Abstract
The production of neutral distilled spirits is increasing worldwide due to the popularity of beverages such as vodka and gin. Yeast fermentation lies at the heart of such production, but there are salient differences between the yeast strains employed for neutral spirits, as compared to those used in whisky, rum, and brandy fermentation. For example, the former white spirit processes aim to minimise the synthesis of flavour-active volatile compounds (or congeners), whilst the opposite is true for more flavoursome brown spirits such as whisky. This paper describes the raw materials, yeasts, and fermentation conditions involved in neutral spirit production processes and discusses challenges and opportunities in such technology, including exciting new developments regarding strategies to improve yeast strains.
1. Introduction
Alcohol fermentation by yeast represents the largest of global bioprocesses and the most profitable. In addition to the production of all fermented alcoholic beverages (beers, wines, and distilled spirits), yeasts are also responsible for the billions of litres of fuel alcohol (bioethanol) produced throughout the world. For distilled spirits, the yeast Saccharomyces cerevisiae is the premier microbial ethanologen due to its propensity to efficiently ferment sugars into ethanol, carbon dioxide, and flavour-active metabolites. The latter appear in subsequent distillates and are known as congeners that dictate the flavour and aroma characteristics of many distilled spirits, including whiskies, rums, and brandies. Neutral spirit refers to potable fermentation-derived ethanol that forms the base alcohol in white spirits, such as vodka and gin, that have seen increases in worldwide production in recent years.
This paper describes the yeasts and fermentation strategies employed to make neutral spirits. Neutral spirits are characterised by being notably very low in flavour congeners, and we emphasised the importance of yeast-strain selection and fermentation conditions for minimising congener production whilst maximising ethanol production. Regarding the latter, new opportunities for fermentation optimization through yeast-strain engineering strategies were discussed. As intimated by Pauley and Maskell [1], the information on the role of yeast fermentation in neutral spirit production has hitherto not been reported in detail, and the contribution of yeast to consumer experiences of gin and vodka have been “shrouded in mystery”. This article aimed to provide further insight into yeast fermentation in the production of the neutral alcohol bases for these major global distilled spirits.
2. Overview of Neutral Distilled Spirits Production
2.1. What Are “Neutral Distilled Spirits”?
A neutral spirit (or neutral alcohol) may be defined as a fermentation-derived distilled spirit product with a minimum ethanol content of 95–96% v/v that lacks distinctive aroma and taste. In the US, a neutral spirit is distilled at (or above) 96% alcohol by volume (ABV), whilst in the EU, the minimum alcoholic strength following distillation is 96% ABV [2]. Regarding its organoleptic characteristics, a neutral spirit should have no discernible taste, other than the starting raw materials, which should be of agricultural origin. These raw materials are typically cereals such as wheat, resulting in products referred to as neutral grain spirits (NGSs) or grain neutral spirits (GNSs). Other raw materials can also be used, including grapes, potatoes, molasses (either from sugarcane or sugar beets), and cheese whey (see Section 2.3). A neutral spirit is generally the most common base alcohol for the production of gin, vodka, and other flavoured spirit beverages. The earlier production stages for gin and vodka are relatively similar [3,4]. The majority of gin is produced through flavouring of a neutral spirit with botanicals such as juniper, coriander, angelica, and others. This is achieved either by compounding botanicals in neutral spirits (maceration) or by re-distillation in the presence of the botanicals (maceration prior to distillation or vapour infusion) [5,6]. For vodka, the subsequent removal of congeners takes place post-distillation and typically by charcoal filtration [7,8]. Therefore, yeast alcohol production kinetics and yields are much more important than congener biosynthesis for producing the base neutral spirit for gin and vodka, as compared to other distilled beverages [1].
In the European Union, the chemical properties of a spirit (referred to as the ethyl alcohol of agricultural origin [2]) destined for vodka, gin, or other botanically flavoured spirits (for example, kummel and akvavit) production and the allowable levels of specific congeners are defined and summarised in Table 1.

Table 1.
Requirements of neutral spirit constituents used in the production of spirit drinks. The requirements listed are in accordance with European Union regulations (EU 2019/787 Ethyl Alcohol of Agricultural Origin) (Adapted from [2]).
2.2. Process Overview
A general scheme summarising starch and sugar processing into neutral alcohol, including the role of yeasts in the production of diverse alcoholic beverages, is outlined in Figure 1. The diversity of products is reflected by the diversity of starting raw materials, but the common denominator in all these beverages is yeast fermentation.
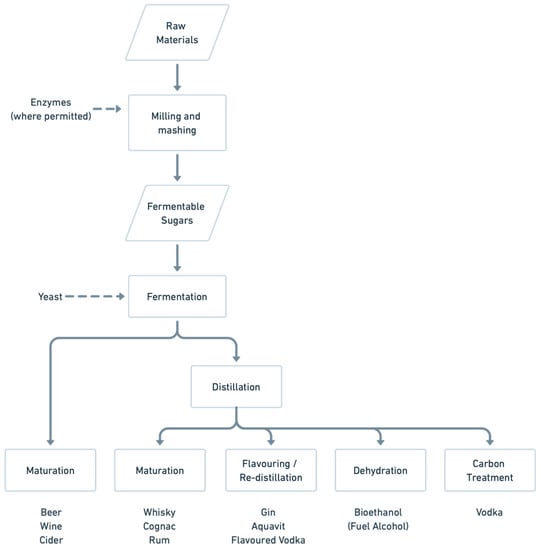
Figure 1.
Summary of the production of diverse alcoholic beverages. The role of yeast, particularly S. cerevisiae, is pivotal in conversion of fermentable sugars to ethanol in all the beverages indicated. (Adapted from [9]).
For the production of neutral spirits destined for both gin and vodka production, careful consideration of raw materials, yeast-strain selection, fermentation conditions, and distillation techniques are important.
2.3. Substrates and Wort Preparation for Fermentation
Regarding the starting carbohydrate materials for yeast fermentation, these can either be starch-based or sugar-based, and examples are provided in Table 2. Although this Table shows the typical spirit types produced from each starting material, it is possible to produce a neutral spirit from any substrate if the correct distillation processes are followed (see Section 2.5). Despite the resulting spirit being classified as neutral, different substrates can still introduce different qualities into the final spirit for sensory perceptions, such as mouthfeel and organoleptic characteristics.

Table 2.
Some fermentation media for the production of distilled spirits. (Adapted from [10]).
Processing of starch-based sources is more involved, as compared to sugar-based sources since the former necessitates pre-hydrolysis, whilst the latter does not. For example, molasses requires relatively straightforward pasteurisation, dilution, and nutrient supplementation prior to fermentation without polysaccharide pre-hydrolysis. Molasses, a by-product of sugar refining, has also been effectively fermented and distilled to produce rums and neutral spirits. Sugarcane molasses-based distilleries for the production of rectified ethanol are commonplace in many countries, notably Brazil and India (e.g., [11]). Similarly, fermentable sugars for fruit-based spirits (glucose, fructose, and sucrose) are simply extracted by physical means (e.g., pressing of grapes to yield wine musts).
Being a polymer of glucose that cannot be fermented directly by yeast, starch requires hydrolysis (or amylolysis) for the conversion to fermentable sugars. Such processes firstly involve particle size reduction by milling, cutting, pressing, or grinding, depending on the starting raw material. This is then followed by mixing with water, unless the raw material is of a sufficiently high moisture content, and high-temperature hydration to gelatinise the starch. The temperature at which gelatinisation occurs, that is, the hydration, swelling, and bursting of the granules into which the starch is packed, is specific to each raw material. In the subsequent mashing stage, starch-degrading enzymes (amylases) then liberate the fermentable sugars into a liquid, known as wort. Note, that for Scotch whisky production, exogenous enzymes (commercially available) are not allowed to be used due to legislation, and therefore, the saccharification of cereal starch is accomplished solely by the amylolytic enzymes present in barley malt. These are known as endogenous enzymes. The sugars fermented by yeast, predominantly glucose, maltose and maltotriose, are liberated from starch during the mashing stage by barley malt enzymes. However, for spirits to be used for gin and vodka production, namely grain neutral spirit (GNS), exogenous enzymes are allowed for conversion of starch (mainly from wheat or maize) into fermentable glucose. Starch is formed of two main structural components, amylose and amylopectin. Amylose is linear, whereas amylopectin is highly branched. Therefore, the enzymes applied must be able to hydrolyse both linear (α 1–4) and branched (α 1–6) glucosidic linkages. Such starch-degrading enzymes are α-amylase and amyloglucosidase (also known as glucoamylase), respectively. The enzymes can further be applied to aid in the processability of the wort. For example, a β-glucanase can be applied when high β-glucans concentrations could cause wort run-off problems due to their high viscosity. Irrespective of the enzyme sources for cereal-wort production, starch degradation processes do not stop when the wort leaves the mashing vessel. This is because distiller’s wort is not boiled following mashing, as in beer production. Consequently, the residual malt or exogenous enzymes continue their amylolysis during fermentation, and this resembles the simultaneous saccharification and fermentation (SSF) processes typically found in bioethanol (fuel alcohol) distilleries that process maize and wheat [12].
Although cereals are the most common substrate utilised in the production of neutral spirits, likely due to being cost-effective products of local agriculture, some distillers use alternative, non-cereal sources of starch-based spirits. For example, potatoes can be used [13], but the basic steps of reducing particle size, starch gelatinisation, and mashing remain the same, including milling, cooking with alpha-amylase, cooling, and the addition of amyloglucosidase prior to yeast fermentation. Following mashing and starch conversion, cooling the wort to suitable temperatures (e.g., 20–30 °C) then takes place to facilitate active yeast fermentation. Selected distilling strains of S. cerevisiae typically produce a fermented wash at 8–10% v/v ethanol.
An interesting raw material for neutral spirit production is cheese whey. This liquid is a by-product of the cheese-making process and is rich in lactose, as well as other nutrients, for yeast fermentation. Although S. cerevisiae is unable to ferment lactose, other yeasts, notably Kluyveromyces marxianus, are able to do so, and this property has been exploited by several large-scale distillery operations worldwide (e.g., in Ireland, New Zealand, Turkey). See O’Shea [14], Tomaszewska and Bialonczyk [15], and Hughes et al. [16] for further information on cheese-whey-based spirits.
2.4. Fermentation Process Control
Although low congener-producing yeast strains are typically selected for the production of neutral spirits, the control of the fermentation temperatures and duration, together with other processing parameters (e.g., vessel pressure), can further help reduce flavour formation. The principal components analysis (PCA), as presented in Figure 2, highlighted the effect of temperature on congener production with distinct congener groups found at each of the fermentation temperatures. A substantial decrease in the concentration of a large number of congeners was apparent as fermentation temperatures increased. Regarding fermentation times, these should be minimised to curtail the development of congeners in neutral spirits, as compared to the situation with prolonged fermentation typified by more flavoursome spirits, such as malt whisky.
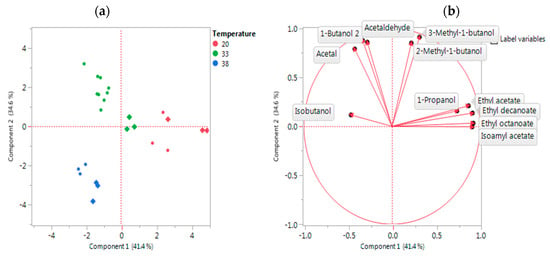
Figure 2.
PCA of congener data obtained following corn-whisky fermentation at 20 °C, 33 °C, and 38 °C using a neutral yeast strain (circles) and a whisky yeast strain (diamonds). (a) Scores plot (b) Loadings plot. Analysis courtesy of Lallemand Biofuels & Distilled Spirits R&D, Montreal, QC, Canada.
2.5. Neutral Spirit Distillation
A neutral spirit is produced by removing most of the congeners during the distillation of the fermented wash. Multiple distillation steps using a multi-column continuous system is typically used to achieve the desired concentration of alcohol and the level of congeners. Following the primary distillation that consists of a stripping and rectifying column, the spirit may be processed through an extractive distillation step (hydro-selection), a secondary distillation (stripper and rectifier), fusel-oil recovery, and a methanol reduction step. Table 3 demonstrates the efficiency of a multi-column distillation system for producing a spirit with barely any detectable congeners present. In comparison, a pot-distilled new-make whisky spirit is typically rich in congeners. The reader is directed to Murray [17] for more comprehensive coverage of distillation technology for distilled spirit production.

Table 3.
Congener profile of new-make whisky and neutral spirits. Values are presented as grams per hectolitre of alcohol. Values with the symbol < are the test-detectable limits and indicate that the compound was not detected. Analysis courtesy of Tatlock & Thomson (by Leven, UK) on samples provided by Arbikie Distilling Ltd. (Arbroath, UK).
2.6. Distilling Yeasts: General Characteristics
Yeasts are single-celled micro-fungi that have widespread uses in industry. The reader is directed to additional published reference material for detailed coverage of the biochemistry and industrial applications of yeast [18]. The species of yeast that predominates in neutral spirit fermentation is S. cerevisiae. This yeast is also primarily involved in fermentation for the production of most other alcoholic beverages, including beer, wine, cider, and matured distilled spirits, such as whisky, rum, and brandy. Different strains of S. cerevisiae have been selected for specific applications in fermented beverage production, and although this yeast predominates in distilled beverage fermentation, other yeast species have occasionally been employed, such as Schizosaccharomyces pombe and Kluyveromyces marxianus, as outlined in Table 4.

Table 4.
Yeasts employed for production of distilled spirits. (Adapted from [19]).
2.7. Properties of Yeast for Neutral Spirit Production
The general desirable characteristics of distilling strains of yeast exploited for production of different distilled spirits are outlined in Table 5. When considering yeasts used for the production of flavoursome distilled spirits (e.g., malt whisky), rather than neutral alcohol destined for gin and vodka, the desired yeast attributes have been focused on congener flavour development, rather than ethanol yield [21]. Conversely, for the production of neutral spirits, the desired attributes of yeasts include rapid fermentation with high alcohol but low congener yield and tolerance to environmental stresses. The physiological and biochemical characteristics of a distilling yeast strain for neutral spirits are strongly influenced by the chosen fermentable substrate, which could either be sugar-based (e.g., molasses) or starch-based (e.g., wheat).

Table 5.
Distilling yeast desired attributes: qualitative criteria (adapted from [22]).
The following salient features of yeasts to be employed specifically for neutral spirit fermentation should be considered:
- Fast utilisation of all available fermentable sugars
- Production of high alcohol yields at the end of fermentation
- Good thermotolerance (e.g., ability to ferment at temperatures as high as 37 °C)
- Non-flocculent behaviour (aggregation of yeast cells into sedimentary clumps)
- Tolerance to osmotic stress (e.g., high-sugar molasses and high-gravity cereal wort)
- Low production of flavour compounds (congeners)
Regarding the latter attribute, several commercially produced yeasts are available that inherently synthesise low levels of congeners, such as higher alcohols, aldehydes, and esters. Although higher alcohols account for the majority of congeners present, the esters are the most influential congener group formed during fermentation from a flavour perspective. Figure 3 compares the ester profiles of a cereal wort fermented with one such neutral spirit yeast, to a whisky yeast. Although the overall concentration of esters present remained lower when using a neutral yeast, this difference was reduced at higher temperatures.
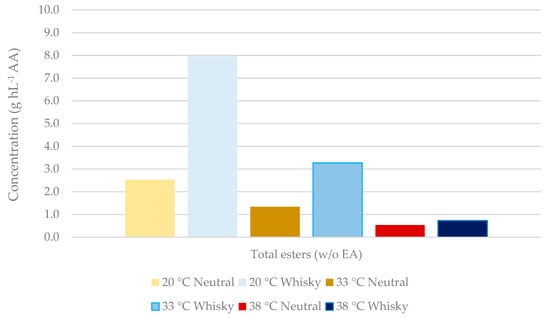
Figure 3.
Effect of yeast strain and temperature on ester formation during corn-whisky fermentation. Congener concentrations were measured in distillates from single distillation with a 30 L capacity hybrid still and are expressed in grams per hectolitre of absolute alcohol (AA, 100% vol. alcohol). EA: ethyl acetate. Esters analysed: isoamyl acetate, ethyl caprylate, ethyl caprate. Analysis courtesy of Lallemand Biofuels & Distilled Spirits R&D, Montreal, QC, Canada.
Distilling yeast strains can be cultured in house or supplied on a commercial basis by yeast supply companies [1]. Some small-scale and artisanal distilleries have adopted spontaneous fermentation that rely on indigenous microbiological flora, such as wild yeasts and bacteria. These microbes are found in the raw materials and are part of the fabric of distillery environments. Spontaneous fermentation have typically been exploited in small distilleries in Mexico (for Tequila and Mezcal production) and in Brazil (for Cachaça production), and their products are typically rich in congeners. Therefore, this is not an ideal practice for the production of a neutral spirit. In addition to S. cerevisiae, several non-Saccharomyces yeasts can be used as pure starter cultures or can occur naturally in beverage fermentation. For example, Schiz. pombe was found in molasses fermentation for rum production, and K. marxianus strains can ferment lactose in cheese-whey to produce white spirits, such as vodka and gin. Several wild yeast species, such as non-distilling strains of S. cerevisiae, Pichia membranefaciens, Torulaspora delbrueckii, and Candida species, can also be found in some distillery fermentations [9]. Although the levels of such yeasts can be deleterious in affecting fermentation progress, they are usually controlled at low cell numbers due to the predominance of S. cerevisiae as the main yeast strain if used at high starting cell densities during production. However, another wild yeast, Dekkera bruxellensis (anamorph Brettanomyces bruxellensis), was found to dominate and out-compete S. cerevisiae in wheat-based distillery fermentation [23].
2.8. Yeast Handling/Management
As indicated above, distilleries can choose between freshly propagated and commercially supplied pure-cultured strains of S. cerevisiae. The latter may be supplied in compressed (cake) form, liquid (cream) form, or in dried form (Table 6 and [21,24]).

Table 6.
Distilling yeast formats.
The pitching rate (inoculum) is generally around 107 cells/mL. Distilled spirit producers, as compared to brewers and winemakers, do not recycle yeast, and instead, the fermented wash, including the yeast, is frequently distilled. This results in the concomitant destruction of yeast cells, thus necessitating supplies of freshly propagated or freshly supplied yeast for subsequent fermentation. The distillation of yeast-containing fermented wash also contributes flavour to the resultant spirit, which is undesirable for neutral alcohol production.
Typically, distilled spirit fermentations are allowed to proceed for a few days without precise temperature control. The pH is also not commonly controlled in a cereal-mash fermentation. The pH prior to fermentation is typically 5–5.5 pH and can fall to a pH of 4.2–4.5 by the end of fermentation. Yeast viability declines at the end of fermentation due to the combination of a low pH, temperatures > 30 °C, and a high final ethanol concentration. These additional reasons are why yeast is not recycled by distilleries.
For all types of industrial fermentation processes that use yeast, it is important to ensure the yeast strain employed has good viability and vitality and is a pure culture. The latter means that the strain of yeast is genetically pure and that the culture population is free from contaminating micro-organisms, including other yeast strains. Some (larger) distilleries have suitable equipment to propagate enough pure yeast aerobically to pitch (inoculate) into the distillery’s fermenters. Note, that yeast propagation is different from fermentation. The former relies on the yeast’s respiratory capabilities to generate sufficient energy (in the form of ATP) from the aerobic metabolism of sugar, whilst the latter produces ethanol and CO2 under anaerobic conditions with lower energy generation.
Yeast strain purity, viability, and vitality are key analytical parameters to ensure consistent fermentation [25]. Therefore, a pitching yeast contaminated by lactic-acid bacteria is considered to have poor viability; if it were to be used to initiate a distillery fermentation, a sluggish fermentation with low alcohol yield would be expected.
2.9. Alcoholic Fermentation
Yeasts ferment sugars into alcohol, carbon dioxide, and secondary metabolites, as they reproduce and grow under anaerobic conditions. The reproduction of S. cerevisiae is by a mechanism known as budding. Figure 4 summarises the main pathways adopted by yeast when converting sugars into alcohol under anaerobic conditions. Glycolysis, also known as the Embden–Meyerhof–Parnas pathway, involves the action of 10 cytosolic enzymes in yeast that degrades glucose (and other fermentable sugars) into pyruvate with the production of energy (2ATP) for yeast cell growth. Full details of the glycolysis pathway, including the enzymes and the substrates, can be found in any biochemistry and distilled spirits textbooks (e.g., [19,26,27]). The final pathway is fermentation, which involves two yeast enzymes that convert pyruvate into ethanol and carbon dioxide as the yeast regenerates NAD to control the redox balance. The key fermentative enzymes in question are the pyruvate decarboxylase and the alcohol dehydrogenase.
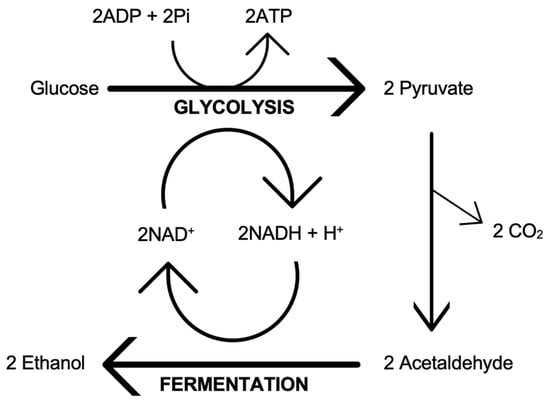
Figure 4.
Summary of glycolysis and fermentation.
The principal fermentable sugars found in cereal wort are glucose, maltose, and maltotriose, whilst those found in molasses are predominantly sucrose. The initial concentration of these sugars dictates the final yield of ethanol at the end of fermentation [9]. Theoretical yields can be calculated utilising the Gay-Lussac equation, which states that 1 g glucose yields 0.511 g of ethanol. In a 100% efficient system, 1 tonne of starch liberates 1.111 tonnes of glucose (due to the addition of 1 molecule of water per 1 molecule of glucose formed) which, in turn, produces 567.7 kg of ethanol or 719 L of pure ethanol. In practice, yields are typically lower than these estimates as a result of processing and equipment inefficiencies.
2.10. Secondary Metabolic Products of Yeast
The primary products of yeast fermentation are ethanol and carbon dioxide. Secondary yeast metabolites are minor compounds produced in much lower concentrations. Nevertheless, a large percentage of the flavour congeners in distilled spirits is contributed by the secondary metabolites produced by yeast during fermentation, as summarised in Figure 5.
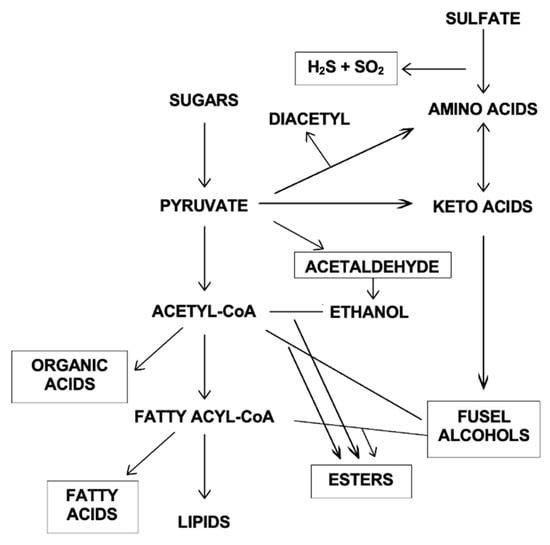
Figure 5.
Outline of metabolic inter-relationships in the synthesis of secondary yeast metabolites.
As compared to a malt-based spirit, grain neutral spirits tend to be quite “clean” and contain relatively low levels of flavour congeners. Figure 3 and Table 3 present congener profiles to demonstrate the impact of yeast and distillation techniques on the presence of flavour congeners.
The flavour (taste and aroma) characteristics of yeast-derived flavour congeners in distilled spirits are summarised in Table 7. The principal flavour congeners in distilled spirits include fatty acids and their esters (e.g., ethyl caprate, ethyl laurate), organic acids (e.g., succinic acid), higher alcohols (e.g., n-propanol, isoamylalcohol), aldehydes (e.g., acetaldehyde), and vicinal diketones (diacetyl). Additional information regarding the origins and identification of different flavour congeners in distilled spirits can be found in [9,17,19,20,26,27,28,29].

Table 7.
Important yeast-derived flavour congeners in distilled spirits. (Adapted from [10]).
Esters are especially important flavour congeners in alcohol distillates due to their fruity and floral characteristics. Ester production by yeast during fermentation is facilitated by condensation reactions between alcohols and acyl CoAs. Ethyl acetate is the predominant ester produced, with isoamyl acetate formed at lower concentrations to impart a fruity (banana) aroma to spirits. However, certain esters, notably ethyl lactate, are linked to lactic acid bacterial metabolism. Another group of flavour congeners derived from yeast metabolism that are important in distilled spirits are higher alcohols. Their production by yeast is related to the concentrations of amino acids in a fermentation medium. For example, phenylalanine stimulates phenylethanol production, giving rise to a rose-like aroma.
In many distilled spirits, yeast-derived congeners are beneficial contributors to the flavour characteristics of distillates, if controlled within certain limits. The choice of the specific strain of distiller’s yeast, together with the contributions from other micro-organisms, such as lactic-acid bacteria, are important in dictating the final flavour and aroma characteristics of the distilled spirits. Furthermore, for the production of neutral spirits, the yeast strain selected, together with the distillation stage, are crucial for minimising the concentration of these flavour congeners.
2.11. Yeast Strain Improvement Strategies
Walker and Walker [30] discussed various yeast-strain-engineering strategies for the enhancement of alcoholic fermentation performance in both beverage and biofuel ethanol. For the latter, de Silva Fernandez et al. [31] discussed approaches to improve yeasts for converting sugar, starch, and cellulosic substrates to bioethanol as a renewable transport fuel. There were similarities in the desired attributes of yeast strains for biofuel and neutral spirit applications. Most notably, this included the minimal biosynthesis of glycerol as well as the other secondary metabolites, such as higher alcohols, acids, and esters. Moreover, the production of a flavour-less distillate in both cases would be a common goal. For both biofuels and distilled spirits, yeast-strain improvement can be accomplished via physiological, genetic, and molecular and synthetic biological strategies. Very high yields of ethanol (>20% v/v) have been achieved using cereal starches in large-scale bioethanol (fuel alcohol) distilleries, for example, in the United States. This was possible by employing various yeast physiological and process-engineering approaches, including very-high-gravity fermentation; stress-tolerant yeasts; optimised nitrogen and mineral nutrition; temperature staging; and simultaneous saccharification and fermentation [32]. Such approaches have the potential for increasing ethanol yields for neutral spirit producers. The adaptive evolution of yeast strains with improved fermentation characteristics and increased tolerance to stress have also been employed. This involves growing yeast, preferably in chemostat cultures, under a particular selective pressure (e.g., increased ethanol concentrations) to select for faster growing spontaneous mutants. This has proved successful to enhance yeast substrate utilisation, ethanol production, and stress tolerance. Such cell-engineering approaches may be applicable for achieving high ethanol yields for neutral spirit producers.
Regarding more directed genetic-based yeast-strain improvements, these involve either classical genetics (e.g., strain hybridisation), genetic engineering (recombinant DNA technology), gene editing (via CRISPR/Cas9), or synthetic biology. Figure 6 summarises some of the strain improvement strategies for industrial yeasts based on molecular genetic approaches that have been discussed by Argyros and Stonehouse [33], for yeasts for bioethanol production. However, the improved yeast traits, as shown in Figure 6, represented the desired characteristics for both biofuel and distilled spirit fermentation. For example, the exploitation of genetically engineered yeast strains to utilise raw starch could circumvent the need to use exogenous amylolytic enzymes to saccharify starch, and such strains were successfully developed [34]. Particularly concerning neutral alcohol, one approach potentially involved a reduction in glycerol biosynthesis, but a suppression of the pathways leading to other by-products were feasible and would be highly beneficial. Regarding glycerol, this represented a significant metabolic product of yeast fermentation that could result in corresponding losses in ethanol yields (around 4%) by S. cerevisiae [35]. To counteract this, van Aalst et al. [36] described how to alter the energy coupling in anaerobic yeast fermentation to reduce or eliminate glycerol biosynthesis whilst, at the same time, maximising alcohol yields. This would also benefit neutral spirit producers.
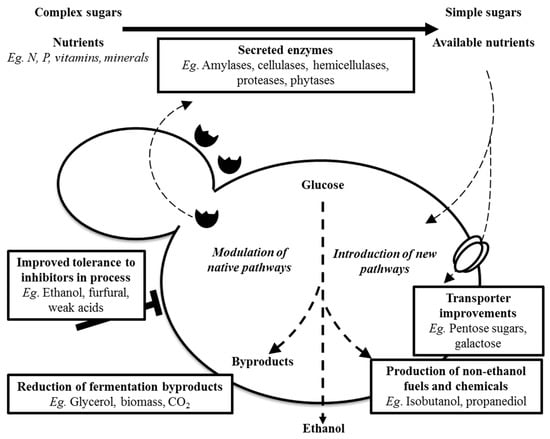
Figure 6.
Yeast strain improvement opportunities (adapted from [33]).
Gene-editing technologies using CRISPR/Cas9 in yeast have been well established [37] and applied to confer ethanol stress tolerance in S. cerevisiae [38]. Deparis et al. [39] discussed how other industrially relevant traits could be introduced into yeast using gene and pathway engineering.
Regarding synthetic biological approaches, Walker and Pretorius [40] discussed the opportunities to engineer wine yeast communities for specific fermentation attributes, and similar approaches have been feasible for developing new distilling yeasts, including strains with specific characteristics for neutral spirit fermentation. The synthetic biological tool SCRaMbLE (Synthetic Chromosome Recombination and Modification by LoxP-mediated Evolution) is a method that allows yeast to optimise metabolic pathways [41], potentially for ethanol fermentation. It could also be used to improve tolerance to fermentation stress factors, such as ethanol toxicity and elevated temperatures [42]. When applied to S. cerevisiae, SCRaMbLE was a powerful tool for increasing the industrial fermentation productivity [43]. Nevertheless, SCRaMbLE has been performed primarily on laboratory yeast strains. However, recent studies [44,45] have indicated the potential to transition synthetic biological approaches in order to improve the characteristics of industrial S. cerevisiae strains with polyploid genotypes. The synthetic biology approach in yeast showed it was possible to completely replace the yeast glycolysis pathway with alternative pathways (e.g., Kuijpers et al., [46]). Therefore, one could envisage future synthetic yeast variants containing synthetic, modular pathways, resulting in new and more efficient functions, including reduced congener biosynthesis, which would be pertinent for neutral spirit production.
Although synthetic biological approaches for improving beverage yeast characteristics have yet to be proven in scaled-up industrial fermentation, the technology for neutral alcohol production processes has great potential. This is particularly pertinent for the development of novel yeast strains with a reduced biosynthesis of secondary fermentation metabolites, notably glycerol. Nevertheless, in addition to the technology constraints, the negative consumer perception of genetically modified or synthetic microbe variants in food and drink production may prove a difficult challenge to overcome (e.g., [47,48]).
3. Conclusions
This paper discussed the desired properties of yeasts and the fermentation processes employed for the production of neutral distilled spirits. Due to the increasing global production of spirits such as gin and vodka, it is important for producers of the base alcohol for such beverages to select the best strains of S. cerevisiae for optimum fermentation performance with the appropriate carbohydrate substrate. The yeast properties in question include, but are not limited to, the fast utilisation of available sugars and high ethanol yields, with the reduced biosynthesis of secondary flavour metabolites. Commercial yeast companies can supply suitable yeast strains for neutral spirit production, and further options are available via strain engineering to achieve yeast attributes, resulting in the desired characteristics in the distillates. Considering what the future may hold regarding novel next-generation ethanologenic yeasts, one intriguing scenario includes the possibility of curtailing the production of carbon dioxide whilst augmenting ethanol biosynthesis during fermentation by S. cerevisiae. Several molecular genetic strategies for developing CO2-fixing yeasts and potentially achieving such sustainable outcomes have already been reported [49,50,51,52]. Some of the strategies for improved yeast strains, as highlighted in this paper, should point the way for future improvements for neutral spirit producers.
Author Contributions
Conceptualization, K.B. and G.W.; writing—original draft preparation, G.W.; writing—review and editing, K.B. and G.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within this article.
Acknowledgments
We thank the following for their technical support regarding some of the analyses presented in this paper: Tatlock & Thomson Ltd., By Leven, Scotland, and Lallemand Biofuels & Distilled Spirits R&D, Montreal, QC, Canada.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pauley, M.; Maskell, D. Mini-review: The role of Saccharomyces cerevisiae in the Production of Gin and Vodka. Beverages 2017, 3, 13. [Google Scholar] [CrossRef]
- Council Regulation (EU) 2019/787 on the Definition, Description, Presentation and Labelling of Spirit Drinks, the Use of the Names of Spirit Drinks in the Presentation and Labelling of Other Foodstuffs, the Protection of Geographical Indications for Spirit Drinks, the Use of Ethyl alcohol and Distillates of Agricultural Origin in Alcoholic Beverages, and Repealing Regulation (EC) No 110/2008′ (2019) Official Journal L130. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R0787&rid=6 (accessed on 3 April 2021).
- Aylott, R.I. Gina and Vodka. In Production, Technology and Innovation; Bryce, J.H., Piggot, J.R., Stewart, G.G., Eds.; Nottingham University Press: Nottingham, UK, 2008; pp. 299–303. [Google Scholar]
- Murtagh, J.E. Production of neutral spirits and preparation of gin and vodka. In The Alcohol Textbook: A Reference for the Beverage, Fuel and Industrial Alcohol Industries, 3rd ed.; Jaques, K.A., Lyons, T.P., Kelsall, D.R., Eds.; Nottingham University Press: Nottingham, UK, 1999; pp. 195–210. [Google Scholar]
- Smith, D.T. The Gin Dictionary; Octopus Publishing: London, UK, 2018; ISBN 9781784724894. [Google Scholar]
- Greer, D.; Pfahl, L.; Rieck, J.; Daniels, T.; Garza, O. Comparison of a novel distillation method in a model gin system using liquid/liquid extraction. J. Agric. Food Chem. 2008, 56, 9030–9036. [Google Scholar] [CrossRef]
- Perederii, M.A.; Tsodikov, M.V.; Uvarov, V.I. Purification of aqueous alcohol solutions in two-bed adsorber filters. Solid Fuel Chem. 2011, 45, 34–38. [Google Scholar] [CrossRef]
- Siristova, L.; Prinosilova, S.; Ridellova, K.; Hajslova, J.; Melzoch, K. Changes in quality parameters of vodka filtered through activated charcoal. Czech J. Food Sci. 2012, 30, 474–482. [Google Scholar] [CrossRef]
- Walker, G. Physiology of ethanol-producing yeasts. In The Alcohol Textbook, 6th ed.; Walker, G.M., Abbas, C., Ingledew, W.M., Pilgrim, C., Eds.; Ethanol Technology Institute: Duluth, Georgia, 2017; Chapter 17; pp. 257–271. [Google Scholar]
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the Production of Fermented Beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Manickam, P.; Muthukaruppan, V. Bioprocessing of Cane Molasses to Produce Ethanol and Its Derived Products from South Indian Distillery. In Emerging Eco-Friendly Green Technologies for Wastewater Treatment. Microorganisms for Sustainability; Bharagava, R., Ed.; Springer: Singapore, 2020; Volume 18. [Google Scholar] [CrossRef]
- Walker, G.M. Fuel alcohol: Current production and future challenges. J. Inst. Brew. 2011, 117, 3–22. [Google Scholar] [CrossRef]
- Menezes, A.G.T.; Menezes, E.G.T.; Alves, J.G.L.F.; Rodriguez, L.F.; das Gracas Cardosa, M. Vodka production from potato (Solanum tuberosum L.) using three Saccharomyces cerevisiae isolates. J. Inst. Brew. 2016, 122, 76–83. [Google Scholar] [CrossRef]
- O’Shea, J. Whey alcohol-a viable outlet for whey? In The Alcohol Textbook, 4th ed.; Jacques, K.A., Lyons, T.P., Kelsall, D.R., Eds.; Nottingham University Press: Nottingham, UK, 2003; pp. 65–73. ISBN 1-897676-13-1. [Google Scholar]
- Tomaszewska, M.; Bialonczyk, L. Ethanol production from whey in a bioreactor coupled with direct contact membrane distillation. Catal. Today 2016, 268, 156–163. [Google Scholar] [CrossRef]
- Hughes, P.; Risner, D.; Goddik, L.M. Whey to Vodka. In Whey–Biological Properties and Alternative Uses; Gigli, I., Ed.; Interopen Publications: London, UK, 2019. [Google Scholar] [CrossRef]
- Murray, D.M. Distillation practices for beverage alcohol and impact on flavour. In The Alcohol Textbook, 6th ed.; Walker, G.M., Abbas, C., Ingledew, W.M., Pilgrim, C., Eds.; Ethanol Technology Institute: Duluth, GA, USA, 2017; Chapter 29; pp. 455–469. [Google Scholar]
- Walker, G.M. Yeast Physiology and Biotechnology; J. Wiley & Sons: Chichester, UK; New York, NY, USA, 1998. [Google Scholar]
- Goodall, I.; Fotheringham, R.; Murray, D.; Speers, A.; Walker, G.M. Distilled Spirits. In Future Challenges & New Solutions, Proceedings of the 5th Worldwide Conference on Distilled Spirits, Glasgow, UK, 8–11 September 2014; Context Publishers: Nottingham, UK, 2015; ISBN 9781899043712. [Google Scholar]
- Walker, G.M.; Lappe-Oliveras, P.; Moreno-Terrazas, R.; Kirchmayr, M.; Arellano-Plaza, M.; Gschaedler-Mathis, A. Yeasts associated with the production of distilled beverages. In Yeasts in the Production of Wine; Romano, P., Cianni, M., Fleet, G.H., Eds.; Springer Nature: New York, NY, USA, 2019; pp. 477–512. ISBN 978-1-4939-9780-0. [Google Scholar] [CrossRef]
- Walker, G.; Hill, A. Saccharomyces cerevisiae in the Production of Whisk(e)y. Beverages 2016, 2, 38. [Google Scholar] [CrossRef]
- Walker, G.M.; Bringhurst, T.; Brosnan, J.; Jack, F. Selecting new distilling yeasts for improved fermentation and for sustainability. In Distilled Spirits. Science and Sustainability [Proceedings of the 4th Worldwide Conference on Distilled Spirits, Edinburgh]; Walker, G.M., Goodall, I., Fotheringham, R., Murray, D., Eds.; Nottingham University Press: Nottingham, UK, 2012; pp. 127–136. [Google Scholar]
- Passoth, V.; Blomqvist, J.; Schnurer, J. Dekkera bruxellensis and Lactobacillus vini form a stable ethanol-producing consortium in a commercial alcohol production process. Appl. Environ. Microbiol. 2007, 73, 4354–4356. [Google Scholar] [CrossRef]
- Cheung, A.W.Y.; Brosnan, J.M.; Phister, T.; Smart, K.A. Impact of dried, creamed and cake supply formats on the genetic variation and ethanol tolerance of three Saccharomyces cerevisiae distilling strains. J. Inst. Brew. 2012, 118, 152–162. [Google Scholar] [CrossRef]
- Walker, G.M. Yeast vitality and stress responses: Novel investigative approaches. In Yeast Flocculation, Vitality and Viability. Proceedings of the 2nd International Brewers Symposium; Speers, A., Ed.; ASBC/MBAA Press: St Paul, MN, USA, 2012; pp. 85–95. ISBN 978-0-9787726-4-2. [Google Scholar]
- Walker, G.M.; Hughes, P.S. (Eds.) Distilled Spirits. In New Horizons: Energy, Environment & Enlightenment [Proceedings of the 3rd Worldwide Conference on Distilled Spirits, Edinburgh]; Nottingham University Press: Nottingham, UK, 2010; ISBN 978-1-907284-45-8. [Google Scholar]
- Russell, I.; Stewart, G.G. Whisky Technology, Production and Marketing, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; ISBN 978-0-12-401735-1. [Google Scholar]
- Plutowska, B.; Biernacka, P.; Wardencki, W. Identification of Volatile Compounds in Raw Spirits of Different Organoleptic Quality. J. Inst. Brew. 2010, 116, 433–439. [Google Scholar] [CrossRef]
- Biernacka, P.; Wardencki, W. Volatile composition of raw spirits of different botanical origin. J. Inst. Brew. 2012, 118, 393–400. [Google Scholar] [CrossRef]
- Walker, G.M.; Walker, R.S.K. Enhancing yeast alcoholic fermentations. Adv. Appl. Microbiol. 2018, 105, 87–129. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.D.S.; de Souza, S.; Carneiro, L.M.; Silva, J.P.A.; de Souza, J.V.B.; Batista, J.D.S. Current Ethanol Production Requirements for the Yeast Saccharomyces cerevisiae. Int. J. Microbiol. 2022, 2022, 1–14. [Google Scholar] [CrossRef]
- Ingledew, W.M. Very high gravity (VHG) and associated new technologies for fuel alcohol production. In The Alcohol Textbook, 6th ed.; Walker, G.M., Abbas, C., Ingledew, W.M., Pilgrim, C., Eds.; Ethanol Technology Institute: Duluth, GA, USA, 2017; p. 363e376. ISBN 978-0-692-93088-5. [Google Scholar]
- Argyros, A.D.; Stonehouse, E.A. Yeast strain development for alcohol production. In The Alcohol Textbook, 6th ed.; Walker, G.M., Abbas, C., Ingledew, W.M., Pilgrim, C., Eds.; Ethanol Technology Institute: Duluth, GA, USA, 2017; Chapter 19; pp. 287–297. [Google Scholar]
- Gorgens, J.F.; Bressler, D.C.; van Rensburg, E. Engineering Saccharomyces cerevisiae for direct conversion of raw, uncooked or granular starch to ethanol. Crit. Rev. Biotechnol. 2015, 35, 369–391. [Google Scholar] [CrossRef]
- Nissen, T.L.; Hamann, C.W.; Kielland-Brandt, M.C.; Nielsen, J.; Villadsen, J. Anaerobic and aerobic batch cultivations of Saccharomyces cerevisiae mutants impaired in glycerol synthesis. Yeast 2000, 16, 463–474. [Google Scholar] [CrossRef]
- van Aalst, A.C.; de Valk, S.C.; van Gulik, W.M.; Jansen, M.L.; Pronk, J.T.; Mans, R. Pathway engineering strategies for improved product yield in yeast-based industrial ethanol production. Synth. Syst. Biotechnol. 2022, 7, 554–566. [Google Scholar] [CrossRef]
- Stovicek, V.; Holkenbrink, C.; Borodina, I. CRISPR/Cas system for yeast genome engineering: Advances and applications. FEMS Yeast Res. 2017, 17, fox030. [Google Scholar] [CrossRef]
- Swinnen, S.; Schaerlaekens, K.; Pais, T.; Claesen, J.; Hubmann, G.; Yang, Y.; Demeke, M.; Foulquié-Moreno, M.R.; Goovaerts, A.; Souvereyns, K.; et al. Identification of novel causative genes determining the complex trait of high ethanol tolerance in yeast using pooled-segregant whole-genome sequence analysis. Genome Res. 2012, 22, 975–984. [Google Scholar] [CrossRef]
- Deparis, Q.; Claes, A.; Foulquié-Moreno, M.R.; Thevelein, J.M. Engineering tolerance to industrially relevant stress factors in yeast cell factories. FEMS Yeast Res. 2017, 17. [Google Scholar] [CrossRef]
- Walker, R.S.K.; Pretorius, I.S. Synthetic biology for the engineering of complex wine yeast communities. Nat. Food 2022, 3, 249–254. [Google Scholar] [CrossRef]
- Dymond, J.S.; Richardson, S.M.; Coombes, C.E.; Babatz, T.; Muller, H.; Annaluru, N.; Blake, W.J.; Schwerzmann, J.W.; Dai, J.; Lindstrom, D.L.; et al. Synthetic chromosome arms function in yeast and generate phenotypic diversity by design. Nature 2011, 477, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wang, L.; Wang, Y.; Zhang, W.; Guo, Y.; Shen, Y.; Jiang, L.; Wu, Q.; Zhang, C.; Cai, Y.; et al. Identifying and characterizing SCRaMbLEd synthetic yeast using ReSCuES. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Wu, Y.; Li, B.-Z.; Mitchell, L.A.; Liu, H.; Pan, S.; Wang, J.; Zhang, H.-R.; Jia, N.; Li, B.; et al. Precise control of SCRaMbLE in synthetic haploid and diploid yeast. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Kutyna, D.R.; Onetto, C.A.; Williams, T.C.; Goold, H.D.; Paulsen, I.T.; Pretorius, I.S.; Johnson, D.L.; Borneman, A.R. Construction of a synthetic Saccharomyces cerevisiae pan-genome neo-chromosome. Nat. Commun. 2022, 13, 1–9. [Google Scholar] [CrossRef]
- Williams, T.C.; Kroukamp, H.; Xu, X.; Wightman, E.L.I.; Llorente, B.; Borneman, A.R.; Carpenter, A.C.; Van Wyk, N.; Espinosa, M.I.; Daniel, E.L.; et al. Laboratory evolution and polyploid SCRaMbLE reveal genomic plasticity to synthetic chromosome defects and rearrangements. bioRxiv 2022. [Google Scholar] [CrossRef]
- Kuijpers, N.G.; Solis-Escalante, D.; Luttik, M.A.; Bisschops, M.M.; Boonekamp, F.J.; van den Broek, M.; Pronk, J.T.; Daran, J.M.; Daran-Lapujade, P. Pathway swapping: Toward modular engineering of essential cellular processes. Proc. Natl. Acad. Sci. USA 2016, 113, 15060e15065. [Google Scholar] [CrossRef]
- Grunert, K.G.; Bredahl, L.; Scholderer, J. Four questions on European consumers’ attitudes toward the use of genetic modification in food production. Innov. Food Sci. Emerg. Technol. 2003, 4, 435–445. [Google Scholar] [CrossRef]
- Frewer, J.; van der Lans, L.A.; Fischer, A.R.H.; Reinders, M.L.; Menozzi, D.; Zhang, X.; van den Berg, L.; Zimmerman, K.L. Public perception of agri-food applications of genetic modification—A systematic review and meta-analysis. Trends Food Sci. Technol. 2013, 30, 142–152. [Google Scholar] [CrossRef]
- Kim, S.R.; Kim, S.-K.; Seo, S.-O.; Park, S.; Shin, J.; Kim, J.-S.; Park, B.-R.; Jin, Y.-S.; Chang, P.-S.; Park, Y.-C. Yeast metabolic engineering for carbon dioxide fixation and its application. Bioresour. Technol. 2021, 346, 126349. [Google Scholar] [CrossRef]
- Van Aalst, A.C.A.; Mans, R.; Pronk, J.T. An engineered non-oxidative glycolytic bypass based on Calvin-cycleenzymes enables anaerobic co-fermentation of glucose and sorbitol by Saccharomyces cerevisiae. Biotechnol. Biofuels Bioprod. 2022, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Malubhoy, Z.; Bahia, F.M.; de Valk, S.C.; de Hulster, E.; Rendulić, T.; Ortiz, J.P.R.; Xiberras, J.; Klein, M.; Mans, R.; Nevoigt, E. Carbon dioxide fixation via production of succinic acid from glycerol in engineered Saccharomyces cerevisiae. Microb. Cell Factories 2022, 21, 102. [Google Scholar] [CrossRef] [PubMed]
- Guadalupe-Medina, V.; Wisselink, H.W.; Luttik, M.A.; de Hulster, E.; Daran, J.M.; Pronk, J.T.; van Maris, A.J. Carbon dioxide fixation by Calvin cycle enzymes improves ethanol yield in yeast. Biotechnol. Biofuels 2013, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).