Design and Development of a Flexible 3D-Printed Endoscopic Grasping Instrument
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrument Design
2.1.1. Grasping Forceps
2.1.2. Bending Section
2.1.3. Flexible Shaft
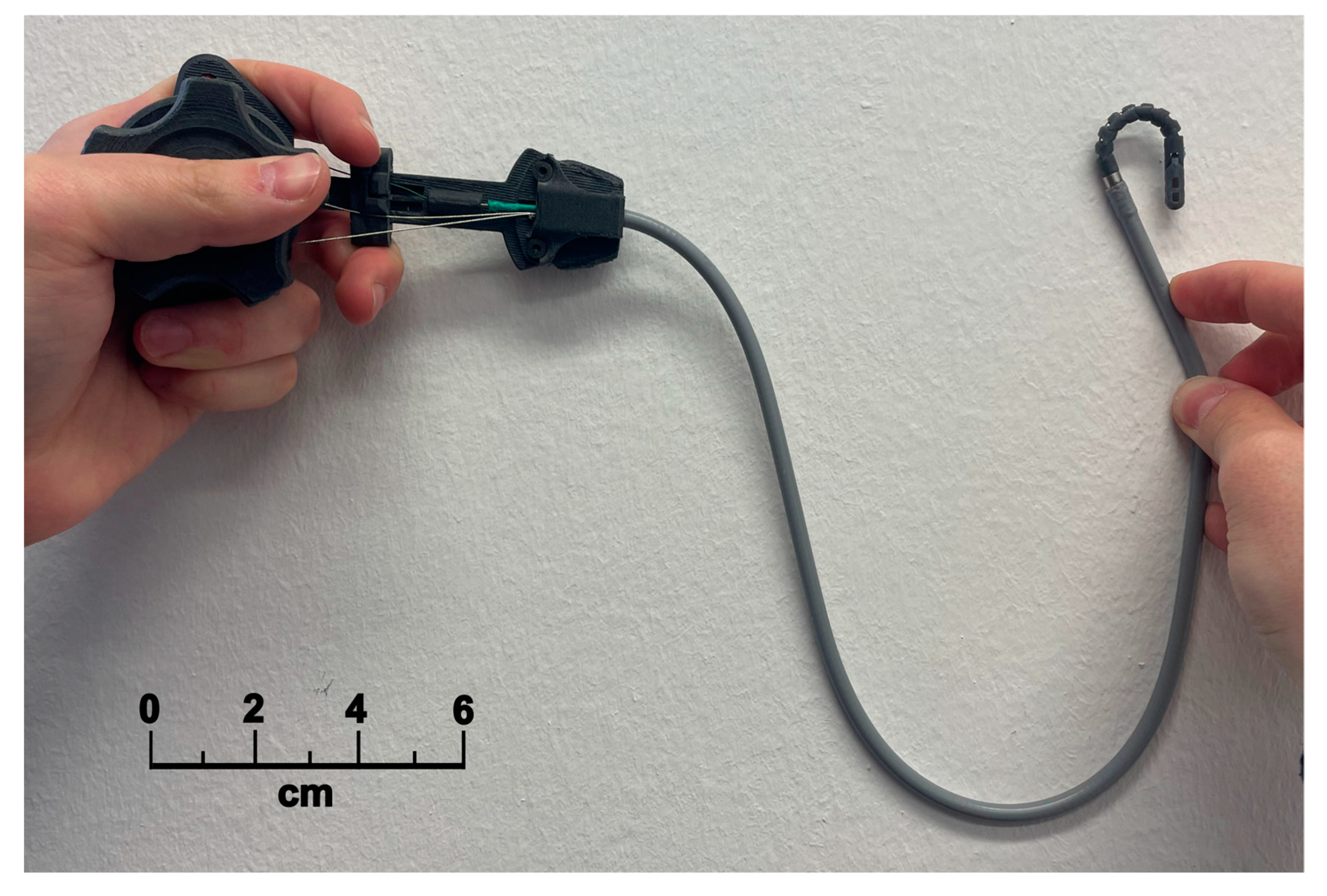
2.1.4. Control Handle
2.2. Prototype Development
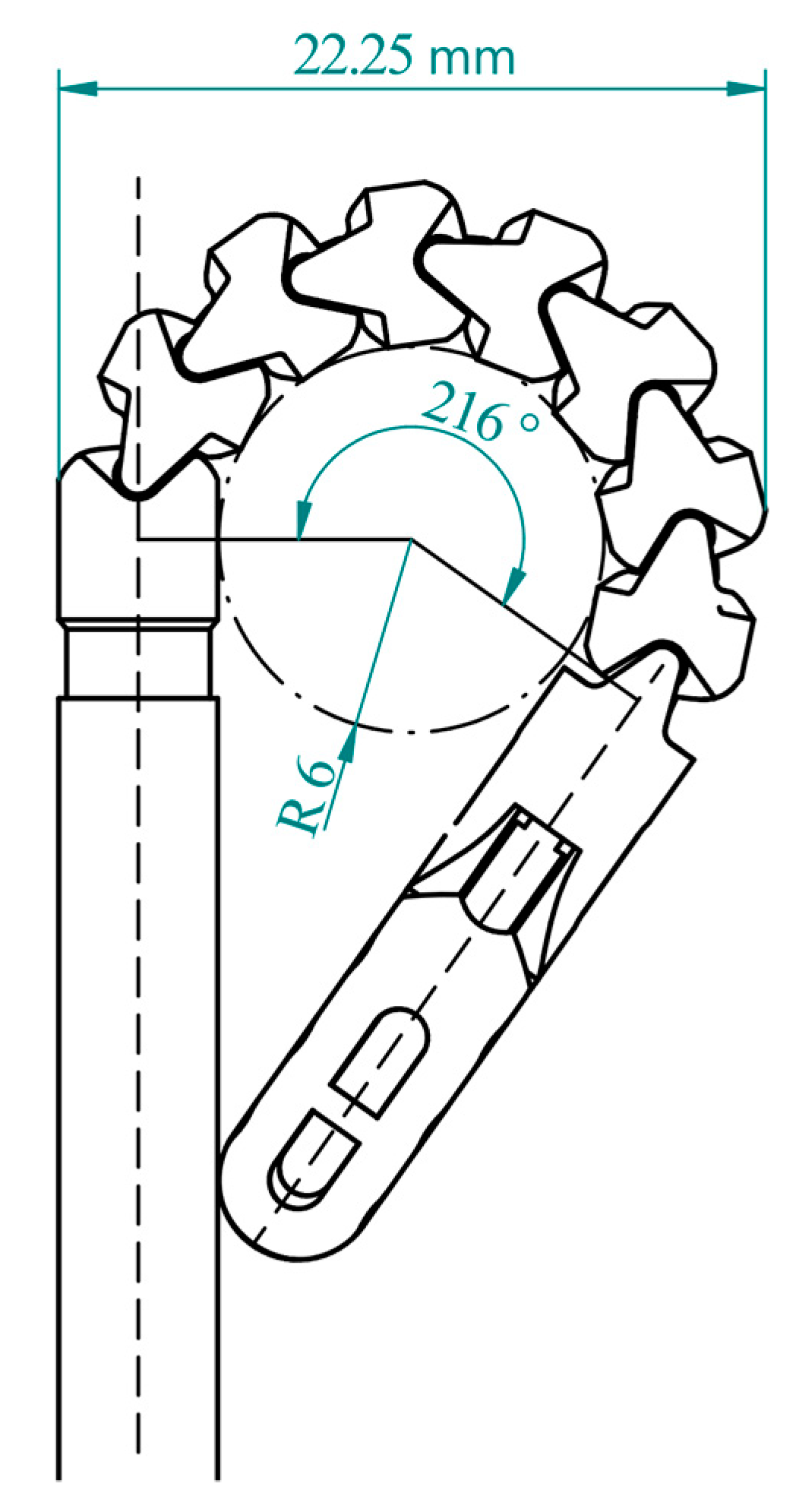
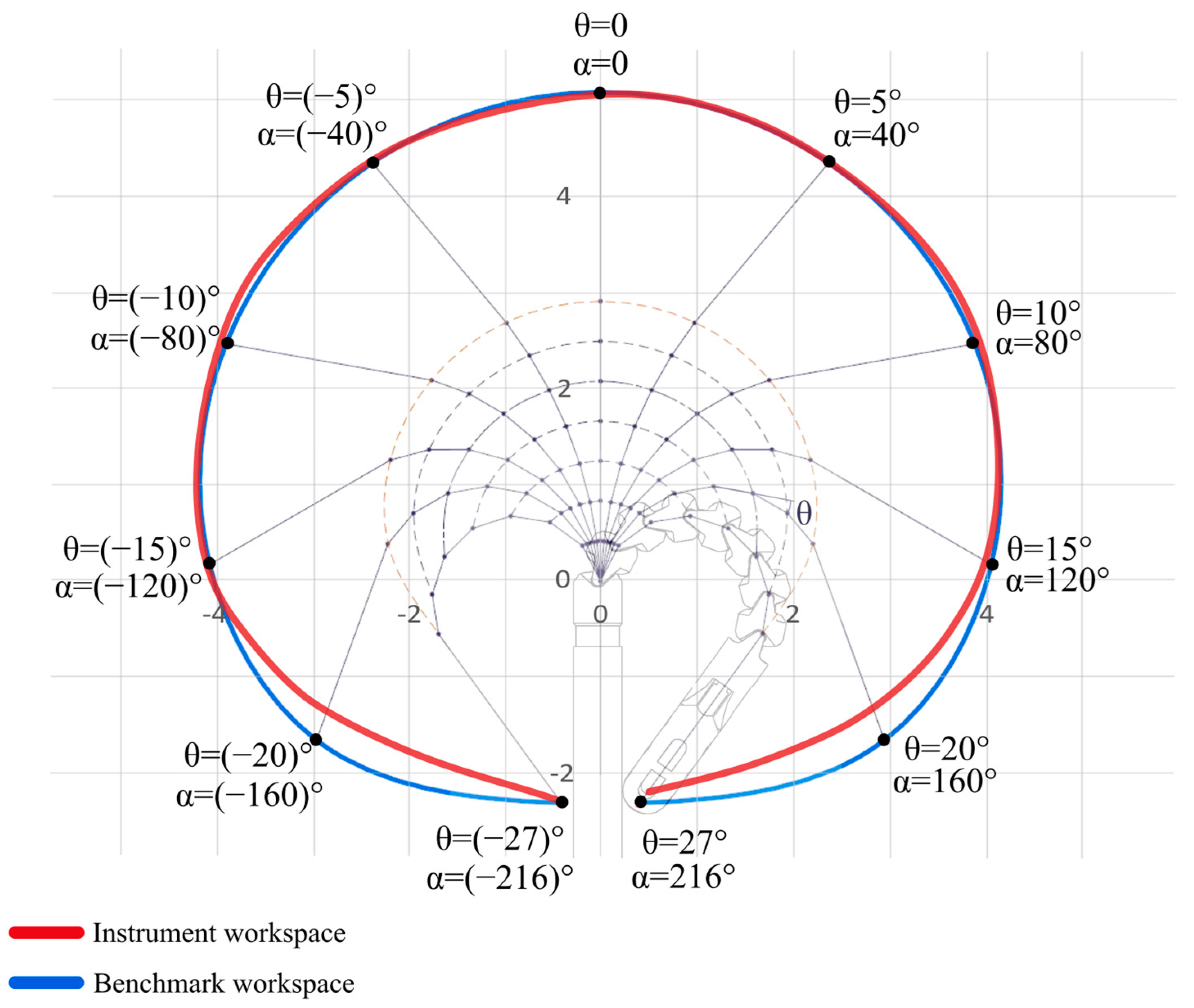
2.3. Workspace of the Distal Tip
2.4. Measurement of the Bending Force
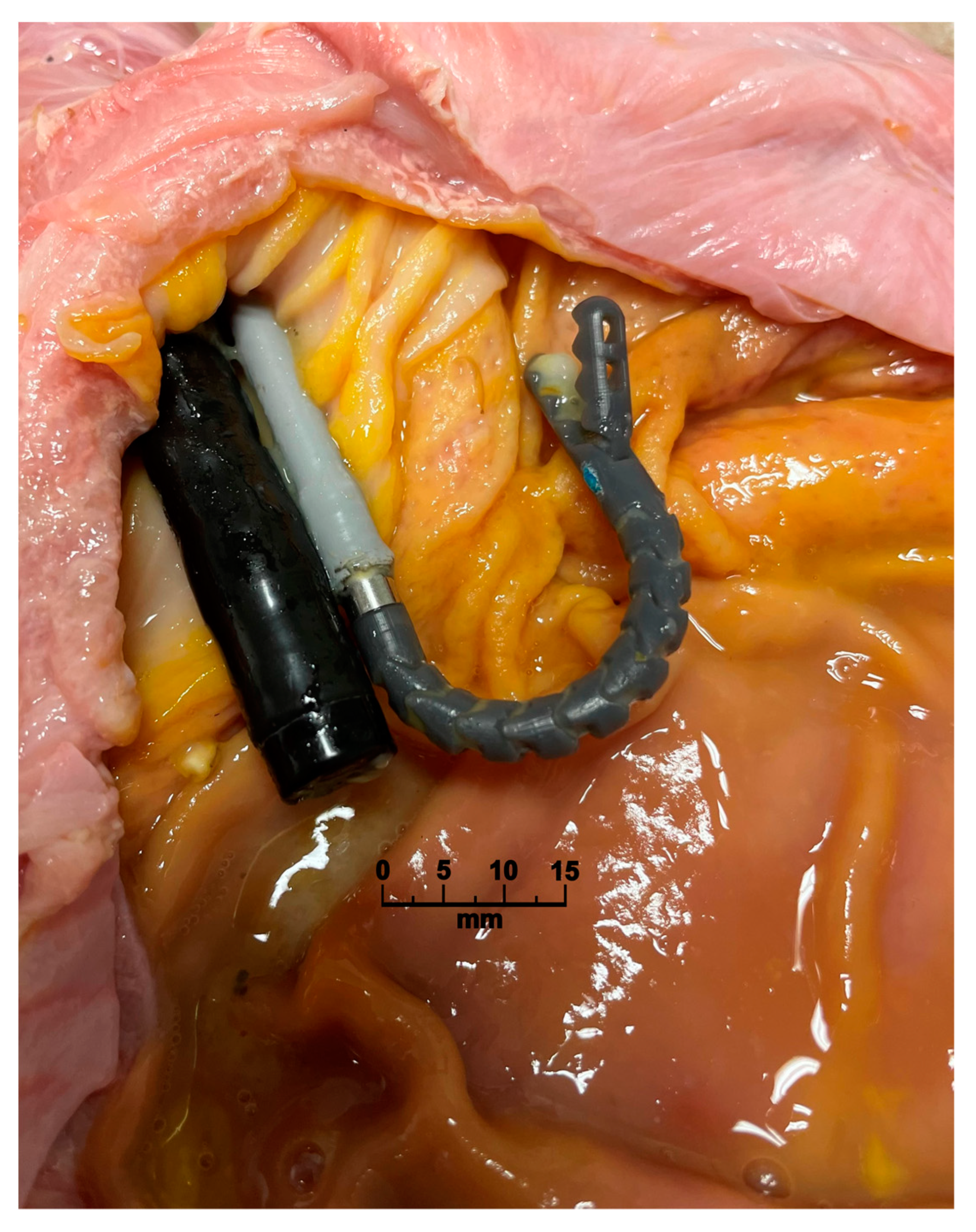
2.5. Performance Assessment in an Upper GI Porcine Model
- Flexibility—the ability of the instrument to bend around the anatomical landmarks inside the porcine model.
- Resistance to buckling—the ability to avoid bending (buckling) when pushed through the organ or when advancing an object to the leak-opening area.
- Friction between instrument and organ surface.
- The maneuverability of the distal tip inside the porcine model.
- Torsional stiffness—the ability of the instrument to transfer the rotational movement from the handle to the distal tip.
2.6. Time Necessary to Perform Interventional Endoscopic Tasks in the Porcine Model
- The time needed to insert and transport the object to the dedicated region. The sponge was grasped outside the porcine model and inserted together with the instrument and gastroscope until reaching the leak-opening region.
- The time required to maneuver and place the object into the cavity space. When the dedicated region was reached, and leak opening was visible, the instrument would be bent into the cavity and used to fixate the sponge inside.
- The time needed to grab and retract the object from the organ. The instrument was inserted into the organ without the sponge and then maneuvered into the cavity. Once the instrument was positioned, the already-placed sponge was grasped and completely removed from the organ.
2.7. Data Analysis
2.8. Availability of the Instrument
3. Results
3.1. Workspace of the Distal Tip
3.2. Measurement of the Bending Force
3.3. Performance Assessment in the Upper GI Porcine Model
3.4. Time Necessary to Perform Interventional Endoscopic Tasks in the Porcine Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neuhaus, H.; Costamagna, G.; Devière, J.; Fockens, P.; Ponchon, T.; Rösch, T. Endoscopic Submucosal Dissection (ESD) of Early Neoplastic Gastric Lesions Using a New Double-Channel Endoscope (the “R-Scope”). Endoscopy 2006, 38, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Ell, C.; May, A.; Pech, O.; Gossner, L.; Guenter, E.; Behrens, A.; Nachbar, L.; Huijsmans, J.; Vieth, M.; Stolte, M. Curative Endoscopic Resection of Early Esophageal Adenocarcinomas (Barrett’s Cancer). Gastrointest. Endosc. 2007, 65, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Fukuzawa, M.; Matsuda, T.; Fukunaga, S.; Sakamoto, T.; Uraoka, T.; Nakajima, T.; Ikehara, H.; Fu, K.-I.; Itoi, T.; et al. Clinical Outcome of Endoscopic Submucosal Dissection versus Endoscopic Mucosal Resection of Large Colorectal Tumors as Determined by Curative Resection. Surg. Endosc. 2010, 24, 343–352. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Dinis-Ribeiro, M.; Ponchon, T.; Repici, A.; Vieth, M.; De Ceglie, A.; Amato, A.; Berr, F.; Bhandari, P.; Bialek, A.; et al. Endoscopic Submucosal Dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015, 47, 829–854. [Google Scholar] [CrossRef]
- Stavropoulos, S.N.; Modayil, R.; Friedel, D. Current Applications of Endoscopic Suturing. WJGE 2015, 7, 777. [Google Scholar] [CrossRef]
- Schmidt, A.; Fuchs, K.-H.; Caca, K.; Küllmer, A.; Meining, A. The Endoscopic Treatment of Iatrogenic Gastrointestinal Perforation; Deutsches Ärzteblatt International: Cologne, Germany, 2016. [Google Scholar]
- Meier, B.; Schmidt, A.; Glaser, N.; Meining, A.; Walter, B.; Wannhoff, A.; Riecken, B.; Caca, K. Endoscopic Full-Thickness Resection of Gastric Subepithelial Tumors with the GFTRD-System: A Prospective Pilot Study (RESET Trial). Surg. Endosc. 2020, 34, 853–860. [Google Scholar] [CrossRef]
- Race, A.; Horgan, S. Overview of Current Robotic Technology. In Innovative Endoscopic and Surgical Technology in the GI Tract; Horgan, S., Fuchs, K.-H., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–17. [Google Scholar]
- Meining, A. Endoscopic Platforms. In Innovative Endoscopic and Surgical Technology in the GI Tract; Horgan, S., Fuchs, K.-H., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 313–316. [Google Scholar]
- Kaindlstorfer, A.; Koch, O.O.; Antoniou, S.A.; Asche, K.-U.; Granderath, F.A.; Pointner, R. A Randomized Trial on Endoscopic Full-Thickness Gastroplication Versus Laparoscopic Antireflux Surgery in GERD Patients Without Hiatal Hernias. Surg. Laparosc. Endosc. Percutaneous Tech. 2013, 23, 212–222. [Google Scholar] [CrossRef]
- Kato, M.; Takeuchi, Y.; Hoteya, S.; Oyama, T.; Nonaka, S.; Yoshimizu, S.; Kakushima, N.; Ohata, K.; Yamamoto, H.; Hara, Y.; et al. Outcomes of Endoscopic Resection for Superficial Duodenal Tumors: 10 Years’ Experience in 18 Japanese High Volume Centers. Endoscopy 2021, 54, 663–670. [Google Scholar] [CrossRef]
- Kermavnar, T.; Shannon, A.; O’Sullivan, K.J.; McCarthy, C.; Dunne, C.P.; O’Sullivan, L.W. Three-Dimensional Printing of Medical Devices Used Directly to Treat Patients: A Systematic Review. 3D Print. Addit. Manuf. 2021, 8, 366–408. [Google Scholar] [CrossRef]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Culmone, C.; Lussenburg, K.; Alkemade, J.; Smit, G.; Sakes, A.; Breedveld, P. A Fully 3D-Printed Steerable Instrument for Minimally Invasive Surgery. Materials 2021, 14, 7910. [Google Scholar] [CrossRef] [PubMed]
- Culmone, C.; Henselmans PW, J.; van Starkenburg RI, B.; Breedveld, P. Exploring Non-Assembly 3D Printing for Novel Compliant Surgical Devices. PLoS ONE 2020, 15, e0232952. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Aroom, K.R.; Hawes, H.G.; Gill, B.S.; Love, J. 3D Printed Surgical Instruments: The Design and Fabrication Process. World J. Surg. 2017, 41, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Zizer, E.; Roppenecker, D.; Helmes, F.; Hafner, S.; Krieger, Y.; Lüth, T.; Meining, A. A New 3D-Printed Overtube System for Endoscopic Submucosal Dissection: First Results of a Randomized Study in a Porcine Model. Endoscopy 2016, 48, 765. [Google Scholar] [PubMed]
- Troya, J.; Krenzer, A.; Flisikowski, K.; Sudarevic, B.; Banck, M.; Hann, A.; Puppe, F.; Meining, A. New Concept for Colonoscopy Including Side Optics and Artificial Intelligence. Gastrointest. Endosc. 2022, 95, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Meining, A. Endoneering: A New Perspective for Basic Research in Gastrointestinal Endoscopy. United Eur. Gastroenterol. J. 2020, 8, 241–245. [Google Scholar] [CrossRef]
- Meining, A. Setting the Stage for Research in Endoscopy. United Eur. Gastroenterol. J. 2019, 7, 177–178. [Google Scholar] [CrossRef]
- Walter, B.; Schmidbaur, S.; Rahman, I.; Albers, D.; Schumacher, B.; Meining, A. The BougieCap—A New Method for Endoscopic Treatment of Complex Benign Esophageal Stenosis: Results from a Multicenter Study. Endoscopy 2019, 51, 866–870. [Google Scholar] [CrossRef]
- Walter, B.; Schmidbaur, S.; Krieger, Y.; Meining, A. Improved Endoscopic Resection of Large Flat Lesions and Early Cancers Using an External Additional Working Channel (AWC): A Case Series. Endosc. Int. Open 2019, 7, E298–E301. [Google Scholar] [CrossRef]
- Hann, A.; Walter, B.M.; Epp, S.; Ayoub, Y.K.; Meining, A. The ‘Twist-Needle’—A New Concept for Endoscopic Ultrasound-Guided Fine Needle-Biopsy. Endosc. Int. Open 2019, 7, E1658–E1662. [Google Scholar] [CrossRef]
- Fuchs, K.-H.; Neki, K.; Lee, A.M.; Dominguez, R.; Broderick, R.; Sandler, B.; Horgan, S. New Suturing System for Flexible Endoscopy in The Gastrointestinal Tract. JJGH 2021, 6, 1–6. [Google Scholar]
- Fuchs, K.-H.; Breithaupt, W. Transgastric Small Bowel Resection with the New Multitasking Platform EndoSAMURAITM for Natural Orifice Transluminal Endoscopic Surgery. Surg. Endosc. 2012, 26, 2281–2287. [Google Scholar] [CrossRef]
- Bernhard, L.; Krumpholz, R.; Krieger, Y.; Czempiel, T.; Meining, A.; Navab, N.; Lüth, T.; Wilhelm, D. PLAFOKON: A New Concept for a Patient-Individual and Intervention-Specific Flexible Surgical Platform. Surg. Endosc. 2022, 36, 5303–5312. [Google Scholar] [CrossRef]
- Brecht, S.V.; Stock, M.; Stolzenburg, J.-U.; Lueth, T.C. 3D Printed Single Incision Laparoscopic Manipulator System Adapted to the Required Forces in Laparoscopic Surgery. In Proceedings of the 2019 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Macau, China, 3–8 November 2019; IEEE: Macau, China, 2019; pp. 6296–6301. [Google Scholar]
- Perretta, S.; Dallemagne, B.; Barry, B.; Marescaux, J. The ANUBISCOPE® Flexible Platform Ready for Prime Time: Description of the First Clinical Case. Surg. Endosc. 2013, 27, 2630. [Google Scholar] [CrossRef] [PubMed]
- Meining, A.; Feussner, H.; Swain, P.; Yang, G.; Lehmann, K.; Zorron, R.; Meisner, S.; Ponsky, J.; Martiny, H.; Reddy, N.; et al. Natural-Orifice Transluminal Endoscopic Surgery (NOTES) in Europe: Summary of the Working Group Reports of the Euro-NOTES Meeting 2010. Endoscopy 2011, 43, 140–143. [Google Scholar] [CrossRef]
- Rattner, D.W.; Hawes, R.; Schwaitzberg, S.; Kochman, M.; Swanstrom, L. The Second SAGES/ASGE White Paper on Natural Orifice Transluminal Endoscopic Surgery: 5 Years of Progress. Surg. Endosc. 2011, 25, 2441–2448. [Google Scholar] [CrossRef]
- Fuchs, K.H.; Meining, A.; von Renteln, D.; Fernandez-Esparrach, G.; Breithaupt, W.; Zornig, C.; Lacy, A. Euro-NOTES Status Paper: From the Concept to Clinical Practice. Surg. Endosc. 2013, 27, 1456–1467. [Google Scholar] [CrossRef]
- Fuchs, K.-H.; Broderick, R.C.; Lee, A.M. The Role of Endoscopic Technology in GastrointestinaI Surgery. In Innovative Endoscopic and Surgical Technology in the GI Tract; Horgan, S., Fuchs, K.-H., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 87–95. [Google Scholar]
- Broderick, R.C.; Tsai, C.; Lee, A.M.; Fuchs, K.-H. Endoscopic Surgical Platforms. In Innovative Endoscopic and Surgical Technology in the GI Tract; Horgan, S., Fuchs, K.-H., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 267–279. [Google Scholar]
- Golahmadi, A.K.; Khan, D.Z.; Mylonas, G.P.; Marcus, H.J. Tool-Tissue Forces in Surgery: A Systematic Review. Ann. Med. Surg. 2021, 65, 102268. [Google Scholar] [CrossRef]
- Hu, J.; Liu, T.; Zeng, H.; Chua, M.X.; Katupitiya, J.; Wu, L. Static Modeling of a Class of Stiffness-Adjustable Snake-like Robots with Gravity Compensation. Robotics 2022, 12, 2. [Google Scholar] [CrossRef]
- Jin, L.; Han, L. Kinematic Model and Real-Time Path Generator for a Wire-Driven Surgical Robot Arm with Articulated Joint Structure. Appl. Sci. 2019, 9, 4114. [Google Scholar] [CrossRef]
- Zolfagharian, A.; Bodaghi, M.; Winter, A.; Nahavandi, D. Non-Assembly Spherical Joints 3D-Printed for Soft Robotic Applications. In Proceedings of the 2021 IEEE International Conference on Recent Advances in Systems Science and Engineering (RASSE), Shanghai, China, 12–14 December 2021; IEEE: Shanghai, China, 2021; pp. 1–6. [Google Scholar]
- Werner, Y.B.; Hakanson, B.; Martinek, J.; Repici, A.; von Rahden BH, A.; Bredenoord, A.J.; Bisschops, R.; Messmann, H.; Vollberg, M.C.; Noder, T.; et al. Endoscopic or Surgical Myotomy in Patients with Idiopathic Achalasia. N. Engl. J. Med. 2019, 381, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Yoshida, N.; Nakanishi, H.; Takemura, K.; Yamada, S.; Doyama, H. Recent Traction Methods for Endoscopic Submucosal Dissection. WJG 2016, 22, 5917. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, S.; Hirashita, T.; Hirashita, Y.; Lowenfeld, L.; Gurram, K.C.; Nishimura, M.; Milsom, J.W. Use of an Endoscopic Flexible Grasper as a Traction Tool for Excision of Polyps: Preclinical Trial. Sci. Rep. 2021, 11, 18674. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Ali, F.S.; Zhang, J.; Zhao, L.; Liu, B. Endoscopic Traction Techniques. Am. J. Gastroenterol. 2021, 116, 862–866. [Google Scholar] [CrossRef]
- Thompson, C.C.; Ryou, M.; Soper, N.J.; Hungess, E.S.; Rothstein, R.I.; Swanstrom, L.L. Evaluation of a Manually Driven, Multitasking Platform for Complex Endoluminal and Natural Orifice Transluminal Endoscopic Surgery Applications (with Video). Gastrointest. Endosc. 2009, 70, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Steinemann, D.C.; Müller, P.C.; Apitz, M.; Nickel, F.; Kenngott, H.G.; Müller-Stich, B.P.; Linke, G.R. An Ad Hoc Three Dimensionally Printed Tool Facilitates Intraesophageal Suturing in Experimental Surgery. J. Surg. Res. 2018, 223, 87–93. [Google Scholar] [CrossRef]
- Swaney, P.J.; York, P.A.; Gilbert, H.B.; Burgner-Kahrs, J.; Webster, R.J. Design, Fabrication, and Testing of a Needle-Sized Wrist for Surgical Instruments. J. Med. Devices 2017, 11, 014501. [Google Scholar] [CrossRef]
- Krieger, Y.S.; Roppenecker, D.B.; Kuru, I.; Lueth, T.C. Multi-Arm Snake-like Robot. In Proceedings of the 2017 IEEE International Conference on Robotics and Automation (ICRA), Singapore, 29 May 2017–3 June 2017; IEEE: Singapore, 2017; pp. 2490–2495. [Google Scholar]
- Dearden, J.; Grames, C.; Jensen, B.D.; Magleby, S.P.; Howell, L.L. Inverted L-Arm Gripper Compliant Mechanism. J. Med. Devices 2017, 11, 034502. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Kong, K.; Wang, S. Design of a Dexterous Robotic Surgical Instrument with a Novel Bending Mechanism. Robot. Comput. Surg. 2022, 18, e2334. [Google Scholar] [CrossRef]
- Francis, P.; Eastwood, K.W.; Bodani, V.; Price, K.; Upadhyaya, K.; Podolsky, D.; Azimian, H.; Looi, T.; Drake, J. Miniaturized Instruments for the Da Vinci Research Kit: Design and Implementation of Custom Continuum Tools. IEEE Robot. Automat. Mag. 2017, 24, 24–33. [Google Scholar] [CrossRef]
- Haraguchi, D.; Kanno, T.; Tadano, K.; Kawashima, K. A Pneumatically Driven Surgical Manipulator With a Flexible Distal Joint Capable of Force Sensing. IEEE/ASME Trans. Mechatron. 2015, 20, 2950–2961. [Google Scholar] [CrossRef]
- Peter, S.; Bang, J.Y.; Varadarajulu, S. Single-Use Duodenoscopes: Where Are We and Where Are We Going? Curr. Opin. Gastroenterol. 2021, 37, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Ellrichmann, M.; Eickhoff, A. Einmalprodukte in der Endoskopie—Vom Zubehör bis zu den “single-use scopes”: Die Zukunft? Gastroenterologe 2022, 17, 15–21. [Google Scholar] [CrossRef]
- Carlier, M.; Baboudjian, M.; Govidin, L.; Yahia, M.; Chiappini, J.; Lechevallier, E.; Boissier, R. Urétéroscope souple à usage unique versus réutilisable: Aspects techniques et médico-économiques. Progrès Urol. 2021, 31, 937–942. [Google Scholar] [CrossRef]

| Instrument features | |
| Adequate length to reach the dedicated region, at least 40 cm. | |
| A diameter of 5 mm for intraluminal application alongside an endoscope. | |
| Sufficient strength for movement independent from the endoscope. | |
| Safe introduction and maneuvering inside the GI tract, with smooth parts and surfaces. | |
| Biocompatibility of the parts with contact with organs. | |
| Affordable manufacturing through 3D printing and simple assembly. | |
| Grasping forceps | |
| A smooth shape of the features that are in contact with the mucosal surfaces. | |
| A minimum of 10 N grasping force to manipulate the tissue or a sponge. | |
| Minimal length of non-grasping segments. The total length is less than 25 mm. | |
| Jaw opening angle of at least 60°. | |
| At least 8 mm-long grasping area with a gentle wave-like profile. | |
| Bending section | |
| Planar bending in both directions (left and right). | |
| A high bending angle of at least 180° and a compact bending radius. | |
| Adequate bending force for tissue and object manipulation of more than 5N. | |
| Sufficient strength to withstand the loads introduced during maneuvering. | |
| Flexible shaft | |
| A high degree of flexibility for safe introduction into the dedicated region. | |
| Sufficient torsional stiffness to precisely transfer the hand inputs to the distal tip. | |
| Enough stiffness during pushing when transporting objects (no buckling). | |
| Control handle and tendon wires | |
| Convenient for single-hand use. | |
| Star knob mechanism to control the bending of the distal tip, such as on endoscopes. | |
| Push–pull motion for opening the grasping jaws, like on common biopsy forceps. | |
| Parameter | Median Grade | Grading Range | Short Conclusion |
|---|---|---|---|
| Flexibility | 2 | 2–3 | Adequate |
| Rigidity | 2 | 2–3 | Adequate |
| Torsional stiffness | 2 | 2 | Sufficient |
| Friction | 3 | 2–3 | Adequate |
| Maneuverability | 3 | 3 | Very good |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sudarevic, B.; Troya, J.; Fuchs, K.-H.; Hann, A.; Vereczkei, A.; Meining, A. Design and Development of a Flexible 3D-Printed Endoscopic Grasping Instrument. Appl. Sci. 2023, 13, 5656. https://doi.org/10.3390/app13095656
Sudarevic B, Troya J, Fuchs K-H, Hann A, Vereczkei A, Meining A. Design and Development of a Flexible 3D-Printed Endoscopic Grasping Instrument. Applied Sciences. 2023; 13(9):5656. https://doi.org/10.3390/app13095656
Chicago/Turabian StyleSudarevic, Boban, Joel Troya, Karl-Hermann Fuchs, Alexander Hann, Andras Vereczkei, and Alexander Meining. 2023. "Design and Development of a Flexible 3D-Printed Endoscopic Grasping Instrument" Applied Sciences 13, no. 9: 5656. https://doi.org/10.3390/app13095656
APA StyleSudarevic, B., Troya, J., Fuchs, K.-H., Hann, A., Vereczkei, A., & Meining, A. (2023). Design and Development of a Flexible 3D-Printed Endoscopic Grasping Instrument. Applied Sciences, 13(9), 5656. https://doi.org/10.3390/app13095656







