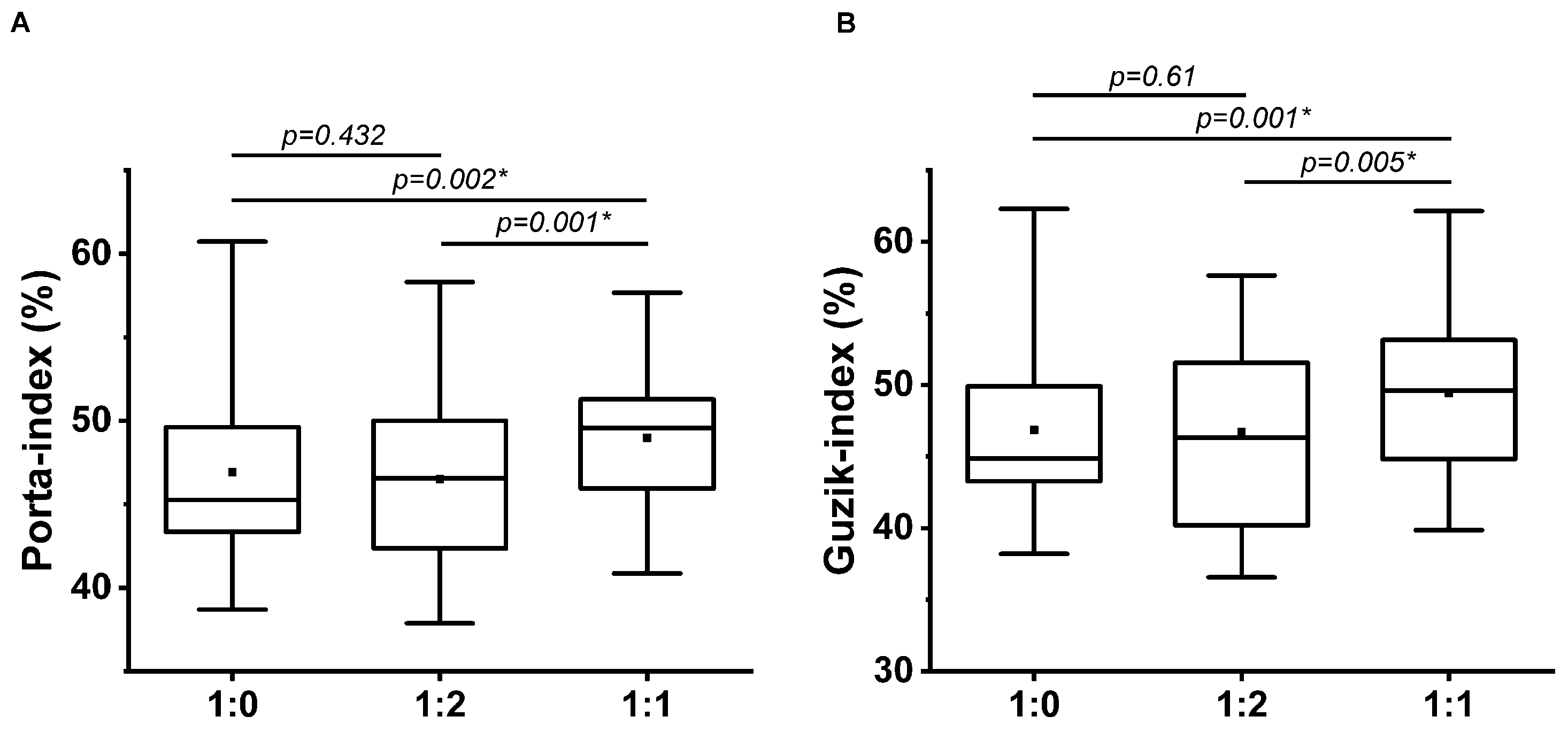Abstract
Background: The development of wearables has facilitated the monitoring of biomedical parameters in everyday life. One of the most common sensors of these gadgets is the photoplethysmograph (PPG); hence, the proper processing and interpretation of the PPG signal are essential. Besides pulse rate detection, these devices—together with an ECG—compute the pulse arrival time (PAT), from which the actual beat-to-beat blood pressure can be estimated. The heart rate shows asymmetrical accelerations and decelerations, quantified by the parameters of heart rate asymmetry (HRA). In the present study, we investigated the influences of different breathing-patterns on the PATs and HRA parameters. Methods: The authors evaluated 5 min simultaneous respiratory-, ECG- and PPG-signal recordings of 35 healthy, young volunteers specifically expressing the following breathing patterns: metronome-controlled inspiration, and both inspiration and expiration controlled at 1:1 and 1:2 ratios, respectively. The records were analyzed by HRVScan_Merge v3.2 software. The PAT values were calculated at eight different reference points. The HRA parameters and the PAT values at different breathing patterns were compared using the Friedman test and post hoc Wilcoxon paired-sample test. Results: Porta- and Guzik-indices significantly increased at 1:1 breathing compared to 1:2 and single-paced breathing. PATs increased significantly in dual-paced series compared to single-paced series at each reference point. Conclusion: Based on our results, the increased PATs at dual-paced versus single-paced breathing may indicate the involvement of cognitive functions. The symmetrical respiration ratio increases the heart rate symmetry; however, this effect is not detectable in the periphery through the PATs.
1. Introduction
The dynamic technological progress in the wearables market opens new fields in well-being and outpatient monitoring. These gadgets are able to collect and evaluate several biological signals over the long-term. The ease of use and convenience of these devices have promoted their popularity at the same time wearables have attracted the attention of both researchers and industry [1].
Photoplethysmography (PPG) is a popular optical method to detect the variations in the light intensity related to the blood volume changes and the tilting of red blood cells due to the blood flow [2]. The PPG sensor consists of a light source and a photodiode. Most wearables (for example, smart watches) are equipped with a reflective PPG-unit, which operates in most cases with a green light, that penetrates through the epidermal and papillary dermal layers of the skin, but not deeper, as in the case of red or near-infrared light [3]. The signal-to-noise ratio is better for the green types compared to the others. In the reflective PPG, the photodiode is positioned next to the light source, on the same side of the tissue or body part and detects the reflected light. On the contrary, in consideration of transmissive PPG devices, the light source and the photosensor are separated by the tissue or body part. These types use mostly red- or infrared light [2,3]. When compared to the ECG, the main advantage of the reflective PPG technique is the single contact surface and the lack of cables, electrodes and potential skin irritation. Recent publications report on the possibility of remote PPG [2]. Shao and co-workers extracted biological signals (heart rate, breathing rate, and pulse transit time) from images captured by a commercial digital camera [4].
The most conventional use of the PPG in clinical practice is to measure blood oxygen saturation and monitor heart rate (HR) [5,6]. Due to its advantages described above, several investigations have focused on improving the signal quality [7,8,9,10], in order to obtain reliable biological data including pulse rate variability (PRV) parameters [11].
The autonomic nervous system can be examined non-invasively through the heart rate variability (HRV). In addition to sympathetic and parasympathetic innervation, numerous mechanisms (autocrine, paracrine, endocrine and mechanical effects) influence the activity of the sinus node (SN) including numerous feedback-loops. The SN summarizes these effects, which are manifested in the instantaneous heart rate. Consequently, the succeeding R-R intervals (RRIs) are not identical and show a beat-to-beat variability. The most spectacular fluctuations are related to the respiration [12].
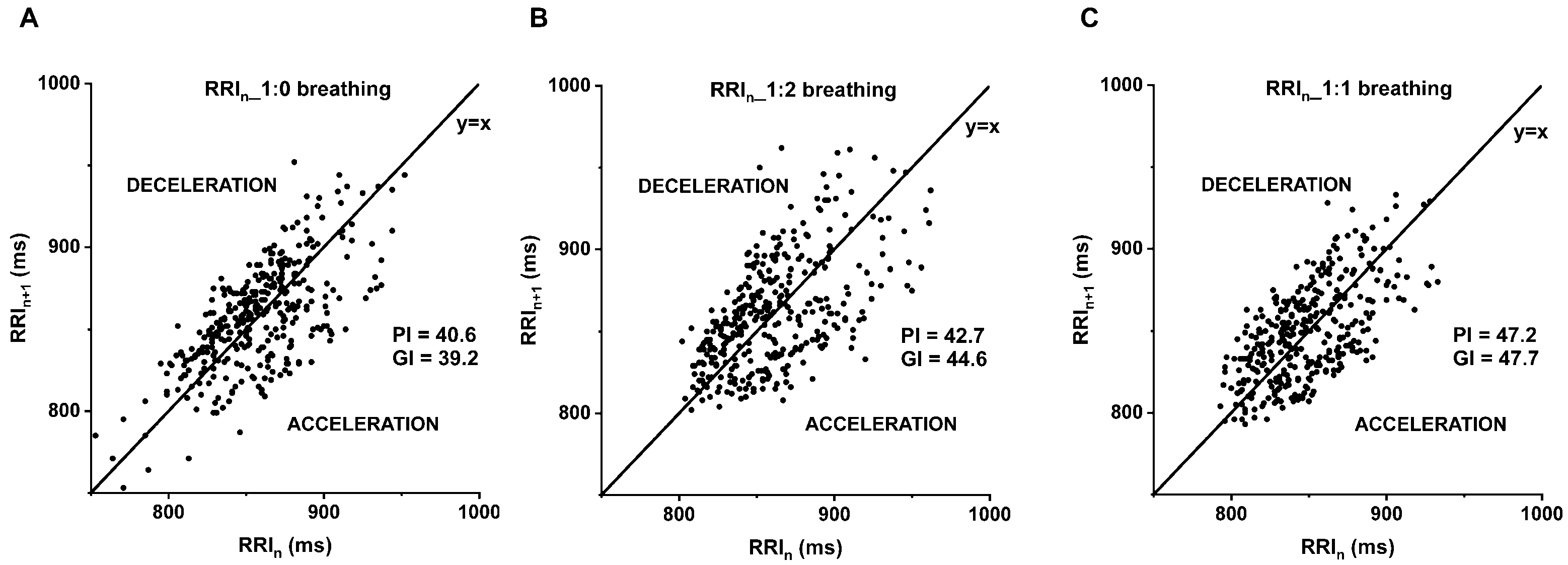
The asymmetry in the accelerations and decelerations of the heart rate can be described by the parameters of heart rate asymmetry (HRA), which is also related to the autonomic modulation of the cardiovascular system [13,14]. The Poincaré plot illustrates the actual RRI against the preceding RRI of the tachogram, resulting in a cloud (Figure 1). In addition to visual assessment, the heart rate asymmetry parameters quantize the evident asymmetry of the cloud [15]: the Guzik-index (GI) [16] is proportional to the contribution of the decelerating cloud to the global variability, related to expiration. Whereas the Porta-index (PI) [17] is proportionate to the number of points in the acceleration cloud, associated to inspiration.
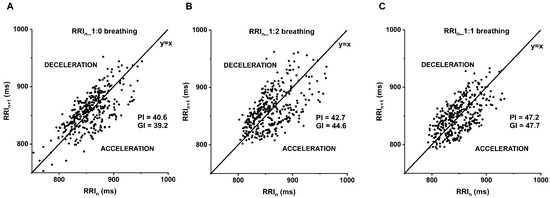
Figure 1.
Poincaré plot of B_SZ_29031990 illustrates the more symmetrical distribution of the deceleration and acceleration of the consecutive RR-interval at 1:1 compared to 1:0 and 1:2 inspiration/expiration (i/e) ratio. (A) 1:0, (B) 1:2 and (C) 1:1 breathing. The graph represents the subsequent RR intervals (Y-axis; RRIn+1) against the current RR intervals (X-axis; RRIn). Porta-index (PI), Guzik-index (GI), under the identity line the heart rate accelerates (ACCELERATION), above the identity line decelerates (DECELERATION).
In the last decades, several studies focused on the comparison of the PPG-based HRV calculation to the “gold standard” ECG-based method [18,19]. Many of them concluded that the PRV is not a surrogate of HRV [19,20,21], at the same time others found HRV and PRV equivalent in certain conditions [7,22]. In most cases, the short-term variability (or beat-to-beat variability) parameters such as RMSSD (root mean square of successive RR-differences) or frequency domain parameters were overestimated from the PPG signal [20]. One possible explanation can be the relation of RRI and the pulse-to-pulse interval (PPI) as detailed in our earlier publication [5]. The difference of consecutive pulse arrival times (PAT, see later in the Introduction) oscillating with breathing is added to the RRIs, resulting in greater fluctuation of PPIs.
In consideration of technical factors, including appropriate sampling frequency, Béres et al. investigated the minimally required temporal resolution of the PPG signals for accurate PRV parameters among healthy volunteers. Their results showed the mean RRI is the most resistant to the low sampling frequency (or high sampling interval), as low as 303 ms sampling may be sufficient to maintain accuracy within the five-minute analysis. In the case of SDNN and RMSSD, a minimum of 50 Hz sampling frequency is required, which can be slightly decreased by interpolation [7]. Another current research paper highlights the importance of the signal-to-noise ratio (SNR) prior to digitization versus the sampling noise due to the low sampling rate [23].
In order to reduce the timing uncertainty of the reference point, it is essential to select the appropriate filtering technique, which minimizes the confounding noise or baseline wandering. Mejía-Mejía and co-workers extracted several time-domain, frequency-domain, and Poincaré plot parameters from simulated PPG signals prior to and following noise contamination and subsequent filtering by the combination of several IIR and FIR low pass and high pass filters. They found a better reproducibility using elliptic IIR filters and equiripple, or Parks–McClellan FIR filters when compared to Butterworth, Hamming window, constrained least squares and least squares filtering. They also suggest a lower low cut-off frequency, except for those PPG signals in which the motion artifacts’ baseline wandering and respiratory noise appear together. In this case, a higher frequency low-pass is required to achieve better results [24].
Several studies focused on selecting the most reliable reference point of the PPG signal, which also affects the accuracy of PRV parameters. Mejía-Mejía et al. [9] found time domain parameters reliable from simulated PPG signals based on the valley point among five complex interbeat-interval detection algorithms. Other authors [25] proved the superiority of middle-amplitude, first derivative peak and the tangent intersection points versus the peak and foot fiducial points in a human study during the head-up tilt test.
In addition to technical and environmental factors influencing the quality of the PPG signal and derived PRV parameters, certain physiological factors such as instantaneous blood pressure, arterial stiffness, and vasomotor activity among others can distort the shape of the peripheral pulse wave, resulting in a constant or variable shift of the reference points [19].
The PAT is the time interval between the defined reference point of the ventricular complex in the ECG signal and a corresponding fiducial point of the simultaneous PPG wave. This time interval consists of the pre-ejection period (PEP), which is the delay between the left ventricular depolarization and the contraction (also known as the electromechanical coupling), and the pulse transit time (PTT) which is the span of time consumed for the ejected blood to travel from the aortic valve to the PPG sensor [26]. In addition to the momentary changes in the preload and afterload, the direct vegetative actions on the left ventricle can also theoretically influence the instantaneous PEP. However, Finnegan and co-workers [26] found only a weak correlation between the PEP to systolic, mean and diastolic BPs; on the contrary, PAT and PTT both showed an excellent correlation to all three BP values in healthy volunteers in the phenylephrine test. The PAT and PTT parameters possess important biological information; one can non-obtrusively estimate arterial blood pressure or arterial stiffness in outpatient follow-up or well-being monitoring. The mean arterial pressure can be successfully calculated from ECG and PPG by an artificial neural network with periodic calibrations during exercise [27]. On the other hand, there are several publications with insufficient correlations of PTT [28] or PAT [29] to cuff-based arterial BP. Further studies are emerging in published literature and elucidate the exact pathomechanism and influencing factors, both from a technical and physiological point of view.
The elongation of PAT at lower respiration frequencies has already been observed by other research groups. Bachler et al. [30] found an increase in the median PAT values during the slow breathing protocol (average 5.4 bpm) compared to the baseline at spontaneous breathing. Their speculation was that slow breathing causes a reduction in blood pressure, which is indicated by the elongation of the PAT values. They observed a further increase in PATs in the unguided recovery phase. Another research group [31] investigated the effects of step-wise controlled breathing on cardiopulmonary coupling (CPC), blood pressure and PTT in healthy volunteers. Their breathing protocol included spontaneous respiration and 14, 12.5, 11, 9.5, 8, 7, 3 bpm with a fixed 1:2 inspiration/expiration (i/e) ratio. The blood pressure was continuously monitored during the measurements. The PAT values were calculated between the R peak of the ECG and the peak of the second derivative of the PPG. They experienced a significant decrease both in systolic and diastolic blood pressure at a breathing frequency of 7 bpm compared to spontaneous breathing, and a significant increase in PAT values. According to their results, a breathing frequency of 11 bpm was considered optimal for their applied breathing pattern, in order to increase CPC and reduce blood pressure. Our research group has already reported the close relationship between the HRA and variable i/e ratios [15,32]. The i/e ratio can vary in different physiological conditions, e.g., during intense exercises [33]. Due to its previously detailed close relationship with breathing, the varying i/e ratio can also influence the PAT, thus leading to an inaccurate estimation of the derived parameters (e.g., blood pressure).
The aim of the present study was to investigate the possible effect of different inspiration/expiration ratios on PAT values, considering several reference points on the PPG signal side. Additionally, the already published [15] respiration-related changes of the HRA parameters were reproduced as “indicators” or “positive controls” of the cardiorespiratory relationship, taking into account the new proband population.
2. Materials and Methods
The ethical approval was granted by the Regional Research Ethics Committee (approval number: 7533-PTE-2018) and was conducted in full accordance with the Declaration of Helsinki and its later amendments.
2.1. The Study Protocol
The data acquisition was performed at the Heart Institute, University of Pécs, and at the Zsigmondy Vilmos Spa Hospital, Harkány, within the sanctity of a calm and quiet room. Speech and movements can influence the hemodynamics and the signal quality, so all volunteers were asked to spend the measurements while at rest and in silence. The smoking and consumption of meals or alcohol- and caffeine-containing drinks were also prohibited two hours prior to the measurements.
During the 15 min-long orthostatic adaptation in a supine position with a 30° raised head, the participants were equipped with sensors (breathing sensor, ECG electrodes and PPG clips), and trained for the device-controlled breathing. The respiratory sensor belt was fitted around the lower chest, and the infrared transmissive PPG sensor was attached to the right earlobe. The four ECG electrodes were positioned on the left and right sides of the upper and lower chest.
Three rounds of 300 s records were acquired from each volunteer with the given breathing patterns controlled by a digital metronome. This triggered inspiration in the first round with a short beep signal with a cycle length of 4500 ms (referred as “1:0” or “single-paced”). In the second and third rounds, both inspiration and expiration were triggered by two short beeps of different frequencies, with an inspiration/expiration ratio of 1:1 and 1:2 within the 4500 ms cycle (hereafter: “dual-paced” or “1:1” and “1:2”); their order was randomized by a coin at each proband. The applied cycle length of 4500 ms equals 0.22 Hz respiration frequency.
2.2. Participants
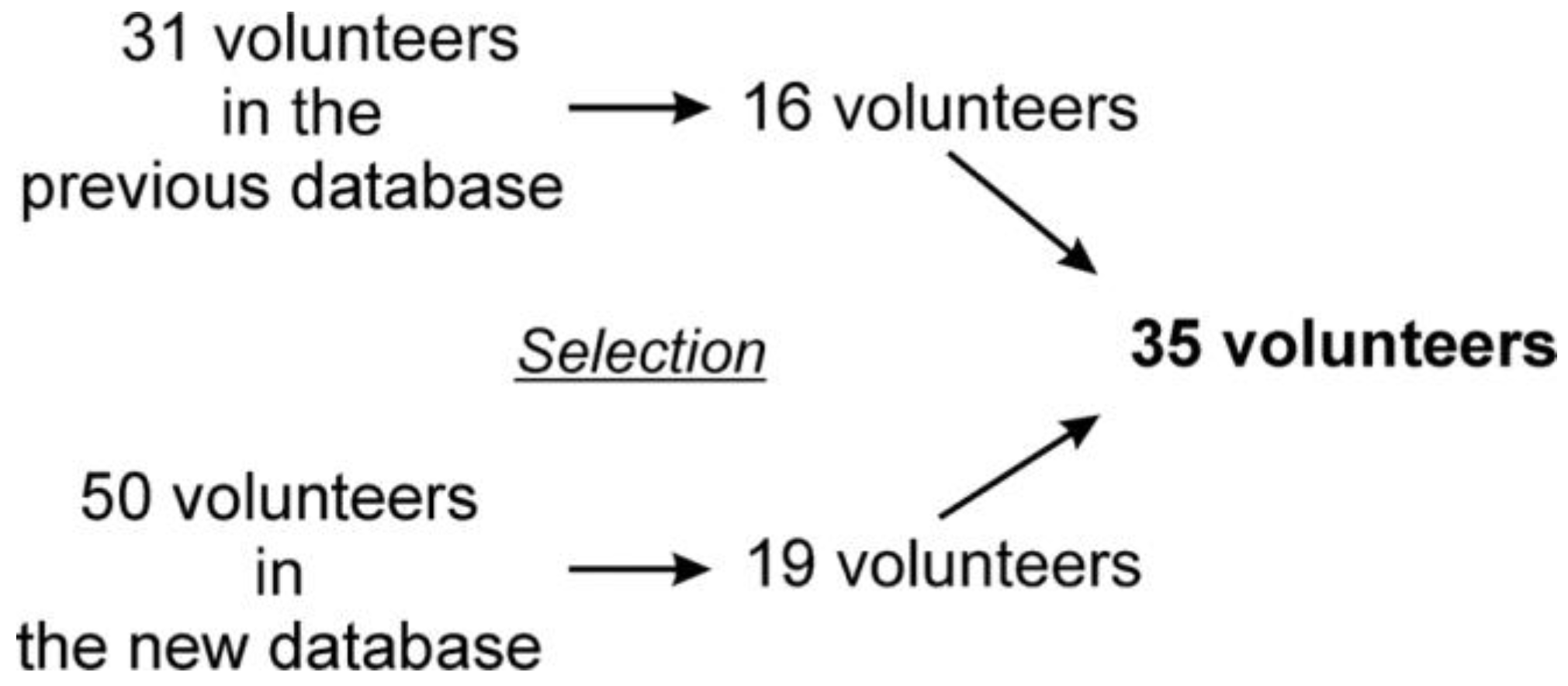
Eighty-one young, healthy and physically active volunteers were enrolled in the study, including 31 measurements from our previous database with nearly the same protocol (approval number: 7535-PTE 2019) [5,7]. All participants were informed regarding the details of the study and all signed a statement of consent. Those volunteers were included, who correctly followed the metronome-controlled breathing. The filtered records that contained more than two non-sinus beats (e.g., extrasystole) or artifacts were rejected. Following careful visual inspection, a total of 35 volunteers were selected for final analysis (Figure 2). Table 1 shows the baseline characteristic of the selected participants.
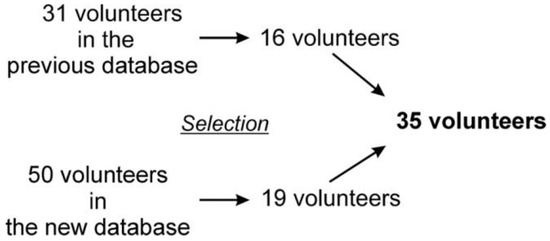
Figure 2.
Composition of the final study group.

Table 1.
The characteristics of the included subjects. Values presented as mean ± standard deviation, if applicable.
2.3. Data Acquisition and PAT Calculation
All data were acquired by the BioSign HRV-Scanner plus Study version 3.05 (BioSign GmbH, Ottenhofen, Germany). The ECG, PPG, and respiratory signals were stored in 16-bit raw files. The signals were sampled at 1 ms (ECG), 2 ms (PPG), and 20 ms (breathing signal) temporal resolution. The data pre-processing and the computation of the PATs and HRA parameters were performed by the HRVScan_Merge v3.2 software. This software was developed by the last author (L. Hejjel), and it has been used in previous studies [5,7]. The signals were cubic spline interpolated to 1 ms resolution, then automatically filtered with a 21 ms wide, single pass moving average filter and a 1001 ms trend removal filter, which subtracts the moving average of the previous and succeeding 500 points [34]. Applying adequate filters is especially important to reduce noise and baseline fluctuations, thus increasing the detection accuracy.
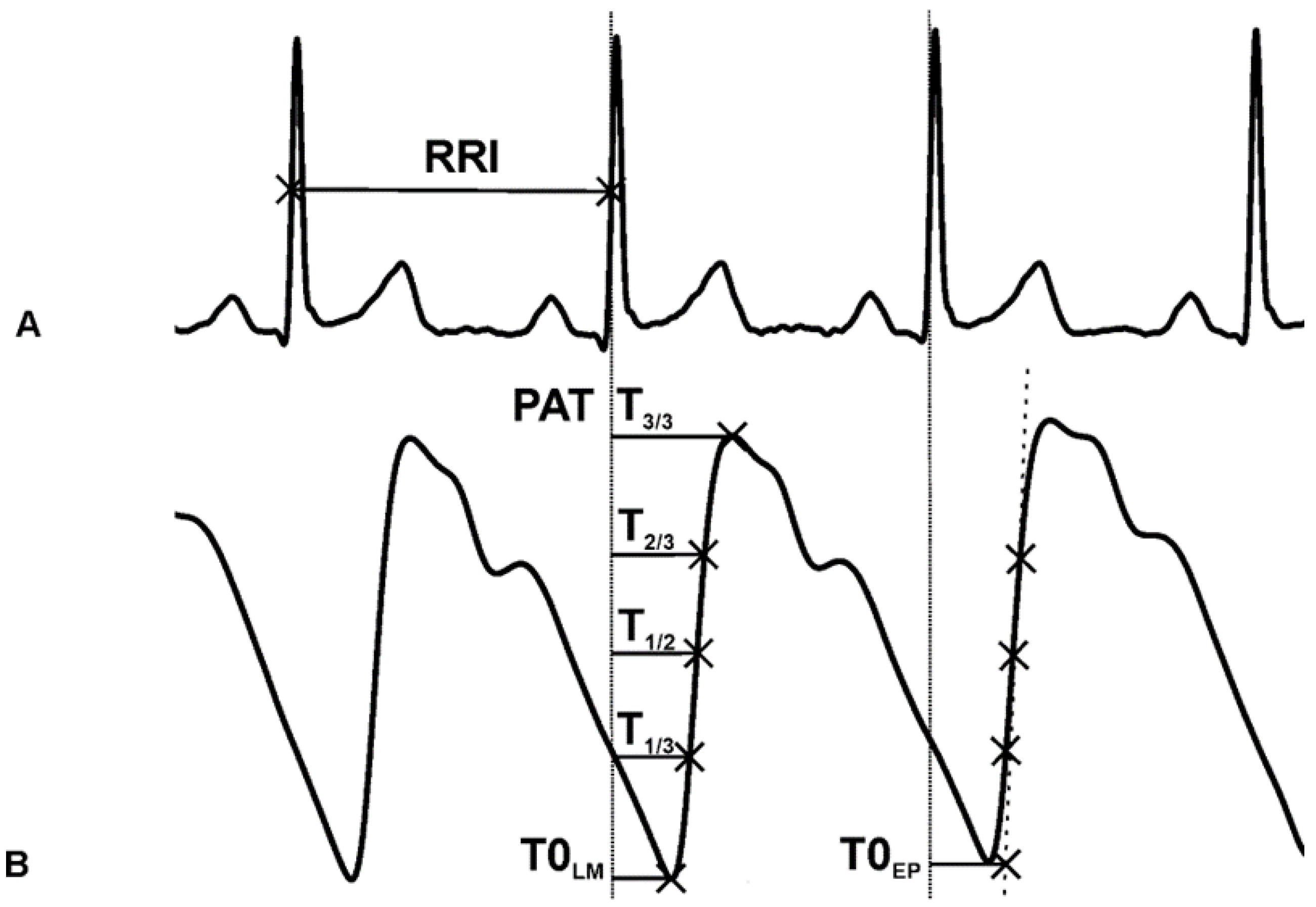
We used the ½-amplitude height of the ascending R-waves (R1/2) on the ECG, as the starting point of the PAT calculation. The detection accuracy is superior at this point, compared to the peak detection methods [35]. We defined eight different points on the PPG signal as a reference for PAT calculation. The software detected the local positive peaks (T3/3), the local minima (TLM), and the 1/3–1/2–2/3 amplitude heights (T1/3, T1/2, T2/3) between the previous two points. The program computed the extrapolated base points by the intersection of the actual linear regression line on the T1/3, T1/2 and T2/3 points and the heights of the preceding local minima. Additionally, the PATs were calculated at the peaks of the first derivative (Tdiff) as well as the smooth derivative (Tsmdiff) [36] of the PPG signal (Figure 3).
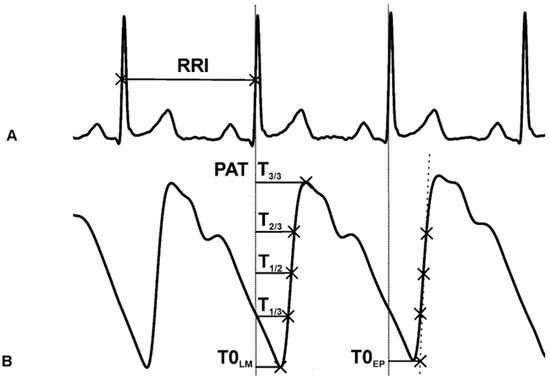
Figure 3.
(A) ECG signal. The starting point for PAT calculation is at the 1/2 amplitude height of the R-wave. (B) PPG signal and the reference points: the peak (T3/3), 2/3 height (T2/3), 1/2 height (T1/2), 1/3 height (T1/3) of the ascending slope, the local minimum (T0LM), the extrapolated point (T0EP), the time interval between T1/3 and T2/3 (T1/3T2/3).
The beat-to-beat time intervals between the corresponding T1/3 and T2/3 points were computed (T1/3T2/3), which are inversely proportional to the steepness of the ascending slope (Figure 3). The derivative as the slope of the PPG signal would not give comparable values since the PPG amplitude is not calibrated or standardized. The HRVScan_Merge v3.2 software automatically calculated the eight different PATs (between the R1/2 and the given PPG reference points) and the T1/3T2/3 intervals at each cardiac cycle.
2.4. Statistical Analysis and Mathematical Background of the HRA Parameters
The i/e-ratio-related changes of PAT and HRA parameters (GI, PI) were compared using the Friedman test as a primary assessment in which p < 0.5 was considered statistically significant. The three possible post hoc Wilcoxon paired-sample tests were performed by each parameter with the following i/e ratio pairs: (1) single-paced to 1:2, (2) single-paced to 1:1, and (3) 1:2 to 1:1 breathing ratios. According to Holm–Bonferroni correction, the post hoc significance level was corrected to p < 0.01667 (0.5/3 = 0.01667) [37]. The Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) with StatistiXL package (v. 2.0, 2008, Broadway-Nedlands, Australia) was used for statistical analysis.
The PI and GI are expressed in the form of percentages. They are calculated using the following Equations (1)–(3) [16,17]:
in which the N(ΔRRi−) represented the number of the points below the identity line (y = x), and N(ΔRRi ≠ 0) is the number of the points that are not on the identity line on the Poincaré plot [17].
in which the Di value is the distance of the ith point from the identity line, Di+ is the distance of the ith point above the y = x line, and N+ represents the number of points above the identity line [16].
3. Results
3.1. The PAT Values at Different Breathing Patterns
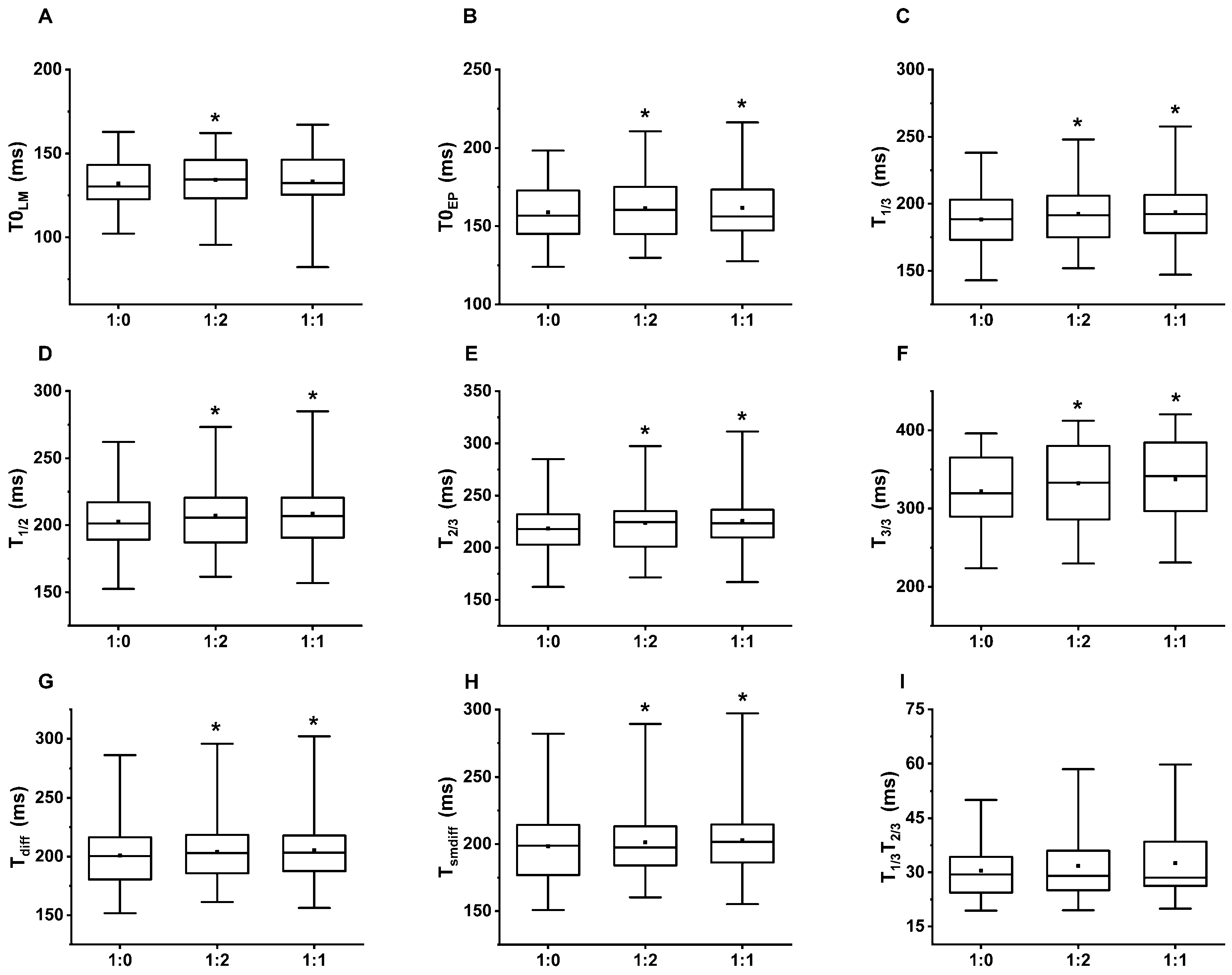
Table 2 summarizes the mean of the 5 min average PAT values by the breathing patterns and the fiducial points. The Friedman test showed a significant difference in the mean PAT regarding the breathing patterns at each fiducial point; however, not at the T1/3T2/3 delay (Table 3). We found a significant increase on the post hoc Wilcoxon test at each of the PAT reference points comparing single-paced breathing to the 1:2 or 1:1 i/e ratio, except PAT-T0LM at the single-paced to 1:1 comparison (Table 4). Figure 4 graphically demonstrates the increased PATs on dual-paced versus single-paced breathing.

Table 2.
The mean of the PAT values (ms) at different fiducial points of the PPG (n = 35): local minimum (T0LM), extrapolated point (T0EP), 1/3 height (T1/3), 1/2 height (T1/2), 2/3 height (T2/3) of the ascending slope, the peak (T3/3), the time interval between T1/3 and T2/3 (T1/3T2/3), the peak of the first derivative (Tdiff) and the smooth derivative (Tsmdiff) of the PPG signal, at different breathing patterns: single-paced breathing (1:0), 1:2, and 1:1 i/e ratio.

Table 3.
The p values of the Friedman tests (n = 35) according to the PAT-fiducial points.

Table 4.
The p values of post hoc Wilcoxon’s paired-sample test (n = 35) comparing the following breathing patterns: single-paced (1:0) to 1:2; and to 1:1 ratio; and 1:2 to 1:1 ratio. The fiducial points of the PPG signal: local minimum (T0LM), extrapolated point (T0EP), 1/3 height (T1/3), 1/2 height (T1/2), 2/3 height (T2/3) of the ascending slope, local maximum (T3/3), the average time interval between T1/3 and T2/3 points (T1/3T2/3), peak of first derivative (Tdiff), and smooth derivative (Tsmdiff).
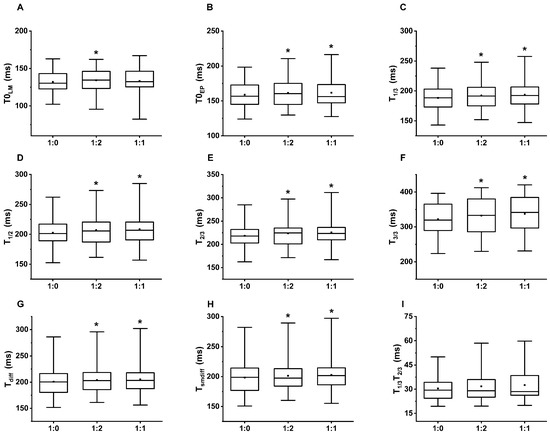
Figure 4.
The box and whiskers diagrams represent the PAT values. The significant difference compared to single paced (1:0) breathing is marked with *. The upper and lower sides of the box are the lower and upper quartiles. The box covers the interquartile interval. The horizontal line that splits the box is the median. The small square within the box is the mean. The whiskers indicate the minimum and maximum values in the study population. (A) PAT were calculated at the local minimum (T0LM), (B) extrapolated point (T0EP), (C) 1/3 height (T1/3), (D) 1/2 height (T1/2), (E) 2/3 height (T2/3) of the ascending slope, (F) local maximum (T3/3), (G) the peak of the first derivative (Tdiff) and (H) the smooth derivative (Tsmdiff) of the PPG signal. (I) There was no significant difference in the time interval between T1/3 and T2/3 (T1/3T2/3) regarding the i/e ratio.
3.2. HRA Parameters at Different Breathing Patterns
Significant differences were found among the breathing patterns by Friedman test at both GI and PI. Post hoc Wilcoxon paired-sample test proved a significant increase at 1:1 i/e ratio breathing compared to single-paced and 1:2 i/e ratios (Table 5, Figure 5), i.e., it is not the number of paces but the i/e ratio that determines the HRA parameters in contrast to the PAT values.

Table 5.
The values of Guzik and Porta indices at different breathing patterns (n = 35): single-paced (1:0), dual-paced at 1:2 and 1:1 i/e ratio.
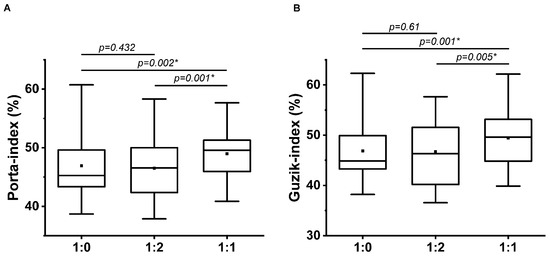
Figure 5.
Guzik (A) and Porta (B) indices at single-paced (1:0), 1:2, and 1:1 breathing ratio (n = 35); * indicates the significant differences by post hoc Wilcoxon Paired-Sample test (p < 0.01667). The upper and lower sides of the box are the lower and upper quartiles. The box covers the interquartile interval. The horizontal line that splits the box is the median. The small square within the box is the mean. The whiskers indicate the minimum and maximum values in the group.
4. Discussion
In the present study, we observed a significant increase in the average PAT values on dual-paced versus single-paced breathing patterns at each reference point except T0LM; however, the tendency was the same. On the contrary, the PI and GI significantly increased on 1:1 dual-paced breathing when compared to single-paced and 1:2 dual-paced respiration. The increased symmetry of breathing results in higher heart rate symmetry based on the HRA parameters. In considering 50.0 as perfect symmetry by PI and GI, the significantly increased parameters on 1:1 breathing versus 1:0 and 1:2 indicate the higher-level of symmetry (PI = 48.98 versus 46.91 and 46.50, respectively; GI = 49.42 versus 46.86 and 46.74, respectively). In support of our previous observations [15], the HRA changes due to respiration patterns reflect a proportional relation between breathing and heart rate control. At a 1:2 breathing ratio, there is relatively less time to inhale compared to 1:1 breathing. Thus, due to the “shorter” inhalation time, relatively fewer beats belong in the acceleration cloud. To preserve the mean heart rate in steady state conditions, the acceleration requires larger steps in “fewer RR intervals” in a 1:2 breathing. The short-term HRA due to breathing-related oscillations of RRIs is mediated by the parasympathetic tone rather than by the sympathetic activity, since the parasympathetic effects on the heart occur almost immediately (0.5 sec), while there is a delay of approximately 5 s prior to the sympathetic effects becoming noticeable [38]. Previous studies have also demonstrated how changes in posture affect the HRA parameters: Pawłowski et al. [39] investigated the effect of the head-up tilt (75°, HUTT) through the changing in HRA (PI, GI) and HRV parameters among healthy individuals. Their results showed that the GI significantly increased during the HUTT, indicating the sensitivity of HRA to orthostatic stress. This phenomenon was explained by the shift in the sympathovagal-balance from the supine position-related vagal saturation by vagal withdrawal, assuring a greater play for the RRI variability associated to respiratory changes. These results are in accordance with the previous study, in which Guzik et al. observed the asymmetry in the RRIs is proportionally more prominent during increased head-up tilt (75° and 90°) when compared to the supine position among healthy adults [17].
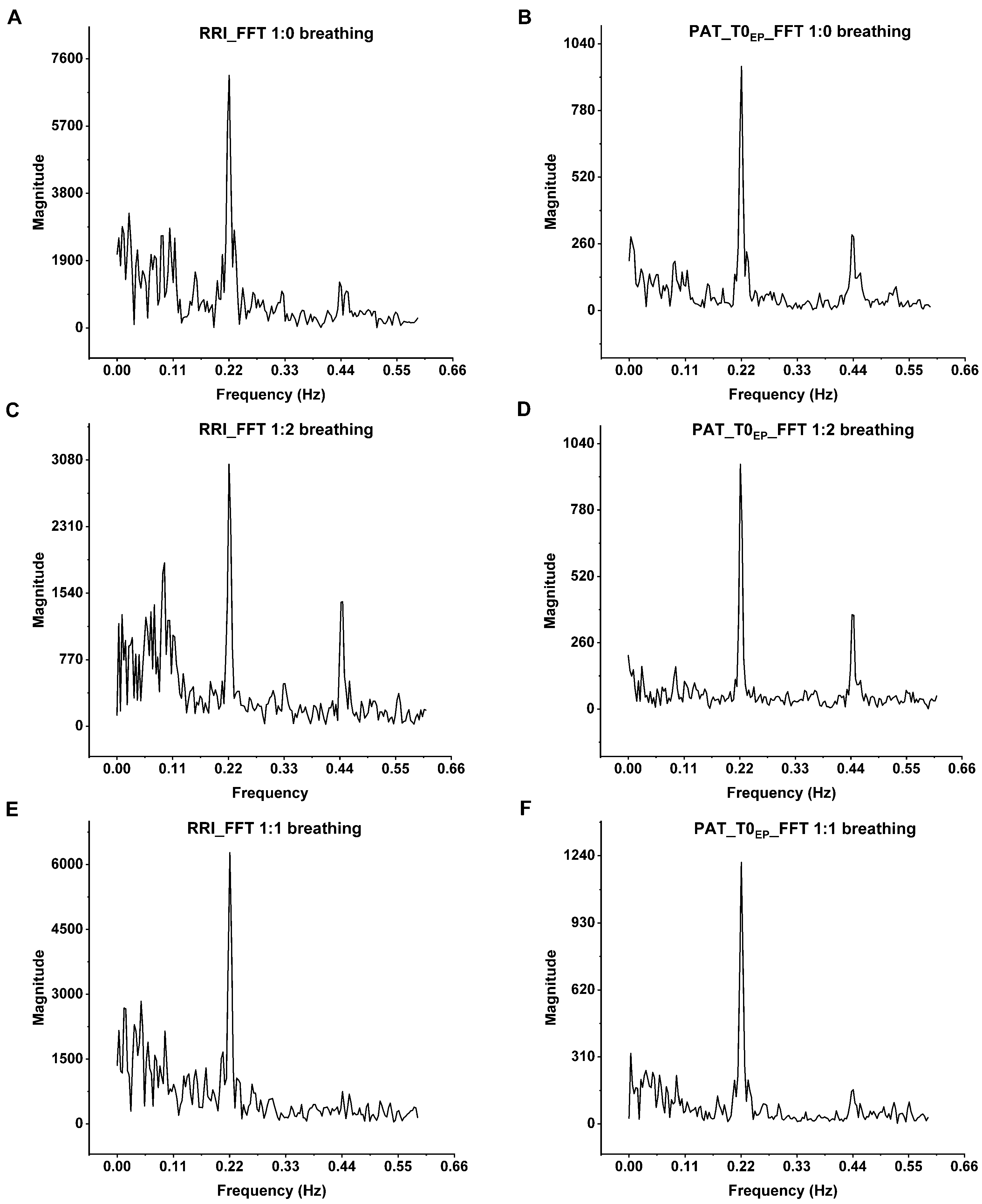
The close relation between the breathing and beat-to-beat PP interval variation has been observed by spectral evaluation of the PPG signal [7]. In the present research, FFT analysis was performed on a volunteer’s 5 min-long ECG recordings at each breathing pattern and the corresponding beat-to-beat PAT values at T0EP, in which the most prominent respiratory oscillation can be detected [5] (Figure 6.). The peak at 0.22 Hz, associated with the 4500 ms respiratory cycle of paced breathing, was noticeable both at RRI and at PATs as well. There is a second harmonic at 0.44 Hz, due to the asymmetry of 1:2 i/e ratio, in contrast to 1:1 breathing, in which the second harmonic nearly disappeared. In addition to the direct connections between the respiratory and circulatory centers, short-term intrathoracic pressure changes affect the heart rate and blood pressure due to respiration through hemodynamic actions on preload and subsequently stroke volume, thus influencing the PEP and PTT, and consequently PAT [40]. Cox et al. [41] investigated the relationship between PAT and BP during physiologically induced BP changes when the pathway contains resistance vessels (PPG) or merely the conducting arteries (radial artery tonometry) to investigate the BP-independent neurological effect on the peripheral vascular resistance. They found no BP-independent autonomic influence on PTT and PAT during rest, cold pressor test, cycling and isometric handgrip exercises.
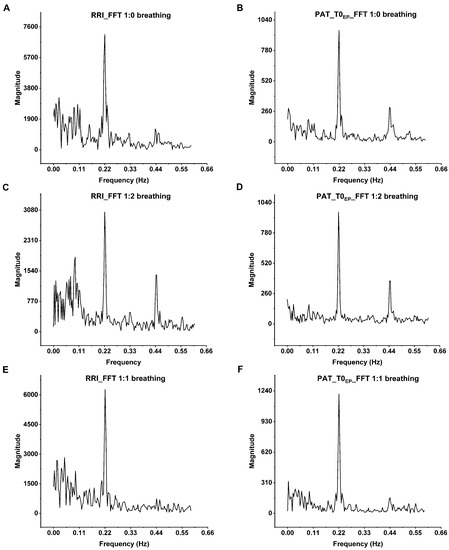
Figure 6.
The spectral analysis of the RRIs and the corresponding PAT values of the volunteer B_B_20000627 at (A,B) single-paced, (C,D) 1:2 and (E,F) 1:1 i/e ratio. The most prominent peak is at 0.22 Hz that corresponds to the respiratory sinus arrhythmia at 4500 ms paced breathing. On the FFT of both the ECG and PAT, there are second harmonics at 0.44 Hz. These harmonics are the most prominent at 1:2 breathing ratio and nearly disappear at 1:1 ratio due to the sinusoidal shape of breathing. The Origin Pro 2021 (OriginLab Corp., Northampton, MA, USA) software was used for the spectral analysis of beat-to-beat RRIs and PAT values.
The main result of the present study substantiates that dual-paced respiration proved to be the reason for the mild, but statistically significant, increase in the PAT values, regardless of the i/e ratio. Currently, we are limited to hypotheses and further investigations are needed to explain the phenomenon. Park et al. investigated the changes in the electroencephalogram (EEG) and HRV parameters during dual-paced breathing at 10 bpm, with a 2.4 s inhalation and 3.6 s-long exhalation period [42]. They found global increases in EEG parameters by the low- and high-frequency alpha power and a locally decreased theta power at dual-paced breathing compared to spontaneous respiration, as well as a significantly increased high-frequency band in the HRV, which reflects the parasympathetic activity. Due to the increase in low-frequency alpha power, they suggested that the paced breathing promotes the internal alertness, indicating a successful meditation. In our study, during dual-paced breathing, volunteers were triggered by two short beeps with different tones at the beginning of inhalation and exhalation. This is similar to the auditory oddball paradigm, in which participants have to discriminate between a standard and a target stimulus from one another and perform the action. Related to the target stimuli, a positive deviation of the electroencephalogram (EEG) signal appears at approximately 300 ms delay, known as P300, which is a component of the event-related potential. It reflects the cognitive process: attention, short-memory, stimulus evaluation and decision-making [43,44]. Several areas of research have focused on the relationship between the autonomic nervous system and the higher-level brain controls. Ito et al. [45] examined the changes in sympathetic nerve activity (SSNA) during the auditory oddball task among healthy volunteers. They found significantly higher incidences of SSNA following the target stimulus. This phenomenon was not detectable in the case of the passive oddball paradigm, during which the subjects had to ignore the auditory signal. They also observed a sympathetic skin response and the reduction of skin blood flow following the SSNA burst. The decrease in skin blood flow may also have occurred during our measurements, which could explain the consequential increase in the PAT values.
In another study, the activation of the anterior cingulate cortex and the cerebellum were observed [46] during stochastic decision-making tasks. The increase in the PAT values on dual-paced respiration may indicate the presence of complex connections between the autonomic nervous system and higher-level brain areas in the explanation; however, further investigations are needed in order to clarify the role of dual-paced vs. single-paced respiration in this context.
Study limitations: The low sample size (n = 35) may be considered as a limitation; however, the differences were proved to be statistically significant. Another shortcoming is the lack of continuous blood pressure measurement. Invasive measurement was not an option, and inflating and deflating the cuff for calibration would have disturbed the resting conditions.
5. Conclusions
According to our knowledge, this was the first study investigating the effects of the inspiration/expiration ratios and the number of respiration pacing pulses on the PAT values, considering multiple fiducial points on the PPG signal. The main finding of this research is that changes in the inspiration/expiration ratio do not have significant effects upon the average PAT values, regardless of the reference points. However, the significant increase in PATs during dual-paced breathing patterns can reflect the reduced blood pressure and/or reduced skin blood flow. The different actions of the single- and dual-paced respirations imply the involvement of higher-order cortical functions and their relationship to the autonomic nervous system. On the contrary, the inspiration to expiration ratio, not the number of pacing triggers, significantly influences the HRA parameters among healthy individuals according to PI and GI. A symmetrical breathing pattern results in greater symmetry in heart rate accelerations and decelerations. We hypothesize that the short-term effects of breathing on HRA are mediated by the vagus nerve via RSA; whereas the actions of respiration on PAT are possibly related to the arterial blood pressure and skin blood flow changes through peripheral sympathetic activity modulated by cognitive levels and not to the intrathoracic pressure variations. Considering the above experiences, these effects should be taken into account in studies examining mental load. Further research is encouraged to elucidate the exact pathomechanism.
Author Contributions
Conceptualization, L.H.; methodology, L.H.; software, L.H.; validation, L.H.; formal analysis, B.E.A. and L.H.; investigation, B.E.A., S.B. and L.H.; resources, L.H.; data curation, B.E.A. and S.B.; writing—original draft preparation, B.E.A. and L.H.; writing—review and editing, L.H.; supervision, L.H.; project administration, B.E.A., S.B. and L.H.; funding acquisition, L.H. All authors have read and agreed to the published version of the manuscript.
Funding
University of Pécs, Medical School, Hungary, provided financial support (3/2016 AOK-KA) for the instrumentation without any other involvement in the study.
Institutional Review Board Statement
The ethical approval was granted by the Regional Research Ethics Committee (approval numbers: 7533-PTE-2018; 7535-PTE 2019) and was conducted in full accordance with the Declaration of Helsinki and its later amendments for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The authors do not have permission to share data.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| BP | blood pressure |
| bpm | breath per minute |
| ECG | electrocardiography/electrocardiogram |
| EEG | electroencephalogram |
| FFT | fast Fourier transformation |
| GI | Guzik-index |
| HR | heart rate |
| HRA | heart rate asymmetry |
| HRV | heart rate variability |
| PAT | pulse arrival time |
| PEP | pre-ejection period |
| PI | Porta-index |
| PPG | photoplethysmography/photoplethysmogram |
| PRV | pulse rate variability |
| PTT | pulse transit time |
| RMSSD | root mean square of successive RR-differences |
| RRI | R-R interval |
References
- Seneviratne, S.; Hu, Y.; Nguyen, T.; Lan, G.; Khalifa, S.; Thilakarathna, K.; Hassan, M.; Seneviratne, A. A Survey of Wearable Devices and Challenges. IEEE Commun. Surv. Tutor 2017, 19, 2573–2620. [Google Scholar] [CrossRef]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, P.A.; Allen, J. Photoplethysmography: Technology, Signal Analysis and Applications; Academic Press: New York, NY, USA, 2021. [Google Scholar]
- Shao, D.; Yang, Y.; Liu, C.; Tsow, F.; Yu, H.; Tao, N. Noncontact monitoring breathing pattern, exhalation flow rate and pulse transit time. IEEE Trans. Biomed. Eng. 2014, 61, 2760–2767. [Google Scholar] [CrossRef] [PubMed]
- Ajtay, B.E.; Béres, S.; Hejjel, L. The oscillating pulse arrival time as a physiological explanation regarding the difference between ECG- and Photoplethysmogram-derived heart rate variability parameters. Biomed. Signal Process. Control. 2023, 79, 104033. [Google Scholar] [CrossRef]
- Nelson, B.W.; Allen, N.B. Accuracy of Consumer Wearable Heart Rate Measurement during an Ecologically Valid 24-Hour Period: Intraindividual Validation Study. JMIR Mhealth Uhealth 2019, 7, e10828. [Google Scholar] [CrossRef]
- Béres, S.; Hejjel, L. The minimal sampling frequency of the photoplethysmogram for accurate pulse rate variability parameters in healthy volunteers. Biomed. Signal Process. Control. 2021, 68, 102589. [Google Scholar] [CrossRef]
- Béres, S.; Holczer, L.; Hejjel, L. On the Minimal Adequate Sampling Frequency of the Photoplethysmogram for Pulse Rate Monitoring and Heart Rate Variability Analysis in Mobile and Wearable Technology. Meas. Sci. Rev. 2019, 19, 232–240. [Google Scholar] [CrossRef]
- Mejía-Mejía, E.; May, J.M.; Kyriacou, P.A. Effects of using different algorithms and fiducial points for the detection of interbeat intervals, and different sampling rates on the assessment of pulse rate variability from photoplethysmography. Comput. Methods Programs Biomed. 2022, 218, 106724. [Google Scholar] [CrossRef]
- Mejia-Mejia, E.; May, J.M.; Kyriacou, P.A. Effect of Filtering of Photoplethysmography Signals in Pulse Rate Variability Analysis. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Jalisco, Mexico, 1–5 November 2021; Volume 2021, pp. 5500–5503. [Google Scholar] [CrossRef]
- Charlton, P.H.; Bonnici, T.; Tarassenko, L.; Alastruey, J.; Clifton, D.A.; Beale, R.; Watkinson, P.J. Extraction of respiratory signals from the electrocardiogram and photoplethysmogram: Technical and physiological determinants. Physiol. Meas. 2017, 38, 669–690. [Google Scholar] [CrossRef]
- Hejjel, L.; Gál, I. Heart rate variability analysis. Acta Physiol. Hung. 2001, 88, 219–230. [Google Scholar] [CrossRef]
- Piskorski, J.; Guzik, P. Asymmetric properties of long-term and total heart rate variability. Med. Biol. Eng. Comput. 2011, 49, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, L.D.; Behnke, M.; Enko, J.; Kosakowski, M.; Hughes, B.M.; Piskorski, J.; Guzik, P. Effects of emotions on heart rate asymmetry. Psychophysiology 2019, 56, e13318. [Google Scholar] [CrossRef] [PubMed]
- Klintworth, A.; Ajtay, Z.; Paljunite, A.; Szabados, S.; Hejjel, L. Heart rate asymmetry follows the inspiration/expiration ratio in healthy volunteers. Physiol. Meas. 2012, 33, 1717–1731. [Google Scholar] [CrossRef]
- Karmakar, C.K.; Khandoker, A.H.; Gubbi, J.; Palaniswami, M. Defining asymmetry in heart rate variability signals using a Poincaré plot. Physiol. Meas. 2009, 30, 1227–1240. [Google Scholar] [CrossRef]
- Porta, A.; Casali, K.; Casali, A.G.; Gnecchi-Ruscone, T.; Tobaldini, E.; Montano, N.; Lange, S.; Geue, D.; Cysarz, D.; Van Leeuwen, P. Temporal asymmetries of short-term heart period variability are linked to autonomic regulation. Am. J. Physiol. Integr. Comp. Physiol. 2008, 295, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Vagedes, J. How accurate is pulse rate variability as an estimate of heart rate variability?: A review on studies comparing photoplethysmographic technology with an electrocardiogram. Int. J. Cardiol. 2013, 166, 15–29. [Google Scholar] [CrossRef]
- Yuda, E.; Shibata, M.; Ogata, Y.; Ueda, N.; Yambe, T.; Yoshizawa, M.; Hayano, J. Pulse rate variability: A new biomarker, not a surrogate for heart rate variability. J. Physiol. Anthr. 2020, 39, 21. [Google Scholar] [CrossRef] [PubMed]
- Bánhalmi, A.; Borbás, J.; Fidrich, M.; Bilicki, V.; Gingl, Z.; Rudas, L. Analysis of a Pulse Rate Variability Measurement Using a Smartphone Camera. J. Health Eng. 2018, 2018, 4038034. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Lin, Y.-Y.; Shih, C.-C.; Kuo, C.-D. Relationship between Heart Rate Variability and Pulse Rate Variability Measures in Patients after Coronary Artery Bypass Graft Surgery. Front. Cardiovasc. Med. 2021, 8, 1892. [Google Scholar] [CrossRef]
- Shi, P.; Hu, S.; Zhu, Y. A preliminary attempt to understand compatibility of photoplethysmographic pulse rate variability with electrocardiogramic heart rate variability. J. Med. Biol. Eng. 2008, 28, 173–180. [Google Scholar]
- Zaunseder, S.; Vehkaoja, A.; Fleischhauer, V.; Antink, C.H. Signal-to-noise ratio is more important than sampling rate in beat-to-beat interval estimation from optical sensors. Biomed. Signal Process. Control. 2022, 74, 103538. [Google Scholar] [CrossRef]
- Mejía-Mejía, E.; Kyriacou, P.A. Effects of noise and filtering strategies on the extraction of pulse rate variability from photoplethysmograms. Biomed. Signal Process. Control. 2023, 80, 104291. [Google Scholar] [CrossRef]
- Peralta, E.; Lazaro, J.; Bailon, R.; Marozas, V.; Gil, E. Optimal fiducial points for pulse rate variability analysis from forehead and finger photoplethysmographic signals. Physiol. Meas. 2019, 40, 025007. [Google Scholar] [CrossRef]
- Finnegan, E.; Davidson, S.; Harford, M.; Jorge, J.; Watkinson, P.; Young, D.; Tarassenko, L.; Villarroel, M. Pulse arrival time as a surrogate of blood pressure. Sci. Rep. 2021, 11, 22767. [Google Scholar] [CrossRef] [PubMed]
- Landry, C.; Hedge, E.T.; Hughson, R.L.; Peterson, S.D.; Arami, A. Cuffless Blood Pressure Estimation during Moderate- and Heavy-Intensity Exercise Using Wearable ECG and PPG. IEEE J. Biomed. Health Inform. 2022, 26, 5942–5952. [Google Scholar] [CrossRef] [PubMed]
- Shin, H. A novel method for non-invasive blood pressure estimation based on continuous pulse transit time: An observational study. Psychophysiology 2023, 60, e14173. [Google Scholar] [CrossRef]
- Louka, K.; Cox, J.; Tan, I.; Avolio, A.P.; O’Rourke, M.F.; Butlin, M. An investigation of the individualized, two-point calibration method for cuffless blood pressure estimation using pulse arrival time: An historical perspective using the Casio BP-100 digital watch. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Jalisco, Mexico, 26 July 2021; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2021; pp. 7493–7496. [Google Scholar] [CrossRef]
- Bachler, M.; Sehnert, W.; Mikisek, I.; Wassertheurer, S.; Mengden, T. Non-invasive quantification of the effect of device-guided slow breathing with direct feedback to the patient to reduce blood pressure. Physiol. Meas. 2020, 41, 104002. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, B.; Wu, H.; Chai, X.; Wang, W.; Peng, C.-K. Effects of slow and regular breathing exercise on cardiopulmonary coupling and blood pressure. Med. Biol. Eng. Comput. 2017, 55, 327–341. [Google Scholar] [CrossRef]
- Béres, S.; Németh, A.; Ajtay, Z.; Kiss, I.; Németh, B.; Hejjel, L. Cellular phone irradiation of the head affects heart rate variability depending on inspiration/expiration ratio. In Vivo 2018, 32, 1145–1153. [Google Scholar] [CrossRef]
- Lind, F.; Hesser, C.M. Breathing pattern and lung volumes during exercise. Acta Physiol. Scand. 1984, 120, 123–129. [Google Scholar] [CrossRef]
- Chen, Y.; Li, D.; Li, Y.; Ma, X.; Wei, J. Use Moving Average Filter to Reduce Noises in Wearable PPG during Continuous Monitoring. In Lecture Notes of the Institute for Computer Sciences, Social-Informatics and Telecommunications Engineering, LNICST; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 193–203. [Google Scholar] [CrossRef]
- Hamilton, P.S.; Tompkins, W.J. Quantitative Investigation of QRS Detection Rules Using the MIT/BIH Arrhythmia Database. IEEE Trans. Biomed. Eng. 1986, 12, 1157–1165. [Google Scholar] [CrossRef]
- Holoborodko, P. Smooth Noise-Robust Differentiators. Available online: http://www.holoborodko.com/pavel/numerical-methods/numerical-derivative/smooth-low-noise-differentiators/ (accessed on 12 December 2021).
- Abdi, H. Bonferroni and Šidák corrections for multiple comparisons. Encycl. Meas. Stat. 2007, 3, 2007. [Google Scholar]
- Guzik, P.; Piskorski, J.; Krauze, T.; Wykretowicz, A.; Wysocki, H. Heart rate asymmetry by Poincaré plots of RR intervals. Biomed. Tech. 2006, 51, 272–275. [Google Scholar] [CrossRef]
- Pawłowski, R.; Buszko, K.; Newton, J.L.; Kujawski, S.; Zalewski, P. Heart Rate Asymmetry Analysis during Head-Up Tilt Test in Healthy Men. Front. Physiol. 2021, 12, 495. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.A.; Santarelli, D.M.; O’rourke, D. The physiological effects of slow breathing in the healthy human. Breathe 2017, 13, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Avolio, A.P.; Louka, K.; Shirbani, F.; Tan, I.; Butlin, M. Blood pressure-independent neurogenic effect on conductance and resistance vessels: A consideration for cuffless blood pressure measurement? In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Virtual, Jalisco, Mexico, 1–5 November 2021; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2021; pp. 7485–7488. [Google Scholar] [CrossRef]
- Park, Y.-J. Clinical utility of paced breathing as a concentration meditation practice. Complement. Ther. Med. 2012, 20, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Picton, T.W. The P300 Wave of the Human Event-Related Potential. J. Clin. Neurophysiol. 1992, 9, 456–479. [Google Scholar] [CrossRef]
- Ajtay, Z.; Kellényi, L.; Hejjel, L.; Solymos, A.; Németh, A.; Bártfai, I.; Kovács, N.; Cziráki, A.; Papp, L. Simple and choice reaction times are prolonged following extracorporeal circulation: A potential method for the assessment of acute neurocognitive deficit. Med. Sci. Monit. 2009, 15, CR470–CR476. [Google Scholar] [PubMed]
- Ito, H.; Sugiyama, Y.; Mano, T.; Okada, H.; Matsukawa, T.; Iwase, S. Skin sympathetic nerve activity and event-related potentials during auditory oddball paradigms. J. Auton. Nerv. Syst. 1996, 60, 129–135. [Google Scholar] [CrossRef]
- Ohira, H.; Ichikawa, N.; Nomura, M.; Isowa, T.; Kimura, K.; Kanayama, N.; Fukuyama, S.; Shinoda, J.; Yamada, J. Brain and autonomic association accompanying stochastic decision-making. Neuroimage 2010, 49, 1024–1037. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).