Spontaneous Osteogenic Potential of Periosteum after Segmental Mandibulectomy in Patients with Medication-Related Osteonecrosis of the Jaw (MRONJ): A Retrospective Study of 14 Cases
Abstract
1. Introduction
2. Materials and Methods
- Current or previous treatment with antiresorptive therapy alone or in combination with immune modulators or antiangiogenic medications.
- Exposed bone or bone that can be probed through an intraoral or extraoral fistula(e) in the maxillofacial region persisting for more than 8 weeks.
- No history of radiation therapy to the jaws or metastatic disease to the jaws.
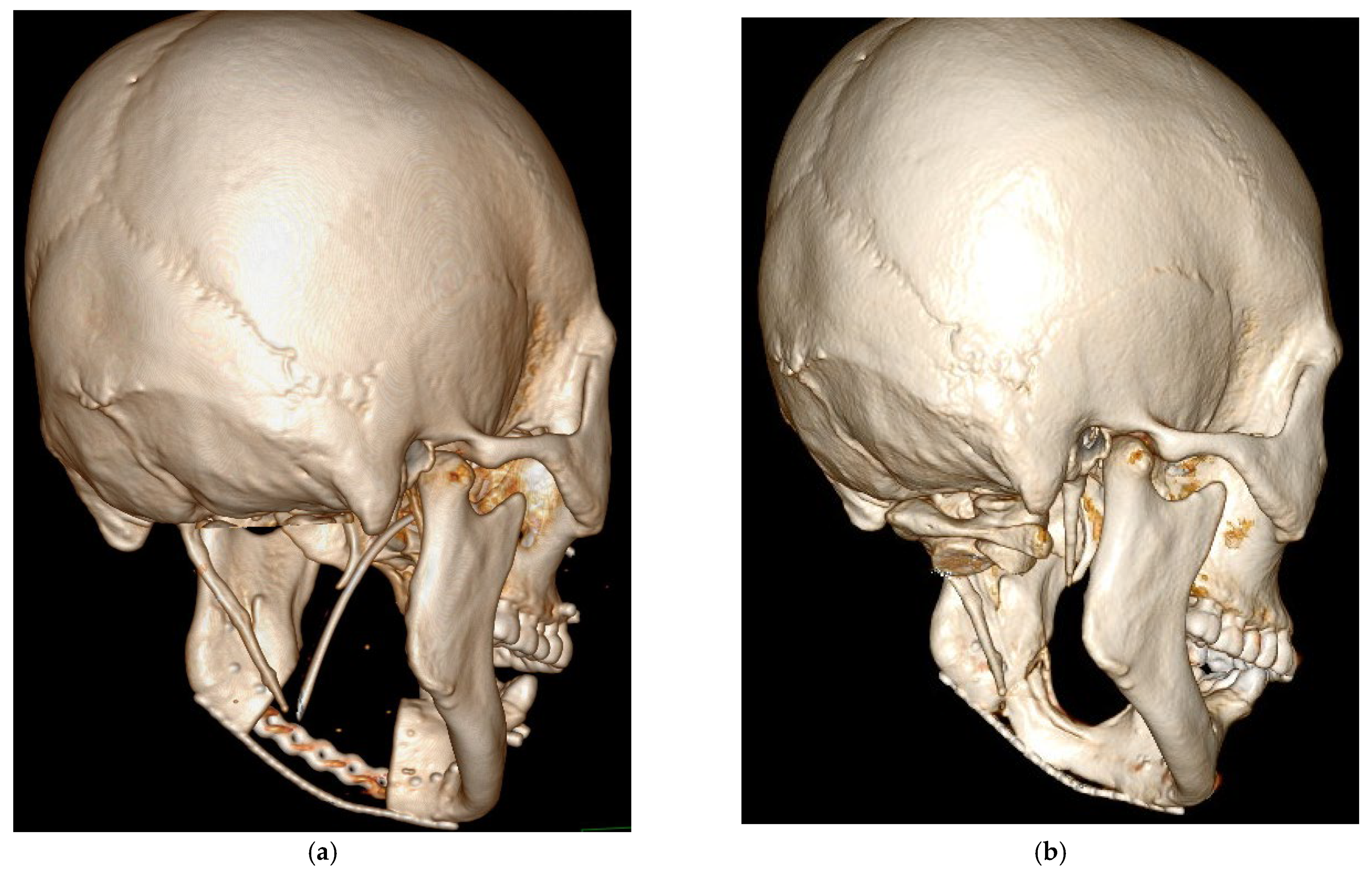
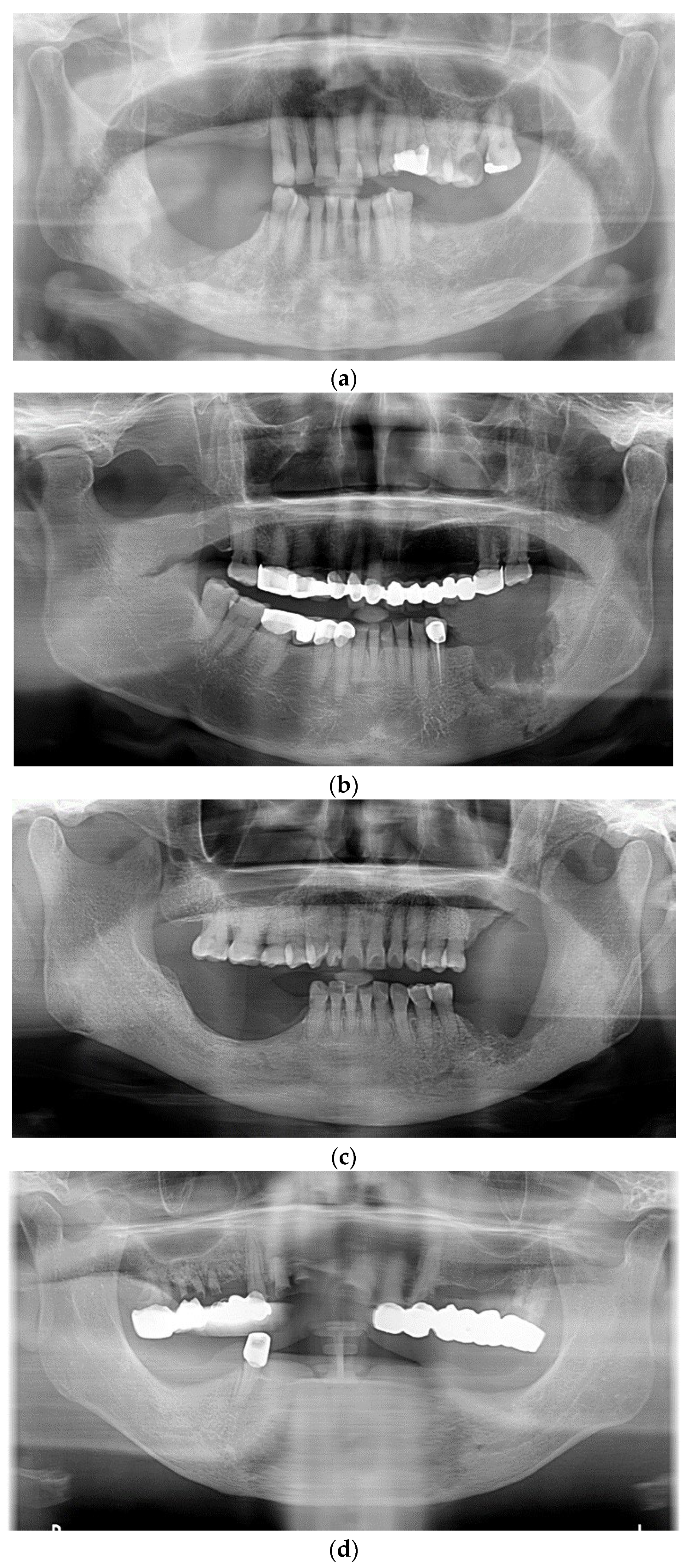
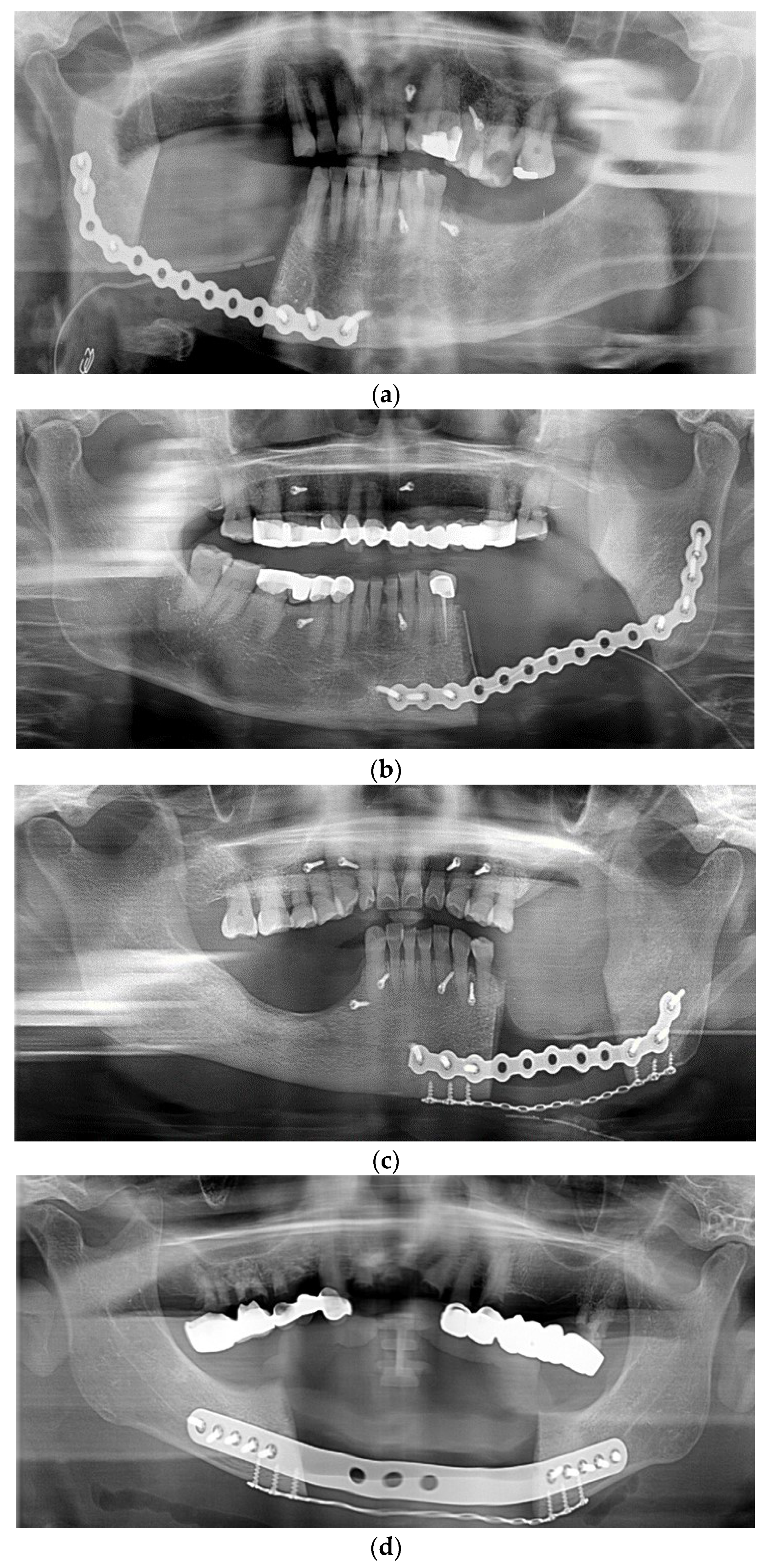
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Mehrotra, B.; Rosenberg, T.J.; Engroff, S.L. Osteonecrosis of the jaws associated with the use of bisphosphonates: A review of 63 cases. J. Oral Maxillofac. Surg. 2004, 62, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Aghaloo, T.L.; Dry, S.M.; Mallya, S.; Tetradis, S. Osteonecrosis of the jaw in a patient on denosumab. J. Oral Maxillofac. Surg. 2014, 72, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Saag, K.G.; Petersen, J.; Brandi, M.L.; Karaplis, A.C.; Lorentzon, M.; Thomas, T.; Maddox, J.; Fan, M.; Meisner, P.D.; Grauer, A. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N. Engl. J. Med. 2017, 377, 1417–1427. [Google Scholar] [CrossRef]
- Estilo, C.L.; Fornier, M.; Farooki, A.; Carlson, D.; Bohle, G., 3rd; Huryn, J.M. Osteonecrosis of the jaw related to bevacizumab. J. Clin. Oncol. 2008, 26, 4037–4038. [Google Scholar] [CrossRef]
- Sivolella, S.; Lumachi, F.; Stellini, E.; Favero, L. Denosumab and anti-angiogenetic drug-related osteonecrosis of the jaw: An uncommon but potentially severe disease. Anticancer Res. 2013, 33, 1793–1797. [Google Scholar]
- Alsalleeh, F.; Keippel, J.; Adams, L.; Bavitz, B. Bisphosphonate-associated osteonecrosis of jaw reoccurrence after methotrexate therapy: A case report. J. Endod. 2014, 40, 1505–1507. [Google Scholar] [CrossRef]
- Henien, M.; Carey, B.; Hullah, E.; Sproat, C.; Patel, V. Methotrexate-associated osteonecrosis of the jaw: A report of two cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 124, e283–e287. [Google Scholar] [CrossRef]
- Hamadeh, I.S.; Ngwa, B.A.; Gong, Y. Drug induced osteonecrosis of the jaw. Cancer Treat. Rev. 2015, 41, 455–464. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws—2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef] [PubMed]
- Ristow, O.; Rückschloß, T.; Müller, M.; Berger, M.; Kargus, S.; Pautke, C. Is the conservative non-surgical management of medication-related osteonecrosis of the jaw an appropriate treatment option for early stages? A long-term single-center cohort study. J. Craniomaxillofac. Surg. 2019, 47, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Wilde, F.; Heufelder, M.; Winter, K.; Hendricks, J.; Frerich, B.; Schramm, A. The role of surgical therapy in the management of intravenous bisphosphonates-related osteonecrosis of the jaw. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 111, 153–163. [Google Scholar] [CrossRef]
- Kyrgidis, A.; Vahtsevanos, K.; Koloutsos, G.; Andreadis, C.; Boukovinas, I.; Teleioudis, Z. Bisphosphonate-related osteonecrosis of the jaws: A case-control study of risk factors in breast cancer patients. J. Clin. Oncol. 2008, 26, 4634–4638. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Warraich, R.; Kokemüller, H.; Lemound, J.; Essig, H.; Tavassol, F. Reconstruction of mandibular defects-clinical retrospective research over a 10-year period. Head Neck Oncol. 2011, 3, 23. [Google Scholar] [CrossRef]
- Sacco, R.; Sacco, N.; Hamid, U.; Ali, S.H.; Singh, M.; Blythe, J.S.J. Microsurgical Reconstruction of the Jaws Using Vascularised Free Flap Technique in Patients with Medication-Related Osteonecrosis: A Systematic Review. BioMed Res. Int. 2018, 2018, 9858921. [Google Scholar] [CrossRef]
- Verhelst, P.J.; Dons, F.; Van Bever, P.J.; Schoenaers, J.; Nanhekhan, L.; Politis, C. Fibula Free Flap in Head and Neck Reconstruction: Identifying Risk Factors for Flap Failure and Analysis of Postoperative Complications in a Low Volume Setting. Craniomaxillofac. Trauma Reconstr. 2019, 12, 183–192. [Google Scholar] [CrossRef]
- Akinbami, B.O. Reconstruction of Continuity Defects of the Mandible with Non-vascularized Bone Grafts. Systematic literature review. Craniomaxillofac. Trauma Reconstr. 2016, 9, 195–205. [Google Scholar] [CrossRef]
- Sun, S.H.; Tsai, W.W.; Shiu, S.I.; Chen, C.H. Induced membrane technique for large bone defects: A systematic review and individual participant data meta-analysis. Medicine 2022, 101, e29292. [Google Scholar] [CrossRef]
- Hibi, H.; Ueda, M. Supraperiosteal transport distraction osteogenesis for reconstructing a segmental defect of the mandible. J. Oral Maxillofac. Surg. 2011, 69, 742–746. [Google Scholar] [CrossRef]
- Bede, S.Y.H.; Ismael, W.K.; Hashim, E.A. Reconstruction plate-related complications in mandibular continuity defects. Oral Maxillofac. Surg. 2019, 23, 193–199. [Google Scholar] [CrossRef]
- Anyanechi, C.E.; Saheeb, B.D.; Bassey, G.O. Spontaneous bone regeneration after segmental mandibular resection: A retrospective study of 13 cases. Int. J. Oral Maxillofac. Surg. 2016, 45, 1268–1272. [Google Scholar] [CrossRef] [PubMed]
- Goh, B.T.; Lee, S.; Tideman, H.; Stoelinga, P.J. Mandibular reconstruction in adults: A review. Int. J. Oral Maxillofac. Surg. 2008, 37, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Rattan, V.; Jolly, S.S.; Sharma, V.K.; Mubashir, M.M. Spontaneous Regeneration of Bone in Segmental Mandibular Defect. J. Maxillofac. Oral Surg. 2019, 18, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Almansoori, A.A.; Choung, H.W.; Kim, B.; Park, J.Y.; Kim, S.M.; Lee, J.H. Fracture of Standard Titanium Mandibular Reconstruction Plates and Preliminary Study of Three-Dimensional Printed Reconstruction Plates. J. Oral Maxillofac. Surg. 2020, 78, 153–166. [Google Scholar] [CrossRef]
- Boyne, P.J. The restoration of resected mandibles in children without the use of bone grafts. Head Neck Surg. 1983, 6, 626–631. [Google Scholar] [CrossRef]
- Lemperle, S.M.; Calhoun, C.J.; Curran, R.W.; Holmes, R.E. Bony healing of large cranial and mandibular defects protected from soft-tissue interposition: A comparative study of spontaneous bone regeneration, osteoconduction, and cancellous autografting in dogs. Plast. Reconstr. Surg. 1998, 101, 660–672. [Google Scholar] [CrossRef]
- Sherris, D.A.; Murakami, C.S.; Larrabee, W.F., Jr.; Bruce, A.G. Mandibular reconstruction with transforming growth factor-beta1. Laryngoscope 1998, 108, 368–372. [Google Scholar] [CrossRef]
- Ishimaru, M.; Ono, S.; Morita, K.; Matsui, H.; Hagiwara, Y.; Yasunaga, H. Prevalence, Incidence Rate, and Risk Factors of Medication-Related Osteonecrosis of the Jaw in Patients with Osteoporosis and Cancer: A Nationwide Population-Based Study in Japan. J. Oral Maxillofac. Surg. 2022, 80, 714–727. [Google Scholar] [CrossRef]
- Aljohani, S.; Fliefel, R.; Ihbe, J.; Kühnisch, J.; Ehrenfeld, M.; Otto, S. What is the effect of anti-resorptive drugs (ARDs) on the development of medication-related osteonecrosis of the jaw (MRONJ) in osteoporosis patients: A systematic review. J. Craniomaxillofac. Surg. 2017, 45, 1493–1502. [Google Scholar] [CrossRef]
- Govaerts, D.; Piccart, F.; Ockerman, A.; Coropciuc, R.; Politis, C.; Jacobs, R. Adjuvant therapies for MRONJ: A systematic review. Bone 2020, 141, 115676. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.; Barone, S.; Giudice, C.; Bennardo, F.; Fortunato, L. Can platelet-rich fibrin improve healing after surgical treatment of medication-related osteonecrosis of the jaw? A pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Ristow, O.; Otto, S.; Geiß, C.; Kehl, V.; Berger, M.; Troeltzsch, M.; Koerdt, S.; Hohlweg-Majert, B.; Freudlsperger, C.; Pautke, C. Comparison of auto-fluorescence and tetracycline fluorescence for guided bone surgery of medication-related osteonecrosis of the jaw: A randomized controlled feasibility study. Int. J. Oral Maxillofac. Surg. 2017, 46, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Merigo, E.; Cella, L.; Oppici, A.; Cristina Arbasi, M.; Clini, F.; Fontana, M.; Fornaini, C. Combined Approach to Treat Medication-Related Osteonecrosis of the Jaws. J. Lasers Med. Sci. 2018, 9, 92–100. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Scribante, A. Oral Microbiota in Patients with Peri-Implant Disease: A Narrative Review. Appl. Sci. 2022, 12, 3250. [Google Scholar] [CrossRef]
- Vale, G.C.; Mayer, M.P.A. Effect of probiotic Lactobacillus rhamnosus by-products on gingival epithelial cells challenged with Porphyromonas gingivalis. Arch. Oral. Biol. 2021, 128, 105174. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Cuggia, G.; Scribante, A. Domiciliary Use of Chlorhexidine vs. Postbiotic Gels in Patients with Peri-Implant Mucositis: A Split-Mouth Randomized Clinical Trial. Appl. Sci. 2022, 12, 2800. [Google Scholar] [CrossRef]
- Yamada, S.I.; Kurita, H.; Kondo, E.; Suzuki, S.; Nishimaki, F.; Yoshimura, N.; Morioka, M.; Ishii, S.; Kamata, T. Treatment outcomes and prognostic factors of medication-related osteonecrosis of the jaw: A case- and literature-based review. Clin. Oral Investig. 2019, 23, 3203–3211. [Google Scholar] [CrossRef]

| No. | Age (Year) | Follow-Up Period (Month) | Gender | New Bone Formation | Bony Bridge Formation | R-Plate Fracture | Medication Type | Duration |
|---|---|---|---|---|---|---|---|---|
| 1 | 73.76 | 50.08 | Female | Present | Present | None | Both | >4 years |
| 2 | 73.48 | 45.42 | Female | Present | Present | None | Bisphosphonates | >4 years |
| 3 | 80.72 | 41.25 | Male | Present | Absent | Present | Bisphosphonates | 2 years |
| 4 | 70.77 | 37.00 | Female | Present | Present | None | Bisphosphonates | 3 years |
| 5 | 71.70 | 32.42 | Female | Present | Present | None | Bisphosphonates | 3 years |
| 6 | 85.01 | 28.67 | Female | Present | Absent | None | Bisphosphonates | >4 years |
| 7 | 83.12 | 25.17 | Female | Present | Absent | None | Bisphosphonates | >4 years |
| 8 | 78.39 | 22.75 | Female | Present | Absent | None | Bisphosphonates | 2 years |
| 9 | 71.72 | 22.67 | Female | Present | Present | None | Bisphosphonates | >4 years |
| 10 | 50.27 | 19.08 | Female | Present | Present | None | Bisphosphonates | 3 years |
| 11 | 62.50 | 16.92 | Male | Present | Present | Present | Denosumab | >4 years |
| 12 | 78.98 | 16.25 | Female | Present | Present | None | Bisphosphonates | 1 year |
| 13 | 87.19 | 14.58 | Female | Present | Present | None | Bisphosphonates | 1 year |
| 14 | 79.22 | 12.67 | Female | Present | Present | None | Denosumab | 2 years |
| Characteristic | |
|---|---|
| Age, y, mean ± SD | 74.8 ± 9.3 |
| Range, y | 50.3–87.2 |
| Follow-up period, m, mean ± SD | 27.5 ± 11.6 |
| Range, m | 12.7–48.3 |
| Gender, n (%) | |
| Male | 2 (14.3%) |
| Female | 12 (85.7%) |
| New bone formation, n (%) | |
| Present | 14 (100%) |
| Absent | 0 (0%) |
| Bony bridge formation, n (%) | |
| Present | 10 (71.4%) |
| Absent | 4 (28.6%) |
| R-plate fracture, n (%) | |
| None | 12 (85.7%) |
| Present | 2 (14.3%) |
| Medication type | |
| Bisphosphonates | 11 (78.6%) |
| Denosumab | 2 (14.3%) |
| Both | 1 (7.1%) |
| Duration of medication | |
| >4 years | 7 (50%) |
| <4 years | 7 (50%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, Y.; Fang, Y.-Q.; Lee, S.; Lee, C. Spontaneous Osteogenic Potential of Periosteum after Segmental Mandibulectomy in Patients with Medication-Related Osteonecrosis of the Jaw (MRONJ): A Retrospective Study of 14 Cases. Appl. Sci. 2023, 13, 5426. https://doi.org/10.3390/app13095426
Kwon Y, Fang Y-Q, Lee S, Lee C. Spontaneous Osteogenic Potential of Periosteum after Segmental Mandibulectomy in Patients with Medication-Related Osteonecrosis of the Jaw (MRONJ): A Retrospective Study of 14 Cases. Applied Sciences. 2023; 13(9):5426. https://doi.org/10.3390/app13095426
Chicago/Turabian StyleKwon, Youngmin, Yi-Qin Fang, Seungjin Lee, and Chunui Lee. 2023. "Spontaneous Osteogenic Potential of Periosteum after Segmental Mandibulectomy in Patients with Medication-Related Osteonecrosis of the Jaw (MRONJ): A Retrospective Study of 14 Cases" Applied Sciences 13, no. 9: 5426. https://doi.org/10.3390/app13095426
APA StyleKwon, Y., Fang, Y.-Q., Lee, S., & Lee, C. (2023). Spontaneous Osteogenic Potential of Periosteum after Segmental Mandibulectomy in Patients with Medication-Related Osteonecrosis of the Jaw (MRONJ): A Retrospective Study of 14 Cases. Applied Sciences, 13(9), 5426. https://doi.org/10.3390/app13095426







