individuals with knee osteoarthritis performing the five times sit-to-stand test. Gait Posture 2018, 62, 140–145. [Google Scholar] [CrossRef]
- Hubley-Kozey, C.L.; Hatfield, G.L.; Astephen Wilson, J.L.; Dunbar, M.J. Alterations in neuromuscular patterns between pre and one-year post-total knee arthroplasty. Clin. Biomech. 2010, 25, 995–1002. [Google Scholar] [CrossRef]
- Davidson, B.S.; Judd, D.L.; Thomas, A.C.; Mizner, R.L.; Eckhoff, D.G.; Stevens-Lapsley, J.E. Muscle activation and coactivation during five-time-sit-to-stand movement in patients undergoing total knee arthroplasty. J. Electromyogr. Kinesiol. 2013, 23, 1485–1493. [Google Scholar] [CrossRef]
- Lester, D.K.; Shantharam, R.; Zhang, K. Dynamic electromyography after cruciate-retaining total knee arthroplasty revealed a threefold quadriceps demand compared with the contralateral normal knee. J. Arthroplast. 2013, 28, 557–562. [Google Scholar] [CrossRef]
- Kajiwara, M.; Kanamori, A.; Kadone, H.; Endo, Y.; Kobayashi, Y.; Hyodo, K.; Takahashi, T.; Arai, N.; Taniguchi, Y.; Yoshioka, T.; et al. Knee biomechanics changes under dual task during single-leg drop landing. J. Exp. Orthop. 2019, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Rainoldi, A.; Melchiorri, G.; Caruso, I. A method for positioning electrodes during surface EMG recordings in lower limb muscles. J. Neurosci. Methods 2004, 134, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Salvadore, G.; Meere, P.A.; Verstraete, M.A.; Victor, J.; Walker, P.S. Laxity and contact forces of total knee designed for anatomic motion: A cadaveric study. Knee 2018, 25, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, S.J.; Reisman, D.S.; Snyder-Mackler, L. Persistence of altered movement patterns during a sit-to-stand task 1 year following unilateral total knee arthroplasty. Phys. Ther. 2008, 88, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Mizner, R.L.; Snyder-Mackler, L. Altered loading during walking and sit-to-stand is affected by quadriceps weakness after total knee arthroplasty. J. Orthop. Res. 2005, 23, 1083–1090. [Google Scholar] [CrossRef]
- Boonstra, M.C.; Schwering, P.J.; De Waal Malefijt, M.C.; Verdonschot, N. Sit-to-stand movement as a performance-based measure for patients with total knee arthroplasty. Phys. Ther. 2010, 90, 149–156. [Google Scholar] [CrossRef]
- Farquhar, S.J.; Kaufman, K.R.; Snyder-Mackler, L. Sit-to-stand 3 months after unilateral total knee arthroplasty: Comparison of self-selected and constrained conditions. Gait Posture 2009, 30, 187–191. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Simpson, K.J.; Ferrara, M.S.; Chamnongkich, S.; Kinsey, T.; Mahoney, O.M. Biomechanical differences exhibited during sit-to-stand between total knee arthroplasty designs of varying radii. J. Arthroplast. 2006, 21, 1193–1199. [Google Scholar] [CrossRef]
- Evangelista, P.J.; Laster, S.K.; Lenz, N.M.; Sheth, N.P.; Schwarzkopf, R. A Computer Model of Mid-Flexion Instability in a Balanced Total Knee Arthroplasty. J. Arthroplast. 2018, 33, S265–S269. [Google Scholar] [CrossRef]
- Victor, J.; Bellemans, J. Physiologic kinematics as a concept for better flexion in TKA. Clin. Orthop. Relat. Res. 2006, 452, 53–58. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).