Abstract
According to a recent report by the WHO, 50–80 million people suffer with infertility. Amongst these populations, male counterparts account for 20–50% of infertility cases. The aetiology of infertility in men includes many factors such as psychological issues, lifestyle and environmental factors, hormonal disorders and chromosomal abnormalities. The pathophysiology of these aetiologies may be initiated by a local inflammatory reaction increasing reactive oxygen species (ROS) production, which can negatively affect the male reproductive system by altering the hypothalamic–pituitary–gonadotropin axis (HPG axis). Alteration of the HPG axis may affect testicular steroidogenesis, spermatogenesis, the Leydig cells and Sertoli cells, leading to poor semen quality. The prevalence of male infertility underscores the need for a thorough scientific investigation to identify treatable or reversible factors using plant adjuvants with antioxidative properties. Therefore, this review aims to provide an overview of the currently available knowledge on the aetiologies of male reproductive dysfunction, emphasising infertility risk factors, as well as elucidating the possible ways by which readily available alternatives, such as Moringa oleifera leaves, may mitigate male infertility by highlighting its role on the oxidative stress parameters, reproductive hormonal levels, testicular steroidogenesis and spermatogenesis, gene expression, weight and morphology of the testis and sperm parameters.
1. Introduction
A global challenge affecting humankind lies in understanding, preventing and managing ever-increasing male infertility. The world health organization (WHO) defines infertility as the inability to conceive after a year of regular unprotected sexual intercourse [1]. Infertility affects all genders, and its prevalence among couples ranges from 10 to 15% globally, with male factors contributing 20–50% of all infertility cases [2,3]. In addition, male infertility causes substantial psychological and social distress and imposes a considerable economic burden on the health care system [3].
Many causes and risk factors contribute to the overarching incidence of male infertility, graded as idiopathic, acquired and congenital [3]. Previous reports estimate that about 30–50% of male infertility patients are idiopathic [2,4]. In addition, infertile couples experience many emotions, including depression, anger and shame. Furthermore, they are sometimes mocked, embarrassed and even pressured by peers, friends and parents, particularly in societies with high expectations for bearing children after marriage [4]. Acquired factors of male infertility include varicocele, testicular trauma, testicular torsion, recurrent urogenital infections and acquired secondary hypogonadism [5]. Among these factors, varicocele is the most common cause of infertility in men, with a prevalence of 40% [5]. In addition, congenital causes of male infertility arise from the congenital bilateral absence of the vas deferens associated with cystic fibrosis, gene mutations and chromosomal abnormalities leading to the deterioration of testicular function and Y chromosome microdeletions resulting in isolated spermatogenic defects [6]. These risk factors may also cause excessive accumulation of reactive oxygen species (ROS) in cells and induce damage to the reproductive function by releasing inflammatory cytokines and, eventually, oxidative stress [7]. ROS affect sperm parameters directly or indirectly by impairing male reproductive hormones, cells and organs [7].
Some factors leading to male infertility can be surgically reversed or therapeutically ameliorated with drugs [8]. However, treatment options depend on the cause of male infertility, the patient’s age, financial status, facilities available in a designated hospital and expertise [9]. The primary treatment for male factor infertility is intrauterine insemination (IUI), tubal and male ejaculatory duct cryosurgery, in-vitro fertilisation (IVF) and embryo transfer with or without intracytoplasmic sperm injection (ICSI) [9]. In addition, psychosexual counselling, vacuum constriction device (penis pump), diet, exercise, weight loss, approved phosphodiesterase type 5 (PDE5) inhibitors drugs, including Viagra (Sildenafil), Cialis (Tadalafil) and Levitra (Vardenafil) and Stendra (Avanafil), apomorphine and intracavernosal injection therapies as well as medicinal plants, such as Pausinystalia yohimbe and Tribulus terrestris, are used for the treatment of erectile dysfunction [8,10]. Furthermore, testosterone replacement therapy (TRT), aromatase inhibitors and human chorionic gonadotrophin (hCG) therapy [11,12] are used for the treatment of primary hypogonadism. Most of these male infertility treatment options have been deemed adequate. However, they also have their downsides regarding side effects, costs, invasiveness of the administration method and availability, particularly in African countries [8,9,11,12]. Therefore, medicinal herbs may be recommended to treat male reproductive impairment, as these herbs enhance reproductive functions [13,14].
M. oleifera (drumstick tree, horseradish tree, ben-oil tree or kelor tree) is of the family ‘Moringaceae’ [15,16,17]. It originates from the sub-Himalayan tracts of India, Pakistan, Bangladesh and Afghanistan and can grow in subtropical and tropical climates [18]. In Southern Asia and Middle East Africa, the tree is mainly cultivated for its various nutraceutical and medicinal properties contained in different parts of the tree, such as seeds, roots, flowers, pots and leaves [19]. In vivo and in vitro studies have been conducted to determine the effects of M. oleifera leaves on the male reproductive system [14,20,21,22,23]. This review highlights the mitigating action of M. oleifera on some of the risk factors that lead to male infertility.
2. Male Infertility
There is increasing evidence of the progressive decline in human fertility in both developed and developing countries, with a variable prevalence [2]. For instance, the prevalence estimate of infertility is 6% in the United States of America (USA), 10–15% in the United Kingdom (UK) and 20–46% in sub-Saharan Africa, with the African continent having an overall infertility rate of 41.91% among males and females, in which male infertility contributes to 22.26% [3,6,24].
Male infertility is commonly caused by reduced semen quality characterised by low sperm count (oligozoospermia), impaired motility of the sperm (asthenozoospermia), decreased vitality of the sperm (necrozoospermia), impaired morphology of the sperm (teratozoospermia) or a combination of these parameters termed oligoasthenoteratozoospermia and azoospermia [2]. In total, 90% of male infertility problems are linked to sperm count, and there is a link between reduced sperm count and reduced semen quality [6]. In addition, a cohort study of South African men of reproductive age indicated in 34.2% of the sub-fertile men, 11.9% had severe azoospermia [25].
Several risk factors can disrupt semen quality leading to semen-related abnormalities [6]. Oxidative stress has been linked to male infertility due to enhanced reactive oxygen species (ROS) production or decreased antioxidants. ROS leads to infertility through two fundamental mechanisms. Firstly, by inducing sperm membrane damage, which decreases sperm motility and its fertilisation capacity, as well as by altering the DNA molecule of sperm, resulting in the passage of defective paternal DNA on to the conceptus [7].
3. Oxidative Stress
Oxidative stress (OS) refers to an imbalance between the production and accumulation of ROS and the biological system’s ability to detoxify these ROS in cells and tissues [26]. These ROS are radical and nonradical oxygen species that form through partial oxygen reduction within the mitochondria [27]. Oxidative stress contributes to multiple pathological conditions and diseases [28]. For example, moderate oxidative stress may cause cell dysfunction and altered behaviour, such as accelerated senescence, abnormal proliferation, dysregulated inflammatory responses and cell tumorigenesis. In contrast, high OS usually causes cell death (e.g., oncosis, apoptosis and autophagy) [29].
Cells have an antioxidant defence system that is made up of enzymes (e.g., superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR) and non-enzymatic molecules (e.g., ascorbic acid, tocopherol, carotene) that can neutralise or scavenge ROS [30]. However, with the abundance of exogenous and endogenous sources of ROS, reproductive cells, in particular, lose the ability to balance the levels of oxidants and antioxidants over time, leading to damage to cellular constituents, such as lipids, DNA and proteins [31,32], which further complicates the functions of the reproductive cells and lead to male infertility [33].
For instance, OS is a significant cause of sperm cell dysfunction and contributes to the aetiology of male infertility due to the impairment of both spermatozoa’s structural and functional integrity [34,35]. It disrupts the integrity of the DNA because of concurrent damage to proteins and lipids present in the sperm cell plasma membrane, affecting cell membrane fluidity and permeability [36]. The presence of high levels of DNA damage in human spermatozoa has been correlated with adverse clinical outcomes, including recurrent pregnancy loss, dominant genetic disorders and infertility.
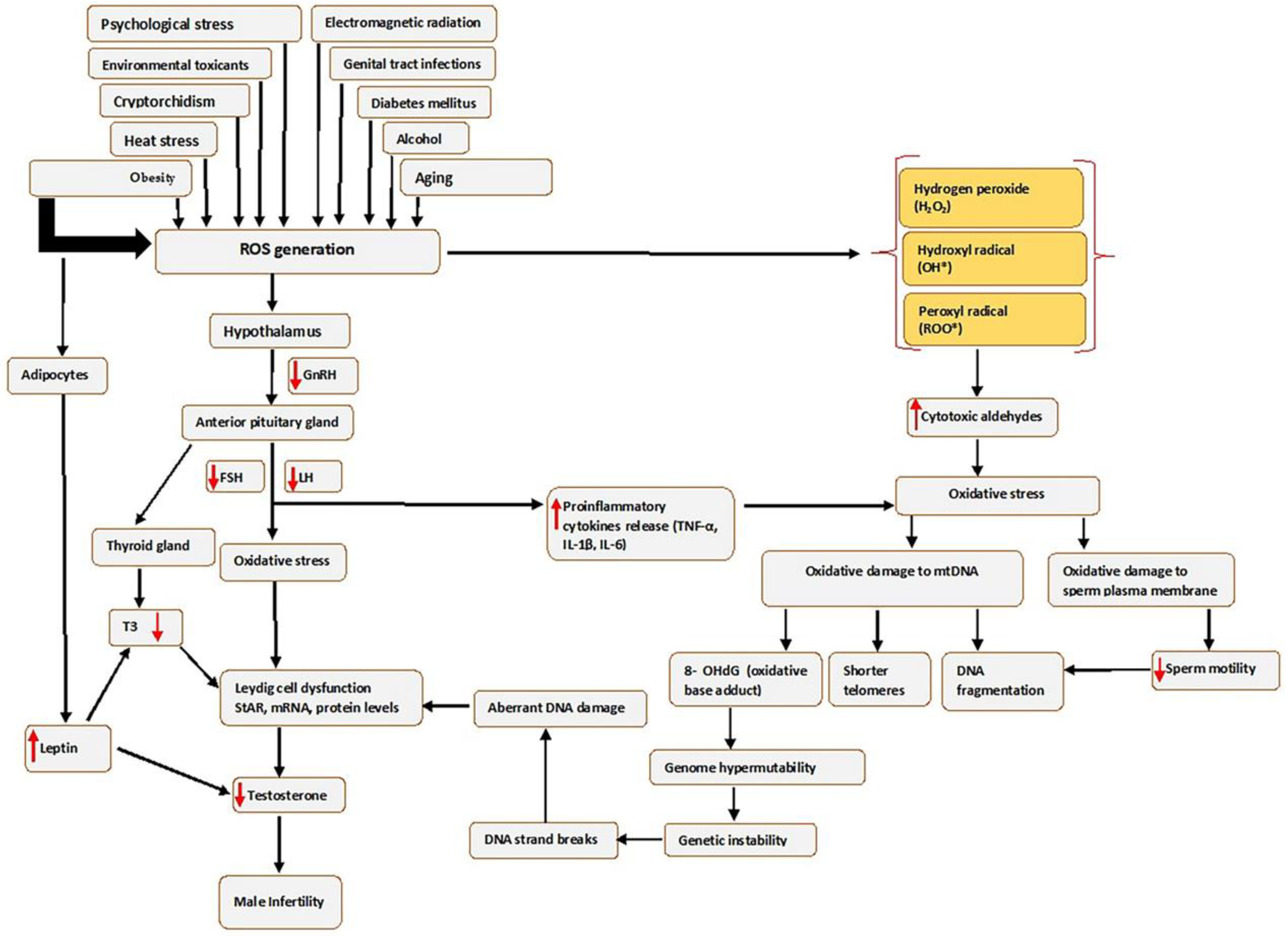
Oxidative stress affects sperm function in two ways: by damaging the sperm nuclear and mitochondrial DNA (mtDNA), which is associated with shorter telomere length, formation of the oxidative base adduct 8-hydroxy-deoxyguanine (8-OHdG) and fragmentation of mitochondrial DNA; or by damaging the sperm plasma membrane and thus affecting sperm motility and its ability to fuse with the oocyte often leading to genome hypermutability, genetic instability, single-strand and double-strand breaks, aberrant DNA damage, Leydig cell dysfunction and finally leading to infertility (see Figure 1).
Figure 1.
Causes of seminal oxidative stress and oxidative DNA damage. Various factors can lead to or affect the generation of reactive oxygen species (ROS) in the male germ line, which creates oxidative stress. Abbreviations: FSH-Follicle stimulating hormone; IL-6-interleukin 6; LH-luteinizing hormone; mRNA-messenger RNA; ROS, Reactive oxygen species; StAR-steroidogenic acute regulatory protein; TNF-α-Tumour necrosis factor alpha; T3, Triiodothyronine; 8 OHdG-8-hydroxy-2′-deoxyguanosine. Red arrows pointing up indicates increase; red arrows pointing downwards indicates a decrease.
Furthermore, the risk factors that increase ROS in the male genital tract can create an imbalance in the production of oxidants and the scavenging capacity of antioxidant enzymes, consequently leading to OS, as illustrated in Figure 1. In addition, high levels of ROS may disrupt the hormonal balance that regulates male reproductive functions by acting on the hypothalamic–pituitary–gonadotropic (HPG) axis, thus reducing the secretion of LH and FSH from the anterior pituitary gland [37]. The reduced LH secretion results in failure to stimulate Leydig cells to produce sufficient testosterone [38], while reduced FSH negatively affects the release of androgen-binding protein (ABP), which helps in concentrating testosterone. This causes an overall decrease in Leydig cells’ function and, in turn, affects the proteins mediating cholesterol uptake into the mitochondria, such as the steroidogenic acute regulatory (StAR) protein, or by increasing concentrations of inflammatory cytokines [39], and consequently decreasing circulating testosterone [40], which results in unregulated spermatogenesis and suppression of sexual behaviour [7].
In addition, obesity results in the excessive production of ROS by stimulating the adipocyte cells to produce leptin, the critical regulatory adipokine [41]. The increased leptin secretion may also decrease testosterone production by the Leydig cells through altering the endocrine regulation [42], which is mediated primarily through the hypothalamic–pituitary–gonadal (HPG) axis [43], which affects the release of hypothalamic GnRH, FSH and LH. This will activate OS through cellular metabolisms, negatively impacting the differentiation processes of germ cells. The obesity-induced testicular OS explains this scenario.
Moreover, obesity may induce testicular OS via other potential mechanisms, such as increased fatty acid oxidation in mitochondria and peroxisomes by adipose tissue, which may lead to a higher generation of ROS. Thus, more elevated ROS mediates oxidative damage to biomolecules that include lipids, proteins and DNA. These cause oxidation of polyunsaturated fatty acids in the sperm membrane, loss of the mitochondrial membrane potential and single- and double-strand sperm DNA fragmentation (SDF) [44]. OS may also impact the hypothalamic–pituitary–thyroid (HPT) axis by decreasing the secretion of triiodothyronine (T3) and triiodothyronine (T4). Reduction in T3 lowers the levels of StAR, mRNA and protein in Leydig cells and reduces the generation of testosterone [45].
Spermatozoa are susceptible to OS because their plasma membrane contains an abundance of polyunsaturated fatty acids (PUFAs). The PUFAs in sperm are required to create fluidity, which is crucial for sperm motility, acrosome reaction and egg fertilisation [46]. An increase in unsaturated fatty acid content is associated with ROS generation that results in a decline in sperm motility [47] either through the ability of H2O2 to diffuse across the membranes and inhibit the activity of several enzymes crucial for the sperm movement [48], or through inhibition of phosphorylation of axonemal proteins and subsequent sperm immobilisation [49]. High levels of ROS in the spermatozoa perpetuate a lipid peroxidation (LPO) cascade and ultimately drive these cells into a state of oxidative disintegration of DNA and proteins [50].
3.1. DNA Damage in Reproductive Cells
Reactive oxygen species cause various types of damage to DNA [1] due to their ability to oxidise the guanine base, which yields a pre-mutagenic 8-oxo-7,8-dihydroguanine (8-oxoG) [51]. 8-oxo-7,8-dihydroguanine (8-oxoG) can convert into single or double-strand breaks (SSBs or DSBs) [52]. DNA double-strand breaks induce several chromatin changes in the promoter, initiating permanent gene silencing in a small fraction of the repaired genes [53]. In the Leydig cells, the exogenous sources of ROS, such as heavy metals, particularly cadmium, showed that DNA damage caused by the accumulation of ROS might affect the expression and catalytic reactions of steroidogenic enzymes, such as 3βB-HSD, which is crucial for testosterone synthesis along with 17βB-HSD and P450c17A [54]. In addition, DNA damage in male germ cells impairs spermatogenesis, and mitochondrial DNA damage in sperm cells negatively affects motility associated with male infertility [55].
3.2. Lipid Peroxidation in Reproductive Cells
Lipid peroxidation (LPO) refers to the process whereby oxidants such as ROS attack the lipids, particularly the PUFAs, that involve hydrogen removal from carbon [56]. The LPO produces lipid hydroperoxides (LOOHs) as main products, and aldehydes, such as malondialdehyde (MDA), propanal, hexanal, and 4-hydroxynonenal (4-HNE), as secondary products [57]. In addition, lipid hydroperoxides may decompose in vivo through one-electron reduction and participate in initiation or propagation steps, induce new lipid hydroperoxides, and feed the lipid peroxidation process. All these mechanisms can contribute to peroxidative damage induction and loss of cell viability with increasing 7-OOH concentration in reproductive cells [58]. In spermatozoa, MDA molecules penetrate the cell membrane structure and impair the symmetric distribution of lipid membrane components. Lipid peroxidation disrupts the middle section of the sperm cell and causes loss of acrosome capacity for fertilisation [59].
3.3. Protein Oxidation in Reproductive Cells
Oxidative stress also causes damage to proteins, which involves modification of amino acids in a site-specific manner, fragmentation of the peptide chain, aggregation of the cross-linked reaction products, alteration of the electric charge, inactivation of an enzyme and susceptibility to proteolysis [26,56], which could disrupt enzymes responsible for steroidogenesis within the Leydig cells [33]. In addition, proteins can be damaged through post-translational modification processes such as oxidation facilitated by ROS [60]. For example, a study by Diemer et al. [61] demonstrated that ROS inhibited StAR protein post-transcriptionally in MA-10 Leydig cells, which inhibits cholesterol transport into the mitochondria and inhibits steroidogenesis.
4. Moringa oleifera
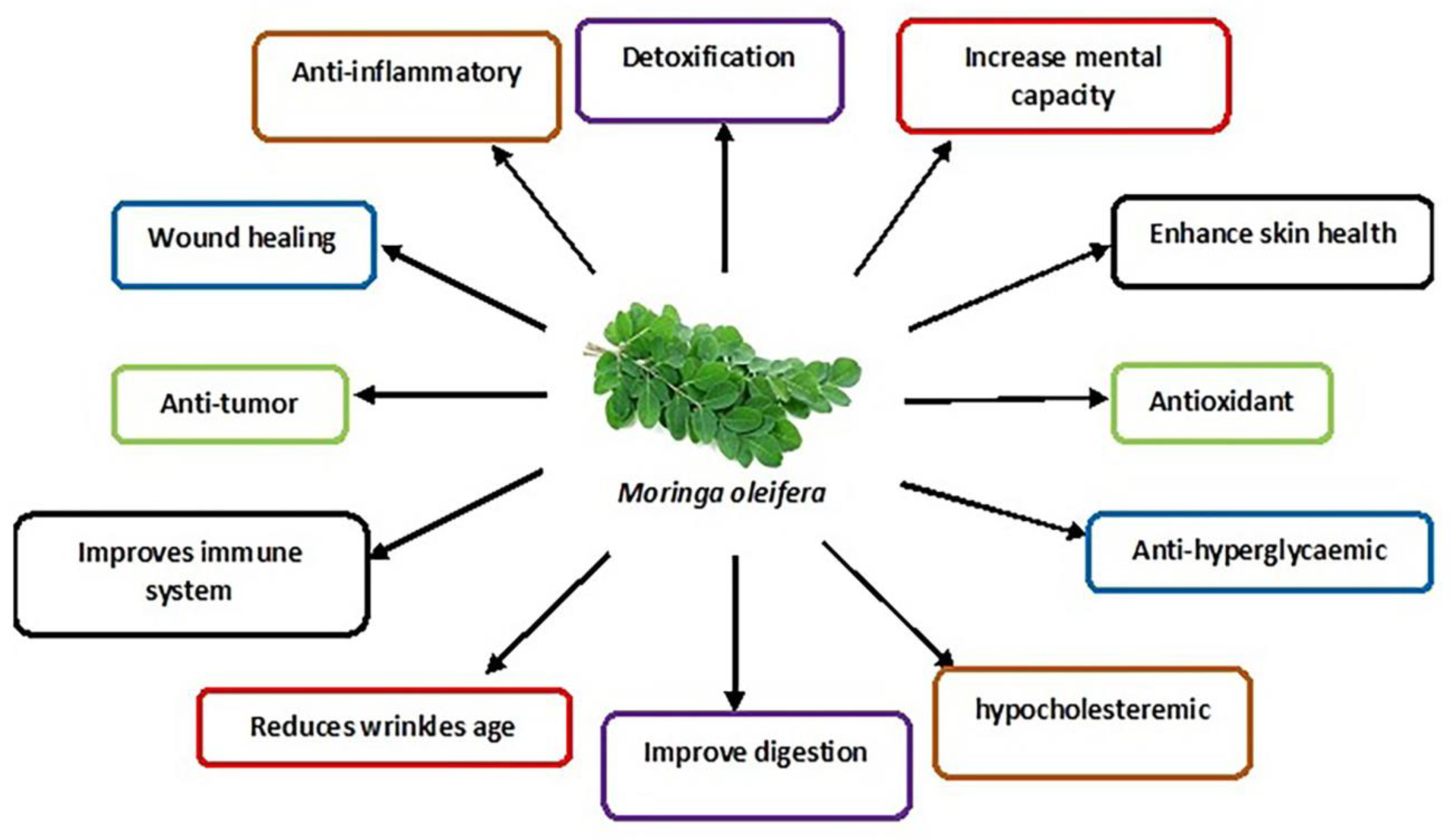
The M. oleifera tree is mainly cultivated for its various uses as an essential herb due to its nutraceutical and medicinal properties [62]. The different parts of the tree, such as the roots, flowers, fruits, seeds and leaves, are traditionally used to treat abdominal tumours, hysteria, scurvy, paralysis, helminthic bladder, prostate problems, sores and skin infections [19]. In addition, its leaves are commonly used as they contain many bioactive compounds such as nutrients and phytonutrients [63]. The properties of M. oleifera leaves are summarised in Figure 2.
Figure 2.
Schematic diagram showing various medicinal uses of M. oleifera leaves.
These nutrients include proteins, vitamins (E, C, beta-carotene, B-6), minerals (calcium, phosphorus, magnesium etc.) and fatty acids [64]. The intrinsic bioactive phytonutrients include flavonoids, phenolic acids and glycosides. Other groups include saponins, alkaloids, tannins, isothiocyanate and glucosinolate [17]. The flavonoid group mainly include quercetin and kaempferol, with a concentration of up to 137.81 and 106.75 mg/g, respectively, found in the M. oleifera leaves. The phenolic acid in the M. oleifera leaves includes ferulic acid, gallic acid, vanillic and ellagic acid, with chlorogenic acid being the most abundant [14,65].
M. oleifera leaves show significant protective effects against many diseases [16] and the widely persistent environmental toxins that disrupt cellular metabolic function [66]. M. oleifera is even used to treat neuro-dysfunctional diseases such as Alzheimer’s, ischaemic stroke and epilepsy [14]. Studies have also confirmed its potential treatment in chronic diseases such as diabetes mellitus (insulin resistance and hyperglycaemia) [67] and high blood pressure [64]. In addition, M. oleifera is a preventive strategy for various conditions and diseases, e.g., preventing testosterone-induced benign prostate hyperplasia [67]. Furthermore, M. oleifera has been used to enhance male sexual functions, including libido, erectile dysfunction and testicular injury [14,68], the symptoms of primary hypogonadism that mostly leads to male infertility.
5. Studies on the Effects of M. oleifera Leaf Extracts on Male Reproductive Function
5.1. Sperm Parameters
M. oleifera significantly affects the improvement of sperm characteristics; this is demonstrated by the Egyptian buffalo bulls fed with 4% and 8% concentrations of M. oleifera leaves in their diet [69]. Furthermore, M. oleifera has been shown to reduce sperm abnormality in male Swiss mice fed with 4% and 8% M. oleifera in their diet [70]. In addition, rabbit bucks administered with 200 and 400 mg/kg B.W. showed an increase in sperm viability, sperm membrane integrity and sperm motility [20]. Finally, Bali bulls fed with a diet containing 15% M. oleifera demonstrated increased sperm motility [14].
5.2. Hormonal Levels
M. oleifera significantly increased serum testosterone levels and gene expressions for luteinizing hormone and follicle-stimulating hormone in Rabbit bucks [20]. However, in buffalo bulls [69] and Bali bulls [14], there is only an increase in testosterone with no effect on LH and FSH. Contrastingly, M. oleifera leaf extract increased the concentrations of FSH and LH in New Zealand white (NZW) rabbit bucks. The extracts also increased semen volume, sperm count and motility [71].
5.3. Testis
Administration of M. oleifera improved the testicular structure of rabbit bucks. High doses of moringa leaves led to germinal hyperactivity of cells, such as the increase in the number of cells at all stages of spermatogenesis, increased density of spermatids, prominence of spermatozoa in the lumen of seminiferous tubules and wider interstitial areas with normal Leydig cells compared to non-supplemented rabbit bucks [20]. Additionally, a significant increase in seminiferous tubule diameter, height and epithelium area, type A spermatogonia and spermatogenic efficiency, as well as the increased number of Sertoli cells and total spermatogenic cells was demonstrated following treatment in male rats [72]. In addition, a significant increase in relative testis, epididymis and seminal vesicle weight, and diameter of the seminiferous tubules was observed in male-treated mice [21].
5.4. Male Reproductive Cells: Leydig Cells and Sperm Cells
M. oleifera leaf extracts (10, 50, 100, 250, 500 and 1000 ug/mL) on TM3 Leydig cells increased the levels of testosterone under stimulatory conditions of hCG. However, the testosterone increase was seen only in 500 and 1000 ug/mL concentrations under basal conditions [22]. In addition, M. oleifera also demonstrated its antioxidative effects by increasing the glutathione concentration in the cells exposed to 250 ug/mL of the extract [22].
M. oleifera leaf extracts were also observed on sperm cells with varying concentrations (0.625; 6.25; 62.5; 625 ug/mL). The findings indicated that M. oleifera inhibited the formation of sperm intracellular ROS at 62.5 and 625 ug/mL, reduced the percentage of sperm with DNA fragmentation and increased the percentage of incapacitated and intact acrosome spermatozoa at 625 ug/mL [73].
6. Effects of M. oleifera Leaf Extracts on Male Reproductive System Constituents following Exposure to Male Infertility Risk Factors
Table 1 summarises the effect of the M. oleifera on the male reproductive system following exposure to various infertility risk factors.

Table 1.
Effects of M. oleifera leaves on damage induced by male infertility risk factors in the male reproductive system.
6.1. Environmental Toxicants and Heavy Metals
Environmental toxicants have adverse effects on male reproductive potential. For example, these contaminants affect the quality of the semen. In addition, they disrupt the Leydig and Sertoli cells, disrupting DNA integrity, hormone biosynthesis, gene expression and epigenetic modifications [7]. These contaminants also reduce testis size, leading to testicular dysfunction [11]. Table 2 summarises the respective treatment of M. oleifera on different models, length and mode of treatment.

Table 2.
Male infertility risk factors studied, M. oleifera extract, model of the study, length and mode of treatment.
Some environmental toxicants damage the reproductive system via the induction of oxidative stress [11]. M. oleifera leaf extracts in animal models exposed to environmental toxicants have demonstrated their antioxidative capacity by significantly increasing superoxide dismutase (SOD) and catalase (CAT) levels and reducing malondialdehyde (MDA) molecules [85]. M. oleifera also increased the testicular levels of glutathione peroxidase (GPx) [81,84] and reduced glutathione S-transferase (GST) activity [82].
M. oleifera leaf extract increased the concentration of serum testosterone, FSH and LH [84,85]. M. oleifera also increased testicular testosterone levels in male rats [81,82]. Additionally, M. oleifera leaf extracts upregulated CYP11A1 and HSD17B3 genes of the steroidogenesis hormones downregulated CYP19A1 aromatase gene [84] and upregulated StAR and cytochrome p450c17 subfamily A (CYP17A) genes [82].
M. oleifera leaf extracts also demonstrated its beneficial effects on semen quality of male rats by increasing the percentage of viable and normal spermatozoa [85], sperm count, sperm motility and reduced sperm abnormality [82,84] and reduced sperms with morphological damage [81].
M. oleifera leaf extracts in male rats exposed to environmental toxicants increased the weight, volume of the testis [81] and gonadosomatic index [84]. The extracts also preserved the Leydig cells and prevented the disruption of the seminiferous tubules by heavy metals [83]. More so, M. oleifera leaf extract increased elongated spermatids and spermatozoa in the seminiferous tubules. The epididymal histological integrity and sperm density were also improved, while congestion and interstitial oedema reduction were noted [82]. Lastly, M. oleifera leaf extract reduced the vacuolisation of germinal epithelium [84].
6.2. Electromagnetic Radiations
There is evidence the long-term exposure to electromagnetic radiation causes an increase in the production of reactive oxygen species (ROS) in organs of the reproductive system [7]. These radiations have been reported to reduce sperm motility, cause sperm defects, increase peroxidation, histological aberrations in the testicular tissue and testicular tissue atrophy [97]. Additionally, these radiations affect the HPA axis by increasing the adrenocorticotropic hormone (ACTH) secretion from the anterior pituitary gland, which stimulates the production of cortisol form the adrenal cortex [7]. Cortisol then suppresses testosterone secretion from the Leydig cells [98].
Studies on the effects of M. oleifera leaf extracts on male Wistar rats exposed to electromagnetic radiation have demonstrated a reduction in oxidative damage in vivo (see Table 1), as indicated by the reduced MDA, increased CAT and SOD activities [78,79]. In addition, M. oleifera leaf extracts also increased serum testosterone levels [79], increased sperm parameters (epididymal sperm count and motility) and reduced sperm defects (pyriform head, detached head, coiled tails and multiple abnormalities) [78].
In another study, M. oleifera leaf extracts on adult white albino male rats exposed to an electromagnetic field reduced the degeneration of some parts of the seminiferous tubules. As a result, they increased the number of Leydig cells [80]. In addition, the testes’ morphology was almost normal, as seen in the histoarchitecture of the seminiferous tubules and Sertoli cells [80].
6.3. Heat Stress
Heat stress on the testis enhances the production of ROS and reduces the enzyme defence system activities, reducing the semen quality of males. Heat stress also increases NADPH oxidase activity and disrupts the homeostasis of mitochondria of the reproductive cells [7]. Additionally, heat stress negatively impacts the male HPG-axis through impairment of the normal release of hypothalamic GnRH and LH and FSH from the pituitary gland, decreasing serum circulating testosterone. In the testis, heat stress leads to Leydig cell apoptosis and the reduction in testicular testosterone synthesis [7].
A study conducted on human subjects to determine the correlation between heat stress and semen quality among male workers in the steel industry found that workers exposed to heat had poor semen quality compared to unexposed subjects [11]. Additional studies observed that increased scrotal temperature in fertile men decreases sperm quality and temper with sperm morphology [11].
A study on the effects of M. oleifera extract (100, 200 and 400 mg/kg) on adult male Wistar rats exposed to heat stress for 14 days indicated an improvement in tubular epithelial cells, germinal Sertoli cells, spermatogonia, spermatocytes, early spermatids, late spermatids and spermatozoa, which led to an improvement of the histopathology of the testis [76].
Another study on Spanish maternal line (V-line) rabbit bucks showed an increase in hormonal testosterone levels and increased sperm quality, including increased sperm concentration with intact acrosome, total sperm output, motility and viability [74]. Additionally, rabbit bucks orally supplemented with M. oleifera at different concentrations (50, 100 and 150 mg/kg) for 12 weeks had a lower rectal temperature [74]. In White rats exposed to hot temperatures, M. oleifera prevented the harmful effects of high temperature on Leydig cells and Sertoli cells, indicated by the reduction of testicular cell pyknosis [77].
6.4. Obesity
Obesity affects the male reproductive function by increasing the leptin hormonal level, which activates the HPG axis by stimulating the secretion of GnRH, LH and FSH. However, leptin can also negatively affect the male reproductive function by suppressing testosterone secretion through its receptor isoforms in the gonads [7].
The peripheral adipose tissue in obese individuals can increase the aromatisation of serum testosterone into oestradiol, suppressing the HPG axis through negative feedback inhibition [99]. Increased oestradiol is commonly seen in obese people, and reduces sex hormone-binding globulin (SHBG), FSH and inhibin B [100]. Hence, obesity is positively correlated with reduced testicular volume, semen quality, spermatogenesis, hypogonadism and erectile dysfunction [100,101]. Testicular damage in patients with high body mass index (BMIs) includes alterations in sperm parameters, notably reduced sperm count, concentration, motility, progressiveness, disturbed sperm morphology and sperm DNA fragmentation [7,101].
Some damaging effects of obesity on male reproductive functions may be attributed to the accumulation of ROS. Increased levels of ROS in obese people and obesity-induced animal models are correlated with impairment of testicular function and the HPA-axis [7]. In induced obese rats, M. oleifera leaf extracts reduced the damaging effects of oxidative stress by lowering MDA concentration, which is a marker of lipid peroxidation. In addition, they increased antioxidant enzymes such as SOD, CAT and GSH [86].
Additionally, M. oleifera leaf extract (300 mg/kg) increased serum testosterone, FSH and LH, sperm count and sperm motility. In contrast, immotile spermatozoa and primary and secondary sperm abnormalities decreased in induced obese rats [86]. Therefore, M. oleifera leaf extracts could be a potential solution to fertility problems that are associated with obesity.
6.5. Diabetes Mellitus
It is estimated that about 382 million people globally are living with diabetes mellitus [102]. Diabetes mellitus can result in various medical complications; however, the most common complication is male fertility [103]. Studies have shown that 51% of diabetic males show signs of infertility, which indicates that diabetes mellitus leads to alterations in male reproductive function [102,103].
The serum levels of insulin and glucose in diabetic males affect the maturation of sperm cells on the acrosome and plasma membrane [102], which subsequently affects sperm viability, motility and morphology [104]. Insulin and glucose levels also play essential roles in the sugar movement in sperm cells, which indirectly control motility during capacitation and fertilisation [103].
Glucose is a crucial requirement in spermatogenesis and acrosome reaction and is diffused into the mammalian testicular cells via a concentration gradient utilising specific enzyme transporter known as glucose transporters (GLUTs). The family of these glucose transporters comprises 14 members, categorised into groups of three based on similarities of their sequences. Therefore, reduced insulin levels result in the inactivity of GLUT2 function, which results in low glucose levels and subsequent low energy, thereby reducing the rate of spermatogenesis and motility [105]. Furthermore, M. oleifera leaf extracts in male animal models exposed to diabetic conditions show its ameliorative effects on the male reproductive system.
M. oleifera leaf extracts on male albino Swiss mice exposed to diabetic conditions for 21 days increased sperm count and motility [89]. Diabetes mellitus can affect spermatogenesis by indirectly affecting the epithelium of the seminiferous tubules and reducing FSH [7]. Reduced FSH result in poor activation of Sertoli cells, which leads to impaired spermatogenesis [103]. In some cases of diabetes mellitus (T2DM), there is inhibition of the HPG-axis, which leads to low production of FSH and LH in response to GnRH, as well as reduced testosterone levels as a result of diminishing Leydig cells of the testis [7]. Fortunately, M. oleifera has an androgenic effect on the male reproductive system in vitro [22]. M. oleifera leaf extract on male Wistar rats induced with diabetes increased LH, FSH and testosterone levels, which were reduced by the damaging effects of diabetes mellitus [88].
Oxidative stress is regarded as the leading cause of reproductive deficiency in patients with diabetes mellitus [106]. Diabetes mellitus induces oxidative stress through elevated levels of ROS, lipid peroxidation [102] and reduced total antioxidant capacity (TAC) in the seminal fluid [107]. This increases the DNA fragmentation of sperms, alteration in ATP synthesis of the mitochondria and decreases sperm motility [102]. M. oleifera leaf extract reduced lipid peroxidation and increased the capacity of the antioxidant defensive system by increasing SOD, CAT, GSH and ascorbic acid in the testes of diabetes mellitus-induced rats. This indicates that M. oleifera may ameliorate the effects of diabetes on male fertility through the enhancement of the antioxidant defence system of the testis [88].
Hyperglycaemic conditions have also been correlated with decreased germ cell population, epithelial cell clusters, reduced stereocilia and lipid vacuolisation in the testes [88]. A reduced seminal vesicle weight, mass and weight of the testis, number of Leydig cells, diameter of the seminiferous tubules and height of the germinal epithelium in hyperglycaemic conditions were also shown in another study [102]. M. oleifera showed ameliorative effects on the testicular histology of diabetic rats by increasing the mean number of spermatogonia in the seminiferous tubules and increasing the population of the round (normal) spermatids. M. oleifera also increased the diameter of the seminiferous tubules, the nuclear diameter of the Leydig cells [88] and the weight of the epididymis [89].
6.6. Therapy and Medications: Highly Active Antiretroviral Therapy
The increment in global access to highly active antiretroviral therapy (HAART) has significantly improved the management of HIV/AIDS [108]. HAART positively impacts AIDS patients’ survival with a considerable decline in morbidity and a reduction in new HIV cases. HAART involves a combination of two or more antiretroviral drugs to treat HIV infection. The drugs mainly combined are nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) and protease inhibitors (P.I.s) or non-nucleoside reverse transcriptase inhibitors (NNRTIs). The first line of HIV-1 defence contains a combination of two NRTIs (tenofovir and emtricitabine) and NNRTIs (efavirenz) [109]. Despite its high success rate, chronic use of NRTIs is followed by many adverse effects. Clinical practice and experimental studies have shown the negative consequence of the long-term use of HAART on male fertility [110]. The use of HAART leads to extensive apoptotic degeneration of germinal cells and metabolic disruption of testicular cells. HAART is also extensively involved in generating free radicals leading to oxidative stress damage. Studies on the animal model showed HAART administration reduced sperm parameters by distortion of testicular cell architecture and induction of DNA fragmentation of the testicular tissue. Hence, disturbing spermatogenesis and steroid synthesis [111]. It has also been indicated that exposure to HAART causes degeneration of the seminiferous tubules, spermatids necrosis and deformation of spermatocytes [112].
Long-term exposure to HAART in patients with HIV-1 impairs sperm parameters by causing mitochondrial DNA depletion [109]. NRTIs prevent the replication of the virus; however, they also cause the depletion of mitochondrial DNA by inhibiting DNA polymerase, which leads to a decrease in the production of proteins involved in the electron transport chain, such as cytochrome c oxidase [113]. Hence, this causes damage to sperm motility and progressiveness [114]. Additionally, in HIV-1-infected men, HAART results in lower ejaculate volume and abnormal morphology of spermatozoa [114]. Impairment of sperm parameters may also be due to increased testicular oxidative stress induced by HAART [112].
M. oleifera increased the levels of FSH, LH and testosterone hormone in male Wistar rats exposed to HAART therapy. M. oleifera also reduced the percentage of sperm with abnormal morphology and increased semen quality, characterised by fast sperm progressivity, increased sperm volume, increased motility of sperm and increased sperm count and viability [90]. Additionally, M. oleifera (100 and 300 mg/kg) increased the testicular weight and restored the microarchitecture of testicular morphology altered during HAART treatment [90]. Therefore, M. oleifera shows predominant effects in alleviating the side effects of HAART therapy.
6.7. Cryptorchidism
Cryptorchidism is characterised by the failure of one or both testes to descend into the bottom of the scrotum. Instead, the testes are located along the usual route of testicular descent, which may have an intra-abdominal, inguinal, suprascrotal or high scrotal position. The abnormal positioning of the testis induces hyperthermia, which is detrimental to the process of spermatogenesis [115]. There is evidence that 10% of infertile men are cryptorchid, and 98% of these cryptorchid men are azoospermic. In patients with cryptorchidism, there is also a prominent depletion of the Leydig cells each month due to insufficient secretion of gonadotropic hormones [116]. There is also impairment of germ cell maturation, reduced sperm cell density and tubular and interstitial damage [117].
The temperature in undescended testicles might influence the testicular environment and spermatogenesis through oxidative stress induction [118]. Fortunately, M. oleifera leaves exhibit enormous antioxidant properties. In male Wistar rats induced with bilateral cryptorchidism, M. oleifera leaf extract increased SOD activity, reduced GGT activity [95] and the levels of MDA [94]. This leads to increased sperm count, germ cell count, testicular testosterone and testicular weight [95]
Histological evaluation of the testes of cryptorchid rats indicated a widened seminiferous tubule lumen with an absence of spermatozoa strands and degenerative alterations in the epithelium of the seminiferous tubules, which was suggested by indistinct Sertoli cells and loss of germ cells. Administration of a low dose of M. oleifera improved the appearance of the testes. At the same time, a high dose of M. oleifera attenuated the appearance of the seminiferous epithelium and interstitium with mild degenerative changes characterised by very few tubules with normal germ cell layers visible [95].
6.8. Psychological Stress
Psychological stress has been revealed as one of the causes of idiopathic male infertility, confirmed by studies based on the correlation between stress and impaired semen quality [119]. Psychological stress is explained as a less comfortable surge of emotions coupled with alterations in an individual’s biochemistry, physiology and behaviour [120]. These alterations lead to changes in reproductive functions, reducing libido, sexual performance and overall functions of the reproductive system [119].
M. oleifera leaf extracts in male Wistar rats subjected to the 12 h immobilisation of stress for seven days improved sexual performance by decreasing the intromission latency and increasing intromission frequency on the stressed male rats, and also suppressed the activity of monoamine and phosphodiesterase type 5 (PDE-5) [92].
Psychological stress in animals and humans increases corticosterone and cortisol, respectively, and increases apoptosis of the Leydig cells, subsequently reducing testosterone levels; this leads to changes in Sertoli cells and the blood–testis barrier, causing spermatogenesis arrest [7,120]. Treatment of stressed rats with M. oleifera reduced corticosterone and increased testosterone levels, number of Leydig cells and spermatozoa. In addition, M. oleifera in these stressed rats resulted in a more organised seminiferous epithelium with more interstitial Leydig cells and more spermatozoa in the seminiferous tubule lumen [92].
6.9. Food Additives
In many studies, men who consume healthy food such as fish, fruits, vegetables, legumes, whole grains, and omega-3- and omega-6-fatty acids have increased semen quality as compared to men consuming caffeine, red meat, processed meat, pizza, sugary drinks, and sweets, etc. in their diet [121]. The latter-mentioned foods typically contain monosodium glutamate, a widely used food additive found in many ingredients and processed foods that can be obtained in every market and grocery store [122].
Monosodium glutamate (MSG) has been associated with different toxicities and linked to obesity, metabolic disorders, and detrimental effects on the reproductive organs [122]. Monosodium glutamate toxicity can be linked to its ability to act on the glutamate receptors and release neurotransmitters playing a crucial role in normal pathological and physiological processes [123]. Due to the ability of MSG to influence cells of the reproductive system, MSG can cause sperm alterations, histological alterations, hormonal imbalance, and oxidative damage, which eventually leads to abnormalities in reproductive function [123].
Additionally, MSG leads to sperm membrane dysfunction, sperm DNA damage and sperm motility impairment [124]. These can be attributed to the effects of MSG in the induction of oxidative stress within the testes, marked by increased MDA and reduced GSH [125]. A study on male mice orally administered with monosodium glutamate food additive for 30 days showed a reduction in sperm motility of the rats, which was improved with the administration of M. oleifera leaf extracts at 300 and 600 mg/kg doses [96]. Although this is the only study on the effects of M. oleifera on monosodium glutamate-induced reduction of sperm quality, M. oleifera can serve as a potential treatment for male infertility that might occur as a result of poor diet.
6.10. Alcohol
Chronic abuse of alcohol can eventually lead to atrophy of the testicles, germ cell degeneration, reduced lumen size of the seminiferous tubules, an abundance of lipid droplets and apoptosis of the Sertoli cells [7]. Sertoli cells are the most prone to damage by alcohol due to their cross-links with the Leydig cells, which are also affected by the effects of alcohol, leading to the disruption of male fertility [7]. Male rats exposed to alcohol showed variously atrophied and damaged cells in the seminiferous tubules, depleted spermatogonia, spermatocytes, spermatids, spermatozoa, and the lumen filled with semen, degenerated intratesticular Leydig cells and interstitium [91].
Administration of M. oleifera leaves (400 mg/kg) on male Wistar rats exposed to alcohol resulted in the normal histological architecture of the testis indicated by numerous seminiferous tubules containing swollen myoid living cells, spermatogenic living cells, spermatogonia, spermatocytes, spermatids, spermatozoa, the lumen filled with semen and normal interstitial Leydig cells in between the seminiferous tubules [91]. These results demonstrated the protective action of M. oleifera leaves and its preventive and reversibility of alcohol-induced testicular injuries.
6.11. Ageing
The ageing process may be correlated with an increase in endogenous sources of ROS, which diminishes the anti-oxidant enzyme activities, and Leydig cells are mainly prone to this effect. Excessive growth in ROS leads to alterations of the DNA and membrane potential in Leydig cells, disrupting the synthesis of testosterone [7]. Ageing also diminishes the number of LH receptors on each of the Leydig cells, leading to the inability of the LH to activate the StAR gene, leading to less production of testosterone [7].
It has already been established that reduced testosterone synthesis leads to the loss of Sertoli cell function in spermatogenesis [7], indicating that the effects of ageing on the Leydig cells may indirectly affect spermatogenesis leading to disruption of sperm count as well as sperm morphology. The study of M. oleifera on the sperm count and morphology in old rats (18–19 months old) showed an improvement in sperm count and morphology after oral administration of M. oleifera, which indicates its beneficial effects in the treatment of reproductive changes induced by ageing [93].
7. Mechanism of Action of M. oleifera Extract on Oxidative Stress and Male Fertility
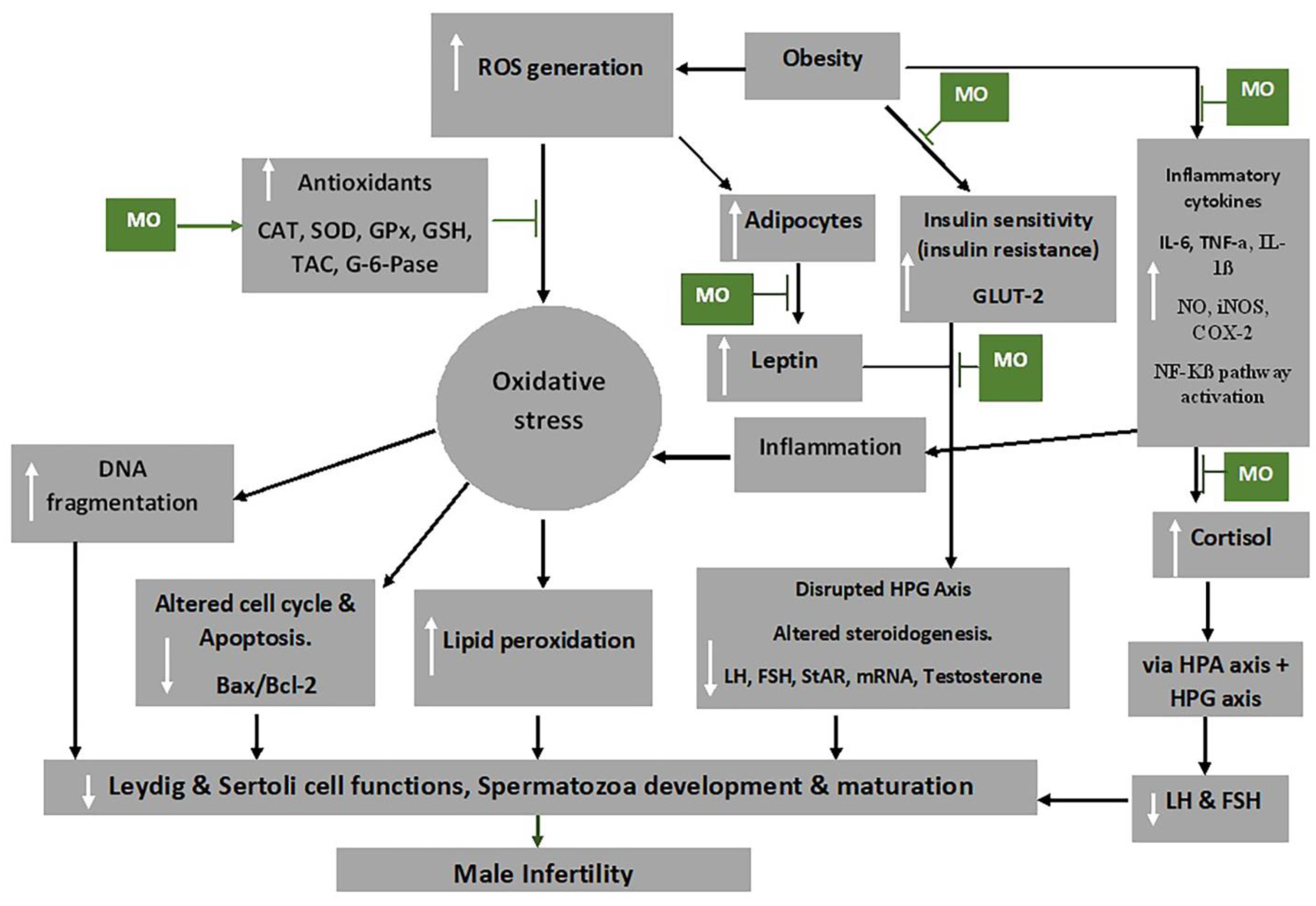
Moringa oleifera extracts possess anti-oxidative, anti-inflammatory, anti-diabetic, anti-obesity and anti-apoptotic properties [86,126], which have been attributed to its polyphenols, flavonoids (particularly quercetin and kaempferol), phenolic acids, caffeoylquinic acid and isothiocyanates [127,128]. The alkaloids, flavonoids, saponins, triterpenoids/steroids and tannins in M. oleifera extract are powerful anti-oxidants that acts to prevent new free radicals and chain reactions and protects the cells from oxidative damage [129]. Figure 3 shows some mechanisms through which M. oleifera extract inhibit the damaging effects of oxidative stress, thereby preventing male infertility.
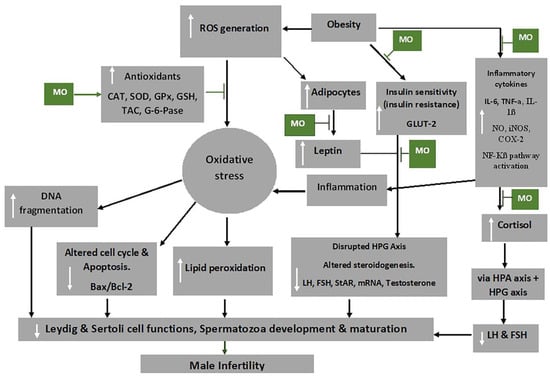
Figure 3.
Mechanism of action of Moringa oleifera (MO) extract on oxidative stress and male infertility. Abbreviations: CAT-catalase; FSH-follicle stimulating hormone; COX-2-cyclooxygenase -2; GLUT2-glucose transporter 2; G-6-pase-glucose 6-phosphatase; GPx-glutathione peroxidase; GSH-glutathione; HPA-hypothalamic–pituitary–adrenal axis; HPG-hypothalamic–pituitary–gonadal axis; IL-6-interleukin 6; iNOS-inducible nitric oxide synthase; LH-luteinizing hormone; mRNA-messenger RNA; NF-Kβ-nuclear factor kappa-light-chain-enhancer of activated B cells; NO-nitric oxide; ROS-reactive oxygen species; SOD-superoxide dismutase; StAR-steroidogenic acute regulatory protein, TAC-total anti-oxidant capacity; TNF-α-tumour necrosis factor alpha. White arrows pointing up indicates increase; white arrows pointing downwards indicates a decrease.  Indicated the regulatory/inhibitory role of Moringa oleifera extract.
Indicated the regulatory/inhibitory role of Moringa oleifera extract.
 Indicated the regulatory/inhibitory role of Moringa oleifera extract.
Indicated the regulatory/inhibitory role of Moringa oleifera extract.
Obesity activates the adipocytes to further produce leptin and could lead to leptin resistance, which has been shown to inhibit the GnRH neurons due to the suppression of KISS1 neuron activities and increased NPY levels [130]. This, consequently, affects the HPG axis, by the impairment of the release of GnRH, FSH and LH, and ultimately impairs the functions of the reproductive cells, testosterone release and development and maturation of the spermatozoa [131]. Besides the reduction of food intake, the anti-obesity and anti-hyperglycaemic activities of M. oleifera are brought about by the reduction of leptin levels by down regulating mRNA expression of leptin and resistin [132]. Additionally, hyperglycaemia may occur due to oxidative stress associated with obesity as it impedes the functioning of insulin and glucose utilisation by peripheral tissue [133]. Flavonoids, particularly quercetin, in the extracts has been shown to act as an apical inhibitor of glucose transporter 2, demonstrating its anti-hyperglycaemic activity [134]. Furthermore, the anti-hyperglycaemic activity of the plant extract is noted by the inhibition of α-glucosidase, pancreatic α-amylase and intestinal sucrose [135].
Inflammatory cytokines, including IL-1β and TNF-α, can increase the production of prostaglandin E2 (PGE-2), nitric oxide (NO), inducible NO synthase (iNOS), cyclooxygenase-2 (COX-2) and microsomal PGE synthase-1 (mPGES-1) as well as their expression in target cells [136]. M. oleifera has also been shown to reduce the production of inflammatory cytokines, such as of TNF-α and IL-6 [126], as well as inhibit the expression of RelA, a gene involved in NF-kB p65 signalling during inflammation [137].
Cortisol and leptin, respectively, produce a primary and secondary negative feedback mechanism for the HPA axis [138], which is critical in maintaining equilibrium under stressful conditions. Chronic psychological stress, for instance, can bring about dysregulation of cortisol [139]. The high levels of ROS result in the release of cortisol (stress hormone), which is usually activated by the HPA axis. The HPA axis, through its communication with the HPG axis, decreases the release of LH, FSH and, ultimately, testosterone [38,140]. In addition, the HPT axis is also affected by oxidative stress and consequently decreases T3 production from the thyroid gland and decreases the circulating testosterone through HPT–HPG axes cross-talk [7].
High levels of ROS disrupt the inner and outer mitochondrial membrane by the induction of cytochrome c protein and activation of caspases and apoptosis [141]. Moringa oleifera extract down regulates caspase 3 and the activation of pathways of NF-kB and phosphatidylinositide 3-kinase/protein kinase B (P13K/AKT) and suppress testicular apoptosis by down regulating Bax expression [70,142,143], thereby preventing male infertility. NF-kB promotes the transcription of genes engaged in apoptosis of male germ cells, which may result from stimulation of Bax/Bcl2 and initiation of caspases [144]. In addition, it has been demonstrated that mechanisms that reduce ROS production ensure that the Bcl-2 inhibitor gene of apoptosis protects the cells [141].
8. Conclusions
Pre-testicular activities (such as hormonal regulation by the endocrine system), testicular activities (such as spermatogenesis, steroidogenesis, Leydig cell, germ cell and Sertoli cell proliferation) and post-testicular activities (such as ejaculation and erectile activities) can be affected by the disrupted balance between the reactive oxygen species and the anti-oxidant defence system in the male reproductive system. The unregulated generation of reactive oxygen species from endogenous and exogenous sources can interfere with the HPG axis and all pathways of the male reproductive system, leading to a reduction in semen quality, which causes male infertility. However, M. oleifera can alleviate all the impacts of reactive oxygen species on the male reproductive system and improve semen quality, increasing libido, erection and ejaculatory function by directly acting on the pathways of male reproductive function or utilising its anti-oxidative, anti-inflammatory, anti-hyperglycaemic and anti-apoptotic properties without toxicities at correct doses. Hence, M. oleifera may be a potential alternative to treating male infertility.
Author Contributions
K.M. prepared the draft of the review paper. U.O., N.B.T. and E.M. reviewed the draft. C.S.O. reviewed the draft and prepared the final document. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval not applicable for this study due to it being a review.
Informed Consent Statement
Not applicable as a review.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alahmar, A.T. Role of Oxidative Stress in Male Infertility: An Updated Review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Öztekin, Ü.; Caniklioğlu, M.; Sarı, S.; Selmi, V.; Gürel, A.; Işıkay, L. Evaluation of Male Infertility Prevalence with Clinical Outcomes in Middle Anatolian Region. Cureus 2019, 11, e5122. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef]
- Deka, P.K.; Sarma, S. Psychological aspects of infertility. Br. J. Med. Pract. 2010, 3, 336. [Google Scholar]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.-L.; Henkel, R.; Vij, S.; Arafa, M.; Selvam, M.K.P.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef]
- Sharma, A. Male Infertility; Evidences, Risk Factors, Causes, Diagnosis and Management in Human. Ann. Clin. Lab. Res. 2017, 5, 188. [Google Scholar] [CrossRef]
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Sengupta, P.; Durairajanayagam, D.; Henkel, R.; Sadeghi, M.R. Reactive oxygen species and male reproductive hormones. Reprod. Biol. Endocrinol. 2018, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Abdillahi, H.; Van Staden, J. South African plants and male reproductive healthcare: Conception and contraception. J. Ethnopharmacol. 2012, 143, 475–480. [Google Scholar] [CrossRef]
- Nwajiaku, L.A.; Mbachu, I.I.; Ikeako, L. Prevalence, Clinical Pattern and Major Causes of Male Infertility in Nnewi, South East Nigeria: A Five Year Review. Afrimedic J. 2012, 3, 16–19. [Google Scholar]
- Retzler, K. Erectile Dysfunction: A Review of Comprehensive Treatment Options for Optimal Outcome. J. Restor. Med. 2019, 8, e20190104. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, N.; Thakur, D.S.; Patidar, A. Male hypogonadism: Symptoms and treatment. J. Adv. Pharm. Technol. Res. 2010, 1, 297. [Google Scholar] [CrossRef]
- Khodamoradi, K.; Khosravizadeh, Z.; Parmar, M.; Kuchakulla, M.; Ramasamy, R.; Arora, H. Exogenous testosterone replacement therapy versus raising endogenous testosterone levels: Current and future prospects. F&S Rev. 2021, 2, 32–42. [Google Scholar] [CrossRef]
- Opuwari, C.S.; Moundipa, P.F. Herbal medicine used to treat andrological problems: Africa. In Herbal Medicine in Andrology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 83–91. [Google Scholar] [CrossRef]
- Syarifuddin, N.; Toleng, A.; Rahardja, D.; Ismartoyo, I.; Yusuf, M. Improving Libido and Sperm Quality of Bali Bulls by Supplementation of Moringa oleifera Leaves. Media Peternak. 2017, 40, 88–93. [Google Scholar] [CrossRef]
- Chatterjee, P.; Chatterjee, P.K.; Mallya, R.; Mithra, P.; Vinodini, N. Moringa oleifera Leaf Extract: Beneficial Effects on Cadmium Induced Toxicities—A Review. J. Clin. Diagn. Res. JCDR 2017, 11, CE01–CE04. [Google Scholar] [CrossRef]
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci. Hum. Wellness 2016, 5, 49–56. [Google Scholar] [CrossRef]
- Susanto, H.; Taufiq, A.; Sunaryono, S.; Soontaranon, S.; Hariyanto, Y.A.; Mawardi, A.I.; Adreyanto, N.G.; Yunisa, D.T.; Rufiandita, F.; Nizarghazi, F.; et al. Moringa oleifera Leaf Powder Madura Variety: Characterization and Biomaterials Property for Biomedical and Nanotechnology Application. J. Phys. Conf. Ser. 2018, 1093, 012007. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Buso, P.; Radice, M.; Dissette, V.; Lampronti, I.; Gambari, R.; Manfredini, S.; Vertuani, S. Moringa oleifera Leaf Extracts as Multifunctional Ingredients for “Natural and Organic” Sunscreens and Photoprotective Preparations. Molecules 2018, 23, 664. [Google Scholar] [CrossRef]
- Ishola, I.O.; Yemitan, K.O.; Afolayan, O.O.; Anunobi, C.C.; Durojaiye, T.E. Potential of Moringa oleifera in the Treatment of Benign Prostate Hyperplasia: Role of Antioxidant Defence Systems. Med. Princ. Pract. 2018, 27, 15–22. [Google Scholar] [CrossRef]
- Khalifa, W.H.; Ibrahim, F.M.; El Makawy, A.I.; Sharaf, H.A.; Khalil, W.K.B.; Maghraby, N.A. Safety And Fertility Enhancing Role Of Moringa Oleifera Leaves Aqueous Extract In New Zealand Rabbit Bucks. Int. J. Pharm. 2016, 6, 156–168. [Google Scholar]
- Cajuday, L.A.; Pocsidio, G.L. Effects of Moringa oleifera Lam. (Moringaceae) on the reproduction of male mice (Mus musculus). J. Med. Plants Res. 2010, 4, 1115–1121. [Google Scholar]
- Opuwari, C.S.; Matshipi, M.N.; Phaahla, M.K.; Setumo, M.A.; Moraswi, R.T.; Zitha, A.A.; Offor, U.; Choma, S.S.R. Androgenic effect of aqueous leaf extract of Moringa oleifera on Leydig TM3 cells in vitro. Andrologia 2020, 52, e13825. [Google Scholar] [CrossRef]
- Moichela, F.T. In Vitro Effects of Aqueous Leaf Extract of Moringa oleifera on Human Sperm. Master’s Thesis, University of Limpopo, Mankweng, South Africa, 2020; p. 210. [Google Scholar]
- Eze, U.A.; Okonofua, F.E. High Prevalence of Male Infertility in Africa: Are Mycotoxins to Blame? Afr. J. Reprod. Health 2015, 19, 9–17. [Google Scholar] [PubMed]
- Zarrabi, A.D.; Kruger, T.F. The challenges of supporting male infertility treatment in South Africa. Nat. Rev. Urol. 2018, 15, 719–720. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Li, R.; Jia, Z.; Trush, M.A. Defining ROS in biology and medicine. React. Oxyg. Species Apex NC 2016, 1, 9–21. [Google Scholar] [CrossRef]
- Bouchez, C.; Devin, A. Mitochondrial Biogenesis and Mitochondrial Reactive Oxygen Species (ROS): A Complex Relationship Regulated by the cAMP/PKA Signaling Pathway. Cells 2019, 8, 287. [Google Scholar] [CrossRef]
- Beattie, M.C.; Chen, H.; Fan, J.; Papadopoulos, V.; Miller, P.; Zirkin, B.R. Aging and Luteinizing Hormone Effects on Reactive Oxygen Species Production and DNA Damage in Rat Leydig Cells1. Biol. Reprod. 2013, 88, 100. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Ye, L.; Zirkin, B.; Chen, H. Steroidogenesis in Leydig cells: Effects of aging and environmental factors. Reproduction 2017, 154, R111–R122. [Google Scholar] [CrossRef]
- Baskaran, S.; Finelli, R.; Agarwal, A.; Henkel, R. Reactive oxygen species in male reproduction: A boon or a bane? Andrologia 2020, 53, e13577. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Durairajanayagam, D.; Du Plessis, S.S. Utility of antioxidants during assisted reproductive techniques: An evidence based review. Reprod. Biol. Endocrinol. 2014, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Baker, M.A. Oxidative stress, sperm survival and fertility control. Mol. Cell. Endocrinol. 2006, 250, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Spiers, J.G.; Chen, H.-J.C.; Sernia, C.; Lavidis, N.A. Activation of the hypothalamic-pituitary-adrenal stress axis induces cellular oxidative stress. Front. Neurosci. 2015, 8, 456. [Google Scholar] [CrossRef]
- Greifová, H.; Jambor, T.; Tokárová, K.; Speváková, I.; Knížatová, N.; Lukáč, N. Resveratrol attenuates hydrogen peroxide-induced oxidative stress in TM3 Leydig cells in vitro. J. Environ. Sci. Health Part A 2020, 55, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Leisegang, K.; Henkel, R. The in vitro modulation of steroidogenesis by inflammatory cytokines and insulin in TM3 Leydig cells. Reprod. Biol. Endocrinol. 2018, 16, 26. [Google Scholar] [CrossRef]
- Tugaeva, K.V.; Sluchanko, N.N. Steroidogenic acute regulatory protein: Structure, functioning, and regulation. Biochem. Mosc. 2019, 84, 233–253. [Google Scholar] [CrossRef]
- Ahima, R.S. Revisiting leptin’s role in obesity and weight loss. J. Clin. Investig. 2008, 118, 2380–2383. [Google Scholar] [CrossRef]
- Caprio, M.; Isidori, A.M.; Carta, A.R.; Moretti, C.; Dufau, M.L.; Fabbri, A. Expression of Functional Leptin Receptors in Rodent Leydig Cells1. Endocrinology 1999, 140, 4939–4947. [Google Scholar] [CrossRef]
- Agarwal, A.; Dutta, S. Obesity. In Male Infertility; Parekattil, S.J., Esteves, S.C., Agarwal, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 497–508. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Agarwal, A.; Henkel, R.; Finelli, R.; Robert, K.A.; Iovine, C.; Baskaran, S. The effect of oxidative and reductive stress on semen parameters and functions of physiologically normal human spermatozoa. Free Radic. Biol. Med. 2020, 152, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.J.; Stocco, D.M. The steroidogenic acute regulatory protein (StAR). In Cholesterol Transporters of the START Domain Protein Family in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2014; pp. 15–47. [Google Scholar]
- Takeshima, T.; Kuroda, S.; Yumura, Y. Reactive Oxygen Species and Sperm Cells. In Reactive Oxygen Species (ROS) in Living Cells; Filip, C., Albu, E., Eds.; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Kurkowska, W.; Bogacz, A.; Janiszewska, M.; Gabryś, E.; Tiszler, M.; Bellanti, F.; Kasperczyk, S.; Machoń-Grecka, A.; Dobrakowski, M.; Kasperczyk, A. Oxidative Stress is Associated with Reduced Sperm Motility in Normal Semen. Am. J. Men’s Health 2020, 14, 1557988320939731. [Google Scholar] [CrossRef] [PubMed]
- Pujianto, D.A.; Oktarina, M.; Sharaswati, I.A.S.; Yulhasri, Y. Hydrogen peroxide has adverse effects on human sperm quality parameters, induces apoptosis, and reduces survival. J. Hum. Reprod. Sci. 2021, 14, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Jr, S.T.P.; Gummow, B.; Parker, A.J.; Paris, D.B.B.P. Antioxidant supplementation mitigates DNA damage in boar (Sus scrofa domesticus) spermatozoa induced by tropical summer. PLoS ONE 2019, 14, e0216143. [Google Scholar] [CrossRef]
- Juan, C.; de la Lastra, J.P.; Plou, F.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Wang, R.; Hao, W.; Pan, L.; Boldogh, I.; Ba, X. The roles of base excision repair enzyme OGG1 in gene expression. Cell. Mol. Life Sci. 2018, 75, 3741–3750. [Google Scholar] [CrossRef]
- Baquero, J.M.; Benítez-Buelga, C.; Rajagopal, V.; Zhenjun, Z.; Torres-Ruiz, R.; Müller, S.; Hanna, B.M.F.; Loseva, O.; Wallner, O.; Michel, M.; et al. Small molecule inhibitor of OGG1 blocks oxidative DNA damage repair at telomeres and potentiates methotrexate anticancer effects. Sci. Rep. 2021, 11, 3490. [Google Scholar] [CrossRef]
- Khobta, A.; Epe, B. Interactions between DNA damage, repair, and transcription. Mutat. Res. Mol. Mech. Mutagen. 2012, 736, 5–14. [Google Scholar] [CrossRef]
- Liu, Q.; Gu, J.H.; Yuan, Y.; Liu, X.Z.; Wang, Y.J.; Wang, H.D.; Liu, Z.P. Effect of cadmium on rat Leydig cell testosterone production and DNA integrity in vitro. Biomed. Environ. Sci. 2013, 26, 769–773. [Google Scholar]
- Bibov, M.Y.; Kuzmin, A.V.; Alexandrova, A.A.; Chistyakov, V.A.; Dobaeva, N.M.; Kundupyan, O.L. Role of the reactive oxygen species induced DNA damage in human spermatozoa dysfunction. AME Med. J. 2018, 3, 19. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef] [PubMed]
- Korytowski, W.; Pilat, A.; Schmitt, J.C.; Girotti, A.W. Deleterious Cholesterol Hydroperoxide Trafficking in Steroidogenic Acute Regulatory (StAR) Protein-expressing MA-10 Leydig Cells. J. Biol. Chem. 2013, 288, 11509–11519. [Google Scholar] [CrossRef]
- Asadi, N. The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in Improving it: A Review. J. Clin. Diagn. Res. 2017, 11, IE01–IE05. [Google Scholar] [CrossRef] [PubMed]
- Kehm, R.; Baldensperger, T.; Raupbach, J.; Höhn, A. Protein oxidation—Formation mechanisms, detection and relevance as biomarkers in human diseases. Redox Biol. 2021, 42, 101901. [Google Scholar] [CrossRef] [PubMed]
- Diemer, T.; Allen, J.A.; Hales, K.H.; Hales, D.B. Reactive Oxygen Disrupts Mitochondria in MA-10 Tumor Leydig Cells and Inhibits Steroidogenic Acute Regulatory (StAR) Protein and Steroidogenesis. Endocrinology 2003, 144, 2882–2891. [Google Scholar] [CrossRef] [PubMed]
- Abd, H.H.; Ahmed, H.A.; Mutar, T.F. Moringa oleifera leaves extract modulates toxicity, sperms alterations, oxidative stress, and testicular damage induced by tramadol in male rats. Toxicol. Res. 2020, 9, 101–106. [Google Scholar] [CrossRef]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive Components in Moringa oleifera Leaves Protect against Chronic Disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef]
- Sun, M.C.; Ruhomally, Z.B.; Boojhawon, R.; Neergheen-Bhujun, V.S. Consumption of Moringa oleifera Lam Leaves Lowers Postprandial Blood Pressure. J. Am. Coll. Nutr. 2020, 39, 54–62. [Google Scholar] [CrossRef]
- Manisha, N.; Rajak, R.; Jat, D. Oxidative stress and antioxidants: An overview. Int. J. Adv. Res. Rev. 2017, 2, 110–119. [Google Scholar]
- El-Sheikh, S.; Khairy, M.; Fadil, H.A.; Abo-Elmaaty, A. Ameliorative Effect of Moringa oleifera Extract on Male Fertility in Paroxetine Treated Rats. Zagazig Vet. J. 2016, 44, 244–250. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Ahmed, M.A.; El Sayed, R.A. Molecular effects of Moringa leaf extract on insulin resistance and reproductive function in hyperinsulinemic male rats. J. Diabetes Metab. Disord. 2019, 18, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Sharmila; Prabsattroo, T.; Wattanathorn, J.; Iamsa-Ard, S.; Muchimapura, S.; Thukhammee, W. Moringa oleifera Leaves Extract Attenuates Male Sexual Dysfunction. Am. J. Neurosci. 2012, 3, 17–24. [Google Scholar] [CrossRef]
- Wafa, W.M.; El-Nagar, H.A.; Gabr, A.A.; Rezk, M.M. Impact of Dietary Moringa oleifera Leaves Supplementation on Semen Characteristics, Oxidative Stress, Physiological Response and Blood Parameters of Heat Stressed Buffalo Bulls. J. Anim. Poult. Prod. 2017, 8, 367–379. [Google Scholar] [CrossRef]
- Zeng, B.; Luo, J.; Wang, P.; Yang, L.; Chen, T.; Sun, J.; Xie, M.; Li, M.; Zhang, H.; He, J.; et al. The beneficial effects of Moringa oleifera leaf on reproductive performance in mice. Food Sci. Nutr. 2019, 7, 738–746. [Google Scholar] [CrossRef]
- Ajuogu, P.K.; Mgbere, O.O.; Bila, D.S.; McFarlane, J.R. Hormonal changes, semen quality and variance in reproductive activity outcomes of post pubertal rabbits fed Moringa oleifera Lam. leaf powder. J. Ethnopharmacol. 2018, 233, 80–86. [Google Scholar] [CrossRef]
- Laoung-On, J.; Saenphet, K.; Jaikang, C.; Sudwan, P. Effect of Moringa oleifera Lam. Leaf Tea on Sexual Behavior and Reproductive Function in Male Rats. Plants 2021, 10, 2019. [Google Scholar] [CrossRef]
- Moichela, F.T.; Adefolaju, G.A.; Henkel, R.R.; Opuwari, C.S. Aqueous leaf extract of Moringa oleifera reduced intracellular ROS production, DNA fragmentation and acrosome reaction in Human spermatozoa in vitro. Andrologia 2021, 53, e13903. [Google Scholar] [CrossRef]
- El-Desoky, N.; Hashem, N.; Elkomy, A.; Abo-Elezz, Z. Physiological response and semen quality of rabbit bucks supplemented with Moringa leaves ethanolic extract during summer season. Animal 2017, 11, 1549–1557. [Google Scholar] [CrossRef]
- Ewuola, E.O.; Adeyemi, A.A.; Adeyinka, A.D.; Akabuike, C.F. Potential of Moringa oleifera leaf meal in improving reproductive efficiency of rabbit bucks in hot climate. Niger. J. Anim. Sci. 2019, 21, 80–86. [Google Scholar] [CrossRef]
- Hanafi, A.; Fadholly, A.; Utomo, B.; Sudjarwo, S.A.; Yunus, M.; Hariadi, M.; Legowo, D. Effects of Moringa oleifera L. Extract on leydig and sertoli cells induced high Temperature on Rattus norvegicus. Res. J. Pharm. Technol. 2020, 13, 3361–3364. [Google Scholar] [CrossRef]
- Hidayat, N.; Utomo, B.; Budiarto, R.K.; Legowo, D.; Safitri, E. Effect of grant leaf extract (Moringa oleifera lam) on histopathological featureof white rat (Rattus Norvegicus) testis exposed hot temperature. Pollut. Res. 2020, 39, 1251–1255. [Google Scholar]
- Bin-Meferij, M.M.; El-Kott, A.F. The radioprotective effects of Moringa oleifera against mobile phone electromagnetic radiation-induced infertility in rats. Int. J. Clin. Exp. Med. 2015, 8, 12487–12497. [Google Scholar] [PubMed]
- Ramalingam, S.; Suriyakumari, K.V.P.; Philip, X.C. The Effect of Ethanolic Extract of Moringa oleifera Leaves on 4 G-Cell Phone-EMR-Induced Oxidative Stresses Associated with Altered Sperm Count in Pre-Pubertal Wistar Rats. Ann. Rom. Soc. Cell Biol. 2021, 25, 3226–3239. [Google Scholar]
- Salama, A.A.; Elsaeid, A.A.; Awad, O.M. Effect of Moringa oleifera leaves extract against electromagnetic field impairments on hemoglobin and testes of rat. J. Biosci. Appl. Res. 2020, 6, 132–141. [Google Scholar] [CrossRef]
- Akunna, G.G.; Ogunmodede, O.S.; Saalu, C.L.; Ogunlade, B.; Bello, A.J.; Salawu, E.O. Ameliorative effect of Moringa oleifera (drumstick) leaf extracts on chromium-induced testicular toxicity in rat testes. World J. Life Sci. Med. Res. 2012, 2, 20. [Google Scholar]
- Elblehi, S.S.; El Euony, O.I.; El-Nahas, A.F. Partial ameliorative effect of Moringa leaf ethanolic extract on the reproductive toxicity and the expression of steroidogenic genes induced by subchronic cadmium in male rats. Environ. Sci. Pollut. Res. 2019, 26, 23306–23318. [Google Scholar] [CrossRef]
- Owolabi, J.O.; Ghazal, O.K.; Williams, F.E.; Ayodele, E.O. Effects of Moringa oleifera (Drumstick) Leaf Extracts on Lead-Induced Testicular Toxicity in Adult Wistar Rat (Rattus Novergicus). Int. J. Biotech. Biomed. Res. 2012, 2, 4003–4009. [Google Scholar]
- Mansour, M.; Arisha, A.; Algamal, M.; Elsayed, A.; Saad, S.; El Bohi, K. Effect of Moringa oleifera Leaves Extract -SeNPs Conjugate Administration on Testicular Toxicity Induced by Melamine in Rats. 2020. Available online: https://www.semanticscholar.org/paper/Effect-of-Moringa-oleifera-Leaves-Extract-SeNPs-on-Mansour-Arisha/57e9bb5ffdf6942389c0af4f71a127e1df9758d8 (accessed on 18 August 2022).
- Ododo, A.; Ojeka, S.O.; Dapper, V.D. Ameliorative Effect of Aqueous Leaf Extract of Moringa oleifera on Reproductive Function Following Cadmium Chloride Induced Oxidative Stress in Male Wistar Rats. Not. Sci. Biol. 2019, 11, 352–357. [Google Scholar] [CrossRef]
- Alkafafy, M.E.; Sayed, S.M.; El-Shehawi, A.M.; El-Shazly, S.; Farouk, S.; Alotaibi, S.S.; Madkour, D.A.; Orabi, S.H.; Elbaz, H.T.; Ahmed, M.M. Moringa oleifera ethanolic extract ameliorates the testicular dysfunction resulted from HFD-induced obesity rat model. Andrologia 2021, 53, e14126. [Google Scholar] [CrossRef]
- Juan, C.A. Moringa protein drink increases testosterone and anabolic status of men with hyperlipidemia: A randomized controlled study. Turk. J. Kinesiol. 2021, 7, 1–15. [Google Scholar] [CrossRef]
- Jangir, R.N.; Jain, G.C. Ameliorative Effect of Moringa oleifera Lam. Leaves Extract on the Sex Hormone Profile and Testicular Dysfunctions in Streptozotocin-induced Diabetic Wistar Rats. Pharmacogn. Res. 2022, 14, 225–232. [Google Scholar] [CrossRef]
- Priyadarshani, N.; Varma, M.C. Effect of Moringa oleifera leaf powder on sperm count, histology of testis and epididymis of hyperglycaemic mice Mus musculus. Am. Int. J. Res. Form. Appl. Nat. Sci. 2014, 7, 7–13. [Google Scholar]
- Ogunlade, B.; Jeje, S.O.; Adelakun, S.A.; Akingbade, G.T. Moringa oleifera restored semen quality, hormonal profile, and testicular morphology against Highly Active Antiretroviral Therapy- induced toxicity in adult male Wistar rats. JBRA Assist. Reprod. 2022, 26, 3. [Google Scholar] [CrossRef]
- Bassey, R.B.; Bala, D.N.; Edagha, I.A.; Peter, A.I. The effect of ethanolic extract of Moringa oleifera on alcohol-induced testicular histopathologies in pre-pubertal albino Wistar rats. Biol. Med. 2013, 5, 40. [Google Scholar]
- Prabsattroo, T.; Wattanathorn, J.; Iamsaard, S.; Somsapt, P.; Sritragool, O.; Thukhummee, W.; Muchimapura, S. Moringa oleifera extract enhances sexual performance in stressed rats. J. Zhejiang Univ. B 2015, 16, 179–190. [Google Scholar] [CrossRef]
- Widiastini, L.P.; Karuniadi, I.G.A.M.; Tangkas, M. Ethanol Extract of Moringa oleifera Increased the Number of Spermatozoa and Improved Sperm Morphology of Old Rattus norvegicus. J. Bioteknol. Biosains Indones. JBBI 2022, 9, 11–19. [Google Scholar] [CrossRef]
- Afolabi, A.; Aderoju, H.; Alagbonsi, A. Effects of methanolic extract of Moringa oleifera leave on semen and biochemical parameters in cryptorchid rats. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 230–235. [Google Scholar] [CrossRef]
- Afolabi, A.O.; Olotu, O.O.; Alagbonsi, I.A. Vitamins E and C Alleviate the Germ Cell Loss and Oxidative Stress in Cryptorchidism When Administered Separately but Not When Combined in Rats. ISRN Pharmacol. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Nugraha, I.S.; Wibisono, D.S.; Saraswati, I.; Juniarto, A.Z. The Effect of Moringa Leaf Extract (Moringa oleifera L) against Motility of Spermatozoa Mice Exposed to Monosodium Glutamate. Indones. J. Urol. 2022, 29, 41–46. [Google Scholar] [CrossRef]
- Adah, A.; Adah, D.; Biobaku, K.; Adeyemi, A. Effects of electromagnetic radiations on the male reproductive system. Anat. J. Afr. 2018, 7, 1152–1161. [Google Scholar] [CrossRef]
- Brownlee, K.K.; Moore, A.W.; Hackney, A.C. Relationship between circulating cortisol and testosterone: Influence of physical exercise. J. Sports Sci. Med. 2005, 4, 76–83. [Google Scholar] [PubMed]
- Kahn, B.E.; Brannigan, R.E. Obesity and male infertility. Curr. Opin. Urol. 2017, 27, 441–445. [Google Scholar] [CrossRef]
- Craig, J.R.; Jenkins, T.G.; Carrell, D.T.; Hotaling, J.M. Obesity, male infertility, and the sperm epigenome. Fertil. Steril. 2017, 107, 848–859. [Google Scholar] [CrossRef]
- Katib, A. Mechanisms linking obesity with male infertility. Cent. Eur. J. Urol. 2015, 68, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-F.; Ding, G.-L.; Liu, Y.; Liu, M.-E.; Pan, J.-X.; Guo, M.-X.; Sheng, J.-Z. The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis. Asian J. Androl. 2015, 17, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Martins, A.; Rato, L.; Moreira, P.; Socorro, S.; Oliveira, P. Molecular mechanisms beyond glucose transport in diabetes-related male infertility. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2013, 1832, 626–635. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.A.; Vicari, E.S.D.; D’Agata, R.; Calogero, A.E. Diabetes Mellitus and Sperm Parameters. J. Androl. 2012, 33, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Temidayo, S.O.; Stefan, S.D.P. Diabetes mellitus and male infertility. Asian Pac. J. Reprod. 2018, 7, 6. [Google Scholar] [CrossRef]
- Ma, J.; Han, R.; Deng, P.; Qi, Y.; Liu, W.; Cui, T.; Wang, S. Effect of diabetes mellitus on semen quality. Int. J. Clin. Exp. Med. 2020, 13, 7910–7919. [Google Scholar]
- Kumar, S.; Thaker, R.; Verma, V.; Gor, M.; Agarwal, R.; Mishra, V. Occupational, Environmental exposure, and Lifestyle factors: Declining Male Reproductive Health. J. Gynecol. Infertil. 2018, 1, 30. [Google Scholar]
- Arenas-Pinto, A.; Milinkovic, A.; Peppa, D.; McKendry, A.; Maini, M.; Gilson, R. Systemic inflammation and residual viraemia in HIV-positive adults on protease inhibitor monotherapy: A cross-sectional study. BMC Infect. Dis. 2015, 15, 138. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Honma, M.; Kimura, Y.; Abe, H. Structure, Synthesis and Inhibition Mechanism of Nucleoside Analogues as HIV-1 Reverse Transcriptase Inhibitors (NRTIs). ChemMedChem 2021, 16, 743–766. [Google Scholar] [CrossRef] [PubMed]
- Azu, O.O.; Naidu, E.C.S.; Naidu, J.S.; Masia, T.; Nzemande, N.F.; Chuturgoon, A.; Singh, S. Testicular histomorphologic and stereological alterations following short-term treatment with highly active antiretroviral drugs (HAART) in an experimental animal model. Andrology 2014, 2, 772–779. [Google Scholar] [CrossRef]
- Akhigbe, R.; Hamed, M.; Aremu, A. HAART exacerbates testicular damage and impaired spermatogenesis in anti-Koch-treated rats via dysregulation of lactate transport and glutathione content. Reprod. Toxicol. 2021, 103, 96–107. [Google Scholar] [CrossRef]
- Oyeyipo, I.P.; Skosana, B.T.; Everson, F.P.; Strijdom, H.; Du Plessis, S. Highly Active Antiretroviral Therapy Alters Sperm Parameters and Testicular Antioxidant Status in Diet-Induced Obese Rats. Toxicol. Res. 2018, 34, 41–48. [Google Scholar] [CrossRef]
- Azu, O.O. Highly Active Antiretroviral Therapy (HAART) and Testicular Morphology: Current Status and a Case for a Stereologic Approach. J. Androl. 2012, 33, 1130–1142. [Google Scholar] [CrossRef]
- Kehl, S.; Weigel, M.; Müller, D.; Gentili, M.; Hornemann, A.; Sütterlin, M. HIV-infection and modern antiretroviral therapy impair sperm quality. Arch. Gynecol. Obstet. 2011, 284, 229–233. [Google Scholar] [CrossRef]
- Cobellis, G.; Noviello, C.; Nino, F.; Romano, M.; Mariscoli, F.; Martino, A.; Parmeggiani, P.; Papparella, A. Spermatogenesis and Cryptorchidism. Front. Endocrinol. 2014, 5, 63. [Google Scholar] [CrossRef]
- Chung, E.; Brock, G.B. Cryptorchidism and its impact on male fertility: A state of art review of current literature. Can. Urol. Assoc. J. 2011, 5, 210–214. [Google Scholar] [CrossRef]
- Fawzy, F.; Hussein, A.; Eid, M.M.; El Kashash, A.M.; Salem, H.K. Cryptorchidism and Fertility. Clin. Med. Insights: Reprod. Health 2015, 9, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Hu, X.-Q.; Xiao, L.-J.; Hu, Z.-Y.; Guo, J.; Zhang, K.-Y.; Song, X.-X.; Liu, Y.-X. An oligonucleotide microarray study on gene expression profile in mouse testis of experimental cryptorchidism. Front. Biosci. 2006, 11, 2465–2482. [Google Scholar] [CrossRef] [PubMed]
- Ilacqua, A.; Izzo, G.; Emerenziani, G.P.; Baldari, C.; Aversa, A. Lifestyle and fertility: The influence of stress and quality of life on male fertility. Reprod. Biol. Endocrinol. 2018, 16, 115. [Google Scholar] [CrossRef] [PubMed]
- Nargund, V.H. Effects of psychological stress on male fertility. Nat. Rev. Urol. 2015, 12, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Skoracka, K.; Eder, P.; Łykowska-Szuber, L.; Dobrowolska, A.; Krela-Kaźmierczak, I. Diet and Nutritional Factors in Male (In)fertility—Underestimated Factors. J. Clin. Med. 2020, 9, 1400. [Google Scholar] [CrossRef]
- Niaz, K.; Zaplatic, E.; Spoor, J. Extensive use of monosodium glutamate: A threat to public health? EXCLI J. 2018, 17, 273–278. [Google Scholar] [CrossRef]
- Kayode, O.T.; Rotimi, D.E.; Kayode, A.A.A.; Olaolu, T.D.; Adeyemi, O.S. Monosodium Glutamate (MSG)-Induced Male Reproductive Dysfunction: A Mini Review. Toxics 2020, 8, 7. [Google Scholar] [CrossRef]
- El-Sawy, H.B.I.; Soliman, M.M.; El-Shazly, S.A.; Ali, H. A-M. Protective effects of camel milk and vitamin E against monosodium glutamate induced biochemical and testicular dysfunctions. Prog. Nutr. 2018, 20, 76–85. [Google Scholar] [CrossRef]
- Khaled, F.A.; Yousef, M.I.; Kamel, K.I. The protective role of propolis against the reproductive toxicity of mono-sodium glutamine in male rabbits. Int. J. Chem. Stud. 2016, 4, 4–9. [Google Scholar]
- Kilany, O.E.; Abdelrazek, H.M.; Aldayel, T.S.; Abdo, S.; Mahmoud, M. Anti-obesity potential of Moringa olifera seed extract and lycopene on high fat diet induced obesity in male Sprauge Dawely rats. Saudi J. Biol. Sci. 2020, 27, 2733–2746. [Google Scholar] [CrossRef]
- Vargas-Sánchez, K.; Garay-Jaramillo, E.; González-Reyes, R.E. Effects of Moringa oleifera on Glycaemia and Insulin Levels: A Review of Animal and Human Studies. Nutrients 2019, 11, 2907. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Singh, B.R.; Singh, R.L.; Prakash, D.; Dhakarey, R.; Upadhyay, G.; Singh, H.B. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem. Toxicol. 2009, 47, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Widiastini, L.P.; Karuniadi, I.A.M.; Karmaya, I.N.M.; Widhiantara, I.G. The potential of a Balinese traditional medicine kelor leaves (Moringa oleifera) for male infertility treatment: A mini review. Indian J. Forensic Med. Toxicol. 2022, 16, 734–742. [Google Scholar]
- Ojeda, S.R.; Lomniczi, A.; Mastronardi, C.; Heger, S.; Roth, C.; Parent, A.-S.; Matagne, V.; Mungenast, A.E. Minireview: The Neuroendocrine Regulation of Puberty: Is the Time Ripe for a Systems Biology Approach? Endocrinology 2006, 147, 1166–1174. [Google Scholar] [CrossRef]
- Leisegang, K.; Sengupta, P.; Agarwal, A.; Henkel, R. Obesity and male infertility: Mechanisms and management. Andrologia 2020, 53, e13617. [Google Scholar] [CrossRef]
- Metwally, F.M.; Rashad, H.M.; Ahmed, H.H.; Mahmoud, A.A.; Raouf, E.R.A.; Abdalla, A.M. Molecular mechanisms of the anti-obesity potential effect of Moringa oleifera in the experimental model. Asian Pac. J. Trop. Biomed. 2017, 7, 214–221. [Google Scholar] [CrossRef]
- Ceriello, A.; Motz, E. Is Oxidative Stress the Pathogenic Mechanism Underlying Insulin Resistance, Diabetes, and Cardiovascular Disease? The Common Soil Hypothesis Revisited. Arter. Thromb. Vasc. Biol. 2004, 24, 816–823. [Google Scholar] [CrossRef]
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.-H.; Kruhlak, M.; Levine, M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007, 21, 366–377. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Chanathong, B. Alpha-glucosidase inhibitory activity and lipid-lowering mechanisms of Moringa oleifera leaf extract. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 803–808. [Google Scholar]
- Kou, X.; Qi, S.; Dai, W.; Luo, L.; Yin, Z. Arctigenin inhibits lipopolysaccharide-induced iNOS expression in RAW264.7 cells through suppressing JAK-STAT signal pathway. Int. Immunopharmacol. 2011, 11, 1095–1102. [Google Scholar] [CrossRef]
- Kooltheat, N.; Sranujit, R.P.; Chumark, P.; Potup, P.; Laytragoon-Lewin, N.; Usuwanthim, K. An Ethyl Acetate Fraction of Moringa oleifera Lam. Inhibits Human Macrophage Cytokine Production Induced by Cigarette Smoke. Nutrients 2014, 6, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Aschbacher, K.; Rodriguez-Fernandez, M.; Van Wietmarschen, H.; Tomiyama, A.J.; Jain, S.; Epel, E.; Doyle, F.J.; Van Der Greef, J. The hypothalamic–pituitary–adrenal–leptin axis and metabolic health: A systems approach to resilience, robustness and control. Interface Focus 2014, 4, 20140020. [Google Scholar] [CrossRef] [PubMed]
- Aschbacher, K.; O’donovan, A.; Wolkowitz, O.M.; Dhabhar, F.S.; Su, Y.; Epel, E. Good stress, bad stress and oxidative stress: Insights from anticipatory cortisol reactivity. Psychoneuroendocrinology 2013, 38, 1698–1708. [Google Scholar] [CrossRef]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Makker, K.; Agarwal, A.; Sharma, R. Oxidative stress & male infertility. Indian J. Med. Res. 2009, 129, 357–367. [Google Scholar] [PubMed]
- Jung, I.L. Soluble Extract from Moringa oleifera Leaves with a New Anticancer Activity. PLoS ONE 2014, 9, e95492. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Li, B.; Olayanju, J.B.; Drake, J.M.; Chen, N. Nutraceutical or Pharmacological Potential of Moringa oleifera Lam. Nutrients 2018, 10, 343. [Google Scholar] [CrossRef]
- Orrenius, S.; Nicotera, P.; Zhivotovsky, B. Cell Death Mechanisms and Their Implications in Toxicology. Toxicol. Sci. Off. J. Soc. Toxicol. 2011, 119, 3–19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).