1. Introduction
Living organisms are exposed to magnetic fields, the intensities of which are under the Ampère law that links magnetic intensity to current intensity. Variable magnetic fields are responsible for the generation of electric fields known as inducted fields; they differ from electrostatic fields and are explained by the Faraday law [
1,
2,
3].
Electric fields generally interact with electric charges of invested bodies, both fixed and moving, according to the Coulomb law, which determines a change on system energy. Instead, the magnetic field only interacts with moving charges, spin, and magnetic moment, applying a force on the charges according to the Lorentz law.
Electric negative charges are tightly bound to their atoms and are responsible for the magnetic moment of the matter, biological or otherwise. The exhibited magnetic moment of the matter is regulated by quantum mechanics and is responsible for molecular ferromagnetism and para-magnetism. All matter is subjected to such phenomena. In this frame also operates diamagnetism. This—a much less evident phenomenon—is another characteristic of all molecules and is sensitive to the derivative of the magnetic flux because of the Lentz law. Thus, significant effects are evident at high frequencies.
Almost all biological matter can be considered paramagnetic, and its interaction with a magnetic field leads to a mechanical moment corresponding to the product
, where
is the matter magnetic moment and
the external magnetic field [
4]. Inside the matter, the mechanical moment can vary the orientation of the molecules, especially the less bound, and changes the energy of the molecule expressed as
[
4]. To study the effects of fields on biological substrates is not an easy task. Generally, it is assumed that the mechanical moment induced by a magnetic field can influence the energetic state and, so, also the charge transport through the cells. Considering the magnetic moment caused by the molecule electrons only, the spin of which is ±½, the energy variation, according to Equation (1), can be positive or negative; namely:
where
is the factor of Landé, which is close to 2.00 for free electrons and for most organic radicals [
4],
μB is the magnet of Bohr,
is the magnetic field, and
is the quantum constant.
On Earth, a (geo)magnetic field is present, which is relevant for living organisms, although its intensity is very low, ~50 μT, as is its variation during the day and year. Nowadays, more intense fields operate in the environment. In laboratory experiments, they may range from a few 0.001 to 3 T.
Previously, we studied the effects of magnetic fields on the morphology and locomotor (posture, swimming, and circling) behavior of zebrafish (
Danio rerio), a well-recognized model from vertebrate biology to ecotoxicology (and nowadays widely used as a model also in biomedical research) in larvae at 5 days post-fertilization (dpf) [
5]. Zebrafish embryos were exposed to static and four frequency fields up to 900 MHz for up to 5 days. The magnetic field intensity ranged from 240 nT to 40 mT. In this study, we extend the previous work to define the effects of the same stimuli on zebrafish body pigmentation [
6], which results from the precise arrangement of three main classes of pigment cells: black melanophores, yellow xanthophores, and iridescent iridophores. Here, we emphasize the changes in the melanophores. The survival rate is also studied.
2. Materials and Methods
2.1. Static Magnetic Field (B0) Irradiation
A simple magnet was sufficient to provide a static magnetic field. We used a magnetic disc 4 cm in diameter (
Supplementary Figure S1). To irradiate the samples contained in the Petri dishes (5.5 cm in diameter), we placed this last on the magnet on the north side. The magnetic field intensity at ~0.5 cm from the magnet plane was ~40 mT, as well as near the edges of Petri dish. The inner diameter of the Petri dish is ~5.2 cm and the maximum distance from the magnet becomes about 0.8 cm. However, let us again assume uniform exposure to the magnetic field because it is ensured by the Brownian motion of the samples and by the care taken in placing the embryos in the center of the Petri dish.
2.2. Extremely Low Frequency (ELF) Magnetic Field Irradiation
The extremely low-frequency magnetic field is an alternating field by 0.2 Hz frequency (±40 mT peak). It was constructed with four static magnets made of discs of 4 cm in diameter (
Supplementary Figure S2). The magnets are placed along a circumference supported by two ferromagnetic strips. The support is moved by a low-frequency electric motor of ~0.1 Hz, which resulted in a field frequency of ~0.2 Hz.
2.3. Low Frequency (LF) Electromagnetic Field Irradiation
To obtain variable and intense magnetic fields is rather difficult since high and variable currents, ~10 A, are necessary. Generally, the common radio frequency (RF) devices generate low currents, so it was necessary to create an ad hoc generator. Therefore, we made a solenoid with an internal diameter of ~5 cm and a height of 11 cm with a number of turns, 18, to get low inductance and low resistance, which favors a high current. The picture of the device is shown in
Supplementary Figure S3. The value of the inductance was ~4 μH. The latter coupled in parallel with a capacitor form an oscillating
LC circuit. To obtain a frequency of
, it is necessary to use a capacitor with capacitance of ~
. To reach a low frequency of ~270 kHz, we used a capacitor, the nominal value of which was 100 nF.
The real LC circuits is dispersive, namely, they are unable to maintain continuum oscillations. Thus, it is necessary to restore the energy lost per cycle. To achieve this goal, the LC circuit was connected to ground by a switch circuit formed by three transistors in the emitter mode. A continuum voltage, +V, fed the circuit. All transistor bases were connected to a low power RF generator, the signal of which was in resonance with the current of the LC circuit. The same RF signal was also used as an oscilloscope trigger.
Moreover, to measure the magnetic field, as there are no gaussmeters for magnetic fields variable to hundreds of kHz, we opted to use a secondary inductor concatenated with the principal one and closed on an infinite impedance. We call it a probe. The latter consists of four turns, and the attenuation coefficient depends on frequency value. At 270 kHz, the attenuation coefficient was Att = IB/VL~0.38 A/V, with IB the solenoid current and VL the output voltage delivered from the probe. According to the Ampère law, the magnetic field inside the inductor is controlled by the relation B = IB · 206 × 10−6 T. Therefore, with 6 V of output voltage delivered by the probe, VL, the maximum magnetic field that resulted was 470 μT.
The probe output signal
VL is shown in
Supplementary Figure S4. In the same figure, it is possible to see the trigger signal.
The measurements were made with a LeCroy WaveSurfer 422 oscilloscope, 2 GS/s with 200 MHz band limitation (LeCroy Corporation, New York, NY, USA).
2.4. Very-High-Frequency (VHF) Electromagnetic Field Irradiation
The range of frequency of hundreds MHz is mainly used for radio transmission and telephone communication, so it is widespread in the environment. To explore its effect on biological samples, suitable devices are necessary such as transmission lines. For our purpose, the geometry of the line we built was flat and was designed with a height of
h = 1.35 cm, width of
a = 9 cm, and length of 20 cm. The length of the line does not affect the line efficiency, and it allows the processing of multiple samples simultaneously. The
RF generator was a ROHDE & SCHWARZ SM 300 with an output impedance of 50 Ω (Rohde & Schwarz Italia, Rome, Italy). Its maximum output power was 20 mW. The characteristic impedance of a flat line, neglecting the radiation that escapes laterally, is looked at in the following formula [
7]:
where
L and
C are the inductance and capacitance for length unit, respectively, and ε
0 and μ
0 are the electrical permittivity and magnetic permeability, respectively. To avoid signal reflections, the characteristic impedance of the line must approximate that of the generator. From Equation (2), the line has an impedance of 56.6 Ω in vacuum, which involves a reflection of the signal of 6.2%. This value is rather small, but if we concentrate on the growth medium made mostly of water, the characteristic impedance again decreases, as well as the reflection coefficient of the field.
Consequently, a high-frequency 50 Ω cable connected the input line to the generator, while the line output was closed to a load of 50 Ω using
resistors (
Supplementary Figure S5). These last are non-inductive resistors.
The wavelength at 100 MHz in vacuum is ~3 m, and such a value makes secure the transversal uniformity of the fields inside the line. The applied input signal was 1 V, and the correspondent electric field was ~71 V/m, while the magnetic field that resulted was of ~240 nT.
The measurements at 100 MHz this time were made with a LeCroy Wavepro 7100 fast oscilloscope, 200 GS/s with 1 GHz band limitation (LeCroy Corporation, New York, NY, USA).
2.5. Ultra-High-Frequency (UHF) Electromagnetic Field Irradiation
To perform treatments at ultra-high frequency (UHF), 900 MHz, we used a short flat transmission line with edges modified to limit the effects of stray irradiations. To preserve the characteristic impedance of the system, the dimensions of the line were, again, h = 1.35 cm and a = 9 cm, while the length was only 12 cm. It was closed on a load of 50 Ω using resistors, like the VHF flat line. The RF generator was a ROHDE & SCHWARZ SMF 100 A. Its output power was 20 W. The expression of the characteristic impedance of the flat line is again expressed by Equation (2).
The wavelength at 900 MHz in vacuum is ~0.33 m. Therefore, to limit the lateral irradiation and make secure the transversal uniformity of the fields inside the line, it was necessary to modify the conductors bending the lateral outline (
Supplementary Figure S6).
The applied input signal was again 1 V, and the correspondent electric field again 71 V/m. The generated magnetic field was always 240 nT. All measurements were made again with the Le Croy Wavepro 7100 fast oscilloscope (Chestrut Ridge, NY, 10977, USA).
The main radiation characteristics associated with the static, low-, and high- (radio) frequency magnetic fields used in this work are reported in
Table 1.
2.6. Zebrafish Maintenance and Treatments
The simple devices and experimental sets presented above were made to optimize their use on zebrafish. To date, zebrafish have already been used to study the effects of magnetic fields [
8,
9,
10].
Adult wild-type zebrafish (AB line) were maintained under standard rearing conditions in plastic tanks at 27–28 °C at the Department of Biological and Environmental Sciences and Technologies, University of Salento (Lecce, Italy). The fish were kept to a constant 14 h light: 10 h darkness photoperiod and fed thrice daily with commercial fish diet (ZEBRAFEED, Sparos I&D; Olhão, Portugal). Animal care and procedures were in accordance with the U.S. National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and European Community Directive 2010/63/EU.
Fertilized eggs (diameter ~0.6 mm) were collected within 1–2 h (not later than the 8- to 16-cell stage) of natural spawning and then transferred into Petri dishes (Corning, Milano, Italy) with deionized water added with commercial Instant Ocean (Blacksburg, VA, USA) sea salts (60 mg of Instant Ocean per liter of water; pH range 6.8–7.4) [
11]. All experiments were performed in Petri dishes with a water column height of about 3 mm and a constant 14 h light:10 h darkness photoperiod for the entire duration of the test. With the fertilized eggs subjected to Brownian motion, the mechanical moment direction changed continually. Since, during the experiments, the fertilized eggs developed from embryos to larvae, we measured the effects of radiations during the early embryonic-to-larval development stages up to 5 dpf.
Within 1–2 h post-fertilization (hpf), developing embryos (diameter ~0.9 mm) were separated into six groups. After 1–2 hpf, zebrafish embryos were exposed under the magnetic fields and radio frequencies for 5 days (~120 hpf). Specifically, five groups were subjected each to a single stress: static (0 Hz), extremely low-frequency (0.2 Hz), low-frequency (270 kHz), very-high-frequency (100 MHz), and ultra-high-frequency (900 MHz) field irradiation; the sixth group of untreated embryos was used as control (number of eggs
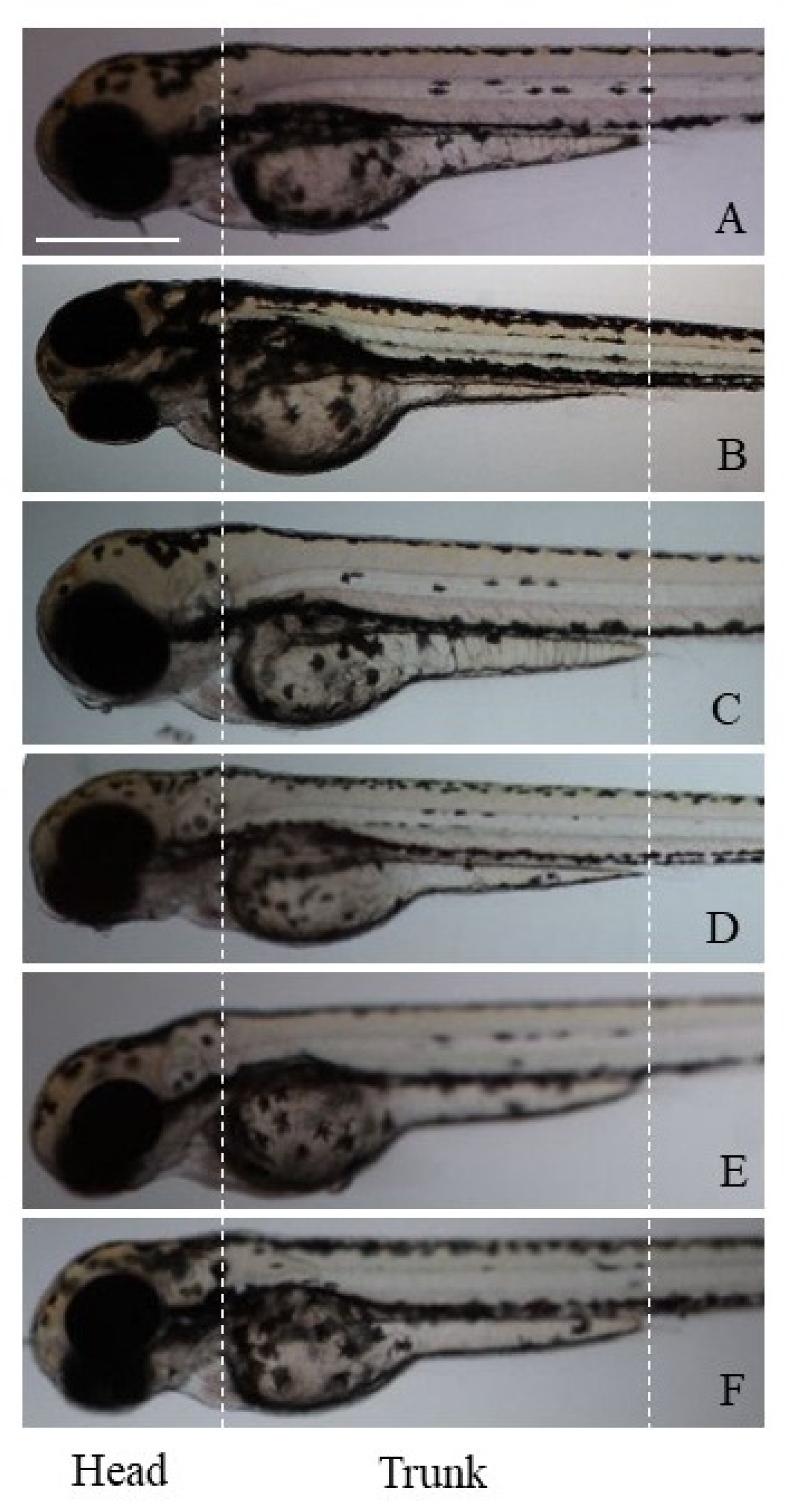
n = 10 for each condition; number of experiments = 3). At the end of the experiments (length ~3.9 mm for untreated larvae), larvae were anesthetized with tricaine methanesulfonate (MS-222; SIGMA-Aldrich Chemical Co., London, UK) and observed at low magnification (10×) using a Nikon AZ100 stereomicroscope equipped with the Nikon NIS-Elements D suite for image capture. For our study, head and trunk pigmentation (morphology of melanophores) was analyzed By ImageJ software v 1.8.0 (
https://imagej.net/ImageJ; Wayne Rasband, National Institutes of Health, Bethesda, MD, USA), the total count and area of melanophore were analyzed. Data are presented as mean ±SE (standard error of the mean). The statistical analysis was performed using an unpaired
t-test, considering
p < 0.05 significant (* =
p < 0.05; ** =
p < 0.01; ***
p < 0.001). Images of zebrafish larvae on different magnetic field irradiations to evaluate the different pigmentation are shown in
Figure 1.
3. Results
In a previous work, we showed that zebrafish larvae were affected by the exposure to different irradiations in terms of both developmental delay, alterations of body structures, and survival rate [
5]. Here, we analyzed the anatomical and morphological alterations of the pigment melanophores in zebrafish larvae after 5 days of exposure (
Table 2).
Magnetic field irradiations induced different effects on the zebrafish body pigmentation. In fact, compared to the controls in the static exposure group (B
0, 0 Hz), the number of head and trunk (black) melanophores was not significantly changed, but a statistically different increase in the area of these cells was found (
Figure 1B). No morphological changes of melanophores were observed.
The exposure to the extremely low-frequency magnetic fields (ELF, 0.2 Hz) determined milder effects on the pigmentation. In fact, the larvae showed a pigmentation pattern like the controls, in terms of number, area, and morphology of melanophores, which appeared dense and rounded (
Figure 1C).
Exposure to the low-frequency magnetic field (LF, 270 kHz) induced a significant reduction in the melanophore area in both zebrafish head and trunk, and an increase in the number of melanophores located specifically on the trunk was also observed (
Figure 1D).
Again, the very-high-frequency (VHF, 100 MHz) and the ultra-high-frequency (UHF, 900 MHz) fields significantly reduced the melanophore area only in the larval head, whereas the number of melanophores was not altered along the animal skin (
Figure 1E,F).
The effects of all frequencies tested were evident on the melanophore morphology, as they induced a transition from a dense/rounded to a loose/stellate cell phenotype (
Figure 1D–F).
4. Discussion
This work shows the effects of different physical stresses, consisting of one static, two low-frequency and two high- (radio) frequency magnetic fields on zebrafish development. Controlled magnetic fields were generated by using controlled magnetic devices, which made controlled experimental setups available for systematic analyses on aquatic biological samples. Since five experimental devices for the generation of static, extremely low-, low-, very-high-, and ultra-high-frequency magnetic fields were contemporarily available, it was possible to analyze at the same time the effects of five types of irradiations on the same biological model and the same experimental animal batch. The main radiation properties associated with the various magnetic fields are summarized in
Table 1.
When biological samples were exposed to different fields, variable and often conflicting results were obtained, this depending on type and properties (static vs. other characteristics) of the radiation used, type and biological state (animals vs. plants, eukaryotes vs. prokaryotes, embryos vs. adults, etc.) of the organism, and the time and/or duration of the physical insult [
13,
14]. Here, we discuss the zebrafish model and its embryonic/early larval stages only. Under our experimental setups, we tested zebrafish embryos that were exposed to the irradiation procedures continuously since the beginning of their embryonic development (fertilization) up to 5 dpf. This means that they were subjected to the radiation effects for all their embryonic/early larval development.
In a previous study, we observed that while the effects on the embryonic mortality seemed limited (i.e., fish embryos stayed alive during the experiment and only in one case the number of survivors become lower than the control), the effects on the time and plan of development seemed more evident [
5]. One of the most relevant observations in our previous study was the developmental delay, which—together with the limited effect on mortality—has already been observed in zebrafish embryos exposed at both strong static (0 Hz, 9 T) [
10] and low alternating (50 Hz, 1 T) [
15] magnetic fields. In addition, in the current study, we prove that magnetic fields and radio frequencies affect zebrafish body pigmentation, altering the morphology and morphometric characteristics of black melanophores.
4.1. Static Magnetic Field
Zebrafish embryos/larvae exposed continually to the static magnetic field (40 mT) did not show increased mortality but showed a slight delay in development and a reduction in swimming activity and circling motion. In addition, there was slight evidence of malformations in the spine development, and thus, curved larvae could appear. Pericardial oedemas were also evident in developing larvae [
5]. Moreover, treated zebrafish larvae did not show any differences in the developing melanophores, one of the three pigment cell types responsible for zebrafish stripes [
16]. These data agree well with those of Ge et al. [
10] on zebrafish embryos exposed from fertilization to 24 hpf to a 9 T static magnetic field, which resulted in neither lethal nor teratogenic effects. However, the static magnetic field delayed the developmental pace of the whole animal, as indicated by slower hatching, pharyngeal development, and body growth, altered gene expression, and worse performance in behavior tests compared to control. Whether the development-delaying effect is caused by the interference of the static magnetic field in microtubule and spindle positioning during mitosis, especially in early cleavages [
10], remains an open question. Shorter exposures (e.g., 2 h) associated with stronger static magnetic fields (e.g., 14 T)—as requested in magnetic resonance imaging (MRI)—results in stronger biological effects (e.g., otolith fusion), suggesting a possible role of magnetic field intensities in the manifestation of the biological effect [
17].
4.2. Extremely Low-Frequency Magnetic and Low-Frequency Electromagnetic Fields
Zebrafish embryos/early larvae exposed to the very-low-frequency (0.2 Hz, 40 mT) magnetic field did not show increased mortality but showed a delay in development, and therefore, larvae appeared slightly shorter than the controls. Exposure had limited effects on motion behavior (with three-quarters of the larvae showing normal movements and the remaining one-quarter showing hypo-neuromuscular activity). On the other side, zebrafish embryos/larvae exposed to a low-frequency (270 kHz, 470 μT) magnetic field showed—the only case in our study—increased mortality and a delay in development with respect to control. All larvae exposed to low-frequency magnetic fields showed normal movement. Nevertheless, some evident deformities may occur in terms of spinal curvatures. Notably, our findings correlate well with the literature dealing with low-frequency magnetic fields (<300 Hz). Among these, those of natural or artificial origin, such as solar activity, geomagnetic fields, residential electric equipment, nearby power, and overhead high-voltage transmission lines and domestic installations, are included. Skauli et al. [
15] report that exposure to a 50 Hz magnetic field at 1.0 mT delays the progress of zebrafish embryo hatching. Furthermore, exposure to magnetic fields at a lower intensity of 0.2 mT results in a delay in hatching, decrease in heart rate, and increase in apoptosis [
17]. In this case, more evident effects on zebrafish pigmentation were observed only in the low-frequency group, supporting the hypothesis of alterations in zebrafish development. Absence or delay in pigmentation is, in fact, one of the markers of zebrafish stress and/or toxicity, according to Test Guideline 236 (TG236) [
12]. Notably, in the work by Li et al. [
18], embryos were exposed to a 50 Hz sinusoidal magnetic field with intensities of 30, 100, 200, 400, and 800 μT for 96 h. Continuous exposure caused delayed hatching and decreased heart rate at the early developmental stages of zebrafish embryos, whereas no significant differences in embryo mortality and abnormality were observed. Moreover, acridine orange staining assays showed notable signals of apoptosis mainly in the ventral fin and spinal column, and the transcription of apoptosis-related genes was significantly upregulated in exposed embryos.
4.3. Radio-Frequency Electromagnetic Fields
Zebrafish embryos/early larvae exposed to both very-high- (100 MHz, 240 nT) and ultra-high- (900 MHz, 240 nT) frequency magnetic fields did not show increased mortality, but a slight delay in development with respect to control. Both radio frequencies caused abnormal swimming behavior because of hypo-neuromuscular activity, slow swimming speed and failure to maintain posture, even in the absence of spinal curvatures. Limited pericardial oedema could also be present in the developing larvae. In this case, alterations in development pigmentation were found only in the zebrafish head. These findings largely match with results from other groups. For example, Piccinetti et al. [
19] showed that at 100 MHz, an electromagnetic field affects various aspects of zebrafish embryonic development from 24 to 72 hpf. At the 48 hpf stage, reduced growth, increased transcription of oxidative stress genes, the onset of apoptotic/autophagic processes, and modification in cholesterol metabolism have been detected. In addition, zebrafish embryos face stress induced by radiation by triggering detoxification mechanisms.
Our results are worthy, but require more follow-ups and data collection, such as histopathological examinations of the neuromuscular tissue to appreciate the lesions, to fully address the question of the effects of a magnetic field in the zebrafish model. In this context, the fact that the zebrafish, as well as other fish, do sense the geomagnetic field (which is known as ‘magnetoreception’), as well as other natural magnetic fields [
2,
20,
21,
22], is not to be underestimated. In this way, our results can contribute to the advancement of research on the effects of magnetic fields on biological matter. Moreover, our findings can be beneficial for fully assessing the role of magnetic radiations on humans and, thus, be useful for their implications in medicine (as e.g., for MRI).
In a perspective, the rapid deployment of the 5G transmission mode of the telecommunications industry will have to be considered. Since exposures to radio-frequency radiations, >2.4 GHz, are still uncommon, concerns about their potential health impacts are ongoing. The embryonic zebrafish has already been used to assess the impacts of a 3.5 GHz radio-frequency radiation on biological matter [
23]. Exposing developing zebrafish from 6 to 48 hpf and measuring a battery of morphological and behavioral endpoints at 120 hpf revealed no significant impacts on mortality, morphology, or photomotor response and a modest inhibition of startle response. This suggests that cell-phone radiations at low-GHz-level frequencies are likely benign on the zebrafish model, with subtle sensorimotor effects.