Abstract
The positive airway pressure (PAP) is a gold standard in therapy for obstructive sleep apnea (OSA) patients, though weight loss is among the most effective supportive therapeutic methods. The aim of the study is to conduct a systematic review of randomized controlled trials (RCTs) of diet therapy interventions for OSA patients treated with PAP. The systematic review was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42023398374). The 1436 records were screened and five records were included identified as RCTs available within PubMed and Web of Science databases until 1 February 2023. Two researchers independently conducted identification, screening, inclusion, and evaluation of RCTs, using the revised Cochrane risk-of-bias tool for randomized trials. The studies were conducted in groups with patients treated or intended to be treated with CPAP, while patients with moderate-to-severe or severe OSA were included. Within all included studies, an experimental diet was compared with a control group; however, various interventions were applied, including dietary intervention, which was compared with no intervention, and dietary and physical activity intervention, which was only compared with general advice. The applied intervention included various dietary approaches, such as various energy deficits or dietary health-promoting behaviors. The monitored variables included apnea–hypopnea index (AHI), sleep time, sleep efficiency, sleep latency, oxygen desaturation episodes, oxygen saturation, OSA type, Epworth Sleepiness Scale score, Pittsburgh Sleep Quality Index, and quality of life. For the majority of studies, some concerns were defined for the risk of bias; however, for one study the risk was high. Three studies supported the positive influence of diet therapy, one study somewhat supported it and one study did not support it. It may be stated that for excessive body mass individuals, even if PAP is applied, the diet therapy should be included in order to reduce body mass, reduce the symptoms of OSA, and improve the quality of life. This therapeutic option should be applied, even if no effect on OSA is noted, as body mass reduction have multiple positive effects, which may also influence the quality of life.
1. Introduction
The obstructive sleep apnea is a disturbance characterized by a fragmented and nonrestorative sleep, which results from episodes of either complete or partial collapse of the airway, accompanied by decrease in oxygen saturation or arousal from sleep [1]. In spite of the fact that its epidemiology is monitored from 90s only, currently it is observed that the prevalence of the obstructive sleep apnea may be estimated as 3–7% of general population, with predisposing factors listed as: older age, male sex, excessive body mass, family history of obstructive sleep apnea, being after menopause (especially for women without hormone-replacement therapy), craniofacial abnormalities, tobacco smoking, and alcohol consumption [2].
If untreated, the obstructive sleep apnea may lead to both short-term and long-term health consequences; short-term consequences include excessive sleepiness during day, fatigue, nocturia, morning headache, irritability, and memory impairment, while long-term consequences include increased risk of cardiovascular diseases, metabolic disorders, cognitive impairment, and depression [3]. As a result, it also influences the quality of life, reducing both physical and mental functions [4].
In the treatment of obstructive sleep apnea, the positive airway pressure (PAP) is the gold standard; it is commonly applied as the continuous PAP (CPAP), which stabilizes the upper airway with constant positive pressure while using a mask interface, but also as the bi-level PAP, which uses various pressures for the inspiratory and expiratory cycle (for inhalation and exhalation) [5]. The systematic review and meta-analysis by Labarca et al. [6] revealed that in patients with obstructive sleep apnea and hypertension, the CPAP therapy reduces blood pressure, especially nighttime blood pressure. Similarly, the meta-analysis by Khan et al. [7] indicated that while applying CPAP for more than 4 h per night, the risk of major cardiovascular events is reduced by 57%.
Nonetheless, it must be also considered that for PAP or CPAP treatment, the adherence is crucial as it is indicated that in case of low adherence, the potential benefits of PAP therapy are not obtained [8]. In a large retrospective study, it was observed that low adherence is associated with increased hospital all-cause and cardiovascular-cause readmission [9]. Within the other retrospective study, it was observed that this therapy may result in a decrease in healthcare resource utilization, though this reduction is observable in patients who adhere to therapy [10]. The PAP or CPAP treatment adherence may differ between countries [11]; at the same time, other factors influencing adherence include hypertension, sleep posture, and apnea–hypopnea index (AHI) reduction obtained during treatment [12]. Moreover, barriers to obtaining required adherence include: inability to afford a PAP or PAP device (if not covered); perception of symptom reduction, resulting in belief in no need for treatment; and dissatisfaction with treatment [13], arising from physical barriers (including mask leaks, and dry throat and nose) and psychological barriers (including anxiety, claustrophobia and, insomnia) [14].
Taking this into account, in obstructive sleep apnea the other therapeutic methods are also applied and are sometimes even more important; examples include surgical treatment [15] and other methods applied mainly as supportive therapies, such as oral appliances, weight loss, or positional therapy [5]. Among the listed methods, the weight loss is indicated to be beneficial and recommended for all overweight or obese individuals with obstructive sleep apnea due to the fact that the association between excessive body mass and obstructive sleep apnea is obvious; thus, the potential of body mass reduction is significant [16]. Taking this into account, it is recommended within Clinical Guidelines for the Management and Long-term Care of Obstructive Sleep Apnea in Adults by the American Academy of Sleep Medicine for overweight and obese patients with obstructive sleep apnea to obtain weight loss [17].
This proposal was confirmed within the systematic review and meta-analysis by Carneiro-Barrera et al. [18], which assessed the effectiveness of weight loss and other lifestyle interventions for obstructive sleep apnea patients, as it proved that lifestyle interventions significantly reduce apnea–hypopnea index (AHI) and excessive daytime sleepiness. However, it must be emphasized that the systematic review mentioned [18] included both studies with and without PAP applied, as well as the various dietary and lifestyle interventions. The randomized controlled trials included by Carneiro-Barrera et al. [18], were applying various diets, which may be incomparable, including energy deficit of 500 kcal [19] or 800 kcal per day [20], individual sessions with dieticians based on food habit changes [21] or minor energy deficit and food habit changes [22], very low calorie diets (VLCD) based on dedicated preparations [23,24], or intensive interventions developed for diabetic individuals based on dedicated preparations [25].
Taking this into account, there is a need to compare the effectiveness of various diet therapy interventions for obstructive sleep apnea syndrome patients treated with PAP as a gold standard in therapy [5]. Based on the present knowledge, the aim of this study was to conduct a systematic review of randomized controlled trials (RCTs) of diet therapy interventions for obstructive sleep apnea syndrome patients treated with positive airway pressure.
2. Materials and Methods
2.1. Design and Registeration of the Systematic Review
The protocol of the systematic review for literature searching, publications screening, studies inclusion, and data reporting was developed in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) [26] statement. The systematic review was registered under no CRD42023398374 in the International Prospective Register of Systematic Reviews (PROSPERO).
The PubMed and Web of Science databases were screened to include RCTs published up to 1 February 2023. The included studies were related to the diet therapy of obstructive sleep apnea syndrome treated with PAP.
2.2. Elecrtronic Search Strategy and Eligibility Assessment
The electronic literature searching strategies for PubMed and Web of Science databases are presented in Table 1. The initial searching was conducted to find manuscripts published in English that presented the original studies.

Table 1.
Electronic literature searching strategies for PubMed and Web of Science databases applied within this study.
Afterwards, the screening and inclusion were conducted based on the following inclusion criteria:
- –
- Study conducted in adult participants;
- –
- Study conducted in a population of patients with obstructive sleep apnea diagnosed;
- –
- Study conducted in patients treated with PAP;
- –
- Study of any kind of dietary intervention;
- –
- Effect of dietary intervention compared with the effect observed for control group with no dietary intervention or with any other kind of dietary intervention;
- –
- Obstructive sleep apnea monitored using any objective or subjective method of assessment;
- –
- Study presenting results of RCT.
The following exclusion criteria were applied:
- –
- Study conducted in population of pregnant or lactating women;
- –
- Study conducted in population with any concurrent disease diagnosed, other than excessive body mass (overweight/obesity);
- –
- Study conducted in population with any mental health problems, eating disorders, or intellectual disabilities;
- –
- Study conducted in animal model.
The population, intervention/exposure, comparator, outcome, and study design (PICOS) criteria were applied within the study as presented in Table 2.

Table 2.
Population, intervention/exposure, comparator, outcome, and study design (PICOS) criteria applied within this study.
The duplicated records were manually removed after searching. Two researchers independently analyzed the titles of articles, abstracts of articles (records included based on titles), and full texts of articles (records included based on abstracts) on the basis of inclusion and exclusion criteria. Until consensus was achieved, any disagreement was discussed if necessary. The full texts of the articles defined as potentially eligible based on titles and abstracts were searched within electronic databases and libraries and, if necessary, the corresponding authors were contacted.
Moreover, in order to identify the RCTs with diet therapy for obstructive sleep apnea syndrome patients conducted only for participants treated with PAP, the corresponding authors were contacted, if necessary. They were asked about the studied group in order to include only those studies in which all participants were treated with PAP; those with participants treated without PAP, with mixed populations, or with no defined mode of therapy were excluded [20,22,24,27,28,29,30,31,32,33,34,35].
The procedure of identification, screening, and inclusion of RCTs applied within the study is presented in Figure 1.
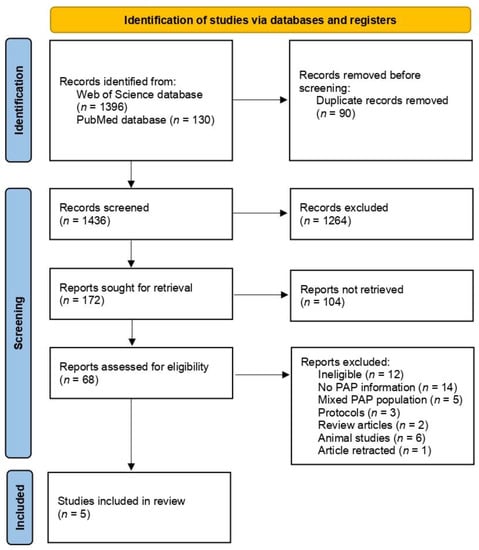
Figure 1.
Procedure of identification, screening, and inclusion of randomized controlled trials applied within this study.
2.3. Data Extracion and Risk of Bias Assessment
After including studies, the following information was extracted from each retrieved article:
- –
- General characteristics of the study and studied population, including: country; detailed location of the study; general description of the studied population; period of the study; number of participants (males and females); age; and inclusion and exclusion criteria for the study;
- –
- Intervention applied, including: studied groups; description of dietary interventions; duration of intervention; and monitored variables;
- –
- Results and conclusions of the study.
Two researchers independently extracted information and, until consensus was achieved, any disagreement was discussed, if necessary. In order to find the required information, the articles full texts were searched and the corresponding authors were contacted, if necessary, while the data provided on request are indicated within the study.
The included studies were evaluated using the revised Cochrane risk-of-bias tool for randomized trials [36], along with the RoB 2 tool [37]. Bias was classified as: low risk; some concerns; and high risk of bias based on individual assessments for 5 domains (randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result) with final overall risk also assessed [38]. Two researchers independently evaluated studies and, until consensus was achieved, any disagreement was discussed, if necessary.
3. Results
The general characteristics of the randomized controlled trials included in the study [23,39,40,41,42] and studied populations are presented in Table 3. Only studies clearly defined as those conducted in a population of patients with PAP treatment applied were intended to be included; thus, numerous studies with mixed populations with and without PAP treatment were excluded [22,32,33,34]. Similarly, if not obstructive sleep apnea was monitored, but the concurrent diseases and symptoms or other factors only, it was not included [43,44,45]. All the included studies were conducted in developed European countries: Spain [40,41], Sweden [23], the UK [39], and Italy [42]. The studies were conducted in groups with patients treated with CPAP [23,39,40,41] or intended to be treated with CPAP [42]; patients with moderate-to-severe [23,39,41] or severe obstructive sleep apnea were included [40,42]. The additional elements taken into account within the general characteristics of the studied groups were male gender [23,41], obesity [40,42], and being scheduled for bariatric surgery [42]. The populations studied within the included trials were of a medium size of either less than 50 [40] or more than 50 participants [23,39,41,42]. The studied participants were mainly in their 40s [23,40,42] or 50s [41]. Among the additional inclusion criteria, there was a criterion based on body mass index (BMI); only overweight and obese participants [41], obese participants [23,39,40], or obesity class two and three participants were qualified [42]. Similarly, for some studies the inclusion criteria included having CPAP treatment conducted for at least 6 months [12,41] or not smoking [42].

Table 3.
General characteristics of randomized controlled trials included in this study and studied populations.
The interventions applied within the randomized controlled trials included in the study and accompanied by the conclusions of the study regarding the influence of diet therapy are presented in Table 4. The conclusions of the study and the influence of diet therapy were formulated based on the results and conclusions formulated within the randomized controlled trials included in the study (Supplementary Table S1). Within all included studies, an experimental diet was compared with the control group; however, various interventions were applied, including: dietary intervention, which was compared with no intervention [23,42], and dietary and physical activity intervention, which was compared with general advice [39,40,41]. At the same time, the applied intervention included various dietary approaches, such as various energy deficits [23,40,42] or dietary health-promoting behaviors [39,41]. The monitored variables included apnea–hypopnea index (AHI) [23,40,41,42], sleep time [23,40,41], sleep efficiency [40,41], sleep latency [41], oxygen desaturation episodes [39,41], oxygen saturation [23,41], obstructive sleep apnea type (positional/not) [40], Epworth Sleepiness Scale score [23,40,41], Pittsburgh Sleep Quality Index [41], and quality of life (EuroQol EQ5D-3L VAS) [39].

Table 4.
Interventions applied within randomized controlled trials included in this study, accompanied by the conclusions of this study for influence of diet therapy.
The assessment of the risk of bias, conducted based on the revised Cochrane risk-of-bias tool for randomized trials, within the randomized controlled trials included in the study, is presented in Table 5. For the risk of bias, the majority of studies defined some concerns [23,39,41,42] resulting from the randomization process (D1 domain) [39,42], deviations from the intended interventions (D2 domain) [23], or selection of the reported result (D5 domain) [23,39,40,42]. For one study, the risk of bias was assessed as high [40]; this risk resulted from the randomization process (D1 domain) and deviations from the intended interventions (D2 domain). The risk of bias was not associated with the results as only for one study did they not support the positive influence of diet therapy (a study for which some concerns were indicated) [42]; for another study, the results were defined as somewhat supporting the positive influence of diet therapy (another study for which some concerns were indicated) [39]. At the same time, three studies supported the positive influence of diet therapy, including both of those for which some concerns were indicated [23,41], and this study of a high risk of bias [40].

Table 5.
Assessment of risk of bias, conducted based on revised Cochrane risk-of-bias tool for randomized trials, within randomized controlled trials included in this study.
4. Discussion
As observed within the gathered studies, no negative effect of applied diet therapies was observed in the studied populations of obstructive sleep apnea syndrome patients treated with PAP, independently from the applied mode of diet therapy. Moreover, three studies supported the positive effect of diet therapy [23,40,41] and one study somewhat supported it [39], while only one study did not support it [44]. Taking this into account, it may be indicated that application of a diet therapy should be recommended and if possible, the PAP should not be applied alone; rather, it should be applied with the body mass reduction as it may provide additional benefits associated with reduced body mass, reduced symptoms of obstructive sleep apnea, and improved quality of life.
The observations formulated within the included studies [23,39,40,41,42] result from the association between obstructive sleep apnea syndrome and excessive body mass, which is among the condition’s major predisposing factors [2]. It is associated with the role of pharyngeal narrowing and closure during sleep as its four pathogenetic mechanisms are indicated as resulting from narrow, crowded, or collapsible upper airway anatomical compromise, ineffective pharyngeal dilator muscle functioning, low threshold for arousal to airway narrowing, and unstable control of breathing during sleep [3]. As a result of pharyngeal narrowing and closure, whether complete or partial, the apnea or hypopnea, respectively, are observed, leading to disturbances in gas exchange, oxygen desaturation, hypercapnia, and sleep fragmentation [47]. The described changes do not only result from independent processes, but they may be influenced by being overweight and obese as the excessive body mass associated with central distribution of fat tissue leads to reduced lung volume and, as a result, influences the stability of respiratory control [47]. Similarly, it is indicated that the neck circumference is a factor associated with severity of obstructive sleep apnea syndrome; this issue is an independent risk factor for experiencing a severe course of the disease [48].
Based on the described mechanisms and taking into account the increasing prevalence of excessive body mass, especially in countries of a higher socioeconomic status [49], the increasing prevalence of obstructive sleep apnea may be also supposed for the future. Taking this into account, the researchers emphasize the need to screen excessive body mass populations for obstructive sleep apnea in order to treat affected individuals effectively [50].
However, it must be indicated that obstructive sleep apnea is not the only condition to result from excessive body mass; other diseases resulting from excessive body mass and obstructive sleep apnea may be similarly treated with body mass reduction as a supportive therapy. For example, hypertension can result from excessive body mass [51] and obstructive sleep apnea [52]. Similarly, for obese patients increased risk of stroke may be stated while, depending on the metabolic consequences of obesity [53], obstructive sleep apnea can be associated with a high risk of stroke among patients with coronary artery disease [54]. Such associations between excessive body mass, the development of cardiovascular problems, and obstructive sleep apnea indicates that excessive body mass is not only the source of the problem, but also a means of finding a potential solution. At the same time, treatment for obstructive sleep apnea leads to the reduction in cardiovascular problems, indicating that the mentioned conditions should be treated in combination [55]. Similarly, it is indicated that obstructive sleep apnea is related to other diseases resulting from excessive body mass, such as diabetes; obstructive sleep apnea is associated with marked insulin resistance in adipose tissue triglyceride lipolysis and glucose uptake into skeletal muscles and adipose tissue [56]. However, it is emphasized that benefits from diet therapy in obstructive sleep apnea extend beyond the recognized benefits of weight reduction [57].
Taking this into account, it may be indicated that while PAP must be applied as the gold standard in obstructive sleep apnea therapy, the body mass reduction (not applied instead of PAP, but accompanying it) may correct existing problems by reduction in central fat tissue deposits and resultant lungs volume increase [47].
At the same time, it must be indicated that numerous factors predisposing obstructive sleep apnea development are fixed and unchangeable, such as older age, male sex, family history of obstructive sleep apnea, being post-menopausal, and craniofacial abnormalities, so the modifiable habits must be considered during therapy as excessive body mass, tobacco smoking, and alcohol consumption [2]. Taking this into account, behavioral therapy, including not only body mass reduction but also stopping smoking and reducing alcohol consumption, may be most recommended approach to support PAP treatment.
Although the study revealed interesting observations, some limitations should also be listed. The quantity of the included studies, as well as their quality and heterogenicity, are the major problems. Only five studies met the inclusion criteria, while a lot of studies were excluded; moreover, within the studied groups various therapeutic modes were applied. The small number of included studies are incomparable, with various dietary options studied and diverse variables monitored; thus, they cannot be treated as a homogenous sample of studies. Lastly, as shown in the conducted systematic review, there were some problems with the risk of bias within all the included studies.
5. Conclusions
Based on the gathered studies assessing the diet therapy interventions for obstructive sleep apnea syndrome patients treated with PAP, it may be stated that for excessive body mass individuals, even if PAP is applied, the diet therapy should be included in order to reduce body mass and the symptoms of obstructive sleep apnea and improve the quality of life. As the majority of studies indicated that diet therapy is beneficial as a supporting treatment and that body mass reduction may influence the symptoms of the disease, this therapeutic option should be applied; even if no effect on obstructive sleep apnea is noted, body mass reduction has multiple positive effects, which may also influence quality of life.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13085105/s1, Supplementary Table S1. The results and conclusions formulated within the randomized controlled trials included to the study.
Author Contributions
Conceptualization, D.G. (Dominika Guzek) and D.G. (Dominika Głąbska); methodology, D.G. (Dominika Guzek) and D.G. (Dominika Głąbska); formal analysis, D.G. (Dominika Guzek) and D.G. (Dominika Głąbska); investigation, D.G. (Dominika Guzek) and D.G. (Dominika Głąbska); writing—original draft preparation D.G. (Dominika Guzek) and D.G. (Dominika Głąbska); writing—review and editing, D.G. (Dominika Guzek) and D.G. (Dominika Głąbska). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Polish Ministry of Education and Science within funds of Institute of Human Nutrition Sciences, Warsaw University of Life Sciences (WULS), for scientific research.
Institutional Review Board Statement
The literature search was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42023398374).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Slowik, J.M.; Sankari, A.; Collen, J.F. Obstructive Sleep Apnea. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459252/?report=classic (accessed on 9 March 2023).
- Punjabi, N.M. The epidemiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 15, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.M.; Carter, S.G.; Carberry, J.C.; Eckert, D.J. Obstructive sleep apnea: Current perspectives. Nat. Sci. Sleep 2018, 23, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Coman, A.C.; Borzan, C.; Vesa, C.S.; Todea, D.A. Obstructive sleep apnea syndrome and the quality of life. Clujul. Med. 2016, 89, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Pavwoski, P.; Shelgikar, A.V. Treatment options for obstructive sleep apnea. Neurol. Clin. Pract. 2017, 7, 77–85. [Google Scholar] [CrossRef]
- Labarca, G.; Schmidt, A.; Dreyse, J.; Jorquera, J.; Enos, D.; Torres, G.; Barbe, F. Efficacy of continuous positive airway pressure (CPAP) in patients with obstructive sleep apnea (OSA) and resistant hypertension (RH): Systematic review and meta-analysis. Sleep Med. Rev. 2021, 58, 101446. [Google Scholar] [CrossRef]
- Khan, S.U.; Duran, C.A.; Rahman, H.; Lekkala, M.; Saleem, M.A.; Kaluski, E. A meta-analysis of continuous positive airway pressure therapy in prevention of cardiovascular events in patients with obstructive sleep apnoea. Eur. Heart J. 2018, 21, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
- Derose, S.F.; Zhou, H.; Huang, B.Z.; Manthena, P.; Hwang, D.; Shi, J.M. Does Providing Positive Airway Pressure for Sleep Apnea Change Health Care Utilization? Med. Care 2018, 56, 901–907. [Google Scholar] [CrossRef]
- Truong, K.K.; De Jardin, R.; Massoudi, N.; Hashemzadeh, M.; Jafari, B. Nonadherence to CPAP Associated With Increased 30-Day Hospital Readmissions. J. Clin. Sleep Med. 2018, 15, 183–189. [Google Scholar] [CrossRef]
- Walter, R.J.; Hagedorn, S.I.; Lettieri, C.J. Impact of diagnosing and treating obstructive sleep apnea on healthcare utilization. Sleep Med. 2017, 38, 73–77. [Google Scholar] [CrossRef]
- Drager, L.F.; Malhotra, A.; Yan, Y.; Pépin, J.L.; Armitstead, J.P.; Woehrle, H.; Nunez, C.M.; Cistulli, P.A.; Benjafield, A.V.; medXcloud group. Adherence with positive airway pressure therapy for obstructive sleep apnea in developing vs. developed countries: A big data study. J. Clin. Sleep Med. 2021, 1, 703–709. [Google Scholar] [CrossRef]
- Kim, J.H.; Kwon, M.S.; Song, H.M.; Lee, B.J.; Jang, Y.J.; Chung, Y.S. Compliance with positive airway pressure treatment for obstructive sleep apnea. Clin. Exp. Otorhinolaryngol. 2009, 2, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, L.; Phillips, D.; Khazaie, H. Barriers to acceptance and adherence to continuous positive airway pressure therapy in patients with obstructive sleep apnea: A report from Kermanshah province, western Iran. Patient Prefer. Adherence 2018, 20, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.A.; Purcell, N.; Sarmiento, K.F.; Neylan, T.C.; Maguen, S. Barriers to positive airway pressure adherence among veterans with sleep apnea: A mixed methods study. Transl. Behav. Med. 2022, 17, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Iannella, G.; Lechien, J.R.; Perrone, T.; Meccariello, G.; Cammaroto, G.; Cannavicci, A.; Burgio, L.; Maniaci, A.; Cocuzza, S.; Di Luca, M.; et al. Barbed reposition pharyngoplasty (BRP) in obstructive sleep apnea treatment: State of the art. Am. J. Otolaryngol. 2022, 43, 103197. [Google Scholar] [CrossRef]
- Jehan, S.; Zizi, F.; Pandi-Perumal, S.R.; Wall, S.; Auguste, E.; Myers, A.K.; Jean-Louis, G.; McFarlane, S.I. Obstructive Sleep Apnea and Obesity: Implications for Public Health. Sleep Med. Disord. 2017, 1, 00019. [Google Scholar] [PubMed]
- Epstein, L.J.; Kristo, D.; Strollo, P.J., Jr.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, E.M.; et al. Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. 2009, 15, 263–276. [Google Scholar]
- Carneiro-Barrera, A.; Díaz-Román, A.; Guillén-Riquelme, A.; Buela-Casal, G. Weight loss and lifestyle interventions for obstructive sleep apnoea in adults: Systematic review and meta-analysis. Obes. Rev. 2019, 20, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Maki-Nunes, C.; Toschi-Dias, E.; Cepeda, F.X.; Rondon, M.U.; Alves, M.J.; Fraga, R.F.; Braga, A.M.; Aguilar, A.M.; Amaro, A.C.; Drager, L.F.; et al. Diet and exercise improve chemoreflex sensitivity in patients with metabolic syndrome and obstructive sleep apnea. Obesity 2015, 23, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.F.; Araújo Lda, S.; Kaiser, S.E.; Sanjuliani, A.F.; Klein, M.R. The effects of moderate energy restriction on apnoea severity and CVD risk factors in obese patients with obstructive sleep apnoea. Br. J. Nutr. 2015, 28, 2022–2031. [Google Scholar] [CrossRef] [PubMed]
- Igelström, H.; Åsenlöf, P.; Emtner, M.; Lindberg, E. Improvement in obstructive sleep apnea after a tailored behavioural sleep medicine intervention targeting healthy eating and physical activity: A randomised controlled trial. Sleep Breath 2018, 22, 653–661. [Google Scholar] [CrossRef]
- Ng, S.S.S.; Chan, R.S.M.; Woo, J.; Chan, T.O.; Cheung, B.H.K.; Sea, M.M.M.; To, K.W.; Chan, K.K.P.; Ngai, J.; Yip, W.H.; et al. A Randomized Controlled Study to Examine the Effect of a Lifestyle Modification Program in OSA. Chest 2015, 148, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Johansson, K.; Neovius, M.; Lagerros, Y.T.; Harlid, R.; Rössner, S.; Granath, F.; Hemmingsson, E. Effect of a very low energy diet on moderate and severe obstructive sleep apnoea in obese men: A randomised controlled trial. BMJ 2009, 3, b4609. [Google Scholar] [CrossRef]
- Tuomilehto, H.P.; Seppä, J.M.; Partinen, M.M.; Peltonen, M.; Gylling, H.; Tuomilehto, J.O.; Vanninen, E.J.; Kokkarinen, J.; Sahlman, J.K.; Martikainen, T.; et al. Kuopio Sleep Apnea Group. Lifestyle intervention with weight reduction: First-line treatment in mild obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2009, 15, 320–327. [Google Scholar] [CrossRef]
- Foster, G.D.; Borradaile, K.E.; Sanders, M.H.; Millman, R.; Zammit, G.; Newman, A.B.; Wadden, T.A.; Kelley, D.; Wing, R.R.; Pi-Sunyer, F.X.; et al. Sleep AHEAD Research Group of Look AHEAD Research Group. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: The Sleep AHEAD study. Arch. Intern. Med. 2009, 28, 1619–1626. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e100009. [Google Scholar] [CrossRef] [PubMed]
- Fiori, C.Z.; Martinez, D.; Montanari, C.C.; Lopez, P.; Camargo, R.; Sezerá, L.; Gonçalves, S.C.; Fuchs, F.D. Diuretic or sodium-restricted diet for obstructive sleep apnea-a randomized trial. Sleep 2018, 1, 41. [Google Scholar] [CrossRef] [PubMed]
- de Melo, C.M.; Dos Santos Quaresma, M.V.L.; Del Re, M.P.; Ribeiro, S.M.L.; Moreira Antunes, H.K.; Togeiro, S.M.; Tufik, S.; de Mello, M.T. One-month of a low-energy diet, with no additional effect of high-protein, reduces Obstructive Sleep Apnea severity and improve metabolic parameters in obese males. Clin. Nutr. ESPEN 2021, 42, 82–89. [Google Scholar] [CrossRef]
- Trzepizur, W.; Bironneau, V.; Recoquillon, S.; Priou, P.; Meslier, N.; Hamel, J.F.; Henni, S.; Darsonval, A.; Messaoudi, K.; Martínez, M.C.; et al. Polyphenols Have No Impact on Endothelial Function in Patients with Obstructive Sleep Apnea: A Randomized Controlled Trial. J. Nutr. 2018, 1, 581–586. [Google Scholar] [CrossRef]
- Sahlman, J.; Seppä, J.; Herder, C.; Peltonen, M.; Peuhkurinen, K.; Gylling, H.; Vanninen, E.; Tukiainen, H.; Punnonen, K.; Partinen, M.; et al. Effect of weight loss on inflammation in patients with mild obstructive sleep apnea. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 583–590. [Google Scholar] [CrossRef]
- Nerfeldt, P.; Nilsson, B.Y.; Uddén, J.; Rössner, S.; Friberg, D. Weight reduction improves nocturnal respiration in obese sleep apnoea patients-A randomized controlled pilot study. Obes. Res. Clin. Pract. 2008, 2, 71–142. [Google Scholar] [CrossRef]
- Kerley, C.P.; Hutchinson, K.; Bramham, J.; McGowan, A.; Faul, J.; Cormican, L. Vitamin D Improves Selected Metabolic Parameters but Not Neuropsychological or Quality of Life Indices in OSA: A Pilot Study. J. Clin. Sleep. Med. 2017, 15, 19–26. [Google Scholar] [CrossRef]
- Georgoulis, M.; Yiannakouris, N.; Kechribari, I.; Lamprou, K.; Perraki, E.; Vagiakis, E.; Kontogianni, M.D. Cardiometabolic Benefits of a Weight-Loss Mediterranean Diet/Lifestyle Intervention in Patients with Obstructive Sleep Apnea: The “MIMOSA” Randomized Clinical Trial. Nutrients 2020, 28, 1570. [Google Scholar] [CrossRef] [PubMed]
- Georgoulis, M.; Yiannakouris, N.; Tenta, R.; Fragopoulou, E.; Kechribari, I.; Lamprou, K.; Perraki, E.; Vagiakis, E.; Kontogianni, M.D. A weight-loss Mediterranean diet/lifestyle intervention ameliorates inflammation and oxidative stress in patients with obstructive sleep apnea: Results of the “MIMOSA” randomized clinical trial. Eur. J. Nutr. 2021, 2021, 3799–3810. [Google Scholar] [CrossRef]
- Georgoulis, M.; Yiannakouris, N.; Kechribari, I.; Lamprou, K.; Perraki, E.; Vagiakis, E.; Kontogianni, M.D. The effectiveness of a weight-loss Mediterranean diet/lifestyle intervention in the management of obstructive sleep apnea: Results of the “MIMOSA” randomized clinical trial. Clin. Nutr. 2021, 40, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Assessing Risk of Bias in Non-Randomized Studies. Chapter 13.5.2.3. Available online: http://handbook-5-1.cochrane.org/ (accessed on 28 February 2023).
- RoB 2: A Revised Cochrane Risk-of-Bias Tool for Randomized Trials. Available online: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials (accessed on 28 February 2023).
- Minozzi, S.; Cinquini, M.; Gianola, S.; Gonzalez-Lorenzo, M.; Banzi, R. The revised Cochrane risk of bias tool for randomized trials (RoB 2) showed low interrater reliability and challenges in its application. J. Clin. Epidemiol. 2020, 126, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.; Tew, G.A.; Copeland, R.J.; Stout, M.; Billings, C.G.; Saxton, J.M.; Winter, E.M.; Bianchi, S.M. Effects of a pragmatic lifestyle intervention for reducing body mass in obese adults with obstructive sleep apnoea: A randomised controlled trial. Biomed. Res. Int. 2014, 2014, 102164. [Google Scholar] [CrossRef]
- López-Padrós, C.; Salord, N.; Alves, C.; Vilarrasa, N.; Gasa, M.; Planas, R.; Montsserrat, M.; Virgili, M.N.; Rodríguez, C.; Pérez-Ramos, S.; et al. Effectiveness of an intensive weight-loss program for severe OSA in patients undergoing CPAP treatment: A randomized controlled trial. J. Clin. Sleep. Med. 2020, 15, 503–514. [Google Scholar] [CrossRef]
- Carneiro-Barrera, A.; Amaro-Gahete, F.J.; Guillén-Riquelme, A.; Jurado-Fasoli, L.; Sáez-Roca, G.; Martín-Carrasco, C.; Buela-Casal, G.; Ruiz, J.R. Effect of an Interdisciplinary Weight Loss and Lifestyle Intervention on Obstructive Sleep Apnea Severity: The INTERAPNEA Randomized Clinical Trial. JAMA Netw. Open 2022, 1, e228212. [Google Scholar] [CrossRef]
- Schiavo, L.; Pierro, R.; Asteria, C.; Calabrese, P.; Di Biasio, A.; Coluzzi, I.; Severino, L.; Giovanelli, A.; Pilone, V.; Silecchia, G. Low-Calorie Ketogenic Diet with Continuous Positive Airway Pressure to Alleviate Severe Obstructive Sleep Apnea Syndrome in Patients with Obesity Scheduled for Bariatric/Metabolic Surgery: A Pilot, Prospective, Randomized Multicenter Comparative Study. Obes. Surg. 2022, 32, 634–642. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Gurubhagavatula, I.; Teff, K.; Rader, D.J.; Wadden, T.A.; Townsend, R.; Foster, G.D.; Maislin, G.; Saif, H.; Broderick, P.; et al. CPAP, weight loss, or both for obstructive sleep apnea. N. Engl. J. Med. 2014, 12, 2265–2275. [Google Scholar] [CrossRef]
- Barceló, A.; Morell-Garcia, D.; Salord, N.; Esquinas, C.; Pérez, G.; Pérez, A.; Monasterio, C.; Gasa, M.; Fortuna, A.M.; Montserrat, J.M.; et al. A randomized controlled trial: Branched-chain amino acid levels and glucose metabolism in patients with obesity and sleep apnea. J. Sleep Res. 2017, 26, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Carneiro-Barrera, A.; Amaro-Gahete, F.J.; Jurado-Fasoli, L.; Sáez-Roca, G.; Martín-Carrasco, C.; Tinahones, F.J.; Ruiz, J.R. Effect of a Weight Loss and Lifestyle Intervention on Dietary Behavior in Men with Obstructive Sleep Apnea: The INTERAPNEA Trial. Nutrients 2022, 30, 2731. [Google Scholar] [CrossRef] [PubMed]
- Carneiro-Barrera, A.; Amaro-Gahete, F.J.; Díaz-Román, A.; Guillén-Riquelme, A.; Jurado-Fasoli, L.; Sáez-Roca, G.; Martín-Carrasco, C.; Ruiz, J.R.; Buela-Casal, G. Interdisciplinary Weight Loss and Lifestyle Intervention for Obstructive Sleep Apnoea in Adults: Rationale, Design and Methodology of the INTERAPNEA Study. Nutrients 2019, 15, 2227. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.S.; McSharry, D.G.; Malhotra, A. Adult obstructive sleep apnoea. Lancet 2014, 22, 736–747. [Google Scholar] [CrossRef]
- Ahbab, S.; Ataoğlu, H.E.; Tuna, M.; Karasulu, L.; Cetin, F.; Temiz, L.U.; Yenigün, M. Neck circumference, metabolic syndrome and obstructive sleep apnea syndrome; evaluation of possible linkage. Med. Sci. Monit. 2013, 13, 111–117. [Google Scholar] [CrossRef]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 6, 706978. [Google Scholar] [CrossRef]
- Sankri-Tarbichi, A.G. Obstructive sleep apnea-hypopnea syndrome: Etiology and diagnosis. Avicenna J. Med. 2012, 2, 3–8. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Picca, A.; Tosato, M.; Martone, A.M.; Ortolani, E.; Sisto, A.; D’Angelo, E.; Serafini, E.; Desideri, G.; et al. Body Mass Index is Strongly Associated with Hypertension: Results from the Longevity Check-up 7+ Study. Nutrients 2018, 13, 1976. [Google Scholar] [CrossRef]
- Benjamin, J.A.; Lewis, K.E. Sleep-disordered breathing and cardiovascular disease. Postgrad. Med. J. 2008, 84, 15–22. [Google Scholar] [CrossRef]
- Horn, J.W.; Feng, T.; Mørkedal, B.; Strand, L.B.; Horn, J.; Mukamal, K.; Janszky, I. Obesity and Risk for First Ischemic Stroke Depends on Metabolic Syndrome: The HUNT Study. Stroke 2021, 52, 3555–3561. [Google Scholar] [CrossRef]
- Valham, F.; Mooe, T.; Rabben, T.; Stenlund, H.; Wiklund, U.; Franklin, K.A. Increased risk of stroke in patients with coronary artery disease and sleep apnea: A 10-year follow-up. Circulation 2008, 26, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.J. Sleep Apnea and Cardiovascular Disease. Curr. Diab. Rep. 2021, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.E.; van Vliet, S.; Cao, C.; Patterson, B.W.; Reeds, D.N.; Laforest, R.; Gropler, R.J.; Ju, Y.S.; Mittendorfer, B. Effect of obstructive sleep apnea on glucose metabolism. Eur. J. Endocrinol. 2022, 23, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Dobrosielski, D.A.; Papandreou, C.; Patil, S.P.; Salas-Salvadó, J. Diet and exercise in the management of obstructive sleep apnoea and cardiovascular disease risk. Eur. Respir. Rev. 2017, 28, 160110. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
































