Flux Behaviour, Rejection and Concentration Factors, and Energy Demand during Ultrafiltration of Sweet Buttermilk
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Membrane System
2.2.2. Calculation of the Characteristics of the Ultrafiltration Process
- Volume reduction ratio
- Transmembrane pressure
- Permeate flux
- Rejection factor
- Concentration factor
- Energy demand
2.2.3. Methods for Analyzing the Composition of the Initial Buttermilk, Retentates and Permeate, and Acidity during Ultrafiltration
- ➢
- Dry matter content—using ISO 6731:2010 [28];
- ➢
- Total protein content—by ISO 8968-1:2014 [29];
- ➢
- Fat content—according to ISO 2446:2008 [30];
- ➢
- Ash content—according to BS 6154:1974 [31];
- ➢
- Phospholipid composition—extraction of the buttermilk was made using a mixture of chloroform and methanol (2:1, v/v) [32]. Precipitation of the phospholipids from the mixture was performed with acetone and then two-dimensional thin-layer chromatography (TLC) was used for the isolation of the individual phospholipids [33]. The identification was made by comparison of the Rf values with authentic standards. The spots of phospholipids were scrapped and mineralized with sulphuric acid and perchloric, 1:1 (v/v), and the quantification was made spectrophotometrically at 700 nm [34].
- ➢
- Active acidity (pH)—using a pen-type pH meter PH—03 [I] (Hinotek Lab, Ningbo, China);
- ➢
- Titratable acidity—according to ISO 6091:2010|IDF 86:2010 [35].
2.2.4. Statistical Analysis
3. Results and Discussion
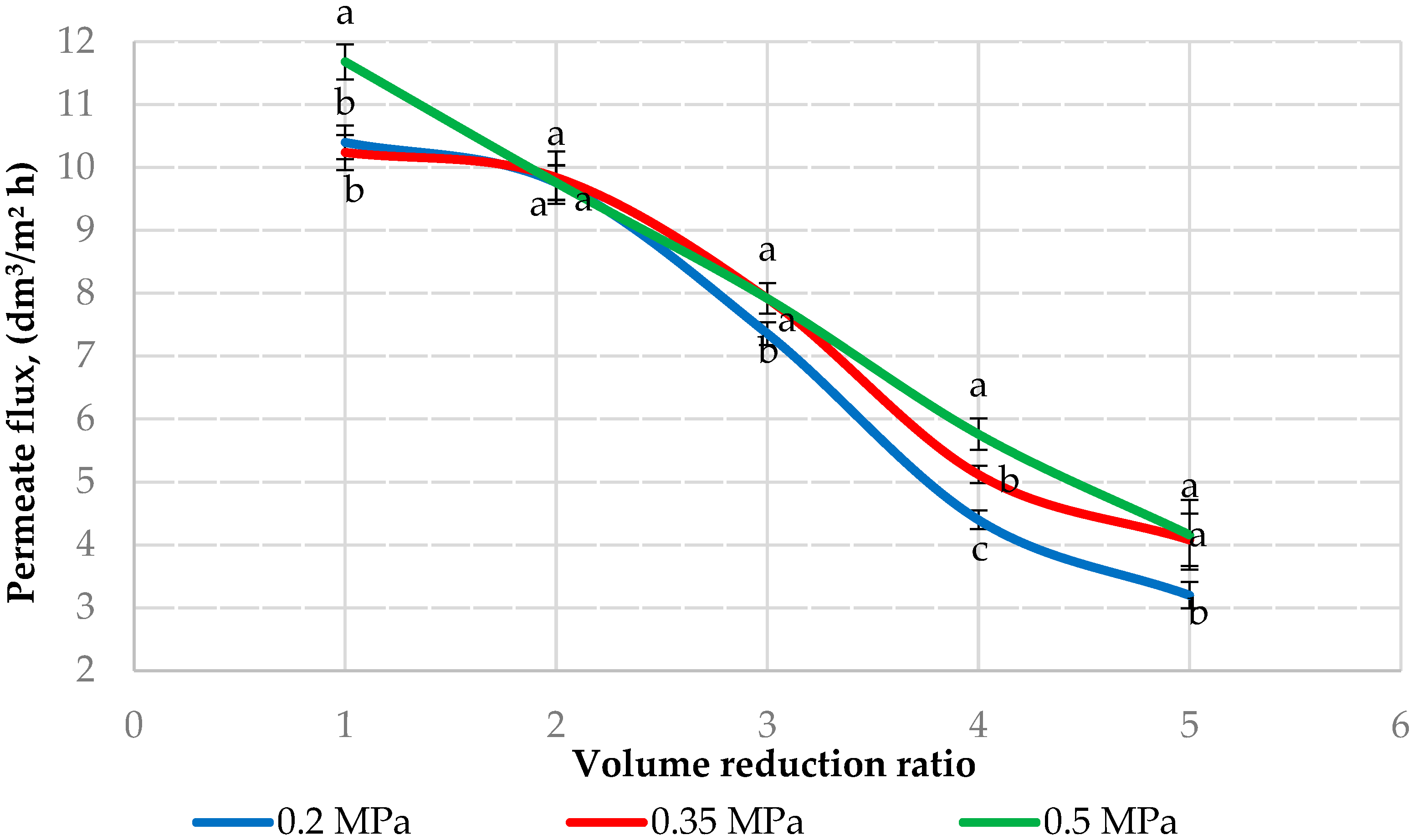
3.1. Flux Behaviour
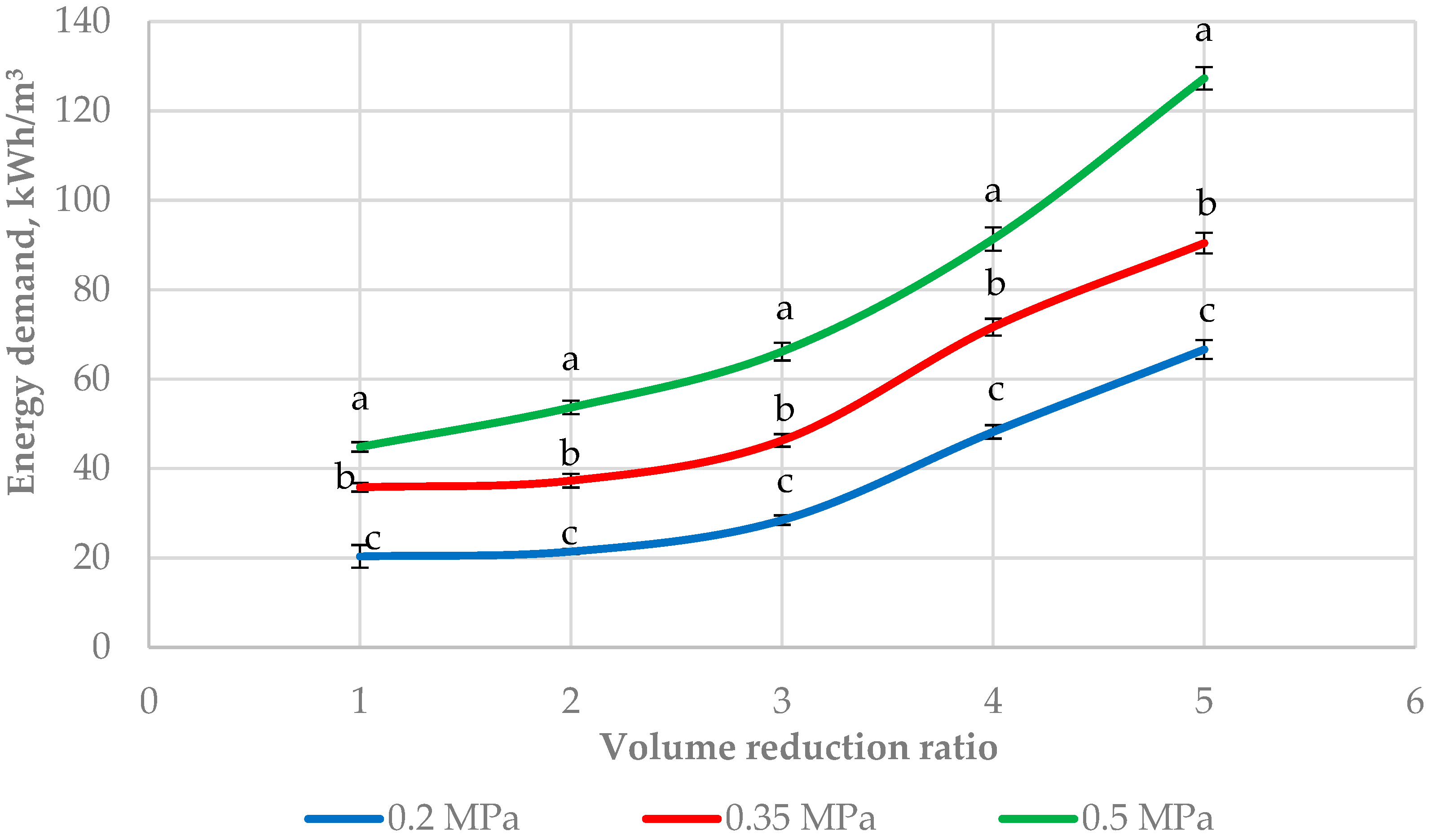
3.2. Energy Demand
3.3. Active and Titratable Acidity
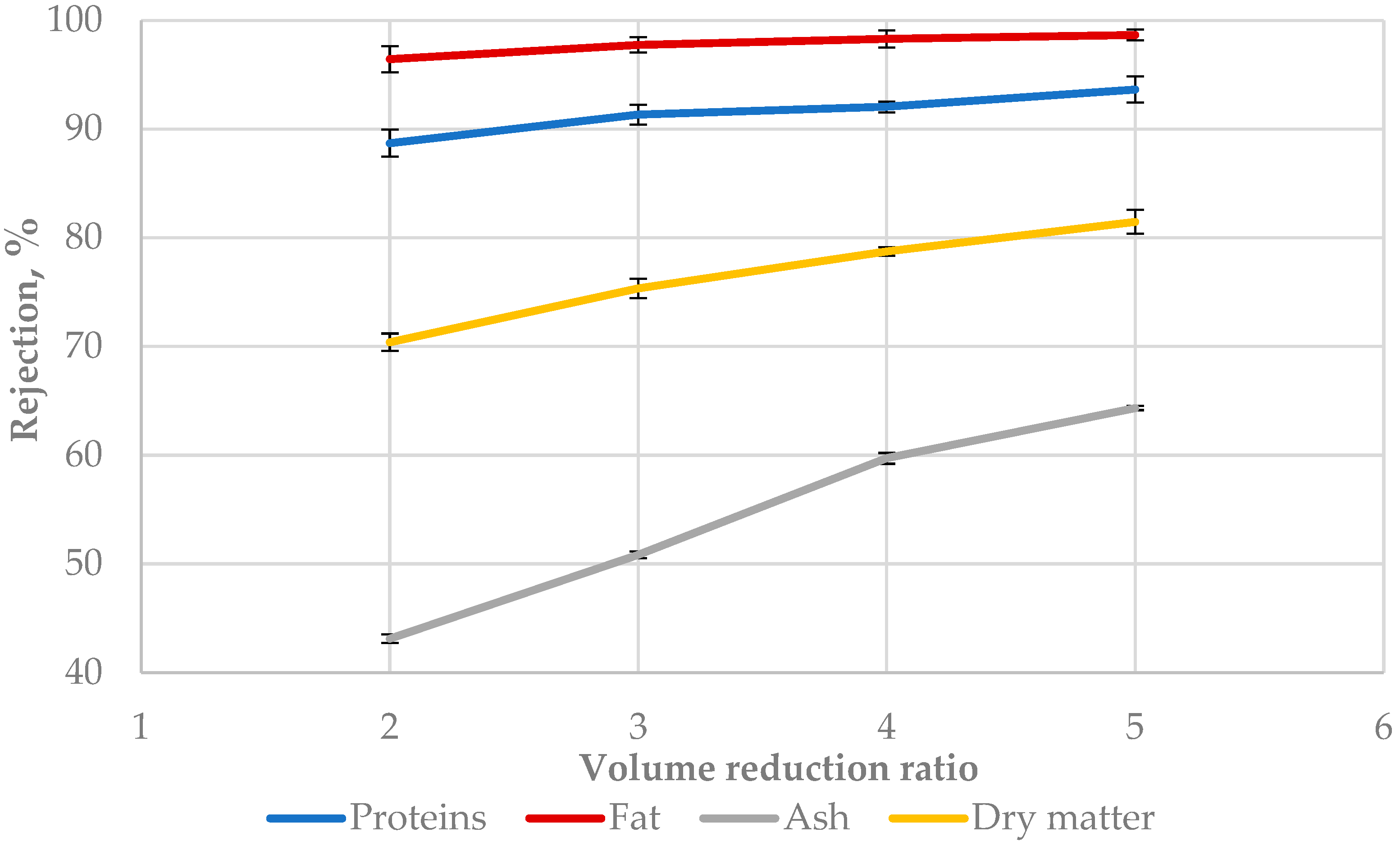
3.4. Rejection Factor
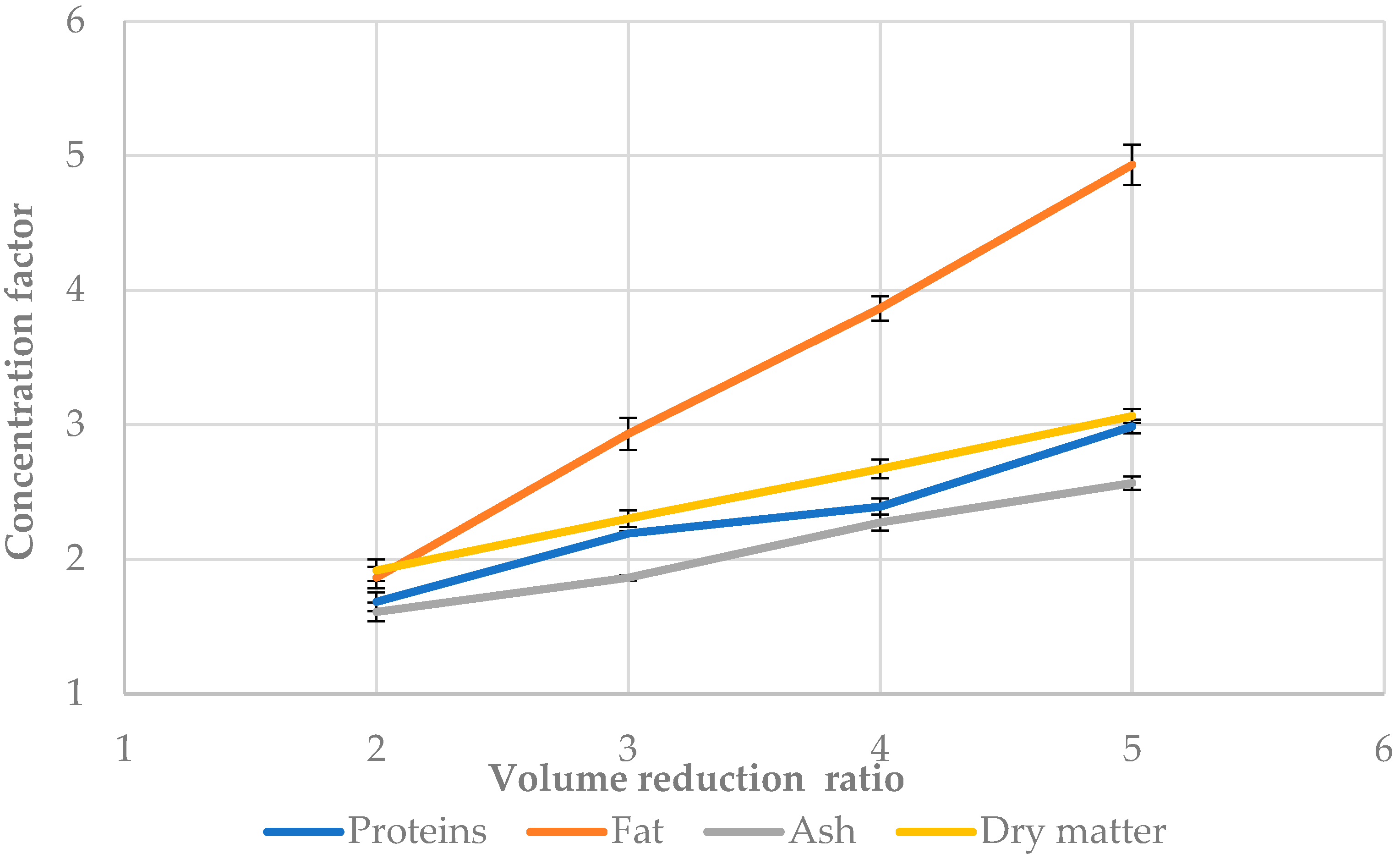
3.5. Concentration Factor
3.6. Phospholipid Composition
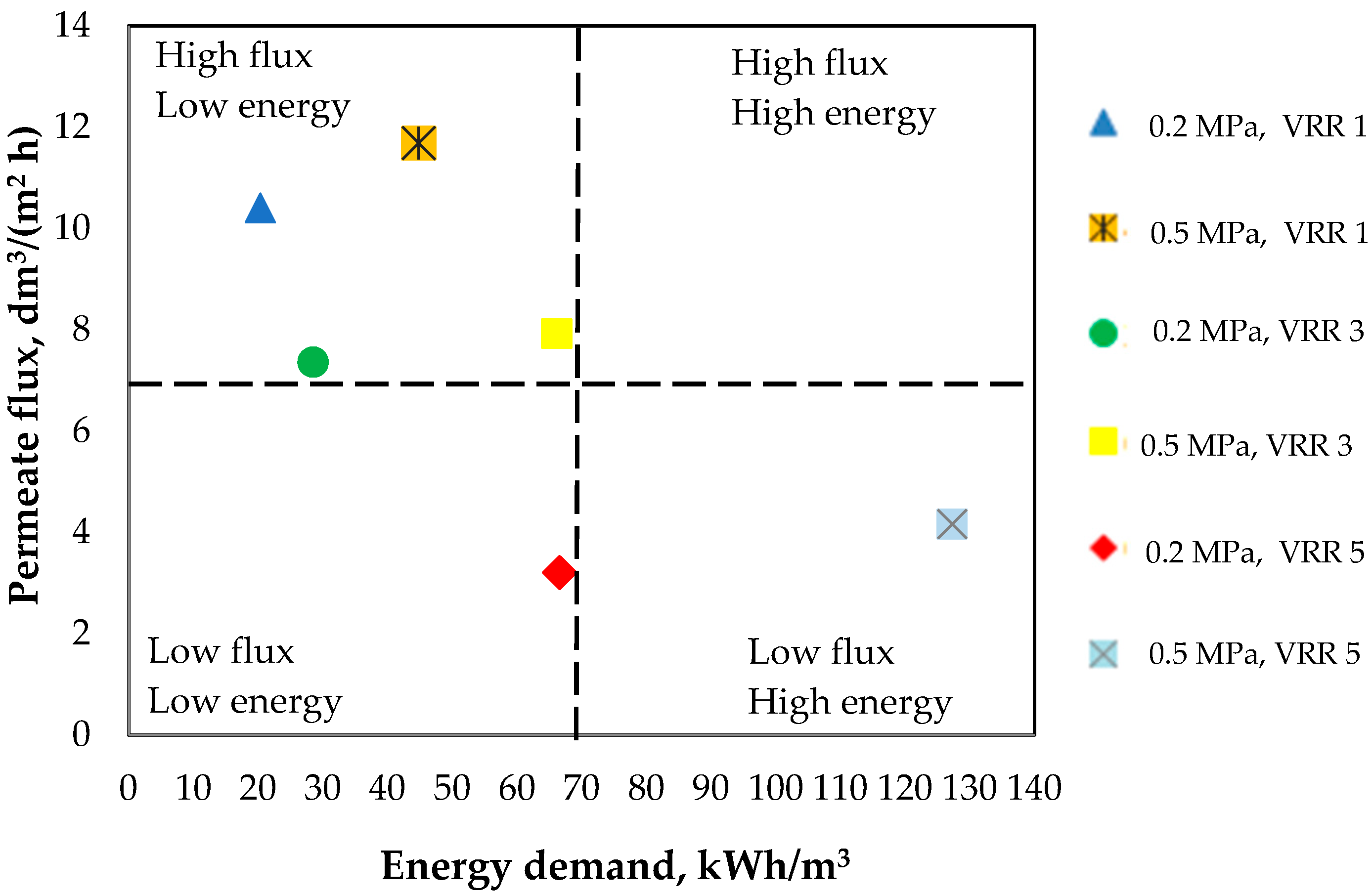
3.7. Choice of Working Conditions for Future Application in the Dairy Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreiro, T.; Martínez, S.; Gayoso, L.; Rodríguez-Otero, J.L. Evolution of phospholipid contents during the production of quark cheese from buttermilk. J. Dairy Sci. 2016, 99, 4154–4159. [Google Scholar] [CrossRef] [PubMed]
- Vanderghem, C.; Francis, F.; Danthine, S.; Deroanne, C.; Paquot, M.; De Pauw, E.; Blecker, C. Study on the susceptibility of the bovine milk fat globule membrane proteins to enzymatic hydrolysis and organization of some of the proteins. Int. Dairy J. 2011, 21, 312–318. [Google Scholar] [CrossRef]
- Conway, V.; Gauthier, S.F.; Pouliot, Y. Buttermilk: Much more than a source of milk phospholipids. Anim. Front. 2014, 4, 44–51. [Google Scholar] [CrossRef]
- Costa, M.R.; Elias-Argote, X.E.; Jiménez-Flores, R.; Gigante, M.L. Use of ultrafiltration and supercritical fluid extraction to obtain a whey buttermilk powder enriched in milk fat globule membrane phospholipids. Int. Dairy J. 2010, 20, 598–602. [Google Scholar] [CrossRef]
- El-Loly, M. Composition, properties and nutritional aspects of milk fat globule membrane—A review. Pol. J. Food Nutr. Sci. 2011, 61, 7–32. [Google Scholar] [CrossRef]
- Contarini, G.; Povolo, M. Phospholipids in milk fat: Composition, biological and technological significance, and analytical strategies. Int. J. Mol. Sci. 2013, 14, 2808–2831. [Google Scholar] [CrossRef]
- Dewettinck, K.; Rombaut, R.; Thienpont, N.; Le, T.T.; Messens, K.; Van Camp, J. Nutritional and technological aspects of milk fat globule membrane material. Int. Dairy J. 2008, 18, 436–457. [Google Scholar] [CrossRef]
- Lean, I.J. Diseases of Dairy Animals; Non-Infectious Diseases: Pregnancy Toxemia. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Fox, P.F., McSweeney, P.L.H., Eds.; Academic Press: San Diego, CA, USA, 2011; Volume 2, pp. 246–249. [Google Scholar]
- Chen, G.Q.; Leong, T.S.H.; Kentish, S.E.; Ashokkumar, M.; Martin, G.J.O. Separation of Functional Molecules in Food by Membrane Technology; Elsevier Science Ltd.: Cambridge, MA, USA, 2019. [Google Scholar]
- Tamime, A.Y. Membrane Processing: Dairy and Beverage Applications; John & Wiley Sons Ltd.: West Sussex, UK, 2013. [Google Scholar]
- Corredig, M.; Roesch, R.R.; Dalgleish, D.G. Production of a novel ingredient from buttermilk. J. Dairy Sci. 2003, 86, 2744–2750. [Google Scholar] [CrossRef] [PubMed]
- Morin, P.; Pouliot, Y.; Jiménez-Flores, R. A comparative study of the fractionation of regular buttermilk and whey buttermilk by microfiltration. J. Food Eng. 2006, 77, 521–528. [Google Scholar] [CrossRef]
- Rombaut, R.; Dejonkheere, V.; Dewettinck, K. Filtration of milk fat globule membrane fragments from acid buttermilk cheese whey. J. Dairy Sci. 2007, 90, 1662–1673. [Google Scholar] [CrossRef]
- Svanborg, S.; Johansen, A.G.; Abrahamsen, R.K.; Skeie, S.B. The composition and functional properties of whey protein concentrates produced from buttermilk are comparable with those of whey protein concentrates produced from skimmed milk. J. Dairy Sci. 2015, 98, 5829–5840. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.R.; Pires, A.F.; Marnotes, N.G.; Gomes, D.G.; Henriques, M.F.; Pereira, C.D. Dairy by-products concentrated by ultrafiltration used as ingredients in the production of reduced fat washed curd cheese. Foods 2020, 9, 1020. [Google Scholar] [CrossRef] [PubMed]
- Mistry, V.V.; Metzger, L.E.; Maubois, J.L. Use of ultrafiltered sweet buttermilk in the manufacture of reduced fat cheddar cheese. J. Dairy Sci. 1996, 79, 1137–1145. [Google Scholar] [CrossRef]
- Raval, D.M.; Mistry, V.V. Application of ultrafiltered sweet buttermilk in the manufacture of reduced fat process cheese. J. Dairy Sci. 1999, 82, 2334–2343. [Google Scholar] [CrossRef]
- Méthot-Hains, S.; Benoit, S.; Bouchard, C.; Doyen, A.; Bazinet, L.; Pouliot, Y. Effect of transmembrane pressure control on energy efficiency during skim milk concentration by ultrafiltration at 10 and 50 °C. J. Dairy Sci. 2016, 99, 8655–8664. [Google Scholar] [CrossRef]
- Konrad, G.; Kleinschmidt, T.; Lorenz, C. Ultrafiltration of whey buttermilk to obtain a phospholipid concentrate. Int. Dairy J. 2013, 30, 39–44. [Google Scholar] [CrossRef]
- Ng, K.S.Y.; Haribabu, M.; Harvie, D.J.E.; Dunstan, D.E.; Martin, G.J.O. Mechanisms of flux decline in skim milk ultrafiltration: A review. J. Membr Sci. 2017, 523, 144–162. [Google Scholar] [CrossRef]
- Youravong, W.; Lewis, M.J.; Grandison, A.S. Critical flux in ultrafiltration of skimmed milk. Food Bioprod. Process. 2003, 81, 303–308. [Google Scholar] [CrossRef]
- Bahnasawy, H.; Shenana, M. Flux behavior and energy consumption of ultrafiltration (UF) process of milk. Aust. J. Agric. Eng. 2010, 1, 54–65. [Google Scholar]
- Dushkova, M.; Mihalev, K.; Dinchev, A.; Vasilev, K.; Georgiev, D.; Terziyska, M. Concentration of polyphenolic antioxidants in apple juice and extraxt using ultrafiltration. Membranes 2022, 12, 1032. [Google Scholar] [CrossRef]
- Laborie, S.; Cabassud, C.; Durand-Bourlier, L.; Laine, J.M. Fouling control by air sparging inside hollow fiber membranes—Effects on energy consumption. Desalination 1998, 118, 189–196. [Google Scholar] [CrossRef]
- Mercier, M.; Fonade, C.; Lafforgue-Delorme, C. How slug flow can enhance the ultrafiltration flux in mineral tubular membranes. J. Membr. Sci. 1997, 128, 103–113. [Google Scholar] [CrossRef]
- Verberk, J.Q.C.; Van Dijk, J.C. Air sparging in capillary nanofiltration. J. Membr. Sci. 2006, 284, 339–351. [Google Scholar] [CrossRef]
- Thuvander, J.; Arkell, A.; Jönsson, A.S. Reduction of energy consumption by use of air sparking during ultrafiltration of alkali-extracted wheat bran hemicelluloses. Chem. Eng. Res. Des. 2018, 138, 43–50. [Google Scholar] [CrossRef]
- ISO 6731:2010; Milk, Cream and Evaporated Milk—Determination of Total Solids Content. International Organization for Standardization: Geneva, Switzerland, 2010.
- ISO 8968-1:2014; Milk and Milk Products—Determination of Nitrogen Content—Part 1: Kjeldahl Principle and Crude Protein Calculation. International Organization for Standardization: Geneva, Switzerland, 2014.
- ISO 2446:2008; Milk Determination of Fat Content. International Organization for Standardization: Geneva, Switzerland, 2008.
- Bulgarian Standard BS 6154:1974; Methods for Determination of Ash Content. Bulgarian Institute for Standardization: Sofia, Bulgaria, 1974.
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Schneiter, R.; Daum, G. Analysis of yeast lipids. In Methods in Molecular Biology, 2nd ed.; Walker, J.M., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2006; pp. 75–84. [Google Scholar]
- ISO 10540-1:02014; Animal and Vegetable Fats and Oils. Determination of Phosphorus Content. Part 1: Colorimetric Method. International Organization for Standardization: Geneva, Switzerland, 2014.
- ISO 6091:2010/IDF 86:2010; Dried Milk—Detremination of Titratable Acidity. International Organization for Standardization: Geneva, Switzerland, 2010.
- Luo, X.; Ramchandran, L.; Vasiljevic, T. Lower ultrafiltration temperature improves membrane performance and emulsifying properties of milk protein concentrates. Dairy Sci. Technol. 2015, 95, 15–31. [Google Scholar] [CrossRef]
- Domagala, J.; Kupiec, B.E. Changes in texture of yoghurt from ultrafiltered goat’s milk as influenced by different membrane types. Electron. J. Pol. Agric. Univ. 2003, 6. [Google Scholar]
- Jahadi, M.; Ehsani, M.R.; Paidari, S. Characterization of milk proteins in ultrafiltration permeate and their rejection coefficients. J. Food Biosci. Technol. 2018, 8, 49–54. [Google Scholar]
- Moreno-Montoro, M.; Olalla, M.; Giménez-Martínez, R.; Bergillos-Meca, T.; Ruiz-López, M.; Cabrera-Vique, C.; Artacho, R.; Navarro-Alarcón, M. Ultrafiltration of skim goat’s milk increases its nutritional value by concentrating nonfat solids such as proteins, Ca, P, Mg, and Zn. J. Dairy Sci. 2015, 98, 7628–7634. [Google Scholar] [CrossRef]
- Meena, P.K.; Gupta, V.K.; Meena, G.S.; Raju, P.N.; Parmar, P.T. Application of ultrafiltration technique for the quality improvement of Dahi. J. Food Sci. Technol. 2015, 52, 7974–7983. [Google Scholar] [CrossRef]
- Macedo, A.; Pinho, M.; Duarte, E. Application of ultrafiltration for valorization of ovine cheese whey. Proc. Eng. 2012, 44, 1949–1950. [Google Scholar] [CrossRef]
- Catarino, I.; Martins, A.P.L.; Duarte, E.; Prudêncio, E.S.; Pinho, M.N. Rennet coagulation of sheep milk processed by ultrafiltration at low concentration factors. J. Food Eng. 2013, 114, 249–254. [Google Scholar] [CrossRef]
- Sergius-Ronot, M.; Suwal, S.; Shama, S.; Chamberland, J.; Unger, S.; O’Connor, D.L.; Pouliot, Y.; Doyen, A. The ultrafiltration molecular weight cut-off has a limited effect on the concentration and protein profile during preparation of human milk protein concentrates. J. Dairy Sci. 2021, 104, 3820–3831. [Google Scholar] [CrossRef] [PubMed]
- Britten, M.; Lamothe, S.; Robitaille, G. Effect of cream treatment on phospholipids and protein recovery in butter-making process. Int. J. Food. Sci. Technol. 2008, 43, 651–657. [Google Scholar] [CrossRef]
- Rombaut, R.; Dewettinck, K.; Van Camp, J. Phospho- and sphingolipid content of selected dairy products as determined by HPLC coupled to an evaporative light scattering detector (HPLC- ELSD). J. Food Compos. Anal. 2007, 20, 308–312. [Google Scholar] [CrossRef]
| Sample | Buttermilk | VRR 2 | VRR 3 | VRR 4 | VRR 5 |
|---|---|---|---|---|---|
| pH | 6.29 ± 0.05 a | 6.24 ± 0.03 a | 6.16 ± 0.025 b | 6.12 ± 0.04 b | 5.98 ± 0.03 c |
| Titratable acidity (% lactic acid) | 0.180 ± 0.015 a | 0.270 ± 0.02 b | 0.405 ± 0.008 c | 0.495 ± 0.011 d | 0.675 ± 0.010 e |
| Component | Power Model | Correlation Coefficient R |
|---|---|---|
| Proteins | P = 85.447 × VRR0.0563 | 0.987 |
| Fat | F = 94.901 × VRR0.0249 | 0.985 |
| Ash | A = 31.511 × VRR0.4482 | 0.997 |
| Dry matter | DM = 63.108 × VRR0.1594 | 0.999 |
| Component | Linear Model | Correlation Coefficient R |
|---|---|---|
| Proteins | P = 0.4108 × VRR + 0.8768 | 0.984 |
| Fat | F = 1.0133 × VRR − 0.1467 | 0.999 |
| Ash | A = 0.3284 × VRR + 0.9295 | 0.996 |
| Dry matter | DM = 0.381 × VRR + 1.1571 | 0.999 |
| Phospholipids, mg/100 g | Initial Buttermilk | Retentate at VRR 2 | Retentate at VRR 3 | Retentate at VRR 4 | Retentate at VRR 5 |
|---|---|---|---|---|---|
| Lysophosphatidylethanolamine | 2.6 ± 0.2 a | 9.5 ± 0.4 b | 9.8 ± 0.6 b | 11.0 ± 0.5 c | 11.5 ± 0.4 c |
| Lysophosphatidylcholine | 3.9 ± 0.1 a | 8.3 ± 0.2 b | 8.7 ± 0.6 b | 9.1 ± 0.3 b | 10.0 ± 0.2 c |
| Phosphatidylserine | 8.4 ± 0.2 a | 22.6 ± 0.9 b | 23.0 ± 0.3 b | 25.0 ± 0.6 c | 26.5 ± 0.5 d |
| Phosphatidylinositol | 23.1 ± 0.5 a | 35.9 ± 0.7 b | 67.3 ± 0.6 c | 71.0 ± 0.9 d | 73.5 ± 1.0 e |
| Phosphatidylcholine | 26.7 ± 0.5 a | 47.5 ± 1.3 b | 69.6 ± 1.8 c | 72.1 ± 1.2 d | 74.2 ± 1.4 d |
| Sphingomyelin | 17.1 ± 0.2 a | 18.6 ± 0.4 b | 31.6 ± 1.2 c | 41.0 ± 1.0 d | 46.1 ± 0.4 e |
| Phosphatidylethanolamine | 28.5 ± 0.6 a | 63.0 ± 0.7 b | 70.0 ± 1.5 c | 78.0 ± 0.6 d | 80.6 ± 0.4 e |
| Diphosphatidylglycerol | 4.4 ± 0.2 a | 5.7 ± 0.2 b | 6.6 ± 0.6 c | 7.1 ± 0.2 c | 8.1 ± 0.3 d |
| Phosphatidic acids | 7.8 ± 0.2 a | 8.5 ± 0.5 a | 9.9 ± 0.6 b | 11.1 ± 0.3 c | 11.7 ± 0.5 c |
| Total phospholipids | 123 ± 3 a | 220 ± 5 b | 296 ± 8 c | 325 ± 7 d | 342 ± 8 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dushkova, M.; Ivanova, M.; Trublet, L.; Petkova, Z.; Teneva, O.; Miteva-Petrova, M.; Desseva, I.; Mihaylova, D. Flux Behaviour, Rejection and Concentration Factors, and Energy Demand during Ultrafiltration of Sweet Buttermilk. Appl. Sci. 2023, 13, 3804. https://doi.org/10.3390/app13063804
Dushkova M, Ivanova M, Trublet L, Petkova Z, Teneva O, Miteva-Petrova M, Desseva I, Mihaylova D. Flux Behaviour, Rejection and Concentration Factors, and Energy Demand during Ultrafiltration of Sweet Buttermilk. Applied Sciences. 2023; 13(6):3804. https://doi.org/10.3390/app13063804
Chicago/Turabian StyleDushkova, Mariya, Mihaela Ivanova, Luca Trublet, Zhana Petkova, Olga Teneva, Milena Miteva-Petrova, Ivelina Desseva, and Dasha Mihaylova. 2023. "Flux Behaviour, Rejection and Concentration Factors, and Energy Demand during Ultrafiltration of Sweet Buttermilk" Applied Sciences 13, no. 6: 3804. https://doi.org/10.3390/app13063804
APA StyleDushkova, M., Ivanova, M., Trublet, L., Petkova, Z., Teneva, O., Miteva-Petrova, M., Desseva, I., & Mihaylova, D. (2023). Flux Behaviour, Rejection and Concentration Factors, and Energy Demand during Ultrafiltration of Sweet Buttermilk. Applied Sciences, 13(6), 3804. https://doi.org/10.3390/app13063804









