Rare Variants Residing in Novel Cis-Acting Element in Visual System Homeobox 1 and Their Contribution in the Pathogenesis of Keratoconus
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and DNA Isolation
2.2. VSX1 Sequencing
2.3. In Silico Analysis
3. Results
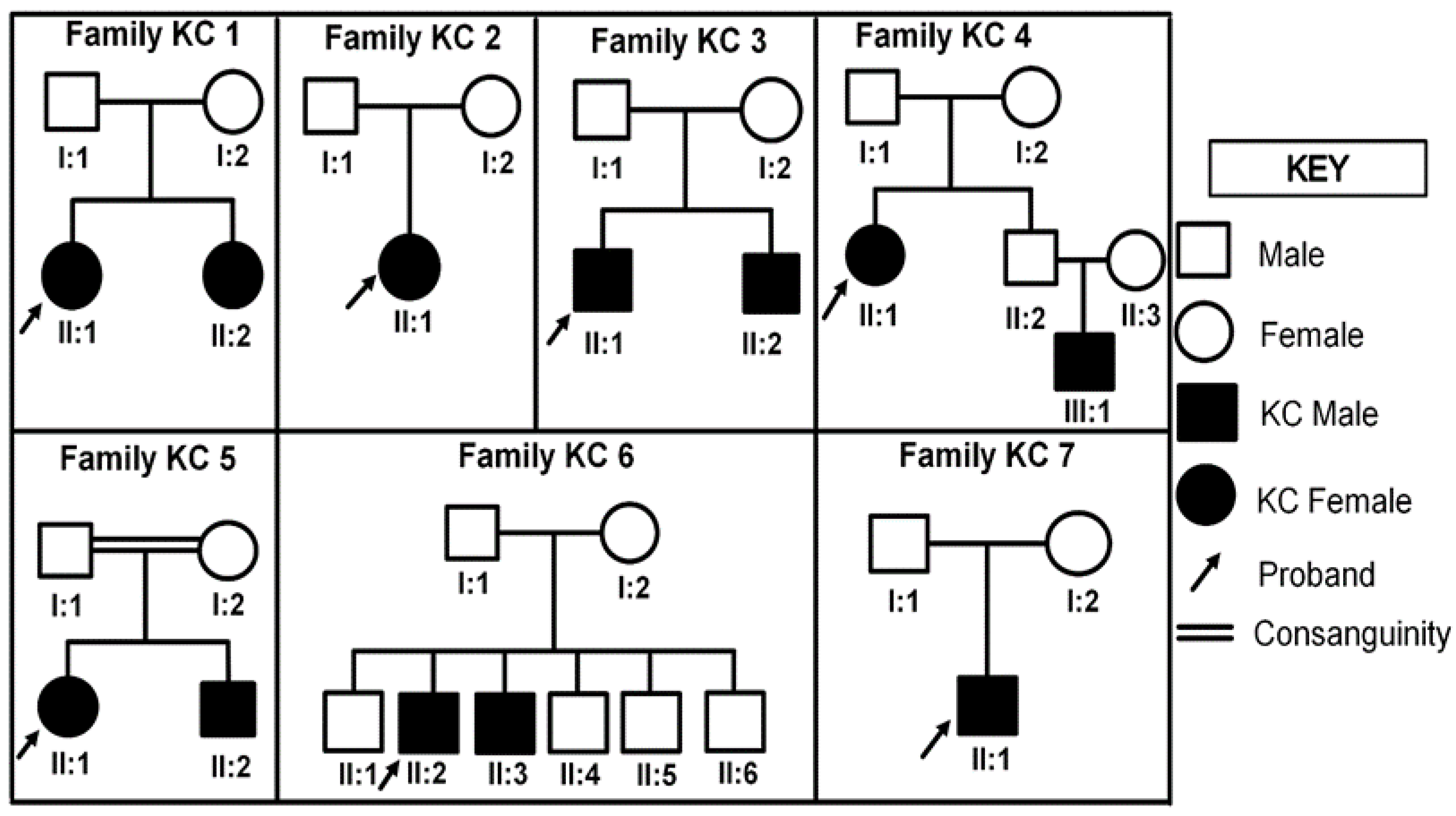
3.1. Pedigree Analysis
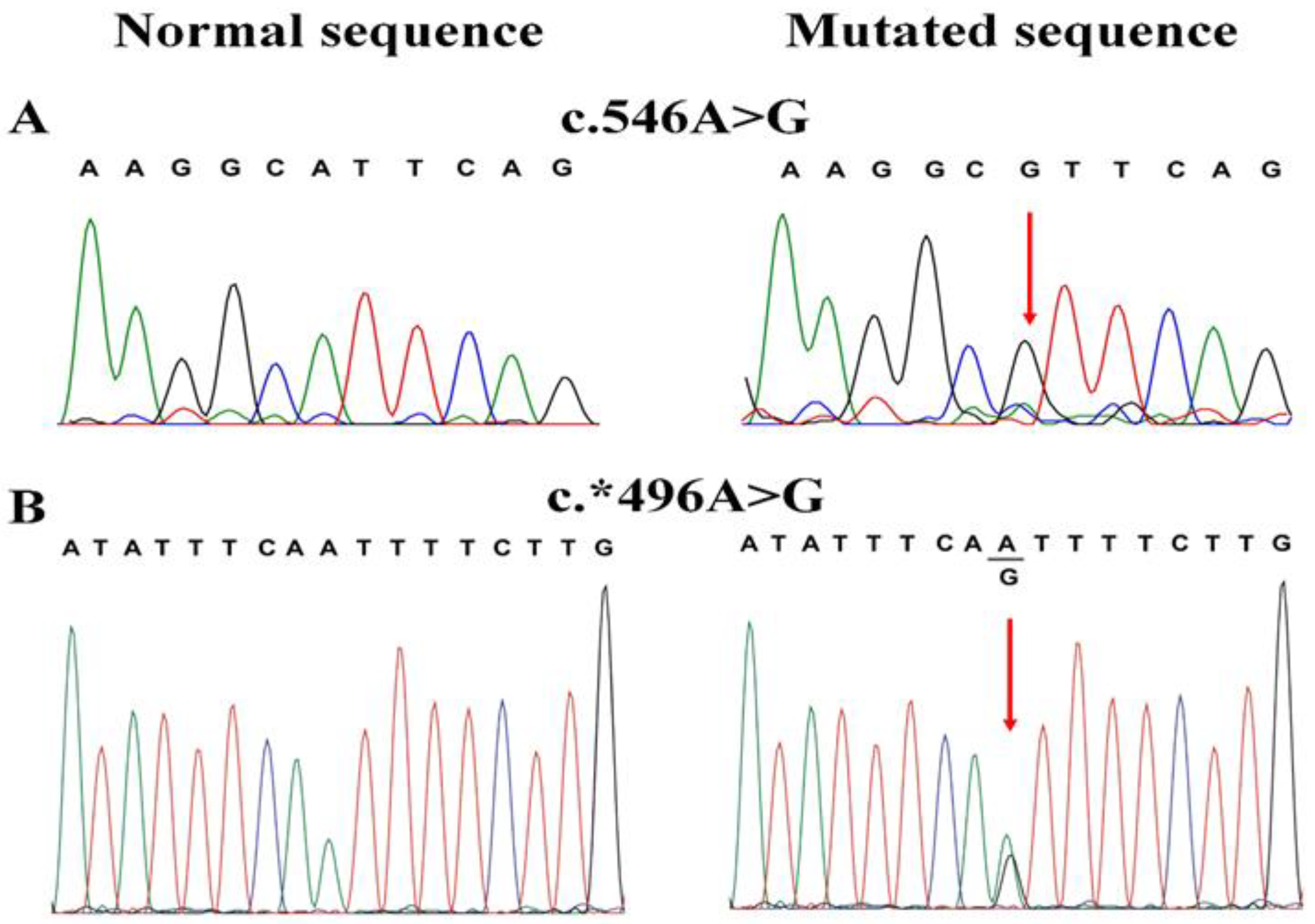
3.2. Screening of VSX1
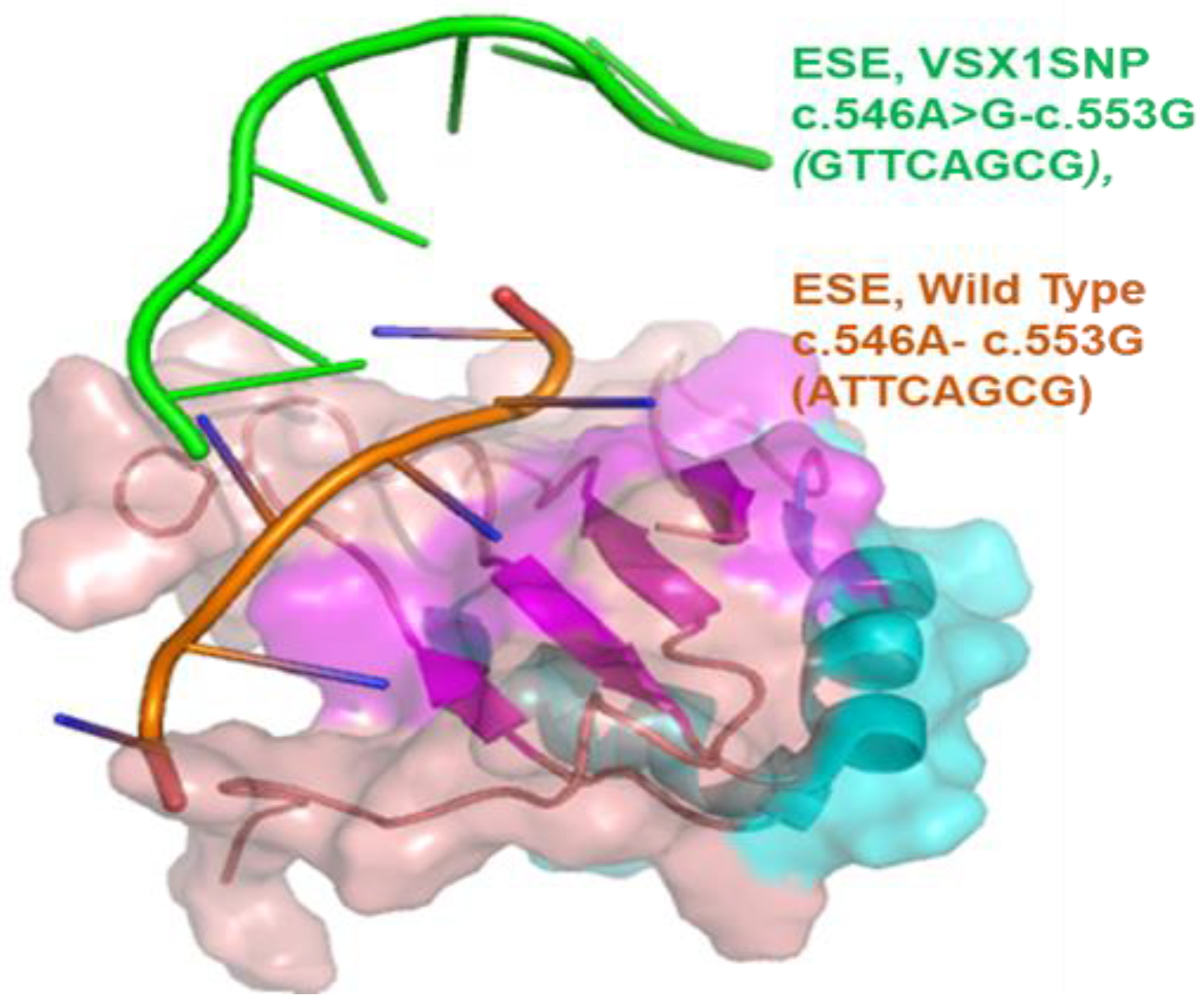
3.3. In Silico Analysis of c.546A>G
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rabinowitz, Y.S. Keratoconus. Surv. Ophthalmol. 1998, 42, 297–319. [Google Scholar] [CrossRef] [PubMed]
- Atilano, S.R.; Coskun, P.; Chwa, M.; Jordan, N.; Reddy, V.; Le, K.; Wallace, D.C.; Kenney, M.C. Accumulation of Mitochondrial DNA Damage in Keratoconus Corneas. Investig. Opthalmology Vis. Sci. 2005, 46, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Avetisov, S.; Mamikonian, V.; Novikov, I. The role of tear pH values and Cu-cofactor of lysyl oxidase activity in the pathogenesis of keratoconus. Vestn. Oftalmol. 2011, 2, 3–8. [Google Scholar]
- Romero-Jiménez, M.; Santodomingo-Rubido, J.; Wolffsohn, J.S. Keratoconus: A review. Contact Lens Anterior Eye 2010, 33, 157–166. [Google Scholar] [CrossRef]
- Li, X.; Yang, H.; Rabinowitz, Y.S. Longitudinal study of keratoconus progression. Exp. Eye Res. 2007, 85, 502–507. [Google Scholar] [CrossRef]
- Mohd-Ali, B.; Liew, L.Y.; Tai, H.J.; Wong, Y.Y. Tears evaluation of one sample of keratoconus patients in Kuala Lumpur. Med. J. Malays. 2011, 66, 53. [Google Scholar]
- Hashemi, H.; Beiranvand, A.; Khabazkhoob, M.; Asgari, S.; Emamian, M.H.; Shariati, M.; Fotouhi, A. Prevalence of Keratoconus in a Population-based Study in Shahroud. Cornea 2013, 32, 1441–1445. [Google Scholar] [CrossRef]
- Kennedy, R.H.; Bourne, W.M.; Dyer, J.A. A 48-Year Clinical and Epidemiologic Study of Keratoconus. Am. J. Ophthalmol. 1986, 101, 267–273. [Google Scholar] [CrossRef]
- Netto EA, T.; Al-Otaibi, W.M.; Hafezi, N.L.; Kling, S.; Al-Farhan, H.M.; Randleman, J.B.; Hafezi, F. Prevalence of keratoconus in paediatric patients in Riyadh, Saudi Arabia. Br. J. Ophthalmol. 2018, 102, 1436. [Google Scholar] [CrossRef]
- Krachmer, J.H.; Feder, R.S.; Belin, M.W. Keratoconus and related noninflammatory corneal thinning disorders. Surv. Ophthalmol. 1984, 28, 293–322. [Google Scholar] [CrossRef]
- Lim, N.; Vogt, U. Characteristics and functional outcomes of 130 patients with keratoconus attending a specialist contact lens clinic. Eye 2002, 16, 54–59. [Google Scholar] [CrossRef]
- Owens, H.; Gamble, G. A Profile of Keratoconus in New Zealand. Cornea 2003, 22, 122–125. [Google Scholar] [CrossRef]
- Tyynismaa, H.; Sistonen, P.; Tuupanen, S.; Tervo, T.; Dammert, A.; Latvala, T.; Alitalo, T. A locus for autosomal dominant keratoconus: Linkage to 16q22.3-q23.1 in Finnish families. Investig. Opthalmology Vis. Sci. 2002, 43, 3160–3164. [Google Scholar]
- Wang, Y.; Rabinowitz, Y.S.; Rotter, J.I.; Yang, H. Genetic epidemiological study of keratoconus: Evidence for major gene determination. Am. J. Med. Genet. 2000, 93, 403–409. [Google Scholar] [CrossRef]
- Tuft, S.J.; Hassan, H.; George, S.; Frazer, D.G.; Willoughby, C.; Liskova, P. Keratoconus in 18 pairs of twins. Acta Ophthalmol. 2012, 90, e482–e486. [Google Scholar] [CrossRef]
- Nowak, D.M.; Gajecka, M. The genetics of keratoconus. Middle East Afr. J. Ophthalmol. 2011, 18, 2. [Google Scholar]
- Chow, R.L.; Snow, B.; Novak, J.; Looser, J.; Freund, C.; Vidgen, D.; Ploder, L.; McInnes, R.R. Vsx1, a rapidly evolving paired -like homeobox gene expressed in cone bipolar cells. Mech. Dev. 2001, 109, 315–322. [Google Scholar] [CrossRef]
- Hayashi, T.; Huang, J.; Deeb, S.S. RINX(VSX1), a Novel Homeobox Gene Expressed in the Inner Nuclear Layer of the Adult Retina. Genomics 2000, 67, 128–139. [Google Scholar] [CrossRef]
- Semina, E.; Mintz-Hittner, H.; Murray, J. Isolation and Characterization of a Novel Human paired-like Homeodomain-Containing Transcription Factor Gene, VSX1, Expressed in Ocular Tissues. Genomics 2000, 63, 289–293. [Google Scholar] [CrossRef]
- Arbab, M.; Tahir, S.; Niazi, M.K.; Ishaq, M.; Hussain, A.; Siddique, P.M.; Saeed, S.; Khan, W.A.; Qamar, R.; Butt, A.M.; et al. TNF-α Genetic Predisposition and Higher Expression of Inflammatory Pathway Components in Keratoconus. Investig. Opthalmology Vis. Sci. 2017, 58, 3481–3487. [Google Scholar] [CrossRef]
- Desmet, F.-O.; Hamroun, D.; Lalande, M.; Collod-Béroud, G.; Claustres, M.; Béroud, C. Human Splicing Finder: An online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009, 37, e67. [Google Scholar] [CrossRef] [PubMed]
- Cartegni, L.; Wang, J.; Zhu, Z.; Zhang, M.Q.; Krainer, A.R. ESEfinder: A web resource to identify exonic splicing enhancers. Nucleic Acids Res. 2003, 31, 3568–3571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, W.-H.; Krainer, A.R.; Zhang, M.Q. RNA landscape of evolution for optimal exon and intron discrimination. Proc. Natl. Acad. Sci. USA 2008, 105, 5797–5802. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, W.; Yeo, E.; Yeh, R.; Goldstein, P.; Mawson, M.; Sharp, P.A.; Burge, C.B. RESCUE-ESE identifies candidate exonic splicing enhancers in vertebrate exons. Nucleic Acids Res. 2004, 32, W187–W190. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, M.; Iovino, N.; Unnerstall, U.; Gaul, U.; Segal, E. The role of site accessibility in microRNA target recognition. Nat. Genet. 2007, 39, 1278–1284. [Google Scholar] [CrossRef]
- Enright, A.J.; John, B.; Gaul, U.; Tuschl, T.; Sander, C.; Marks, D.S. MicroRNA targets in Drosophila. Genome Biol. 2003, 5, R1. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Chien, C.-H.; Jen, K.-H. RegRNA: An integrated web server for identifying regulatory RNA motifs and elements. Nucleic Acids Res. 2006, 34, W429–W434. [Google Scholar] [CrossRef]
- Aldave, A.J.; Yellore, V.S.; Salem, A.K.; Yoo, G.L.; Rayner, S.A.; Yang, H.; Tang, G.Y.; Piconell, Y.; Rabinowitz, Y.S. NoVSX1Gene Mutations Associated with Keratoconus. Investig. Opthalmology Vis. Sci. 2006, 47, 2820–2822. [Google Scholar] [CrossRef]
- Liskova, P.; Ebenezer, N.D.; Hysi, P.G.; Gwilliam, R.; El-Ashry, M.F.; Moodaley, L.C.; Hau, S.; Twa, M.; Tuft, S.J.; Bhatacharya, S.S. Molecular analysis of the VSX1 gene in familial keratoconus. Mol. Vis. 2007, 13, 1887–1891. [Google Scholar]
- Tang, Y.G.; Picornell, Y.; Su, X.; Li, X.; Yang, H.; Rabinowitz, Y.S. Three VSX1 Gene Mutations, L159M, R166W, and H244R, Are Not Associated with Keratoconus. Cornea 2008, 27, 189–192. [Google Scholar] [CrossRef]
- Štabuc-Šilih, M.; Stražišar, M.; Hawlina, M.; Glavac, D. Absence of pathogenic mutations in VSX1 and SOD1 genes in patients with keratoconus. Cornea 2010, 29, 172–176. [Google Scholar] [CrossRef]
- Verma, A.; Das, M.; Srinivasan, M.; Prajna, N.V.; Sundaresan, P. Investigation of VSX1 sequence variants in South Indian patients with sporadic cases of keratoconus. BMC Res. Notes 2013, 6, 103–105. [Google Scholar] [CrossRef]
- Tanwar, M.; Kumar, M.; Nayak, B.; Pathak, D.; Sharma, N.; Titiyal, J.S.; Dada, R. VSX1 gene analysis in keratoconus. Mol. Vis. 2010, 16, 2395–2401. [Google Scholar]
- Héon, E.; Greenberg, A.; Kopp, K.K.; Rootman, D.; Vincent, A.L.; Billingsley, G.; Priston, M.; Dorval, K.M.; Chow, R.L.; McInnes, R.R.; et al. VSX1: A gene for posterior polymorphous dystrophy and keratoconus. Hum. Mol. Genet. 2002, 11, 1029–1036. [Google Scholar] [CrossRef]
- Cartegni, L.; Chew, S.L.; Krainer, A. Listening to silence and understanding nonsense: Exonic mutations that affect splicing. Nat. Rev. Genet. 2002, 3, 285–298. [Google Scholar] [CrossRef]
- Blencowe, B.J. Exonic splicing enhancers: Mechanism of action, diversity and role in human genetic diseases. Trends Biochem. Sci. 2000, 25, 106–110. [Google Scholar] [CrossRef]
- Cáceres, J.F.; Kornblihtt, A.R. Alternative splicing: Multiple control mechanisms and involvement in human disease. Trends Genet. 2002, 18, 186–193. [Google Scholar] [CrossRef]
- Furuta, M.; Kozaki, K.-I.; Tanaka, S.; Arii, S.; Imoto, I.; Inazawa, J. miR-124 and miR-203 are epigenetically silenced tumor-suppressive microRNAs in hepatocellular carcinoma. Carcinogenesis 2010, 31, 766–776. [Google Scholar] [CrossRef]
- Saini, S.; Majid, S.; Yamamura, S.; Tabatabai, L.; Suh, S.O.; Shahryari, V.; Chen, Y.; Deng, G.; Tanaka, Y.; Dahiya, R. Regulatory Role of mir-203 in Prostate Cancer Progression and MetastasismiR-203 in Metastatic Prostate Cancer. Clin. Cancer Res. 2011, 17, 5287–5298. [Google Scholar] [CrossRef]
- Viticchiè, G.; Lena, A.M.; Latina, A.; Formosa, A.; Gregersen, L.H.; Lund, A.H.; Bernardini, S.; Mauriello, A.; Miano, R.; Spagnoli, L.G.; et al. MiR-203 controls proliferation, migration and invasive potential of prostate cancer cell lines. Cell Cycle 2011, 10, 1121–1131. [Google Scholar] [CrossRef]
- Meng, Q.-L.; Liu, F.; Yang, X.-Y.; Liu, X.-M.; Zhang, X.; Zhang, C.; Zhang, Z.-D. Identification of latent tuberculosis infection-related microRNAs in human U937 macrophages expressing Mycobacterium tuberculosis Hsp16.3. BMC Microbiol. 2014, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, P.; Tandon, R.; Dube, D.; Kaur, P.; Sharma, A. Familial segregation of a VSX1 mutation adds a new dimension to its role in the causation of keratoconus. Mol. Vis. 2011, 17, 481–485. [Google Scholar] [PubMed]
- Dong, Q.; Gu, Y.; Groome, L.J.; Wang, Y. OS073. Over-expression of MIRNA-203 results in increased inflammatory response in endothelial cells: A mechanism of increased endothelial inflammatory response in preeclampsia. Pregnancy Hypertens. 2012, 2, 217. [Google Scholar] [CrossRef] [PubMed]
- Reyhan, A.; Karadağ, A.; Çınar, Ş.Ş. Assessing the role of systemic inflammation in the etiopathogenesis of advanced stage keratoconus. Indian J. Ophthalmol. 2021, 69, 2658. [Google Scholar] [CrossRef]
- Nichani, P.A.H.; Solomon, B.; Trinh, T.; Mimouni, M.; Rootman, D.; Singal, N.; Chan, C.C. Investigating the role of inflammation in keratoconus: A retrospective analysis of 551 eyes. Eur. J. Ophthalmol. 2022, 33, 35–43. [Google Scholar] [CrossRef]
| Exons | Primer Sequence | Annealing (°C) | Product Size (bp) | MgCl2 (mM) | Primer (µM) |
|---|---|---|---|---|---|
| Exon 1_1 | F: 5′-TTTCGAGGGACAGGCAGAC-3′ R: 5′-AGGTCCGTGATGGCGAAG-3′ | 60 | 449 | 2 | 0.4 |
| Exon 1_2 | F: 5′-TGCTTGCTAAGGAACCATGAC-3′ R: 5′-TCAGAGCCTAGGGGACAGG-3′ | 61 | 489 | 2.5 | 0.32 |
| Exon 2 | F: 5′-AATGCTGGCTCATACTGTAAAC-3′ R: 5′-AACCAGGAAACCACTGGG-3′ | 58 | 327 | 2.5 | 0.4 |
| Exon 3 | F: 5′-AGCAGAGGAAGCAGGCAC-3′ R: 5′-CTATGCAAAGGGAGCGTG-3′ | 58 | 332 | 2.5 | 0.4 |
| Exon 4 | F: 5′-ATCATGCTCGGGAGAGAAG-3′ R: 5′-TTGCTTTGCTTTGGAAATG-3′ | 58 | 391 | 3 | 0.4 |
| Exon 5_1 | F: 5′-CCCCAGAGATAGGCACTGAC-3′ R: 5′-TGCCAGTGAGGAATATGCAC-3′ | 58 | 470 | 3 | 0.4 |
| Exon 5_2 | F: 5′-GCAGGAGACCAAGAAAGTGC-3′ R: 5′-CTCAAATGATGCCCAGCAG-3′ | 58 | 416 | 2.5 | 0.4 |
| Exon 5_3 | F: 5′-ATGCCACTTGCTTTAAGAGG-3′ R: 5′-TGCAGAAACGACTAGAGTATGG-3′ | 58 | 464 | 3 | 0.4 |
| Exon 5_4 | F: 5′-TACCTTGAACTTGGCCTTGG-3′ R: 5′-TGGCTGGGATCAGAGATAGTG-3′ | 58 | 391 | 2.5 | 0.4 |
| Predicted Signal | Prediction Algorithm | cDNA Position | Interpretation |
|---|---|---|---|
| ESE Site Broken | 1. ESE-Finder-SRp40 | 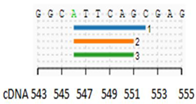 | Alteration of an exonic ESE site. Potential alteration of spllicing |
| 2. RESCUE ESE Hexamers | |||
| 3. EIEs from Zhang et al. [23] |
| Type | SRSF Binding Properties | |||
|---|---|---|---|---|
| Biding Motif | SRSF Protein | Binding Score | BS Threshold | |
| VSX1 Wild Type | c.546A-c.553G ATTCAGCG | SRSF5 | 3.07 | 2.67 |
| VSX1 SNP | c.546A>G-c.553G GTTCAGCG | SRSF2 | 3.20 | 2.38 |
| miRNA | Wild and Mutant Type Sequences | miRANDA | PITA | |||
|---|---|---|---|---|---|---|
| Binds | Binding Free Energy (kcal/mol) | Score | Binds | ΔΔG | ||
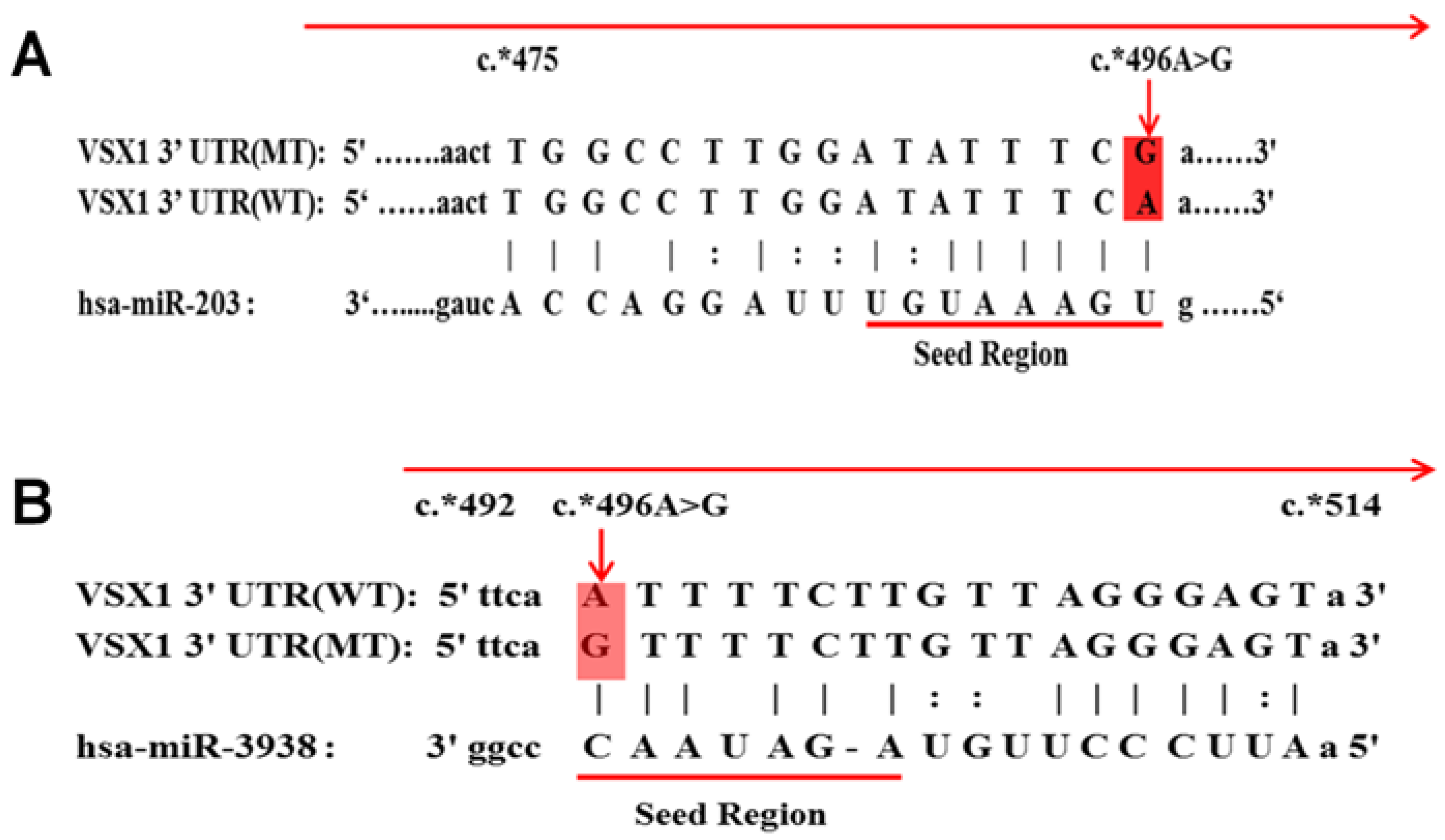
| miRNA-203 | VSX1 3′UTR(WT) c* 496 A | ✓ | −15.50 | 150 | ✓ | −9.35 |
| VSX1 3′UTR(MT) c* 496 A>G | X | 0 | 0 | X | 0 | |
| miRNA-3938 | VSX1 3′UTR(WT) c* 496 A | X | 0 | 0 | X | 0 |
| VSX1 3′UTR(MT) c* 496 A>G | ✓ | −12.00 | 140 | ✓ | −7.65 | |
| Ethnicity | Study Type and Cohort Size | Isolated/Familial Cohort | Technique | Identified Variant(s) | No.of Patient(s) | Variant Type | Reference |
|---|---|---|---|---|---|---|---|
| Caucasian | Association study. 100 unrelated KC Patients | Isolated cohort | Sanger Sequencing | p.Asp144Glu p.Ser6Ser p.Pro58Pro p.Arg131Ser p.Ala182Ala | 1 4 2 1 51 | Non-synonymous Synonymous Synonymous Non-synonymous Synonymous | [28] |
| Caucasian+Asian +African | 85 Probands | Familial | Sanger Sequencing | p.D144E c.504-10G>A 504-24C>T | 1 1 3 | Non-synonymous Intronic Intronic | [29] |
| Whites+ Hispanics+Others | Association study with 77 unrelated KC Patients and 75 families | Isolated cohort+familial cohort | ARMS-PCR and RFLP | p.H244R p.L159M | 3 5 | Non-synonymous Non-synonymous | [30] |
| Slovenian | 113 patients with sporadic and familial KC | Isolated cohort+familial cohort | Sanger Sequencing | p.S6S p.A128A p.D144E 504-24C.T 627+23G.A | 21 35 1 0 44 | Synonymous Synonymous Non-synonymous Intronic Intronic | [31] |
| Indian | 117 sporadic cases of keratoconus | Isolated cohort | Sanger Sequencing | p.A182A c.627+23G>A c.627+84T>A c.504-24C>T | 7 3 9 7 | Non-synonymous Intronic Intronic Intronic | [32] |
| Indian | 50 sporadic cases | Isolated cohort | Sanger Sequencing | p.R217H p.P237P | 1 3 | Non-synonymous Synonymous | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alswailmi, F.K.; Malik, R.K.; Parrey, M.U.R.; Siddiqi, A.R.; Karimulla, S.; Alanezi, A.A.; Qamar, R.; Azam, M.; Ahmad, A. Rare Variants Residing in Novel Cis-Acting Element in Visual System Homeobox 1 and Their Contribution in the Pathogenesis of Keratoconus. Appl. Sci. 2023, 13, 4888. https://doi.org/10.3390/app13084888
Alswailmi FK, Malik RK, Parrey MUR, Siddiqi AR, Karimulla S, Alanezi AA, Qamar R, Azam M, Ahmad A. Rare Variants Residing in Novel Cis-Acting Element in Visual System Homeobox 1 and Their Contribution in the Pathogenesis of Keratoconus. Applied Sciences. 2023; 13(8):4888. https://doi.org/10.3390/app13084888
Chicago/Turabian StyleAlswailmi, Farhan Khashim, Rida Khursheed Malik, Mujeeb Ur Rehman Parrey, Abdul Rauf Siddiqi, Shaik Karimulla, Abdulkareem A. Alanezi, Raheel Qamar, Maleeha Azam, and Ashfaq Ahmad. 2023. "Rare Variants Residing in Novel Cis-Acting Element in Visual System Homeobox 1 and Their Contribution in the Pathogenesis of Keratoconus" Applied Sciences 13, no. 8: 4888. https://doi.org/10.3390/app13084888
APA StyleAlswailmi, F. K., Malik, R. K., Parrey, M. U. R., Siddiqi, A. R., Karimulla, S., Alanezi, A. A., Qamar, R., Azam, M., & Ahmad, A. (2023). Rare Variants Residing in Novel Cis-Acting Element in Visual System Homeobox 1 and Their Contribution in the Pathogenesis of Keratoconus. Applied Sciences, 13(8), 4888. https://doi.org/10.3390/app13084888







