Abstract
Dermal papilla cells (DPCs) are a rich source of nutrients and secrete multiple growth factors that can affect hair growth. As oxidative stress leads to hair loss in humans, it is considered to be one of the factors that can impair the function of DPCs. Herb-derived phytochemicals exhibit potent antioxidant activities; therefore, this study investigated whether a set of essential oils (lavender, lemongrass, rosemary, and chamomile oils) promote the hair-growth activity of DPCs. Intracellular reactive oxygen species (ROS) increased markedly in ultraviolet B-irradiated DPCs (50 mJ/cm2) and were efficiently blocked by essential oils. Essential oils upregulated the mRNA and protein levels of phase II enzymes (detoxifying and antioxidant), including heme oxygenase-1, NAD(P)H quinone oxidoreductase-1, and glutathione S-transferase pi. They also upregulated and activated nuclear factor E2-related factor 2, an essential transcription factor for phase II enzymes. Regarding biomarkers for hair growth, essential oils significantly increased vascular endothelial cell growth factor and insulin-like growth factor-1 mRNA levels. In conclusion, phytochemicals in essential oils enhance hair growth through ROS-scavenging activity in DPCs.
1. Introduction
The hair follicle is a complex structure made up of epithelial (the matrix, the inner root sheath (IRS), and the outer root sheath (ORS) and dermal (the dermis) components (dermal papilla and connective tissue sheaths) [1]. The hair bulb is the primary structure at the base of the hair follicle, where the oval and indented dermal papilla (DP) is found. It contains blood vessels that deliver nutrients and energy necessary for hair growth [2]. In addition to being programmed for cyclical regeneration, hair follicles undergo a growth phase (anagen), a regression phase (catagen), and a relatively quiescent phase (telogen) [3]. When high levels of reactive oxygen species (ROS) are encountered, the DP is exposed to a stressful environment. Antioxidant systems should be able to efficiently remove these ROS.
Oxidative stress in hair follicles has been proposed to be a causative factor in the induction of different disorders that result in hair loss [4]. An oxidant/antioxidant imbalance is a common feature in autoimmune disorders, including alopecia areata (AA) [5]. AA was reported to be induced by a redox imbalance, which is mediated by abnormal T-cells-mediated adaptive immunity [6,7]. High levels of oxidative stress were reported to trigger abnormal innate immune responses in AA [8,9]. As biomarkers for oxidative stress, lipid peroxides and their degradation product malondialdehyde were found to be increased in AA lesions [10,11,12]. Antioxidant defense system impairment has been consistently reported to be a causal factor of AA. Superoxide dismutase (SOD) activity has been shown to decrease in serum and erythrocytes of AA patients [9,12]. Additionally, SOD activity inversely correlates with the severity or duration of AA and was found to be further reduced in the most severe form of AA or AA of longer duration (≥6 months) [13]. Glutathione peroxidase, which converts hydrogen peroxide to water and oxygen in the presence of glutathione, is reduced in both scalp tissues and plasma and erythrocytes of patients with AA. Tocopherol and β-carotene, which are essential fat-soluble vitamins, function as important protectors of the cell membrane against lipid peroxidation, and they interact preferentially with free radicals, such as lipid peroxyl radicals [14]. Decreased levels of vitamin E and β-carotene in AA tissue or circulating blood have also been reported [10,15]. In androgenetic alopecia (AGA), the most common form of male baldness, the balding scalp was found to have been exposed to high levels of oxidative stress, which cause the secretion of TGF-β, a hair growth inhibitory factor [16]. These studies suggest that oxidative stress in hair follicles is involved in the pathogenesis of hair loss.
As a master regulator of redox homeostasis, nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2) performs detoxifying and antioxidant activities in the skin [17]. This transcription factor regulates a set of genes that includes heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase-1, SOD, glutathione S-transferase (GST), and glutamate-cysteine ligase, which are involved in the detoxification and antioxidant defense [18]. The group of NRF2-mediated phase II detoxifying and antioxidant (phase II enzymes) comprises of factors essential for maintaining normal cellular integrity in the skin and hair follicles. These follicle cells and the scalp are continually exposed to harmful conditions. Therefore, NRF2-dependent protection from oxidative stress in hair follicles could be essential for their maintenance to ensure normal function and integrity.
Dermal papilla cells (DPCs), the major cellular component of DP, play a crucial role in morphogenesis and hair growth cycling. Recently, DPCs have been extensively used in in vitro screening models to evaluate the efficacy of agents tested for their hair growth-modulating activities. DPCs produce numerous soluble factors, such as vascular endothelial cell growth factor (VEGF), insulin-like growth factor-1 (IGF-1), hepatocyte growth factor, transforming growth factor-β1 (TGF-β1), and others [19], that are essential for the development and maintenance of hair follicles. It has been reported that VEGF and IGF-1 are essential growth factors for DPCs and biomarkers for hair growth [20,21]. VEGF is a growth factor that regulates hair morphogenesis and hair growth by promoting angiogenesis, which restores the oxygen supply to tissues. IGF-1 signaling has been identified as a crucial mitogenic and morphogenetic regulator in the biology of hair follicles, influencing follicular proliferation, tissue remodeling, and the hair growth cycle. In contrast, dysfunctional DPCs that produce pro-inflammatory and chemo-attractive cytokines can result in keratinocyte damage in the hair matrix, and, consequently, hair growth disorders [22]. It is believed that the interaction between the epidermal and dermal components of the hair follicle is essential for maintaining hair growth [23,24]. Consequently, ORS keratinocyte cultures have been used in secondary in vitro screening models to examine hair growth [25].
Phytochemicals are compounds derived from different parts of plants, including the roots, leaves, flowers, and seeds. Phytochemicals contain polyphenols as active ingredients. They can be classified into flavonoids (catechins, isoflavones, proanthocyanidins, and anthocyanins) or non-flavonoids (phenolic acids, benzoic acids, stilbene, and resveratrol). They have been widely studied due to their potent antioxidant activity, which can protect skin from ROS-induced skin inflammation, photoaging, and photocarcinogenesis [26]. Altogether, the ROS-mediated activation of the immune system in the DPCs has been implicated in the pathogenesis of hair loss, suggesting that phytochemicals in essential oils could prevent ROS-mediated hair loss in humans [27]. This study expects the phytochemicals in essential oils to promote hair growth through the ROS-scavenging activity of DPC.
2. Materials and Methods
2.1. Two-Dimensional (2D) and Three-Dimensional (3D) DPC Cultures
DPCs were purchased from PromoCell (PromoCell GmbH, Heidelberg, Germany).
2.1.1. 2D-DPC Cultures
The DPCs were grown in follicle dermal papilla cell growth medium of the follicle supplemented with human dermal papilla cell growth medium and antibiotics (10,000 U/mL penicillin and 100 mg/mL streptomycin) in a 5% CO2 incubator. Passages 2 through 7 were used for all experiments [28].
2.1.2. 3D-DPC Cultures
Using the RAFT™ 3D cell culture system and 24-well plates, three-dimensional cultures were prepared according to the manufacturer’s instructions (Lonza, Walkersville, MD, USA). Briefly, cells (4.5 × 105 cells/mL) and neutralized collagen were mixed, dispensed, and incubated to allow the formation of a cell-seeded hydrogel. Fully loaded media comprising of 10 × MEM and collagen solution mix were added to each well, and plates were placed in the incubator (Medi40, Thermo Scientific™) under standard culture conditions. The 3D cultures were maintained for 6 days before being serum-starved for 24 h before treatment. Essential plant oils were diluted in serum-free medium at a range of concentrations immediately prior to treatment, and the treated cells were incubated for 72 h. Controls were treated with serum-free medium only. For 3D cultures, the cell-seeded hydrogel was liquified using Cell Recovery Solution (Sigma-Aldrich, St. Louis, MO, USA) and shaken at 37 °C. Cell spheroids were collected by centrifuging at 10,000 rpm for 15 min [28].
2.2. Preparation of Essential Oils Derived from Herbs
Lavender, lemongrass, rosemary, and chamomile were grown and harvested in Namwon province (Jeollabuk-do, Republic of Korea). Essential oils from each plant were extracted by steam distillation using 250 L steam distillation units. The collected material was distilled for 1 h with boiling until all the oil was recovered. Essential oil samples were kept at −5 °C. The total phenolic content in the herbal extracts was determined using the Folin–Ciocalteu’s phenol reagent. The absorbance was measured at 750 nm with an enzyme-linked immunosorbent assay (ELISA) reader (Molecular Devices, Menlo Park, CA, USA). Each sample’s total phenolic concentration was expressed as mg of gallic acid equivalent/kg of extract.
2.3. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium Bromide (MTT) Assay
To verify cell viability, the DPCs were seeded in 96-well plates and treated with different concentrations of essential plant oils for 24 h. Cell viability was tested using the colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) kit (Chemicon International Inc., Billerica, MA, USA) [28].
2.4. Measurement of Ultraviolet B (UVB)-Induced Intracellular ROS Levels
Fluorescent 2′,7’-dichlorodihydrofluorescein diacetate (DCF-DA; Invitrogen, Carlsbad, CA, USA) was used to detect UVB-induced intracellular ROS, and positive fluorescence signals were analyzed using a fluorescence-activated cell sorter (FACS; FACS Calibur; Becton Dickinson; San Jose, CA, USA) [29]. The DPCs were seeded at a density of 1 × 106 cells per well in each well of a 60 mm culture plate and treated with four different essential oils for 1 h. To induce ROS production, DPCs were exposed to 50 mJ/cm2 of UVB radiation once in the presence of herb-derived essential oils. The cells were then labeled with 10 mM DCF-DA at 37 °C for 30 min before Argon laser (488 nm) and red diode laser (635 nm) were used for FACS analysis of single-cell suspensions diluted in phosphate-buffered saline (PBS).
2.5. Confocal Microscopy for Detecting NRF2 Expression in DPCs
The DPCs were seeded at a density of 4 × 104 cells per well in a slide chamber and then treated with essential oils for 24 h. Following washing with PBS, the cells were fixed with 4% paraformaldehyde in PBS for 10 min, then permeabilized with PBS containing 0.5% TritonX-100 (PBS-T) for 15 min. An anti-NRF2 antibody (sc-722, 1:100; Santa Cruz Biotechnology, Dallas, TX, USA) was added to the cells and incubated for 1 h in PBS-T containing 1% bovine serum albumin, followed by overnight incubation at 4 °C with the Alexa Fluor 488 goat anti-rabbit IgG (A11034, H + L, 1:500; Invitrogen, Carlsbad, CA, USA). Cell staining was visualized by confocal microscopy with a 20× objective on a laser scanning microscope (LSM510; Carl Zeiss, Jena, Germany) and analyzed using LSM 5 browser imaging software [29].
2.6. Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) for NRF2, Phase II Enzymes Genes, and the Hair Growth Factors Genes VEGF and IGF1 in DPCs
Total RNA was extracted with an RNeasy mini kit (Qiagen, Fremont, CA, USA), and 1 mg of total RNA was reverse-transcribed with an Omniscript RT kit (Qiagen). RT-PCR was performed with the primers described in Table 1 using a HiPi Plus 5× PCR Premix (ELPIS, Daejeon, Republic of Korea) [29]. The PCR products were analyzed in 1.5% agarose gels. This was followed by staining with Sybr Safe DNA gel stain buffer (Invitrogen, Carlsbad, CA, USA). For quantification, endogenous glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to normalize the mRNA levels for each tested molecule.

Table 1.
Human Primer sequences for reverse transcriptase-polymerase chain reaction (RT-PCR).
2.7. Real-Time Quantitative RT-PCR (qRT-PCR) for NRF2 and Phase II Enzyme Genes in 3D-DPC Cultures
Real-time RT-PCR was performed in triplicate with the HOT FIREPol EvaGreen® qPCR Mix Plus (Solis BioDyne, Tartu, Estonia) using a Rotor-Gene 3000 (Corbett Research, Cambridge, UK) [29]. Thermal cycling conditions were 15 min at 95 °C, followed by 50 cycles of 95 °C for 10 s, 58 °C for 20 s, and 72 °C for 30 s. The relative abundance of a given transcript was estimated using the condition, followed by normalization to GAPDH. The real-time PCR primers used were the same as those used for conventional RT-PCR.
2.8. ELISA Assay to Determine VEGF and IGF-1 Protein Levels in 3D-DPC Cultures
To measure the VEGF and IGF-1 protein levels in a 3D culture medium, 500 μL of culture supernatant was harvested from cultured cells. Commercial ELISA kits were used according to the manufacturer’s protocols to quantify human VEGF (DVE00, R&D Systems, Inc., Minneapolis, MN, USA) and IGF-1 (DG-100, R&D Systems, Inc., Minneapolis, MN, USA). The optical density was measured at 450 nm using an ELISA reader (Molecular Devices, Sunnyvale, CA, USA) [30].
2.9. Statistical Analyses
All experiments were performed in triplicate and the results were expressed as mean ± standard deviation. Comparisons between samples were carried out using the Student’s t-test, and statistical significance was considered to be p < 0.05.
3. Results
3.1. Extraction of Herb-Derived Essential Oils
The tested essential oils were extracted by steam distillation, and the final polyphenol content was determined by ELISA to be 1.0 GAE mg/kg for lavender oil (essential), 1.9 GAE mg/kg for lemongrass (essential) oil, 0.5 GAE mg/kg for rosemary oil, and 17.9 GAE mg/kg for chamomile oil (essential). According to mass spectrometric analysis, the primary phytochemicals in lavender oil were ascorbic acid and retinol; in lemongrass oil, they were retinoic acid, retinol, and beta-carotene; in rosemary oil, they were retinoic acid, retinol, and retinal; and in chamomile oil, it was β-carotene [31].
In preliminary experiments, an MTT assay was performed to determine the optimal concentration of each essential oil. These results indicated that the optimal dose ranges without cytotoxicity were 0.0025–0.01% (vol/vol) for lavender oil, 0.0012–0.005% for lemongrass oil, 0.005–0.02% for rosemary oil, and 0.005–0.02% for chamomile oil (data not shown). The following experiments were performed with essential oils at three concentrations within these ranges.
3.2. The ROS-Scavenging Activity of Herb-Derived Essential Oils in UVB-Irradiated DPCs. DCF-DA Signals Are Reduced to Confirm Anti-Oxidant Activity
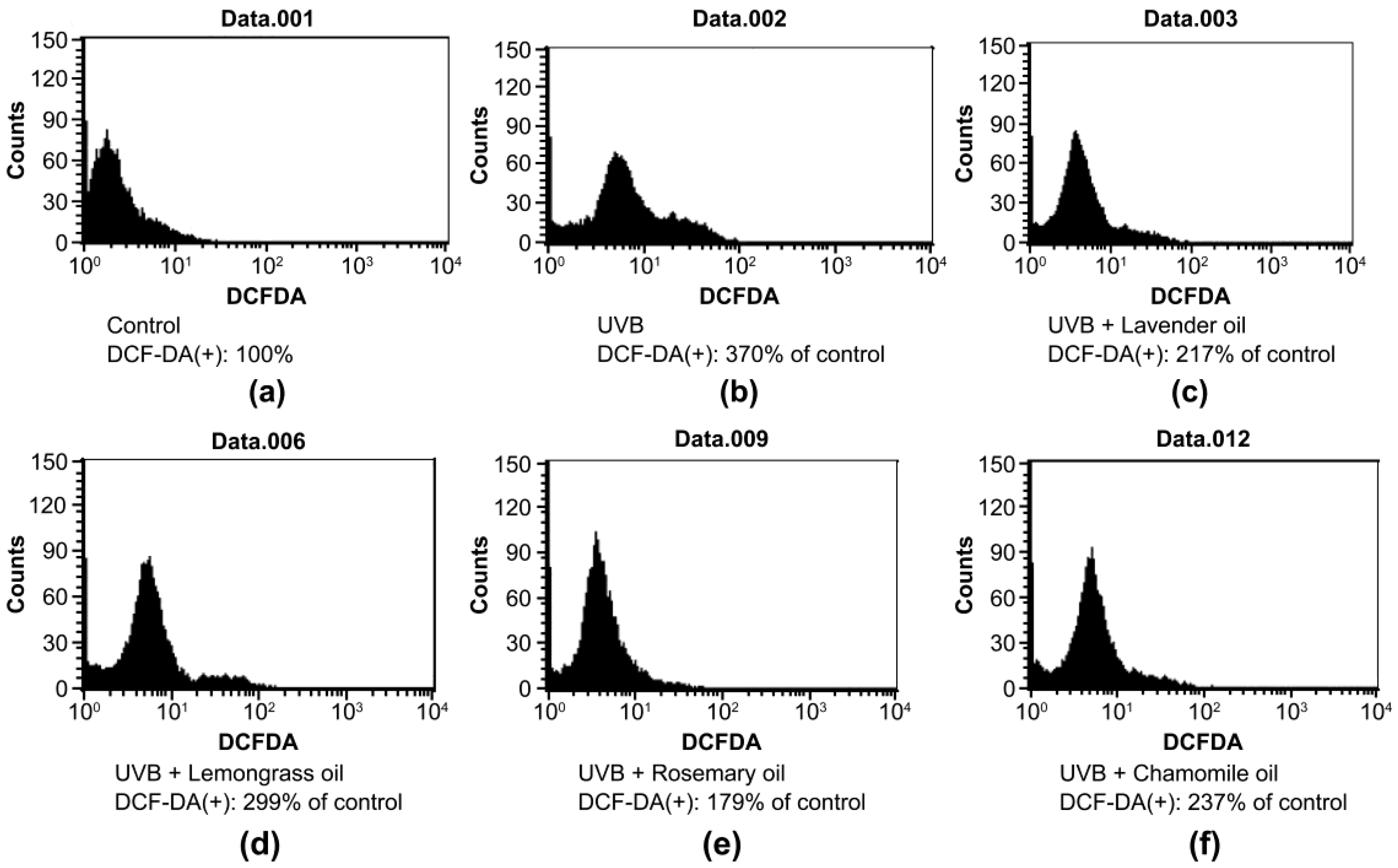
In UVB 50 mJ/cm2-irradiated DPCs, increased DCF-DA signal indicating ROS level increased significantly (370% of the non-irradiated control). UVB-induced ROS production was blocked by essential oils with different potencies of ROS-scavenging activity as follows: 41.3% ± 5.1% reduction by 0.01% lavender oil, 19.2% ± 3.3% reduction by 0.005% lemongrass oil, 50.4% ± 1.2% reduction by 0.01% rosemary oil, and 51.6% ± 3.9% reduction by 0.01% chamomile oil, respectively (Figure 1).
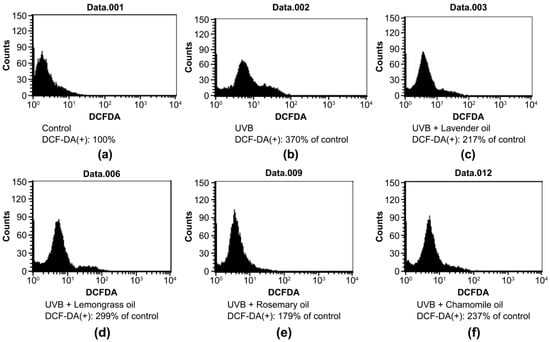
Figure 1.
Effect of essential oils on ROS production in UVB-irradiated DPCs. FACS analysis to detect the DCF-DA-positive cells was performed. (a) Control DPC. (b) UVB-irradiated DPC; and (c) Lavender oil-treated, UVB-irradiated DPC. (d) Lemongrass oil-treated, UVB-irradiated DPC. (e) Rosemary oil-treated, UVB-irradiated DPC. (f) Chamomile oil-treated, UVB-irradiated DPC. The average number of DCF-DA-positive cells shown in (a) is designated as 100%.
3.3. Upregulation of NRF2 by Herb-Derived Essential Oils in DPCs
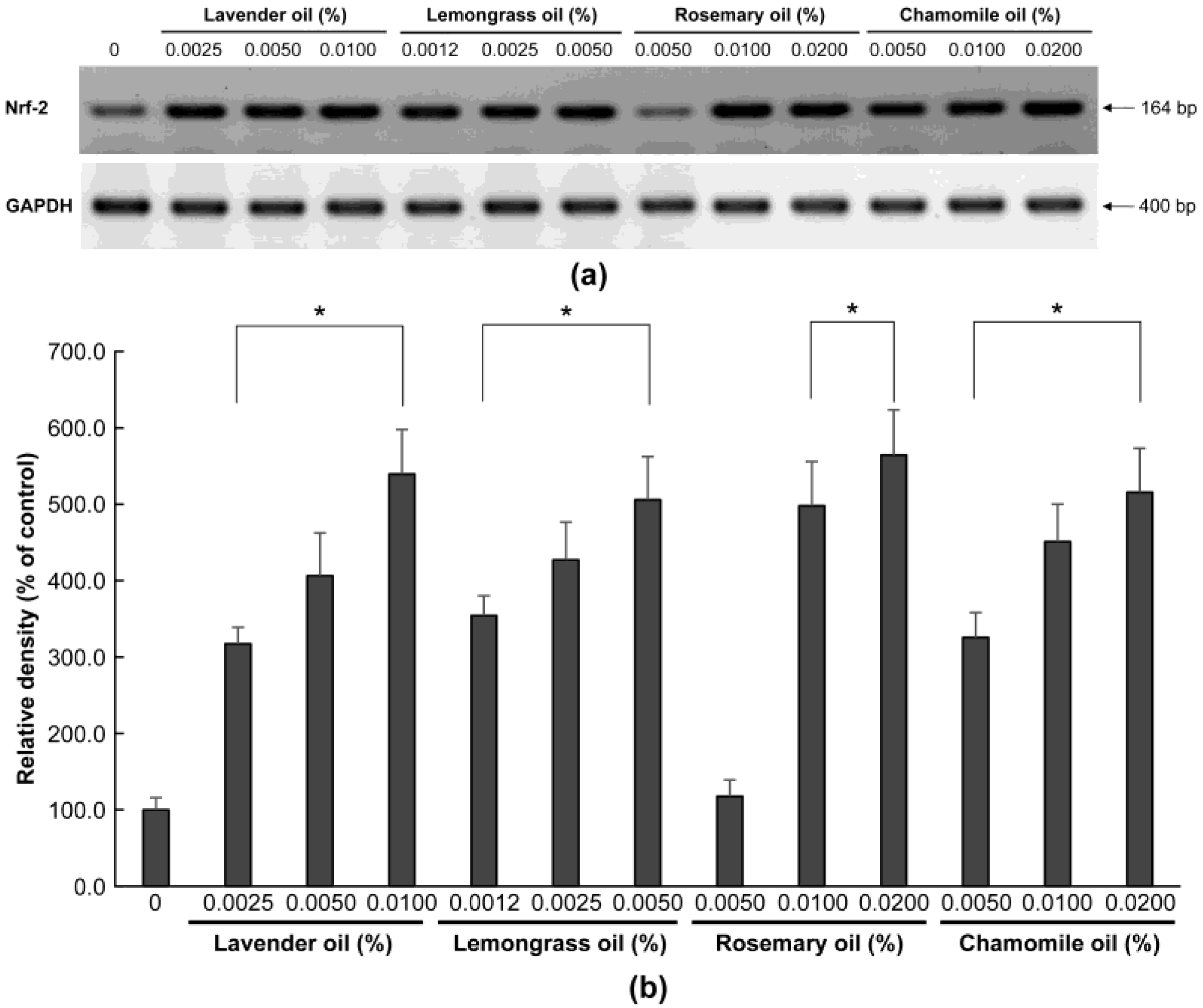
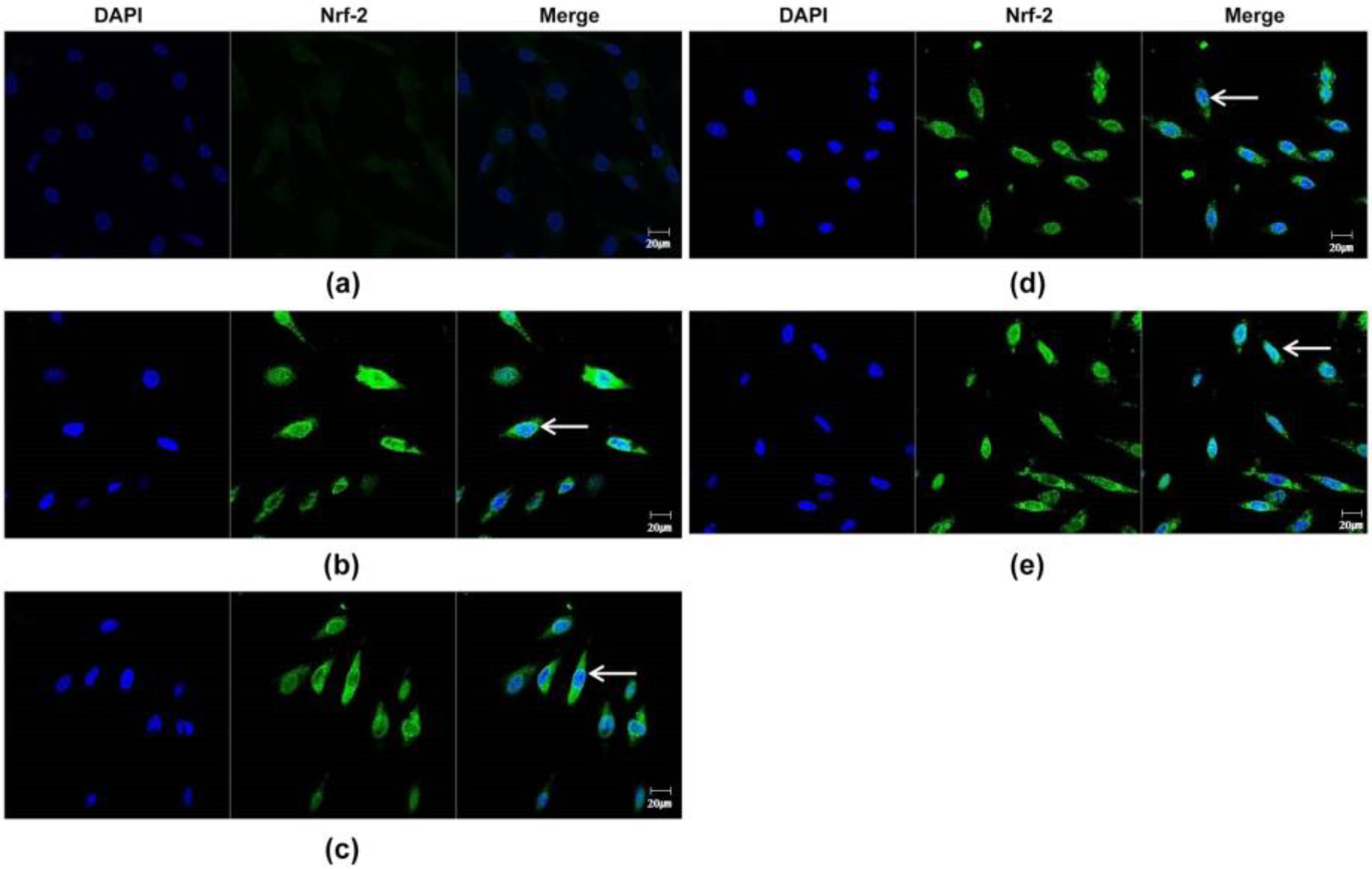
To examine the antioxidant properties of essential oils, their ability to modulate NRF2 in DPCs was evaluated. In RT-PCR experiments followed by densitometric analysis, all essential oils dose-dependently increased NRF2 mRNA levels (p < 0.01) (Figure 2a,b). Essential oils increased NRF2 protein expression in the cytoplasm and nucleus of DPCs, as determined by immunocytochemical staining (Figure 3). In particular, NRF2 immunoreactivity was detected in the nuclei of DPCs treated with essential oils, indicating that the essential oils tested had the potential to activate NRF2 in DPCs (Figure 3, arrow).
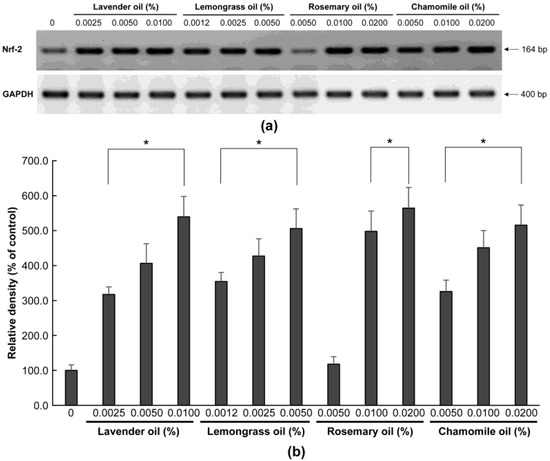
Figure 2.
Dose-dependent induction of NRF-2 mRNA expression in essential oils-treated DPC. (a) The DPCs were treated with lavender oil (0.025–0.01%), lemongrass oil (0.0012–0.005%), rosemary oil (0.005–0.02%), or chamomile oil (0.005–0.02%). GAPDH was used as an internal standard to quantify the loading dose of mRNA. The figure shows representative results of triplicate RT-PCR experiments. (b) NRF-2 mRNA level is quantified by densitometric analysis * p < 0.05.
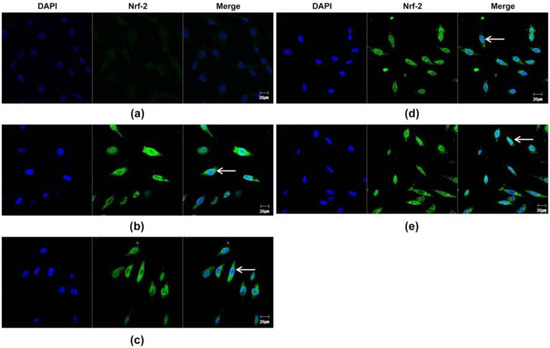
Figure 3.
Confocal microscopic detection of Nrf-2-positive fluorescence in plant essential oils-treated DPC. (a) Nrf2-positive fluorescence signals from non-treated DPCs (control). (b) Nrf2-positive fluorescence signals from lavender-treated DPCs. (c) Nrf2-positive fluorescence signals from lemongrass-treated DPCs. (d) Nrf2-positive fluorescence signals from rosemary-treated DPCs. (e) Nrf2-positive fluorescence signals from chamomile-treated DPCs. The arrow represents nuclear translocation of Nrf2. Scale bar: 20 μm.
3.4. Upregulation of Phase II Enzymes by Herb-Derived Essential Oils in DPCs
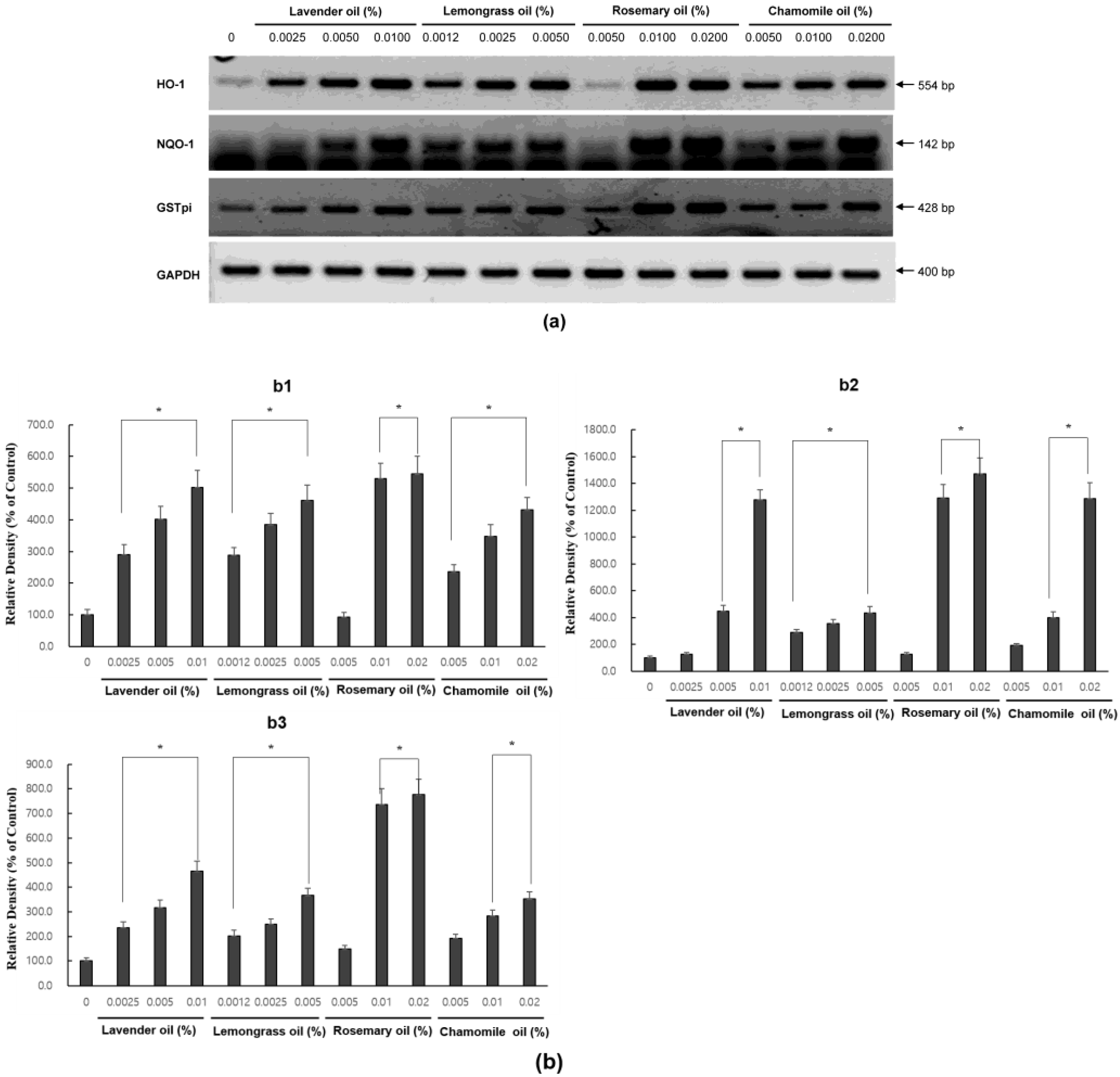
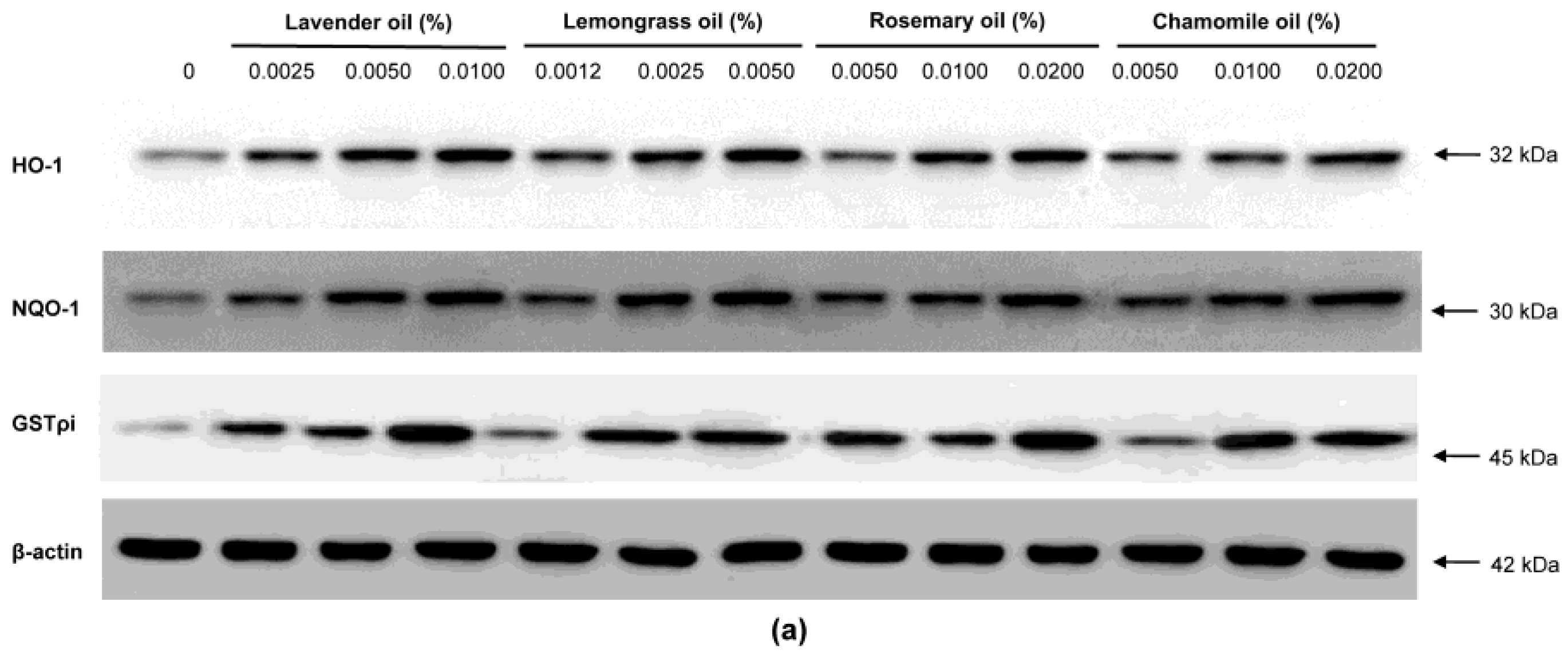
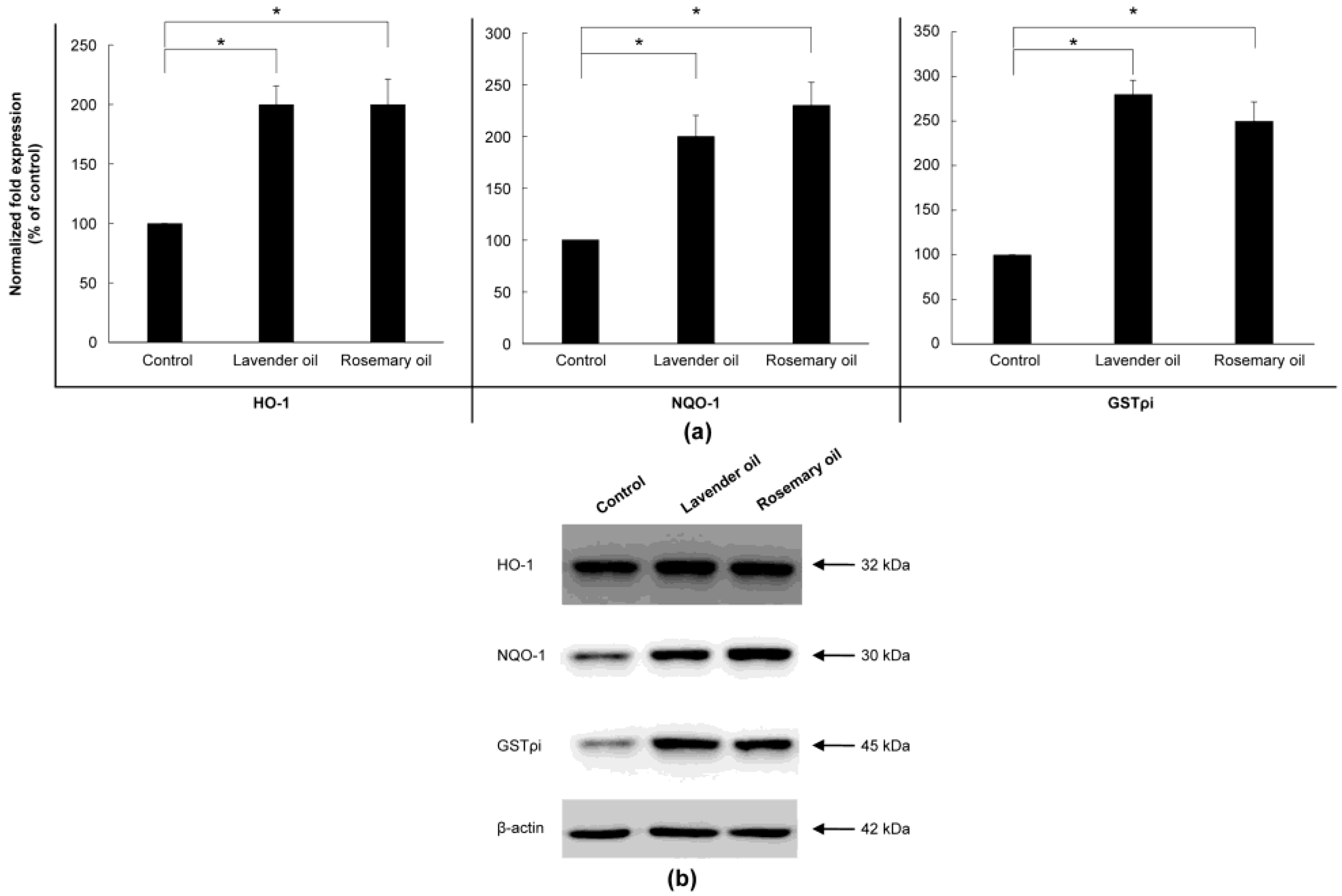
Since all essential oils had the potential to activate NRF2, further experiments were performed to test whether they could upregulate the expression of downstream target antioxidant proteins. As representative antioxidant proteins of phase II enzymes, we focused on HO-1, NQO-1, and GSTpi expression in DPCs. In RT-PCR experiments followed by densitometric analysis, the mRNA expression levels of our phase II enzyme genes were found to be upregulated in a dose-dependent manner (Figure 4a,b). Consistently, Western immunoblots demonstrated that essential oils upregulated HO-1, NQO-1, and GSTpi protein levels in a dose-dependent manner (Figure 5a,b). These results show that a set of phase II enzymes expressed in human DPCs were upregulated by essential oils, suggesting that DPCs express sufficient NRF2 and downstream target proteins to cope with the oxidative stress.
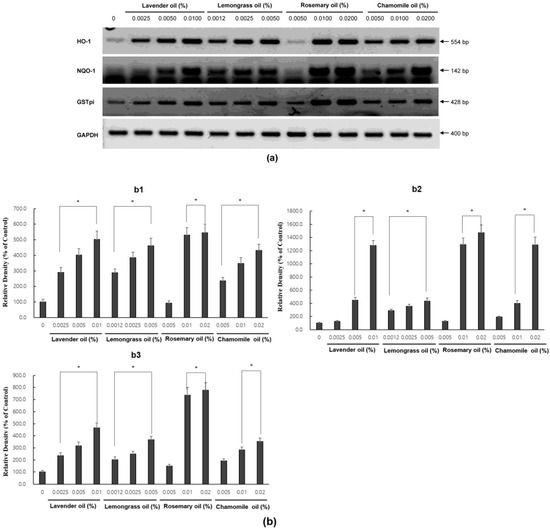
Figure 4.
Dose-dependent induction of the mRNA expression of phase II enzymes in essential oils-treated DPC. (a) The DPCs were treated with lavender oil (0.025–0.01%), lemongrass oil (0.0012–0.005%), rosemary oil (0.005–0.02%), and chamomile oil (0.005–0.02%). The figure shows representative results from triplicate RT-PCR experiments. (b) Phase II enzymes mRNA level are quantified by densitometric analysis * p < 0.05.
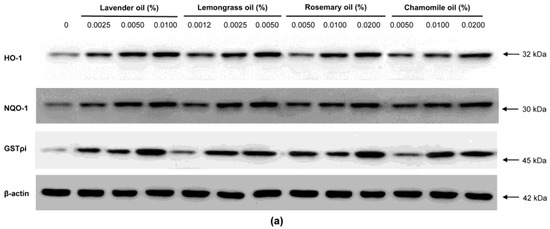
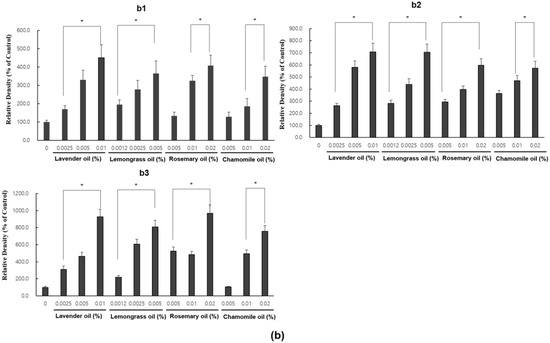
Figure 5.
Dose-dependent induction of the protein expression of phase II enzymes measured by Western immunoblots in essential oils-treated DPC. (a) The DPCs were treated with lavender oil (0.025–0.01%), lemongrass oil (0.0012–0.005%), rosemary oil (0.005–0.02%), and chamomile oil (0.005–0.02%). The figure shows representative results of triplicate experiments. (b) Phase II enzymes protein level are quantified by densitometric analysis * p < 0.05.
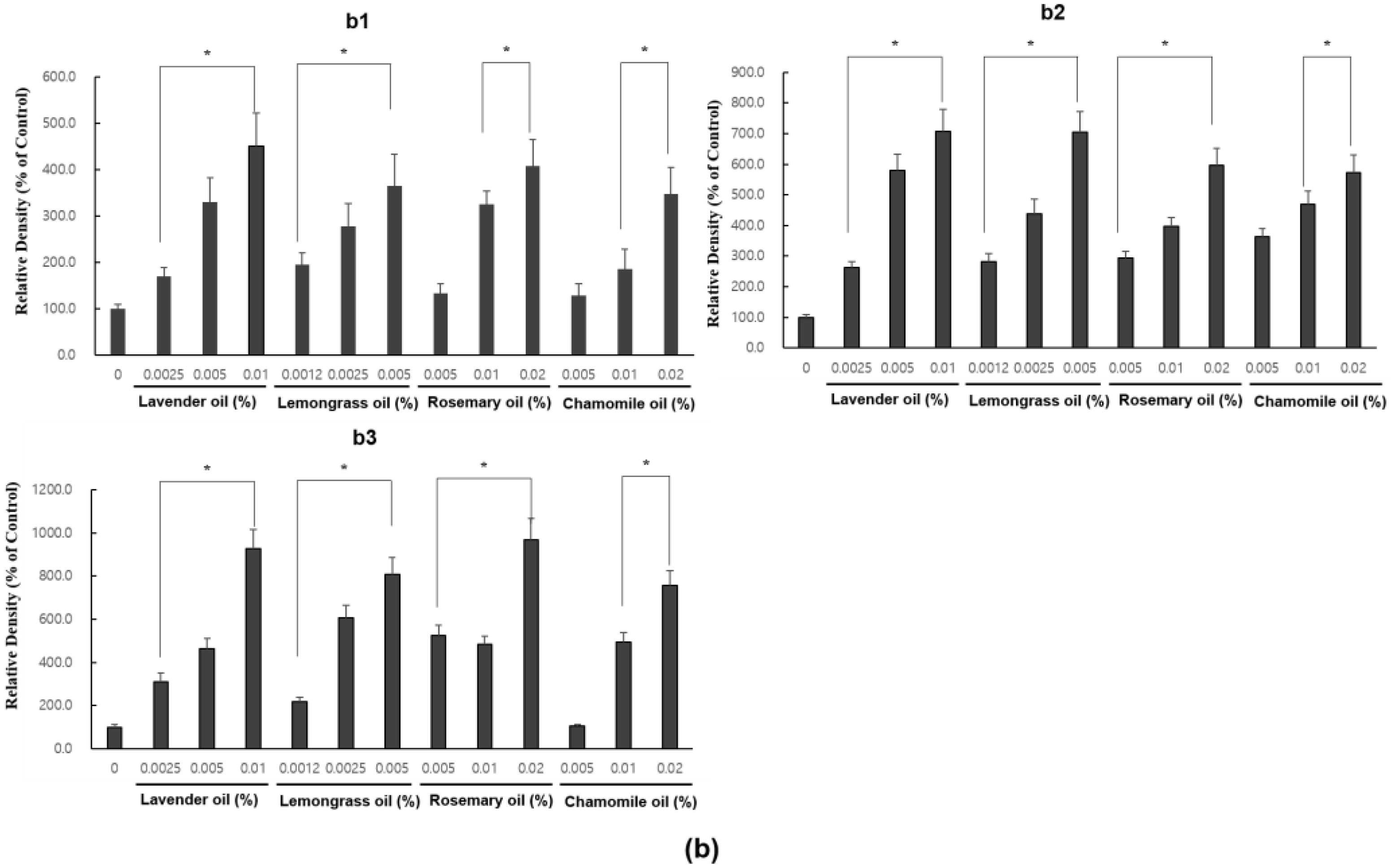
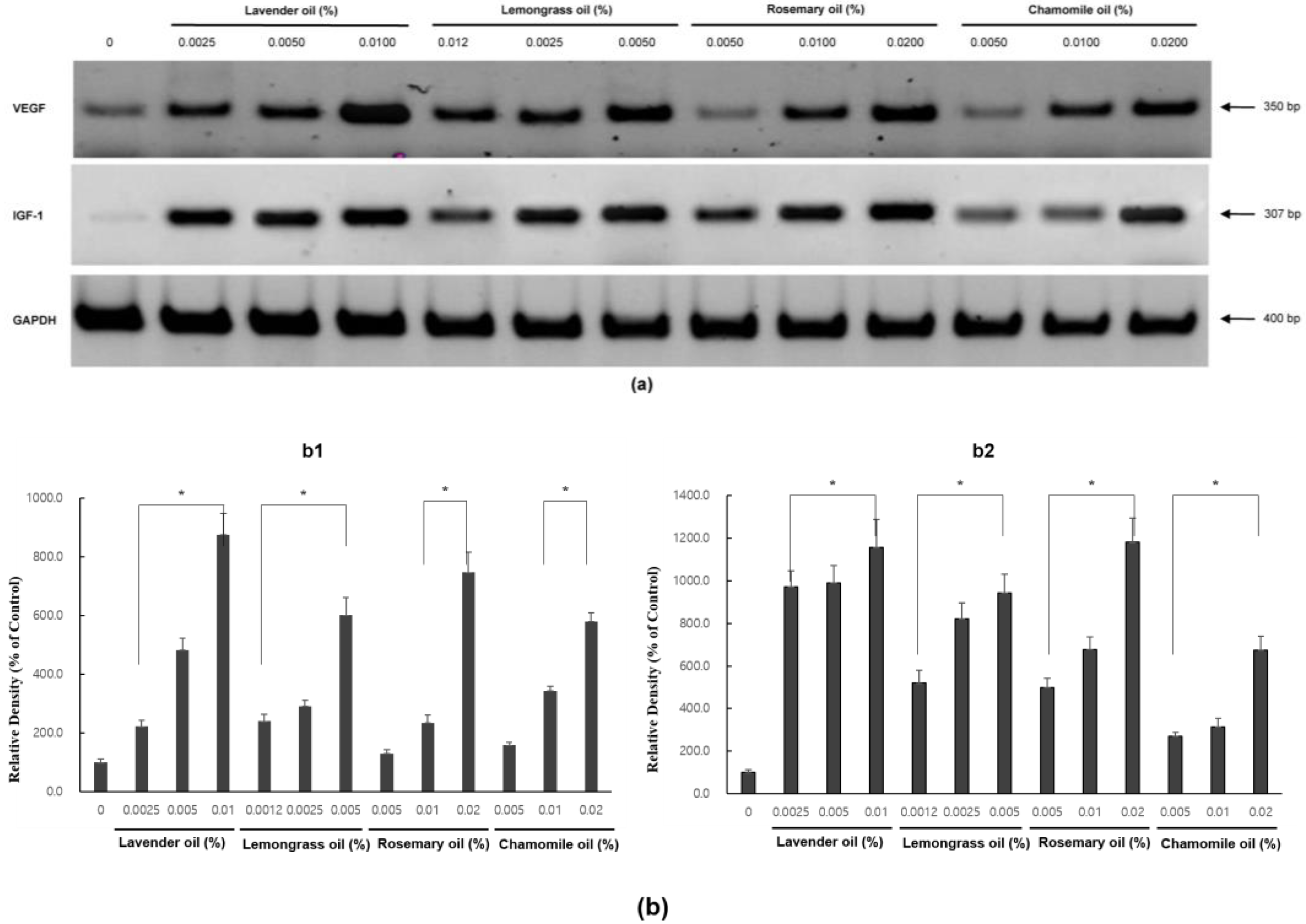
3.5. Upregulation of VEGF and IGF1, Hair Growth Factors by Herb-Derived Essential Oils in DPC Cultures
In RT-PCR experiments, all essential oils were found to upregulate VEGF and IGF1 mRNA expression levels in a dose-dependent manner (p < 0.01) (Figure 6a,b).
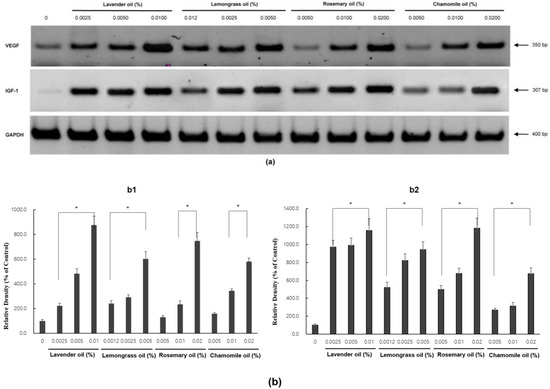
Figure 6.
Dose-dependent induction of the mRNA expression of VEGF and IGF-1 in essential oils-treated DPC. (a) The DPCs were treated with lavender oil (0.025–0.01%), lemongrass oil (0.0012–0.005%), rosemary oil (0.005–0.02%), and chamomile oil (0.005–0.02%). The figure shows representative results of triplicate RT-PCR experiments. (b) Hair growth factor (VEGF and IGF-1) mRNA level are quantified by densitometric analysis * p < 0.05.
3.6. Upregulation of NRF2, Phase II Enzymes, and Hair Growth Factors by Herb-Derived Essential Oils in 3D-DPC Culture
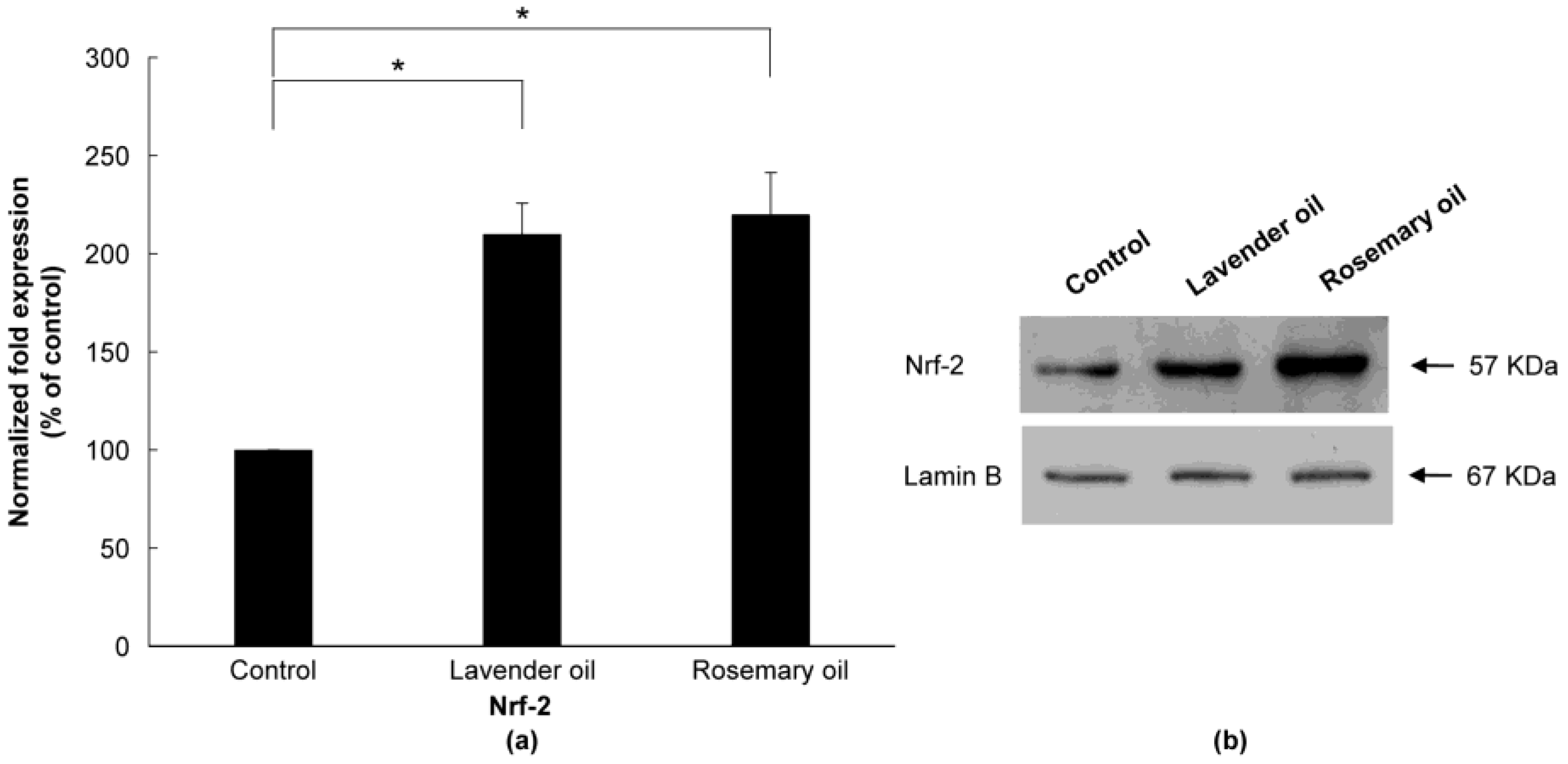
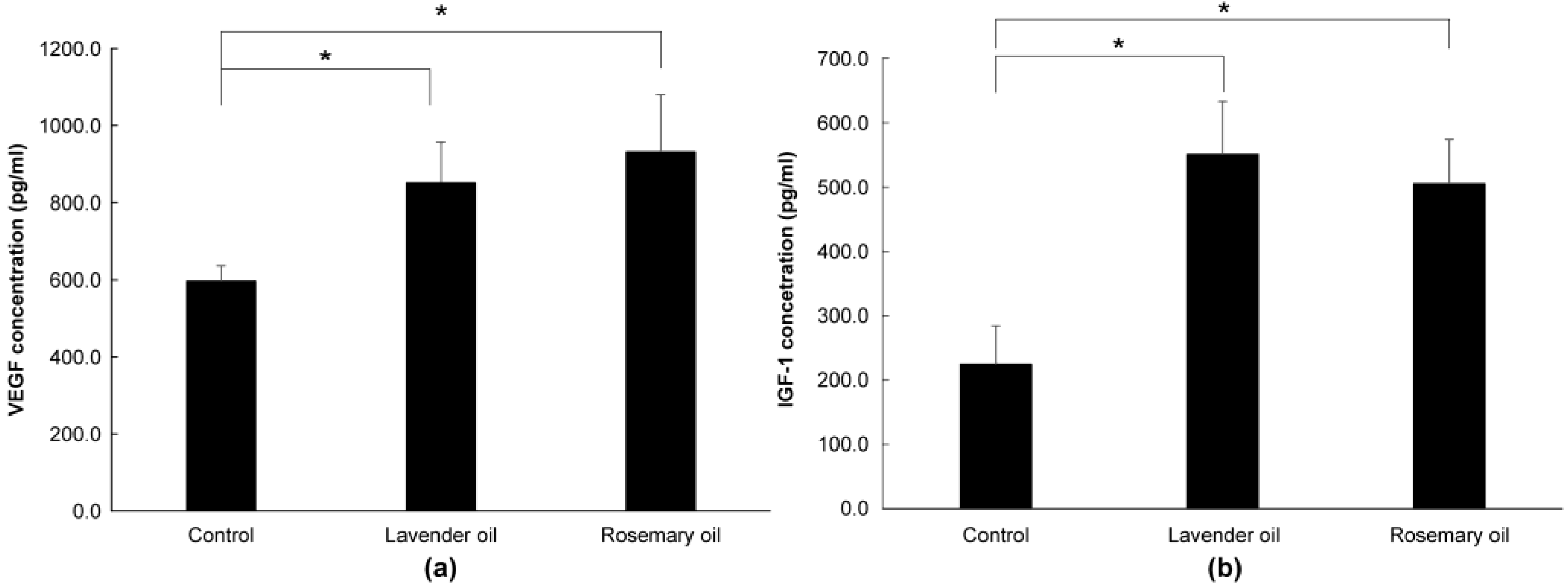
Based on the results above, 0.01% lavender oil and 0.01% rosemary oil, which have been widely used as cosmetic ingredients with suitable safety, were selected for 3D-DPCculture experiments. According to the qRT-PCR and Western immunoblot analyses, both oils upregulated mRNA and protein expression of NRF-2 (Figure 7a,b) and three phase II enzymes (HO-1, NQO-1, and GSTpi) (Figure 8a,b). Subsequently, using ELISA, both oils were shown to upregulate the levels of VEGF and IGF-1 proteins in the supernatants of the 3D-DPC cultures (Figure 9a,b). The results of the 3D culture system further confirmed those of the 2D-DPC culture system.
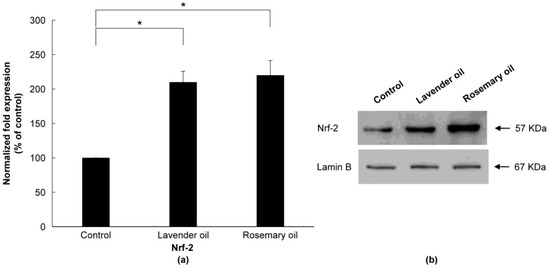
Figure 7.
Upregulation of NRF-2 by lavender (0.01%) and rosemary (0.01%) oils in the 3D-DPC culture system. To confirm the results of the 2D-DPCs culture system, cells were treated with essential oils in the 3D-DPC culture system. (a) NRF-2 mRNA levels are qRT-PCR experiments. * p < 0.01 between the essential oils-treated samples and non-treated control DPCs. (b) NRF-2 protein expression was analyzed by Western immunoblots.
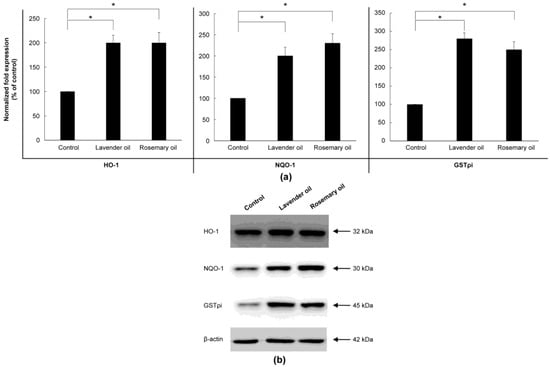
Figure 8.
Upregulation of phase II enzymes by lavender (0.01%) and rosemary (0.01%) oils in the 3D-Dermal papilla cell (3D-DPC) culture system. To confirm the results of the 2D-DPCs culture system, cells were treated with essential oils in the 3D-DPCs culture system. (a) Phase II enzyme mRNA expression was analyzed by qRT-PCR. * p < 0.01 between the essential oils-treated samples and non-treated control DPCs. (b) Phase II enzyme protein expression was analyzed by Western immunoblots.
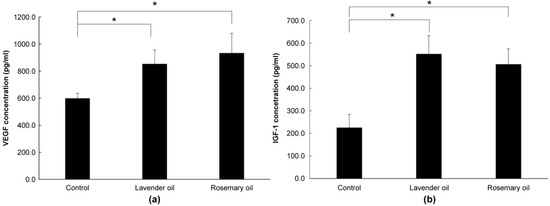
Figure 9.
Upregulation of hair growth factors by lavender (0.01%) and rosemary (0.01%) oils in the 3D-Dermal papilla cell (3D-DPC) culture system. To confirm the results of the 2D-DPCs culture system, cells were treated with essential oils in the 3D-DPCs culture system. (a) VEGF mRNA expression was analyzed by qRT-PCR. * p < 0.01 between the essential oils-treated samples and non-treated control DPCs. (b) IGF-1 mRNA expression was analyzed by qRT-PCR. * p < 0.01 between the essential oils-treated samples and non-treated control DPCs.
4. Discussion
Plant-derived essential oils are a complex mixture of compounds with a low molecular weight that can be extracted using a variety of techniques [32]. During the course of this study, we evaluated a collection of essential oils derived from plant extracts that are widely used and proven to be safe for cosmetic applications.
As a mesenchymal component of hair follicles, the DP found at the base of follicles is crucial for maintaining normal hair growth. DPCs are rich sources of nutrients and secrete multiple growth factors that support the proliferation and growth of follicular epithelial cells [33]. Since DP plays a major role in hair growth and the hair growth cycle, DPCs are exposed to relatively high levels of oxidative stress resulting from active cell metabolism. In fact, a growing body of evidence indicates that ROS are produced by DPC and play an important role in the induction of hair loss (alopecia) through DPC senescence [34,35]. Therefore, DPCs require sophisticated antioxidant protective mechanisms to protect DP from oxidative damage. Furthermore, proper epidermal–dermal interaction is important in the normal development and growth of hair follicles. Furthermore, both DPCs and germinative epidermal cells of the lower follicles are required to induce and stimulate hair growth [36]. In this study, various mechanistic factors for the protection of DP from oxidative stress are introduced, and the role of Nrf-2 in stimulation and antioxidant activity in the skin is demonstrated with essential oils.
To evaluate the ROS-scavenging antioxidant activities of essential oils in DPCs, cells were exposed to 50 mJ/cm2 of UVB light. UV rays are highly desirable experimental inducers of ROS production in skin-related research, because chronic UV irradiation has been found to contribute to photoaging and photocarcinogenesis [26,37].
Through ROS-scavenging in UVB-irradiated human keratinocytes, we discovered in a previous study that essential oils possess potent antioxidant activity [38]. The positive effects of plant-derived essential oils on epidermal keratinocytes and DPCs of human skin provide a promising foundation for the development of new therapeutic agents for hair loss. In this study, antioxidant properties can be confirmed by examining the antioxidant properties of herbal essential oils that promote hair growth through ROS-scavenging activity in DPC.
When exposed to ultraviolet rays, various inflammatory mediators and reactive oxygen specifications (ROS) are produced, resulting in photoaging [39].
The NRF-2 Keap1 pathway is well known as an important regulator of the phase II defense system against oxidative stress. When NRF-2 is activated, cyclotective genes such as HO-1, NQO-1, and GCL are induced, and the production of GCL-induced glutathione in particular affects redox signals. When herbal essential oils are treated with oxidative and eletrophilic stimuli processes, they react to redox reactive cysteine in Keap 1. NRF-2 is released from Keap1 and translated into the nucleus. In the nucleus, NRF-2 is dimerized to the Maf protein and bound to the ARE, and the ARE is located in the promoter of phase II, anti-oxidative gene, triggering the transcription of the ARE-regulated gene. Phase II enzyme such as NQO1, GSTpi, GPX, catalase, and HO-1 is expressed through a series of processes, which has an anti-oxidative effect [39,40,41].
Herbal essential oil increases the expression of anti-oxidant enzymes such as SOD and GPx, and activates NRF-2, resulting in the following phase II enzyme.
In this study, Herbal essential oils upregulate phase II enzyme as well as enzymatic antioxidant in DPC to prove that they have ROS-scavenging activity and antioxidant activity [42]. Herbal essential oil is considered to be an ideal material for stimulating DPS to produce anti-oxidant enzyme by penetrating DP with safe, low-molecular weight.
Hair growth depends on the complex interactions among growth factors and other soluble signals released from DPCs [43]. Furthermore, VEGF is known to play a crucial role in the mediation of angiogenesis during development and in various inflammatory and neoplastic disorders related to neovascularization [44]. In the skin, VEGF has been identified as a major angiogenic factor for vascularization. Moreover, VEGF expression has been reported to be upregulated in the hyperplastic epidermis of patients with psoriasis and in those with healing wounds [45,46]. VEGF also plays an important role in the control of perifollicular vascularization during the hair cycle, and it acts directly on DPCs as an autocrine growth factor [47]. It is also known to stimulate hair growth through a paracrine mechanism. Furthermore, it has been reported to induce increased vascular support to meet highly increased nutritional needs during the anagen growth phase in the proliferative epithelial compartment of the hair follicle. In animal experiments, the expression of VEGF by follicular keratinocytes in ORS was upregulated in association with perifollicular angiogenesis.
In contrast, blockage of VEGF by a neutralizing anti-VEGF antibody led to retardation of hair growth and a reduction in the size of the hair follicle. Tofacitinib-treated mice exhibited a higher rate of hair growth through the upregulated expression of VEGF mRNA and protein [48]. In human hair follicles, strong expression of VEGF mRNA was detected in DPCs in the anagen phase but was found to be reduced during the catagen and telogen phases [49]. Various herbal extracts were tested as VEGF inducers to stimulate hair growth. Asiasari radix extract induced the expression of VEGF in human DPCs [50], Carthamus tinctorius floret extract promoted the proliferation of both DPCs and HaCaT by inducing VEGF and keratinocyte growth factor [51], and Chamaecyparis obtusa essential oils promoted hair growth by inducing VEGF and KGF in mice [52]. In this study, herbal essential oil proved that hair growth factors VEGF and IGF-1-regulation with anti-oxidant activity by modulating ROS-scavenging enzymatic antioxidant and NRF-2-dependent phase II enzyme in DPC. Taken together, phytochemicals derived from plant extracts are expected to have the potential to stimulate hair growth by enhancing the expression of hair follicle growth factors in DPCs. It can be used as a good raw material for antioxidant functionality and hair loss-only cosmetics, and is highly valuable in utilizing functional cosmetics materials that are important for commercialization.
5. Conclusions
In this study, herb-derived essential oils showed potent antioxidant activity produced by upregulating a set of phase II enzymes through the activation of NRF2, an essential transcription factor for phase II enzymes, in DPCs. All our tested essential oils exhibited potent hair growth activity, shown by the upregulation of VEGF and IGF-1, which are biomarkers for hair growth in DPCs. Consistent with our previous study, which indicated that a set of essential oils had the potential to upregulate and activate NRF2 and phase II enzymes in keratinocytes [34], these results indicate that herb-derived essential oils exerted synergistic effects of DPCs and oxidative stress. Additionally, the results of 2D-DPC cultures could be further verified in 3D-DPC cultures. In the future, an ex vivo model of hair follicles should be further studied to confirm the efficacy of candidate phytochemicals in promoting hair growth, which can be developed as new cosmeceutical agents for human hair loss disorders.
Author Contributions
Conceptualization, S.-C.L.; data curation, J.-Y.C.; formal analysis, B.-K.M. and Y.-G.A.; investigation, D.-I.C., J.-Y.C. and S.-J.Y.; resources, D.-I.C. and S.-Y.L.; methodology, D.-I.C. and J.-B.L.; software, B.-K.M. and Y.-G.A.; writing—original draft preparation, D.-I.C.; supervision, S.-Y.L., S.-C.L., S.-J.Y. and J.-B.L.; writing—review and editing, J.-B.L. and S.-C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Technology Development Program (S3311074), funded by the Ministry of SMEs and Startups (Republic of Korea).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data can be provided by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, C.C.; Cotsarelis, G. Review of hair follicle dermal cells. J. Dermatol. Sci. 2010, 57, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Madaan, A.; Verma, R.; Singh, A.T.; Jaggi, M. Review of hair follicle dermal papilla cells as in vitro screening model for hair growth. Int. J. Cosmet. Sci. 2018, 40, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef]
- Jahoda, C.A.B.; Reynolds, A.J. Dermal-epidermal interactions-follicle-derived cell populations in the study of hair-growth mechanisms. J. Investig. Dermatol. 1993, 101, S33–S38. [Google Scholar] [CrossRef]
- Harris, J.E. Cellular stress and innate inflammation in organ-specific autoimmunity: Lessons learned from vitiligo. Immunol. Rev. 2016, 269, 11–25. [Google Scholar] [CrossRef]
- Bakry, O.A.; Elshazly, R.M.A.; Shoeib, M.A.M.; Gooda, A. Oxidative stress in alopecia areata: A case-control study. Am. J. Clin. Dermatol. 2014, 15, 57–64. [Google Scholar] [CrossRef]
- Xing, L.; Dai, Z.; Jabbari, A.; Cerise, J.E.; Higgins, C.A.; Gong, W.; de Jong, A.; Harel, S.; DeStefano, G.M.; Rothman, L.; et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat. Med. 2014, 20, 1043–1049. [Google Scholar] [CrossRef]
- Prie, B.E.; Voiculescu, V.M.; Ionescu-Bozdog, O.B.; Petrutescu, B.; Iosif, L.; Gaman, L.E.; Clatici, V.G.; Stoian, I.; Giurcaneanu, C. Oxidative stress and alopecia areata. J. Med. Life 2015, 8, 43–46. [Google Scholar]
- Yenin, J.Z.; Serarslan, G.; Yönden, Z.; Ulutaş, K.T. Investigation of oxidative stress in patients with alopecia areata and its relationship with disease severity, duration, recurrence and pattern. Clin. Exp. Dermatol. 2015, 40, 617–621. [Google Scholar] [CrossRef]
- Naziroglu, M.; Kokcam, I. Antioxidants and lipid peroxidation status in the blood of patients with alopecia. Cell Biochem. Funct. 2000, 18, 169–173. [Google Scholar] [CrossRef]
- Akar, A.; Arca, E.; Erbil, H.; Akay, C.; Sayal, A.; Gür, A.R. Antioxidant enzymes and lipid peroxidation in the scalp of patients with alopecia areata. J. Dermatol. Sci. 2002, 29, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Koca, R.; Armutcu, F.; Altinyazar, C.; Gürel, A. Evaluation of lipid peroxidation, oxidant/antioxidant status, and serum nitric oxide levels in alopecia areata. Med. Sci. Monit. 2005, 11, CR296–CR299. [Google Scholar] [PubMed]
- Abdel Fattah, N.S.A.; Ebrahim, A.A.; El Okda, E.S. Lipid peroxidation/antioxidant activity in patients with alopecia areata. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.; Picardo, M. Antioxidant activity, lipid peroxidation and skin diseases. What’s new. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 663–669. [Google Scholar] [CrossRef]
- Ramadan, R.; Tawdy, A.; Abdel Hay, R.; Rashed, L.; Tawfik, D. The antioxidant role of paraoxonase 1 and vitamin E in three autoimmune diseases. Skin Pharmacol. Physiol. 2013, 26, 2–7. [Google Scholar] [CrossRef]
- Upton, J.H.; Hannen, R.F.; Bahta, A.W.; Farjo, N.; Farjo, B.; Philpott, M.P. Oxidative stress–associated senescence in dermal papilla cells of men with androgenetic alopecia. J. Investig. Dermatol. 2015, 135, 1244–1252. [Google Scholar] [CrossRef]
- Schäfer, M.; Werner, S. Nrf2–A regulator of keratinocyte redox signaling. Free Radic. Biol. Med. 2015, 88, 243–252. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Fujie, T.; Katoh, S.; Oura, H.; Urano, Y.; Arase, S. The chemotactic effect of a dermal papilla cell–derived factor on outer root sheath cells. J. Dermatol. Sci. 2001, 25, 206–212. [Google Scholar] [CrossRef]
- Yano, K.; Brown, L.F.; Detmar, M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J. Clin. Investig. 2001, 107, 409–417. [Google Scholar] [CrossRef]
- Trüeb, R.M. Further clinical evidence for the effect of IGF-1 on hair growth and alopecia. Skin Appendage Disord. 2018, 4, 90–95. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, A.J.G.; Elliott, K.R.; Messenger, A.G. Cytokines and dermal papilla function in alopecia areata. J. Investig. Dermatol. 1995, 104, S9–S10. [Google Scholar] [CrossRef] [PubMed]
- Jahoda, C.A.B.; Reynolds, A.J. Dermal-epidermal interactions: Adult follicle-derived cell populations and hair growth. Dermatol. Clin. 1996, 14, 573–583. [Google Scholar] [CrossRef]
- Millar, S.E. Molecular mechanisms regulating hair follicle development. J. Investig. Dermatol. 2002, 118, 216–225. [Google Scholar] [CrossRef]
- Fischer, T.W.; Herczeg-Lisztes, E.; Funk, W.; Zillikens, D.; Bíró, T.; Paus, R. Differential effects of caffeine on hair shaft elongation, matrix and outer root sheath keratinocyte proliferation, and transforming growth factor-β2/insulin-like growth factor-1-mediated regulation of the hair cycle in male and female human hair follicles in vitro. Br. J. Dermatol. 2014, 171, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.; Philips, N.; Suárez-Pérez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Jadkauskaite, L.; Coulombe, P.A.; Schäfer, M.; Dinkova-Kostova, A.T.; Paus, R.; Haslam, I.S. Oxidative stress management in the hair follicle: Could targeting NRF2 counter age-related hair disorders and beyond. BioEssays 2017, 39, 1700029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, J.; Fu, D.; Liu, Z.; Wang, H.; Wang, J.; Qu, Q.; Li, K.; Fan, Z.; Hu, Z.; et al. Transcriptome Analysis Reveals an Inhibitory Effect of Dihydrotestosterone-Treated 2D- and 3D-Cultured Dermal Papilla Cells on Hair Follicle Growth. Front. Cell Dev. Biol. 2021, 17, 724310. [Google Scholar] [CrossRef]
- Piao, M.S.; Choi, J.Y.; Lee, D.H.; Yun, S.J.; Lee, J.B.; Lee, S.C. Differentiation-dependent expression of NADP(H): Quinone oxidoreductase-1 via NF-E2 related factor-2 activation in human epidermal keratinocytes. J. Dermatol. Sci. 2011, 62, 147–153. [Google Scholar] [CrossRef]
- Mu, Y.; He, J.; Yan, R.; Hu, X.; Liu, H.; Hao, Z. IGF-1 and VEGF can be used as prognostic indicators for patients with uterine fibroids treated with uterine artery embolization. Exp. Ther. Med. 2016, 11, 645–649. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; Hassan, K.M.; Abdelhamid, A.N.; Yousef, E.E.; Abdellatif, Y.M.R.; Abu-Hussien, S.H.; Naseerm, M.A.; Elshalakany, W.A.; Eldin Darwish, D.B.; Abdulmajeed, A.M.; et al. Exogenous Paclobutrazol Reinforces the Antioxidant and Antimicrobial Properties of Lavender (Lavandula officinalis L.) Oil through Modulating Its Composition of Oxygenated Terpenes. Plants 2022, 19, 1607. [Google Scholar] [CrossRef]
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential oils: Extraction techniques, pharmaceutical and therapeutic potential—A review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Itami, S.; Kurata, S.; Takayasu, S. Androgen induction of follicular epithelial cell growth is mediated via insulin-like growth factor-I from dermal papilla cells. Biochem. Biophys. Res. Commun. 1995, 212, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. Oxidative stress in ageing of hair. Int. J. Trichology 2009, 1, 6–14. [Google Scholar] [CrossRef]
- Bae, S.; Lim, K.M.; Cha, H.J.; An, I.S.; Lee, J.P.; Lee, K.S.; Lee, G.T.; Lee, K.K.; Jung, H.J.; Ahn, K.J.; et al. Arctiin blocks hydrogen peroxide-induced senescence and cell death though microRNA expression changes in human dermal papilla cells. Biol. Res. 2014, 47, 50. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.J.; Jahoda, C.A. Hair matrix germinative epidermal cells confer follicle-inducing capabilities on dermal sheath and high passage papilla cells. Development 1996, 122, 3085–3094. [Google Scholar] [CrossRef] [PubMed]
- Uliasz, A.; Spencer, J.M. Chemoprevention of skin cancer and photoaging. Clin. Dermatol. 2004, 22, 178–182. [Google Scholar] [CrossRef]
- Choi, J.; Yun, S.; Lee, J.; Piao, M.; Park, E.; Lee, S. A set of plant essential oils have antioxidant activity in ultraviolet B-irradiated epidermal keratinocytes by upregulating antioxidant and detoxifying enzymes. J. Investig. Dermatol. 2017, 5, 233–242. [Google Scholar] [CrossRef]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef]
- Furue, M.; Fuyuno, Y.; Mitoma, C.; Uchi, H.; Tsuji, G. Therapeutic agents with AHR inhibiting and NRF2 activating activity for managing chloracne. Antioxidants 2018, 7, 90. [Google Scholar] [CrossRef]
- Scapagnini, G.; Davinelli, S.; Renzo, L.D.; Lorenzo, A.D.; Olarte, H.H.; Micali, G.; Cicero, A.F.; Gonzalez, S. Cocoa bioactive compounds: Significance and potential for the maintenance of skin health. Nutrients 2014, 6, 3202–3213. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, X.; Zhao, S.; Ma, C.; Cui, J.; Zheng, Y. Sulforaphane Protects against Cardiovascular Disease via Nrf2 Activation. Oxid. Med. Cell. Longev. 2015, 2015, 407580. [Google Scholar] [CrossRef] [PubMed]
- Limat, A.; Hunziker, T.; Waelti, E.R.; Inaebnit, S.P.; Wiesmann, U.; Braathen, L.R. Soluble factors from human hair papilla cells and dermal fibroblasts dramatically increase the clonal growth of outer root sheath cells. Arch. Dermatol. Res. 1993, 285, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F.; Brown, L.F.; Detmar, M.; Dvorak, A.M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995, 146, 1029–1039. [Google Scholar] [PubMed]
- Brown, L.F.; Yeo, K.T.; Berse, B.; Yeo, T.K.; Senger, D.R.; Dvorak, H.F.; van de Water, L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J. Exp. Med. 1992, 176, 1375–1379. [Google Scholar] [CrossRef]
- Detmar, M.; Brown, L.F.; Claffey, K.P.; Yeo, K.T.; Kocher, O.; Jackman, R.W.; Berse, B.; Dvorak, H.F. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J. Exp. Med. 1994, 180, 1141–1146. [Google Scholar] [CrossRef]
- Lachgar, S.; Moukadiri, H.; Jonca, F.; Charveron, M.; Bouhaddioui, N.; Gall, Y.; Bonafe, J.L.; Plouët, J. Vascular endothelial growth factor is an autocrine growth factor for hair dermal papilla cells. J. Investig. Dermatol. 1996, 106, 17–23. [Google Scholar] [CrossRef]
- Meephansan, J.; Thummakriengkrai, J.; Ponnikorn, S.; Yingmema, W.; Deenonpoe, R.; Suchonwanit, P. Efficacy of topical tofacitinib in promoting hair growth in non-scarring alopecia: Possible mechanism via VEGF induction. Arch. Dermatol. Res. 2017, 309, 729–738. [Google Scholar] [CrossRef]
- Lachgar, S.; Charveron, M.; Gall, Y.; Bonafe, J.L. Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. Br. J. Dermatol. 1998, 138, 407–411. [Google Scholar] [CrossRef]
- Rho, S.S.; Park, S.J.; Hwang, S.L.; Lee, M.H.; Kim, C.D.; Lee, I.H.; Chang, S.Y.; Rang, M.J. The hair growth promoting effect of Asiasari radix extract and its molecular regulation. J. Dermatol. Sci. 2005, 38, 89–97. [Google Scholar] [CrossRef]
- Junlatat, J.; Sripanidkulchai, B. Hair growth-promoting effect of Carthamus tinctorius floret extract. Phytother. Res. 2014, 28, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.S.; Hong, E.J.; Gwak, K.S.; Park, M.J.; Choi, K.C.; Choi, I.G.; Jang, J.W.; Jeung, E.B. The essential oils of Chamaecyparis obtusa promote hair growth through the induction of vascular endothelial growth factor gene. Fitoterapia 2010, 81, 17–24. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).