Laser Seed Pretreatment Alters the Silybin Content and Anti-Dictyostelium discoideum Cell Growth Activity of Silybum marianum (L.) Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Helium–Neon (He–Ne) Laser Irradiation
2.2. S. marianum Cultivation in a Greenhouse
2.3. Measurement of Photosynthetic Pigment Contents and Attributes of Growth and Yield
2.4. Quantification of Silybin (A + B) in S. marianum Fruits Using High Performance Liquid Chromatography (HPLC)
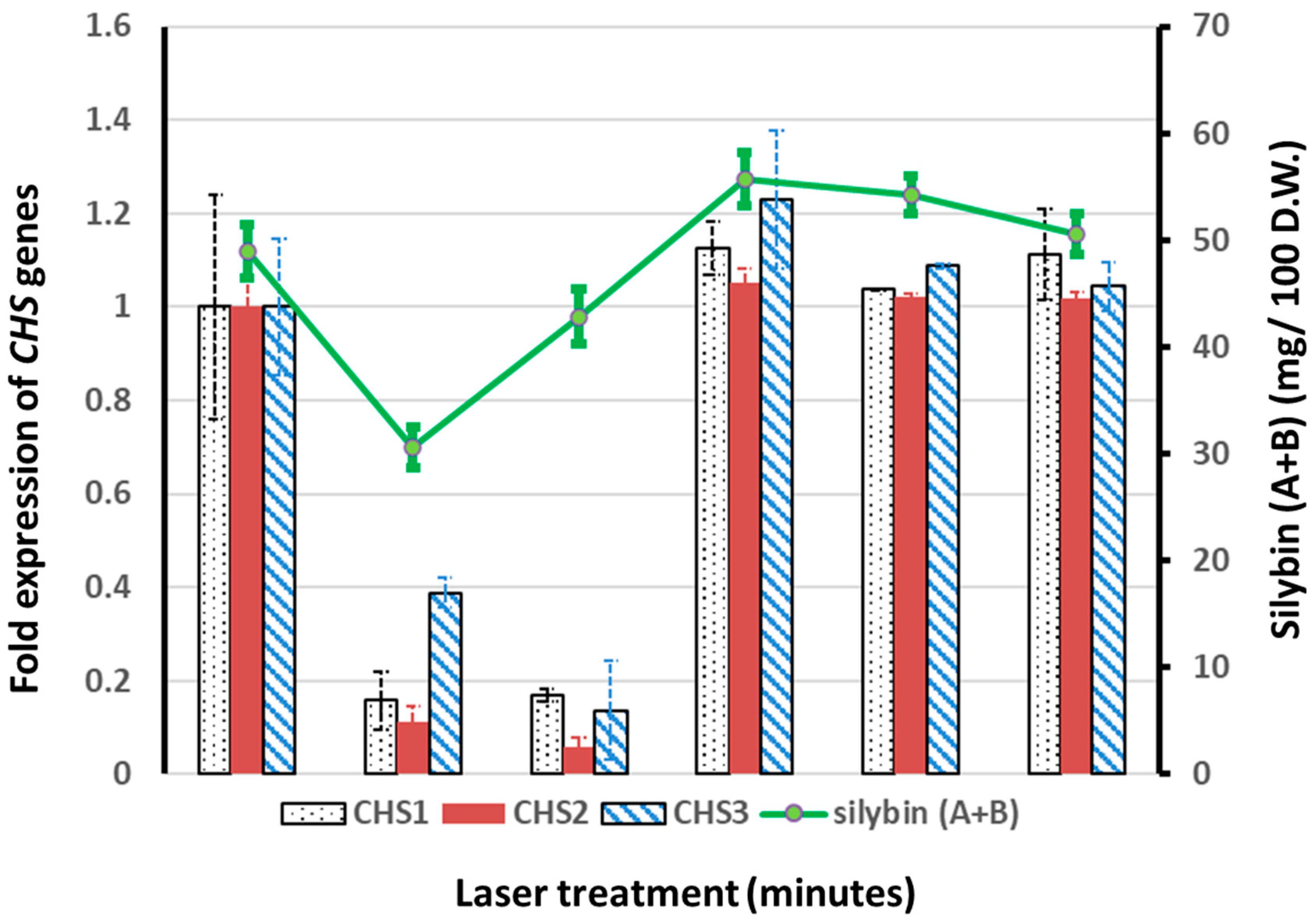
2.5. Gene Expression Analyses of Chalcone Synthase 1, 2, and 3 by Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.6. Assay of S. marianum Fruit Methanolic Extract Bioactivities Using D. discoideum Cell Culture System
2.6.1. Dictyostelium discoideum Cell Lines and Cell Culture
2.6.2. D. discoideum Growth Assay
2.6.3. D. discoideum Development Experiment
2.7. Statistical Analysis
3. Results
3.1. The Effect of He–Ne Laser Pre-Sowing Seed Treatment on the Vegetative Growth and Yield of S. marianum Plants
3.2. Effect of He–Ne Laser Pre-Sowing Seed Treatment on the Leaf Photosynthetic Pigments of S. marianum Plants
3.3. Effect of He–Ne Laser Pre-Sowing Seed Treatment on the Leaf Silybin A + B Contents of S. marianum Plants
3.4. Effect of He–Ne Laser Pre-Sowing Seed Treatment on the Expressions of Chalcone Synthase 1, 2, and 3 Genes in the Petals of S. marianum Plants
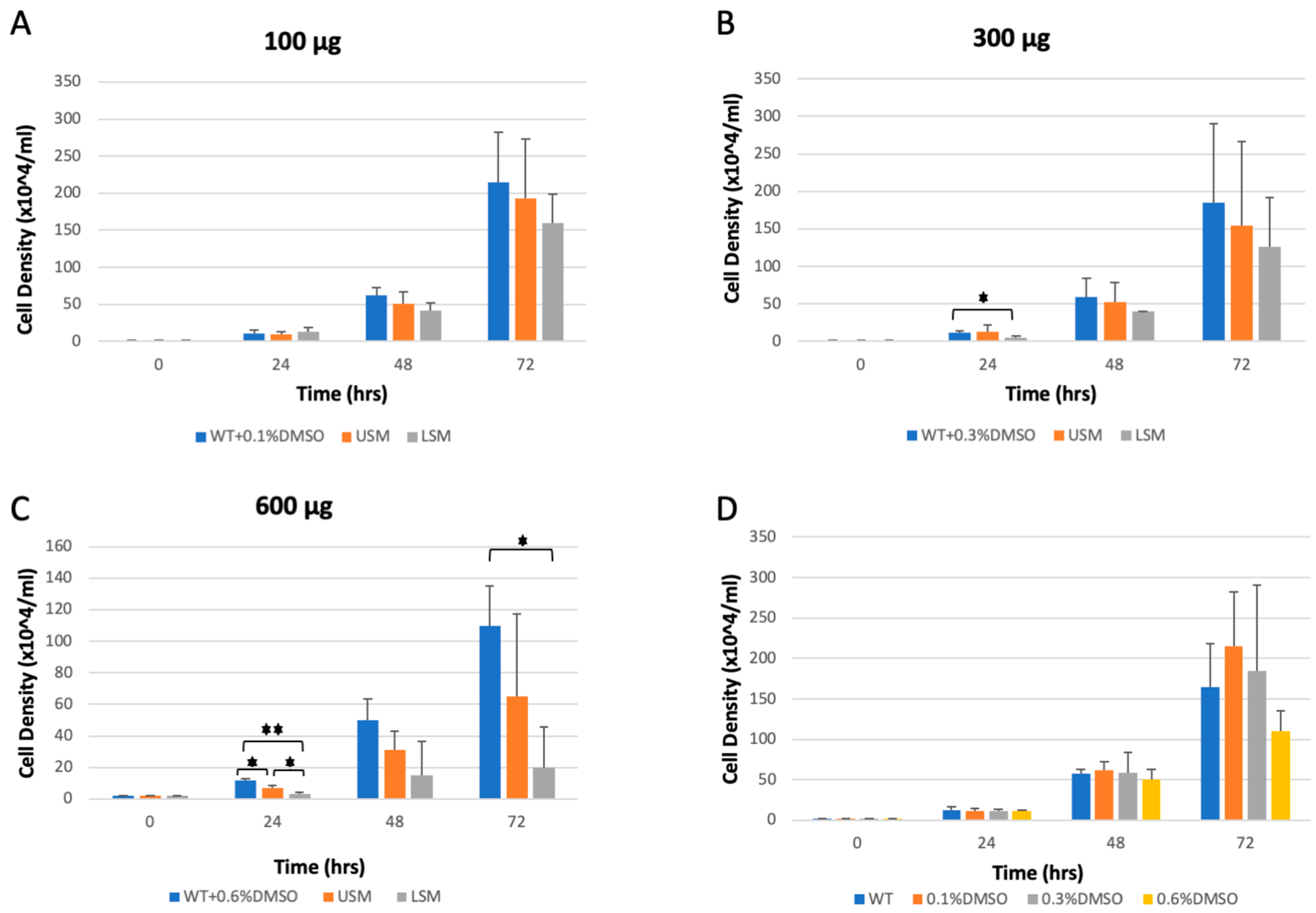
3.5. Effect of He–Ne Laser Pre-Sowing Seed Treatment on the Bioactivities of S. marianum Fruit Extracts
3.5.1. The Anti-Dictyostelium discoideum Cell Growth Activity of S. marianum Fruit Extract
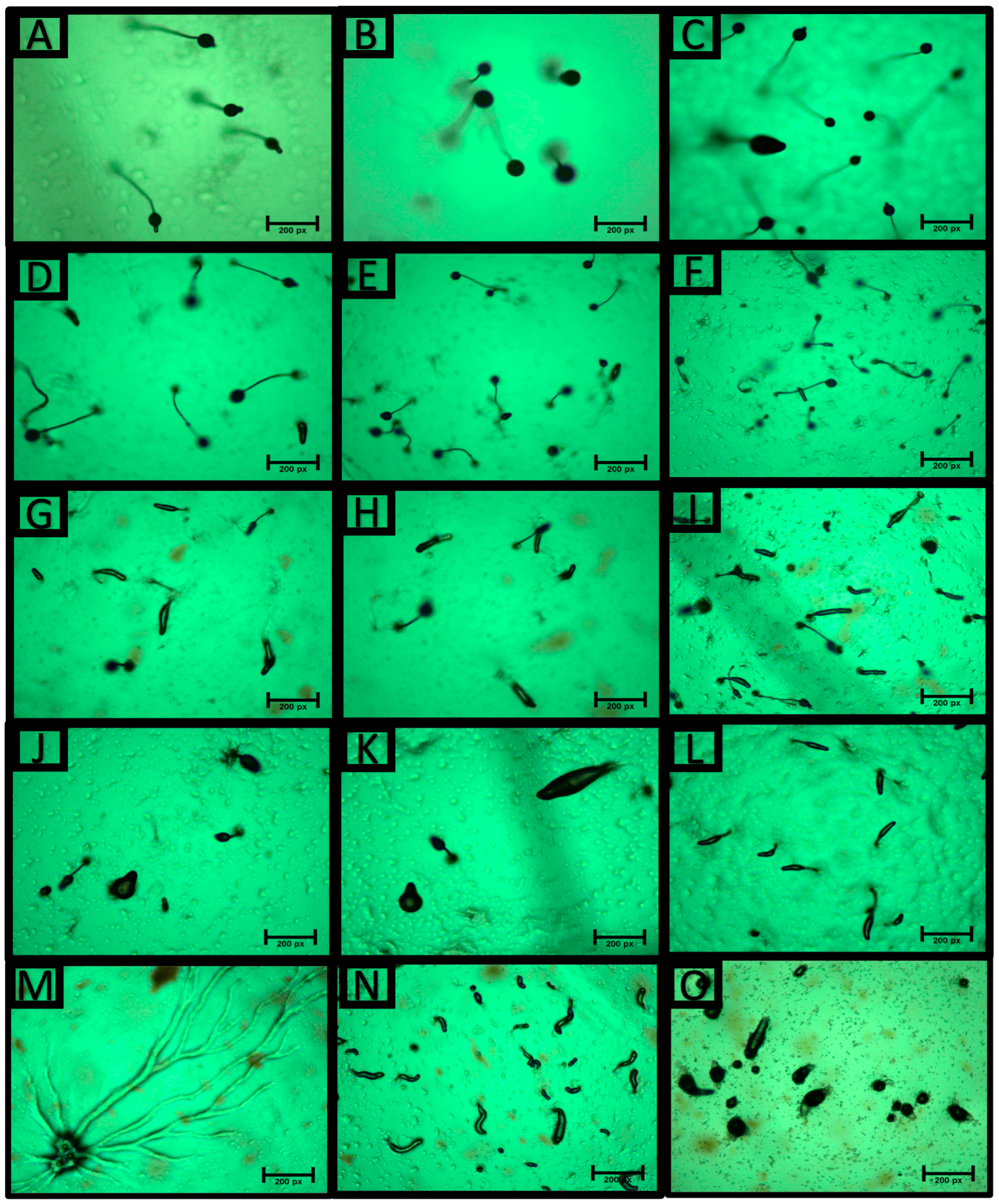
3.5.2. Effect of S. marianum Fruit Extracts on Dictyostelium discoideum Development Life Cycle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dziwulska-Hunek, A.; Szymanek, M.; Stadnik, J. Impact of Pre-Sowing Red Light Treatment of Sweet Corn Seeds on the Quality and Quantity of Yield. Agriculture 2020, 10, 165. [Google Scholar] [CrossRef]
- Krawiec, M.; Dziwulska-Hunek, A.; Kornarzyński, K. The Use of Physical Factors for Seed Quality Improvement of Horticultural Plants. J. Hortic. Res. 2018, 26, 81–94. [Google Scholar] [CrossRef]
- Hernandez, A.C.; Dominguez, P.A.; Cruz, O.A.; Ivanov, R.; Carballo, C.A.; Zepeda, B.R. Laser in Agriculture-Review. Int. Agrophys. 2010, 2010, 407–422. [Google Scholar]
- Podlesna, A.; Gładyszewska, B.; Podlesny, J.; Zgrajka, W. Changes in the Germination Process and Growth of Pea in Effect of Laser Seed Irradiation. Int. Agrophys. 2015, 29, 485–492. [Google Scholar] [CrossRef]
- Krawiec, M.; Dziwulska-Hunek, A.; Sujak, A.; Palonka, S. Laser Irradiation Effects on Scorzonera (Scorzonera hispanica L.) Seed Germination and Seedling Emergence. Acta Sci. Pol. Hortorum Cultus 2015, 14, 145–158. [Google Scholar]
- Dziwulska-Hunek, A.; Krawiec, M.; Sujak, A. Laser Light Stimulation Effects on Scorzonera hispanica L. Seeds Germination, Field Emergence and Photosynthetic Pigments Content. J. Hortic. Res. 2016, 24, 57–62. [Google Scholar] [CrossRef]
- Podleśny, J.; Stochmal, A.; Podleśna, A.; Misiak, L.E. Effect of Laser Light Treatment on Some Biochemical and Physiological Processes in Seeds and Seedlings of White Lupine and Faba Bean. Plant Growth Regul. 2012, 67, 227–233. [Google Scholar] [CrossRef]
- Karkanis, A.; Bilalis, D.; Efthimiadou, A. Cultivation of Milk Thistle (Silybum marianum L. Gaertn.), a Medicinal Weed. Ind. Crops Prod. 2011, 34, 825–830. [Google Scholar] [CrossRef]
- Crocenzi, F.; Roma, M. Silymarin as a New Hepatoprotective Agent in Experimental Cholestasis: New Possibilities for an Ancient Medication. Curr. Med. Chem. 2006, 13, 1055–1074. [Google Scholar] [CrossRef]
- Ou, Q.; Weng, Y.; Wang, S.; Zhao, Y.; Zhang, F.; Zhou, J.; Wu, X. Silybin Alleviates Hepatic Steatosis and Fibrosis in NASH Mice by Inhibiting Oxidative Stress and Involvement with the Nf-ΚB Pathway. Dig. Dis. Sci. 2018, 63, 3398–3408. [Google Scholar] [CrossRef]
- El-Sherif, F.; Khattab, S.; Ibrahim, A.K.; Ahmed, S.A. Improved Silymarin Content in Elicited Multiple Shoot Cultures of Silybum marianum L. Physiol. Mol. Biol. Plants 2013, 19, 127–136. [Google Scholar] [CrossRef] [PubMed]
- El-Garhy, H.A.S.; Khattab, S.; Moustafa, M.M.A.; Abou Ali, R.; Abdel Azeiz, A.Z.; Elhalwagi, A.; El-Sherif, F. Silybin Content and Overexpression of Chalcone Synthase Genes in Silybum marianum L. Plants under Abiotic Elicitation. Plant Physiol. Biochem. 2016, 108, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Firouzi, A.; Mohammadi, S.A.; Khosrowchahli, M.; Movafeghi, A.; Hasanloo, T. Enhancement of Silymarin Production in Cell Culture of Silybum marianum (L) Gaertn by Elicitation and Precursor Feeding. J. Herbs. Spices Med. Plants 2013, 19, 262–274. [Google Scholar] [CrossRef]
- Madrid, E.; Corchete, P.N. Silymarin Secretion and Its Elicitation by Methyl Jasmonate in Cell Cultures of Silybum marianum Is Mediated by Phospholipase D-Phosphatidic Acid. J. Exp. Bot. 2010, 61, 747–754. [Google Scholar] [CrossRef]
- Banisharif, A.; Amooaghaie, R. The Effect of Laser Pretreatment on Seed Germination Indices and α-Amylase Activity and Growth of Silybum marianum L. Seedling in Normal Condition and under Pb Stress. J. Plant Res. (Iran. J. Biol.) 2020, 33, 285–298. [Google Scholar]
- Sepideh, S.; Shobbar, S.Z.; Ebrahimi, M.; Hasanloo, T.; Sadat-Noori, S.-A.; Tirnaz, S. Chalcone Synthase Genes from Milk Thistle (Silybum marianum): Isolation and Expression Analysis. J. Genet. 2015, 94, 611–617. [Google Scholar] [CrossRef]
- Drouet, S.; Tungmunnithum, D.; Lainé, É.; Hano, C. Gene Expression Analysis and Metabolite Profiling of Silymarin Biosynthesis during Milk Thistle (Silybum marianum (L.) Gaertn.) Fruit Ripening. Int. J. Mol. Sci. 2020, 21, 4730. [Google Scholar] [CrossRef]
- Martín-González, J.; Montero-Bullón, J.F.; Lacal, J. Dictyostelium Discoideum as a Non-Mammalian Biomedical Model. Microb. Biotechnol. 2021, 14, 111–125. [Google Scholar] [CrossRef]
- Schaf, J.; Damstra-Oddy, J.; Williams, R.S.B. Dictyostelium Discoideum as a Pharmacological Model System to Study the Mechanisms of Medicinal Drugs and Natural Products. Int. J. Dev. Biol. 2019, 63, 541–550. [Google Scholar] [CrossRef]
- El-Sherif, F.; Yap, Y.-K.; Ibrahim, H.I. Laser Irradiation Induces DNA Polymorphism and Alters Phytochemicals Compositions as Well as Growth and Yield of Curcuma longa. J. Dis. Med. Plants 2019, 5, 29–38. [Google Scholar] [CrossRef]
- Yap, Y.K.; El-sherif, F.; Habib, E.S.; Khattab, S. Moringa Oleifera Leaf Extract Enhanced Growth, Yield and Silybin Content While Mitigating Salt-induced Adverse Effects on the Growth of Silybum marianum. Agronomy 2021, 11, 2500. [Google Scholar] [CrossRef]
- Cocorocchio, M.; Baldwin, A.J.; Stewart, B.; Kim, L.; Harwood, A.J.; Thompson, C.R.L.; Andrews, P.L.R.; Williams, R.S.B. Curcumin and Derivatives Function through Protein Phosphatase 2A and Presenilin Orthologues in Dictyostelium Discoideum. DMM Dis. Model. Mech. 2018, 11, dmm032375. [Google Scholar] [CrossRef] [PubMed]
- StatSoft. STATISTICA Fur Windows; StatSoft: Tulsa, OK, USA, 2001. [Google Scholar]
- Urva; Shafique, H.; Jamil, Y.; ul Haq, Z.; Mujahid, T.; Khan, A.U.; Iqbal, M.; Abbas, M. Low Power Continuous Wave-Laser Seed Irradiation Effect on Moringa Oleifera Germination, Seedling Growth and Biochemical Attributes. J. Photochem. Photobiol. B 2017, 170, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Asghar, T.; Jamil, Y.; Iqbal, M.; Zia-ul-Haq; Abbas, M. Laser Light and Magnetic Field Stimulation Effect on Biochemical, Enzymes Activities and Chlorophyll Contents in Soybean Seeds and Seedlings during Early Growth Stages. J. Photochem. Photobiol. B 2016, 165, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Hanafiah, M.M.; Taha ZAeyad, A.I.; Said, M.N.M. Effect of Low-Intensity Laser Irradiation on Field Performance of Maize (Zea mays L.) Emergence, Phenological and Seed Quality Characteristics. Appl. Ecol. Environ. Res. 2020, 18, 6009–6923. [Google Scholar] [CrossRef]
- Podleoeny, J. Effect of Laser Irradiation on the Biochemical Changes in Seeds and the Accumulation of Dry Matter in the Faba Bean. Int. Agrophys. 2002, 16, 209–213. [Google Scholar]
- Musznski, S.; Gladyszewska, B. He-Ne Laser Irradiation Effect on Radish Seeds with Selected Germination Indices. Int. Agrophys. 2008, 22, 151–157. [Google Scholar]
- Osman, Y.A.H.; El Tobgy, K.M.K.; Sayed, E.; El Sherbini, A. Effect of Laser Radiation Treatments on Growth, Yield and Chemical Constituents of Fennel and Coriander Plants. J. Appl. Sci. Res. 2009, 5, 244–252. [Google Scholar]
- El Tobgy, K.M.K.; Osman, Y.A.H.; Sayed, E.; El Sherbini, A. Effect of Laser Radiation on Growth, Yield and Chemical Constituents of Anise and Cumin Plants. J. Appl. Sci. Res. 2009, 5, 522–528. [Google Scholar]
- Ali, S.I.; Gaafar, A.A.; Metwally, S.A.; Habba, I.E.; Khalek, M.R.A. The Reactive Influences of Pre-Sowing He-Ne Laser Seed Irradiation and Drought Stress on Growth, Fatty Acids, Phenolic Ingredients, and Antioxidant Properties of Celosia Argentea. Sci. Hortic. 2020, 261, 108989. [Google Scholar] [CrossRef]
- Agarwal, C.; Wadhwa, R.; Deep, G.; Biedermann, D.; Gažák, R.; Křen, V.; Agarwal, R. Anti-Cancer Efficacy of Silybin Derivatives—A Structure-Activity Relationship. PLoS ONE 2013, 8, e60074. [Google Scholar] [CrossRef] [PubMed]
- Deep, G.; Agarwal, R. Antimetastatic Efficacy of Silibinin: Molecular Mechanisms and Therapeutic Potential against Cancer. Cancer Metastasis Rev. 2010, 29, 447–463. [Google Scholar] [CrossRef] [PubMed]
| Laser Treatments (Min) | Plant Height (cm) | No. of Leaves (n) | No. of Branches (n) | Root Dry Weight (g) | Aerial Part Dry Weight (g) |
|---|---|---|---|---|---|
| Control | 52.3 d * | 37.0 e | 5.4 b | 3.6 c | 51.2 d |
| 2 | 93.5 ab | 51.2 c | 6.8 a | 10.2 a | 89.6 b |
| 4 | 96.2 a | 54.2 c | 3.6 d | 11.5 a | 61.0 c |
| 6 | 91.5 b | 59.7 b | 4.7 c | 7.4 b | 66.0 c |
| 8 | 62.5 c | 48.5 d | 3.5 d | 3.4 c | 44.2 e |
| 10 | 95.8 a | 74.5 a | 5.5 b | 8.6 ab | 92.0 a |
| Laser Treatments (Min) | Capitula Number (n) | Fruit Dry Weight (g) |
|---|---|---|
| Control | 3.7 d * | 9.4 e |
| 2 | 11.5 a | 40.6 ab |
| 4 | 9.6 b | 41.6 ab |
| 6 | 8.8 b | 26.5 d |
| 8 | 5.5 c | 34.9 c |
| 10 | 12.0 a | 49.8 a |
| Laser Treatments (Min) | Chl a (mg/100 g F.W.) | Chl b (mg/100 g F.W.) | Carotenoids (mg/100 g F.W.) |
|---|---|---|---|
| Control | 41.29 c * | 8.91 c | 36.37 d |
| 2 | 54.84 a | 10.90 b | 47.98 a |
| 4 | 50.07 ab | 10.77 b | 38.38 c |
| 6 | 43.11 c | 10.82 b | 40.26 ab |
| 8 | 41.59 c | 10.67 b | 37.74 c |
| 10 | 55.17 a | 12.28 a | 49.50 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Sherif, F.; Yap, Y.-K.; Alamer, S.; Althumairy, D.; Khattab, S. Laser Seed Pretreatment Alters the Silybin Content and Anti-Dictyostelium discoideum Cell Growth Activity of Silybum marianum (L.) Fruit. Appl. Sci. 2023, 13, 3546. https://doi.org/10.3390/app13063546
El Sherif F, Yap Y-K, Alamer S, Althumairy D, Khattab S. Laser Seed Pretreatment Alters the Silybin Content and Anti-Dictyostelium discoideum Cell Growth Activity of Silybum marianum (L.) Fruit. Applied Sciences. 2023; 13(6):3546. https://doi.org/10.3390/app13063546
Chicago/Turabian StyleEl Sherif, Fadia, Yun-Kiam Yap, Sarah Alamer, Duaa Althumairy, and Salah Khattab. 2023. "Laser Seed Pretreatment Alters the Silybin Content and Anti-Dictyostelium discoideum Cell Growth Activity of Silybum marianum (L.) Fruit" Applied Sciences 13, no. 6: 3546. https://doi.org/10.3390/app13063546
APA StyleEl Sherif, F., Yap, Y.-K., Alamer, S., Althumairy, D., & Khattab, S. (2023). Laser Seed Pretreatment Alters the Silybin Content and Anti-Dictyostelium discoideum Cell Growth Activity of Silybum marianum (L.) Fruit. Applied Sciences, 13(6), 3546. https://doi.org/10.3390/app13063546







