Abstract
This work aimed to study the flux behavior, rejection and concentration factors, and energy demand to establish the optimal conditions during ultrafiltration of sweet buttermilk to produce ice cream. The experiments were conducted with a UF25-PAN membrane at a transmembrane pressure of 0.2, 0.35 and 0.5 MPa, and a volume reduction ratio (VRR) of 2, 3, 4, and 5. Total protein, fat, ash, and dry matter contents, phospholipid composition, and acidity of retentates and permeate were determined. The increase in the VRR led to a decrease in the permeate flux and an increase in the energy demand, rejection, and concentration factors of the main components of sweet buttermilk. The highest values of rejection and concentration factors established for fat were 98.65% and 4.93, respectively. The permeate flux and energy demand increased with the rise in the transmembrane pressure. The total phospholipids increased 2.8 times at VRR 5 compared to the initial buttermilk. The use of VRR 3 gave the best ratio between the permeate flux, the energy demand, and relatively high values of concentration and rejection factors. The use of VRR 5 will enrich the ice cream to the greatest extent to obtain a product with the highest level of biologically active substances (proteins, phospholipids, minerals).
1. Introduction
Traditional buttermilk presents the aqueous phase separated when the cream is transformed into butter. It involves all water-soluble components of the cream (proteins, lactose, minerals), but also phospholipids located in the membrane of the milk fat globules [1]. According to [2], the phospholipid content in buttermilk is higher than in milk because the separated membranes from the milk fat globules mainly pass to the aqueous phase.
The compounds of the milk fat globule membrane (MFGM) were related to various health benefits [3,4]. Many previous pieces of research [2,5,6] were notified that the MFGM lipids in buttermilk have cholesterol-reducing, anti-inflammatory, chemotherapeutic, and anti-neurodegenerative effects due to the phospholipids. Sweet buttermilk is rich in MFGM components. The MFGM contains about 70% proteins, 25% phospholipids, neutral lipids, cholesterol, and water [7]. Depending on the type, the proteins vary between 25% and 70%. Minor proteins are also important bioactive components.
Membrane technologies can be successfully used for the separation, concentration, and purification of milk components [8,9]. They have many advantages compared to the traditional separation and concentration methods: environmental friendliness, lower power consumption, relatively low costs, yield enhancement, a better quality of the final product, and usage of room temperature during the process to keep heat-sensitive ingredients [10]. They can be successfully applied when the basic characteristics (flux, rejection, concentration, energy consumption) of the membrane process are well-known, as well as the dependence of these characteristics on some working factors. The membrane type, the transmembrane pressure, the temperature, the volumetric flow rate, the velocity, etc., affect the efficiency of the process. Therefore, it is important to know the dependence of the main characteristics of each membrane process on the working factors to choose the best conditions for the production of some food products.
Membrane technologies were used to concentrate the phospholipids from buttermilk [11,12]. Microfiltration causes some performance difficulties because the size distribution of casein micelles and milk fat globule membrane fragments are very similar [13]. The authors in [14] used a combination of microfiltration and ultrafiltration to obtain whey protein concentrates from buttermilk and skimmed milk. Ultrafiltration is used for the concentration of buttermilk as the ingredients were used in the production of reduced-fat washed curd cheese [15], reduced-fat Cheddar cheese [16], and reduced-fat process cheese [17]. According to the authors in [18], ultrafiltration was widely used for the concentration of various milk components. Ultrafiltration is also used to obtain a phospholipid concentrate from whey buttermilk [19]. The same authors include diafiltration to increase the phospholipid content in the concentrate.
The permeate flux is an important technical characteristic of the membrane processes. Membrane fouling is a serious problem in all pressure-driven membrane processes and will reduce the flux of the membrane [20]. It limits the efficiency of the process. The fouling depends on the different components present in the feed solution (casein micelles, whey proteins, minerals, etc.) [21]. The energy demand is also an important technical and economical characteristic of each production process like the permeate flux, especially for pressure-driven processes where ultrafiltration belongs [22]. Energy consumption is inevitable for human existence. Therefore, it is environmentally friendly to search for an opportunity to reduce it at each industrial case and process.
We found no literature data on the flux behavior, energy demand, rejection, and concentration factors during ultrafiltration of sweet buttermilk to produce ice cream, which was the aim of this study.
2. Materials and Methods
2.1. Materials
Sweet buttermilk supplied by dairy enterprise “Mlechni produkti”, Manole, Bulgaria was used in this study. The composition of initial buttermilk was analyzed according to dry matter, protein, fat, ash, phospholipids content, and acidity. The methods used are described in Section 2.2.3. The initial buttermilk contained 2.70% total proteins, 0.75% fat, 0.475% ash, and 8.93% dry matter. The buttermilk was pasteurized at 85 °C for 10 min before ultrafiltration.
2.2. Methods
2.2.1. Membrane System
The membrane experiment was performed on a laboratory system with a plate and frame module with a membrane area of 1250 cm2 [23]. Polyacrylonitrile asymmetric membrane UF25-PAN with molecular weight cut-off (MWCO) of 25 kDa was used for ultrafiltration. The membranes were produced at the University “Prof. Dr. Asen Zlatarov”, Bulgaria. The initial volume of the feed buttermilk (VF) was 6 L. The working conditions during the experiment were transmembrane pressure of 0.2, 0.35 and 0.5 MPa, a temperature of 50 °C, a feed flow rate of 330 L/h, and a volume reduction ratio of 2, 3, 4, and 5. The temperature was chosen to preserve heat-sensitive substances such as proteins and is also the maximal working temperature given by the producers of the membrane. The membrane system worked with the recirculation of the feed solution (buttermilk). The cleaning of the membranes was carried out with NaOH 0.5%, at a temperature of 50 °C, a pressure of 0.2 MPa, with a circulation time of 30 min, and a further final rinsing with distilled water.
2.2.2. Calculation of the Characteristics of the Ultrafiltration Process
- Volume reduction ratio
The volume reduction ratio (VRR) was calculated by the following equation:
where VF is the volume of the feed buttermilk, dm3; VR is the volume of the retentate obtained during ultrafiltration, dm3.
- Transmembrane pressure
The transmembrane pressure (p, MPa) was calculated by the following formula:
where p1 is the feed stream’s inlet pressure, Pa; p2 is the concentrate stream pressure, Pa; p3 is the permeate stream pressure, Pa.
- Permeate flux
The permeate flux (J, L/(m2 h)) characterizes the volume of permeate generated per unit area of membrane per unit of time. It was calculated according to the following formula:
where V is the volume of the permeate, L; A is the membrane area, m2; t is the time, h.
- Rejection factor
The rejection factor (R, %) of the membrane according to the main compounds in buttermilk was calculated using the following formula:
where CP is the concentration of the compound in the permeate, %; CR is the concentration of the compound in the retentate, %.
- Concentration factor
For calculating the concentration factor (CF) of the main components in buttermilk, the following equation was used:
where CR is the concentration of the compound present in the retentate, %; CO is the concentration of the compound present in the feed buttermilk, %.
- Energy demand
For the calculation of the energy demand, it is necessary to know the value of the power required by the pump (Wpump, W). It was estimated using Equation (6) [24,25,26].
where p1 is the feed stream’s inlet pressure without considering the hydraulic resistances because they were negligibly small, Pa; Qfeed is the volumetric flow rate, (m3/s); ηpump is the pump efficiency, ηpump = 0.7 according to [22,26,27], and type and state of the pump.
The energy demand per m3 of permeate (E, kWh/m3) was determined [27]:
where J is the permeate flux according to Equation (3), m3/(h m2); A is the membrane area, m2.
2.2.3. Methods for Analyzing the Composition of the Initial Buttermilk, Retentates and Permeate, and Acidity during Ultrafiltration
The initial buttermilk, retentates, and permeate were analyzed according to dry matter, protein, fat, ash, and phospholipids content using the following methods:
- ➢
- Dry matter content—using ISO 6731:2010 [28];
- ➢
- Total protein content—by ISO 8968-1:2014 [29];
- ➢
- Fat content—according to ISO 2446:2008 [30];
- ➢
- Ash content—according to BS 6154:1974 [31];
- ➢
- Phospholipid composition—extraction of the buttermilk was made using a mixture of chloroform and methanol (2:1, v/v) [32]. Precipitation of the phospholipids from the mixture was performed with acetone and then two-dimensional thin-layer chromatography (TLC) was used for the isolation of the individual phospholipids [33]. The identification was made by comparison of the Rf values with authentic standards. The spots of phospholipids were scrapped and mineralized with sulphuric acid and perchloric, 1:1 (v/v), and the quantification was made spectrophotometrically at 700 nm [34].
The active and titratable acidity were determined as follows:
- ➢
- Active acidity (pH)—using a pen-type pH meter PH—03 [I] (Hinotek Lab, Ningbo, China);
- ➢
- Titratable acidity—according to ISO 6091:2010|IDF 86:2010 [35].
2.2.4. Statistical Analysis
The results were presented as mean values from three determinations. For comparison of the experimental values, Fisher’s least significant difference test using a 0.05 level of significance was applied in Excel 2010 using a one-way analysis of variance (one-way ANOVA).
3. Results and Discussion
3.1. Flux Behaviour
Figure 1 presents the permeate flux depending on the VRR during ultrafiltration of sweet buttermilk at different transmembrane pressures. The permeate flux decreased with the decrease in the transmembrane pressure (p < 0.05) except at VRR 2 where the values were statistically equal (p > 0.05). Similar results were obtained in [22] where the authors found that the operational pressure has a great effect on the ultrafiltration performance. They established the highest permeate flux at 6 bar, and the lowest at 3 bar during ultrafiltration of a mixture of cow’s and buffalo’s kinds of milk (1:1). These authors found a similar trend for the retentate flux. Our results showed a decrease in the rise in VRR (p < 0.05). The rise of the concentration of the solution causes an increase in the dynamic viscosity, thus reducing the mass transfer coefficient and the flux decreases. The permeate flux decrease is also due to the concentration polarization and membrane fouling. During membrane filtration, an accumulation of retained colloidal particles at the membrane surface is observed leading to a rise of a concentration gradient of compounds perpendicular to the membrane, called concentration polarization [10]. The membrane fouling is provoked by the direct interaction of the colloidal particles with the membrane and is a different phenomenon from concentration polarization. The fouling depends on the milk components (casein micelles, whey proteins, minerals, etc.) [20]. The authors in [19] found a significant decrease in the permeate flux from the beginning to VRR 2 during ultrafiltration of whey buttermilk with membranes of 30, 50, 100, and 300 kDa, after that the decrease was not so pronounced. The researchers in [21] established that the permeate flux increased significantly from 0.25 to 1 bar, then remained relatively constant. The authors in [18] reported that the temperature (10 and 50 °C) of the skim milk had a significant effect on the permeate flux when an ultrafiltration membrane of 10 kDa was used. The same authors found that the pressure adjustment did not affect the permeate flux at a temperature of 10 °C. According to [36], the rate of the permeate flux decline was more rapid during ultrafiltration at 50 °C in comparison with 15 °C. These authors attributed this phenomenon to the rise in calcium and protein deposition on the membrane surface at 50 °C.
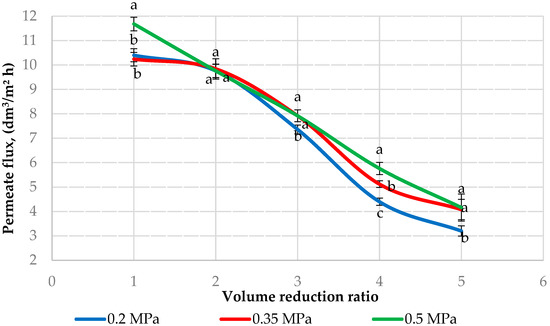
Figure 1.
Effect of volume reduction ratio on the permeation flux during ultrafiltration of sweet buttermilk. Different lowercase letters (a, b, c) show a significant difference between the permeate flux at 0.2, 0.35, and 0.5 MPa (p < 0.05).
3.2. Energy Demand
The effect of the VRR and the transmembrane pressure on the energy demand is presented in Figure 2. The increase in the pressure led to an increase in the energy demand (p < 0.05) at all VRRs. The lowest value was observed at 0.2 MPa and VRR 1 (20.37 kWh/m3), and the highest was at 0.5 MPa and VRR 5 (127.31 kWh/m3). The pressure rise leads to an increase in the power required by the pump, and thus the energy consumption increases [22]. The same authors reported that the ultrafiltration process consumed more energy at higher operational pressures which is in accordance with our results. According to [18], the pressure (0.465 and 0.672 MPa) had no significant effect on the energy consumption at both temperatures studied (10 and 50 °C). The same authors found that only temperature significantly affected (p < 0.01) the total energy requirement and this effect was higher at 10 °C than at 50 °C. An explanation for this consists of the fluid viscosity which decreases with the temperature rise. Our investigation showed a significant difference (p < 0.05) in energy demand at different pressures. This could be explained by the different levels of pressure investigated in this research. It is also well known that at high transmembrane pressure, the concentration polarization is more pronounced, and flux starts to form a plateau with increasing pressure [20]. This phenomenon is probably reflected in energy consumption at high pressures. The energy demand was also influenced by the volume reduction ratio. A higher level of ultrafiltration concentration increased the processing time and energy consumption of the pump. Moreover, the fluid viscosity increases when the VRR increases because of the rise in the dry matter content. This was confirmed in [37] where the authors established higher values of density and viscosity of retentates from goat’s milk obtained with three ultrafiltration membranes of 10, 25, and 100 kDa.
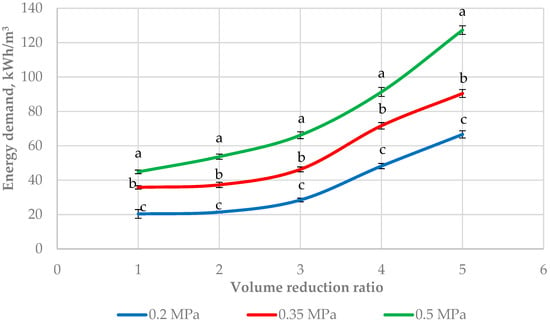
Figure 2.
Effect of volume reduction ratio on the energy demand during ultrafiltration of sweet buttermilk. Different lowercase letters (a, b, c) show a significant difference between the energy demand at 0.2, 0.35, and 0.5 MPa (p < 0.05).
3.3. Active and Titratable Acidity
According to Table 1, there is an increase in the titratable acidity as a function of the VRR, from 0.180% lactic acid for initial buttermilk to 0.405% lactic acid for VRR 3 and up to 0.675% lactic acid for VRR 5. This increase can be explained by the fact that the acidity is due to the presence of proteins, phosphate salts, and citric acid, a small amount of dissolved carbon dioxide and organic acids present in buttermilk, and concentrating these components leads to an increase in lactic acid content [37]. However, the main cause of this increase is the ultrafiltration concentration of the proteins presented in buttermilk. The main objective of this process is to concentrate caseins which are acidic proteins with an isoelectric pH of 4.6, thus increasing the protein concentration from 1.69 at VRR 2 to 2.99 at VRR 5. Our results showed that a big part of the proteins in buttermilk was concentrated by ultrafiltration because the protein content in the permeate was 0.36%. This low protein content may be explained by the fact that the permeate contains a part of the soluble proteins of buttermilk which could not be retained by the ultrafiltration membranes because of their solubility and molecular weight. Our results showed a slight decrease in the pH from 6.29 (initial buttermilk) to 5.98 at VRR 5. There is no significant difference between the values of pH of initial buttermilk and VRR 2, as well as at VRR 3 and 4. This small decrease can be explained by the total ash content designating the mineral substances of the product. Depending on the ionic composition of the minerals, they are influenced directly by the pH. Thus, by concentrating the minerals in buttermilk from 1.6 times at VRR 2 to 2.57 times at VRR 5, the pH decreases.

Table 1.
Titratable and active (pH) acidity of retentates at different volume reduction ratios.
3.4. Rejection Factor
The rejection factor characterizes the possibility of the membrane to retain the compounds presented in the feed solution. Figure 3 presents the rejection of the main components at different VRRs. The highest values were obtained for fat, followed by proteins and ash. This could be explained by the phenomenon that ultrafiltration membranes retain the high molecular weight compounds, while water and the low molecular weight compounds pass through the membrane [38]. Milk fat is the highest molecular weight substance in milk and dairy products, followed by proteins, lactose, and mineral substances. The increase in the VRR led to a rise in the rejection of different buttermilk components. The rejection of ash increased by 1.49 times when the VRR increased from 2 to 5, followed by dry matter by 1.15 times, proteins by 1.05 times, and fat by 1.02 times. The correlation between the rejection and VRR can be described by the power models shown in Table 2. Many authors found that the dry matter, total proteins, fat, and ash in different types of milk increased when the volume reduction increased during ultrafiltration [37,39,40]. The same trend is now observed when sweet buttermilk is subjected to ultrafiltration. The authors in [37] found that the molecular weight cut-off of the membrane affected the milk compound’s change during ultrafiltration.
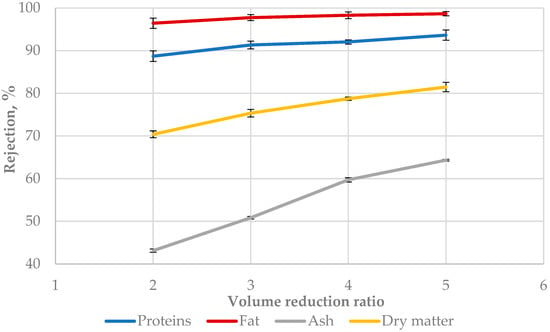
Figure 3.
Rejection at different volume reduction ratios during ultrafiltration of sweet buttermilk.

Table 2.
Power models for rejection factor of buttermilk components depending on the volume reduction ratio.
3.5. Concentration Factor
The concentration factor is an important characteristic of the membrane process because it shows the level of concentration in the retentate of each compound present in the feed buttermilk. The effect of the VRR on the concentration factor during ultrafiltration of sweet buttermilk is shown in Figure 4. The highest values of the concentration factor were obtained for fat, followed by dry matter, proteins, and ash. The correlation between the concentration factor and VRR is linear which is proved by the models shown in Table 3. The researchers in [41] found a correlation between the concentration factor and VRR for other types of dairy products. An increase in the concentration of protein and calcium with the increase in the volumetric concentration factor (VCF) during ultrafiltration of skimmed sheep milk was established by [42]. Our work shows a linear dependence of the concentration factor of all buttermilk compounds studied on the VRR. According to the authors in [43], the concentration of the protein fraction increased by a factor of 3 in the retentate during ultrafiltration until the volume concentration factor of 4. Concerning fats, under the same working conditions, they increased by 3.5 times. The concentration of buttermilk twice almost doubled its fat content using an ultrafiltration membrane of 50 kDa [1]. Our results showed similar results when sweet buttermilk was subjected to ultrafiltration—the concentration factor for proteins was 2.39, while it was 3.87 for fats. The concentration of fats and proteins in retentates obtained by ultrafiltration will be suitable for the production of many dairy products, for example, cheeses where these components are main, as well as ice cream.
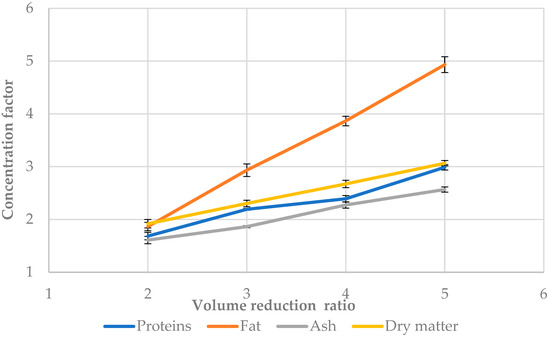
Figure 4.
Concentration factor at different volume reduction ratios during ultrafiltration of sweet buttermilk.

Table 3.
Linear models for concentration factor of buttermilk compounds depending on the volume reduction ratio.
3.6. Phospholipid Composition
The phospholipid composition is an important indicator from the technological point of view because it will show the necessity of usage of the concentrated buttermilk for the production of dairy products. The phospholipid composition of buttermilk retentates is given in Table 4. The total phospholipids increased with the increase in VRR 1.8, 2.4, 2.6, and 2.8 times, respectively. The main components in the phospholipid fraction were phosphatidylethanolamine (23.3%) and phosphatidylcholine (21.8%), followed by phosphatidylinositol (18.8%) and sphingomyelin (13.9%). The authors in [44] established that the total phosphatidylethanolamine, phosphatidylinositol, phosphatidylcholine, phosphatidylserine, and sphingomyelin contents of buttermilk were 39.0, 8.9, 24.4, 8.3 and 19.3%, and those reported by [45] were 46.5, 9.6, 20.7, 9.3 and 13.9%. The authors in [1] also established that the main phospholipids in the raw material, intermediate and final products in the production of buttermilk quark were phosphatidylethanolamine and phosphatidylcholine, and total phospholipid content increased from 154 mg/100 g (in the buttermilk) to 232.2 mg/100 g (in the concentrated buttermilk) at double concentration. They established that the highest increase was observed for phosphatidylcholine and phosphatidylinositol at VRR 2. In our results at the same VRR, the highest concentrations were for phosphatidylcholine, lysophosphatidylethanolamine, and phosphatidylserine. The lowest increase was for diphosphatidylglycerol. The rise in the phospholipid concentrations together with the concentrations factors of the main components (Figure 4) demonstrates the suitability to use concentrated buttermilk by ultrafiltration in the production of dairy products to enrich them with biologically active substances.

Table 4.
Phospholipid composition of buttermilk retentates.
3.7. Choice of Working Conditions for Future Application in the Dairy Production
Figure 5 presents the relationship between the permeate flux and energy demand at different working conditions (transmembrane pressure and VRR). The working field was separated into four quadrants with different combinations of permeate flux and energy demand. The most desirable quadrant with the most efficient conditions was this where the permeate flux is maximal and the energy is minimal. In this quadrant, there are four experimental points corresponding to these criteria. Two of them concern VRR 1, i.e., the beginning of the ultrafiltration process where there is no concentration of the sweet buttermilk. The two others concern VRR 3. Between them, it is more suitable to choose the point which concerns 0.2 MPa and VRR 3 because here the energy is 2.32 times lower than at the point concerning 0.5 MPa and VRR 3. At the same time, comparing the other two points characterizing low permeate flux-low energy demand and low permeate flux-high energy demand, we can conclude that is more suitable to apply a transmembrane pressure of 0.2 MPa and VRR 5 compared to 0.5 MPa and VRR 5 to obtain a low value of energy demand and lower production costs. However, the high value of VRR (5) will assure a high level of proteins, fats, ash, and dry matter contents, as well as total phospholipids, i.e., will enrich the ice cream which will be produced, and to a greater extent obtain a final dairy product with the highest level of biologically active substances (proteins, phospholipids, minerals). To establish which of the two variants is more appropriate from the point of view of the final dairy product, more extensive studies should be done and the technological characteristics of the products obtained should be compared.
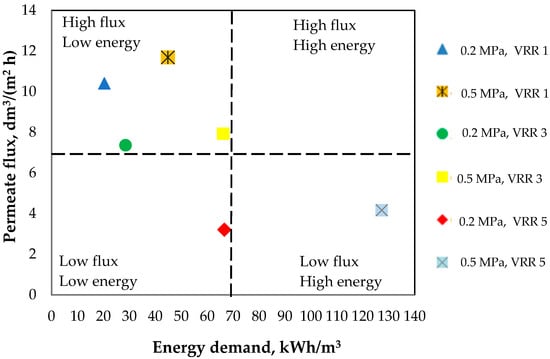
Figure 5.
Relationship between the permeate flux and energy demand at different working conditions (transmembrane pressure and volume reduction ratio).
4. Conclusions
The increase in the volume reduction ratio led to a decrease in the permeate flux and an increase in the energy demand, rejection, and concentration factors of the main compounds of sweet buttermilk. The permeate flux and energy demand increased with the rise in the transmembrane pressure. The rise in the phospholipid’s concentration together with the concentration factors of the main compounds demonstrates the suitability of using concentrated buttermilk by ultrafiltration in the production of dairy products to enrich them with biologically active substances. We recommend using a volume reduction ratio of 3 and 5 at a transmembrane pressure of 0.2 MPa to obtain an ice cream from ultrafiltered sweet buttermilk. The use of VRR 3 gave the best ratio between the permeate flux, the energy demand, and relatively high values of concentration and rejection factors. The application of VRR 5 will enrich the ice cream to the greatest extent to obtain a product with the highest level of bioactive components (proteins, phospholipids, minerals).
Author Contributions
Conceptualization, M.D. and M.I.; methodology, M.D. and M.I.; software, M.D. and L.T.; validation, M.D., M.I. and L.T.; formal analysis, M.D., M.I., L.T., Z.P., O.T. and M.M.-P.; investigation, M.D., M.I. and L.T.; resources, M.D., M.I. and L.T.; data curation, M.D., M.I. and L.T.; writing—original draft preparation, M.D., M.I. and L.T.; writing—review and editing, M.D., M.I., Z.P., O.T., M.M.-P., I.D. and D.M.; visualization, M.D.; supervision, M.D. and M.I.; project administration, M.D. and M.I.; funding acquisition, M.D., I.D. and D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ferreiro, T.; Martínez, S.; Gayoso, L.; Rodríguez-Otero, J.L. Evolution of phospholipid contents during the production of quark cheese from buttermilk. J. Dairy Sci. 2016, 99, 4154–4159. [Google Scholar] [CrossRef] [PubMed]
- Vanderghem, C.; Francis, F.; Danthine, S.; Deroanne, C.; Paquot, M.; De Pauw, E.; Blecker, C. Study on the susceptibility of the bovine milk fat globule membrane proteins to enzymatic hydrolysis and organization of some of the proteins. Int. Dairy J. 2011, 21, 312–318. [Google Scholar] [CrossRef]
- Conway, V.; Gauthier, S.F.; Pouliot, Y. Buttermilk: Much more than a source of milk phospholipids. Anim. Front. 2014, 4, 44–51. [Google Scholar] [CrossRef]
- Costa, M.R.; Elias-Argote, X.E.; Jiménez-Flores, R.; Gigante, M.L. Use of ultrafiltration and supercritical fluid extraction to obtain a whey buttermilk powder enriched in milk fat globule membrane phospholipids. Int. Dairy J. 2010, 20, 598–602. [Google Scholar] [CrossRef]
- El-Loly, M. Composition, properties and nutritional aspects of milk fat globule membrane—A review. Pol. J. Food Nutr. Sci. 2011, 61, 7–32. [Google Scholar] [CrossRef]
- Contarini, G.; Povolo, M. Phospholipids in milk fat: Composition, biological and technological significance, and analytical strategies. Int. J. Mol. Sci. 2013, 14, 2808–2831. [Google Scholar] [CrossRef]
- Dewettinck, K.; Rombaut, R.; Thienpont, N.; Le, T.T.; Messens, K.; Van Camp, J. Nutritional and technological aspects of milk fat globule membrane material. Int. Dairy J. 2008, 18, 436–457. [Google Scholar] [CrossRef]
- Lean, I.J. Diseases of Dairy Animals; Non-Infectious Diseases: Pregnancy Toxemia. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Fox, P.F., McSweeney, P.L.H., Eds.; Academic Press: San Diego, CA, USA, 2011; Volume 2, pp. 246–249. [Google Scholar]
- Chen, G.Q.; Leong, T.S.H.; Kentish, S.E.; Ashokkumar, M.; Martin, G.J.O. Separation of Functional Molecules in Food by Membrane Technology; Elsevier Science Ltd.: Cambridge, MA, USA, 2019. [Google Scholar]
- Tamime, A.Y. Membrane Processing: Dairy and Beverage Applications; John & Wiley Sons Ltd.: West Sussex, UK, 2013. [Google Scholar]
- Corredig, M.; Roesch, R.R.; Dalgleish, D.G. Production of a novel ingredient from buttermilk. J. Dairy Sci. 2003, 86, 2744–2750. [Google Scholar] [CrossRef] [PubMed]
- Morin, P.; Pouliot, Y.; Jiménez-Flores, R. A comparative study of the fractionation of regular buttermilk and whey buttermilk by microfiltration. J. Food Eng. 2006, 77, 521–528. [Google Scholar] [CrossRef]
- Rombaut, R.; Dejonkheere, V.; Dewettinck, K. Filtration of milk fat globule membrane fragments from acid buttermilk cheese whey. J. Dairy Sci. 2007, 90, 1662–1673. [Google Scholar] [CrossRef]
- Svanborg, S.; Johansen, A.G.; Abrahamsen, R.K.; Skeie, S.B. The composition and functional properties of whey protein concentrates produced from buttermilk are comparable with those of whey protein concentrates produced from skimmed milk. J. Dairy Sci. 2015, 98, 5829–5840. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.R.; Pires, A.F.; Marnotes, N.G.; Gomes, D.G.; Henriques, M.F.; Pereira, C.D. Dairy by-products concentrated by ultrafiltration used as ingredients in the production of reduced fat washed curd cheese. Foods 2020, 9, 1020. [Google Scholar] [CrossRef] [PubMed]
- Mistry, V.V.; Metzger, L.E.; Maubois, J.L. Use of ultrafiltered sweet buttermilk in the manufacture of reduced fat cheddar cheese. J. Dairy Sci. 1996, 79, 1137–1145. [Google Scholar] [CrossRef]
- Raval, D.M.; Mistry, V.V. Application of ultrafiltered sweet buttermilk in the manufacture of reduced fat process cheese. J. Dairy Sci. 1999, 82, 2334–2343. [Google Scholar] [CrossRef]
- Méthot-Hains, S.; Benoit, S.; Bouchard, C.; Doyen, A.; Bazinet, L.; Pouliot, Y. Effect of transmembrane pressure control on energy efficiency during skim milk concentration by ultrafiltration at 10 and 50 °C. J. Dairy Sci. 2016, 99, 8655–8664. [Google Scholar] [CrossRef]
- Konrad, G.; Kleinschmidt, T.; Lorenz, C. Ultrafiltration of whey buttermilk to obtain a phospholipid concentrate. Int. Dairy J. 2013, 30, 39–44. [Google Scholar] [CrossRef]
- Ng, K.S.Y.; Haribabu, M.; Harvie, D.J.E.; Dunstan, D.E.; Martin, G.J.O. Mechanisms of flux decline in skim milk ultrafiltration: A review. J. Membr Sci. 2017, 523, 144–162. [Google Scholar] [CrossRef]
- Youravong, W.; Lewis, M.J.; Grandison, A.S. Critical flux in ultrafiltration of skimmed milk. Food Bioprod. Process. 2003, 81, 303–308. [Google Scholar] [CrossRef]
- Bahnasawy, H.; Shenana, M. Flux behavior and energy consumption of ultrafiltration (UF) process of milk. Aust. J. Agric. Eng. 2010, 1, 54–65. [Google Scholar]
- Dushkova, M.; Mihalev, K.; Dinchev, A.; Vasilev, K.; Georgiev, D.; Terziyska, M. Concentration of polyphenolic antioxidants in apple juice and extraxt using ultrafiltration. Membranes 2022, 12, 1032. [Google Scholar] [CrossRef]
- Laborie, S.; Cabassud, C.; Durand-Bourlier, L.; Laine, J.M. Fouling control by air sparging inside hollow fiber membranes—Effects on energy consumption. Desalination 1998, 118, 189–196. [Google Scholar] [CrossRef]
- Mercier, M.; Fonade, C.; Lafforgue-Delorme, C. How slug flow can enhance the ultrafiltration flux in mineral tubular membranes. J. Membr. Sci. 1997, 128, 103–113. [Google Scholar] [CrossRef]
- Verberk, J.Q.C.; Van Dijk, J.C. Air sparging in capillary nanofiltration. J. Membr. Sci. 2006, 284, 339–351. [Google Scholar] [CrossRef]
- Thuvander, J.; Arkell, A.; Jönsson, A.S. Reduction of energy consumption by use of air sparking during ultrafiltration of alkali-extracted wheat bran hemicelluloses. Chem. Eng. Res. Des. 2018, 138, 43–50. [Google Scholar] [CrossRef]
- ISO 6731:2010; Milk, Cream and Evaporated Milk—Determination of Total Solids Content. International Organization for Standardization: Geneva, Switzerland, 2010.
- ISO 8968-1:2014; Milk and Milk Products—Determination of Nitrogen Content—Part 1: Kjeldahl Principle and Crude Protein Calculation. International Organization for Standardization: Geneva, Switzerland, 2014.
- ISO 2446:2008; Milk Determination of Fat Content. International Organization for Standardization: Geneva, Switzerland, 2008.
- Bulgarian Standard BS 6154:1974; Methods for Determination of Ash Content. Bulgarian Institute for Standardization: Sofia, Bulgaria, 1974.
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Schneiter, R.; Daum, G. Analysis of yeast lipids. In Methods in Molecular Biology, 2nd ed.; Walker, J.M., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2006; pp. 75–84. [Google Scholar]
- ISO 10540-1:02014; Animal and Vegetable Fats and Oils. Determination of Phosphorus Content. Part 1: Colorimetric Method. International Organization for Standardization: Geneva, Switzerland, 2014.
- ISO 6091:2010/IDF 86:2010; Dried Milk—Detremination of Titratable Acidity. International Organization for Standardization: Geneva, Switzerland, 2010.
- Luo, X.; Ramchandran, L.; Vasiljevic, T. Lower ultrafiltration temperature improves membrane performance and emulsifying properties of milk protein concentrates. Dairy Sci. Technol. 2015, 95, 15–31. [Google Scholar] [CrossRef]
- Domagala, J.; Kupiec, B.E. Changes in texture of yoghurt from ultrafiltered goat’s milk as influenced by different membrane types. Electron. J. Pol. Agric. Univ. 2003, 6. [Google Scholar]
- Jahadi, M.; Ehsani, M.R.; Paidari, S. Characterization of milk proteins in ultrafiltration permeate and their rejection coefficients. J. Food Biosci. Technol. 2018, 8, 49–54. [Google Scholar]
- Moreno-Montoro, M.; Olalla, M.; Giménez-Martínez, R.; Bergillos-Meca, T.; Ruiz-López, M.; Cabrera-Vique, C.; Artacho, R.; Navarro-Alarcón, M. Ultrafiltration of skim goat’s milk increases its nutritional value by concentrating nonfat solids such as proteins, Ca, P, Mg, and Zn. J. Dairy Sci. 2015, 98, 7628–7634. [Google Scholar] [CrossRef]
- Meena, P.K.; Gupta, V.K.; Meena, G.S.; Raju, P.N.; Parmar, P.T. Application of ultrafiltration technique for the quality improvement of Dahi. J. Food Sci. Technol. 2015, 52, 7974–7983. [Google Scholar] [CrossRef]
- Macedo, A.; Pinho, M.; Duarte, E. Application of ultrafiltration for valorization of ovine cheese whey. Proc. Eng. 2012, 44, 1949–1950. [Google Scholar] [CrossRef]
- Catarino, I.; Martins, A.P.L.; Duarte, E.; Prudêncio, E.S.; Pinho, M.N. Rennet coagulation of sheep milk processed by ultrafiltration at low concentration factors. J. Food Eng. 2013, 114, 249–254. [Google Scholar] [CrossRef]
- Sergius-Ronot, M.; Suwal, S.; Shama, S.; Chamberland, J.; Unger, S.; O’Connor, D.L.; Pouliot, Y.; Doyen, A. The ultrafiltration molecular weight cut-off has a limited effect on the concentration and protein profile during preparation of human milk protein concentrates. J. Dairy Sci. 2021, 104, 3820–3831. [Google Scholar] [CrossRef] [PubMed]
- Britten, M.; Lamothe, S.; Robitaille, G. Effect of cream treatment on phospholipids and protein recovery in butter-making process. Int. J. Food. Sci. Technol. 2008, 43, 651–657. [Google Scholar] [CrossRef]
- Rombaut, R.; Dewettinck, K.; Van Camp, J. Phospho- and sphingolipid content of selected dairy products as determined by HPLC coupled to an evaporative light scattering detector (HPLC- ELSD). J. Food Compos. Anal. 2007, 20, 308–312. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).