Material Design in Implantable Biosensors toward Future Personalized Diagnostics and Treatments
Abstract
1. Introduction
2. Implantable Biosensors and Their Design

3. Classification of Implantable Biosensors Based on Material Design
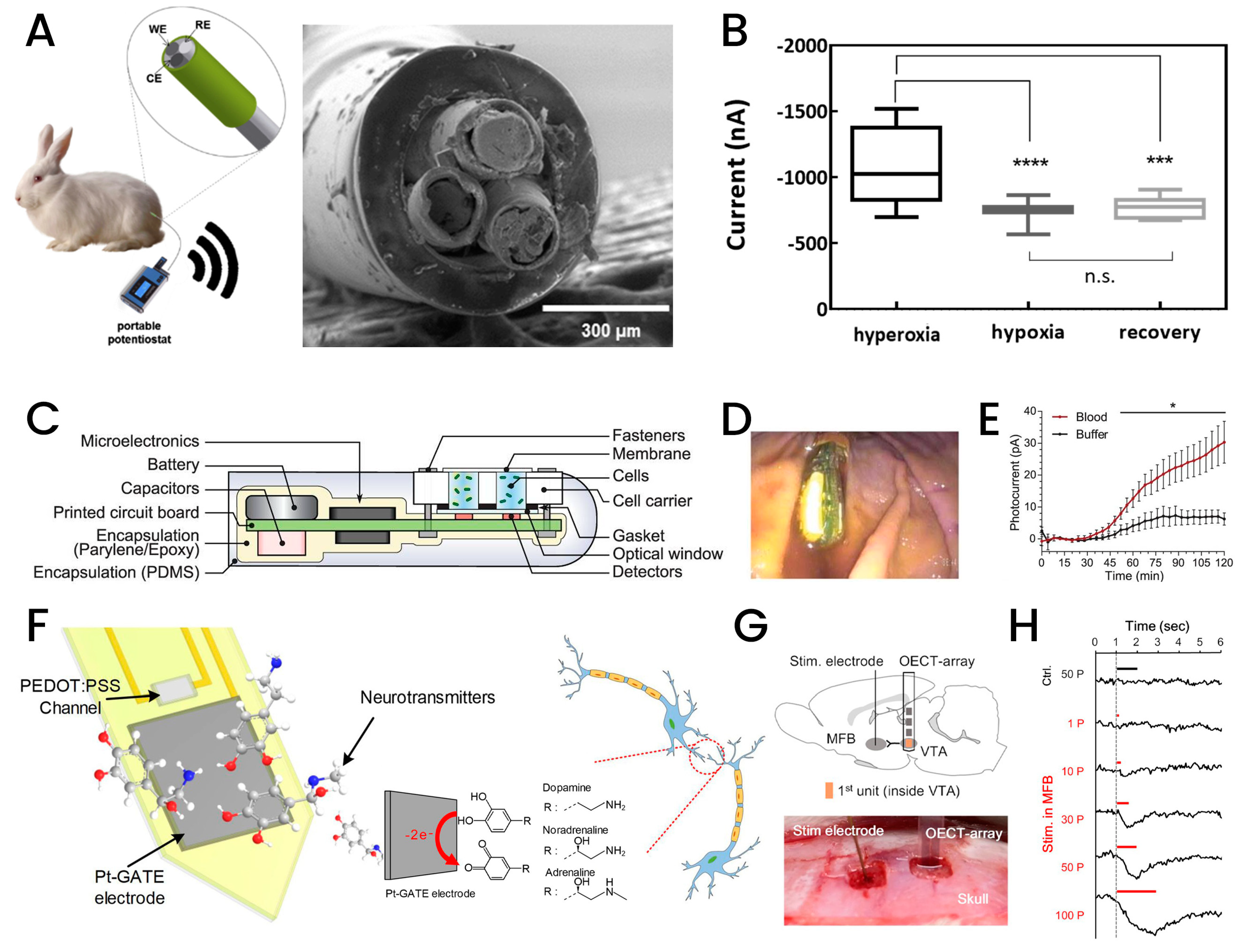
3.1. Electrochemical Active-Based Implantable Biosensors
3.2. Nanomaterial-Based Implantable Biosensors
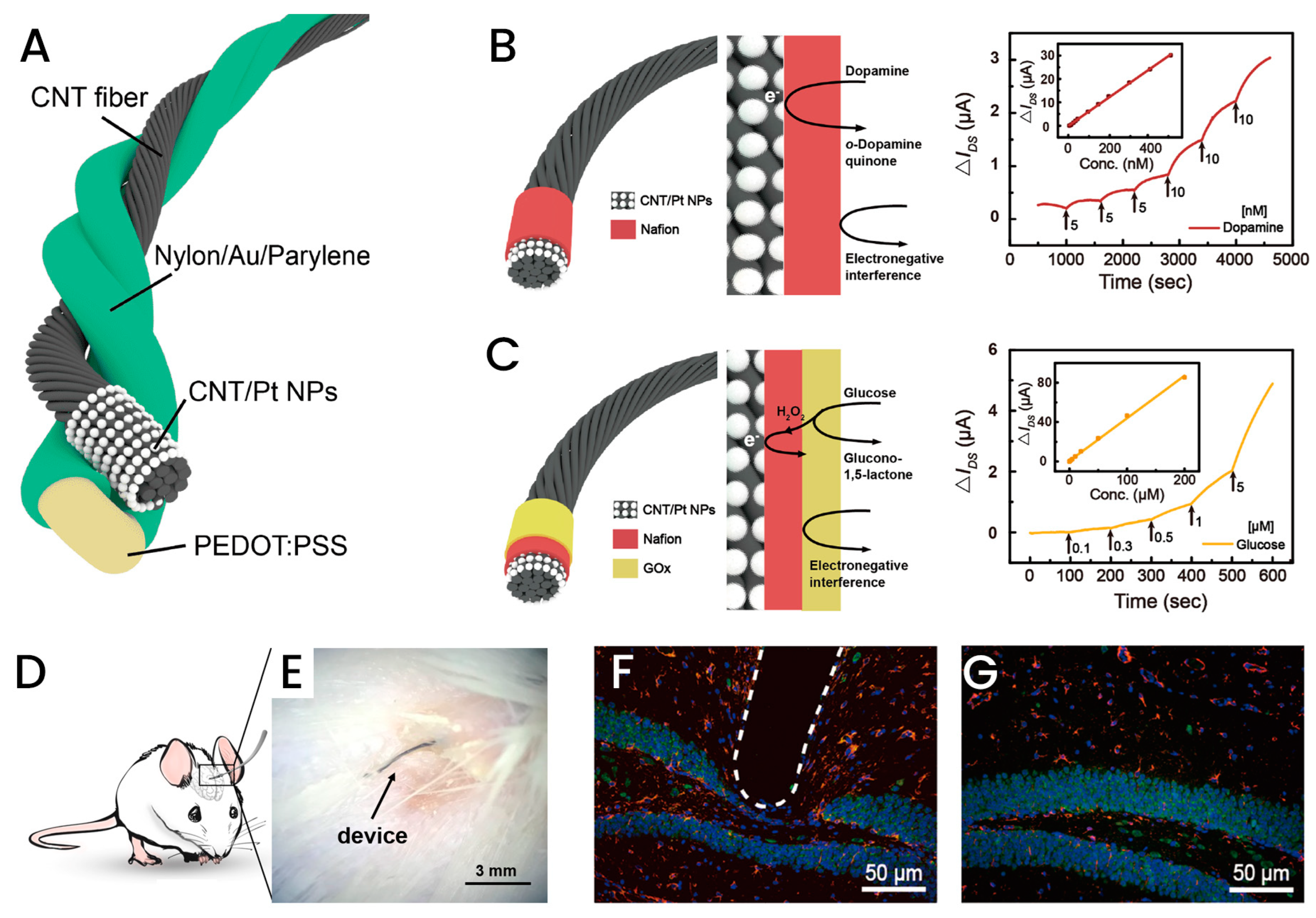
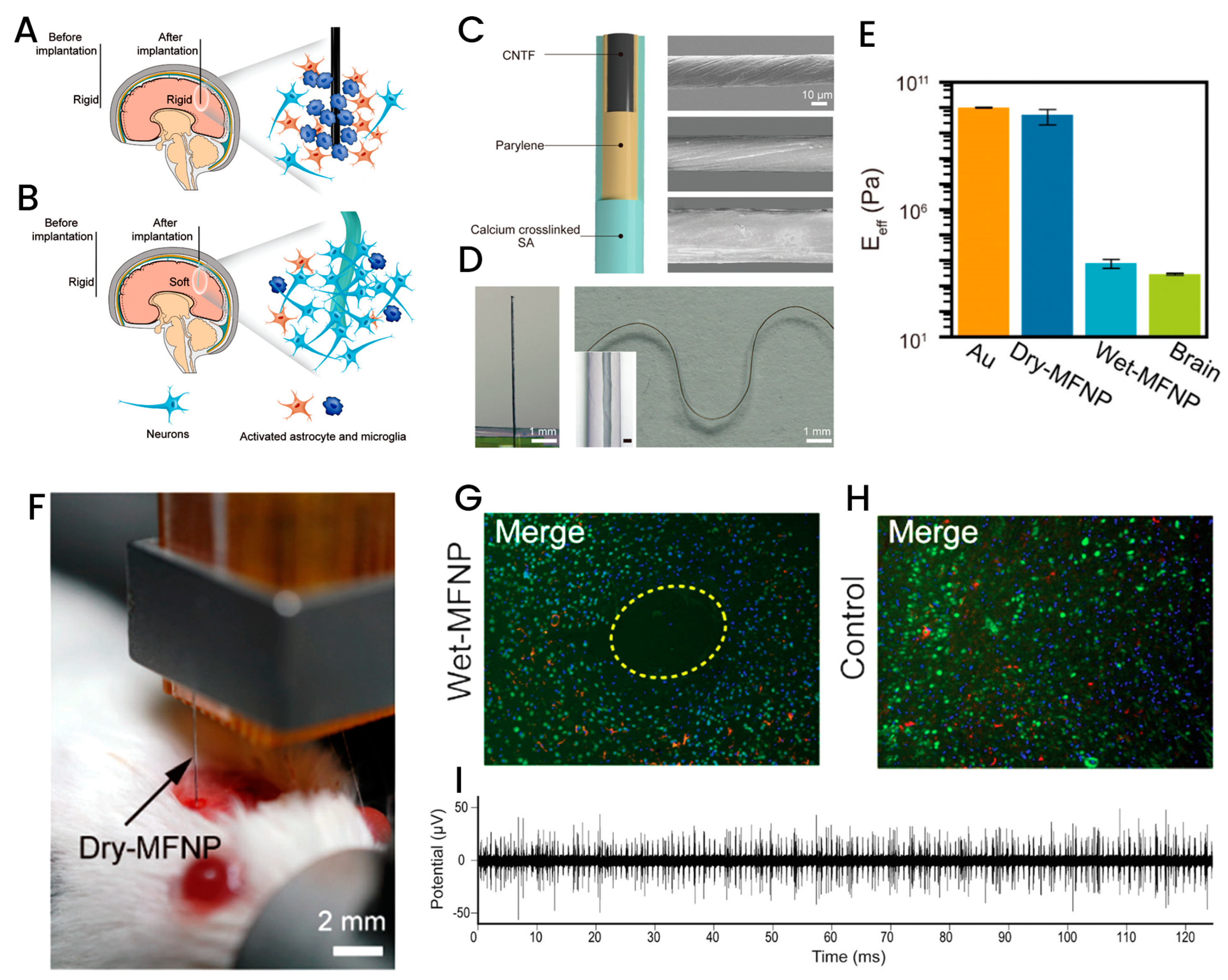
3.3. Fiber-Based Implantable Biosensors
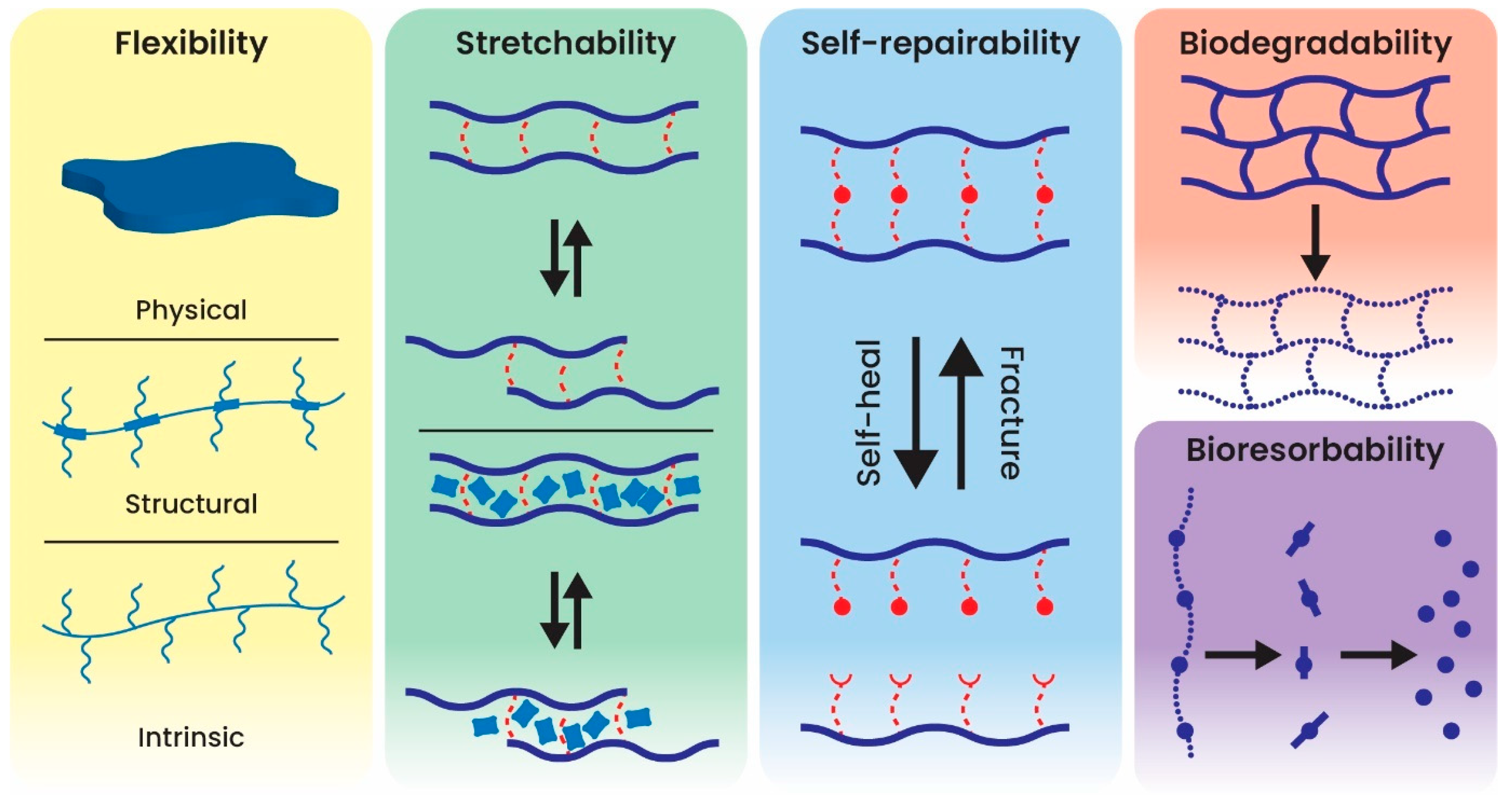
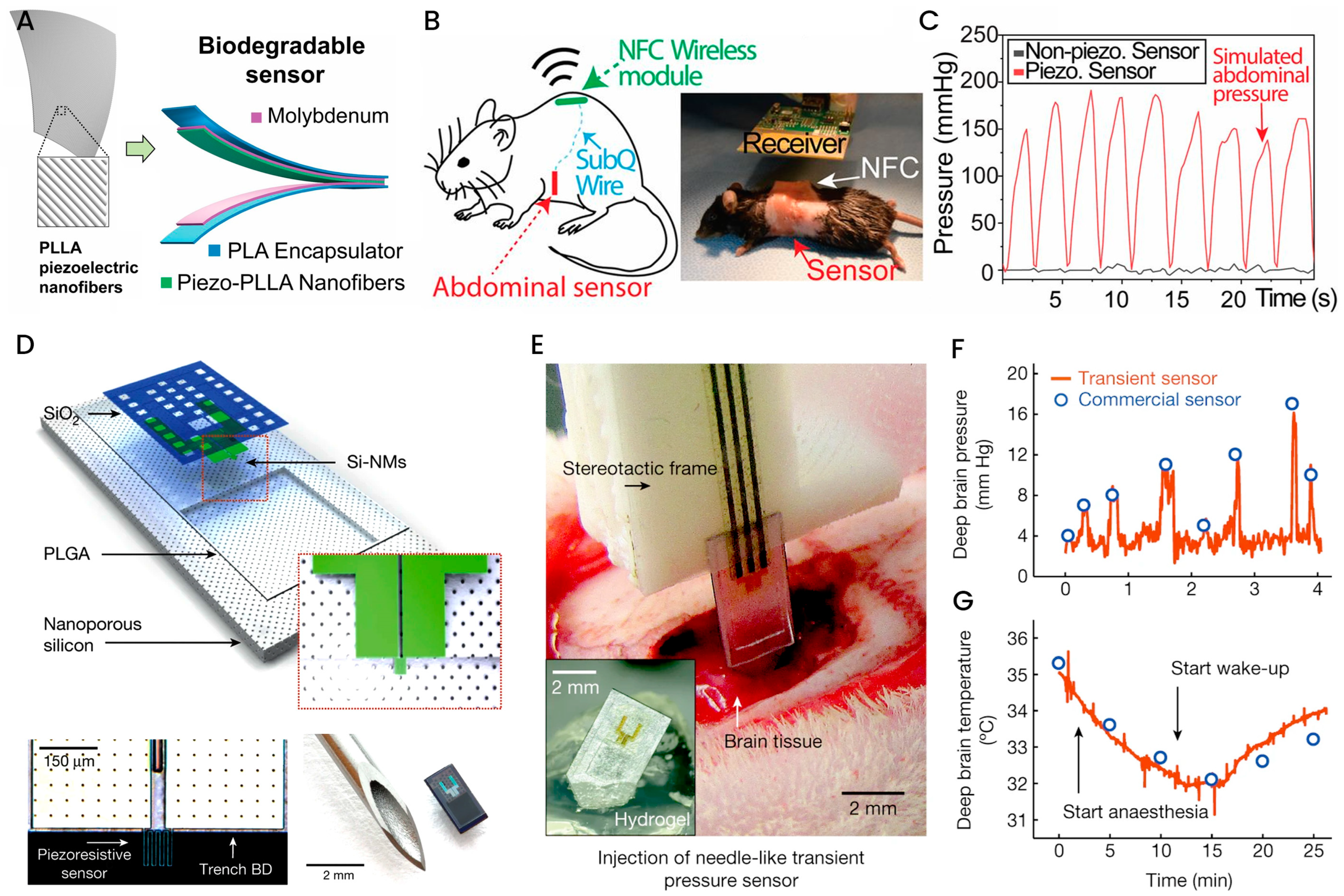
3.4. Polymer-Based Implantable Biosensors
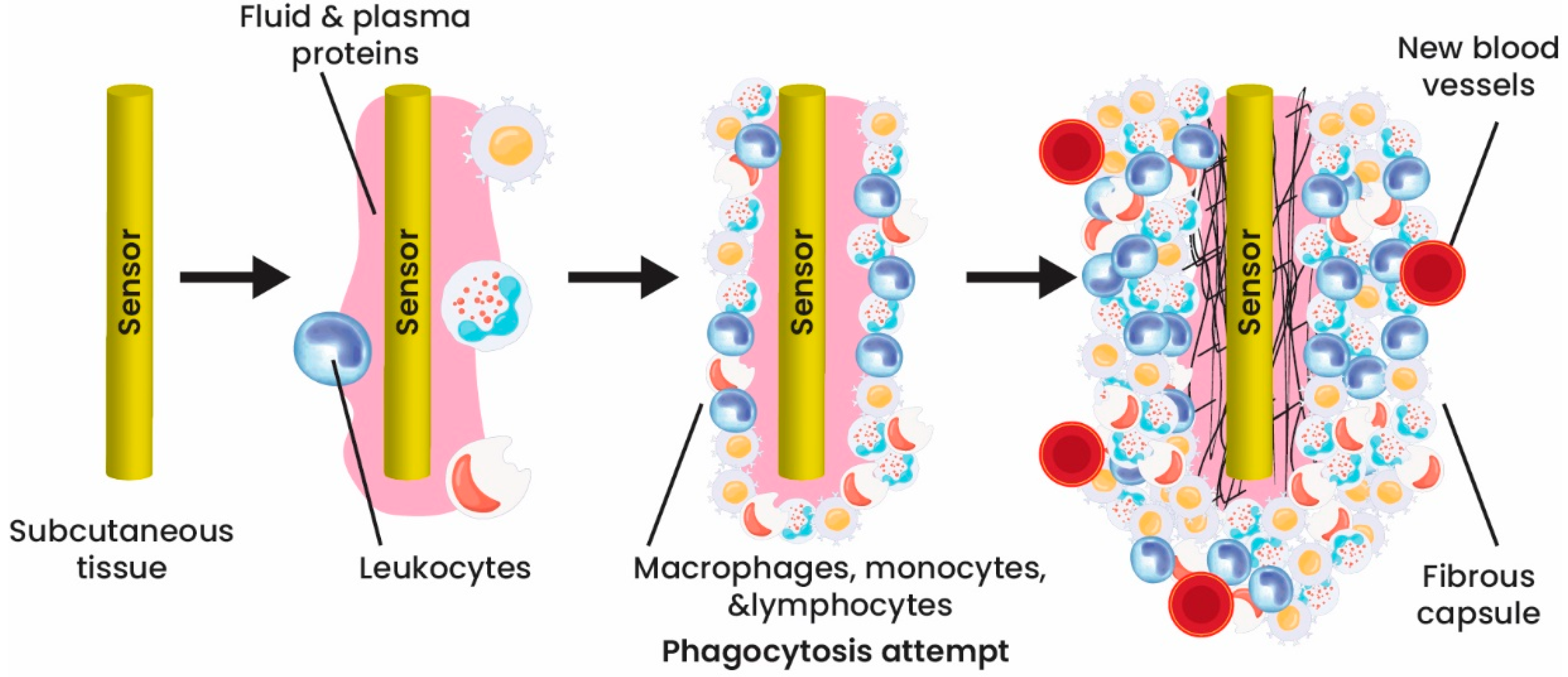
4. Coating Implantable Biosensors
5. Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Boutry, C.M.; Beker, L.; Kaizawa, Y.; Vassos, C.; Tran, H.; Hinckley, A.C.; Pfattner, R.; Niu, S.; Li, J.; Claverie, J.; et al. Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nat. Biomed. Eng. 2019, 3, 47–57. [Google Scholar] [CrossRef]
- Lee, J.; Cho, H.R.; Cha, G.D.; Seo, H.; Lee, S.; Park, C.K.; Kim, J.W.; Qiao, S.; Wang, L.; Kang, D.; et al. Flexible, sticky, and biodegradable wireless device for drug delivery to brain tumors. Nat. Commun. 2019, 10, 5205. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, J.; Liu, C.; Carlisle, J.A.; Mech, B.; Greenberg, R.; Guven, D.; Freda, R.; Humayun, M.S.; Weiland, J.; et al. In vitro and in vivo evaluation of ultrananocrystalline diamond for coating of implantable retinal microchips. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 77, 273–281. [Google Scholar] [CrossRef]
- Ben-Menachem, E.; Manon-Espaillat, R.; Ristanovic, R.; Wilder, B.J.; Stefan, H.; Mirza, W.; Tarver, W.B.; Wernicke, J.F. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia 1994, 35, 616–626. [Google Scholar] [CrossRef]
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef]
- Rose, E.A.; Gelijns, A.C.; Moskowitz, A.J.; Heitjan, D.F.; Stevenson, L.W.; Dembitsky, W.; Long, J.W.; Ascheim, D.D.; Tierney, A.R.; Levitan, R.G.; et al. Long-term use of a left ventricular assist device for end-stage heart failure. N. Engl. J. Med. 2001, 345, 1435–1443. [Google Scholar] [CrossRef]
- Sanders, G.D.; Hlatky, M.A.; Owens, D.K. Cost-effectiveness of implantable cardioverter-defibrillators. N. Engl. J. Med. 2005, 353, 1471–1480. [Google Scholar] [CrossRef]
- Song, E.; Li, J.; Won, S.M.; Bai, W.; Rogers, J.A. Materials for flexible bioelectronic systems as chronic neural interfaces. Nat. Mater. 2020, 19, 590–603. [Google Scholar] [CrossRef]
- Mei, X.; Ye, D.; Zhang, F.; Di, C.-a. Implantable application of polymer-based biosensors. J. Polym. Sci. 2021, 60, 328–347. [Google Scholar] [CrossRef]
- Byun, S.H.; Sim, J.Y.; Zhou, Z.; Lee, J.; Qazi, R.; Walicki, M.C.; Parker, K.E.; Haney, M.P.; Choi, S.H.; Shon, A.; et al. Mechanically transformative electronics, sensors, and implantable devices. Sci. Adv. 2019, 5, eaay0418. [Google Scholar] [CrossRef]
- Lim, H.R.; Kim, H.S.; Qazi, R.; Kwon, Y.T.; Jeong, J.W.; Yeo, W.H. Advanced Soft Materials, Sensor Integrations, and Applications of Wearable Flexible Hybrid Electronics in Healthcare, Energy, and Environment. Adv. Mater. 2020, 32, e1901924. [Google Scholar] [CrossRef]
- Gray, M.; Meehan, J.; Ward, C.; Langdon, S.P.; Kunkler, I.H.; Murray, A.; Argyle, D. Implantable biosensors and their contribution to the future of precision medicine. Vet. J. 2018, 239, 21–29. [Google Scholar] [CrossRef]
- Ashraf, D.; Gehad Ismail, S.; Aboul Ella, H. The Impact of Implantable Sensors in Biomedical Technology on the Future of Healthcare Systems. In Intelligent Pervasive Computing Systems for Smarter Healthcare; Wiley: New York, NY, USA, 2019; pp. 67–89. [Google Scholar] [CrossRef]
- Hwang, S.W.; Tao, H.; Kim, D.H.; Cheng, H.; Song, J.K.; Rill, E.; Brenckle, M.A.; Panilaitis, B.; Won, S.M.; Kim, Y.S.; et al. A physically transient form of silicon electronics. Science 2012, 337, 1640–1644. [Google Scholar] [CrossRef]
- Koo, J.; MacEwan, M.R.; Kang, S.K.; Won, S.M.; Stephen, M.; Gamble, P.; Xie, Z.; Yan, Y.; Chen, Y.Y.; Shin, J.; et al. Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy. Nat. Med. 2018, 24, 1830–1836. [Google Scholar] [CrossRef]
- Kang, J.; Son, D.; Wang, G.N.; Liu, Y.; Lopez, J.; Kim, Y.; Oh, J.Y.; Katsumata, T.; Mun, J.; Lee, Y.; et al. Tough and Water-Insensitive Self-Healing Elastomer for Robust Electronic Skin. Adv. Mater. 2018, 30, e1706846. [Google Scholar] [CrossRef]
- Kang, J.; Tok, J.B.H.; Bao, Z.A. Self-healing soft electronics. Nat. Electron. 2019, 2, 144–150. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Song, S.; Kang, J.; Tsao, Y.; Chen, S.; Mottini, V.; McConnell, K.; Xu, W.; Zheng, Y.Q.; et al. Morphing electronics enable neuromodulation in growing tissue. Nat. Biotechnol. 2020, 38, 1031–1036. [Google Scholar] [CrossRef]
- Jeong, J.W.; McCall, J.G.; Shin, G.; Zhang, Y.; Al-Hasani, R.; Kim, M.; Li, S.; Sim, J.Y.; Jang, K.I.; Shi, Y.; et al. Wireless Optofluidic Systems for Programmable In Vivo Pharmacology and Optogenetics. Cell 2015, 162, 662–674. [Google Scholar] [CrossRef]
- Kang, S.K.; Murphy, R.K.; Hwang, S.W.; Lee, S.M.; Harburg, D.V.; Krueger, N.A.; Shin, J.; Gamble, P.; Cheng, H.; Yu, S.; et al. Bioresorbable silicon electronic sensors for the brain. Nature 2016, 530, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.D.; Karipott, S.S.; Wang, Y.; Ong, K.G. Wireless Technologies for Implantable Devices. Sensors 2020, 20, 4604. [Google Scholar] [CrossRef] [PubMed]
- Guillou, P.J.; Quirke, P.; Thorpe, H.; Walker, J.; Jayne, D.G.; Smith, A.M.; Heath, R.M.; Brown, J.M.; for the MRC CLASICC Trial Group. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): Multicentre, randomised controlled trial. Lancet 2005, 365, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, T.M.; Cheng, Z.; Hong, G.; Zhou, T.; Jin, L.; Duvvuri, M.; Jiang, Z.; Kruskal, P.; Xie, C.; et al. Syringe-injectable electronics. Nat. Nanotechnol. 2015, 10, 629–636. [Google Scholar] [CrossRef]
- Montgomery, M.; Ahadian, S.; Davenport Huyer, L.; Lo Rito, M.; Civitarese, R.A.; Vanderlaan, R.D.; Wu, J.; Reis, L.A.; Momen, A.; Akbari, S.; et al. Flexible shape-memory scaffold for minimally invasive delivery of functional tissues. Nat. Mater. 2017, 16, 1038–1046. [Google Scholar] [CrossRef]
- Qazi, R.; Gomez, A.M.; Castro, D.C.; Zou, Z.; Sim, J.Y.; Xiong, Y.; Abdo, J.; Kim, C.Y.; Anderson, A.; Lohner, F.; et al. Wireless optofluidic brain probes for chronic neuropharmacology and photostimulation. Nat. Biomed. Eng. 2019, 3, 655–669. [Google Scholar] [CrossRef]
- Whyte, W.; Roche, E.T.; Varela, C.E.; Mendez, K.; Islam, S.; O’Neill, H.; Weafer, F.; Shirazi, R.N.; Weaver, J.C.; Vasilyev, N.V.; et al. Sustained release of targeted cardiac therapy with a replenishable implanted epicardial reservoir. Nat. Biomed. Eng. 2018, 2, 416–428. [Google Scholar] [CrossRef]
- Kim, D.H.; Lu, N.; Ghaffari, R.; Kim, Y.S.; Lee, S.P.; Xu, L.; Wu, J.; Kim, R.H.; Song, J.; Liu, Z.; et al. Materials for multifunctional balloon catheters with capabilities in cardiac electrophysiological mapping and ablation therapy. Nat. Mater. 2011, 10, 316–323. [Google Scholar] [CrossRef]
- Swan, H.J.; Ganz, W.; Forrester, J.; Marcus, H.; Diamond, G.; Chonette, D. Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N. Engl. J. Med. 1970, 283, 447–451. [Google Scholar] [CrossRef]
- Park, S.; Guo, Y.; Jia, X.; Choe, H.K.; Grena, B.; Kang, J.; Park, J.; Lu, C.; Canales, A.; Chen, R.; et al. One-step optogenetics with multifunctional flexible polymer fibers. Nat. Neurosci. 2017, 20, 612–619. [Google Scholar] [CrossRef]
- Wang, S.; Xu, J.; Wang, W.; Wang, G.-J.N.; Rastak, R.; Molina-Lopez, F.; Chung, J.W.; Niu, S.; Feig, V.R.; Lopez, J.; et al. Skin electronics from scalable fabrication of an intrinsically stretchable transistor array. Nature 2018, 555, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.N.; Park, S.I.; Qazi, R.; Zou, Z.; Mickle, A.D.; Grajales-Reyes, J.G.; Jang, K.-I.; Gereau IV, R.W.; Xiao, J.; Rogers, J.A.; et al. Miniaturized, Battery-Free Optofluidic Systems with Potential for Wireless Pharmacology and Optogenetics. Small 2018, 14, 1702479. [Google Scholar] [CrossRef]
- Lu, T.; Ji, S.; Jin, W.; Yang, Q.; Luo, Q.; Ren, T.L. Biocompatible and Long-Term Monitoring Strategies of Wearable, Ingestible and Implantable Biosensors: Reform the Next Generation Healthcare. Sensors 2023, 23, 2991. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Akoum, N.; McGann, C.; Vergara, G.; Badger, T.; Ranjan, R.; Mahnkopf, C.; Kholmovski, E.; Macleod, R.; Marrouche, N. Atrial fibrosis quantified using late gadolinium enhancement MRI is associated with sinus node dysfunction requiring pacemaker implant. J. Cardiovasc. Electrophysiol. 2012, 23, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Celiz, A.D.; Yang, J.; Yang, Q.; Wamala, I.; Whyte, W.; Seo, B.R.; Vasilyev, N.V.; Vlassak, J.J.; Suo, Z.; et al. Tough adhesives for diverse wet surfaces. Science 2017, 357, 378–381. [Google Scholar] [CrossRef]
- Yuk, H.; Varela, C.E.; Nabzdyk, C.S.; Mao, X.; Padera, R.F.; Roche, E.T.; Zhao, X. Dry double-sided tape for adhesion of wet tissues and devices. Nature 2019, 575, 169–174. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Z.; Jiang, S.; Yan, B.; Shi, X.; Xie, Y.; Huang, X.; Yu, Z.; Liu, H.; Weng, S.; et al. A shape-memory and spiral light-emitting device for precise multisite stimulation of nerve bundles. Nat. Commun. 2019, 10, 2790. [Google Scholar] [CrossRef]
- Mendelson, Y. Biomedical Sensors. In Introduction to Biomedical Engineering; Enderle, J.D., Bronzino, J.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 609–666. [Google Scholar] [CrossRef]
- Long, N.; Yu, B.; Moussy, Y.; Moussy, F. Strategies for testing long-term transcutaneous amperometric glucose sensors. Diabetes Technol. Ther. 2005, 7, 927–936. [Google Scholar] [CrossRef]
- Frost, M.C.; Meyerhoff, M.E. Implantable chemical sensors for real-time clinical monitoring: Progress and challenges. Curr. Opin. Chem. Biol. 2002, 6, 633–641. [Google Scholar] [CrossRef]
- Zhang, X. Real time and in vivo monitoring of nitric oxide by electrochemical sensors—From dream to reality. Front. Biosci. 2004, 9, 3434–3446. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.D.; Greenberg, R.J. Microsensors and microbiosensors for retinal implants. Front. Biosci.-Landmark 2005, 10, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, M.; Kros, A.; Sprakel, V.; Lutterman, J.A.; Nolte, R.J.; Jansen, J.A. Biocompatibility evaluation of sol-gel coatings for subcutaneously implantable glucose sensors. Biomaterials 2000, 21, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ozlu, B.; Eom, T.; Martin, D.C.; Shim, B.S. Electrically conducting polymers for bio-interfacing electronics: From neural and cardiac interfaces to bone and artificial tissue biomaterials. Biosens. Bioelectron. 2020, 170, 112620. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Conducting Polymers in the Design of Biosensors and Biofuel Cells. Polymers 2021, 13, 49. [Google Scholar] [CrossRef]
- Sharkawy, A.A.; Klitzman, B.; Truskey, G.A.; Reichert, W.M. Engineering the tissue which encapsulates subcutaneous implants. III. Effective tissue response times. J. Biomed. Mater. Res. 1998, 40, 598–605. [Google Scholar] [CrossRef]
- Updike, S.J.; Shults, M.; Rhodes, R. Principles of long-term fully implanted sensors with emphasis on radiotelemetric monitoring of blood glucose from inside a subcutaneous foreign body capsule (FBC). In Biosensors in the Body: Continuous In Vivo Monitoring; Wiley: Hoboken, NJ, USA, 1997; pp. 117–137. [Google Scholar]
- Srinivasan, S.; Sawyer, P.N. Role of surface charge of the blood vessel wall, blood cells, and prosthetic materials in intravascular thrombosis. J. Colloid Interface Sci. 1970, 32, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Ainslie, K.M.; Desai, T.A. Microfabricated implants for applications in therapeutic delivery, tissue engineering, and biosensing. Lab Chip 2008, 8, 1864–1878. [Google Scholar] [CrossRef]
- Popat, K.C.; Eltgroth, M.; Latempa, T.J.; Grimes, C.A.; Desai, T.A. Decreased Staphylococcus epidermis adhesion and increased osteoblast functionality on antibiotic-loaded titania nanotubes. Biomaterials 2007, 28, 4880–4888. [Google Scholar] [CrossRef]
- Martin, F.; Walczak, R.; Boiarski, A.; Cohen, M.; West, T.; Cosentino, C.; Shapiro, J.; Ferrari, M. Tailoring width of microfabricated nanochannels to solute size can be used to control diffusion kinetics. J. Control. Release 2005, 102, 123–133. [Google Scholar] [CrossRef]
- Adiga, S.P.; Curtiss, L.A.; Elam, J.W.; Pellin, M.J.; Shih, C.C.; Shih, C.M.; Lin, S.J.; Su, Y.Y.; Gittard, S.A.; Zhang, J.; et al. Nanoporous materials for biomedical devices. JOM 2008, 60, 26–32. [Google Scholar] [CrossRef]
- Yeh, P.Y.; Le, Y.; Kizhakkedathu, J.N.; Chiao, M. An investigation of vibration-induced protein desorption mechanism using a micromachined membrane and PZT plate. Biomed. Microdevices 2008, 10, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Asuri, P.; Karajanagi, S.S.; Kane, R.S.; Dordick, J.S. Polymer-nanotube-enzyme composites as active antifouling films. Small 2007, 3, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Guo, Z.; Song, J.J.; Huang, Q.A.; Zhu, S.W.; Huang, X.J.; Wei, Y. Tunable nanogap devices for ultra-sensitive electrochemical impedance biosensing. Anal. Chim. Acta 2016, 905, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lee, C. NEMS diaphragm sensors integrated with triple-nano-ring resonator. Sens. Actuator A Phys. 2011, 172, 61–68. [Google Scholar] [CrossRef]
- Webster, T.J.; Waid, M.C.; McKenzie, J.L.; Price, R.L.; Ejiofor, J.U. Nano-biotechnology: Carbon nanofibres as improved neural and orthopaedic implants. Nanotechnology 2004, 15, 9. [Google Scholar] [CrossRef]
- Mozafari, M.; Moztarzadeh, F.; Seifalian, A.M.; Tayebi, L. Self-assembly of PbS hollow sphere quantum dots via gas-bubble technique for early cancer diagnosis. J. Lumin. 2013, 133, 188–193. [Google Scholar] [CrossRef]
- Smith, G.C.; Chamberlain, L.; Faxius, L.; Johnston, G.W.; Jin, S.; Bjursten, L.M. Soft tissue response to titanium dioxide nanotube modified implants. Acta Biomater. 2011, 7, 3209–3215. [Google Scholar] [CrossRef]
- Cheng, L.; Yuan, M.M.; Gu, L.; Wang, Z.; Qin, Y.; Jing, T.; Wang, Z.L. Wireless, power-free and implantable nanosystem for resistance-based biodetection. Nano Energy 2015, 15, 598–606. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, T.; Zwang, T.J.; Hong, G.; Zhao, Y.; Viveros, R.D.; Fu, T.M.; Gao, T.; Lieber, C.M. Bioinspired neuron-like electronics. Nat. Mater. 2019, 18, 510–517. [Google Scholar] [CrossRef]
- Lee, J.; Ihle, S.J.; Pellegrino, G.S.; Kim, H.; Yea, J.; Jeon, C.Y.; Son, H.C.; Jin, C.; Eberli, D.; Schmid, F.; et al. Stretchable and suturable fibre sensors for wireless monitoring of connective tissue strain. Nat. Electron. 2021, 4, 291–301. [Google Scholar] [CrossRef]
- Wang, L.; Xie, S.; Wang, Z.; Liu, F.; Yang, Y.; Tang, C.; Wu, X.; Liu, P.; Li, Y.; Saiyin, H.; et al. Functionalized helical fibre bundles of carbon nanotubes as electrochemical sensors for long-term in vivo monitoring of multiple disease biomarkers. Nat. Biomed. Eng. 2020, 4, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, D.; Nikiforidis, G.; Savva, A.; Giugni, A.; Wustoni, S.; Palanisamy, T.; Chen, X.; Maria, I.P.; Di Fabrizio, E.; Costa, P.; et al. Biofuel powered glucose detection in bodily fluids with an n-type conjugated polymer. Nat. Mater. 2020, 19, 456–463. [Google Scholar] [CrossRef]
- Wu, X.Y.; Feng, J.Y.; Deng, J.; Cui, Z.C.; Wang, L.Y.; Xie, S.L.; Chen, C.R.; Tang, C.Q.; Han, Z.Q.; Yu, H.B.; et al. Fiber-shaped organic electrochemical transistors for biochemical detections with high sensitivity and stability. Sci. China Chem. 2020, 63, 1281–1288. [Google Scholar] [CrossRef]
- Tang, C.; Xie, S.; Wang, M.; Feng, J.; Han, Z.; Wu, X.; Wang, L.; Chen, C.; Wang, J.; Jiang, L.; et al. A fiber-shaped neural probe with alterable elastic moduli for direct implantation and stable electronic-brain interfaces. J. Mater. Chem. B 2020, 8, 4387–4394. [Google Scholar] [CrossRef]
- Curry, E.J.; Le, T.T.; Das, R.; Ke, K.; Santorella, E.M.; Paul, D.; Chorsi, M.T.; Tran, K.T.M.; Baroody, J.; Borges, E.R.; et al. Biodegradable nanofiber-based piezoelectric transducer. Proc. Natl. Acad. Sci. USA 2020, 117, 214–220. [Google Scholar] [CrossRef]
- Kim, I.; Fok, H.H.; Li, Y.; Jackson, T.N.; Gluckman, B.J. Polymer substrate temperature sensor array for brain interfaces. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 3286–3289. [Google Scholar] [CrossRef]
- Rivas, L.; Dulay, S.; Miserere, S.; Pla, L.; Marin, S.B.; Parra, J.; Eixarch, E.; Gratacos, E.; Illa, M.; Mir, M.; et al. Micro-needle implantable electrochemical oxygen sensor: Ex-vivo and in-vivo studies. Biosens. Bioelectron. 2020, 153, 112028. [Google Scholar] [CrossRef]
- Dulay, S.; Rivas, L.; Miserere, S.; Pla, L.; Berdun, S.; Parra, J.; Eixarch, E.; Gratacos, E.; Illa, M.; Mir, M.; et al. in vivo Monitoring with micro-implantable hypoxia sensor based on tissue acidosis. Talanta 2021, 226, 122045. [Google Scholar] [CrossRef]
- Sun, K.; Ding, Z.; Zhang, J.; Chen, H.; Qin, Y.; Xu, S.; Wu, C.; Yu, J.; Chiu, D.T. Enhancing the Long-Term Stability of a Polymer Dot Glucose Transducer by Using an Enzymatic Cascade Reaction System. Adv. Healthc. Mater. 2021, 10, e2001019. [Google Scholar] [CrossRef]
- Mimee, M.; Nadeau, P.; Hayward, A.; Carim, S.; Flanagan, S.; Jerger, L.; Collins, J.; McDonnell, S.; Swartwout, R.; Citorik, R.J.; et al. An ingestible bacterial-electronic system to monitor gastrointestinal health. Science 2018, 360, 915–918. [Google Scholar] [CrossRef]
- Zhang, L.; Han, Y.; Zhao, F.; Shi, G.; Tian, Y. A selective and accurate ratiometric electrochemical biosensor for monitoring of Cu2+ ions in a rat brain. Anal. Chem. 2015, 87, 2931–2936. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Wang, N.; Lin, X.; Wang, Z.; Zhao, X.; Fang, P.; Yue, H.; Kim, J.; Luo, J.; Cui, S.; et al. Organic electrochemical transistor arrays for real-time mapping of evoked neurotransmitter release in vivo. eLife 2020, 9, e50345. [Google Scholar] [CrossRef] [PubMed]
- Morais, J.M.; Papadimitrakopoulos, F.; Burgess, D.J. Biomaterials/tissue interactions: Possible solutions to overcome foreign body response. AAPS J. 2010, 12, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.P.; Pathak, C.P.; Heller, A.; Hubbell, J.A. Photo-crosslinked copolymers of 2-hydroxyethyl methacrylate, poly(ethylene glycol) tetra-acrylate and ethylene dimethacrylate for improving biocompatibility of biosensors. Biomaterials 1995, 16, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, N.; Reichert, M. Methods for reducing biosensor membrane biofouling. Colloids Surf. B Biointerfaces 2000, 18, 197–219. [Google Scholar] [CrossRef]
- Norton, L.W.; Koschwanez, H.E.; Wisniewski, N.A.; Klitzman, B.; Reichert, W.M. Vascular endothelial growth factor and dexamethasone release from nonfouling sensor coatings affect the foreign body response. J. Biomed. Mater. Res. A 2007, 81, 858–869. [Google Scholar] [CrossRef]
- Vaddiraju, S.; Wang, Y.; Qiang, L.; Burgess, D.J.; Papadimitrakopoulos, F. Microsphere erosion in outer hydrogel membranes creating macroscopic porosity to counter biofouling-induced sensor degradation. Anal. Chem. 2012, 84, 8837–8845. [Google Scholar] [CrossRef]
- Ward, W.K.; Slobodzian, E.P.; Tiekotter, K.L.; Wood, M.D. The effect of microgeometry, implant thickness and polyurethane chemistry on the foreign body response to subcutaneous implants. Biomaterials 2002, 23, 4185–4192. [Google Scholar] [CrossRef]
- Lee, D.; Park, K.; Seo, J. Recent Advances in Anti-inflammatory Strategies for Implantable Biosensors and Medical Implants. BioChip J. 2020, 14, 48–62. [Google Scholar] [CrossRef]
- Webb, R.C.; Bonifas, A.P.; Behnaz, A.; Zhang, Y.; Yu, K.J.; Cheng, H.; Shi, M.; Bian, Z.; Liu, Z.; Kim, Y.S.; et al. Ultrathin conformal devices for precise and continuous thermal characterization of human skin. Nat. Mater. 2013, 12, 938–944. [Google Scholar] [CrossRef]
- Rebelo, R.; Barbosa, A.I.; Correlo, V.M.; Reis, R.L. An Outlook on Implantable Biosensors for Personalized Medicine. Engineering 2021, 7, 1696–1699. [Google Scholar] [CrossRef]
- Sadik, O.A.; Aluoch, A.O.; Zhou, A. Status of biomolecular recognition using electrochemical techniques. Biosens. Bioelectron. 2009, 24, 2749–2765. [Google Scholar] [CrossRef] [PubMed]
- Erdem, A.; Karadeniz, H.; Caliskan, A. Single-Walled Carbon Nanotubes Modified Graphite Electrodes for Electrochemical Monitoring of Nucleic Acids and Biomolecular Interactions. Electroanalysis 2009, 21, 464–471. [Google Scholar] [CrossRef]
- Abdur Rahman, A.R.; Justin, G.; Guiseppi-Elie, A. Towards an implantable biochip for glucose and lactate monitoring using microdisc electrode arrays (MDEAs). Biomed. Microdevices 2009, 11, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Steeves, C.A.; Young, Y.L.; Liu, Z.; Bapat, A.; Bhalerao, K.; Soboyejo, A.B.; Soboyejo, W.O. Membrane thickness design of implantable bio-MEMS sensors for the in-situ monitoring of blood flow. J. Mater. Sci. Mater. Med. 2007, 18, 25–37. [Google Scholar] [CrossRef]



| Clinical Application | Advantages | Disadvantages |
|---|---|---|
| Brain stimulator |
|
|
| Heart failure monitoring |
|
|
| Blood glucose level |
|
|
| Cancer treatment |
|
|
| Post-market surveillance |
|
|
| Human factor studies |
|
|
| Nanomaterial | Outcome | Ref. |
|---|---|---|
| Tunable gold nanogap | Ultra-sensitive electrochemical impedance biosensor for detection of streptavidin. | [57] |
| Si and Si/SiO2 | Round diaphragm pressure sensors. | [58] |
| Carbon nanofiber | Nano-implant for neural tissues monitoring, diagnosis, and treatment. | [59] |
| PbS hollow sphere QDs | Early-stage cancer diagnostics and treatment of ophthalmic diseases. | [60] |
| Ultrananocrystalline diamond | Implantable retinal microchip. | [4] |
| TiO2 nanotubes | Soft-tissue responsive sensor. | [61] |
| BZT-BCT NWs/PDMs nanocomposite | Biodection of resistance with an implantable, wireless, and power-free nanosystem. | [62] |
| PLGA nanoporous Si composite | Bioresorbable sensor for the brain. | [22] |
| Aspect | Impact on Healthcare Providers | Impact on General Public |
|---|---|---|
| Daily monitoring of patients’ physiology | By receiving daily information on patients’ physiology, practitioners can decrease the volume of patients at congested hospitals and health centers while diagnosing health conditions at an earlier stage. | Implantable biosensors can provide people with firsthand knowledge of which specific behaviors could adversely impact their health, leading to increased self-awareness and behavioral change. |
| Personalized medicine | The collection of patient information from biosensors would enable patient stratification, leading to more effective treatments and facilitating the creation of a predictive, preventive, and participatory medical follow-up system. | The discovery of more efficient medical treatments based on patients’ physiological, genetic, and demographic characteristics through the use of implantable biosensors would result in better healthcare outcomes. |
| Big data analysis | The patient information gleaned from biosensors could be utilized for big data analysis, where variables such as socio-demographics, medical conditions, genetics, and treatments would be scrutinized, leading to the identification of new trends. | Better understanding of the relationship between lifestyle and health. |
| Feeling of control | Implantable biosensors can empower individuals to take charge of their health, thereby reducing their stress levels, a known contributing factor to various chronic conditions. | Improved mental health and wellbeing. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghorbanizamani, F.; Moulahoum, H.; Guler Celik, E.; Timur, S. Material Design in Implantable Biosensors toward Future Personalized Diagnostics and Treatments. Appl. Sci. 2023, 13, 4630. https://doi.org/10.3390/app13074630
Ghorbanizamani F, Moulahoum H, Guler Celik E, Timur S. Material Design in Implantable Biosensors toward Future Personalized Diagnostics and Treatments. Applied Sciences. 2023; 13(7):4630. https://doi.org/10.3390/app13074630
Chicago/Turabian StyleGhorbanizamani, Faezeh, Hichem Moulahoum, Emine Guler Celik, and Suna Timur. 2023. "Material Design in Implantable Biosensors toward Future Personalized Diagnostics and Treatments" Applied Sciences 13, no. 7: 4630. https://doi.org/10.3390/app13074630
APA StyleGhorbanizamani, F., Moulahoum, H., Guler Celik, E., & Timur, S. (2023). Material Design in Implantable Biosensors toward Future Personalized Diagnostics and Treatments. Applied Sciences, 13(7), 4630. https://doi.org/10.3390/app13074630






