Abstract
Recently, the combined application of synergistic therapies for photodynamic antimicrobial chemotherapy has become important to obtain more efficient results. The synergism between two sensitizers, rose bengal (RB) and chlorin e6 (Ce6), excited by two different methods, was evaluated as a novel approach to both photodynamic and sonodynamic therapy against methicillin-resistant Staphylococcus aureus. The sonostability and singlet oxygen generation (with 1,3-diphenylisobenzofuran for RB and tetrathiafulvalene for Ce6) were measured under sonication (1 MHz, 3 W) using a spectrophotometer. RB and Ce6 remained stable during sonication. RB was a more efficient sonosensitizer than Ce6. The dual synergism between RB and Ce6 was noticed, achieving a >3 log reduction for molar ratios RB:Ce6 of 1:1 and 1:3, while, alone, the sensitizers excited with ultrasound and light, respectively, achieved only ca. a 1 log reduction.
1. Introduction
Antibiotic-resistant infections are a growing global problem for primary care and hospital medicine. They are a therapeutic challenge and a considerable cost to social security systems [1]. Every year there are new strains resistant to antibiotics, while pan-resistant strains pose a particular threat [2]. In the next 30 years, there is a danger that most known therapies will no longer be sufficiently effective against the most common bacterial infectious diseases [3]. From this perspective, infections caused by methicillin-resistant Staphylococcus aureus (MRSA) are becoming particularly important. Currently, MRSA causes the majority of skin and soft tissue infections in the United States of America and about half of the European infections [4]. At the same time, MRSA infections are characterized by significantly higher mortality than infections caused by other S. aureus isolates [5,6]. This strain can cause systemic infections as well as superficial ones. It is mainly associated with hospital-acquired (HA)-MRSA, particularly affecting the elderly and immunocompromised people. In recent years, it has been observed that MRSA infections also affect healthy people, including professional athletes [7]. This fact emphasizes the importance of the problem and the epidemic potential of this strain.
S. aureus resistance can arise through natural selection or horizontal plasmid transfer [8,9]. MRSA shows resistance to beta-lactam antibiotics except for a new class of cephalosporins with anti-MRSA activity, which is a significant difficulty in treatment. Currently, the principal medications used are ceftaroline, ceftobiprole, glycopeptide, lipopeptide, and oxazolidinone antibiotics [4]. On the other hand, several new strains of MRSA have already been isolated and revealed resistance to antibiotics, even to those used so far such as vancomycin and teicoplanin [10]. It suggests the possibility of further, rapid development of resistance to current treatments. Faced with these issues, it will become necessary to find new alternatives—PACT. The beginning of PACT dates back to the early 20th century when, thanks to the observations of von Tappeiner and Jesionek, it was discovered that certain compounds under the influence of visible light are able to kill bacteria. As a result of further experiments, it was found that reactive oxygen species (ROS) were the factor determining the death of bacteria. In photodynamic therapy, the interaction of three separately non-toxic components is crucial: a photosensitizer (PS), molecular oxygen, and light with a wavelength appropriate to the PS used. The great benefits of this therapy is its multi-targeting nature, non-specificity, and non-invasiveness. Additional advantages of PACT include the possibility of combining it with other therapeutic methods, its non-interference with parallel pharmacological treatment (e.g., antibiotic therapy), the option of repeated irradiation, and its low side effects and high safety profile. Moreover, photodynamic therapy may in the coming decades become the answer to the problem of antibiotic resistance in superficial infections, including infections with multidrug-resistant bacteria. It is extremely important that microorganisms have not yet developed specific defence mechanisms against singlet oxygen—the basic factor responsible for antimicrobial activity. The main cause of PACT failure is usually not bacterial resistance but problems with the delivery of the photosensitizer. The PS is often unable to penetrate inside the cell, and external activity may be insufficient to kill microbes. This fact emphasizes that the search for new PSs with optimal properties and the development of the existing ones will be essential in the near future [11,12,13,14,15,16].
The article presents an entirely new approach to MRSA eradication. A possible option is the combination of photodynamic therapy (PDT) with sonodynamic therapy (SDT) [17]. Rose bengal, RB (xanthenes), has been known for many years as a dye with potential medical use (Figure 1). Thus far, it has been used primarily as a contrast agent in the diagnosis of liver diseases [18]. For years, it has also been studied as a potential agent in photodynamic therapy [19,20]. Until now, there has been little research into the possibility of using RB as a sonosensitizer (SS). It is worth mentioning that RB has been used at a relatively high concentration (10–15 µM) [21]. Chlorine e6, Ce6 (chlorins), has a long tradition of use in PDT against cancer and some bacteria and fungi. Unfortunately, also in this case, to achieve bacterial eradication, high concentrations (≥128 μM) are required [22]. At these concentrations side effects could be expected in potential clinical practice. The dualistic approach, using both PDT and SDT, significantly reduces the concentration of active substances. At the same time, it does not limit the mechanism of action, only the effect based on reactive oxygen species and also to the thermal effects caused by pyrolysis (a phenomenon characteristic of SDT) [21].
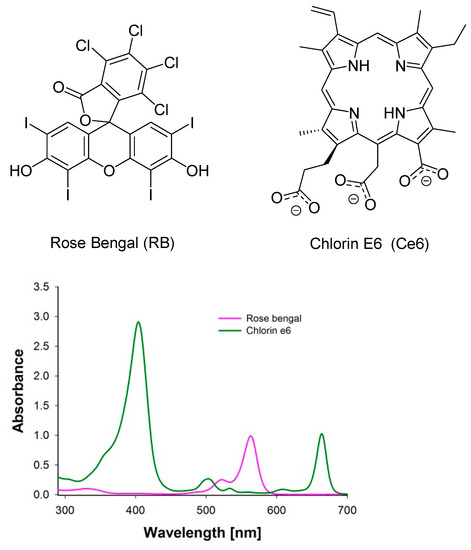
Figure 1.
Molecular structure of rose bengal (RB) and chlorin e6 (Ce6) and their absorption spectra.
In the present study, the ROS production potential of RB and Ce6 upon excitation with ultrasound was evaluated. Next, the dual synergism based on two different sensitizers and two different excitation methods against MRSA was assessed. The RB was used as an SS, while the Ce6 was utilized as a PS. The obtained results, combined with the established position of both dyes and the well-known safety profile, allow for the further development of the therapy based on the combination of PDT and SDT.
2. Material and Methods
2.1. General
RB was purchased from Merck (Darmstadt, Germany), and Ce6 from Bertin Bioreagent (Montigny le Bretonneux, France).
2.2. Physicochemical Properties
2.2.1. Stability Measurements under Sonication
Measurements in a quartz cuvette (l = 1 cm) were performed. The samples were aerated, the cuvette was sealed, and shaken before each spectrum recording (Shimadzu U-1900 spectrophotometer). The sample was sonicated in the ultrasonic apparatus (developed by the Institute of Fundamental Technological Research, Polish Academy of Sciences, Warsaw, Poland), equipped with a 1 MHz ultrasonic head with burst mode (40% duty cycle, 3 W, 1 MHz) for 5 min, divided into one-minute intervals. The experiments were performed as a set of ten independent measurements. The mean of the obtained data was taken to the kinetics calculations.
2.2.2. Singlet Oxygen Generation by Ultrasound Excitation
Measurements in a quartz cuvette (l = 1 cm) were performed. A mixture of sensitizer and quencher (DPBF—1,3-diphenyloisobenzofuran or TTF—tetrathiafulvalene) solution was aerated. Next, the cuvette was sealed and shaken before each spectrum recording (Shimadzu U-1900 spectrophotometer). The mixture was sonicated in the ultrasonic apparatus (constructed by the Institute of Fundamental Technological Research, Polish Academy of Sciences, Warsaw, Poland), equipped with an ultrasonic head with burst mode (40% duty cycle, 3 W, 1 MHz) for 5 min, divided into one-minute intervals. The experiment was performed as a set of ten independent measurements. The mean of the obtained data was taken to the kinetics calculations.
2.2.3. RB-Ce6 Interaction Assessment by Job Method
Stock solutions of the compounds in DMSO or water with 1% DMSO were prepared. Next, appropriate volumes of each solution were diluted with the solvent to achieve the host to guest ratio and then mixed. Subsequently, a quartz cuvette with two cells (l1 = l2 = 0.5 cm) was filled with diluted solutions and sealed with plugs. The cuvette was placed in a spectrophotometer, and UV spectra of the non-mixed solutions were obtained. In the next step, the cuvette was shaken vigorously to mix the solutions, and the UV spectra of the mixed solutions were also recorded. The measurements were repeated for ratios from 0 to 1.
2.3. In Vitro Photodynamic Activity against Bacteria
2.3.1. Microbial Cultures
Gram-positive bacteria of methicillin-resistant Staphylococcus aureus (clinical strain) were used in the experiment. It was grown in BHI broth (bioMerieux, Marcy-l’Étoile, France) at 36 ± 1 °C for approximately 24 h. After multiplication, the bacteria were centrifuged (3000 rpm for 15 min) and then harvested. In the next step, the bacteria were resuspended in 1.5 mL of saline and then diluted to a final concentration of about 107 CFU/mL.
2.3.2. Determination of the Dark Toxicity of RB and Ce6 to MRSA
A total of 150 µL of the bacterial suspension prepared as described above was placed in each well of a 96-well plate. Then 150 µL aqueous solutions of RB or Ce6 at concentrations of 2 × 10−4, 2 × 10−5, and 2 × 10−6 M were added to the bacteria. The samples prepared in this way were incubated for 200 min. After this time, the bacterial cultures, along with the dyes, were plated on tryptic soy agar (TSA) plates. After approximately 24 h of incubation at a constant temperature of 36 ± 1 °C, the colony-forming units (CFU) were counted. The reduction in the number of bacteria was calculated relative to a control in which the dye solution was replaced with a physiological saline solution.
2.3.3. Light-Dependent Activity of Ce6
The evaluation of the photodynamic activity of Ce6 was performed analogously to the dark test. In total, 150 µL of an adequately prepared bacterial suspension and 150 µL of a dye at the concentration of 2 × 10−5 and 2 × 10−6 M were exposed to light with a length of 660 nm (epiLED, Wroclaw, Poland) and intensity of 50 and 100 J/cm2. In parallel, a control sample was prepared consisting of a suspension of bacteria and saline. Then, analogously to the toxicity test, the bacteria were plated on TSA, incubated, and the number of colony was counted. On this basis, the number of viable bacteria and the log reduction were calculated.
2.3.4. Ultrasound-Dependent Activity of RB
Amounts of 150 µL of bacterial suspension and 150 µL of RB at the concentration of 2 × 10−6 M were placed in a 96-well plate. Then they were subjected to ultrasound with a frequency of 1 MHz (40% duty cycle) with a total energy of 256 and 512 J/cm2 (ultrasound equipment for biological trials was constructed by the Institute of Fundamental Technological Research, Polish Academy of Sciences, Warsaw, Poland, and applied previously in anticancer experiments in vitro [23]). At the same time, a control probe was carried out with the bacteria and saline alone. Then, the bacteria were plated on TSA and incubated in the same way as in the toxicity test. The number of bacteria was counted, and the log reduction was determined.
2.3.5. Synergy Test
The bacteria were prepared in five sets of samples. Sample A (only RB at 1.0 × 10−6 M) was subjected to ultrasound only. Samples B (RB at 7.5 × 10−7 M and Ce6 at 2.5 × 10−7 M), C (RB at 5.0 × 10−7 M and Ce6 at 5.0 × 10−7 M), D (RB at 2.5 × 10−7 M and Ce6 at 7.5 × 10−7 M) were subjected to ultrasounds and further immediately to light at a wavelength of 660 nm. Sample E (Ce6 at 1.0 × 10−6 M) was subjected only to light irradiation. The control experiments were performed as follows: sample F (light control), sample G (ultrasounds control), sample H (ultrasounds + light control), and sample I (bacterial growth control). Light at a maximum of 660 nm and a light dose of 50 J/cm2 was used. Ultrasound irradiation provided a dose of 512 J/cm2 (1 MHz, 40% duty cycle). The samples were then plated on TSA and incubated for 24 h at 36 ± 1 °C. After this time, the the number of bacteria was counted, and the log reduction was calculated. A dark toxicity control was also performed.
2.3.6. Statistical Analysis
Data represent the mean from the experiment performed in triplicate. The unpaired Student’s t-test and U Mann-Whitney test were used to establish the significance of differences between groups. A probability value (p) of less than 0.05 was considered significantly different. Statistical analysis was performed with the STATISTICA software, v.13.0.
3. Results and Discussion
The physicochemical properties of RB and Ce6 were evaluated. Firstly, the stability under sonication (1 MHz, 3 W) of the sensitizers was assessed, and no changes were noticed in the UV-Vis spectra. Therefore, it was concluded that the studied sensitizers are stable under ultrasounds used in the measurements. Next, the reactive oxygen species (ROS) production by ultrasound-excited sensitizers was estimated via the decomposition process of DPBF and TTF by monitoring the disappearing characteristic absorption bands, at 417 nm (Figure 2) and 317 nm, respectively. DPBF and TTF are well-known ROS chemical traps for singlet oxygen and other ROS [24,25]. To avoid overlapping the trap and sensitizer bands, the RB with the DPBF and the Ce6 with the TTF were mixed. There were also performed studies of singlet oxygen trap stability under sonication. It was concluded that TTF reveals high stability, whereas DPBF, as a very reactive molecule, is less stable (Table 1).
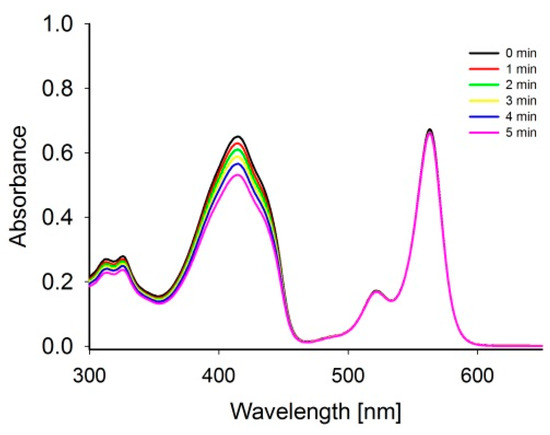
Figure 2.
Spectra changes in the RB and DPBF mixture in DMF under sonication with 1 MHz, 3 W and duty cycle 40% during the experiment.

Table 1.
Kinetic parameters of DPBF and TTF decomposition upon sonication in DMF solution alone and in the presence of Ce6 and RB. The stability parameters of RB and Ce6 upon sonication were also shown.
Due to the complexity of the interaction between the sonosensitizer and the surrounding molecules of the sample including the molecular oxygen, solvent, and sonosensitizer itself [21,26], the calculation of the quantum yields of formation singlet oxygen was abandoned. However, the kinetic parameters of the decomposition of DPBF and TTF in the presence of sensisitizer and ultrasounds were calculated (Table 1). According to the studies reported before, the reaction between singlet oxygen and traps follows first-order kinetics [27] (Figure 3). The first-order kinetic equation (1) was applied for the calculations.
ln[A0] = kt + ln[A]
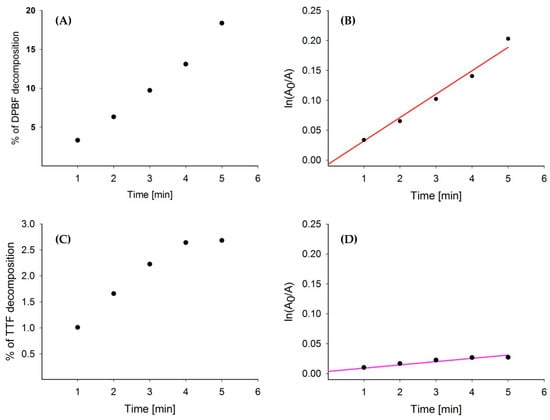
Figure 3.
Effect of mixtures RB + DPBF and Ce6 + TTF sonication with 1 MHz, 3 W (40% duty cycle) vs. time: (A) % DPBF decomposed in the presence of RB; (B) kinetic curve of DPBF decomposition by RB (red); (C) % TTF decomposed in the presence of Ce6; (D) kinetic curve of TTF decomposition by Ce6 (pink).
As shown in Table 1 the improvement in trap decomposition induced by ultrasounds in the presence of RB compared to DPBF alone was noticed. It was concluded that the generated singlet oxygen by RB ultrasound excitation accelerates DPBF decomposition by about 1.8-fold. It enables us to consider RB as an efficient singlet oxygen generator.
Interestingly, under sonication of the mixture of Ce6 and TTF solution, the presence of SS did not significantly improve the decomposition of the singlet oxygen chemical quencher (TTF) (Table 1). This observation enables us to conclude that Ce6 is a much weaker SS in comparison to RB (Figure 3).
The potential interaction between RB and Ce6 in the solution was analyzed by Job’s method. As shown in Figure 4, interactions were excluded and it was confirmed that each sensitizer existed as a separate molecule in the solution.
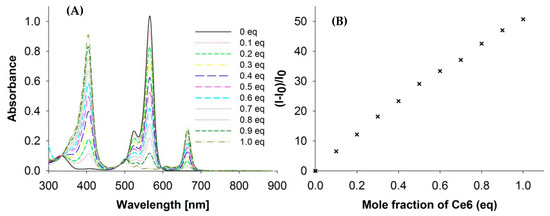
Figure 4.
Job’s plot of RB vs. Ce6; (A) UV-Vis spectra during the addition of Ce6 equivalents to RB; (B) (I−I0)/I0 vs. Ce6 eq during the addition of Ce6 equivalents to RB (concentration of the host—RB—2 × 10−5)—Job’s plot.
The synergy study between PDT and SDT is currently poorly developed [21]. Several experiments evaluating synergism against cancer have emerged in recent years, but in the case of microorganisms, studies are deficient [28,29,30]. Alves et al. investigated the synergy between photo- and sonodynamic therapy against Gram-positive bacterial biofilm (Staphylococcus aureus) with curcumin (80 μM) as a sensitizer. In this study, an aqueous solution of curcumin with 0.5% DMSO was used. The sample containing the bacteria was first sonicated (1 MHz for 32 min) and then irradiated (450 nm, 70 J/cm2) [31]. The results were a 1.67 log reduction for SDT, 2.39 for PDT, and 3.48 for SPDT. However, it should be noted that the applied wavelengths of light was outside the optimal range known as the “therapeutic window” [32]. PDT’s optimal light wavelength range is 600–800 nm [21]. The use of light with a length of less than 600 nm significantly limits the application potential of the method due to the low permeability of this range through human tissues. On the other hand, using wavelengths higher than 800 nm results in the significantly lower efficiency of ROS generation [32].
The current study proposed the use of two popular and well-researched dyes: Ce6 and RB [18]. Both dyes used have numerous advantages. The toxicity of both is well-known and documented. Ce6 is also registered in many countries as a drug utilized in photodynamic therapy against cancer [33]. On the other hand, RB has been used in the past as a contrast agent in liver imaging [19]. In the presented experiment, Ce6 (1 µM), excited with light at the power of 50 J/cm2, resulted in a reduction in MRSA bacteria at the level of 2.43 log (no dark toxicity, Figure 5). Neither the increase in the light dose to 100 J/cm2 or the PS concentration to 10 µM resulted in a statistically significant enhancement of the bactericidal effect against MRSA. Ce6 has been tested in PACT against several microorganisms, including P. acnes, E. coli, C. albicans, S. epidermidis, E. faecalis, E. coli, P. mirabilis, P. aeruginosa, and S. enterica so far [34,35,36,37,38]. Previous research on MRSA performed by Winkler et al. and Park et al. gave confusing results [22,39]. In the case of the first experiment, a 5 log reduction was achieved, but a dose of >128 µM was used [22]. In the dark toxicity tests performed here, such a high concentration of PS was bactericidal against the used strain. Research by Park et al. [39] assessed the bactericidal effect by observing the inhibition zones. This method does not allow for an accurate quantitative assessment of the bactericidal effect. However, irradiation with light with a power of <50 J/cm2 gave only the effect described by the authors as “mild inhibition” [39].
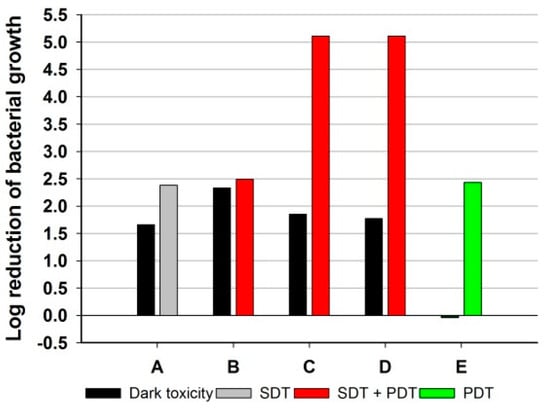
Figure 5.
RB and Ce6 synergy evaluation results (A—only RB at 1.0 × 10−6 M was subjected to ultrasound only; B—RB at 7.5 × 10−7 M and Ce6 at 2.5 × 10−7 M, C—RB at 5.0 × 10−7 M and Ce6 at 5.0 × 10−7 M, D—RB at 2.5 × 10−7 M and Ce6 at 7.5 × 10−7 M were subjected to ultrasounds and further immediately to light; E—Ce6 at 1.0 × 10−6 M was subjected to light only).
RB, a SS, at the concentration of 100 and 10 µM, revealed very strong dark toxicity to the tested strain. Therefore, the concentration was reduced to 1 µM RB (no dark toxicity). The SS in such an amount was subjected to ultrasounds at an energy dose of 256 J/cm2 (40% duty cycle, 3 W, 1 MHz), and a <1 log reduction was noticed as the SDT result. Increasing the ultrasound energy to 512 J/cm2 did not significantly change the antibacterial potential of the therapy. The use of SDT with RB as an SS is relatively innovative. Thus far, only one study by Nakonechny et al. has used pure RB as an SS (15 µM, 28 kHz, 0.84 W/cm2, 100% duty cycle) and reached maximum activity at the level of a 3–4 log reduction in bacterial growth [40]. In the present study, such a high concentration of RB eradicated MRSA without excitation (dark toxicity). The mentioned tendency could be linked with the phenomenon of so-called “collateral sensitivity”, which makes antibiotic-resistant strains more sensitive to other chemicals, which has been described in the literature so far [16,41,42]. It should also be emphasized that the strains used by Nakonechny et al. did not show resistance to methicillin, which may be associated with their greater sensitivity to SDT. Similar observations for PDT have previously been described by Grinholz et al. They confirmed on a representative sample of 80 clinical isolates of S. aureus that those that have methicillin sensitivity are also more susceptible to PDT [43].
To the best of the authors’ knowledge, the phenomenon of dual synergy between SDT and PDT and the two dye classes has not yet been described in the literature. In the experiments, three sets were used with RB and Ce6 in different ratios. Firstly, bacterial suspension was treated with ultrasounds and then with light at a wavelength of 660 nm. It is worth mentioning that irradiation with the light at 660 nm activated only Ce6 and not RB. At this wavelength, RB does not absorb light in opposition to Ce6 (Figure 1).
The sets in molar ratios RB:Ce6 of 1:1 (set C) and 1:3 (set D) proved to be the most effective, and both allowed a maximum reduction of >3 logs against MRSA (Figure 5). Such a result enables them to be considered as highly bactericidal formulations. On the other hand, the combination of two dyes did not result in an increase in dark toxicity compared to reference samples with RB or Ce6 alone. The combination of the two dyes allowed the full potential of dynamic therapy to be realized. The absorption maximum of RB is around 550 nm, which is outside the “therapeutic window” for PDT, so excitation with ultrasound was exceptionally beneficial. Moreover, it has so far been proven that the use of ultrasound in the 20 kHz to 1 MHz range may cause cavitation of the bacterial membrane, thereby increasing the penetration of SS/PS and thus increasing the effectiveness of SPDT [44]. In addition, the occurrence of synergism between the therapies made it possible to effectively reduce the concentration of both sensitizers to 0.5 µM. Such a low concentration significantly reduces the risk of side effects, even local ones.
4. Conclusions
The new phenomenon of dual synergism based on two different sensitizers and two different excitation methods against MRSA was evaluated. The reactive oxygen species (ROS) production by ultrasound-excited sensitizers was assessed. The half-time life of singlet oxygen chemical traps was 18 min for DPBF in the presence of RB and 97.6 min for TTF in the presence of Ce6. It was concluded that Ce6 is a weaker SS in comparison to RB. In the biological activity evaluation, the dual synergism was noticed. MRSA treated with ultrasounds and then with light at a wavelength of 660 nm (maximum of light absorption for RB) in the molar ratios RB:Ce6 of 1:1 and 1:3 allowed the reduction in bacterial growth at the level of >3 logs. However, RB and Ce6 alone, excited with ultrasounds and light, respectively, caused a reduction in bacterial growth of ca. 1 log. This work, thus, presents new insights into the advanced sono-photodynamic properties of sensitizers for efficient synergistic SPDT applications.
Author Contributions
Conceptualization, D.Z. and L.S.; methodology, D.Z., J.D. and L.S.; software, D.Z., E.G. and L.S.; validation, D.Z. and M.W.; formal analysis, D.Z., M.W. and L.S.; investigation, D.Z., M.W., J.D. and M.M.; resources, E.G., D.Z. and L.S.; data curation, D.Z., M.W. and L.S.; writing—original draft preparation, D.Z., E.G. and L.S.; writing—review and editing, E.G. and L.S.; visualization, D.Z., E.G. and L.S.; supervision, D.Z. and L.S.; project administration, D.Z. and L.S.; funding acquisition, E.G. and L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Scientific and Technological Research Council of Turkiye (TÜBİTAK) (Grant No. 122Z689).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Marcin Wysocki is a participant of the STER Internationalisation of Doctoral Schools Programme from NAWA Polish National Agency for Academic Exchange No. PPI/STE/2020/1/00014/DEC/02.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hofer, U. The Cost of Antimicrobial Resistance. Nat. Rev. Microbiol. 2019, 17, 3. [Google Scholar] [CrossRef]
- McAdam, A.J.; Hooper, D.C.; DeMaria, A.; Limbago, B.M.; O’Brien, T.F.; McCaughey, B. Antibiotic Resistance: How Serious Is the Problem, and What Can Be Done? Clin. Chem. 2012, 58, 1182–1186. [Google Scholar] [CrossRef]
- Kåhrström, C.T. Entering a Post-Antibiotic Era? Nat. Rev. Microbiol. 2013, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Righi, E. Safety Profiles of Old and New Antimicrobials for the Treatment of MRSA Infections. Expert Opin. Drug Saf. 2016, 15, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Talan, D.A.; Krishnadasan, A.; Gorwitz, R.J.; Fosheim, G.E.; Limbago, B.; Albrecht, V.; Moran, G.J.; The EMERGEncy ID Net Study Group. Comparison of Staphylococcus Aureus from Skin and Soft-Tissue Infections in US Emergency Department Patients, 2004 and 2008. Clin. Infect. Dis. 2011, 53, 144–149. [Google Scholar] [CrossRef]
- Morrissey, I.; Leakey, A.; Northwood, J.B. In Vitro Activity of Ceftaroline and Comparator Antimicrobials against European and Middle East Isolates from Complicated Skin and Skin-Structure Infections Collected in 2008–2009. Int. J. Antimicrob. Agents 2012, 40, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Redziniak, D.E.; Diduch, D.R.; Turman, K.; Hart, J.; Grindstaff, T.L.; MacKnight, J.M.; Mistry, D.J. Methicillin-Resistant Staphylococcus Aureus (MRSA) in the Athlete. Int. J. Sport. Med. 2009, 30, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Mocan, L.; Tabaran, F.A.; Mocan, T.; Pop, T.; Mosteanu, O.; Agoston-Coldea, L.; Matea, C.T.; Gonciar, D.; Zdrehus, C.; Iancu, C. Laser Thermal Ablation of Multidrug-Resistant Bacteria Using Functionalized Gold Nanoparticles. Int. J. Nurs. 2017, 12, 2255–2263. [Google Scholar] [CrossRef]
- Lindsay, J.A. Hospital-Associated MRSA and Antibiotic Resistance—What Have We Learned from Genomics? Int. J. Med. Microbiol. 2013, 303, 318–323. [Google Scholar] [CrossRef]
- Jian, Y.; Lv, H.; Liu, J.; Huang, Q.; Liu, Y.; Liu, Q.; Li, M. Dynamic Changes of Staphylococcus Aureus Susceptibility to Vancomycin, Teicoplanin, and Linezolid in a Central Teaching Hospital in Shanghai, China, 2008–2018. Front. Microbiol. 2020, 11, 908. [Google Scholar] [CrossRef]
- Sobotta, L.; Ziental, D.; Sniechowska, J.; Dlugaszewska, J.; Potrzebowski, M.J. Lipid Vesicle-Loaded Meso-Substituted Chlorins of High in vitro Antimicrobial Photodynamic Activity. Photochem. Photobiol. Sci. 2019, 18, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, J.; Sobotta, L.; Dlugaszewska, J.; Kryjewski, M.; Mielcarek, J. Menthol Modified Zinc(II) Phthalocyanine Regioisomers and Their Photoinduced Antimicrobial Activity against Staphylococcus Aureus. Dyes Pigments 2021, 193, 109410. [Google Scholar] [CrossRef]
- Stolarska, M.; Glowacka-Sobotta, A.; Ziental, D.; Dlugaszewska, J.; Falkowski, M.; Goslinski, T.; Sobotta, L. Photochemical Properties and Promising Activity against Staphylococci of Sulfanyl Porphyrazines with Dendrimeric Moieties. Inorg. Chim. Acta 2021, 521, 120321. [Google Scholar] [CrossRef]
- Glowacka-Sobotta, A.; Ziental, D.; Sobotta, L. Chapter 12. Porphyrinoids Used for Photodynamic Inactivation against Bacteria. In Applications of Porphyrinoids as Functional Materials; Lang, H., Rueffer, T., Eds.; Smart Materials; Royal Society of Chemistry: Cambridge, UK, 2021; pp. 352–404. ISBN 978-1-83916-188-9. [Google Scholar]
- Ziental, D.; Zajac, J.; Lewandowski, K.; Dlugaszewska, J.; Potrzebowski, M.J.; Sobotta, L. Oxospirochlorins as New Promising Photosensitizers against Priority Pathogens. Dyes Pigments 2022, 201, 110240. [Google Scholar] [CrossRef]
- Ziental, D.; Mlynarczyk, D.T.; Kolasinski, E.; Güzel, E.; Dlugaszewska, J.; Popenda, Ł.; Jurga, S.; Goslinski, T.; Sobotta, L. Zinc(II), Palladium(II), and Metal-Free Phthalocyanines Bearing Nipagin-Functionalized Substituents against Candida Auris and Selected Multidrug-Resistant Microbes. Pharmaceutics 2022, 14, 1686. [Google Scholar] [CrossRef]
- Granados-Tavera, K.; Zambrano-Angulo, M.; Montenegro-Pohlhammer, N.; Yaşa Atmaca, G.; Sobotta, L.; Güzel, E.; Cárdenas-Jirón, G.; Erdoğmuş, A.; Gürek, A.G. Synergistic Effect of Ultrasound and Light to Efficient Singlet Oxygen Formation for Photodynamic Purposes. Dyes Pigments 2023, 210, 110986. [Google Scholar] [CrossRef]
- Ziental, D.; Mlynarczyk, D.T.; Czarczynska-Goslinska, B.; Lewandowski, K.; Sobotta, L. Photosensitizers Mediated Photodynamic Inactivation against Fungi. Nanomaterials 2021, 11, 2883. [Google Scholar] [CrossRef]
- Sobotta, L.; Skupin-Mrugalska, P.; Piskorz, J.; Mielcarek, J. Non-Porphyrinoid Photosensitizers Mediated Photodynamic Inactivation against Bacteria. Dyes Pigments 2019, 163, 337–355. [Google Scholar] [CrossRef]
- Jankun, J. A Thousand Words about the Challenges of Photodynamic Therapy: Challenges of Photodynamic Therapy. JMS 2019, 88, 195–199. [Google Scholar] [CrossRef]
- Wysocki, M.; Czarczynska-Goslinska, B.; Ziental, D.; Michalak, M.; Güzel, E.; Sobotta, L. Excited State and Reactive Oxygen Species against Cancer and Pathogens: A Review on Sonodynamic and Sono-Photodynamic Therapy. ChemMedChem 2022, 17, e202200185. [Google Scholar] [CrossRef]
- Winkler, K.; Simon, C.; Finke, M.; Bleses, K.; Birke, M.; Szentmáry, N.; Hüttenberger, D.; Eppig, T.; Stachon, T.; Langenbucher, A.; et al. Photodynamic Inactivation of Multidrug-Resistant Staphylococcus Aureus by Chlorin E6 and Red Light (λ = 670 Nm). J. Photochem. Photobiol. B Biol. 2016, 162, 340–347. [Google Scholar] [CrossRef]
- Secomski, W.; Bilmin, K.; Kujawska, T.; Nowicki, A.; Grieb, P.; Lewin, P.A. In Vitro Ultrasound Experiments: Standing Wave and Multiple Reflections Influence on the Outcome. Ultrasonics 2017, 77, 203–213. [Google Scholar] [CrossRef]
- Kwak, W.-J.; Kim, H.; Petit, Y.K.; Leypold, C.; Nguyen, T.T.; Mahne, N.; Redfern, P.; Curtiss, L.A.; Jung, H.-G.; Borisov, S.M.; et al. Deactivation of Redox Mediators in Lithium-Oxygen Batteries by Singlet Oxygen. Nat. Commun. 2019, 10, 1380. [Google Scholar] [CrossRef]
- Sobotta, L.; Dlugaszewska, J.; Gierszewski, M.; Tillo, A.; Sikorski, M.; Tykarska, E.; Mielcarek, J.; Goslinski, T. Photodynamic Inactivation of Enterococcus Faecalis by Non-Peripherally Substituted Magnesium Phthalocyanines Entrapped in Lipid Vesicles. J. Photochem. Photobiol. B Biol. 2018, 188, 100–106. [Google Scholar] [CrossRef]
- Giuntini, F.; Foglietta, F.; Marucco, A.M.; Troia, A.; Dezhkunov, N.V.; Pozzoli, A.; Durando, G.; Fenoglio, I.; Serpe, L.; Canaparo, R. Insight into Ultrasound-Mediated Reactive Oxygen Species Generation by Various Metal-Porphyrin Complexes. Free Radic. Biol. Med. 2018, 121, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Seotsanyana-Mokhosi, I.; Kuznetsova, N.; Nyokong, T. Photochemical Studies of Tetra-2, 3-Pyridinoporphyrazines. J. Photochem. Photobiol. A Chem. 2001, 140, 215–222. [Google Scholar] [CrossRef]
- Bakhshizadeh, M.; Moshirian, T.; Esmaily, H.; Rajabi, O.; Nassirli, H.; Sazgarnia, A. Sonophotodynamic Therapy Mediated by Liposomal Zinc Phthalocyanine in a Colon Carcinoma Tumor Model: Role of Irradiating Arrangement. Iran. J. Basic Med. Sci. 2017, 20, 1088–1092. [Google Scholar] [CrossRef]
- Aksel, M.; Bozkurt-Girit, O.; Bilgin, M.D. Pheophorbide A-Mediated Sonodynamic, Photodynamic and Sonophotodynamic Therapies against Prostate Cancer. Photodiagnosis Photodyn. Ther. 2020, 31, 101909. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-X.; Zhu, W.-T.; Hu, J.-H.; Yang, W.; Liu, P.; Liu, Q.-H.; Bai, Y.-X.; Xie, R. Curcumin-Loaded Poly(L-Lactide-Co-Glycolide) Microbubble-Mediated Sono-Photodynamic Therapy in Liver Cancer Cells. Ultrasound Med. Biol. 2020, 46, 2030–2043. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.; Gomes Guimarães, G.; Mayumi Inada, N.; Pratavieira, S.; Salvador Bagnato, V.; Kurachi, C. Strategies to Improve the Antimicrobial Efficacy of Photodynamic, Sonodynamic, and Sonophotodynamic Therapies. Lasers Surg. Med. 2021, 53, 1113–1121. [Google Scholar] [CrossRef]
- Chen, Q.; Dan, H.; Tang, F.; Wang, J.; Li, X.; Cheng, J.; Zhao, H.; Zeng, X. Photodynamic Therapy Guidelines for the Management of Oral Leucoplakia. Int. J. Oral Sci. 2019, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Minamide, T.; Takashima, K.; Nakajo, K.; Kadota, T.; Yoda, Y. Clinical Practice of Photodynamic Therapy Using Talaporfin Sodium for Esophageal Cancer. J. Clin. Med. 2021, 10, 2785. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.-M.; Lee, H.-S.; Jeong, D.; Oh, H.-K.; Ra, K.-H.; Lee, M.-Y. Antimicrobial Photodynamic Therapy Using Chlorin E6 with Halogen Light for Acne Bacteria-Induced Inflammation. Life Sci. 2015, 124, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Deng, D.M.; Wu, Y.; de Oliveira, K.T.; Bagnato, V.S.; Crielaard, W.; Rastelli, A.N. de S. Photodynamic Inactivation Mediated by Methylene Blue or Chlorin E6 against Streptococcus Mutans Biofilm. Photodiagnosis Photodyn. Ther. 2020, 31, 101817. [Google Scholar] [CrossRef]
- Huang, L.; Xuan, Y.; Koide, Y.; Zhiyentayev, T.; Tanaka, M.; Hamblin, M.R. Type I and Type II Mechanisms of Antimicrobial Photodynamic Therapy: An in vitro Study on Gram-Negative and Gram-Positive Bacteria. Lasers Surg. Med. 2012, 44, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Ryu, A.-R.; Jin, S.; Jeon, Y.-M.; Lee, M.-Y. Chlorin E6-Mediated Photodynamic Therapy Suppresses P. Acnes-Induced Inflammatory Response via NFκB and MAPKs Signaling Pathway. PLoS ONE 2017, 12, e0170599. [Google Scholar] [CrossRef]
- Luke-Marshall, N.R.; Hansen, L.A.; Shafirstein, G.; Campagnari, A.A. Antimicrobial Photodynamic Therapy with Chlorin E6 Is Bactericidal against Biofilms of the Primary Human Otopathogens. mSphere 2020, 5, e00492-20. [Google Scholar] [CrossRef]
- Park, J.-H.; Moon, Y.-H.; Bang, I.-S.; Kim, Y.-C.; Kim, S.-A.; Ahn, S.-G.; Yoon, J.-H. Antimicrobial Effect of Photodynamic Therapy Using a Highly Pure Chlorin E6. Lasers Med. Sci. 2010, 25, 705–710. [Google Scholar] [CrossRef]
- Nakonechny, F.; Nisnevitch, M.; Nitzan, Y.; Nisnevitch, M. Sonodynamic Excitation of Rose Bengal for Eradication of Gram-Positive and Gram-Negative Bacteria. BioMed Res. Int. 2013, 2013, 684930. [Google Scholar] [CrossRef]
- Pál, C.; Papp, B.; Lázár, V. Collateral Sensitivity of Antibiotic-Resistant Microbes. Trends Microbiol. 2015, 23, 401–407. [Google Scholar] [CrossRef]
- Chaabane, F.; Graf, A.; Jequier, L.; Coste, A.T. Review on Antifungal Resistance Mechanisms in the Emerging Pathogen Candida Auris. Front. Microbiol. 2019, 10, 2788. [Google Scholar] [CrossRef] [PubMed]
- Grinholc, M.; Szramka, B.; Kurlenda, J.; Graczyk, A.; Bielawski, K.P. Bactericidal Effect of Photodynamic Inactivation against Methicillin-Resistant and Methicillin-Susceptible Staphylococcus Aureus Is Strain-Dependent. J. Photochem. Photobiol. B Biol. 2008, 90, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Serpe, L.; Giuntini, F. Sonodynamic Antimicrobial Chemotherapy: First Steps towards a Sound Approach for Microbe Inactivation. J. Photochem. Photobiol. B Biol. 2015, 150, 44–49. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).