Personalized Plasma Medicine for Cancer: Transforming Treatment Strategies with Mathematical Modeling and Machine Learning Approaches
Abstract
Featured Application
Abstract
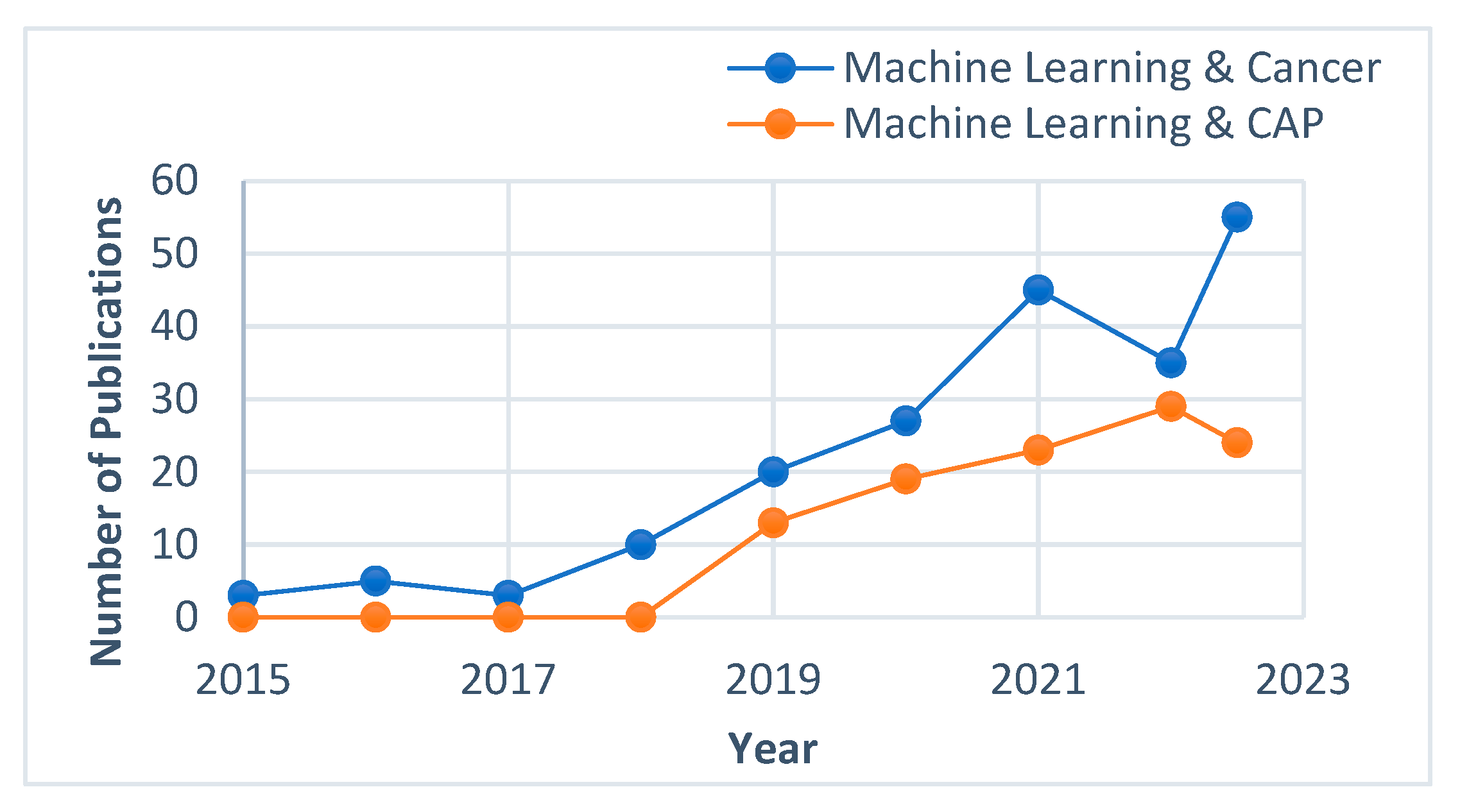
1. Introduction
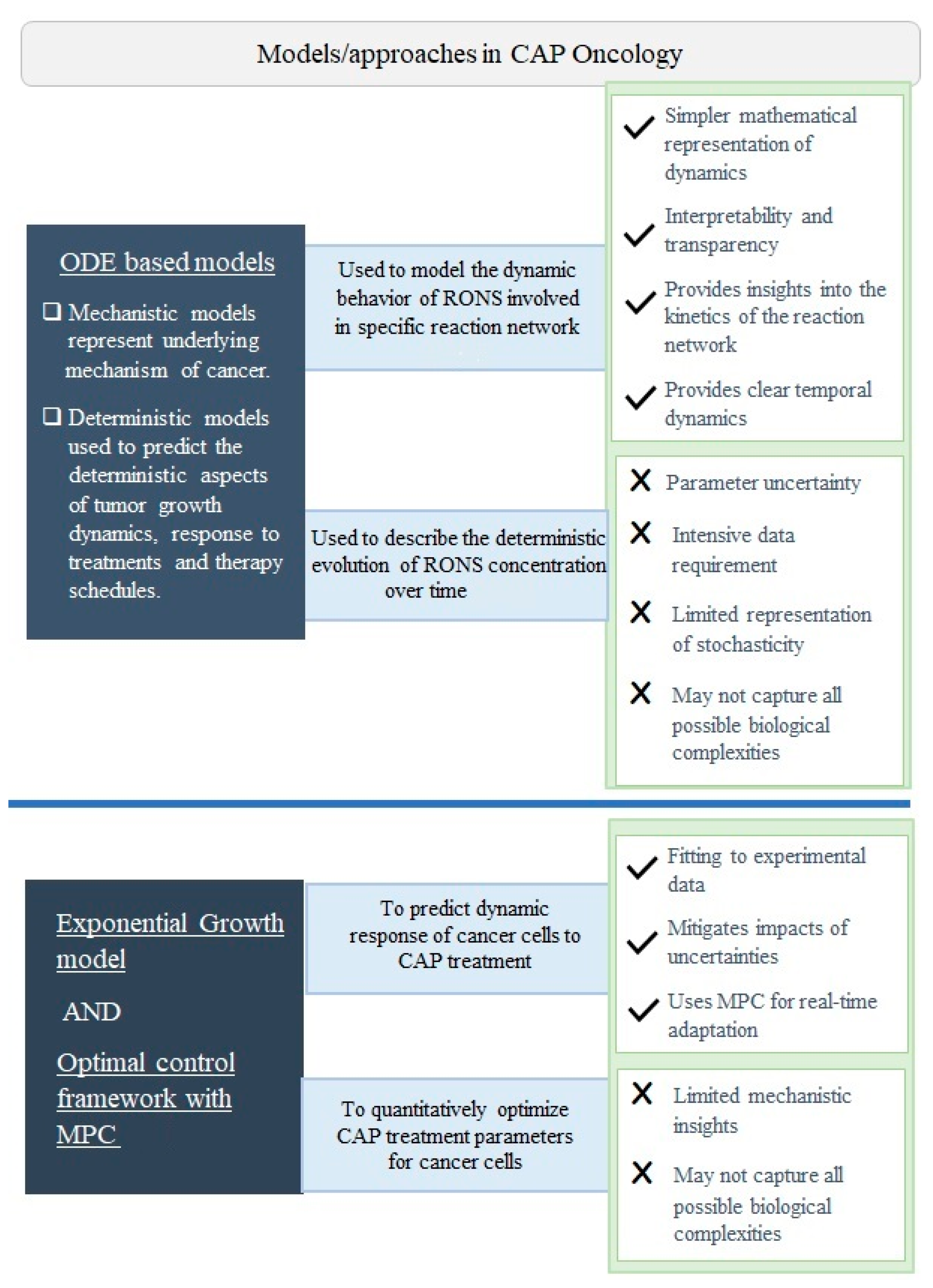
2. Mathematical Modeling
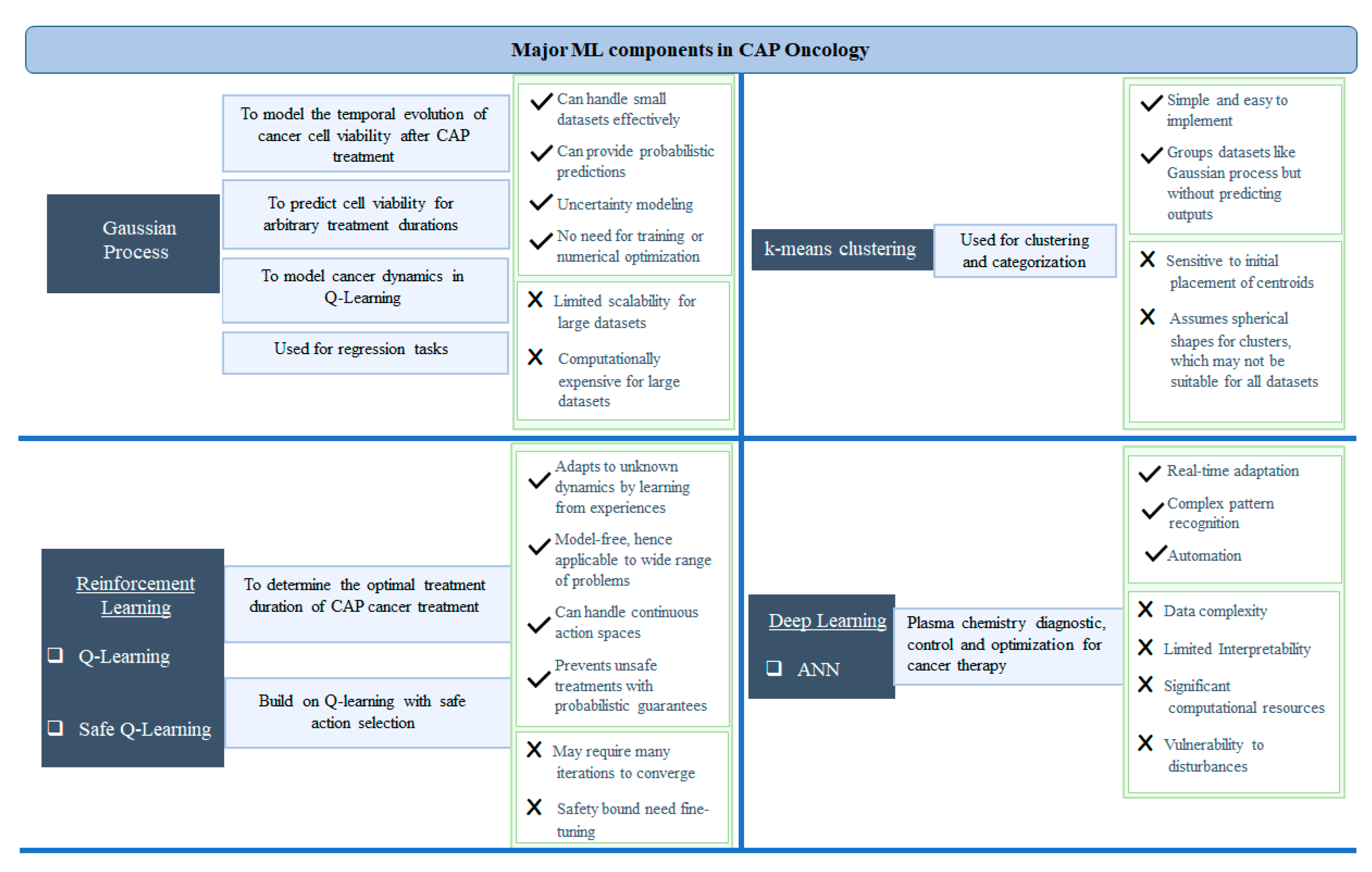
3. ML Techniques for Adaptive Plasma System
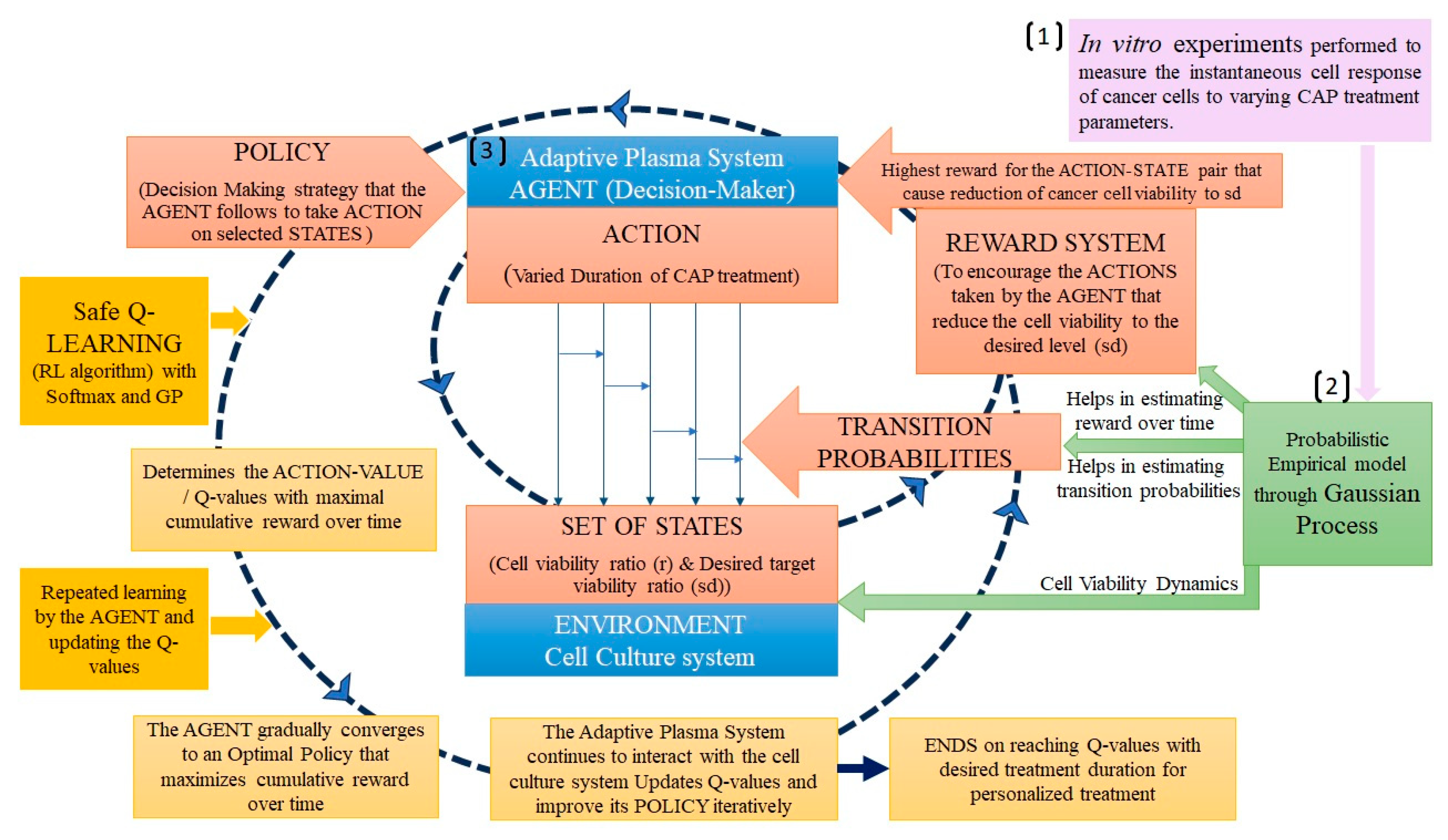
3.1. Reinforcement Learning
3.2. Gaussian Process Regression
3.3. Deep Learning
4. Advanced Algorithms in Real-Time Diagnostics
4.1. Real-Time Diagnosis of Operational Parameters of CAP Sources
4.2. Real-Time Diagnosis of the Cell Responses to CAP Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martines, E. (Ed.) Plasma Technology for Biomedical Applications; MDPI: Cham, Switzerland, 2020. [Google Scholar]
- Duarte, S.; Panariello, B.H.D. Comprehensive Biomedical Applications of Low Temperature Plasmas. Arch. Biochem. Biophys. 2020, 693, 108560. [Google Scholar] [CrossRef]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef] [PubMed]
- Domonkos, M.; Tichá, P.; Trejbal, J.; Demo, P. Applications of Cold Atmospheric Pressure Plasma Technology in Medicine, Agriculture and Food Industry. Appl. Sci. 2021, 11, 4809. [Google Scholar] [CrossRef]
- Boehm, D.; Canal, C. Application of Plasma Technology in Bioscience and Biomedicine. Appl. Sci. 2021, 11, 7203. [Google Scholar] [CrossRef]
- Laroussi, M.; Bekeschus, S.; Keidar, M.; Bogaerts, A.; Fridman, A.; Lu, X.; Ostrikov, K.; Hori, M.; Stapelmann, K.; Miller, V.; et al. Low-Temperature Plasma for Biology, Hygiene, and Medicine: Perspective and Roadmap. IEEE Trans. Radiat. Plasma Med. Sci. 2022, 6, 127–157. [Google Scholar] [CrossRef]
- Mumtaz, S.; Khan, R.; Rana, J.N.; Javed, R.; Iqbal, M.; Choi, E.H.; Han, I. Review on the Biomedical and Environmental Applications of Nonthermal Plasma. Catalysts 2023, 13, 685. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Bezbaruah, R.; Rynjah, D.; Newar, A.; Sengupta, S.; Pegu, P.; Dey, N.; Bora, S.C.; Barman, D. Cold Atmospheric Plasma: A Noteworthy Approach in Medical Science. Sci. Pharm. 2023, 2, 46–76. [Google Scholar] [CrossRef]
- Moszczyńska, J.; Roszek, K.; Wiśniewski, M. Non-Thermal Plasma Application in Medicine—Focus on Reactive Species Involvement. Int. J. Mol. Sci. 2023, 24, 12667. [Google Scholar] [CrossRef]
- Yan, D.; Wang, Q.; Adhikari, M.; Malyavko, A.; Lin, L.; Zolotukhin, D.B.; Yao, X.; Kirschner, M.; Sherman, J.H.; Keidar, M. A Physically Triggered Cell Death via Transbarrier Cold Atmospheric Plasma Cancer Treatment. ACS Appl. Mater. Interfaces 2020, 12, 34548–34563. [Google Scholar] [CrossRef]
- Tornin, J.; Labay, C.; Tampieri, F.; Ginebra, M.-P.; Canal, C. Evaluation of the Effects of Cold Atmospheric Plasma and Plasma-Treated Liquids in Cancer Cell Cultures. Nat. Protoc. 2021, 16, 2826–2850. [Google Scholar] [CrossRef]
- Suenaga, Y.; Takamatsu, T.; Aizawa, T.; Moriya, S.; Matsumura, Y.; Iwasawa, A.; Okino, A. Influence of Controlling Plasma Gas Species and Temperature on Reactive Species and Bactericidal Effect of the Plasma. Appl. Sci. 2021, 11, 11674. [Google Scholar] [CrossRef]
- Feibel, D.; Golda, J.; Held, J.; Awakowicz, P.; Schulz-von der Gathen, V.; Suschek, C.V.; Opländer, C.; Jansen, F. Gas Flow-Dependent Modification of Plasma Chemistry in ΜAPP Jet-Generated Cold Atmospheric Plasma and Its Impact on Human Skin Fibroblasts. Biomedicines 2023, 11, 1242. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Keidar, M. A Map of Control for Cold Atmospheric Plasma Jets: From Physical Mechanisms to Optimizations. Appl. Phys. Rev. 2021, 8, 011306. [Google Scholar] [CrossRef]
- Trelles, J.P. Pattern Formation and Self-Organization in Plasmas Interacting with Surfaces. J. Phys. D Appl. Phys. 2016, 49, 393002. [Google Scholar] [CrossRef]
- Keidar, M. Adaptive and Self-Adaptive Plasma Cancer Therapeutic Platform. U.S. Patent 11517366, 6 December 2022. [Google Scholar]
- Yan, D.; Cui, H.; Zhu, W.; Talbot, A.; Zhang, L.G.; Sherman, J.H.; Keidar, M. The Strong Cell-Based Hydrogen Peroxide Generation Triggered by Cold Atmospheric Plasma. Sci. Rep. 2017, 7, 10831. [Google Scholar] [CrossRef]
- Gjika, E.; Pal-Ghosh, S.; Tang, A.; Kirschner, M.; Tadvalkar, G.; Canady, J.; Stepp, M.A.; Keidar, M. Adaptation of Operational Parameters of Cold Atmospheric Plasma for in vitro Treatment of Cancer Cells. ACS Appl. Mater. Interfaces 2018, 10, 9269–9279. [Google Scholar] [CrossRef]
- Schweigert, I.; Zakrevsky, D.; Gugin, P.; Yelak, E.; Golubitskaya, E.; Troitskaya, O.; Koval, O. Interaction of Cold Atmospheric Argon and Helium Plasma Jets with Bio-Target with Grounded Substrate Beneath. Appl. Sci. 2019, 9, 4528. [Google Scholar] [CrossRef]
- Martinez, L.; Dhruv, A.; Balaras, E.; Keidar, M.; Martinez, L.; Dhruv, A.; Balaras, E.; Keidar, M. On Self Organization: Model for Ionization Wave Propagation with Targets of Varying Electrical Properties. Plasma Sources Sci. Technol. 2022, 31, 035004. [Google Scholar] [CrossRef]
- Keidar, M. Plasma for Cancer Treatment. Plasma Sources Sci. Technol. 2015, 24, 033001. [Google Scholar] [CrossRef]
- Keidar, M. (Ed.) Plasma Cancer Therapy, 1st ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Alzahrani, E.; El-Dessoky, M.M.; Khan, M.A. Mathematical Model to Understand the Dynamics of Cancer, Prevention Diagnosis and Therapy. Mathematics 2023, 11, 1975. [Google Scholar] [CrossRef]
- Oke, S.I.; Matadi, M.B.; Xulu, S.S. Optimal Control Analysis of a Mathematical Model for Breast Cancer. Math. Comput. Appl. 2018, 23, 21. [Google Scholar]
- Shen, J.; Li, L.; Yang, T.; Cohen, P.S.; Sun, G. Biphasic Mathematical Model of Cell--Drug Interaction That Separates Target-Specific and off-Target Inhibition and Suggests Potent Targeted Drug Combinations for Multi-Driver Colorectal Cancer Cells. Cancers 2020, 12, 436. [Google Scholar] [CrossRef] [PubMed]
- Hormuth, D.A.; Jarrett, A.M.; Davis, T.; Yankeelov, T.E. Towards an Image-Informed Mathematical Model of in Vivo Response to Fractionated Radiation Therapy. Cancers 2021, 13, 1765. [Google Scholar] [CrossRef]
- Beck, R.J.; Weigelin, B.; Beltman, J.B. Mathematical Modelling Based on in Vivo Imaging Suggests CD137-Stimulated Cytotoxic T Lymphocytes Exert Superior Tumour Control Due to an Enhanced Antimitotic Effect on Tumour Cells. Cancers 2021, 13, 2567. [Google Scholar] [CrossRef] [PubMed]
- Valle, P.A.; Coria, L.N.; Plata, C. Personalized Immunotherapy Treatment Strategies for a Dynamical System of Chronic Myelogenous Leukemia. Cancers 2021, 13, 2030. [Google Scholar] [CrossRef] [PubMed]
- Anaya, D.A.; Dogra, P.; Wang, Z.; Haider, M.; Ehab, J.; Jeong, D.K.; Ghayouri, M.; Lauwers, G.Y.; Thomas, K.; Kim, R.; et al. A Mathematical Model to Estimate Chemotherapy Concentration at the Tumor-Site and Predict Therapy Response in Colorectal Cancer Patients with Liver Metastases. Cancers 2021, 13, 444. [Google Scholar] [CrossRef]
- Yonekura, Y.; Toki, H.; Watabe, T.; Kaneda-Nakashima, K.; Shirakami, Y.; Ooe, K.; Toyoshima, A.; Nakajima, H.; Tomiyama, N.; Bando, M. Mathematical Model for Evaluation of Tumor Response in Targeted Radionuclide Therapy with 211At Using Implanted Mouse Tumor. Int. J. Mol. Sci. 2022, 23, 15966. [Google Scholar] [CrossRef]
- Ghaffari Laleh, N.; Loeffler, C.M.L.; Grajek, J.; Staňková, K.; Pearson, A.T.; Muti, H.S.; Trautwein, C.; Enderling, H.; Poleszczuk, J.; Kather, J.N. Classical Mathematical Models for Prediction of Response to Chemotherapy and Immunotherapy. PLoS Comput. Biol. 2022, 18, e1009822. [Google Scholar] [CrossRef]
- Italia, M.; Wertheim, K.Y.; Taschner-Mandl, S.; Walker, D.; Dercole, F. Mathematical Model of Clonal Evolution Proposes a Personalised Multi-Modal Therapy for High-Risk Neuroblastoma. Cancers 2023, 15, 1986. [Google Scholar] [CrossRef]
- Jarrett, A.M.; Bloom, M.J.; Godfrey, W.; Syed, A.K.; Ekrut, D.A.; Ehrlich, L.I.; Yankeelov, T.E.; Sorace, A.G. Mathematical Modelling of Trastuzumab-Induced Immune Response in an In Vivo Murine Model of HER2+ Breast Cancer. Math. Med. Biol. A J. IMA 2019, 36, 381–410. [Google Scholar] [CrossRef]
- Budithi, A.; Su, S.; Kirshtein, A.; Shahriyari, L. Data Driven Mathematical Model of FOLFIRI Treatment for Colon Cancer. Cancers 2021, 13, 2632. [Google Scholar] [CrossRef]
- Mohammad Mirzaei, N.; Su, S.; Sofia, D.; Hegarty, M.; Abdel-Rahman, M.H.; Asadpoure, A.; Cebulla, C.M.; Chang, Y.H.; Hao, W.; Jackson, P.R.; et al. A Mathematical Model of Breast Tumor Progression Based on Immune Infiltration. J. Pers. Med. 2021, 11, 1031. [Google Scholar] [CrossRef] [PubMed]
- Bekker, R.A.; Kim, S.; Pilon-Thomas, S.; Enderling, H. Mathematical Modeling of Radiotherapy and Its Impact on Tumor Interactions with the Immune System. Neoplasia 2022, 28, 100796. [Google Scholar] [CrossRef] [PubMed]
- Bitsouni, V.; Tsilidis, V. Mathematical Modeling of Tumor-Immune System Interactions: The Effect of Rituximab on Breast Cancer Immune Response. J. Theor. Biol. 2022, 539, 111001. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Liang, G.; Tian, T.; Zhang, X. Mathematical Modeling and Analysis of Tumor Chemotherapy. Symmetry 2022, 14, 704. [Google Scholar] [CrossRef]
- Jawad, S.; Winter, M.; Rahman, Z.-A.S.A.; Al-Yasir, Y.I.A.; Zeb, A. Dynamical Behavior of a Cancer Growth Model with Chemotherapy and Boosting of the Immune System. Mathematics 2023, 11, 406. [Google Scholar] [CrossRef]
- López-Alvarenga, J.C.; Minzoni-Alessio, A.; Olvera-Chávez, A.; Cruz-Pacheco, G.; Chimal-Eguia, J.C.; Hernández-Ruíz, J.; Álvarez-Blanco, M.A.; Bautista-Hernández, M.Y.; Quispe-Siccha, R.M. A Mathematical Model to Optimize the Neoadjuvant Chemotherapy Treatment Sequence for Triple-Negative Locally Advanced Breast Cancer. Mathematics 2023, 11, 2410. [Google Scholar] [CrossRef]
- León-Triana, O.; Pérez-Martínez, A.; Ramírez-Orellana, M.; Pérez-García, V.M. Dual-Target CAR-Ts with on-and off-Tumour Activity May Override Immune Suppression in Solid Cancers: A Mathematical Proof of Concept. Cancers 2021, 13, 703. [Google Scholar] [CrossRef]
- Sun, X.; Bao, J.; Shao, Y. Mathematical Modeling of Therapy-Induced Cancer Drug Resistance: Connecting Cancer Mechanisms to Population Survival Rates. Sci. Rep. 2016, 6, 22498. [Google Scholar] [CrossRef]
- Adhikarla, V.; Awuah, D.; Brummer, A.B.; Caserta, E.; Krishnan, A.; Pichiorri, F.; Minnix, M.; Shively, J.E.; Wong, J.Y.C.; Wang, X.; et al. A Mathematical Modeling Approach for Targeted Radionuclide and Chimeric Antigen Receptor t Cell Combination Therapy. Cancers 2021, 13, 5171. [Google Scholar] [CrossRef]
- Guzev, E.; Jadhav, S.S.; Hezkiy, E.E.; Sherman, M.Y.; Firer, M.A.; Bunimovich-Mendrazitsky, S. Validation of a Mathematical Model Describing the Dynamics of Chemotherapy for Chronic Lymphocytic Leukemia In Vivo. Cells 2022, 11, 2325. [Google Scholar] [CrossRef]
- Nave, O.P.; Sigron, M. A Mathematical Model for the Treatment of Melanoma with the BRAF/MEK Inhibitor and Anti-PD-1. Appl. Sci. 2022, 12, 12474. [Google Scholar] [CrossRef]
- Nave, O. A Mathematical Model for Treatment Using Chemo-Immunotherapy. Heliyon 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, F.; Yousef, A.; Bilgil, H.; Baleanu, D. A Mathematical Model with Piecewise Constant Arguments of Colorectal Cancer with Chemo-Immunotherapy. Chaos Solitons Fractals 2023, 168, 113207. [Google Scholar] [CrossRef]
- Salim, S.S.; Malinzi, J.; Mureithi, E.; Shaban, N. Mathematical Modelling of Chemovirotherapy Cancer Treatment. Int. J. Model. Simul. 2023, 1–22. [Google Scholar] [CrossRef]
- Kim, Y.; Choe, B.Y.; Suh, T.S.; Sung, W. A Mathematical Model for Predicting Patient Responses to Combined Radiotherapy with CTLA-4 Immune Checkpoint Inhibitors. Cells 2023, 12, 1305. [Google Scholar] [CrossRef] [PubMed]
- Slavkova, K.P.; Patel, S.H.; Cacini, Z.; Kazerouni, A.S.; Gardner, A.L.; Yankeelov, T.E.; Hormuth, D.A. Mathematical Modelling of the Dynamics of Image-Informed Tumor Habitats in a Murine Model of Glioma. Sci. Rep. 2023, 13, 2916. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Thirumalai, D. A Mathematical Model for Phenotypic Heterogeneity in Breast Cancer with Implications for Therapeutic Strategies. J. R. Soc. Interface 2022, 19, 20210803. [Google Scholar] [CrossRef] [PubMed]
- Veith, T.; Schultz, A.; Alahmari, S.; Beck, R.; Johnson, J.; Andor, N. Mathematical Modeling of Clonal Interference by Density-Dependent Selection in Heterogeneous Cancer Cell Lines. Cells 2023, 12, 1849. [Google Scholar] [CrossRef]
- Khailov, E.; Grigorieva, E. Optimal Melanoma Treatment Protocols for a Bilinear Control Model. Mathematics 2023, 11, 3289. [Google Scholar] [CrossRef]
- Le, T.; Su, S.; Shahriyari, L. Investigating Optimal Chemotherapy Options for Osteosarcoma Patients through a Mathematical Model. Cells 2021, 10, 2009. [Google Scholar] [CrossRef]
- Phan, T.; Bennett, J.; Patten, T. Practical Understanding of Cancer Model Identifiability in Clinical Applications. Life 2023, 13, 410. [Google Scholar] [CrossRef]
- Vieira, L.C.; Costa, R.S.; Valério, D. An Overview of Mathematical Modelling in Cancer Research: Fractional Calculus as Modelling Tool. Fractal Fract. 2023, 7, 595. [Google Scholar] [CrossRef]
- Uçar, E.; Özdemir, N. New Fractional Cancer Mathematical Model via IL-10 Cytokine and Anti-PD-L1 Inhibitor. Fractal Fract. 2023, 7, 151. [Google Scholar] [CrossRef]
- Farman, M.; Batool, M.; Nisar, K.S.; Ghaffari, A.S.; Ahmad, A. Controllability and Analysis of Sustainable Approach for Cancer Treatment with Chemotherapy by Using the Fractional Operator. Results Phys. 2023, 51, 106630. [Google Scholar] [CrossRef]
- Alinei-Poiana, T.; Dulf, E.-H.; Kovacs, L. Fractional Calculus in Mathematical Oncology. Sci. Rep. 2023, 13, 10083. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Boulaaras, S.; Jawad, M.; Rajagopal, K. Effect of Virotherapy Treatment on the Dynamics of Tumor Growth through Fractional Calculus. Trans. Inst. Meas. Control 2023, 45, 2981–2996. [Google Scholar] [CrossRef]
- Padder, A.; Almutairi, L.; Qureshi, S.; Soomro, A.; Afroz, A.; Hincal, E.; Tassaddiq, A. Dynamical Analysis of Generalized Tumor Model with Caputo Fractional-Order Derivative. Fractal Fract. 2023, 7, 258. [Google Scholar] [CrossRef]
- Idrees, M.; Alnahdi, A.S.; Jeelani, M.B. Mathematical Modeling of Breast Cancer Based on the Caputo—Fabrizio Fractal-Fractional Derivative. Fractal Fract. 2023, 7, 805. [Google Scholar] [CrossRef]
- Elharrar, X.; Barbolosi, D.; Ciccolini, J.; Meille, C.; Faivre, C.; Lacarelle, B.; André, N.; Barlesi, F. A Phase Ia/Ib Clinical Trial of Metronomic Chemotherapy Based on a Mathematical Model of Oral Vinorelbine in Metastatic Non-Small Cell Lung Cancer and Malignant Pleural Mesothelioma: Rationale and Study Protocol. BMC Cancer 2016, 16, 278. [Google Scholar] [CrossRef]
- Smalley, I.; Kim, E.; Li, J.; Spence, P.; Wyatt, C.J.; Eroglu, Z.; Sondak, V.K.; Messina, J.L.; Babacan, N.A.; Maria-Engler, S.S.; et al. Leveraging Transcriptional Dynamics to Improve BRAF Inhibitor Responses in Melanoma. EBioMedicine 2019, 48, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, N.; Jullion, A.; Ferretti, S.; Fabre, C.; Meille, C. Translational Modeling of Anticancer Efficacy to Predict Clinical Outcomes in a First-in-Human Phase 1 Study of MDM2 Inhibitor HDM201. AAPS J. 2021, 23, 28. [Google Scholar] [CrossRef] [PubMed]
- Brüningk, S.C.; Peacock, J.; Whelan, C.J.; Brady-Nicholls, R.; Yu, H.H.M.; Sahebjam, S.; Enderling, H. Intermittent Radiotherapy as Alternative Treatment for Recurrent High Grade Glioma: A Modeling Study Based on Longitudinal Tumor Measurements. Sci. Rep. 2021, 11, 20219. [Google Scholar] [CrossRef] [PubMed]
- Mathur, D.; Barnett, E.; Scher, H.I.; Xavier, J.B. Optimizing the Future: How Mathematical Models Inform Treatment Schedules for Cancer. Trends Cancer 2022, 8, 506. [Google Scholar] [CrossRef]
- Leder, K.; Pitter, K.; Laplant, Q.; Hambardzumyan, D.; Ross, B.D.; Chan, T.A.; Holland, E.C.; Michor, F. Mathematical Modeling of Pdgf-Driven Glioblastoma Reveals Optimized Radiation Dosing Schedules. Cell 2014, 156, 603–616. [Google Scholar] [CrossRef]
- Dean, J.A.; Tanguturi, S.K.; Cagney, D.; Shin, K.Y.; Youssef, G.; Aizer, A.; Rahman, R.; Hammoudeh, L.; Reardon, D.; Lee, E.; et al. Phase I Study of a Novel Glioblastoma Radiation Therapy Schedule Exploiting Cell-State Plasticity. Neuro. Oncol. 2023, 25, 1100–1112. [Google Scholar] [CrossRef]
- Keidar, M.; Yan, D.; Beilis, I.I.; Trink, B.; Sherman, J.H. Plasmas for Treating Cancer: Opportunities for Adaptive and Self-Adaptive Approaches. Trends Biotechnol. 2018, 36, 586–593. [Google Scholar] [CrossRef]
- Lyu, Y.; Lin, L.; Gjika, E.; Lee, T.; Keidar, M. Mathematical Modeling and Control for Cancer Treatment with Cold Atmospheric Plasma Jet. J. Phys. D Appl. Phys. 2019, 51, 185202. [Google Scholar] [CrossRef]
- Tanaka, H.; Bekeschus, S.; Yan, D.; Hori, M.; Keidar, M.; Laroussi, M. Plasma-Treated Solutions (PTS) in Cancer Therapy. Cancers 2021, 13, 1737. [Google Scholar] [CrossRef]
- Tampieri, F.; Gorbanev, Y.; Sardella, E. Plasma-Treated Liquids in Medicine: Let’s Get Chemical. Plasma Process. Polym. 2023, 20, e2300077. [Google Scholar] [CrossRef]
- Stache, A.B.; Mihăilă, I.; Gerber, I.C.; Dragoș, L.M.; Mihai, C.T.; Ivanov, I.C.; Topală, I.; Gorgan, D.-L. Optimization of Indirect CAP Exposure as an Effective Osteosarcoma Cells Treatment with Cytotoxic Effects. Appl. Sci. 2023, 13, 7803. [Google Scholar] [CrossRef]
- Solé-Martí, X.; Vilella, T.; Labay, C.; Tampieri, F.; Ginebra, M.P.; Canal, C. Thermosensitive Hydrogels to Deliver Reactive Species Generated by Cold Atmospheric Plasma: A Case Study with Methylcellulose. Biomater. Sci. 2022, 10, 3845–3855. [Google Scholar] [CrossRef] [PubMed]
- Malyavko, A.; Yan, D.; Wang, Q.; Klein, A.L.; Patel, K.C.; Sherman, J.H.; Keidar, M. Cold Atmospheric Plasma Cancer Treatment, Direct versus Indirect Approaches. Mater. Adv. 2020, 1, 1494–1505. [Google Scholar] [CrossRef]
- Poramapijitwat, P.; Thana, P.; Sukum, P.; Liangdeng, Y.; Kuensaen, C.; Boonyawan, D. Selective Cytotoxicity of Lung Cancer Cells—A549 and H1299—Induced by Ringer’s Lactate Solution Activated by a Non-Thermal Air Plasma Jet Device, Nightingale®. Plasma Chem. Plasma Process. 2023, 43, 805–830. [Google Scholar] [CrossRef]
- Miebach, L.; Mohamed, H.; Wende, K.; Miller, V.; Bekeschus, S. Pancreatic Cancer Cells Undergo Immunogenic Cell Death upon Exposure to Gas Plasma-Oxidized Ringers Lactate. Cancers 2023, 15, 319. [Google Scholar] [CrossRef] [PubMed]
- Pavlik, T.; Gudkova, V.; Razvolyaeva, D.; Pavlova, M.; Kostukova, N.; Miloykovich, L.; Kolik, L.; Konchekov, E.; Shimanovskii, N. The Role of Autophagy and Apoptosis in the Combined Action of Plasma-Treated Saline, Doxorubicin, and Medroxyprogesterone Acetate on K562 Myeloid Leukaemia Cells. Int. J. Mol. Sci. 2023, 24, 5100. [Google Scholar] [CrossRef]
- Wang, X.; Liu, N.; Liu, J.; Cui, Y.; Wang, X.; Lu, J.; Kang, C.; Gao, L.; Shi, X.; Zhang, G. Comparison of Direct and Indirect Low-Temperature Plasma Triggering Immunogenic Cell Death in B16F10 Melanoma. Plasma Process. Polym. 2023, 20, e2200206. [Google Scholar] [CrossRef]
- Bengtson, C.; Bogaerts, A. On the Anti-Cancer Effect of Cold Atmospheric Plasma and the Possible Role of Catalase-Dependent Apoptotic Pathways. Cells 2020, 9, 2330. [Google Scholar] [CrossRef]
- Bengtson, C.; Bogaerts, A. The Quest to Quantify Selective and Synergistic Effects of Plasma for Cancer Treatment: Insights from Mathematical Modeling. Int. J. Mol. Sci. 2021, 22, 5033. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Bakhrebah, M.A.; AlSaihati, H.; Alhumaid, S.; Alsubki, R.A.; Turkistani, S.A.; Al-Abdulhadi, S.; Aldawood, Y.; Alsaleh, A.A.; Alhashem, Y.N.; et al. Artificial Intelligence for Clinical Diagnosis and Treatment of Prostate Cancer. Cancers 2022, 14, 5595. [Google Scholar] [CrossRef]
- Koteluk, O.; Wartecki, A.; Mazurek, S.; Kołodziejczak, I.; Mackiewicz, A. How Do Machines Learn? Artificial Intelligence as a New Era in Medicine. J. Pers. Med. 2021, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Ercan, U.K.; Özdemir, G.D.; Özdemir, M.A.; Güren, O. Plasma Medicine: The Era of Artificial Intelligence. Plasma Process. Polym. 2023, 20, e2300066. [Google Scholar] [CrossRef]
- Galuzio, P.P.; Cherif, A. Recent Advances and Future Perspectives in the Use of Machine Learning and Mathematical Models in Nephrology. Adv. Chronic Kidney Dis. 2022, 29, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shi, H.; Wang, H. Machine Learning and AI in Cancer Prognosis, Prediction, and Treatment Selection: A Critical Approach. J. Multidiscip. Healthc. 2023, 16, 1779. [Google Scholar] [CrossRef]
- Bonzanini, A.D.; Shao, K.; Graves, D.B.; Hamaguchi, S.; Mesbah, A. Foundations of Machine Learning for Low-Temperature Plasmas: Methods and Case Studies. Plasma Sources Sci. Technol. 2023, 32, 024003. [Google Scholar] [CrossRef]
- Kim, S.; Jung, S.; Park, Y.; Lee, J.; Park, J. Effective Liver Cancer Diagnosis Method Based on Machine Learning Algorithm. In Proceedings of the 2014 7th International Conference on Biomedical Engineering and Informatics, Dalian, China, 14–16 October 2014; pp. 714–718. [Google Scholar]
- Glučina, M.; Lorencin, A.; Anđelić, N.; Lorencin, I. Cervical Cancer Diagnostics Using Machine Learning Algorithms and Class Balancing Techniques. Appl. Sci. 2023, 13, 1061. [Google Scholar] [CrossRef]
- Gawade, S.; Bhansali, A.; Patil, K.; Shaikh, D. Application of the Convolutional Neural Networks and Supervised Deep-Learning Methods for Osteosarcoma Bone Cancer Detection. Healthc. Anal. 2023, 3, 100153. [Google Scholar] [CrossRef]
- Barata, C.; Rotemberg, V.; Codella, N.C.F.; Tschandl, P.; Rinner, C.; Akay, B.N.; Apalla, Z.; Argenziano, G.; Halpern, A.; Lallas, A.; et al. A Reinforcement Learning Model for AI-Based Decision Support in Skin Cancer. Nat. Med. 2023, 29, 1941–1946. [Google Scholar] [CrossRef]
- Afrash, M.R.; Mirbagheri, E.; Mashoufi, M.; Kazemi-Arpanahi, H. Optimizing Prognostic Factors of Five-Year Survival in Gastric Cancer Patients Using Feature Selection Techniques with Machine Learning Algorithms: A Comparative Study. BMC Med. Inform. Decis. Mak. 2023, 23, 54. [Google Scholar] [CrossRef]
- Pais, R.J.; Lopes, F.; Parreira, I.; Silva, M.; Silva, M.; Moutinho, M.G. Predicting Cancer Prognostics from Tumour Transcriptomics Using an Auto Machine Learning Approach. In Medical Sciences Forum; MDPI: Cham, Switzerland, 2023; Volume 22, p. 6. [Google Scholar]
- Bostanci, E.; Kocak, E.; Unal, M.; Guzel, M.S.; Acici, K.; Asuroglu, T. Machine Learning Analysis of RNA-Seq Data for Diagnostic and Prognostic Prediction of Colon Cancer. Sensors 2023, 23, 3080. [Google Scholar] [CrossRef]
- Zhang, K.; Ye, B.; Wu, L.; Ni, S.; Li, Y.; Wang, Q.; Zhang, P.; Wang, D. Machine Learning-Based Prediction of Survival Prognosis in Esophageal Squamous Cell Carcinoma. Sci. Rep. 2023, 13, 13532. [Google Scholar] [CrossRef]
- Wu, R.; Luo, J.; Wan, H.; Zhang, H.; Yuan, Y.; Hu, H.; Feng, J.; Wen, J.; Wang, Y.; Li, J.; et al. Evaluation of Machine Learning Algorithms for the Prognosis of Breast Cancer from the Surveillance, Epidemiology, and end Results Database. PLoS ONE 2023, 18, e0280340. [Google Scholar] [CrossRef] [PubMed]
- Botlagunta, M.; Botlagunta, M.D.; Myneni, M.B.; Lakshmi, D.; Nayyar, A.; Gullapalli, J.S.; Shah, M.A. Classification and Diagnostic Prediction of Breast Cancer Metastasis on Clinical Data Using Machine Learning Algorithms. Sci. Rep. 2023, 13, 485. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Liu, Z.; Liu, J.; Zong, Z.; Chen, Y.; Zhang, Z.; Li, H. Application of Machine Learning Algorithm in Predicting Distant Metastasis of T1 Gastric Cancer. Sci. Rep. 2023, 13, 5741. [Google Scholar] [CrossRef] [PubMed]
- Mengash, H.A.; Alamgeer, M.; Maashi, M.; Othman, M.; Hamza, M.A.; Ibrahim, S.S.; Zamani, A.S.; Yaseen, I. Leveraging Marine Predators Algorithm with Deep Learning for Lung and Colon Cancer Diagnosis. Cancers 2023, 15, 1591. [Google Scholar] [CrossRef]
- Prelaj, A.; Boeri, M.; Robuschi, A.; Ferrara, R.; Proto, C.; Lo Russo, G.; Galli, G.; De Toma, A.; Brambilla, M.; Occhipinti, M.; et al. Machine Learning Using Real-World and Translational Data to Improve Treatment Selection for NSCLC Patients Treated with Immunotherapy. Cancers 2022, 14, 435. [Google Scholar] [CrossRef]
- Kong, J.; Lee, H.; Kim, D.; Han, S.K.; Ha, D.; Shin, K.; Kim, S. Network-Based Machine Learning in Colorectal and Bladder Organoid Models Predicts Anti-Cancer Drug Efficacy in Patients. Nat. Commun. 2020, 11, 5485. [Google Scholar] [CrossRef]
- Chen, S.; Shu, Z.; Li, Y.; Chen, B.; Tang, L.; Mo, W.; Shao, G.; Shao, F. Machine Learning-Based Radiomics Nomogram Using Magnetic Resonance Images for Prediction of Neoadjuvant Chemotherapy Efficacy in Breast Cancer Patients. Front. Oncol. 2020, 10, 1410. [Google Scholar] [CrossRef]
- Shao, Y.; Dang, Y.; Cheng, Y.; Gui, Y.; Chen, X.; Chen, T.; Zeng, Y.; Tan, L.; Zhang, J.; Xiao, M.; et al. Predicting the Efficacy of Neoadjuvant Chemotherapy for Pancreatic Cancer Using Deep Learning of Contrast-Enhanced Ultrasound Videos. Diagnostics 2023, 13, 2183. [Google Scholar] [CrossRef]
- Arezzo, F.; La Forgia, D.; Venerito, V.; Moschetta, M.; Tagliafico, A.S.; Lombardi, C.; Loizzi, V.; Cicinelli, E.; Cormio, G. A Machine Learning Tool to Predict the Response to Neoadjuvant Chemotherapy in Patients with Locally Advanced Cervical Cancer. Appl. Sci. 2021, 11, 823. [Google Scholar] [CrossRef]
- Johannet, P.; Coudray, N.; Donnelly, D.M.; Jour, G.; Illa-Bochaca, I.; Xia, Y.; Johnson, D.B.; Wheless, L.; Patrinely, J.R.; Nomikou, S.; et al. Using Machine Learning Algorithms to Predict Immunotherapy Response in Patients with Advanced Melanoma. Clin. Cancer Res. 2021, 27, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, R.; Basit, S.A.; Shamsi, J.A.; Fan, X.; Nawaz, M.; Yan, H.; Alam, T. Machine Learning Based Personalized Drug Response Prediction for Lung Cancer Patients. Sci. Rep. 2022, 12, 18935. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Ha, D.; Lee, J.; Kim, I.; Park, M.; Im, S.-H.; Shin, K.; Kim, S. Network-Based Machine Learning Approach to Predict Immunotherapy Response in Cancer Patients. Nat. Commun. 2022, 13, 3703. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Bo, Z.; Zhao, Z.; Yang, J.; Yang, Y.; Li, H.; Yang, Y.; Wang, J.; Su, Q.; Wang, J.; et al. Machine Learning to Predict the Response to Lenvatinib Combined with Transarterial Chemoembolization for Unresectable Hepatocellular Carcinoma. Cancers 2023, 15, 625. [Google Scholar] [CrossRef]
- Fujima, N.; Shimizu, Y.; Yoshida, D.; Kano, S.; Mizumachi, T.; Homma, A.; Yasuda, K.; Onimaru, R.; Sakai, O.; Kudo, K.; et al. Machine-Learning-Based Prediction of Treatment Outcomes Using MR Imaging-Derived Quantitative Tumor Information in Patients with Sinonasal Squamous Cell Carcinomas: A Preliminary Study. Cancers 2019, 11, 800. [Google Scholar] [CrossRef]
- Qiao, X.; Gu, X.; Liu, Y.; Shu, X.; Ai, G.; Qian, S.; Liu, L.; He, X.; Zhang, J. MRI Radiomics-Based Machine Learning Models for Ki67 Expression and Gleason Grade Group Prediction in Prostate Cancer. Cancers 2023, 15, 4536. [Google Scholar] [CrossRef]
- Abuhelwa, A.Y.; Kichenadasse, G.; McKinnon, R.A.; Rowland, A.; Hopkins, A.M.; Sorich, M.J. Machine Learning for Prediction of Survival Outcomes with Immune-Checkpoint Inhibitors in Urothelial Cancer. Cancers 2021, 13, 2001. [Google Scholar] [CrossRef]
- Li, Y.; Brendel, M.; Wu, N.; Ge, W.; Zhang, H.; Rietschel, P.; Quek, R.G.W.; Pouliot, J.-F.; Wang, F.; Harnett, J. Machine Learning Models for Identifying Predictors of Clinical Outcomes with First-Line Immune Checkpoint Inhibitor Therapy in Advanced Non-Small Cell Lung Cancer. Sci. Rep. 2022, 12, 17670. [Google Scholar] [CrossRef]
- Qu, J.; Li, C.; Liu, M.; Wang, Y.; Feng, Z.; Li, J.; Wang, W.; Wu, F.; Zhang, S.; Zhao, X. Prognostic Models Using Machine Learning Algorithms and Treatment Outcomes of Occult Breast Cancer Patients. J. Clin. Med. 2023, 12, 3097. [Google Scholar] [CrossRef]
- Savić, M.; Kurbalija, V.; Ilić, M.; Ivanović, M.; Jakovetić, D.; Valachis, A.; Autexier, S.; Rust, J.; Kosmidis, T. The Application of Machine Learning Techniques in Prediction of Quality-of-Life Features for Cancer Patients. Comput. Sci. Inf. Syst. 2023, 20, 381–404. [Google Scholar] [CrossRef]
- Liao, J.; Li, X.; Gan, Y.; Han, S.; Rong, P.; Wang, W.; Li, W.; Zhou, L. Artificial Intelligence Assists Precision Medicine in Cancer Treatment. Front. Oncol. 2023, 12, 998222. [Google Scholar] [CrossRef]
- Charalambous, A.; Dodlek, N. Big Data, Machine Learning, and Artificial Intelligence to Advance Cancer Care: Opportunities and Challenges. In Seminars in Oncology Nursing; WB Saunders: Philadelphia, PA, USA, 2023; Volume 39, p. 151429. [Google Scholar] [CrossRef]
- Hou, Z.; Lee, T.; Keidar, M. Reinforcement Learning with Safe Exploration for Adaptive Plasma Cancer Treatment. IEEE Trans. Radiat. Plasma Med. Sci. 2022, 6, 482–492. [Google Scholar] [CrossRef]
- Bonzanini, A.D.; Shao, K.; Stancampiano, A.; Graves, D.B.; Mesbah, A. Perspectives on Machine Learning-Assisted Plasma Medicine: Toward Automated Plasma Treatment. IEEE Trans. Radiat. Plasma Med. Sci. 2022, 6, 16–32. [Google Scholar] [CrossRef]
- Chan, K.J.; Makrygiorgos, G.; Mesbah, A. Towards Personalized Plasma Medicine via Data-Efficient Adaptation of Fast Deep Learning-Based MPC Policies. In Proceedings of the 2023 American Control Conference (ACC), San Diego, CA, USA, 31 May–2 June 2023; IEEE: New York, NY, USA, 2023; pp. 2769–2775. [Google Scholar] [CrossRef]
- Lin, L.; Yan, D.; Lee, T.; Keidar, M. Self-Adaptive Plasma Chemistry and Intelligent Plasma Medicine. Adv. Intell. Syst. 2022, 4, 2100112. [Google Scholar] [CrossRef]
- Littman, M.L.; Szepesvári, C. A Generalized Reinforcement-Learning Model: Convergence and Applications. In Proceedings of the 13th International Conference on Machine Learning (ICML 1996), Bari, Italy, 3–6 July 1996; Volume 96, pp. 310–318. [Google Scholar]
- Chen, C.L.; Dong, D.Y.; Li, H.X.; Tarn, T.J. Hybrid MDP Based Integrated Hierarchical Q-Learning. Sci. China Inf. Sci. 2011, 54, 2279–2294. [Google Scholar] [CrossRef][Green Version]
- Lin, L.; Hou, Z.; Yao, X.; Liu, Y.; Sirigiri, J.R.; Lee, T.; Keidar, M. Introducing Adaptive Cold Atmospheric Plasma: The Perspective of Adaptive Cold Plasma Cancer Treatments Based on Real-Time Electrochemical Impedance Spectroscopy. Phys. Plasmas 2020, 27, 063501. [Google Scholar] [CrossRef]
- Lin, L.; Keidar, M. Artificial Intelligence without Digital Computers: Programming Matter at a Molecular Scale. Adv. Intell. Syst. 2022, 4, 2200157. [Google Scholar] [CrossRef]
- Gidon, D.; Pei, X.; Bonzanini, A.D.; Graves, D.B.; Mesbah, A. Machine Learning for Real-Time Diagnostics of Cold Atmospheric Plasma Sources. IEEE Trans. Radiat. Plasma Med. Sci. 2019, 3, 597–605. [Google Scholar] [CrossRef]
- Mesbah, A.; Graves, D.B. Machine Learning for Modeling, Diagnostics, and Control of Non-Equilibrium Plasmas. J. Phys. D Appl. Phys. 2019, 52, 30LT02. [Google Scholar] [CrossRef]
- Zaplotnik, R.; Primc, G.; Vesel, A. Optical Emission Spectroscopy as a Diagnostic Tool for Characterization of Atmospheric Plasma Jets. Appl. Sci. 2021, 11, 2275. [Google Scholar] [CrossRef]
- Witman, M.; Gidon, D.; Graves, D.B.; Smit, B.; Mesbah, A. Sim-to-Real Transfer Reinforcement Learning for Control of Thermal Effects of an Atmospheric Pressure Plasma Jet. Plasma Sources Sci. Technol. 2019, 28, 095019. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Gao, S.H.; Ai, F. Efficient Numerical Simulation of Atmospheric Pulsed Discharges by Introducing Deep Learning. Front. Phys. 2023, 11, 50. [Google Scholar] [CrossRef]
- van Der Gaag, T.; Onishi, H.; Akatsuka, H. Arbitrary EEDF Determination of Atmospheric-Pressure Plasma by Applying Machine Learning to OES Measurement. Phys. Plasmas 2021, 28, 033511. [Google Scholar] [CrossRef]
- van Der Gaag, T.; Nezu, A.; Akatsuka, H. Practical Considerations of the Visible Bremsstrahlung Inversion (VBI) Method for Arbitrary EEDF Determination in Cold Atmospheric-Pressure Plasma. Jpn. J. Appl. Phys. 2022, 61, 076004. [Google Scholar] [CrossRef]
- van der Gaag, T.; Nezu, A.; Akatsuka, H. Partial EEDF Analysis and Electron Diagnostics of Atmospheric-Pressure Argon and Argon-Helium DBD Plasma. J. Phys. D Appl. Phys. 2023, 56, 304001. [Google Scholar] [CrossRef]
- Chang, J.; Niu, P.H.; Chen, C.W.; Cheng, Y.C. Using Deep Convolutional Neural Networks to Classify the Discharge Current of a Cold Atmospheric-Pressure Plasma Jet. IEEE Trans. Plasma Sci. 2023, 51, 311–319. [Google Scholar] [CrossRef]
- Lazarus, M.; Yan, D.; Limanowski, R.; Lin, L.; Keidar, M. Recognizing Cold Atmospheric Plasma Plume Using Computer Vision. Plasma 2022, 5, 341–350. [Google Scholar] [CrossRef]
- Lin, L.; Gershman, S.; Raitses, Y.; Keidar, M. Multi-Scale Plasma Chemistry Using Physics-Informed Neural Network. J. Phys. D Appl. Phys. 2023. in review. [Google Scholar]
- Kim, D.H.; Hong, S.J. Use of Plasma Information in Machine-Learning-Based Fault Detection and Classification for Advanced Equipment Control. IEEE Trans. Semicond. Manuf. 2021, 34, 408–419. [Google Scholar] [CrossRef]
- Sebastian, A.; Lipa, D.; Ptasinska, S. DNA Strand Breaks and Denaturation as Probes of Chemical Reactivity versus Thermal Effects of Atmospheric Pressure Plasma Jets. ACS Omega 2022, 8, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Sabrin, S.; Karmokar, D.K.; Karmakar, N.C.; Hong, S.H.; Habibullah, H.; Szili, E.J. Opportunities of Electronic and Optical Sensors in Autonomous Medical Plasma Technologies. ACS Sens. 2023, 8, 974–993. [Google Scholar] [CrossRef] [PubMed]
- Trieschmann, J.; Vialetto, L.; Gergs, T. Machine Learning for Advancing Low-Temperature Plasma Modeling and Simulation. arXiv 2023, arXiv:2307.00131. [Google Scholar]
- Dey, A.; Mitra, A.; Pathak, S.; Prasad, S.; Zhang, A.S.; Zhang, H.; Sun, X.F.; Banerjee, A. Recent Advancements, Limitations, and Future Perspectives of the Use of Personalized Medicine in Treatment of Colon Cancer. Technol. Cancer Res. Treat. 2023, 22, 15330338231178403. [Google Scholar] [CrossRef] [PubMed]
- Aggelopoulos, C.A.; Christodoulou, A.-M.; Tachliabouri, M.; Meropoulis, S.; Christopoulou, M.-E.; Karalis, T.T.; Chatzopoulos, A.; Skandalis, S.S. Cold Atmospheric Plasma Attenuates Breast Cancer Cell Growth through Regulation of Cell Microenvironment Effectors. Front. Oncol. 2022, 11, 826865. [Google Scholar] [CrossRef]
- Byun, J.; Wu, Y.; Lee, J.; Kim, J.S.; Shim, G.; Oh, Y.-K. External Cold Atmospheric Plasma-Responsive on-Site Hydrogel for Remodeling Tumor Immune Microenvironment. Biomaterials 2023, 299, 122162. [Google Scholar] [CrossRef]
- Dai, X.; Zhu, K. Cold Atmospheric Plasma: Novel Opportunities for Tumor Microenvironment Targeting. Cancer Med. 2023, 12, 7189–7206. [Google Scholar] [CrossRef]
- Patrakova, E.; Biryukov, M.; Troitskaya, O.; Gugin, P.; Milakhina, E.; Semenov, D.; Poletaeva, J.; Ryabchikova, E.; Novak, D.; Kryachkova, N.; et al. Chloroquine Enhances Death in Lung Adenocarcinoma A549 Cells Exposed to Cold Atmospheric Plasma Jet. Cells 2023, 12, 290. [Google Scholar] [CrossRef] [PubMed]
- Kniazeva, V.; Tzerkovsky, D.; Baysal, Ö.; Kornev, A.; Roslyakov, E.; Kostevitch, S. Adjuvant Composite Cold Atmospheric Plasma Therapy Increases Antitumoral Effect of Doxorubicin Hydrochloride. Front. Oncol. 2023, 13, 1171042. [Google Scholar] [CrossRef]
- Nitsch, A.; Qarqash, S.; Römer, S.; Schoon, J.; Ekkernkamp, A.; Niethard, M.; Reichert, J.C.; Wassilew, G.I.; Tzvetkov, M.V.; Haralambiev, L. Enhancing the Impact of Chemotherapy on Ewing Sarcoma Cells through Combination with Cold Physical Plasma. Int. J. Mol. Sci. 2023, 24, 8669. [Google Scholar] [CrossRef] [PubMed]
- Soni, V.; Adhikari, M.; Lin, L.; Sherman, J.H.; Keidar, M. Theranostic Potential of Adaptive Cold Atmospheric Plasma with Temozolomide to Checkmate Glioblastoma: An In Vitro Study. Cancers 2022, 14, 3116. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Cao, X.; Shen, B.; Chen, Z.; Chen, G. Injectable Cold Atmospheric Plasma-Activated Immunotherapeutic Hydrogel for Enhanced Cancer Treatment. Biomaterials 2023, 300, 122189. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, Z.; Wen, D.; Wang, Z.; Li, H.; Zeng, Y.; Dotti, G.; Wirz, R.E.; Gu, Z. Transdermal Cold Atmospheric Plasma-Mediated Immune Checkpoint Blockade Therapy. Proc. Natl. Acad. Sci. USA 2020, 117, 3687–3692. [Google Scholar] [CrossRef] [PubMed]
- Momeni, S.; Shanei, A.; Sazgarnia, A.; Attaran, N.; Aledavood, S.A. The Synergistic Effect of Cold Atmospheric Plasma Mediated Gold Nanoparticles Conjugated with Indocyanine Green as an Innovative Approach to Cooperation with Radiotherapy. Cell J. 2023, 25, 51. [Google Scholar]
- Kenari, A.J.; Siadati, S.N.; Abedian, Z.; Sohbatzadeh, F.; Amiri, M.; Gorji, K.E.; Babapour, H.; Zabihi, E.; Ghoreishi, S.M.; Mehraeen, R.; et al. Therapeutic Effect of Cold Atmospheric Plasma and Its Combination with Radiation as a Novel Approach on Inhibiting Cervical Cancer Cell Growth (HeLa Cells). Bioorg. Chem. 2021, 111, 104892. [Google Scholar] [CrossRef]
- Pansare, K.; Vaid, A.; Singh, S.R.; Rane, R.; Visani, A.; Ranjan, M.; Krishna, C.M.; Sarin, R.; Joseph, A. Effect of Cold Atmospheric Plasma Jet and Gamma Radiation Treatments on Gingivobuccal Squamous Cell Carcinoma and Breast Adenocarcinoma Cells. Plasma Chem. Plasma Process. 2021, 42, 163–178. [Google Scholar] [CrossRef]
- Pasqual-Melo, G.; Sagwal, S.K.; Freund, E.; Gandhirajan, R.K.; Frey, B.; von Woedtke, T.; Gaipl, U.; Bekeschus, S. Combination of Gas Plasma and Radiotherapy Has Immunostimulatory Potential and Additive Toxicity in Murine Melanoma Cells In Vitro. Int. J. Mol. Sci. 2020, 21, 1379. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, K.; Manaloto, E.; Casey, A.; Cribaro, G.P.; Byrne, H.J.; Tian, F.; Barcia, C.; Conway, G.E.; Cullen, P.J.; et al. Cold Atmospheric Plasma Induces ATP-Dependent Endocytosis of Nanoparticles and Synergistic U373MG Cancer Cell Death. Sci. Rep. 2018, 8, 5298. [Google Scholar] [CrossRef]
- Li, W.; Yu, H.; Ding, D.; Chen, Z.; Wang, Y.; Wang, S.; Li, X.; Keidar, M.; Zhang, W. Cold Atmospheric Plasma and Iron Oxide-Based Magnetic Nanoparticles for Synergetic Lung Cancer Therapy. Free Radic. Biol. Med. 2019, 130, 71–81. [Google Scholar] [CrossRef]
- Yazdani, Z.; Biparva, P.; Rafiei, A.; Kardan, M.; Hadavi, S. Combination Effect of Cold Atmospheric Plasma with Green Synthesized Zero-Valent Iron Nanoparticles in the Treatment of Melanoma Cancer Model. PLoS ONE 2022, 17, e0279120. [Google Scholar] [CrossRef]
- Qi, M.; Zhao, X.; Zhao, X.; Zhang, H.; Li, Z.; Zhang, X.; Fan, R.; Li, Q.; Zhang, J.; Xu, D. Violet Phosphorene Nanosheets and Cold Atmospheric Plasma for Synergetic Cancer Therapy. Chem. Eng. J. 2023, 475, 145884. [Google Scholar] [CrossRef]
- Fernandes, D.A.; Appak-Baskoy, S.; Berndl, E.; Kolios, M.C. Laser activatable perfluorocarbon bubbles for imaging and therapy through enhanced absorption from coupled silica coated gold nanoparticles. RSC Adv. 2021, 11, 4906–4920. [Google Scholar] [CrossRef] [PubMed]
- Canady, J.; Murthy, S.R.K.; Zhuang, T.; Gitelis, S.; Nissan, A.; Ly, L.; Jones, O.Z.; Cheng, X.; Adileh, M.; Blank, A.T.; et al. The First Cold Atmospheric Plasma Phase I Clinical Trial for the Treatment of Advanced Solid Tumors: A Novel Treatment Arm for Cancer. Cancers 2023, 15, 3688. [Google Scholar] [CrossRef] [PubMed]



| Selected Parameters of the CAP Sources for Real-Time Diagnostics | Input Data Obtained From | ML and Computational Techniques Employed | Reference |
|---|---|---|---|
| Rotational and vibrational temperatures | OES | Linear regression (supervised ML) | [127] |
| Substrate characteristics | OES | k-Means clustering (unsupervised ML) | [127] |
| Separation distance between the electrodes | Electro-acoustic Emission | Gaussian process regression (supervised probabilistic ML) | [127] |
| Electron energy distribution function (EEDF) | OES | Genetic algorithm (metaheuristic algorithm) | [132] |
| EEDF | OES, momentum-transfer cross-section | Visible Bremmsstrahlung inversion (supervised ML) | [133,134] |
| Time-series current signals from APPJ (discharge type and working gas) | Sensors/probes | Convolutional neural networks (DL) | [135] |
| Plasma plume length | Video frames of the plasma plume captured using a camera (iPhone 11) | Computer vision algorithms | [136] |
| Temperature setpoint | Simulated data from thermal dynamics model of plasma–substrate interactions | Reinforcement learning | [130] |
| Self-adaptive plasma chemistry Gas input densities and energy levels | OES | Artificial neural networks (DL), gradual mutation algorithm | [122] |
| Pulse discharge characteristics (current density and gap voltage) | Simulated fluid model data of time and pulse rise rate | Deep neural networks (DL) | [131] |
| Plasma chemistry (tokamak) | FTIR | Physics-informed neural networks | [137] |
| Input Data | Real-Time Diagnostics | Advanced Control and Prediction Methods | Reference |
|---|---|---|---|
| CAP treatment duration and discharge voltage applied | Cell viability luminescence Assay | Model Predictive Control (MPC) | [71] |
| Cancer cell viability ratio | Electrochemical impedance spectroscopy (EIS), operational parameters | GP regression, MPLC | [125] |
| Cancer cell viability ratio | EIS, cell viability assays, operational parameters | GP, safety Q—reinforcement learning | [119] |
| Voltage applied, irradiation time, frequency of the plasma, and flow rate of the feed gas on the extent of DNA damage | Agarose gel electrophoresis, UV fluorescence imaging | Artificial neural networks (supervised DL) Physics-guided neural network (supervised DL) | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramaswamy, V.D.; Keidar, M. Personalized Plasma Medicine for Cancer: Transforming Treatment Strategies with Mathematical Modeling and Machine Learning Approaches. Appl. Sci. 2024, 14, 355. https://doi.org/10.3390/app14010355
Ramaswamy VD, Keidar M. Personalized Plasma Medicine for Cancer: Transforming Treatment Strategies with Mathematical Modeling and Machine Learning Approaches. Applied Sciences. 2024; 14(1):355. https://doi.org/10.3390/app14010355
Chicago/Turabian StyleRamaswamy, Viswambari Devi, and Michael Keidar. 2024. "Personalized Plasma Medicine for Cancer: Transforming Treatment Strategies with Mathematical Modeling and Machine Learning Approaches" Applied Sciences 14, no. 1: 355. https://doi.org/10.3390/app14010355
APA StyleRamaswamy, V. D., & Keidar, M. (2024). Personalized Plasma Medicine for Cancer: Transforming Treatment Strategies with Mathematical Modeling and Machine Learning Approaches. Applied Sciences, 14(1), 355. https://doi.org/10.3390/app14010355








