Abstract
Waste tires from traffic are a well-known environmental problem today. For this reason, the toxicity and potential biodegradation of crushed tires were tested in a respiration test with microorganisms. A non-specific soil microbial community was used. Two experimental designs and their effect on the results were compared—a test with the eluate from tires and a contact test, i.e., the solution containing tire particles during the test. The consumption of dissolved oxygen was measured in the assay over 28 days. The values obtained indicated zero biodegradation of all samples, but the toxicity of the eluates to microorganisms was different depending on whether the microorganisms were exposed only to the leachate or whether tire shred particles were still present in the leachate. In the presence of particles in solutions, the toxicity of the samples for microorganisms was higher. Additionally, the MTT (methyl tetrazolium test) viability assay was performed. The results indicated a 28% inhibition of the viability of microorganisms in samples with tire particles in comparison with eluate, where 9% inhibition was observed. The results confirmed that the contact assay (with the presence of particles) is a more natural and thorough method than the use of leachate.
1. Introduction
Waste tires (passenger and bus, freight transport) from transport have started to be used as a secondary raw material in recent years. They are used, for example, for the production of children’s playgrounds and in the clay of sports grounds [1], or as additional material for building materials [2,3,4,5,6]. Their usability is variable in concrete, soundproof walls and insulation. The greatest amount of re-use of these materials started in the last ten years.
Unfortunately, this is not a material that is flawless from an ecological point of view. Various volatile organic compounds such as polyaromatic hydrocarbons and their heterocyclic derivatives or amines may be released from these materials into the air. On the other hand, some other pollutants, including metals, are not volatile and are not able to (bio)degrade. Therefore, they remain in waste tires for the entire duration of their processing and use in new materials. Their presence in these materials thus continues to be an environmental burden. The organisms that occur in the soil and terrestrial environment are mainly microorganisms [7,8,9,10,11,12,13,14,15,16]. Metals can be a source of nutrients and toxic elements for microorganisms. The origin and fate during the life cycle of tires may be responsible for their different environmental burdens.
Their ecotoxicity was confirmed in several recent studies. A toxic effect was found for water crustacean, freshwater algae, duckweeds, and also soil earthworms and plants [17,18,19,20,21,22,23]. This is likely due to the effect of polycyclic aromatic hydrocarbons (PAHs) or zinc cations, which are most prevalent in waste tires. Most studies were therefore focused only on these polyaromatic hydrocarbons or zinc, as is described in detail in a review by Malik et al. [24]. However, tire rubber is always a mix of variable organic or inorganic compounds (not only PAHs or Zn) [3]. The toxicity of such a matrix (rubber) can be different from the toxicity of individual metals or groups of organic substances (PAHs). However, the problem for the environment consists not only of the presence of heavy metals and organic pollutants that can be leached out by water, but also in the rubber crumb itself. Tires are plastics of artificial origin and they are persistent organic materials. Logically, their chemical or biological degradation is difficult and they decompose very poorly in nature. Generally, it has been proven that plastic products and rubber can remain in nature for tens or hundreds of years [25]. Their chemical degradation is very slow and we do not know much about their fate within the environment. Only isolated experiments have been described for individual species of microorganisms, such as certain soil bacteria, and their applicability in the degradation of polyaromatic hydrocarbons, not rubber as such (e.g., [26,27,28,29,30]).
The question, therefore, remains whether there could be a lack of improvement in this area due to microorganisms that can decompose rubber and potentially releasable pollutants and use them as a source of nutrients. Unfortunately, such studies describing the ability of the naturally-occurring microbial community to degrade pollutants from waste rubber particles have not yet been carried out. The most important aspect is the survival of microorganisms after exposure to rubber particles. This aspect can be studied using certain methodologies, including a respiration test [31] that is suitable for microbial sludge from sewage treatment plants or for non-specific microbial communities of soils and other solid samples. The principle of this method is to compare the consumption of dissolved oxygen by microorganisms over time. If it decreases during the test, it means that the organisms are alive and profiting. If the oxygen value is the same or decreases in different time periods, it means that the organisms are dead or that anaerobic processes are taking place [31].
The viability of microorganisms exposed to waste tires has never been studied. Such additional methods are necessary for the proper evaluation of microorganismal fitness and their ability to fulfil the decomposition function [32,33,34,35,36]. This method is based on the metabolization of a certain substrate (e.g., (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide or (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium chloride) into the final product formazan. The formed formazan occurs in the sample in a form of blue crystals that can be extracted using certain organic solvents such as methanol, ethanol or acetone [37,38]. The intensity of the blue coloration then indicates the intensity of cell survival, as only living cells are able to produce formazan. The MTT assay has been used many times for verification of the viability of prokaryotic and eukaryotic cells in toxicological, ecotoxicological and microbiological studies (e.g., [39,40,41,42,43,44,45]).
A separate type of ecotoxicological study is the design of toxicological biotests. For solid samples, the leachate is always tested without the presence of the original sample of the tested material with microorganisms (e.g., [46,47,48,49,50]). However, this unrealistic experimental design may affect the toxicity level, which deviates from the natural conditions.
For this reason, the toxicity and potential biodegradation of crushed tires to a non-specific soil microbial community were evaluated via respiration and MTT viability assays in the present study. Two designs were compared: (1) pure leachates without the presence of crumb particles and (2) suspensions containing particles during the experiment. Microbial inoculum was deployed into the eluates and the dissolved oxygen and microorganismal viability of the aquatic samples were measured.
2. Materials and Methods
A mix of waste tires from personal transports was used as the tested material. The rubber parts of the tires were crushed to less than 10 mm particles mechanically using a knife mill and kept in a closed plastic sample container under laboratory conditions prior to testing (temperature (20 ± 2) °C, normal pressure, in the dark, 2 months). Microbial inoculum was cultured from agricultural land referred to as loamy sand LUFA 2.2 soil (Speyer Ltd., Speyer, Germany).
A mass of 100 g of rubber particles was used to prepare 1 L of leachates. Distilled water from the workplace of the authors was used for the preparation of the leachates. The distilled water was aerated 24 h before use to prepare the extract. The prepared mixtures were stirred for 24 h on a head-to-heel shaker Reax 20/4. After 24 h, the extracts were filtered through a paper filter (Whatman, Dassel, Germany, grade 6).
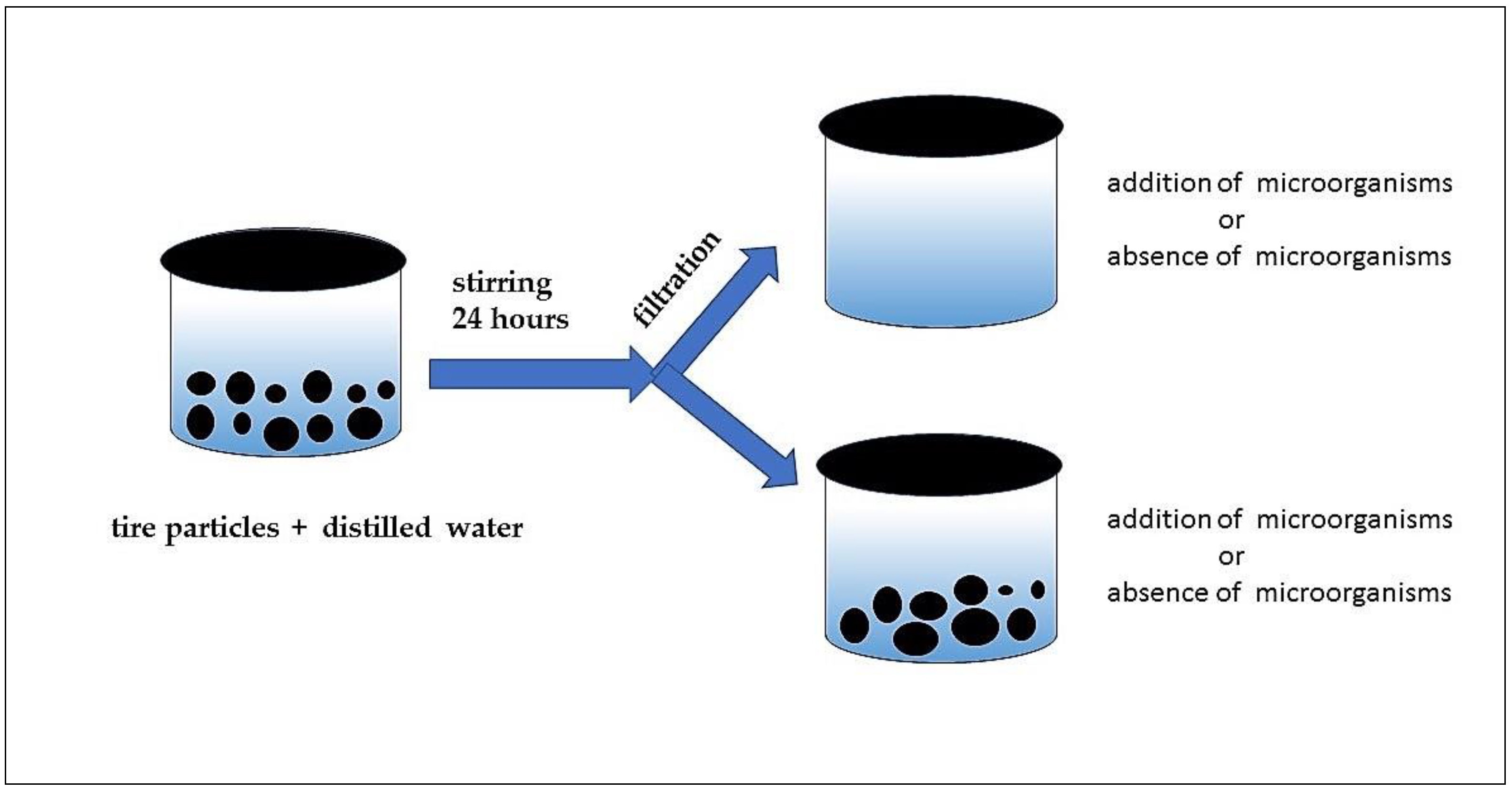
A 100 mL volume of the eluate was spilled into a test glass vessel and 1 mL of unspecific microbial soil community as an aquatic solution was added. Nutrients for microorganisms (1 mL of 1% glucose solution in water) were also pipetted into the tested solutions. The tested samples and controls (1. the eluate samples without microorganisms and nutrients and 2. distilled water with microorganisms and nutrients) were prepared. Three replicates were prepared for the eluates and controls. The experiment lasted 28 days. The dissolved oxygen was measured using an oximeter GOX 20 at the start of the test and on the 7th, 14th, 21st and 28th days of the experiment. The test vessels were covered with their lids and stored in a thermostat (20 ± 2 °C in the dark) (see Figure 1).
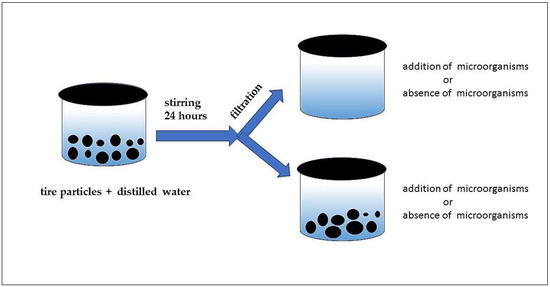
Figure 1.
Scheme of experimental designs (respiration test)—preparation of samples.
Continual leaching of the present particles was performed in a second type of experiment. A 10 g sample of rubber particles was used to prepare 0.1 L of test mixture for one test vessel. The test vessels were stirred for 24 h. After 24 h, the particles were left in the test vessels. A 1 mL volume of a nonspecific microbial soil community as an aquatic solution was added. Nutrients for the microorganisms (1 mL of 1% glucose solution in water) were also pipetted into the tested suspensions. The tested samples and controls (1. the eluate samples without microorganisms and nutrients and 2. distilled water with microorganisms and nutrients) were prepared. Three replicates were prepared for the eluates and controls (see Figure 1). The experiment lasted 28 days. The dissolved oxygen was measured using an oximeter GOX 20 at the start of the test and on the 7th, 14th, 21st and 28th days of the experiment. The tested vessels were covered with their lids and stored in a thermostat (20 ± 2 °C in the dark).
Viability MTT Assay
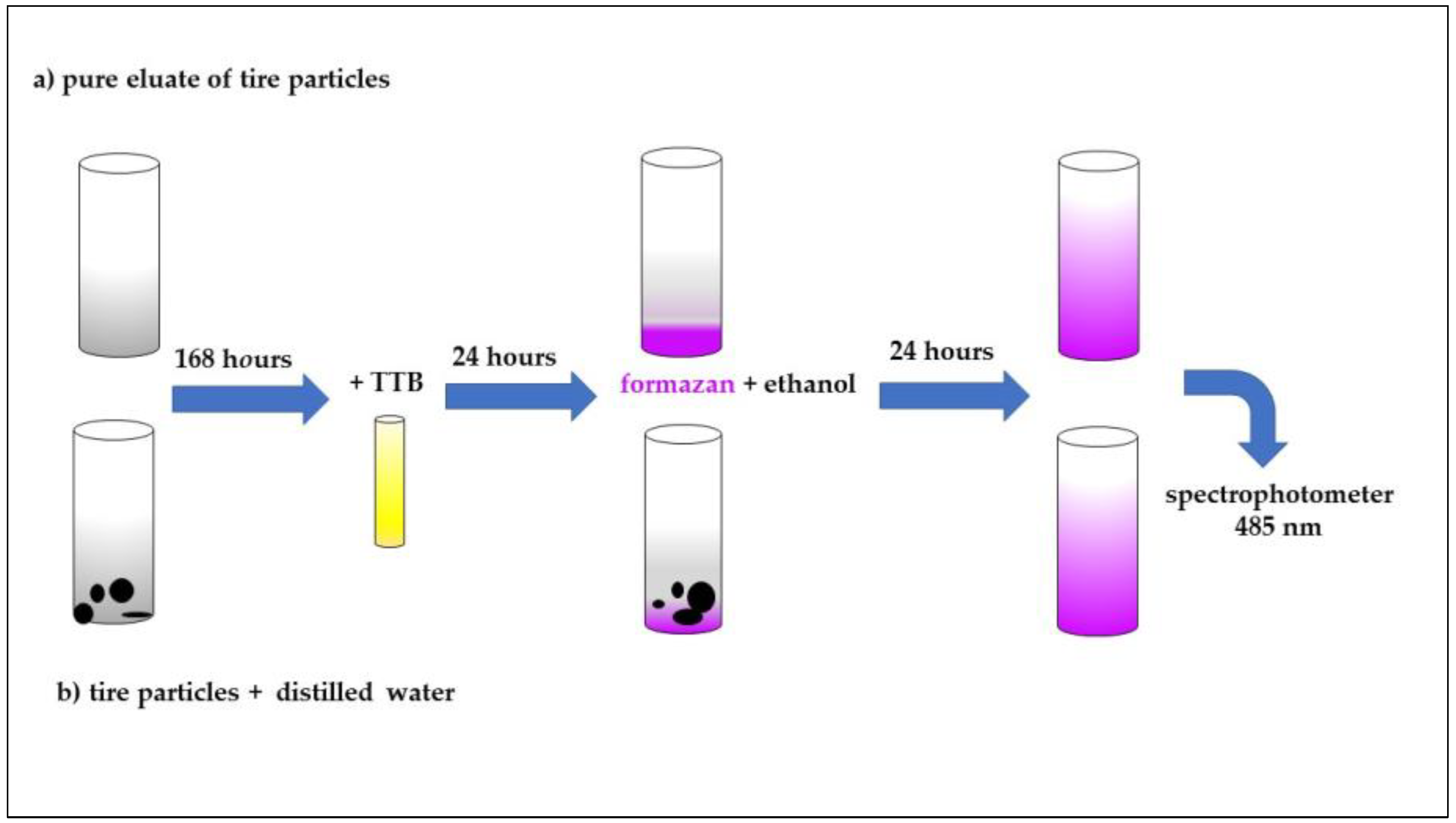
The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) viability assay was performed according to the tested procedures and was modified see Figure 2 for the purposes of this study [37]. A 0.5 mL volume of unspecified soil microorganisms (the same microorganisms used in the respiration test) + 0.15 mL of phosphate buffer + 0.25 mL of sample (eluate) or buffer (control) were mixed in one glass tube. For the suspension, 100 mg of waste tire particles was added to the mixture. Triplicates were performed for the control, eluate and suspension. The samples were incubated in the thermostat (20 ± 2 °C) in the dark for 168 h. 3-[4,5-dimethylthiazol-2-yl]-2,5-difenyltetrazolium bromide (TTB) was added as a substrate (0.1 mL/tube) and the samples were incubated for next hour in the dark. A 7 mL volume of ethanol was then added into each tube. The blue product formazan from the microorganisms was extracted for the next 24 h. The formazan is a product of microorganismal metabolism. Dead microorganisms are not able to produce it and its production is thus an indicator of microorganismal fitness and viability. The levels of formazan were indirectly measured spectrophotometrically at 485 nm. The recommended duration for measurement is 1 h for cultivation of microorganisms at 37 °C. In this experiment, the temperature that was set for the entire experiment, i.e., 25 °C, was used. The formazan was incubated on Tuesdays for 24 h, as it was necessary for sufficient mixing of all suspension components. Some organisms can also live on the surface of the particles, and it would not be possible to achieve the maximum effect and yield of formazan with rapid incubation for 1 h. The incubation times of the formazan with the sample (24 h) were chosen based on previous measurements and experience with this test and solid samples of some natural soils and various building materials (unpublished, own preliminary data).
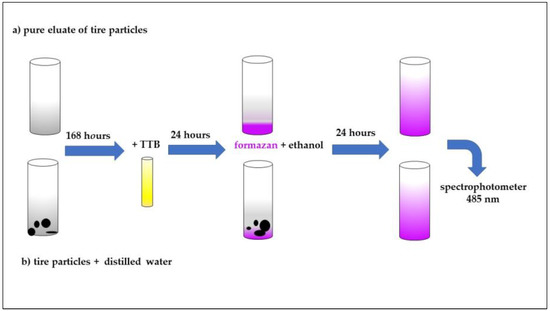
Figure 2.
Scheme of experimental designs (MTT viability assay)—preparation of samples.
The microbial data were evaluated using MS Excel. The oxygen loss (consumption by microorganisms or chemical degradation) during the time period (0 days versus 28 day) was calculated according to Formula (1):
where L—oxygen = oxygen loss in (%), T0 = oxygen level in time 0 and Ta = oxygen level in subsequent time.
The toxicity of the waste tire eluate was calculated according to Formula (2):
where T (%) = toxicity (in%s), mean C(time A) = mean L—oxygen value of control in time A, and mean S(time A) = mean value of the tire sample (the eluate or the suspension) in time A.
The inhibition of toxicity (in%s) was calculated according to Formula (3):
where R − T = rate of toxicity (%), T control (TA) = toxicity value of the control in the time A and T sample (TA) = toxicity value of the sample in the time A.
The oxygen values were evaluated using the one-way analysis of variance (ANOVA) with the GraphPad InStat software (InStat version 3, San Diego, CA, USA). The multiple-comparison Tukey–Kramer test was performed at a 0.05 significance level.
3. Results and Discussion
The authors referred to the levels of heavy metals in the tested tire eluates in one of the previous studies [20]. The concentration of metal leakage from rubber crumbs into water was determined using an inductively coupled plasma optical emission spectrometer Agilent 5110 SVDV device ICP-OES (Agilent technologies, Santa Clara, CA, USA), equipped with a SeaSpray glass concentric nebulizer. The eluate contained a barium (Ba) concentration of 0.021 mg/L and a zinc (Zn) concentration of 0.273 mg/L. The other commonly determined elements (Mn, B, Si, P, Cu, Pb) were under the detection limit [20]. On the other hand, the chemical analysis also confirmed the higher levels of pollutants in solutions more than 1–25 times when the tire particles were in the test solutions for 28 days [51]. The levels of boron, iron and silicon were also under the limit of quantification after 24 h. The barium level was 0.053 mg/L, the manganese level 0.031 mg/L and the zinc concentration 5.670 mg/L [51].
PAHs were detected in units of mg/kg. Only pyrene levels were around 30 mg/kg in the tire particles [20]. However, PAHs are generally volatile compounds and if we prepared the eluate and performed the microbial tests, these substances could enter the air above the leachate or suspension level with the microorganisms in the test vessels. Therefore, it can be assumed that during the test these substances did not have as large of an effect on the obtained toxicity results in the respiration test over 28 days. This means only the metals present could probably serve as a source of toxicity in addition to sugar as food, as they are not able to evaporate in the aquatic or soil environment.
On the other hand, PAHs together with metals may have affected the toxicity and cell survival in the viability assay. It has been proven many times that both inorganic and organic pollutants have an effect on the cellular level and cause damage to membranes, oxidative stress or damage to genetic information [52,53,54,55].
It should be emphasized that no similar study has been published to date. Therefore, it is not possible to compare our data with the values and conclusions of other studies on the same topic.
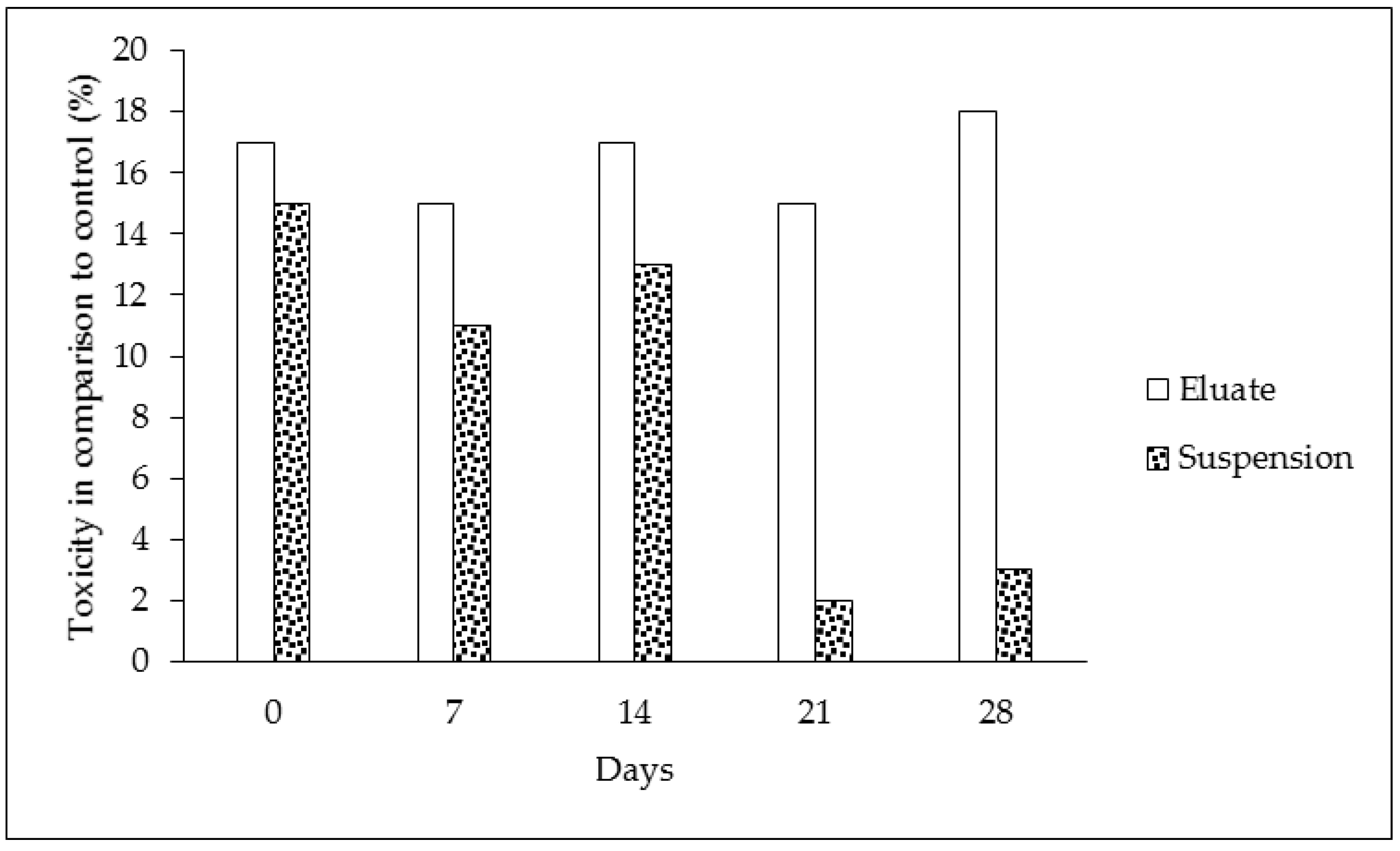
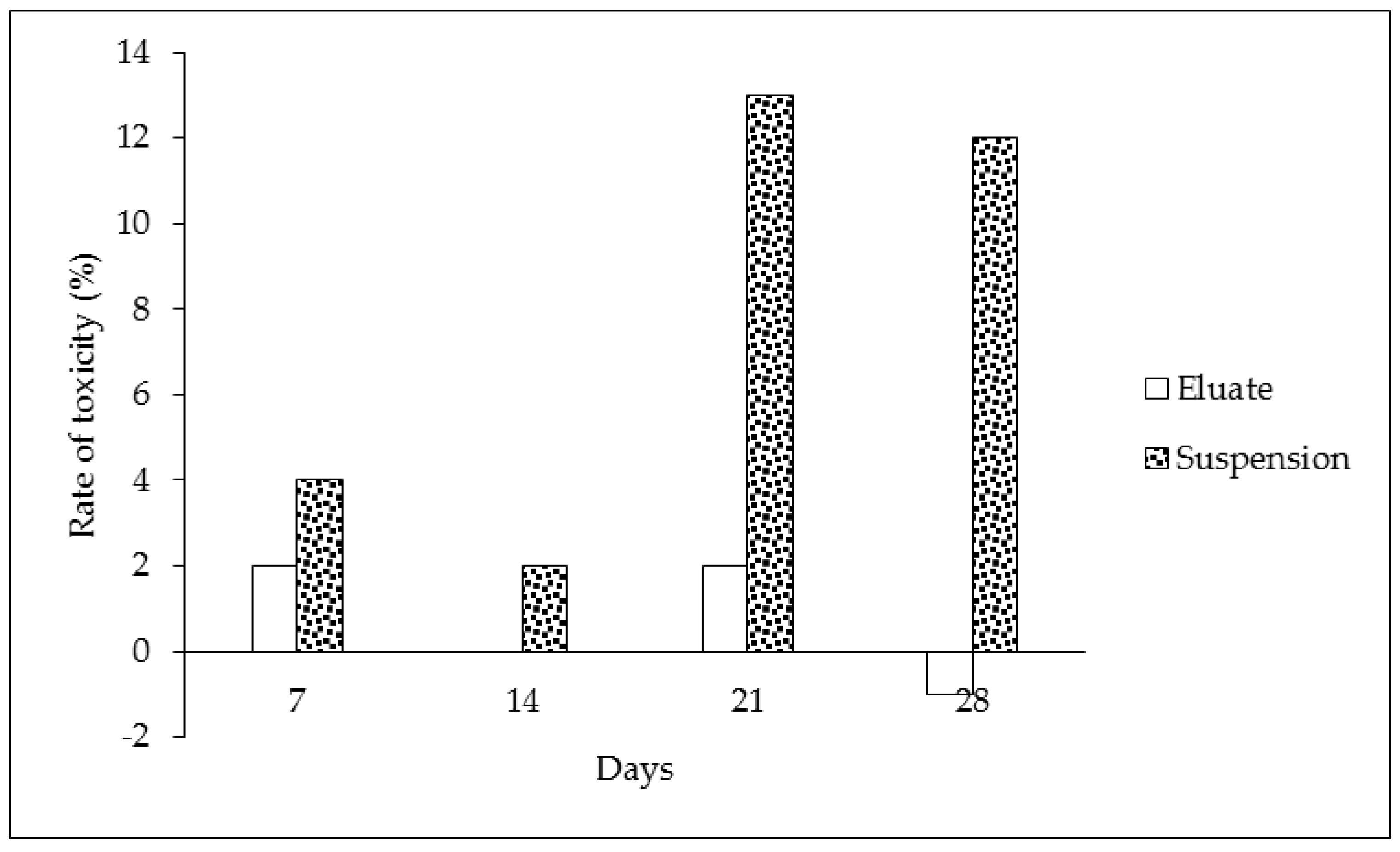
The results of the biodegradation and toxicological respiration test indicate that significant effects were found (see Table 1 and Figure 3). The oxygen loss in the tire eluates without microorganisms showed the same values during the duration of the experiment—these results are expressed in Table 1, part “D”. This indicates that oxygen was not chemically degraded, and the chemical composition of the sample was constant. On the other hand, samples of the eluate containing microorganisms showed decreasing levels of oxygen during the time period. This was caused by microorganisms and their metabolic processes (Table 1, part “B”). The same results were found for control samples with microorganisms but without the tested eluate (Table 1, part “A”). The consumption of oxygen between the control and the eluate sampled did not differ in the case of the eluates, contrary to the suspension of particles (cf. Table 1, parts “B and C”). The lower oxygen consumption indicates that microorganisms were less abundant in the presence of tire particles or that their metabolism was lower in comparison to the control and microorganisms in the clear eluates (cf. Table 1, parts “A, B and C”). The continual involvement of heavy metals and organic pollutants can lead to such conclusions. The toxicity of samples with the prolonged presence of tire particles was confirmed during the course of the experiment (Figure 3 and Figure 4). The biodegradation potential was not recorded because the oxygen values were similar or less for both tire samples in comparison to the control (Figure 4). The statistical analysis confirmed statistically significant differences in the oxygen values between only some of the individual times at a 0.05 significance level. The differences were found between the following pairs: control (Time 0) versus eluate (Time 0 and 28 days), control (Time 7 days) versus eluate (Time 0) at a 0.05 significance level (*) (see Table 2).

Table 1.
Measured values of O2 (mg/L) and their mean + standard deviations (SDs) of the controls and of the tested samples with microbial inoculum and without microbial inoculum.
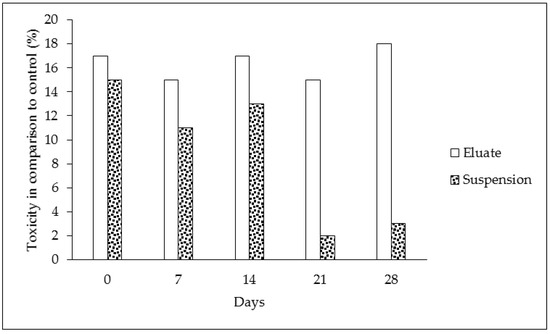
Figure 3.
Toxicity of the samples in comparison to the controls during 28 days of the experiment—day 0, 7, 14, 21 and 28 (the data were calculated according to Formula (2)).
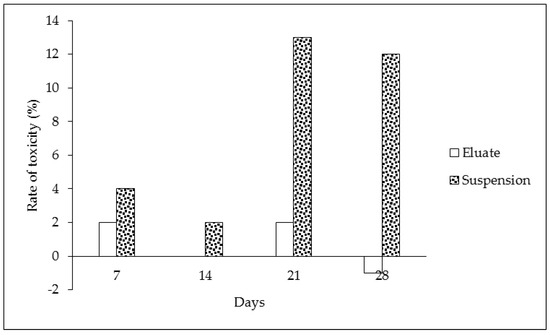
Figure 4.
Rate of toxicity (%) during 7, 14, 21 and 28 days of exposure (the data were calculated according to Formula (3)).

Table 2.
Results of the statistical analysis. One-way analysis of variance (ANOVA) with the GraphPad InStat software was used (InStat version 3, San Diego, CA, USA). The multiple-comparison Tukey–Kramer test was performed at a 0.05 significance level.
The results of the MTT assay confirmed the results of the test with dissolved oxygen. The viability of the microorganisms from suspensions (aquatic solution + own particles) was decreased about 28% in comparison to the control. On the other hand, the viability of the microorganisms in the pure eluates was decreased only about 9% in comparison to the control (see Table 3). The toxicity for microorganisms was thereby confirmed by the second independent assay. The viability test seems to be more sensitive than the respiration inhibition test because the observed toxicity of the eluates was higher in the viability test (compare Figure 2 and Table 3). We can note that the toxicity in the viability tests can be described as an acute toxic effect, at variance with the respiration test, where the chronic effect was observed after 28 days of exposure. It is also a destructive method and it is not possible to monitor the same microbial community during the different times. The respiration test is also a representative of the multi-generation microorganismal method and it is evident (Figure 2) that the microorganismal biomass was reduced over the time period, probably due to gradually released metals from the tire particles.

Table 3.
The absorbances of MTT viability assay after 7 days of exposure. Pure suspension = suspension without microorganism, pure eluate = eluate without microorganisms. The data are expressed as mean values of absorbances with their standard deviations (SDs) and as an inhibition in percentage.
The other question is the usability of own tire particles or elements involving them as a source of nutrients for microorganisms. Generally, in order for microorganisms to live, they require mainly basic nutrients such as carbon, oxygen, sulfur, phosphorus, nitrogen and many microelements. The tire samples tested in the present study contained all of the basic elements. The particles contained about 50% carbon, followed by sulfur (10%), oxygen (26%), zinc (3%), and silicon (2%) [51]. Special attention should be focused on zinc, which is one of the essential elements. Adversely, it is used today in nanoform as an antimicrobial agent in many applications [17,56]. A question worth considering is how much zinc influences the microbial biomass fluctuation during the time period. For this reason, we can assume that its toxicity could be caused mainly by the presence of a relatively high concentration of zinc.
No similar studies have been reported yet and for this reason we cannot compare our data to other literature sources. Some studies only describe the ability of individual bacterial strains to resist PAH toxicity or the ability to gradually biodegrade individual PAHs [17,56,57]. The conclusions of these studies are different, but they are very difficult to put into practice, because in nature there are different microbial communities, not individual species or strains of bacteria. The species diversity of such communities varies over time and within a single locality due to changes in climatic conditions [17,56,57].
The main unique aspect of this study consists of the methodology of the tests. The particles from the tires were analyzed using two types of tests that were not applied simultaneously in the same study to date. The comparison of their results in terms of convenience, applicability and relevance of the obtained data thus led to quite new findings.
A partial unique aspect of the present study is the research and confirmation effect of the design of the experiment on the observed results. If we used only the classical method with leaching, we would not learn about the increased toxicity of the sample for microorganisms during the prolonged presence of particles in water. This method is in any case more ecologically relevant and should be used simultaneously with the leaching or even preferred (if it is possible and the particles do not threaten the model organisms mechanically or do not shade, and thus disrupt photosynthetic processes and the other metabolic actions).
Another other partial unique aspect is the comparison of the acute MTT viability test with the long-lasting respiration test, where the inhibition of respiration was measured in one study. It was evident that the MTT test was more sensitive than the respiration test and this method could, therefore, be more dependent. One of its advantages is also its short duration for estimation and high reliability. On the other hand, it is also a more expensive assay than the respiration test and if used, it will be destructive for the measured microbial cells, which can then not be reused for further measurements. One advantage of the respiration test is its long duration during which the organisms have the opportunity to compensate for some difficulties caused by the stress from the presence of pollutants, which leads to a greater similarity to events that occur spontaneously in nature.
The testing of waste materials, including waste rubber granulates, creates new difficulties and various questions in the subsequent research of such materials for further and repeated use. In every case, reusing such materials should be a better option than putting them in a landfill or burning them, which will create a number of other human and ecological risks due to the fact that a number of other dangerous substances are released.
4. Conclusions
- Waste tires from transport were tested in biodegradation and toxicological experiments with a nonspecific soil microbial community in the present study. The results showed various toxicities but no biodegradation of the waste tires during 28 days of exposure.
- Higher toxicity was found during the respiration inhibition test as well as for the viability assay in the case of suspensions with tire particles than for pure eluates. Such results raise the question of whether such an experimental design should always be used when tests are performed using solid sample leachates, or if both variants should be used together in one experimental design for a more ecologically relevant and realistic comparison.
- The viability tests represented an acute toxic effect and the respiration inhibition test a chronic effect. However, we compared the results from the period of 168 h, and we found that the microorganisms were more sensitive in the short viability test than in the chronic test during the course of 168 h. A similar comparison has never been studied in ecotoxicological papers and as such we are not able to review and discuss these types of results.
- The non-degradability of compounds in the waste eluates indicated that the leachates from waste tires were not subject to degradation. These results were also strongly influenced by a short-term experiment (a duration of only 28 days). Unfortunately, this confirmed the problematic removal of waste tires, which do not undergo environmental degradation. Therefore, the negative ecotoxicological results on soil and aquatic organisms, together with the large leaching of heavy metals or organic pollutants and limited biodegradation, lead to the conclusion that waste tire particles are not a harmless material and their possible use and processing with various techniques needs to be further investigated.
- Other microorganismal or sub-cellular assays should be used for the assessment of toxicity and biodegradability of rubber tires in the future. Another possibility is the use of multi-generation or multi-species tests or in microcosms or mesocosms in aquatic or terrestrial environments. Different species or types of microorganisms should be used as model organisms—mainly yeasts, bacteria, green or blue algae, molds, fungi and their mixtures.
- The practical significance of the conducted research lies in the knowledge that both types of methodologies (eluates + suspension of solid tested particles) are supposed to be used simultaneously. This finding should be reflected in the methodologies used for legislative purposes in the determination of ecotoxicity for microbial communities.
Author Contributions
Conceptualization, K.K.; methodology, K.K.; investigation, K.K.; writing—original draft preparation, K.K., J.F. and R.Č.; writing—review and editing, K.K. and R.Č.; supervision, R.Č. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been supported by the Czech Science Foundation under Project No. FV40054 and the Czech Technical University project No. SGS22/137/OHK1/3T/11.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the reported results can be obtained from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gome, F.B.; Rocha, M.R.; Alves, A.; Ratola, N. A review of potentially harmful chemicals in crumb rubber used in synthetic football pitches. J. Hazard. Mater. 2021, 409, 124998. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, W.; Manalo, A.; Siddique, R.; Mendis, P.; Yan, Z.G.; Wong, H.S.; Lokuge, W.; Aravinthan, T.; Schubel, P. Recycling of landfill wastes (tyres, plastics and glass) in construction—A review on global waste generation, performance, application and future opportunities. Resour. Conserv. Recycl. 2021, 173, 105745. [Google Scholar] [CrossRef]
- Sibeko, M.A.; Adeniji, A.O.; Okoh, O.O.; Hlangothi, S.P. Trends in the management of waste tyres and recent experimental approaches in the analysis of polycyclic aromatic hydrocarbons (PAHs) from rubber crumbs. Environ. Sci. Pollut. Res. 2020, 27, 43553–43568. [Google Scholar] [CrossRef] [PubMed]
- Landi, D.; Gigli, S.; Germani, M.; Marconi, M. Investigating the feasibility of a reuse scenario for textile fibers recovered from end-of-life tyres. Waste Manag. 2018, 75, 187–204. [Google Scholar] [CrossRef]
- Strukar, K.; Sipos, T.K.; Milicevic, L.; Busic, R. Performance of rubber mortars containing silica coated rubber. Eng. Struct. 2019, 188, 452–468. [Google Scholar] [CrossRef]
- Wang, J.Q.; Du, B. Experimental studies of thermal and acoustic properties of recycled aggregate crumb rubber concrete. J. Build. Eng. 2020, 32, 101836. [Google Scholar] [CrossRef]
- De Vries, M.; Claassen, L.; Mennen, M.; Timen, A.; Wierik, M.J.M.; Timmermans, D.R.M. Public perceptions of contentious risk: The case of rubber granulate in the Netherlands. Int. J. Environ. Res. Public Health 2019, 16, 2250. [Google Scholar] [CrossRef]
- Skoczynska, E.; Leonards, P.E.G.; Lompart, M.; de Boer, J. Analysis of recycled rubber: Development of an analytical method and determination of polycyclic aromatic hydrocarbons and heterocyclic aromatic compounds in rubber matrices. Chemosphere 2021, 276, 130076. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Karedal, M.; Nielsen, J.; Adlercreutz, M.; Bergendorf, U.; Strandberg, B.; Antonsson, A.B.; Tinnerberg, H.; Albin, M. Exposure, respiratory symptoms, lung function and inflammation response of road-paving asphalt workers. Occup. Environ. Med. 2018, 75, 494–500. [Google Scholar] [CrossRef]
- Diekmann, A.; Giese, U.; Schaumann, I. Polycyclic aromatic hydrocarbons in consumer goods made from recycled rubber material: A review. Chemosphere 2019, 220, 1163–1178. [Google Scholar] [CrossRef]
- Perkins, B.N.; Inayat-Hussain, S.H.; Deziel, N.C.; Johnson, C.H.; Ferguson, S.S.; Garcia-Milian, R.; Thompson, D.C.; Vasiliou, V. Evaluation of potential carcinogenicity of organic chemicals in synthetic turf crumb rubber. Environ. Res. 2019, 169, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Canepari, S.; Castellano, P.; Astolfi, M.L.; Materazzi, S.; Ferrante, R.; Fiorini, D.; Curini, R. Environ. Biomonitoring of element contamination in bees and beehive products in the Rome province (Italy). Sci. Pollut. Res. 2018, 25, 1448–1459. [Google Scholar] [CrossRef]
- Brandsma, S.H.; Brits, M.; Groenewoud, Q.R.; van Velzen, M.J.M.; Leonards, P.E.G.; de Boert, J. Environ. Chlorinated Paraffins in Car Tires Recycled to Rubber Granulates and Playground Tiles. Sci. Technol. 2019, 53, 7595–7603. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.K.; Lemay, J.C.; Shubin, S.P.; Prueitt, R.L. Comprehensive multipathway risk assessment of chemicals associated with recycled (“crumb”) rubber in synthetic turf fields. Environ. Res. 2018, 160, 256–268. [Google Scholar] [CrossRef]
- Schneider, K.; Bierwisch, A.; Kaiser, E. ERASSTRI—European risk assessment study on synthetic turf rubber infill—Part 3: Exposure and risk characterisation. Sci. Total Environ. 2020, 718, 9. [Google Scholar] [CrossRef] [PubMed]
- Menichini, E.; Abate, V.; Attias, L.; De Luca, S.; Di Domenico, A.; Fochi, I.; Forte, G.; Iacovella, N.; Iamiceli, A.L.; Izzo, P.; et al. Artificial-turf playing fields: Contents of metals, PAHs, PCBs, PCDDs and PCDFs, inhalation exposure to PAHs and related preliminary risk assessment. Sci. Total Environ. 2011, 409, 4950–4957. [Google Scholar] [CrossRef]
- Kruger, O.; Kalbe, U.; Richter, E.; Egeler, P.; Römbke, J.; Berger, W. New approach to the ecotoxicological risk assessment of artificial outdoor sporting grounds. Environ. Pollut. 2013, 175, 69–74. [Google Scholar] [CrossRef]
- Khan, F.R.; Halle, L.L.; Palmquist, A. Acute and long-term toxicity of micronized car tire wear particles to Hyalella azteca. Aquat. Toxicol. 2019, 213, 105216. [Google Scholar] [CrossRef]
- Kobetičová, K.; Fořt, J.; Böhm, M.; Nábělková, J.; Černý, R. Verification of ecological safety of waste tires. Waste Forum 2020, 2, 73–77. [Google Scholar]
- Fořt, J.; Kobetičová, K.; Böhm, M.; Podlesný, J.; Jelínková, V.; Vachtlová, M.; Bureš, F.; Černý, R. Environmental Consequences of Rubber Crumb Application: Soil and Water Pollution. Polymers 2022, 14, 1416. [Google Scholar] [CrossRef]
- Burges, A.; Alkorta, I.; Epelde, L.; Garbisu, C. From phytoremediation of soil contaminants to phytomanagement of ecosystem services in metal contaminated sites. Int. J. Phytoremediat. 2018, 20, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimbabaie, P.; Meeinkuirt, W.; Pichtel, J. Phytoremediation of engineered nanoparticles using aquatic plants: Mechanisms and practical feasibility. J. Environ. Sci. 2020, 93, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Emenike, C.U.; Jayanthi, B.; Agamuthu, P.; Fauziah, S.H. Biotransformation and removal of heavy metals. Environ. Rev. 2018, 26, 156–168. [Google Scholar] [CrossRef]
- Malik, A.; Khan, J.M.; Alhomida, A.S.; Ola, M.S.; Alshehri, M.A.; Ahmad, A. Metal nanoparticles: Biomedical applications and their molecular mechanisms of toxicity. Chem. Pap. 2022, 76, 6073–6095. [Google Scholar] [CrossRef]
- Facciola, A.; Visalli, G.; Ciarello, M.P.; Di Pietro, A. Newly Emerging Airborne Pollutants: Current Knowledge of Health Impact of Micro and Nanoplastics. Int. J. Environ. Res. Public Health 2021, 18, 2997. [Google Scholar] [CrossRef] [PubMed]
- Panver, R.; Mathur, J. Remediation of polycyclic aromatic hydrocarbon-contaminated soils using microbes and nanoparticles: A review. Pedosphere 2023, 33, 93–104. [Google Scholar]
- Singha, L.P.; Pandey, P. Rhizosphere assisted bioengineering approaches for the mitigation of petroleum hydrocarbons contamination in soil. Crit. Rev. Biotechnol. 2021, 41, 749–766. [Google Scholar] [CrossRef]
- Singh, S.K.; Sachan, A. A review on biotransformation of polyaromatic hydrocarbons mediated by biosurfactant producing bacteria. Pet. Sci. Technol. 2021, 40, 2361–2381. [Google Scholar] [CrossRef]
- Ismail, N.A.; Kasmuri, N.; Hamzah, N. Microbial Bioremediation Techniques for Polycyclic Aromatic Hydrocarbon (PAHs)-a Review. Water Air Soil Pollut. 2022, 233, 144. [Google Scholar] [CrossRef]
- Sakshi; Haritash, A.K. A comprehensive review of metabolic and genomic aspects of PAH-degradation. Arch. Microbiol. 2020, 202, 2033–2058. [Google Scholar] [CrossRef]
- OECD Guideline for Testing of Chemicals, Available 3rd April 2023 Online: Test No. 209: Activated Sludge, Respiration Inhibition Test (Carbon and Ammonium Oxidation). 2010. Available online: https://www.oecd.org/env/test-no-209-activated-sludge-respiration-inhibition-test-9789264070080-en.html (accessed on 15 February 2023).
- Benov, L. Improved Formazan Dissolution for Bacterial MTT Assay. Microbiol. Spectr. 2021, 9, 0163721. [Google Scholar] [CrossRef] [PubMed]
- Plumb, J.A.; Milroy, R.; Kaye, S.B. Effects of the pH dependence of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res. 1989, 49, 4435–4440. [Google Scholar] [PubMed]
- Wang, H.; Wang, F.; Tao, X.; Cheng, H. Ammonia-containing dimethyl sulfoxide: An improved solvent for the dissolution of formazan crystals in the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Anal. Biochem. 2012, 421, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Twentyman, P.R.; Luscombe, M.B. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. J. Cancer 1987, 56, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Thakare, A.A.; Gupta, T.; Deewan, R.; Chaudhary, S. Micro and macro-structural properties of waste tyre rubber fibre-reinforced bacterial self-healing mortar. Constr. Build. Mater. 2022, 322, 126459. [Google Scholar] [CrossRef]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef]
- OECD Guideline for Testing of Chemicals, Available 27th February 2023. Available online: https://www.oecd.org/chemicalsafety/testing/TG%20491_Draft%20updated%20Sept%202019_clean.pdf (accessed on 15 February 2023).
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tool since ll biology: New insights in to the ircellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar]
- Porzani, S.; Lima, S.T.; Metcalf, J.S.; Nowruzi, B. In Vivo and In Vitro Toxicity Testing of Cyanobacterial Toxins: A Mini-Review. Rev. Environ. Contam. Toxicol. 2021, 258, 109–150. [Google Scholar]
- Handali, S.; Rezaei, M. Interlink between improved formulations, inhibitory concentrations and cell death mechanism investigations of cytotoxic drugs: What really matters? J. Control. Release 2020, 320, 404–411. [Google Scholar] [CrossRef]
- Da Luz, D.S.; Da Silva, D.G.; Souza, M.M.; Giroldo, D.; Gaspar, M.C.D.G. Efficiency of Neutral Red, Evans Blue and MTT to assess viability of the freshwater microalgae Desmodesmus communis and Pediastrum boryanum. Phycol. Res. 2016, 24, 56–60. [Google Scholar] [CrossRef]
- Isa, H.W.M.; Johari, W.L.W.; Syahir, A.; Abd Shukor, M.Y.; Azwady, A.A.N.; Shaharuddin, N.A.; Muskhazli, M. Development of a Bacterial-based Tetrazolium Dye (MTT) Assay for Monitoring of Heavy Metals. Int. J. Agric. Biol. Eng. 2014, 16, 1123–1128. [Google Scholar]
- Halmi, M.I.E.; Ahmad, F.; Hashim, A.K.; Shamaan, N.A.; Syed, M.A.; Shukor, M.Y. Effect of bacterial growth period on the sensitivity of the MTT assay for silver. J. Environ. Biol. 2014, 35, 353–355. [Google Scholar] [PubMed]
- Pilgard, A.; De Vetter, L.; Van Acker, J.; Westin, M. Toxic hazard of leachates from furfurylated wood: Comparison between two different aquatic organisms. Environ. Toxicol. Chem. 2010, 29, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Wilk, B.K.; Szopinska, M.; Sobaszek, M.; Pierpaoli, M.; Blaszczyk, A.; Luczkiewicz, A.; Fudala-Ksiazek, S. Electrochemical oxidation of landfill leachate using boron-doped diamond anodes: Pollution degradation rate, energy efficiency and toxicity assessment. Environ. Sci. Pollut. Res. 2022, 29, 65625–65641. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, F.H.; Wan, J.; He, J.; Li, Q.; Chen, Q.; Gao, J.; Lin, Y.; Zhang, S. Ecotoxicological bioassays of sediment leachates in a river bed flanked by decommissioned pesticide plants in Nantong City, East China. Environ. Sci. Pollut. Res. 2017, 24, 8541–8550. [Google Scholar] [CrossRef]
- Baysal, A.; Saygin, H.; Ustabasi, G.S. Impact of test conditions on the bacterial bioassay in the presence of TiO2 nanoparticles. J. Chem. Metrol. 2021, 14, 114–124. [Google Scholar] [CrossRef]
- Malara, A.; Oleszczuk, P. Application of a battery of biotests for the determination of leachate toxicity to bacteria and invertebrates from sewage sludge-amended soil. Environ. Sci. Pollut. Res. 2013, 20, 3435–3446. [Google Scholar] [CrossRef]
- Böhm, M.; Kobetičová, K.; Fořt, J.; Černý, R. Determination of metals leakage from rubber granulate. AIP Conf. Proc. 2022, 2425, 150003. [Google Scholar]
- McCarrick, S.; Cunha, V.; Zapletal, O.; Vondracek, J.; Dreij, K. In vitro and in vivo genotoxicity of oxygenated polycyclic aromatic hydrocarbons. Environ. Pollut. 2019, 246, 678–687. [Google Scholar] [CrossRef]
- Toyooka, T.; Ibuki, Y. DNA damage induced by coexposure to PAHs and light. Environ. Toxicol. Pharmacol. 2007, 23, 256–263. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Pang, Q.; Huang, C.M.; Xie, J.; Hu, J.; Wang, L.; Wang, C.C.; Meng, L.; Fan, R. Environmental dose of 16 priority-controlled PAHs mixture induce damages of vascular endothelial cells involved in oxidative stress and inflammation. Toxicol. In Vitro 2022, 79, 105296. [Google Scholar] [CrossRef] [PubMed]
- Misaki, K.; Matsui, S.; Matsuda, T. Metabolic enzyme induction by HepG2 cells exposed to oxygenated and nonoxygenated polycyclic aromatic hydrocarbons. Chem. Res. Toxicol. 2007, 20, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, N.; Shah, A.A.; Qayyum, S.; Hasan, F. Optimization of pH and temperature for degradation of tyre rubber by Bacillus sp. strain S10 isolated from sewage sludge. Int. Biodeterior. Biodegrad. 2015, 103, 154–160. [Google Scholar] [CrossRef]
- Tsuchii, A.; Takeda, K.; Tokiwa, Y. Degradation of the rubber in truck tires by a strain of Nocardia. Biodegradation 1997, 7, 405–413. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).