Optimization of Process Variables for the Sustainable Extraction of Phenolic Compounds from Chicory and Fennel By-Products
Abstract
1. Introduction
2. Materials and Methods
2.1. By-Products and Pre-Treatments
2.2. Conventional Solvent Extraction
2.3. Ultrasound-Assisted Extraction
2.4. Microwave-Assisted Extraction
2.5. Analyses of the Extracts
2.6. Statistical Analysis
3. Results and Discussion
3.1. Results of Conventional Solvent Extraction
3.2. Results of Ultrasound-Assisted Extraction
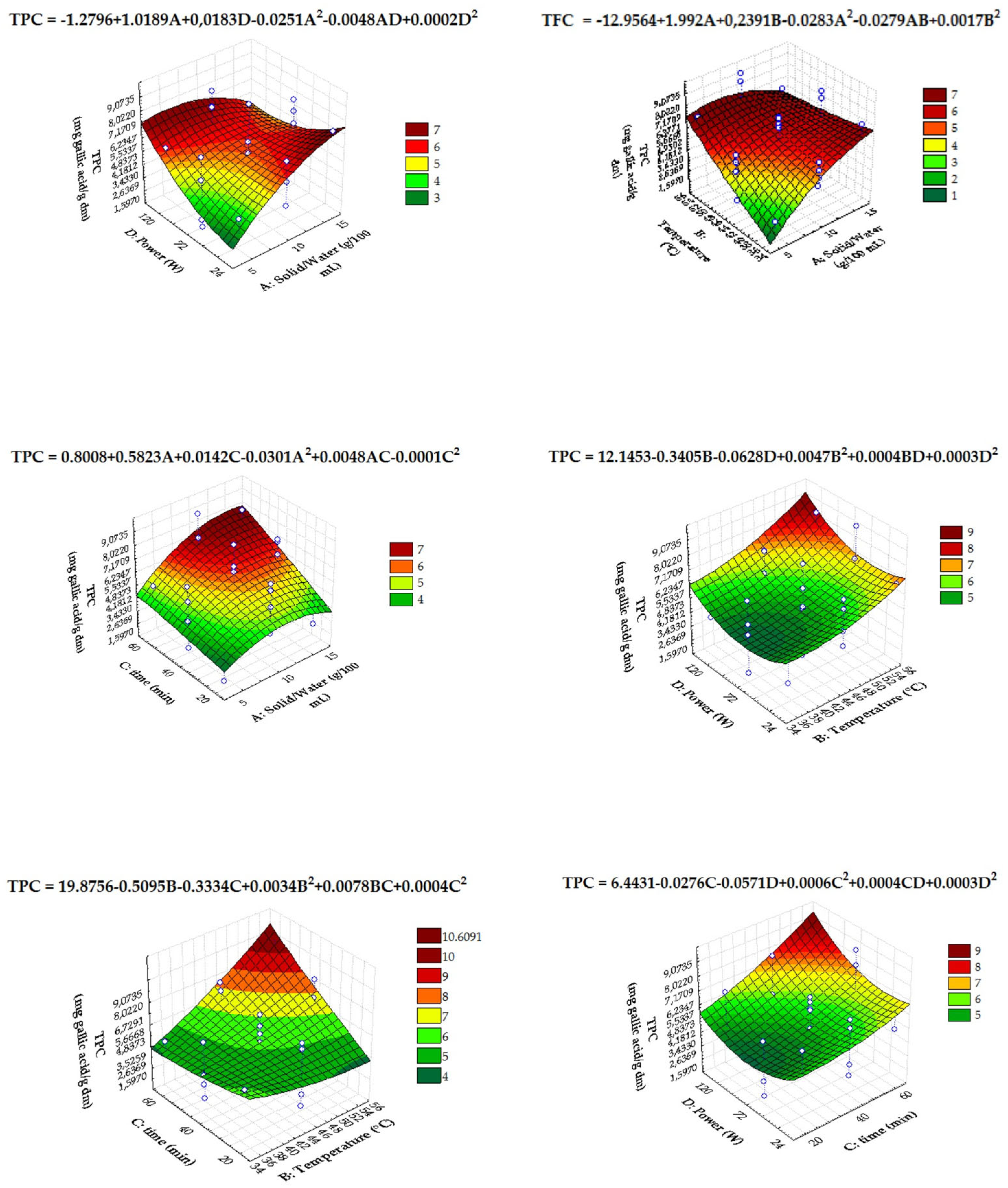
3.2.1. UAE Extracts of Chicory and Fennel By-Products
3.2.2. Predictive Ability of the Models Applied to UAE
3.3. Results of Microwave-Assisted Extraction
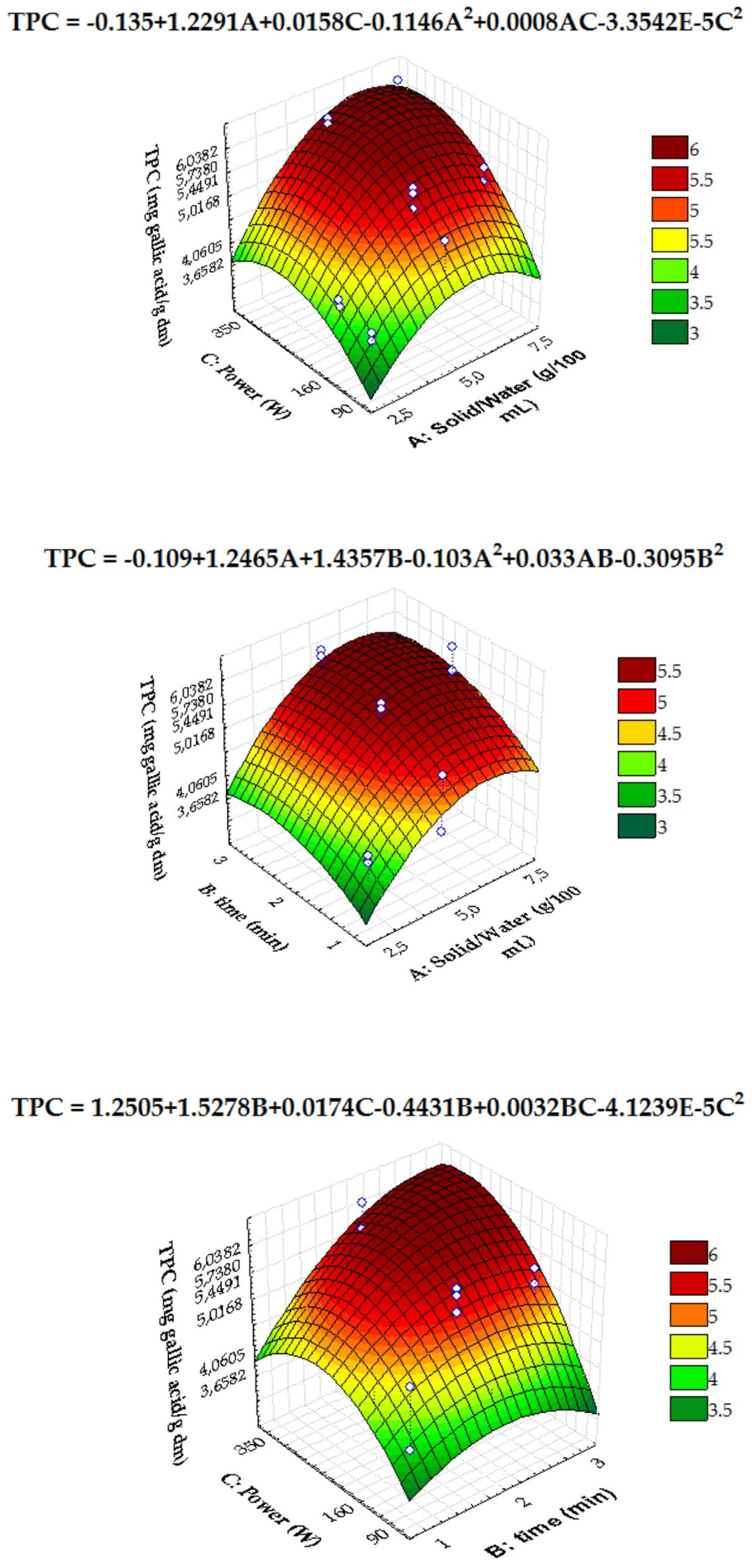
3.3.1. MAE Extracts of Chicory and Fennel By-Products
3.3.2. Predictive ability of the models applied to MAE
3.3.3. Final Consideration on the Influence of the Independent Variables on the Phenolic Content and Antioxidant Activity of the Extracts
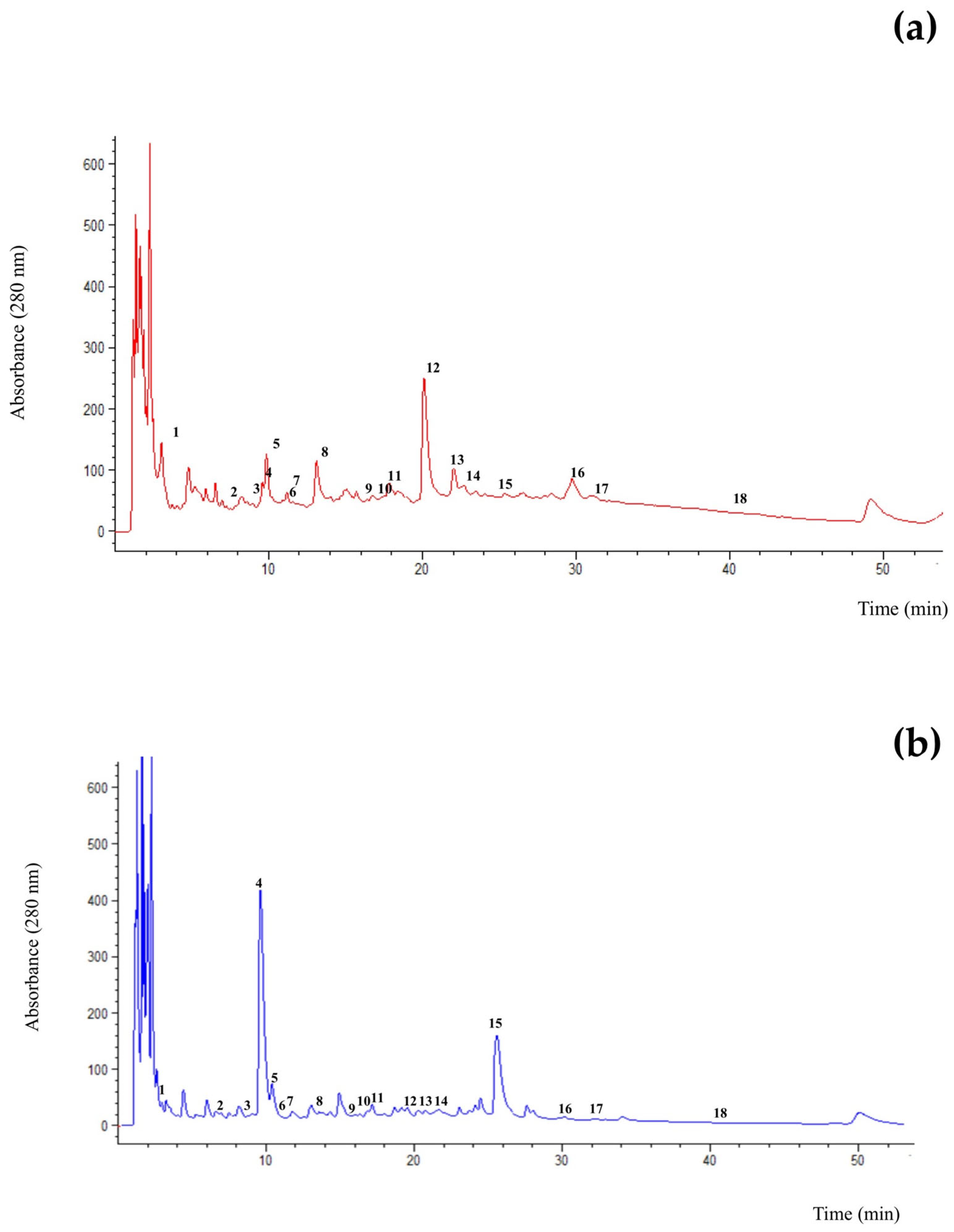
3.4. Phenolic Profiles of the Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| AA | Antioxidant activity |
| ANOVA | Analysis of variance |
| BBD | Box–Behnken design |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FC | Folin–Ciocalteu |
| LSD | Least significant difference |
| MAE | Microwave-assisted extraction |
| RSM | Response surface methodology |
| TPC | Total phenolic content |
| Trolox | 6-hydroxy-2,5,7,8-tetramethylchroman2-carboxylic acid |
| UAE | Ultrasound-assisted extraction |
References
- Gowe, C. Review on potential use of fruit and vegetables by-products as a valuable source of natural food additives. Food Sci. Qual. Manag. 2015, 45, 47–61. [Google Scholar]
- Ayala-Zavala, J.F.; Rosas-Dominquez, C.; Vega-Vega, V.; Gonzalez- Aguilar, G.A. Antioxidant enrichment and antimicrobial protection of fresh-cut fruits using their own by-products: Looking for integral exploitation. J. Food Sci. 2010, 75, R175–R181. [Google Scholar] [CrossRef]
- Dilucia, F.; Lacivita, V.; Conte, A.; Del Nobile, M.A. Sustainable use of fruit and vegetable by-products to enhance food packaging performance. Foods 2020, 9, 857. [Google Scholar] [CrossRef]
- Khan, M.K.I.; Maan, A.A.; Aadil, R.M.; Nazir, A.; Butt, M.S.; Rashid, M.I.; Afzal, M.I. Modelling and kinetic study of microwave assisted drying of ginger and onion with simultaneous extraction of bioactive compounds. Food Sci. Biotechnol. 2020, 29, 513–519. [Google Scholar] [CrossRef]
- Ahmad, T.; Belwal, T.; Li, L.; Ramola, S.; Aadil, R.M.; Abdullah; Xu, Y.; Zisheng, L. Utilization of wastewater from edible oil industry, turning waste into valuable products: A review. Trends Food Sci. Technol. 2020, 99, 21–33. [Google Scholar] [CrossRef]
- Perović, J.; Šaponjac, V.T.; Kojić, J.; Krulj, J.; Moreno, D.A.; García-Viguera, C.; Bodroža-Solarov, M.; Ilić, N. Chicory (Cichorium intybus L.) as a food ingredient—Nutritional composition, bioactivity, safety, and health claims: A review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef]
- Saybel, O.L.; Rendyuk, T.D.; Dargaeva, T.D.; Nikolaev, S.M.; Khobrakova, V.B. Phenolic compounds and immunomodulating activity of chicory (Cichorium intybus L.). Extr. Pharmacogn. J. 2020, 12, 1104–1107. [Google Scholar] [CrossRef]
- Bayazid, A.B.; Park, S.H.; Kim, J.G.; Lim, B.O. Green chicory leaf extract exerts anti-inflammatory effects through suppressing LPS- induced MAPK/NF-κB activation and hepatoprotective activity in vitro. Food Agric. Immunol. 2020, 31, 513–532. [Google Scholar] [CrossRef]
- Street, R.A.; Sidana, J.; Prinsloo, G. Cichorium intybus: Traditional uses, phytochemistry, pharmacology, and toxicology. Evid.-Based Complement. Altern. Med. 2013, 2013, 579319. [Google Scholar] [CrossRef] [PubMed]
- Epure, A.; Pârvu, A.E.; Vlase, L.; Benedec, D.; Hanganu, D.; Gheldiu, A.-M.; Toma, V.A.; Oniga, I. Phytochemical profile, antioxidant, cardioprotective and nephroprotective activity of Romanian chicory extract. Plants 2021, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Kandil, A.S.; Abou-Elella, F.; El Shemy, H.A. Cytotoxic profile activities of ethanolic and methanolic extracts of chicory plant (Cichorium intybus L.). J. Radiat. Res. Appl. Sci. 2019, 12, 106–111. [Google Scholar] [CrossRef]
- Häkkinen, S.T.; Cankar, K.; Nohynek, L.; Suomalainen, M.; van Arkel, J.; Siika-Aho, M.; Twarogowska, A.; Van Droogenbroeck, B.; Oksman-Caldentey, K.M. Enzyme-treated chicory for cosmetics: Application assessment and techno-economic analysis. AMB Express 2022, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Review of the Scientific Evidence on the Physiological Effects of Certain Non-Digestible Carbohydrates. 2018; pp. 1–52. Available online: https://www.fda.gov/food/food-labeling-nutrition/review-scientific-evidence-physiological-effects-certain-non-digestible-carbohydrates (accessed on 1 March 2023).
- Chiboub, W.; Sassi, A.B.; Amina, C.M.; Souilem, F.; El Ayeb, A.; Djlassi, B.; Ascrizzi, R.; Flamini, G.; Harzallah-Skhiri, F. Valorization of the green waste from two varieties of fennel and carrot cultivated in Tunisia by identification of the phytochemical profile and evaluation of the antimicrobial activities of their essentials oils. Chem. Biodivers. 2019, 16, e1800546. [Google Scholar] [CrossRef] [PubMed]
- Malin, V.; Elez Garofulic, I.; Repajc, M.; Zorc, Z.; Pedisc, S.; Sterniša, M.; Smole Možina, S.; Dragovc-Uzelac, V. Phenolic characterization and bioactivity of fennel seed (Foeniculum vulgare mill.) extracts isolated by microwave-assisted and conventional extraction. Processes 2022, 10, 510. [Google Scholar] [CrossRef]
- Salami, M.; Rahimmalek, M.; Ehtemam, M.H. Inhibitory effect of different fennel (Foeniculum vulgare) samples and their phenolic compounds on formation of advanced glycation products and comparison of antimicrobial and antioxidant activities. Food Chem. 2016, 213, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, M.A.; D’Urso, G.; Piacente, S.; Montoro, P. LC-ESI/LTQOrbitrap/MS metabolomic analysis of fennel waste (Foeniculum vulgare Mill.) as a byproduct rich in bioactive compounds. Foods 2021, 10, 1893. [Google Scholar] [CrossRef]
- Zia, S.; Khan, M.R.; Shabbir, M.A.; Aslam Maan, A.; Khan, M.K.I.; Nadeem, M.; Khalil, A.A.; Din, A.; Aadil, R.M. An inclusive overview of advanced thermal and nonthermal extraction techniques for bioactive compounds in food and food-related matrices. Food Rev. Int. 2022, 38, 1166–1196. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Cova, C.M.; Boffa, L.; Pistocchi, M.; Giorgini, S.; Luque, R.; Cravotto, G. Technology and process design for phenols recovery from industrial chicory (Chicorium intybus) leftovers. Molecules 2019, 24, 2681. [Google Scholar] [CrossRef]
- Pradal, D.; Vauchel, P.; Decossin, S.; Dhulster, P.; Dimitrov, K. Kinetics of ultrasound-assisted extraction of antioxidant polyphenols from food by-products: Extraction and energy consumption optimization. Ultrason. Sonochem. 2016, 32, 137–146. [Google Scholar] [CrossRef]
- Urango, A.C.M.; Strieder, M.M.; Silva, E.K.; Meireles, M.A.A. Thermosonication process design for recovering bioactive compounds from fennel: A comparative study with conventional extraction techniques. Appl. Sci. 2021, 11, 12104. [Google Scholar] [CrossRef]
- Diemer, E.; Chadni, M.; Grimi, N.; Ioannou, I. optimization of the accelerated solvent extraction of caffeoylquinic acids from forced chicory roots and antioxidant activity of the resulting extracts. Foods 2022, 11, 3214. [Google Scholar] [CrossRef]
- Saafi, E.B.; El Arem, A.; Issaoui, M.; Hammami, M.; Achour, L. Phenolic content and antioxidant activity of four date palm (Phoenix dactylifera L.) fruit varieties grown in Tunisia. Int. J. Food Sci. Technol. 2009, 44, 2314–2319. [Google Scholar] [CrossRef]
- Rao, P.R.; Rathod, V.K. Mapping study of an ultrasonic bath for the extraction of andrographolide from Andrographis paniculata using ultrasound. Ind. Crops Prod. 2015, 66, 312–318. [Google Scholar] [CrossRef]
- Romani, A.; Vignolini, P.; Isolani, L.; Ieri, F.; HeimLer, D. HPLC-DAD/MS characterization of flavonoids and hydroxycinnamic derivatives in turnip tops (Brassica rapa L. subsp. sylvestris L.). J. Agric. Food Chem. 2006, 54, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.P.; Diñeiro García, Y.; Sánchez, J.M.; Madrera, R.R.; Valles, B.S. Phenolic and antioxidant composition of cider. J. Food Compos. Anal. 2009, 22, 644–648. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Casazza, A.A.; Perego, P. Valorization of olive oil solid waste using high pressure–high temperature reactor. Food Chem. 2011, 128, 704–710. [Google Scholar] [CrossRef]
- Silva, E.K.; Saldaña, M.D. High-Intensity Ultrasound-assisted recovery of cinnamyl alcohol glycosides from Rhodiola rosea roots: Effect of probe diameter on the ultrasound energy performance for the extraction of bioactive compounds. Food Bioprod. Process. 2020, 122, 245–253. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C. The nutritional composition of fennel (Foeniculum vulgare): Shoots, leaves, stems and inflorescences. LWT—Food Sci. Technol. 2010, 43, 814–818. [Google Scholar] [CrossRef]
- Wang, L.F.; Kim, D.M.; Lee, C.Y. Effects of heat processing and storage on flavanols and sensory qualities of green tea beverage. J. Agric. Food Chem. 2000, 48, 4227–4232. [Google Scholar] [CrossRef]
- Akowuah, G.A.; Mariam, A.; Chin, J.H. The effect of extraction temperature on total phenols and antioxidant activity of Gynura procumbens leaf. Pharmacogn. Mag. 2009, 4, 81–85. [Google Scholar]
- Hernández-Carranza, P.; Ávila-Sosa, R.; Guerrero-Beltrán, J.A.; Navarro-Cruz, A.R.; Corona-Jiménez, E.; Ochoa-Velasco, C.E. Optimization of antioxidant compounds extraction from fruit by-products: Apple pomace, orange and banana peel. J. Food Process. Preserv. 2016, 40, 103–115. [Google Scholar] [CrossRef]
- Vauchel, P.; Colli, C.; Pradal, D.; Philippot, M.; Decossin, S.; Dhulster, P.; Dimitrov, K. Comparative LCA of ultrasound-assisted extraction of polyphenols from chicory grounds under different operational conditions. J. Clean. Prod. 2018, 196, 1116–1123. [Google Scholar] [CrossRef]
- Ramic, M.; Vidovic, S.; Zekovic, Z.; Vladic, J.; Cvejin, A.; Pavlic, B. Modeling and optimization of ultrasound-assisted extraction of polyphenolic compounds from Aronia melanocarpa by-products from filter-tea factory. Ultrason. Sonochem. 2015, 23, 360–368. [Google Scholar] [CrossRef]
- Jangra, S.S.; Madan, V.K. Effect of extraction techniques on total phenolics, flavonoids and antioxidant potential of various plant parts of chicory (Cichorium intybus L.). Int. J. Chem. Stud. 2018, 6, 1734–1740. [Google Scholar]
- Di Donato, P.; Taurisano, V.; Tommonaro, G.; Pasquale, V.; Jiménez, J.M.S.; de Pascual-Teresa, S.; Poli, A.; Nicolaus, B. Biological properties of polyphenols extracts from agro industry’s wastes. Waste Biomass Valorization 2018, 9, 1567–1578. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC—Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Akhtar, I.; Javad, S.; Ansari, M.; Ghaffar, N.; Tariq, A. Process optimization for microwave assisted extraction of Foeniculum vulgare Mill using response surface methodology. J. King Saud Univ. Sci. 2020, 32, 1451–1458. [Google Scholar] [CrossRef]
- Boonkird, S.; Phisalaphong, C.; Phisalaphong, M. Ultrasound-assisted extraction of capsaicinoids from Capsicum frutescens on a lab- and pilot-plant scale. Ultrason. Sonochem. 2008, 15, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- d’Alessandro, L.G.; Kriaa, K.; Nikov, I.; Dimitrov, K. Ultrasound assisted extraction of polyphenols from black chokeberry. Sep. Purif. Technol. 2012, 93, 42–47. [Google Scholar] [CrossRef]
- Castaldo, L.; Izzo, L.; De Pascale, S.; Narváez, A.; Rodriguez-Carrasco, Y.; Ritieni, A. Chemical Composition, In vitro bioaccessibility and antioxidant activity of polyphenolic compounds from nutraceutical fennel waste extract. Molecules 2021, 26, 1968. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Blasi, F.; Montesano, D.; Ghisoni, S.; Marcotullio, M.C.; Sabatini, S.; Cossignani, L.; Lucini, L. Impact of conventional/non-conventional extraction methods on the untargeted phenolic profile of Moringa oleifera leaves. Food Res. Int. 2019, 115, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
| Extraction Conditions | Chicory By-Products | Fennel By-Products | ||
|---|---|---|---|---|
| TPC | AA | TPC | AA | |
| Interactive effect of temperature and time of treatment | ||||
| T = 45 °C; t = 30 min | 2.35 ± 0.09 a | 0.0067 ± 0.0005 a | 1.49 ± 0.16 ab | 0.0051 ± 0.0001 a |
| T = 45 °C; t = 60 min | 2.99 ± 0.21 c | 0.0085 ± 0.0004 b | 1.39 ± 0.15 a | 0.0054 ± 0.0000 a |
| T = 45 °C; t = 90 min | 3.93 ± 0.25 d | 0.0124 ± 0.0010 b | 2.09 ± 0.12 b | 0.0074 ± 0.0004 b |
| T = 45 °C; t = 120 min | 2.54 ± 0.16 ab | 0.0090 ± 0.0006 a | 2.78 ± 0.32 c | 0.0095 ± 0.0005 c |
| T = 45 °C; t = 180 min | 2.73 ± 0.23 bc | 0.0126 ± 0.0002 b | 2.85 ± 0.44 c | 0.0108 ± 0.0010 d |
| T = 60 °C; t = 30 min | 5.64 ± 0.33 f | 0.0281 ± 0.0001 c | 5.76 ± 0.13 d | 0.0282 ± 0.0002 f |
| T = 60 °C; t = 60 min | 4.93 ± 0.16 e | 0.0281 ± 0.0000 c | 6.93 ± 0.06 ef | 0.0277 ± 0.0000 e |
| T = 60 °C; t = 90 min | 5.77 ± 0.23 fg | 0.0277 ± 0.0004 c | 7.52 ± 0.85 f | 0.0277 ± 0.0000 e |
| T = 60 °C; t = 120 min | 5.56 ± 0.26 f | 0.0281 ± 0.0000 c | 6.71 ± 0.57 e | 0.0274 ± 0.0000 e |
| T = 60 °C; t = 180 min | 6.14 ± 0.25 g | 0.0280 ± 0.0000 c | 6.29 ± 0.30 de | 0.0275 ± 0.0000 e |
| Significance | * | * | * | * |
| Single effect of temperature | ||||
| T = 45 °C | 2.91 a | 0.0099 a | 2.12 a | 0.0076 a |
| T = 60 °C | 5.61 b | 0.0280 b | 6.64 b | 0.0277 b |
| Significance | * | * | * | * |
| Single effect of time | ||||
| t = 30 min | 3.99 a | 0.0174 a | 3.63 a | 0.0167 a |
| t = 60 min | 3.96 a | 0.0183 ab | 4.16 b | 0.0165 a |
| t = 90 min | 4.85 c | 0.01996 b | 4.80 c | 0.0175 b |
| t = 120 min | 4.05 a | 0.01856 ab | 4.75 c | 0.0185 c |
| t = 180 min | 4.44 b | 0.0203 b | 4.57 bc | 0.0191 d |
| Significance | * | * | * | * |
| Extraction Conditions | Total Phenolic Content (mg Gallic Acid/g gm) | Antioxidant Activity (mmol Trolox/g dm) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chicory By-Product Extracts | Fennel By-Product Extracts | Chicory By-Product Extracts | Fennel By-Product Extracts | ||||||||||||
| Solid/Water (g/100 mL) | T (°C) | t (min) | Power (W) | Observed | Predicted | %var. | Observed | Predicted | %var. | Observed | Predicted | %var. | Observed | Predicted | %var. |
| 10 | 35 | 40 | 120 | 5.25 ± 0.08 | 5.48 | 4.40 | 4.59 ± 0.02 | 4.10 | −10.65 | 0.0203 ± 0.0006 | 0.0181 | −10.85 | 0.0268 ± 0.0000 | 0.0310 | 15.50 |
| 10 | 45 | 60 | 24 | 6.21 ± 0.28 | 7.06 | 13.72 | 5.41 ± 0.24 | 4.80 | −11.30 | 0.0202 ± 0.0002 | 0.0189 | −6.20 | 0.0293 ± 7.8 × 10−5 | 0.0330 | 12.74 |
| 15 | 35 | 40 | 72 | 7.17 ± 0.00 | 6.48 | −9.56 | 5.75 ± 0.11 | 5.86 | 1.83 | 0.0162 ± 0.0002 | 0.0143 | −11.95 | 0.0198 ± 2 × 10−5 | 0.0233 | 17.71 |
| 5 | 45 | 40 | 24 | 4.18 ± 1.12 | 4.14 | −0.96 | 5.00 ± 0.16 | 4.33 | −13.39 | 0.0312 ± 0.0054 | 0.0308 | −1.23 | 0.0534 ± 0.0000 | 0.0512 | −4.06 |
| 5 | 45 | 20 | 72 | 3.60 ± 0.13 | 3.65 | 1.26 | 3.57 ± 0.06 | 3.04 | −14.72 | 0.0319 ± 0.0000 | 0.0308 | −3.39 | 0.0396 ± 0.0010 | 0.0435 | 9.88 |
| 5 | 35 | 40 | 72 | 2.15 ± 0.00 | 2.37 | 10.38 | 2.80 ± 0.03 | 3.04 | 8.74 | 0.0274 ± 0.0003 | 0.0260 | −5.18 | 0.0460 ± 0.0003 | 0.0512 | 11.37 |
| 10 | 45 | 40 | 72 | 4.93 ± 0.20 | 5.52 | 11.88 | 5.17 ± 0.06 | 4.45 | −13.92 | 0.0191 ± 0.0005 | 0.0189 | −0.80 | 0.0268 ± 0.0000 | 0.0310 | 15.50 |
| 10 | 45 | 20 | 24 | 5.73 ± 0.21 | 5.02 | −12.37 | 5.50 ± 0.08 | 4.80 | −12.75 | 0.0200 ± 0.0001 | 0.0189 | −5.26 | 0.0268 ± 0.0000 | 0.0289 | 7.74 |
| 15 | 45 | 60 | 72 | 8.02 ± 0.07 | 7.39 | −7.90 | 5.02 ± 0.18 | 5.86 | 16.64 | 0.0121 ± 0.0001 | 0.0111 | −8.04 | 0.0180 ± 2.8 × 10−5 | 0.0197 | 9.69 |
| 15 | 45 | 40 | 120 | 6.24 ± 0.05 | 6.37 | 2.02 | 6.64 ± 0.22 | 6.44 | −2.96 | 0.0120 ± 0.0000 | 0.0111 | −7.28 | 0.0198 ± 2.4 × 10−5 | 0.0233 | 17.71 |
| 10 | 35 | 20 | 72 | 5.53 ± 0.18 | 5.16 | −6.73 | 5.22 ± 0.00 | 4.45 | −14.75 | 0.0207 ± 0.0004 | 0.0181 | −12.57 | 0.0281 ± 2.0 × 10−5 | 0.0289 | 2.76 |
| 10 | 45 | 40 | 72 | 5.01 ± 0.02 | 5.52 | 10.10 | 5.16 ± 0.10 | 4.45 | −13.76 | 0.0208 ± 0.0002 | 0.0189 | −8.90 | 0.0294 ± 0.0000 | 0.0310 | 5.29 |
| 10 | 55 | 20 | 72 | 3.53 ± 0.08 | 3.83 | 8.57 | 4.01 ± 0.03 | 4.45 | 10.97 | 0.0234 ± 0.0000 | 0.0220 | −5.97 | 0.0270 ± 1.4 × 10−5 | 0.0289 | 6.95 |
| 5 | 55 | 40 | 72 | 6.80 ± 0.20 | 6.96 | 2.34 | 3.34 ± 0.64 | 3.04 | −8.84 | 0.0424 ± 0.0013 | 0.0379 | −10.71 | 0.0509 ± 7.8 × 10−5 | 0.0512 | 0.65 |
| 5 | 45 | 60 | 72 | 5.08 ± 0.07 | 5.69 | 11.94 | 3.28 ± 0.27 | 3.04 | −7.18 | 0.0326 ± 0.0020 | 0.0308 | −5.47 | 0.0522 ± 3.7 × 10−4 | 0.0590 | 12.94 |
| 15 | 45 | 20 | 72 | 4.64 ± 0.07 | 5.35 | 15.20 | 5.71 ± 0.03 | 5.86 | 2.55 | 0.0126 ± 0.0001 | 0.0111 | −11.69 | 0.0239 ± 3.3 × 10−5 | 0.0269 | 12.42 |
| 10 | 35 | 60 | 72 | 4.84 ± 0.00 | 3.70 | −23.55 | 4.35 ± 0.06 | 4.45 | 2.30 | 0.0205 ± 0.0000 | 0.0181 | −11.72 | 0.0295 ± 1.0 × 10−5 | 0.0330 | 11.98 |
| 10 | 45 | 40 | 72 | 6.38 ± 0.50 | 5.52 | −13.54 | 4.11 ± 0.02 | 4.45 | 8.27 | 0.0223 ± 0.0000 | 0.0189 | −15.03 | 0.0296 ± 1.6 × 10−4 | 0.0310 | 4.58 |
| 15 | 55 | 40 | 72 | 6.23 ± 0.27 | 6.25 | 0.27 | 5.12 ± 0.01 | 5.86 | 14,.37 | 0.0121 ± 0.0000 | 0.0102 | −15.73 | 0.0197 ± 6.7 × 10−6 | 0.0233 | 18.30 |
| 10 | 45 | 60 | 120 | 7.27 ± 0.62 | 7.59 | 4.38 | 4.25 ± 0.06 | 4.10 | −3.50 | 0.0212 ± 0.0003 | 0.0189 | −10.62 | 0.0295 ± 5.9 × 10−5 | 0.0330 | 11.98 |
| 15 | 45 | 40 | 24 | 7.37 ± 0.31 | 7.94 | 7.78 | 6.15 ± 0.15 | 5.27 | −14.35 | 0.0121 ± 0.0001 | 0.0111 | −8.04 | 0.0196 ± 4.0 × 10−5 | 0.0233 | 18.91 |
| 10 | 55 | 40 | 120 | 8.40 ± 0.15 | 7.66 | −8.87 | 4.90 ± 0.06 | 4.10 | −16.30 | 0.0234 ± 0.0000 | 0.0220 | −5.97 | 0.0265 ± 2.0 × 10−5 | 0.0310 | 16.81 |
| 10 | 55 | 40 | 24 | 6.76 ± 0.52 | 7.13 | 5.45 | 4.60 ± 0.03 | 4.80 | 4.32 | 0.0248 ± 0.0001 | 0.0220 | −11.27 | 0.0264 ± 1.0 × 10−5 | 0.0310 | 17.25 |
| 10 | 35 | 40 | 24 | 3.43 ± 0.08 | 4.95 | 11.84 | 4.10 ± 0.06 | 4.80 | 17.05 | 0.0211 ± 0.0009 | 0.0181 | −14.23 | 0.0266 ± 2.0 × 10−5 | 0.0310 | 16.37 |
| 10 | 55 | 60 | 72 | 9.07 ± 0.54 | 9.37 | 3.34 | 4.96 ± 0.11 | 4.45 | −10.28 | 0.0235 ± 0.0002 | 0.0220 | −6.37 | 0.0296 ± 1.1 × 10−4 | 0.0330 | 11.60 |
| 10 | 45 | 20 | 120 | 6.23 ± 0.03 | 5.55 | −10.95 | 3.77 ± 0.05 | 4.10 | 8.79 | 0.0170 ± 0.0006 | 0.0189 | 11.46 | 0.0266 ± 2.0 × 10−5 | 0.0289 | 8.55 |
| 5 | 45 | 40 | 120 | 6.67 ± 0.95 | 6.77 | 1.50 | 2.13 ± 0.22 | 1.76 | −17.42 | 0.0354 ± 0.0017 | 0.0308 | −12.95 | 0.0523 ± 3.6 × 10−4 | 0.0512 | −2.04 |
| Mathematical Models | |||||||||||||||
| F | p-value | F | p-value | F | p-value | F | p-value | ||||||||
| Intercept | 0.804268 | 0.10792 | 25.37001 | 0.000048 * | 79.92673 | 0.000000 * | 36.82169 | 0.000006 * | |||||||
| A: solid/water (g/100 mL) | 11.83276 | 0.002592 * | 18.92259 | 0.000310 * | |||||||||||
| B: temperature (°C) | |||||||||||||||
| C: time (min) | 10.10396 | 0.004720 * | 3.90629 | 0.060776 | 14.31691 | 0.001166 * | |||||||||
| D: power (W) | 5.31236 | 0.030989 * | 4.26187 | 0.052190 | |||||||||||
| A * B | 6.53923 | 0.018784 * | 23.36542 | 0.000079 * | |||||||||||
| A * C | 2.89507 | 0.102943 | 11.05817 | 0.003374 * | |||||||||||
| A * D | 4.38972 | 0.049090 * | 4.52823 | 0.044787 * | |||||||||||
| B * C | 13.76017 | 0.001386 * | 3.76534 | 0.065248 | |||||||||||
| B * D | |||||||||||||||
| A2 | 6.05110 | 0.022225 * | 22.16692 | 0.000135 * | |||||||||||
| B2 | 29.09408 | 0.000020 * | |||||||||||||
| C2 | |||||||||||||||
| D2 | 5.44664 | 0.030153 * | 4.47819 | 0.057068 | |||||||||||
| Statistics of the Quadratic Models | |||||||||||||||
| Degree of freedom (df) | 6 | 4 | 4 | 6 | |||||||||||
| F-value | 6.117842 | 1.558337 | 34.88745 | 40.40381 | |||||||||||
| p-value | 0.000909 * | 0.220484 | 0.000000 * | 0.000000 * | |||||||||||
| R2 | 0.8473 | 0.9208 | 0.8638 | 0.9238 | |||||||||||
| Extraction Conditions | Total Phenolic Content (mg Gallic Acid/g gm) | Antioxidant Activity (mmol Trolox/g dm) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chicory By-Product Extracts | Fennel By-Product Extracts | Chicory By-Product Extracts | Fennel By-Product Extracts | |||||||||||
| Solid/Water (g/100 mL) | t (min) | Power (W) | Observed | Predicted | %var. | Observed | Predicted | %var. | Observed | Predicted | %var. | Observed | Predicted | %var. |
| 7.5 | 2 | 350 | 7.74 ± 0.28 | 8.7415 | 12.94 | 5.74 ± 0.35 | 6.08 | 5.94 | 0.0224 ± 0.0002 | 0.0237 | 5.63 | 0.0352 ± 0.0000 | 0.0358 | 1.75 |
| 5 | 2 | 160 | 5.70 ± 0.59 | 5.8946 | 3.41 | 5.02 ± 0.36 | 5.09 | 1.49 | 0.0313 ± 0.0014 | 0.0279 | −10.99 | 0.0528 ± 0.000 | 0.0494 | −6.42 |
| 5 | 3 | 350 | 7.44 ± 0.28 | 8.8431 | 18.86 | 5.48 ± 0.28 | 5.09 | −7.03 | 0.0329 ± 0.0006 | 0.0279 | −15.32 | 0.0426 ± 0.0010 | 0.0494 | 15.99 |
| 7.5 | 2 | 350 | 8.23 ± 0.20 | 8.7415 | 6.21 | 5.88 ± 0.14 | 6.08 | 3.42 | 0.0249 ± 0.0007 | 0.0237 | −4.98 | 0.0349 ± 0.0000 | 0.0358 | 2.62 |
| 7.5 | 3 | 160 | 5.15 ± 0.24 | 5.7929 | 12.48 | 6.73 ± 0.30 | 6.08 | −9.65 | 0.0247 ± 0.0004 | 0.0237 | −4.21 | 0.0353 ± 0.0000 | 0.0358 | 1.46 |
| 2.5 | 1 | 160 | 4.11 ± 0.27 | 4.7912 | 16.58 | 4.95 ± 1.38 | 4.11 | −17.00 | 0.0329 ± 0.0001 | 0.0321 | −2.55 | 0.0531 ± 0.0020 | 0.0576 | 8.42 |
| 5 | 2 | 160 | 5.57 ± 0.10 | 5.8946 | 5.83 | 6.16 ± 0.24 | 5.09 | −17.30 | 0.0293 ± 0.0013 | 0.0279 | −4.91 | 0.0531 ± 0.0000 | 0.0494 | −6.95 |
| 2.5 | 2 | 90 | 3.66 ± 0.33 | 3.7049 | 1.23 | 4.47 ± 0.76 | 4.11 | −8.09 | 0.0286 ± 0.0019 | 0.0321 | 12.10 | 0.0671 ± 0.0000 | 0.0576 | −14.20 |
| 5 | 1 | 90 | 5.02 ± 0.07 | 4.8083 | −4.22 | 4.95 ± 0.09 | 5.09 | 2.92 | 0.0286 ± 0.0007 | 0.0279 | −2.59 | 0.0528 ± 0.0000 | 0.0494 | −6.42 |
| 2.5 | 2 | 90 | 3.84 ± 0.40 | 3.7049 | −3.52 | 3.96 ± 0.19 | 4.11 | 3.75 | 0.0375 ± 0.0022 | 0.0321 | −14.51 | 0.0498 ± 0.0010 | 0.0576 | 15.60 |
| 2.5 | 1 | 160 | 4.06 ± 0.21 | 4.7912 | 18.01 | 3.89 ± 0.14 | 4.11 | 5.61 | 0.0338 ± 0.0003 | 0.0321 | −5.15 | 0.0578 ± 0.0050 | 0.0576 | −0.40 |
| 5 | 3 | 350 | 7.91 ± 0.21 | 8.8431 | 11.80 | 6.33 ± 0.25 | 5.09 | −19.52 | 0.0265 ± 0.0054 | 0.0279 | 5.13 | 0.0527 ± 0.0000 | 0.0494 | −6.24 |
| 7.5 | 3 | 160 | 5.45 ± 0.02 | 5.7929 | 6.29 | 6.01 ± 0.08 | 6.08 | 1.18 | 0.0242 ± 0.0017 | 0.0237 | −2.23 | 0.0352 ± 0.0000 | 0.0358 | 1.75 |
| 5 | 1 | 90 | 4.74 ± 0.26 | 4.8083 | 1.44 | 4.49 ± 0.30 | 5.09 | 13.47 | 0.0255 ± 0.0020 | 0.0279 | 9.25 | 0.0459 ± 0.0020 | 0.0494 | 7.65 |
| 5 | 2 | 160 | 5.26 ± 0.20 | 5.8946 | 12.06 | 5.68 ± 0.30 | 5.09 | −10.31 | 0.0288 ± 0.0014 | 0.0279 | −3.26 | 0.0530 ± 0.0000 | 0.0494 | −6.77 |
| Mathematical Models | ||||||||||||||
| F | p-value | F | p-value | F | p-value | F | p-value | |||||||
| Intercept | 0.94309 | 0.359927 | 11.37521 | 0.004998 * | 130.7809 | 0.000000 * | 648.6510 | 0.000000 * | ||||||
| A: solid/water (g/100 mL) | 15.06193 | 0.004668 * | 5.14653 | 0.040975 * | 7.9544 | 0.014455 * | ||||||||
| B: time (min) | 2.69053 | 0.139576 | ||||||||||||
| C: power (W) | 4.89658 | 0.047826 * | ||||||||||||
| A * B | ||||||||||||||
| A * C | ||||||||||||||
| B * C | ||||||||||||||
| A2 | 10.77116 | 0.011155 * | 38.7114 | 0.000031 * | ||||||||||
| B2 | 2.13493 | 0.182111 | ||||||||||||
| C2 | 2.74431 | 0.136192 | ||||||||||||
| Statistics of the Quadratic Models | ||||||||||||||
| Degree of freedom (df) | 6 | 1 | 1 | 1 | ||||||||||
| F-value | 14.25905 | 5.146529 | 7.954352 | 38.71144 | ||||||||||
| p-value | 0.000696 * | 0.040975 * | 0.014455 * | 0.000031 | ||||||||||
| R2 | 0.9145 | 0.7836 | 0.9796 | 0.7486 | ||||||||||
| Phenolic Compounds (Retention Time in min) | Extraction Techniques | |||||
|---|---|---|---|---|---|---|
| Conventional | UAE | MAE | ||||
| Chicory | Fennel | Chicory | Fennel | Chicory | Fennel | |
| T: 60 °C t: 180 min | T: 60 °C t: 90 min | Solid/Water: 10 g/100 mL T: 55 °C t: 60 min Power: 72 W | Solid/Water: 5 g/100 mL T: 45 °C t: 40 min Power: 24 W | Solid/Water: 7.5 g/100 mL t: 2 min Power: 350 W | Solid/Water: 7.5 g/100 mL t: 3 min Power: 160 W | |
| Gallic acid (3.04) | n.d. a | n.d. A | 0.052 ± 0.010 b | 0.022 A ± 0.001 | 0.011 ± 0.000 a | 0.010 ± 0.000 A |
| 4-Hydoxybenzoic acid (7.20) | 0.058 ± 0.001 b | n.d. A | 0.022 ± 0.001 a | 0.023 ± 0.001 A | 0.010 ± 0.001 a | n.d. A |
| Catechin (8.24) | 0.204 ± 0.003 b | 0.019 ± 0.001 AB | 0.049 ± 0.014 a | n.d. A | 0.030 ± 0.003 a | 0.040 ± 0.002 B |
| Vanillic acid (9.36) | 0.060 ± 0.001 b | 0.085 ± 0.001 B | 0.022 ± 0.002 a | 0.011 ± 0.001 A | 0.010 ± 0.002 a | n.d. A |
| Caffeic acid (10.14) | 0.017 ± 0.001 a | 0.009 ± 0.001 A | 0.031 ± 0.001 a | 0.039 ± 0.001 B | 0.020 ± 0.001 a | 0.053 ± 0.003 B |
| Syringic acid (11.13) | 0.018 ± 0.001 a | n.d. A | n.d. a | n.d. A | n.d. a | 0.012 ± 0.001 A |
| Epicatechin (11.97) | 0.181 ± 0.069 b | 0.077 ± 0.001 A | 0.174 ± 0.033 b | 0.139 ± 0.008 B | 0.100 ± 0.000 a | 3.729 ± 0.072 C |
| Chlorogenic acid (12.35) | n.d. a | n.d. A | 0.321 ± 0.014 b | n.d. A | n.d. a | 0.022 ± 0.000 A |
| Epigallocatechin (16.00) | 0.537 ± 0.001 b | n.d. A | 0.012 ± 0.000 a | n.d. A | n.d. a | 0.100 ± 0.002 B |
| Ferulic acid (16.31) | 0.038 ± 0.001 a | n.d. A | 0.029 ± 0.002 a | 0.038 ± 0.000 B | 0.040 ± 0.001 a | 0.030 ± 0.000 B |
| p-Coumaric acid (16.83) | 0.093 ± 0.005 c | n.d. A | 0.043 ± 0.001 b | 0.018 ± 0.001 A | n.d. a | 0.011 ± 0.000 A |
| Sinapic acid (20.68) | 0.258 ± 0.007 b | 0.161 ± 0.001 B | 0.789 ± 0.035 c | 0.029 ± 0.001 A | n.d. a | 0.010 ± 0.000 A |
| Epigallocatechingallate (21.57) | n.d. a | 0.148 ± 0.001 B | 0.155 ± 0.003 b | n.d. A | n.d. a | n.d. A |
| Rutin (22.14) | 0.355 ± 0.001 c | 0.040 ± 0.001 A | 0.143 ± 0.031 b | 0.025 ± 0.004 A | n.d. a | 0.030 ± 0.002 A |
| Resveratrol (25.90) | 0.032 ± 0.001 b | 0.046 ± 0.001 A | 0.029 ± 0.004 b | 0.047 ± 0.002 A | n.d. a | 0.040 ± 0.001 A |
| Rosmarinic acid (29.50) | n.d. a | n.d. A | 1.527 ± 0.088 b | n.d. A | 0.071 ± 0.011 a | n.d. A |
| Quercetin (31.70) | 0.04 ± 0.002 b | 0.021 ± 0.000 A | 0.082 ± 0.002 c | 0.029 ± 0.000 A | n.d. a | 0.019 ± 0.001 A |
| Kaempferol (40.07) | 0.017 ± 0.001 a | n.d. A | 0.013 ± 0.001 a | n.d. A | n.d. a | 0.060 ± 0.002 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baiano, A.; Romaniello, R.; Giametta, F.; Fiore, A. Optimization of Process Variables for the Sustainable Extraction of Phenolic Compounds from Chicory and Fennel By-Products. Appl. Sci. 2023, 13, 4191. https://doi.org/10.3390/app13074191
Baiano A, Romaniello R, Giametta F, Fiore A. Optimization of Process Variables for the Sustainable Extraction of Phenolic Compounds from Chicory and Fennel By-Products. Applied Sciences. 2023; 13(7):4191. https://doi.org/10.3390/app13074191
Chicago/Turabian StyleBaiano, Antonietta, Roberto Romaniello, Ferruccio Giametta, and Anna Fiore. 2023. "Optimization of Process Variables for the Sustainable Extraction of Phenolic Compounds from Chicory and Fennel By-Products" Applied Sciences 13, no. 7: 4191. https://doi.org/10.3390/app13074191
APA StyleBaiano, A., Romaniello, R., Giametta, F., & Fiore, A. (2023). Optimization of Process Variables for the Sustainable Extraction of Phenolic Compounds from Chicory and Fennel By-Products. Applied Sciences, 13(7), 4191. https://doi.org/10.3390/app13074191








