Abstract
Ibrutinib (ITB) is a specific and novel irreversible inhibitor of Bruton’s tyrosine kinase enzyme, for which reason it exhibits potential chemotherapeutic effects against a few types of B-cell cancers. The objective of this study was to design and characterize the targeted anti-cancer moiety of ITB encapsulated in polymeric nanosponges (IBNS 1-5). The IBNSs were fabricated using the ultrasonication-assisted solvent evaporation technique. They were optimized for robust nanocarriers by varying the ratio of ethylcellulose (50–200 mg), using a constant amount 50 mg of polyvinyl alcohol ((PVA) stabilizer), and drug ITB. Optimized INBS4 containing 50 mg of ITB, PVA, and 162.5 mg of EC was prepared and was studied for anti-cancer potential. Particle analysis and EE and DL calculation of optimized IBNS4 were 640.9 nm, 0.35, −30.2 mV in size, PDI, and ζp, respectively. Physicochemical characterization (FTIR and DSC) studies of IBNS4 showed that the drug was compatible with excipients, and was encapsulated properly within the core of nanosponges. In vitro drug release studies revealed that IBNS4 followed the Higuchi matrix model with anomalous non-Fickian release kinetics. The in vitro diffusion study of I-NS4 exhibited sustained release for 24 h. Enhanced cytotoxicity effects against the MCF-7 observed with the developed NSs (IBNS4) showed 1.96 times more cytotoxic potential compared to the pure drug (ITB).
1. Introduction
Ibrutinib (ITB) is a targeted medicine that identifies and attacks cancer cells without damaging normal cells, thereby reducing lethal side effects. ITB is a specific and novel irreversible inhibitor of Bruton’s tyrosine kinase (BTK) enzyme, making it a potential chemotherapeutic agent for effectively managing some B-cell cancers [1]. ITB (Figure 1) exhibits possible anti-cancer activity by covalently binding to Cystine-serine positioning at 481 (Cys 481), present in the phosphorylation sites of BTK. This leads to the inhibition of kinase activity, perpetual inactivation, and impasse of the signals, which triggers the unrestrained growth and division of carcinogenic cells [2,3,4]. Apart from BTK, ITB has been broadly designated to inhibit the instigation of other kinases, including IL-2-inducible tyrosine kinase (IL-2), B-lymphoid tyrosine kinase (BLK), Taxotere-epirubicin-cyclophosphamide (TEC), Tec family kinases (TFK), Hematopoietic cell kinase (HCK), interleukin-2-inducible T-cell kinase (ITK), Janus kinase 3 (JAK3), and particularly ErbB receptor family, signifying the potential of ITB for further exploration against various types of tumor therapies in the future [5,6]. In addition, ITB was approved by the Food and Drug Administration as an efficient therapeutic agent for treating mantle cell lymphoma (MCL) and chronic lymphocytic leukemia (CLL) [3]. Regulatory agencies have approved the usage of ITB as a therapeutic agent in certain nations for treating CLL, MCL, and Waldenstrom’s macroglobulinemia [4,7,8]. ITB is one of the potential treatment options for gynecological malignancies, including in breasts, as evidenced by various reported investigations [9,10,11].
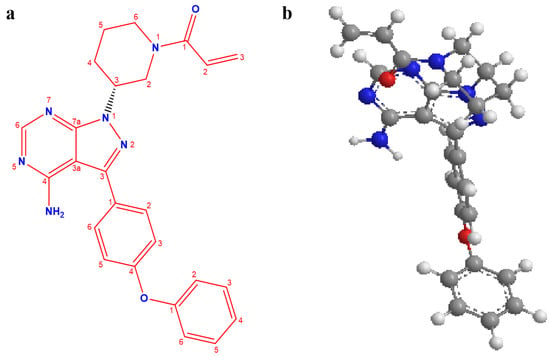
Figure 1.
Chemical structure of ibrutinib: two-dimensional (a) and three-dimensional (b).
As per the reported studies, the bioavailability of ITB has been found to be very low (2.9%). ITB exerts a partition coefficient value of 3.74 and is a weak base showing a pH-dependent solubility leading to low bioavailability and hindering its in vivo anti-cancer effects [12]. Moreover, an increase in pH values probably leads to the precipitation of ITB when it is transported from the stomach to the intestinal environment. In addition, ITB is testified to suffer severe hepatic clearance. Due to first-pass metabolism and low bioavailability, ITB is commercially formulated in higher doses, leading to extensive adverse effects in the GIT [13]. Thus, it becomes necessary to design a substantial alternate formulation of ITB with a sustained release formulation having enhanced oral bioavailability and improved therapeutic efficacy [14,15].
During the last few years, a wide range of nanocarrier-based drug delivery systems have been reported for the effective delivery of various poorly aqueous soluble drugs due to their diversified properties including nanosize, modifiable shape and surface charge, solubilization effect, shielding effects, improved and targeted drug release, enhanced sustained or/and controlled release, and negligible or no toxicity [16]. In particular, the smaller hydrodynamic size of nanocarriers exhibited advantageous effects for their accretion within the tumor microenvironment due to improved permeability and retaining effects, leading to reduced or negligible adverse effects of the drug. Amongst various polymeric nanocarriers, nanosponges (NSs) have been reported as a potential platform for the effective delivery of diverse therapeutics [17,18]. NSs are spherical nanocarriers with extensive hollows and a larger porous surface, which assists for encapsulating both lipophilic and hydrophilic drugs and can be administered orally, parenterally, and topically [19,20,21].
NSs as nanocarrier could be used for various applications, such as encapsulation of fragrance, essential oils, and drugs specifically for targeting at a site for sustained effects and prolonged release behavior. Polymers used for the fabrications of NSs were beta-cyclodextrins with cross-linker diphenyl carbonate, and ethylcellulose (EC), using the polyvinyl alcohol (PVA) as a stabilizer [16,22]. EC is a biocompatible and biodegradable polymer utilized in the pharmaceutical and food industries. NS porousness renders higher encapsulation capacity, ideal for a drug that has poor bioavailability and higher dose, suitably applied for drug delivery systems for sustained and controlled drug mechanisms. NSs prepared with EC and PVA for Olmesartan medoxomil exhibited higher cytotoxicity against A549 lung cancer lines [18]. Muqtader et al. reported that butenafine-loaded NS topical gels showed the maximum flux, sustained release, and effectiveness against the pathogenic fungal strain candida albicans [16].
The prime objective for fabricating stable polymeric nanosponges is to improve the solubility, permeability, and targeted delivery of ibrutinib to improve anti-cancer effects. Moreover, as per our search of the literature, we have not found any study reporting the anti-cancer effects of ibrutinib-loaded polymeric nanosponges against MCF-7 cells with established molecular mechanisms. This study established a robust method for developing ibrutinib-loaded polymeric nanosponges with improved sustained-release properties.
2. Materials and Methods
2.1. Materials
Ibrutinib (ITB) was procured from Mesochem Tech (Beijing, China). Polyvinyl alcohol (PVA), dichloromethane (DCM), and ethylcellulose (EC) were procured from Sigma Aldrich in St. Louis, MI, USA. The solvents and chemicals utilized here were of analytical grade.
2.2. Cell Growth
MCF-7 cell lines were cultured in Roswell Park Memorial Institute (RPMI) 1640 (Sigma Aldrich, USA) supplemented with fetal bovine serum 10% (v/v), streptomycin (100 ng/mL), and penicillin (100 I.U/mL). The cells were then incubated at 37 °C with carbon dioxide (5%). When the cells were 80% confluent, they were sub-cultured to a fresh medium and used for cytotoxicity testing with a Gene-JET RNA Kit and cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, USA).
2.3. Development of Ibrutinib-Loaded Ethylcellulose-Based Nanosponges
Ibrutinib (ITB)-loaded nanosponges (NS) were developed using a probe sonication emulsification solvent evaporation method. Nanosponges were prepared by using varying proportions of EC (50–200 mg), and PVA (0.5%). Table 1 lists the chemical make-up of nanosponges comprising ITB, EC, and PVA. Disperse phase composed of ITB dissolved in 0.5 mL dimethyl sulfoxide and EC in 2 mL of dichloromethane (DCM) were kept on ultrasonication for 1 min. Drug-dissolved EC solution was then added slowly into 10 mL PVA solution dipped with a probe (Probe shaft # 423, Fisher Scientific; Waltham, MA, USA) for 5 min, with power 65% voltage. The o/w type of dispersion (organic phase in aqueous phase) was then transferred to a magnetic stirrer (Isotemp Hot Plate and Stirrer, Fisher Scientific; Waltham, MA, USA) and stirred at 750 rpm overnight in atmospheric conditions. The prepared Ibrutinib-loaded nanosponges (INS) were then centrifuged at 12,000 rpm. Supernatant was preserved for drug encapsulation and loading efficiency. The pellet was washed multiple times with Milli-Q water to remove the surfactant, and lyophilized. Freeze-dried ITB-loaded NSs were then preserved in amber tubular glass vials for further characterizations [23,24].

Table 1.
Composition and physicochemical nanosponge characterizations.
2.4. Particle Characterization: Size, Polydispersity Index (PDI), and Zeta Potential (ζp)
Dynamic light scattering (DLS) and photon correlation spectroscopy (Nano ZS equipment, Malvern Instruments, Malvern, UK) were used to analyze the particle size of the IBNSs at a temperature of 25 ± 2 °C. The samples were dispersed in Milli-Q water to perform particle analysis for each formulation (dilution, 1:200). Samples were ultra-sonicated for 2 min to disperse the agglomerates and filled into the sample holders for size and ζp measurements. The beam of the laser was passed and particles in Brownian motion scattered and average diameter of the particles measured [21,25,26]. Each sample was tested three times (n = 3).
2.5. Entrapment Efficiency and Drug Loading Calculation
An indirect analysis method was used to measure the percent entrapment efficiency (%EE) and drug loading (%DL) of ITB IBNSs. The samples were taken from the freshly prepared dispersion and centrifuged for 20 min at 12,000 rpm. Thereafter, the free drug was detected in the collected supernatant using UV spectroscopy (V-630 Jasco Spectrophotometer, Tokyo, Japan) at a maximum wavelength of 264 nm [27,28]. The %EE and %DL were calculated using the following equation [21]:
2.6. Fourier-Transform Infrared (FTIR) Analysis
To assess ITB’s compatibility with the stabilizers and polymers used to prepare nanosponges, FTIR analysis was carried out. Potassium bromide (KBr) was separately mixed with ITB and optimized IBNS4 before being compressed into a disc and scanned from 4000 to 400 cm−1 to identify the fingerprint region of ITB in IBNS4 (Jasco 4600 Mid-IR FTIR spectrometer, Tokyo, Japan). FTIR is one of the reliable, feasible, and economical techniques for qualitative analysis; the intensity and peak positions directly measure the nature and interactions between the materials [29,30].
2.7. Differential Scanning Calorimetry (DSC) Analysis
DSC performed physicochemical drug–excipient interaction for ITB and IBNS4 was conducted by cramping (5 mg) sample into the hemispherical aluminum pan and putting it on a 20 °C/min heating rate for temperatures ranging from 30 to 250 °C (DSC N-650; Scinco, Seoul, Korea). Nitrogen gas was passed during the testing, and thermal peaks of the samples were processed and collaged using software for interpretation [31].
2.8. XRD Crystallography
Optimized NSs (IBNS4) were characterized by X-ray diffraction (XRD) testing to study the crystal structure of materials. The sample under investigation was loaded onto a sample holder and exposed to ionized radiation (X-ray), which measures the intensity and angle of the scattered X-rays at a 2θ range of 5–75 °C. The resulting XRD diffraction pattern was analyzed and interpreted to identify the material’s crystal structure and physical state properties [29,32,33]. The results of the diffractogram (Siemens D5000 Diffractometer, Tokyo, Japan) are based on the atom and molecular lattice of materials and provide the nature of molecular adduct and degree of crystallinity of the material.
2.9. Scanning Electron Microscopy (SEM)
Optimized NSs (IBNS4) were exposed to a beam of an electron under negative pressure to capture the image using a 20 mA beam current and acceleration voltage of 5 kV to avoid the hazardous impact of an electron beam on the sample (SEM-Ultraplus, Jena, Germany). The sample under test was applied over the thin substrate using conductive adhesive, and dried; gold coating was applied over the sample to improve the sample’s electron conductivity. The sample was then scanned from the desired region and adjusted with coarse and fine adjustments by visualizing on a screen, and images were captured [34].
2.10. In Vitro Drug Release and Mathematical Model Fitting
Using the dialysis bag method at pH 1.2, the in vitro drug release behavior of the pure drug suspension and optimized NSs were evaluated. Gastrointestinal tract acidic pH was reported to be most appropriate for absorption of orally administered ITB [35]. The samples pure ITB suspension and optimized nanosponge IBNS4 (equivalent of 10 mg ITB) were dispersed in 5.0 mL (pH 1.2) and filled in a dialysis bag (molecular weight: 14 kDa) that was closed on both ends, then suspended into a beaker with 50 mL of media (pH 1.2) maintained at 37 ± 2 °C while being stirred magnetically (50 rpm). To keep the medium’s sink condition, 0.5 mL of sample was withdrawn and replenished at predetermined intervals. The drug concentration in the aliquots was evaluated using a Jasco UV/Visible Spectrophotometer V-630, Tokyo, Japan. The in vitro drug release data were obtained by plotting cumulative percent drug release [36,37].
By putting the data in the following equation and performing a regression analysis, the zero-order, first-order, Higuchi, and Korsmeyer–Peppas kinetics models were calculated [23,38]. The corresponding equation for each model is shown as follows:
- Zero-order: Qt = Q0 + k0t
- First-order: logQt = logQ0 − k1t/2.303
- Higuchi: Qt = kHt1/2
- Korsmeyer–Peppas: Mt/M∞ = ktn
where, Qt is the amount of drug dissolved during time t, Q0 is the amount of drug initially dissolved in diffusion medium, k0, k1 and kHt1/2 are the zero-order, first order and Higuchi model rate constants, respectively. Mt and M∞ are cumulative drug release at time t and infinite time, respectively. The t stands for release time, n for the diffusional exponent indicating the release mechanism, and k stands for the rate constant of I-NS structural and geometric features. Additionally, appropriate parameters were plotted in each of the aforementioned models, and the release kinetic behavior of ITB was assessed using the R2 value (coefficient of multiple determination; 0 ≤ R2 ≤ 1). The release mechanisms were also detailed [39] using the values of n = 0.45 (Case I or Fickian diffusion), 0.45 < n < 0.89 (anomalous behavior or non-Fickian transport), n = 0.89 (Case II transport), and n > 0.89 (Super Case II).
2.11. Cytotoxicity Assay against MCF-7
Firstly, MCF-7 cells were cultured in 96-well plates, incubating the cells at a density of 10,000 cells per well in 100 μL of culture medium at 37 °C and a 5% CO2 environment, allowing them to stand for 24 h for plate adherence. These cells were used against the test (IBNS4) and reference (ITB) samples and were tested by keeping alongside negative control for 48 h.
Ibrutinib and an equivalent concentration of IBNS4 were used to incubate the cells. Ibrutinib concentrations ranged from 0.78 to 100 µg/mL, while IBNS4 concentrations ranged from 16.38 to 2100 µg/mL (containing an equivalent amount of the drug). The data demonstrate relative cell viability after treatment since the MTT assay employs mitochondrial activity to distinguish between live and dead cells, which are principally targeted through mitochondria-mediated apoptosis [40,41]. The IC50 values were determined by GraphPad Prism V-5.1 using Log (inhibitor) vs normalized response on a varied slope.
Additionally, each sample’s absorbance was measured at 540 nm with an ELISA microplate reader (Thermo Fisher Scientific, Waltham, MA, USA), and the percentage of cell viability was calculated using the formula shown below:
%Cell Viability = (Mean ABS of treated sample)/(Mean ABS of control) × 100
2.12. Stability Studies Using a Similarity Index
According to ICH recommendations, the optimized formulation IBNS4 was preserved for stability testing at accelerated temperatures (40 ± 2 °C, 75%RH) in a programmable environmental test chamber. The cumulative % drug release and calculation of %EE and %DL were evaluated after 26 weeks. Using the similarity factor (f2), the effects of aging on the drug release were calculated, and the results of EE and DL were compared with the data of before and after stability studies [16,38,42].
3. Results and Discussion
3.1. Development of Ibrutinib-Loaded Ethylcellulose-Based Nanosponges
The developed method and formulation characteristics were optimized by varying the rate-retarding polymer EC concentrations from 50 to 200 mg and keeping the stabilizer (PVA) and ITB concentrations for both constant at 50 mg. The dispersion to aqueous phase ratio (2.5 mL DMSO + DCM and 5 mL PVA aqueous phase) was fixed to 1:2. The most often used method is emulsification and solvent evaporation because of its simplicity and reproducibility. EC concentration influences NS size, whereas PVA modifies the flocculation [16,18]. It was observed that enhancing the concentration of EC increases the size of nanosponges (NSs), and an increase in PVA concentration to an optimum level resulted in a decrease in the formation of floccules; this observation was in agreement with the reported literature.
3.2. Particle Characterization: Size, Polydispersity Index (PDI), and Zeta Potential (ζp)
All the developed NSs were in the nanosize range, narrow in particle size distribution as per PDI. Particle size and PDI of five nanosponges developed, IBNS1-5, were found to be in the range of 328.6–743.6 nm, and 0.38–0.40, respectively, whereas ζp was in between (−10.4 to −30.2). Zeta potential for all NSs was negatively charged, with ≥30 ζpP indicating non-agglomeration and a stable dispersion system (Figure 2). EC about the negative charge over the particles could have resulted in inter-particle repulsion. If the average diameter of the particle ranges from >200 nm to ~1000 nm, it is considered to be the extended stay of EC-based NSs in the body, delivering the drug at the site-specific (cancer region) by increasing the permeability to the cancer cell [18,43].

Figure 2.
Images of optimized nanosponge IBNS4: particle size distribution (a), particle size (b), and zeta potential (c).
3.3. Entrapment Efficiency and Drug Loading Calculation
Calculation of EE (%) and DL (%) of the nanosponges indicates drug entrapment and drug loading in NS (Table 1). EE (%) was found to be in the range of 18.67 ± 1.23%–82.32 ± 1.63%, whereas DL (%) of ITB ranged from 1.16 ± 0.55% to 20.18 ± 0.13%. Results of particle analysis and EE and DL calculation revealed that an increase in the concentration of EC increases the PS, EE, and DL to a certain level (162.5 mg), and thereafter further increase in the EC proportion increases the size but decreases the EE and DL. This could be due to rheological behavior of polymer [44,45]. An increase in viscosity due to an increase in EC leads to a decrease in the diffusion of ITB from the organic phase to the aqueous phases of the stabilizer (PVA) [16].
Therefore, IBNS4 NSs could be selected as the optimized nanocarriers for further characterization. The particle analysis and EE and DL calculation showed IBNS4 composed of ITB (50 mg), EC (162.5 mg), and PVA (50 mg) has 640.9 nm, 0.35, −30.2 mV, regarding size, PDI, and ζp, respectively [23,39].
3.4. Fourier-Transform Infrared (FTIR) Analysis
The FTIR spectrum of ITB had noticeable peaks at 3645.87 cm−1 and 3245.45 cm−1 for N-H stretching vibrations and 3076.56 cm−1 for aromatic C-H stretching vibration, as shown in Figure 3. Peaks at 1265.45 cm−1 and 1576.43 cm−1 could be assigned to carbonyl stretching; more peaks were also placed at 1754.76 cm−1 to 1453.7 cm−1. This indicated alkene and cyano functional group stretching frequency [46,47]. All such peaks were also available in the IBNS4 nanosponges, representing no chemical interaction between the drug and polymers used. Some of the peaks disappeared and broadened in the optimized NSs, suggesting ITB encapsulation within the NSs. Henceforth, the selected NSs could be used in targeted cancer treatment, due to non-chemical incompatibilities.
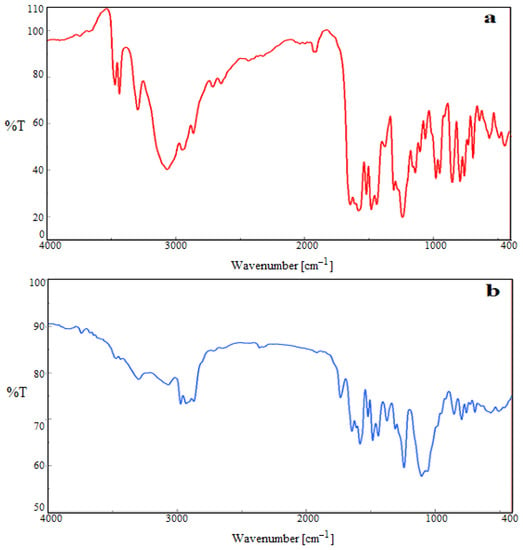
Figure 3.
FTIR spectra of pure ibrutinib ITB (a), and IBNS4 (b).
3.5. Differential Scanning Calorimetry (DSC) Analysis
Thermal peaks of a pure drug (ITB) and nanosponge (IBNS4) were captured and interpreted (Figure 4). The sharp melting point indicates the crystalline nature of the drug at 164.5 °C. This peak disappeared in the optimized nanosponge, signifying an encapsulated drug in the porous EC matrix available in the amorphous form [31]. The endothermic peaks of ITB disappeared in the nanosponge, confirming successful encapsulation of drug.

Figure 4.
DSC thermogram peaks of pure ibrutinib ITB (a) and IBNS4 (b).
3.6. XRD Crystallography
The pure drug (ITB) results showed intense Bragg peaks, due to the crystalline nature of the drug. These peaks intensities were reduced in the optimized nanosponge (IBNS4) due to the energy provided by probe sonication [25]. The drug solubilized in organic solution was added into the aqueous PVA solution that makes the particles stabilized without agglomerations and makes the ITB reduce in nanosize and become amorphous in form, which was again confirmed in the above section. Results of XRD, and DSC collectively suggest that the developed IBNS4 was in an amorphous state, as shown in Figure 5.
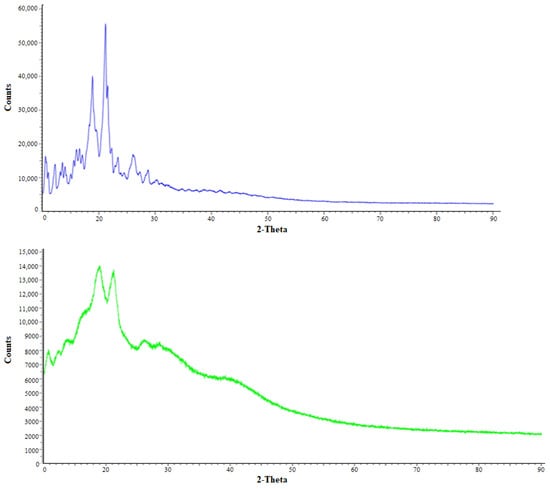
Figure 5.
XRD Bragg peaks of pure ibrutinib ITB (blue) and IBNS4 (green).
3.7. Scanning Electron Microscopy (SEM)
The image of the IBNS4 exhibits holes due to the porousness of the material and some crystals adsorbed over the surface, which could be the stabilizer (PVA) used in the development of NSs [29,48]. Although multiple washing of the NSs was performed with water to remove the excess PVA, some amount remained over the surface and appeared as small flakes over the IBNS4 nanosponges, as shown in Figure 6.

Figure 6.
SEM image of optimized NS IBNS4.
3.8. In Vitro Drug Release and Mathematical Model Fitting
Ethylcellulose acts as the rate-retarding polymer by lowering the diffusion of drugs through the polymer walls/holes. In vitro drug release assay was performed to recognize the release behavior and mechanism of the drug release from the porous matrix in pH 1.2 solution. As shown in Figure 7, in 24 h of study, the release of ITB from optimized IBNS4 was 80.16 ± 5.15%, compared to the pure drug (ITB) with 22.74 ± 1.51%. Initial burst release was observed within the first 6 h with a drug release of 61.80 ± 2.29%, which could be due to the adsorbed drug over the surface of NSs or non-bounded drug-polymer moiety [49]. This type of burst drug release is preferred as per pharmacokinetic studies to initiate the action by reaching the minimum drug concentration, followed by sustained release up to 24 h to prolong the effectiveness of the drug at the site of action and cancer cell [50]. The porous nature of the particle allowed the entry of medium and dissolution of the drug. the effective surface area will also be more due to the porous nature of the particles, and thereby the dissolution was observed to be better compared to the pure IBT. The prolonged release of ITB from optimized IBNS4 could be due to the slow dispersion of aqueous media inside the hydrophobic EC [51].

Figure 7.
Cumulative (%) release of ITB and IBNS4.
Furthermore, the kinetic release study of optimized IBNS4 for different kinetic equations (zero-order, first-order, Higuchi, and Korsmeyer–Peppas) was observed and fitted. The best fit was the Korsmeyer–Peppas model for optimized IBNS4, showing a higher correlation (R2 > 0.90) compared to other models, ensuring the release of ITB from the NSs could be the result of a combination mechanism of diffusion and erosion [52]. Moreover, the Korsmeyer–Peppas model with exponent n < 0.45 denoted that the release is diffusion-controlled, called the Fickian mechanism.
3.9. Cytotoxicity Assay on MCF-7
The optimized nanosponge (IBNS4) exhibited a significant reduction in cell viability (88.24%, 74.27%, 67.10%, 52.84%, 35.55%, 24.86%, 17.12%, and 11.70% at 0.78, 1.56, 3.13, 6.25, 12.50, 25, 50, and 100 µg/mL) in comparison with pure drug IBT (92.29%, 81.49%, 74.43%, 63.86%, 53.94%, 37.73%, 27.17%, and 16% at 0.78, 1.56, 3.13, 6.25, 12.50, 25, 50, and 100 µg/mL), respectively, against MCF-7 cells, as shown in Figure 8 [53]. The half-maximal inhibitory concentration (IC50) was found to be 13.42 and 6.84 for ITB and IBNS4. Developed NSs (IBNS4) had 1.96 times the potential compared to the pure drug (ITB). The reported mechanism of action by BTK inhibitors against the MCF-7 cells is the downregulation of matrix metalloproteinase (MMP) 9 expression by blocking NF-κB and AP-1 activation via the PLCγ/PKC/MAPK and IKK signaling pathways [2,54,55]. The R² value of our cytotoxicity assay was 0.9942 and 0.9929 for ITB and IBNS4, reflecting the data that best fit the regression model.

Figure 8.
Cytotoxicity of pure Ibrutinib (ITB) and optimized ibrutinib-loaded nanosponges (IBNS4) after 48 h of incubation with MCF-7 cells at different concentrations (0.78–100 µg/mL) and control.
3.10. Stability Studies Using a Similarity Index
The results of stability studies showed that the optimized ibrutinib-loaded nanosponge (IBNS4) was stable after 26 weeks, as per ICH. To assess stability, optimized ibrutinib-loaded nanosponges were kept for stability studies at accelerated conditions (40 ± 2 °C, 75%RH). The in vitro release profile, %EE, and %DL of IBNS4 after stability studies (IBNS4-ST) showed no significant difference compared to IBNS4, as shown in Figure 9. The similarity index f2 value for both tests (IBNS4-ST) and reference (IBNS4) calculated was 51.57, within the range 50–100, indicating that the two release profiles are similar [56].
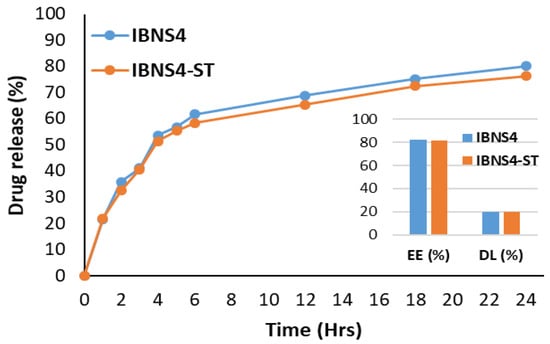
Figure 9.
Cumulative release profile after storage.
The f2 value was calculated by taking the logarithmic reciprocal square root transformation of the sum of squared error, and is a measurement of the similarity in the percent (%) drug release between the test and reference curves.
4. Conclusions
In this study, we aimed to develop ibrutinib-loaded nanosponges for enhanced cytotoxic activity against breast cancer MCF-7 cell lines. Nanosponge was developed by emulsification solvent evaporation technology. Five NSs were developed and characterized for size, PDI, zeta potential FTIR, DSC, XRD, SEM, in vitro drug release, cytotoxicity against MCF-7 cell lines, and stability study. ITB is one of the targeted categories of a moiety that acts as a novel irreversible inhibitor of Bruton’s tyrosine kinase (BTK) enzyme, which suppresses MMP-9, a crucial protein involved in tumor progression and metastasis, including breast cancer. Therefore, ITB-loaded NSs could be effective for breast cancer treatment. Metastasis is a cancer state in which there is a spread of cancer cells into the bones, kidney, brain, liver, and lungs. Breast cancer more easily turns to metastasis and becomes the primary cause of mortality. Ethylcellulose-based NSs loaded with ITB released the drug in a sustained manner for a prolonged period, leading to enhanced cytotoxic effects against the MCF-7 cancer cell lines, and therefore could be used as potential nanocarriers for the effective treatment of breast cancer. The results of the current investigation require further pre-clinical and clinical evaluation.
Author Contributions
Conceptualization, F.F. and M.K.A.; methodology, M.K.A. and F.F.; validation, F.F. and M.K.A.; investigation, F.F. and M.K.A. resources, F.F.; writing—original draft preparation, M.K.A. and F.F.; writing—review and editing, M.K.A.; supervision, F.F.; project administration, F.F.; funding acquisition, F.F. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia, for funding this research work through the project number (IF2/PSAU/2022/03/23127).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia, for funding the research work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sardar, M.; Malik, S.U.; Khan, A.; Idrees, M.; Ahmad, Q.; Sohail, C.; Naseer, R.; Amin, S.; McBride, A.; Abuzar, M.; et al. Efficacy of Ibrutinib-Based Regimen in Chronic Lymphocytic Leukemia: A Systematic Review. J. Hematol. 2019, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kokabee, L.; Kokabee, M.; Conklin, D.S. Bruton’s Tyrosine Kinase and Its Isoforms in Cancer. Front. Cell Dev. Biol. 2021, 9, 668996. [Google Scholar] [CrossRef] [PubMed]
- Broccoli, A.; Argnani, L.; Morigi, A.; Nanni, L.; Casadei, B.; Pellegrini, C.; Stefoni, V.; Zinzani, P.L. Long-Term Efficacy and Safety of Ibrutinib in the Treatment of CLL Patients: A Real Life Experience. J. Clin. Med. 2021, 10, 5845. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.M.; Spurgeon, S.E. Ibrutinib in mantle cell lymphoma patients: Glass half full? Evidence and opinion. Ther. Adv. Hematol. 2015, 6, 242–252. [Google Scholar] [CrossRef]
- Nicolson, P.L.; Hughes, C.E.; Watson, S.; Nock, S.H.; Hardy, A.T.; Watson, C.N.; Montague, S.; Clifford, H.; Huissoon, A.P.; Malcor, J.-D.; et al. Inhibition of Btk by Btk-specific concentrations of ibrutinib and acalabrutinib delays but does not block platelet aggregation mediated by glycoprotein VI. Haematologica 2018, 103, 2097–2108. [Google Scholar] [CrossRef]
- Caldeira, D.; Alves, D.; Costa, J.; Ferreira, J.J.; Pinto, F.J. Ibrutinib increases the risk of hypertension and atrial fibrillation: Systematic review and meta-analysis. PLoS ONE 2019, 14, e0211228. [Google Scholar] [CrossRef]
- de Claro, R.A.; McGinn, K.M.; Verdun, N.; Lee, S.-L.; Chiu, H.-J.; Saber, H.; Brower, M.E.; Chang, C.G.; Pfuma, E.; Habtemariam, B.; et al. FDA Approval: Ibrutinib for Patients with Previously Treated Mantle Cell Lymphoma and Previously Treated Chronic Lymphocytic Leukemia. Clin. Cancer Res. 2015, 21, 3586–3590. [Google Scholar] [CrossRef]
- Burger, J.A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Tedeschi, A.; Bairey, O.; Hillmen, P.; Coutre, S.E.; Devereux, S.; et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia 2019, 34, 787–798. [Google Scholar] [CrossRef]
- Prabaharan, C.B.; Yang, A.B.; Chidambaram, D.; Rajamanickam, K.; Napper, S.; Sakharkar, M.K. Ibrutinib as a potential therapeutic option for HER2 overexpressing breast cancer—The role of STAT3 and p21. Investig. New Drugs 2020, 38, 909–921. [Google Scholar] [CrossRef]
- Varikuti, S.; Singh, B.; Volpedo, G.; Ahirwar, D.K.; Jha, B.K.; Saljoughian, N.; Viana, A.G.; Verma, C.; Hamza, O.; Halsey, G.; et al. Ibrutinib treatment inhibits breast cancer progression and metastasis by inducing conversion of myeloid-derived suppressor cells to dendritic cells. Br. J. Cancer 2020, 122, 1005–1013. [Google Scholar] [CrossRef]
- Metzler, J.M.; Burla, L.; Fink, D.; Imesch, P. Ibrutinib in Gynecological Malignancies and Breast Cancer: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 4154. [Google Scholar] [CrossRef] [PubMed]
- Eisenmann, E.D.; Fu, Q.; Muhowski, E.M.; Jin, Y.; Uddin, M.E.; Garrison, D.A.; Weber, R.H.; Woyach, J.A.; Byrd, J.C.; Sparreboom, A.; et al. Intentional Modulation of Ibrutinib Pharmacokinetics through CYP3A Inhibition. Cancer Res. Commun. 2021, 1, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Hardy-Abeloos, C.; Pinotti, R.; Gabrilove, J. Ibrutinib dose modifications in the management of CLL. J. Hematol. Oncol. 2020, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Allouchery, M.; Tomowiak, C.; Lombard, T.; Pérault-Pochat, M.-C.; Salvo, F. Safety Profile of Ibrutinib: An Analysis of the WHO Pharmacovigilance Database. Front. Pharmacol. 2021, 12, 769315. [Google Scholar] [CrossRef] [PubMed]
- Mehra, N.; Bagal, B.P.; Munot, P.N.; Mirgh, S. Ibrutinib: A narrative drug review. Cancer Res. Stat. Treat. 2020, 3, 767. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Fatima, F.; Anwer, K.; Ibnouf, E.O.; Kalam, M.A.; Alshamsan, A.; Aldawsari, M.F.; Alalaiwe, A.; Ansari, M.J. Formulation and in vitro evaluation of topical nanosponge-based gel containing butenafine for the treatment of fungal skin infection. Saudi Pharm. J. 2021, 29, 467–477. [Google Scholar] [CrossRef]
- Anwer, M.K.; Ahmed, M.M.; Aldawsari, M.F.; Alshahrani, S.; Fatima, F.; Ansari, M.N.; Rehman, N.U.; Al-Shdefat, R.I. Eluxadoline Loaded Solid Lipid Nanoparticles for Improved Colon Targeting in Rat Model of Ulcerative Colitis. Pharmaceuticals 2020, 13, 255. [Google Scholar] [CrossRef]
- Almutairy, B.K.; Alshetaili, A.; Alali, A.S.; Ahmed, M.M.; Anwer, M.K.; Aboudzadeh, M.A. Design of Olmesartan Medoxomil-Loaded Nanosponges for Hypertension and Lung Cancer Treatments. Polymers 2021, 13, 2272. [Google Scholar] [CrossRef]
- Al-Turki, Y.A. Erectile dysfunction among diabetic patients in saudi arabia: A hospital-based primary care study. J. Fam. Community Med. 2007, 14, 19–23. [Google Scholar]
- Iravani, S.; Varma, R.S. Nanosponges for Drug Delivery and Cancer Therapy: Recent Advances. Nanomaterials 2022, 12, 2440. [Google Scholar] [CrossRef]
- Anwer, M.K.; Mohammad, M.; Ezzeldin, E.; Fatima, F.; Alalaiwe, A.; Iqbal, M. Preparation of sustained release apremilast-loaded PLGA nanoparticles: In vitro characterization and in vivo pharmacokinetic study in rats. Int. J. Nanomed. 2019, 14, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Anwer, K.; Ahmed, M.M.; Aldawsari, M.F.; Iqbal, M.; Kumar, V. Preparation and Evaluation of Diosmin-Loaded Diphenylcarbonate-Cross-Linked Cyclodextrin Nanosponges for Breast Cancer Therapy. Pharmaceuticals 2022, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M.; Alnafisah, M.S.; Anwer, M.K.; Fatima, F.; Almutairy, B.K.; Alshahrani, S.M.; Alshetaili, A.S.; Alalaiwe, A.; Fayed, M.H.; Alanazi, A.Z.; et al. Chitosan surface modified PLGA nanoparticles loaded with brigatinib for the treatment of non-small cell lung cancer. J. Polym. Eng. 2019, 39, 909–916. [Google Scholar] [CrossRef]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alsaidan, O.A.; Ahmed, M.M.; Yasir, M.; Warsi, M.H.; Alquraini, A.; Ghoneim, M.M.; Alshehri, S. Development and Optimization of Hybrid Polymeric Nanoparticles of Apigenin: Physicochemical Characterization, Antioxidant Activity and Cytotoxicity Evaluation. Sensors 2022, 22, 1364. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.J.; Anwer, K.; Jamil, S.; Al-Shdefat, R.; Ali, B.E.; Ahmad, M.M. Enhanced oral bioavailability of insulin-loaded solid lipid nanoparticles: Pharmacokinetic bioavailability of insulin-loaded solid lipid nanoparticles in diabetic rats. Drug Deliv. 2016, 23, 1972–1979. [Google Scholar] [CrossRef]
- Fatima, F.; Aldawsari, M.F.; Ahmed, M.M.; Anwer, K.; Naz, M.; Ansari, M.J.; Hamad, A.M.; Zafar, A.; Jafar, M. Green Synthesized Silver Nanoparticles Using Tridax Procumbens for Topical Application: Excision Wound Model and Histopathological Studies. Pharmaceutics 2021, 13, 1754. [Google Scholar] [CrossRef]
- Ansari, M.J.; Ahmed, M.M.; Anwer, K.; Aldawsari, M.F.; Al Shahrani, S.M.; Ahmad, N. Development and Validation of Simple, Rapid and Sensitive High- Performance Liquid Chromatographic Method for the Determination of Butenafine Hydrochloride. J. Pharm. Res. Int. 2020, 32, 116–125. [Google Scholar] [CrossRef]
- Bindu, G.H.; Annapurna, M.M. A sensitive stability indicating RP-HPLC method for the determination of Ibrutinib-An anti-cancer drug. Res. J. Pharm. Technol. 2018, 11, 4587. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Fatima, F.; Kalam, M.A.; Alshamsan, A.; Soliman, G.A.; Shaikh, A.A.; Alshahrani, S.M.; Aldawsari, M.F.; Bhatia, S.; Anwer, K. Development of spray-dried amorphous solid dispersions of tadalafil using glycyrrhizin for enhanced dissolution and aphrodisiac activity in male rats. Saudi Pharm. J. 2020, 28, 1817–1826. [Google Scholar] [CrossRef]
- Anwer, K.; Al-Mansoor, M.A.; Jamil, S.; Al-Shdefat, R.; Ansari, M.N.; Shakeel, F. Development and evaluation of PLGA polymer based nanoparticles of quercetin. Int. J. Biol. Macromol. 2016, 92, 213–219. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Liu, J.; Li, X.L.; Jasti, B.R. Preparation and Characterization of Solid Lipid Nanoparticles Containing Silibinin. Drug Deliv. 2007, 14, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Rani, H.; Singh, V.K.; Asif, S.; Ansari, M.I. Silver Nanoparticle Synthesis and Characterization from leaf Extract of Psoralea Corylifolia (Babchi). Orient. J. Chem. 2018, 34, 2673–2676. [Google Scholar] [CrossRef]
- Alali, A.S.; Kalam, M.A.; Ahmed, M.M.; Aboudzadeh, M.A.; Alhudaithi, S.S.; Anwer, K.; Fatima, F.; Iqbal, M. Nanocrystallization Improves the Solubilization and Cytotoxic Effect of a Poly (ADP-Ribose)-Polymerase-I Inhibitor. Polymers 2022, 14, 4827. [Google Scholar] [CrossRef] [PubMed]
- Dora, C.P.; Trotta, F.; Kushwah, V.; Devasari, N.; Singh, C.; Suresh, S.; Jain, S. Potential of erlotinib cyclodextrin nanosponge complex to enhance solubility, dissolution rate, in vitro cytotoxicity and oral bioavailability. Carbohydr. Polym. 2016, 137, 339–349. [Google Scholar] [CrossRef]
- Ashar, F.; Hani, U.; Osmani, R.A.M.; Kazim, S.M.; Selvamuthukumar, S. Preparation and Optimization of Ibrutinib-Loaded Nanoliposomes Using Response Surface Methodology. Polymers 2022, 14, 3886. [Google Scholar] [CrossRef]
- Heredia, N.S.; Vizuete, K.; Flores-Calero, M.; Pazmiño V, K.; Pilaquinga, F.; Kumar, B.; Debut, A. Comparative statistical analysis of the release kinetics models for nanoprecipitated drug delivery systems based on poly(lactic-co-glycolic acid). PLoS ONE 2022, 17, e0264825. [Google Scholar] [CrossRef]
- Pandav, S.; Naik, J. Preparation and In Vitro Evaluation of Ethylcellulose and Polymethacrylate Resins Loaded Microparticles Containing Hydrophilic Drug. J. Pharm. 2014, 2014, 1–5. [Google Scholar] [CrossRef]
- Alajami, H.N.; Fouad, E.A.; Ashour, A.E.; Kumar, A.; Yassin, A.E.B. Celecoxib-Loaded Solid Lipid Nanoparticles for Colon Delivery: Formulation Optimization and In Vitro Assessment of Anti-Cancer Activity. Pharmaceutics 2022, 14, 131. [Google Scholar] [CrossRef]
- Anwer, M.K.; Mohammad, M.; Iqbal, M.; Ansari, M.N.; Ezzeldin, E.; Fatima, F.; Alshahrani, S.M.; Aldawsari, M.F.; Alalaiwe, A.; Alzahrani, A.A.; et al. Sustained release and enhanced oral bioavailability of rivaroxaban by PLGA nanoparticles with no food effect. J. Thromb. Thrombolysis 2020, 49, 404–412. [Google Scholar] [CrossRef]
- Lerata, M.S.; D’souza, S.; Sibuyi, N.R.; Dube, A.; Meyer, M.; Samaai, T.; Antunes, E.M.; Beukes, D.R. Encapsulation of Variabilin in Stearic Acid Solid Lipid Nanoparticles Enhances Its Anticancer Activity in Vitro. Molecules 2020, 25, 830. [Google Scholar] [CrossRef]
- Pushpalatha, R.; Selvamuthukumar, S.; Kilimozhi, D. Cross-linked, cyclodextrin-based nanosponges for curcumin delivery—Physicochemical characterization, drug release, stability and cytotoxicity. J. Drug Deliv. Sci. Technol. 2018, 45, 45–53. [Google Scholar] [CrossRef]
- Wlodarski, K.; Sawicki, W.; Haber, K.; Knapik, J.; Wojnarowska, Z.; Paluch, M.; Lepek, P.; Hawelek, L.; Tajber, L. Physicochemical properties of tadalafil solid dispersions—Impact of polymer on the apparent solubility and dissolution rate of tadalafil. Eur. J. Pharm. Biopharm. 2015, 94, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.D.; Kurpiers, M.; Götz, R.X.; Zaichik, S.; Hupfauf, A.; Baecker, D.; Gust, R.; Bernkop-Schnürch, A. Phosphorylated PEG-emulsifier: Powerful tool for development of zeta potential changing self-emulsifying drug delivery systems (SEDDS). Eur. J. Pharm. Biopharm. 2020, 150, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Al-Tahami, K. Preparation of Alginate Microspheres for the Delivery of Risperidone. Yemeni J. Med. Sci. 2014, 8, 5. [Google Scholar]
- Parida, P.; Mishra, S.C.; Sahoo, S.; Behera, A.; Nayak, B.P. Development and characterization of ethylcellulose based microsphere for sustained release of nifedipine. J Pharm Anal. 2016, 6, 314–344. [Google Scholar] [CrossRef] [PubMed]
- Anwer, K.; Mohammad, M.; Khalil, N.Y.; Imam, F.; Ansari, M.J.; Aldawsari, M.F.; Shakeel, F.; Iqbal, M. Solubility, thermodynamics and molecular interaction studies of delafloxacin in environmental friendly ionic liquids. J. Mol. Liq. 2020, 305, 112854. [Google Scholar] [CrossRef]
- Swaminathan, S.; Pastero, L.; Serpe, L.; Trotta, F.; Vavia, P.; Aquilano, D.; Trotta, M.; Zara, G.; Cavalli, R. Cyclodextrin-based nanosponges encapsulating camptothecin: Physicochemical characterization, stability and cytotoxicity. Eur. J. Pharm. Biopharm. 2010, 74, 193–201. [Google Scholar] [CrossRef]
- Alshehri, S.M.; Shakeel, F.; Ibrahim, M.A.; Elzayat, E.M.; Altamimi, M.; Mohsin, K.; Almeanazel, O.T.; Alkholief, M.; Alshetaili, A.; Alsulays, B.; et al. Dissolution and bioavailability improvement of bioactive apigenin using solid dispersions prepared by different techniques. Saudi Pharm. J. 2019, 27, 264–273. [Google Scholar] [CrossRef]
- Chan, L.W.; Chow, K.T.; Heng, P.W.S. Investigation of Wetting Behavior of Nonaqueous Ethylcellulose Gel Matrices Using Dynamic Contact Angle. Pharm. Res. 2006, 23, 408–421. [Google Scholar] [CrossRef]
- Singh, P.; Ren, X.; He, Y.; Wu, L.; Wang, C.; Li, H.; Singh, V.; Zhang, J. Fabrication of β-cyclodextrin and sialic acid copolymer by single pot reaction to site specific drug delivery. Arab. J. Chem. 2020, 13, 1397–1405. [Google Scholar] [CrossRef]
- Sarkar, K.; Sadat, S.M.A.; Islam, S.; Jalil, R.-U. Study of Ethyl Cellulose Based Sustained Release Microspheres of Naproxen Sodium. Dhaka Univ. J. Pharm. Sci. 2011, 10, 123–129. [Google Scholar] [CrossRef]
- Ameeduzzafar; Alruwaili, N.K.; Imam, S.S.; Alotaibi, N.H.; Alhakamy, N.A.; Alharbi, K.S.; Alshehri, S.; Afzal, M.; Alenezi, S.K.; Bukhari, S.N.A. Formulation of Chitosan Polymeric Vesicles of Ciprofloxacin for Ocular Delivery: Box-Behnken Optimization, In Vitro Characterization, HET-CAM Irritation, and Antimicrobial Assessment. AAPS PharmSciTech 2020, 21, 167. [Google Scholar] [CrossRef] [PubMed]
- Asfour, M.H.; Mohsen, A.M. Formulation and evaluation of pH-sensitive rutin nanospheres against colon carcinoma using HCT-116 cell line. J. Adv. Res. 2017, 9, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Zi, F.; Yu, L.; Shi, Q.; Tang, A.; Cheng, J. Ibrutinib in CLL/SLL: From bench to bedside (Review). Oncol. Rep. 2019, 42, 2213–2227. [Google Scholar] [CrossRef] [PubMed]
- Godugu, C.; Patel, A.R.; Doddapaneni, R.; Somagoni, J.; Singh, M. Approaches to Improve the Oral Bioavailability and Effects of Novel Anticancer Drugs Berberine and Betulinic Acid. PLoS ONE 2014, 9, e89919. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Anwer, M.K.; Fatima, F.; Aldawsari, M.F.; Alalaiwe, A.; Alali, A.S.; Alharthi, A.I.; Kalam, M.A. Boosting the Anticancer Activity of Sunitinib Malate in Breast Cancer through Lipid Polymer Hybrid Nanoparticles Approach. Polymers 2022, 14, 2459. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).