Production of Sustainable Postbiotics from Sugarcane Straw for Potential Food Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
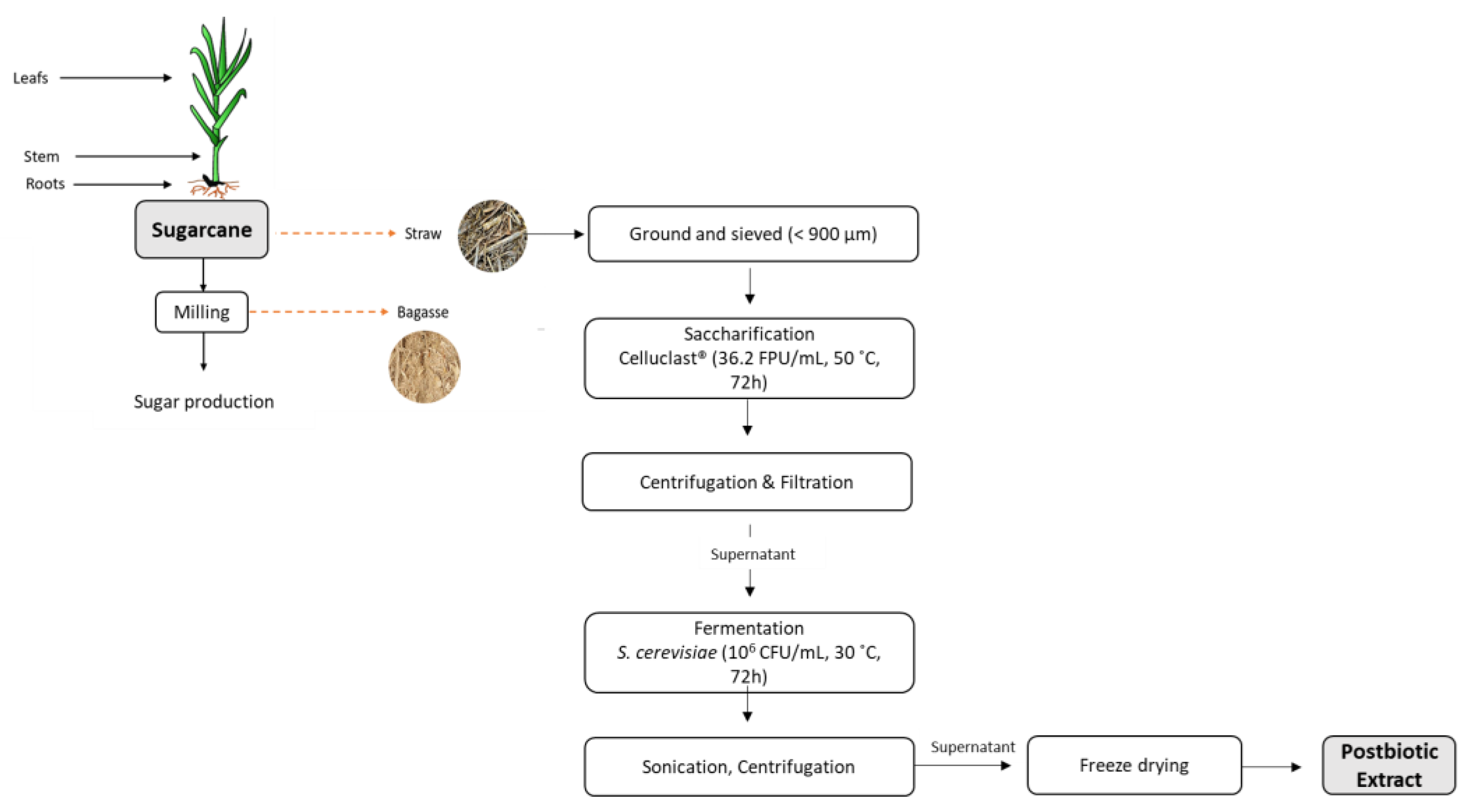
2.2. Production of Postbiotic Extract
2.2.1. Sugarcane Straw Saccharification Conditions
2.2.2. Fermentation Conditions and Extract Production
2.3. Cellular Concentration Measurements
2.4. Postbiotic Extract Chemical Characterization
2.4.1. Total Sugars
2.4.2. Quantification of the Total Phenolic Content
2.4.3. Organic Acids, Sugars, and Short-Chain Fatty Acids Analysis by HPLC-RID
2.4.4. Individual Phenolic Compounds Identification by LC-ESI-QqTOF-HRMS
2.5. Postbiotic Extract Biological Characterization
2.5.1. Impact of the Postbiotic Extract on Gut Microbiota
2.5.2. Radical Scavenging Activity
ABTS Radical Cation
DPPH Radical Cation
Oxygen Radical Absorption Capacity (ORAC)
2.5.3. Cytotoxicity Evaluation
2.5.4. Immunomodulatory Effect
2.6. Statistical Analysis
3. Results and Discussion
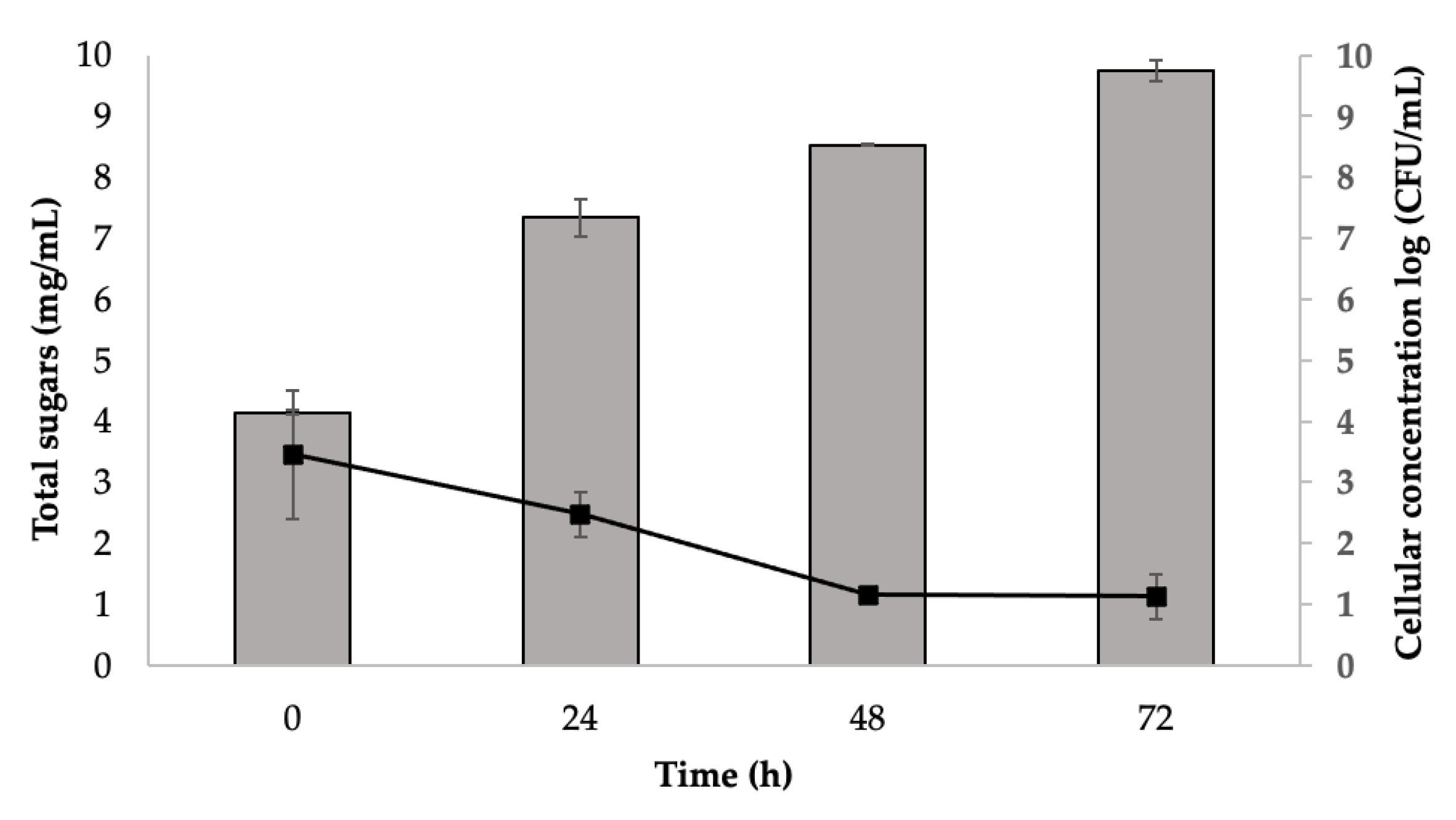
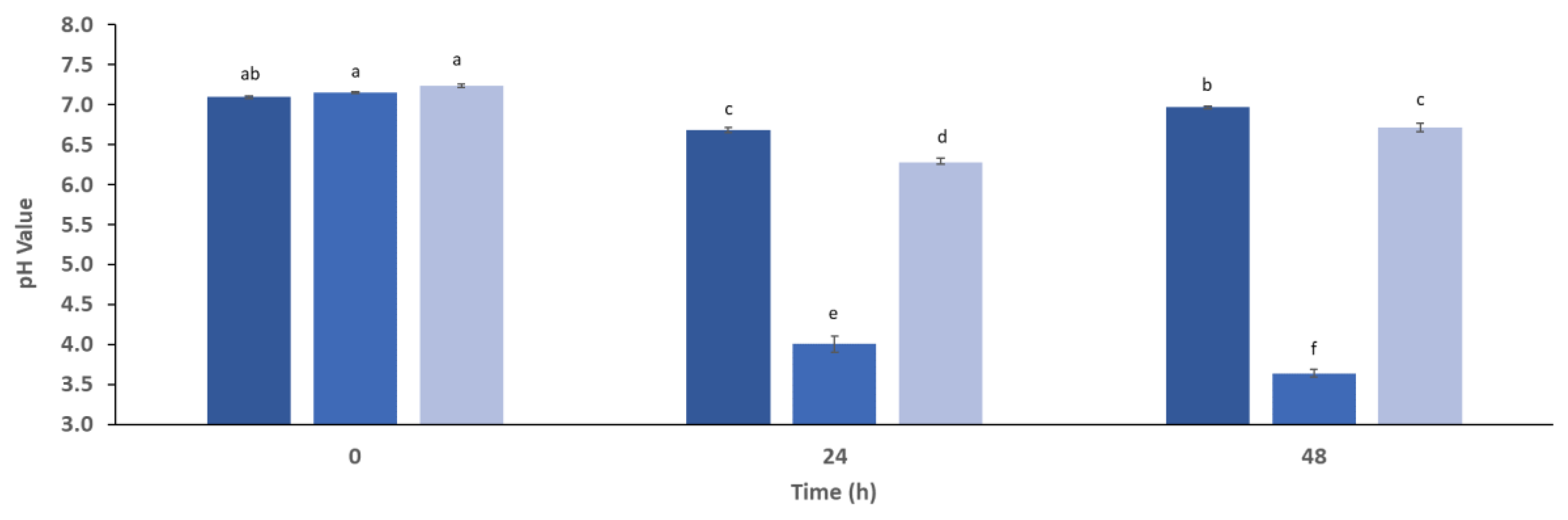
3.1. Saccharification and Fermentation Process
3.2. Postbiotic Extract General Composition
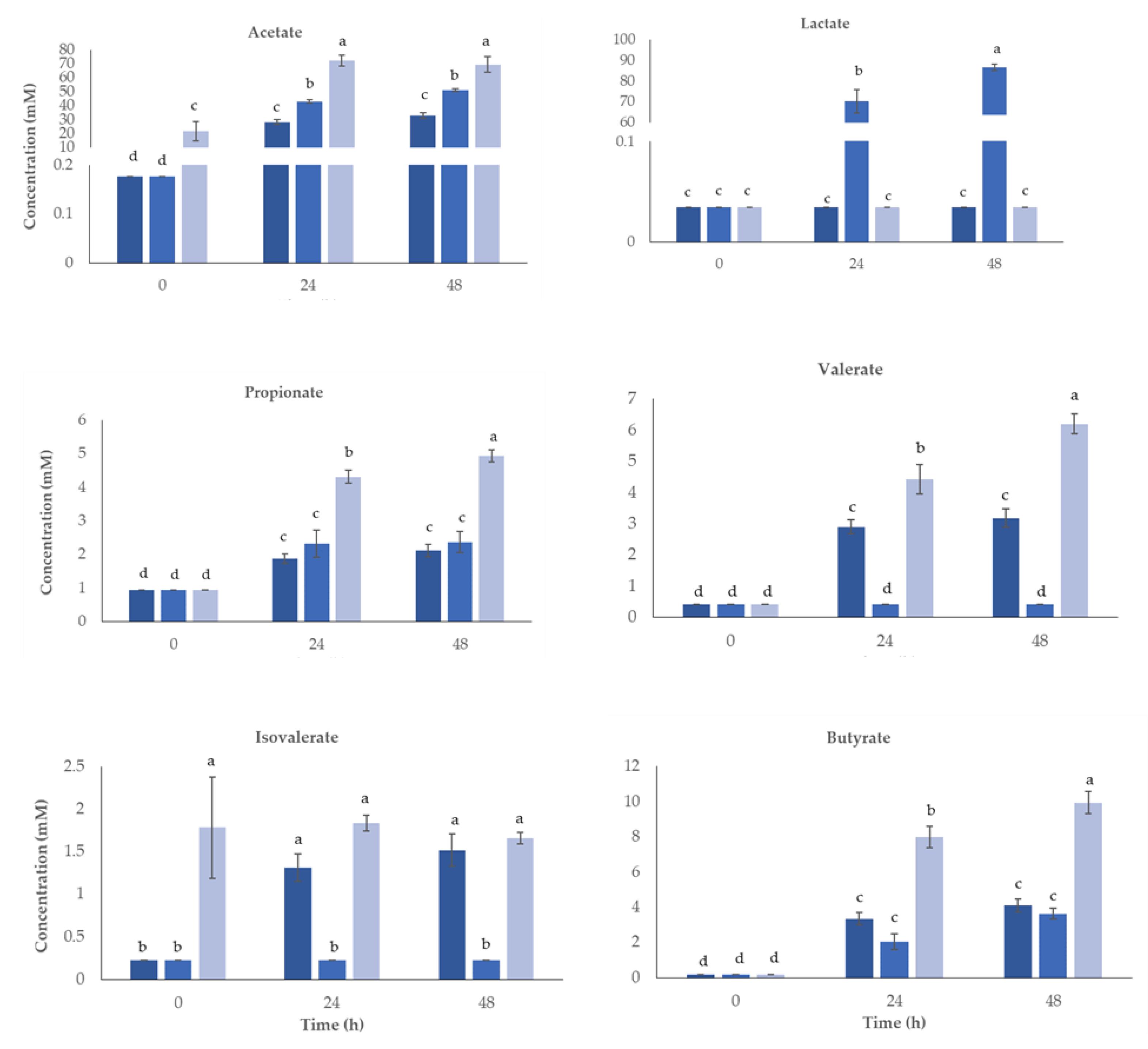
3.3. Organic Acids and Sugars Profiles
3.4. Phenolic Compounds Profile
3.5. Antioxidant Activity
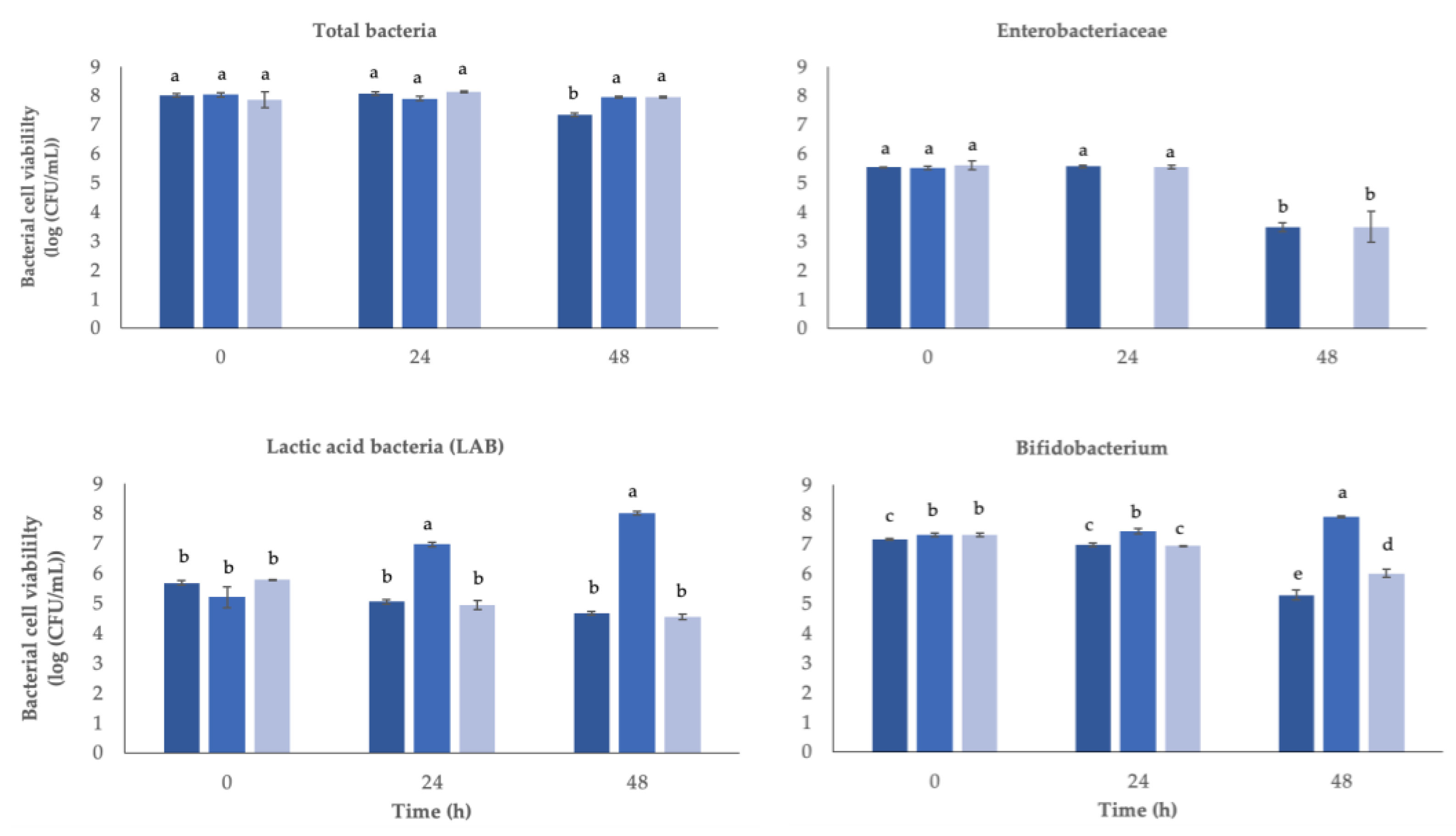
3.6. Impact of Postbiotics on Gut Microbiota Modulation
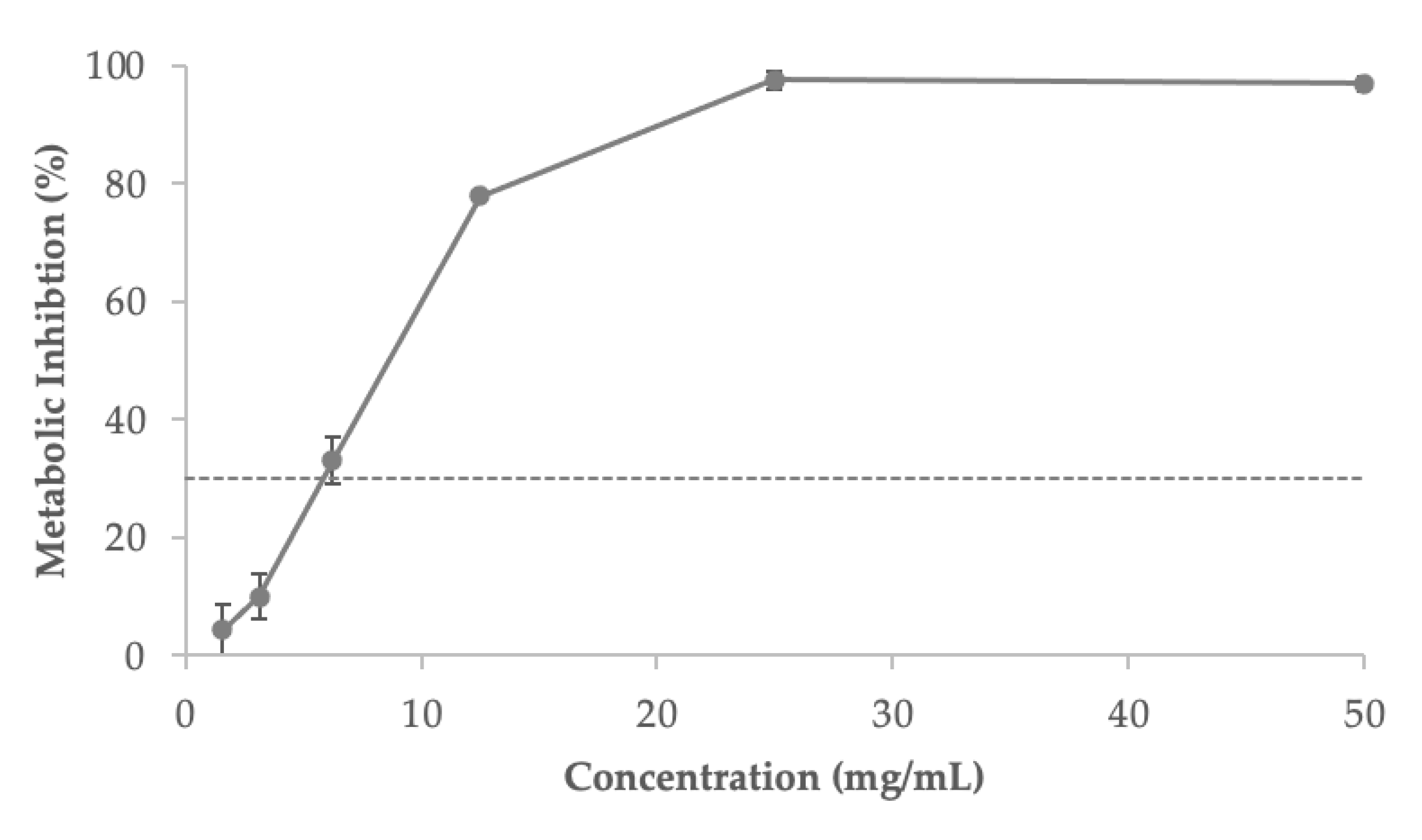
3.7. Influence of the Extract on Cells Viability
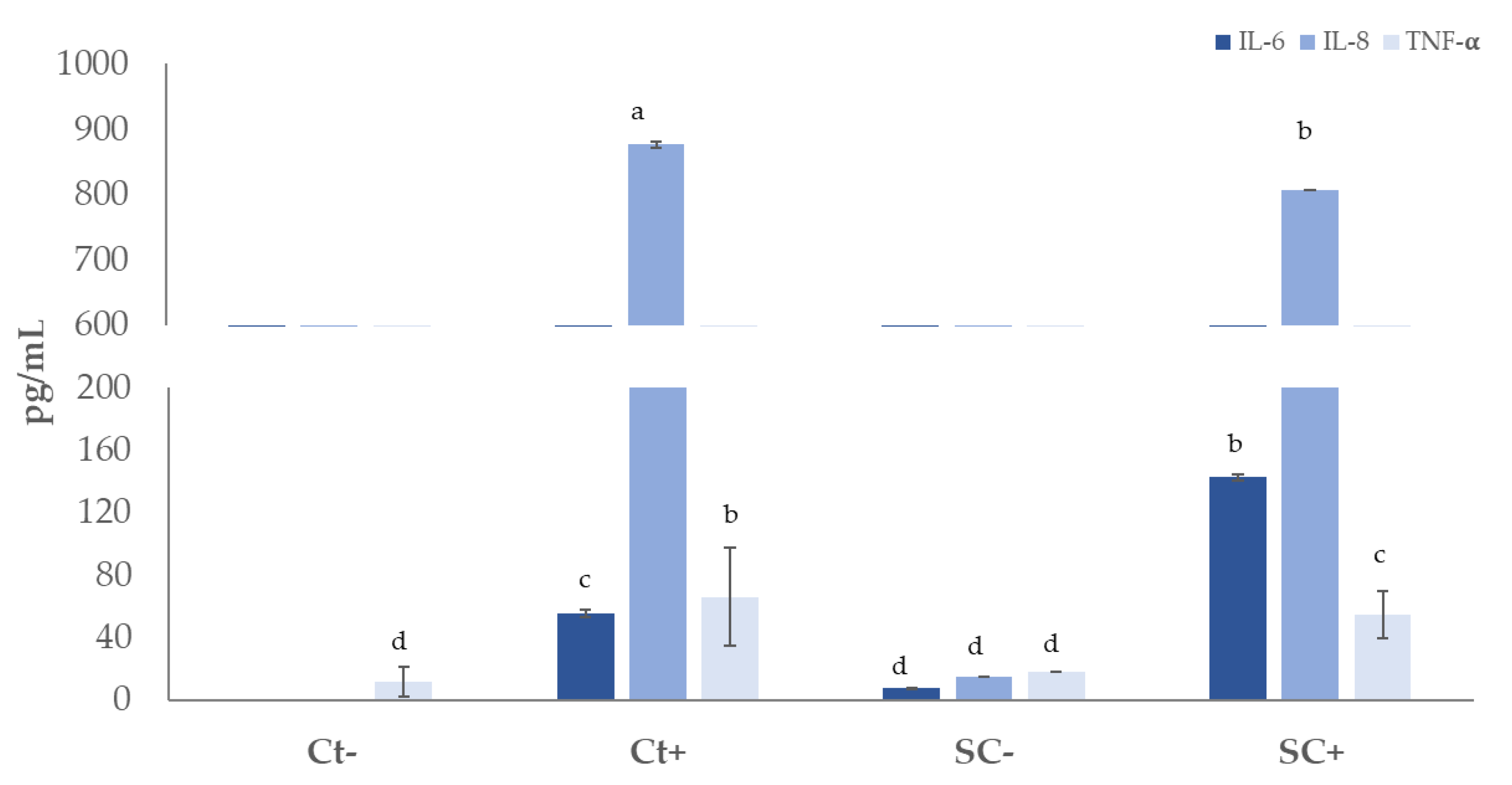
3.8. Anti-Inflammatory Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Sabater, C.; Calvete-Torre, I.; Villamiel, M.; Moreno, F.J.; Margolles, A.; Ruiz, L. Vegetable waste and by-products to feed a healthy gut microbiota: Current evidence, machine learning and computational tools to design novel microbiome-targeted foods. Trends Food Sci. Technol. 2021, 118, 399–417. [Google Scholar] [CrossRef]
- Chaudhari, A.; Dwivedi, M.K. The concept of probiotics, prebiotics, postbiotics, synbiotics, nutribiotics, and pharmabiotics. In Probiotics in the Prevention and Management of Human Diseases; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–11. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A step beyond pre-and probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar]
- Duarte, M.; Oliveira, A.L.; Oliveira, C.; Pintado, M.; Amaro, A.; Madureira, A.R. Current postbiotics in the cosmetic market—An update and development opportunities. Appl. Microbiol. Biotechnol. 2022, 106, 5879–5891. [Google Scholar] [CrossRef]
- Amiri, S.; Rezazadeh-Bari, M.; Alizadeh-Khaledabad, M.; Rezaei-Mokarram, R.; Sowti-Khiabani, M. Fermentation optimization for co-production of postbiotics by Bifidobacterium lactis BB12 in cheese whey. Waste Biomass Valorization 2021, 12, 5869–5884. [Google Scholar] [CrossRef]
- Lee, F.H.; Wan, S.Y.; Foo, H.L.; Loh, T.C.; Mohamad, R.; Abdul Rahim, R.; Idrus, Z. Comparative study of extracellular proteolytic, cellulolytic, and hemicellulolytic enzyme activities and biotransformation of palm kernel cake biomass by lactic acid bacteria isolated from Malaysian foods. Int. J. Mol. Sci. 2019, 20, 4979. [Google Scholar] [CrossRef]
- Lara-Cervantes, T.d.J.; Carrillo-Inungaray, M.L.; Balderas-Hernández, V.E.; Aguilar-Zárate, P.; Veana, F. Valorization of Parmentiera aculeata juice in growth of probiotics in submerged culture and their postbiotic production: A first approach to healthy foods. Arch. Microbiol. 2022, 204, 679. [Google Scholar] [CrossRef]
- Outeiriño, D.; Costa-Trigo, I.; Pinheiro de Souza Oliveira, R.; Pérez Guerra, N.; Salgado, J.M.; Domínguez, J.M. Biorefinery of Brewery Spent Grain by Solid-State Fermentation and Ionic Liquids. Foods 2022, 11, 3711. [Google Scholar] [CrossRef]
- Ali, S.E.; Yuan, Q.; Wang, S.; Farag, M.A. More than sweet: A phytochemical and pharmacological review of sugarcane (Saccharum officinarum L.). Food Biosci. 2021, 44, 101431. [Google Scholar] [CrossRef]
- Saad, M.B.W.; Oliveira, L.R.M.; Cândido, R.G.; Quintana, G.; Rocha, G.J.M.; Gonçalves, A.R. Preliminary studies on fungal treatment of sugarcane straw for organosolv pulping. Enzyme Microb. Technol. 2008, 43, 220–225. [Google Scholar] [CrossRef]
- dos Santos Rocha, M.S.R.; Pratto, B.; de Sousa, R.; Almeida, R.M.R.G.; da Cruz, A.J.G. A kinetic model for hydrothermal pretreatment of sugarcane straw. Bioresour. Technol. 2017, 228, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, M.; Szymańska, K.; Kordala, N.; Dąbrowska, A.; Bednarski, W.; Juszczuk, A. Evaluation of Mucor indicus and Saccharomyces cerevisiae capability to ferment hydrolysates of rape straw and Miscanthus giganteus as affected by the pretreatment method. Bioresour. Technol. 2016, 212, 262–270. [Google Scholar] [CrossRef]
- Ávila, P.F.; Martins, M.; de Almeida Costa, F.A.; Goldbeck, R. Xylooligosaccharides production by commercial enzyme mixture from agricultural wastes and their prebiotic and antioxidant potential. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100234. [Google Scholar] [CrossRef]
- Martins, M.; Silva, K.C.G.; Ávila, P.F.; Sato, A.C.K.; Goldbeck, R. Xylo-oligosaccharide microparticles with synbiotic potential obtained from enzymatic hydrolysis of sugarcane straw. Food Res. Int. 2021, 140, 109827. [Google Scholar] [CrossRef]
- Ávila, P.F.; Martins, M.; Goldbeck, R. Enzymatic Production of Xylooligosaccharides from Alkali-Solubilized Arabinoxylan from Sugarcane Straw and Coffee Husk. BioEnergy Res. 2021, 14, 739–751. [Google Scholar] [CrossRef]
- Scott, K.P.; Grimaldi, R.; Cunningham, M.; Sarbini, S.R.; Wijeyesekera, A.; Tang, M.L.K.; Lee, J.; Yau, Y.F.; Ansell, J.; Theis, S. Developments in understanding and applying prebiotics in research and practice—An ISAPP conference paper. J. Appl. Microbiol. 2020, 128, 934–949. [Google Scholar] [CrossRef]
- da Silva, A.S.; Inoue, H.; Endo, T.; Yano, S.; Bon, E.P.S. Milling pretreatment of sugarcane bagasse and straw for enzymatic hydrolysis and ethanol fermentation. Bioresour. Technol. 2010, 101, 7402–7409. [Google Scholar] [CrossRef]
- Ping, L.; Brosse, N.; Sannigrahi, P.; Ragauskas, A. Evaluation of grape stalks as a bioresource. Ind. Crops Prod. 2011, 33, 200–204. [Google Scholar] [CrossRef]
- Costa, E.M.; Pereira, C.F.; Ribeiro, A.A.; Casanova, F.; Freixo, R.; Pintado, M.; Ramos, O.L. Characterization and Evaluation of Commercial Carboxymethyl Cellulose Potential as an Active Ingredient for Cosmetics. Appl. Sci. 2022, 12, 6560. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- de Carvalho, N.M.; Oliveira, D.L.; Costa, C.M.; Pintado, M.; Madureira, A.R. Can Supplemented Skim Milk (SKM) Boost Your Gut Health? Fermentation 2022, 8, 126. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Barros, A.S.; Silva Ferreira, A.C.; Silva, A.M.S. Influence of the temperature and oxygen exposure in red Port wine: A kinetic approach. Food Res. Int. 2015, 75, 337–347. [Google Scholar] [CrossRef]
- de Carvalho, N.M.; Oliveira, D.L.; Saleh, M.A.D.; Pintado, M.; Madureira, A.R. Preservation of Human Gut Microbiota Inoculums for In Vitro Fermentations Studies. Fermentation 2021, 7, 14. [Google Scholar] [CrossRef]
- de Carvalho, N.M.; Walton, G.E.; Poveda, C.G.; Silva, S.N.; Amorim, M.; Madureira, A.R.; Pintado, M.E.; Gibson, G.R.; Jauregi, P. Study of in vitro digestion of Tenebrio molitor flour for evaluation of its impact on the human gut microbiota. J. Funct. Foods 2019, 59, 101–109. [Google Scholar] [CrossRef]
- Gonçalves, B.; Falco, V.; Moutinho-Pereira, J.; Bacelar, E.; Peixoto, F.; Correia, C. Effects of elevated CO2 on grapevine (Vitis vinifera L.): Volatile composition, phenolic content, and in vitro antioxidant activity of red wine. J. Agric. Food Chem. 2009, 57, 265–273. [Google Scholar] [CrossRef]
- Contreras, M.D.M.; Hernández-Ledesma, B.; Amigo, L.; Martín-Álvarez, P.J.; Recio, I. Production of antioxidant hydrolyzates from a whey protein concentrate with thermolysin: Optimization by response surface methodology. LWT-Food Sci. Technol. 2011, 44, 9–15. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2022.
- Machado, M.; Costa, E.M.; Silva, S.; Rodriguez-Alcalá, L.M.; Gomes, A.M.; Pintado, M. Pomegranate Oil’s Potential as an Anti-Obesity Ingredient. Molecules 2022, 27, 4958. [Google Scholar] [CrossRef]
- Chi, C.-H.; Cho, S.-J. Improvement of bioactivity of soybean meal by solid-state fermentation with Bacillus amyloliquefaciens versus Lactobacillus spp. and Saccharomyces cerevisiae. LWT-Food Sci. Technol. 2016, 68, 619–625. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.M.; Shahidi, F. Importance of insoluble-bound phenolics to antioxidant properties of wheat. J. Agric. Food Chem. 2006, 54, 1256–1264. [Google Scholar] [CrossRef]
- Daroit, D.J.; Silveira, S.T.; Hertz, P.F.; Brandelli, A. Production of extracellular β-glucosidase by Monascus purpureus on different growth substrates. Process Biochem. 2007, 42, 904–908. [Google Scholar] [CrossRef]
- Wang, L.; Wei, W.; Tian, X.; Shi, K.; Wu, Z. Improving bioactivities of polyphenol extracts from Psidium guajava L. leaves through co-fermentation of Monascus anka GIM 3.592 and Saccharomyces cerevisiae GIM 2.139. Ind. Crops Prod. 2016, 94, 206–215. [Google Scholar] [CrossRef]
- Sreeramulu, G.; Zhu, Y.; Knol, W. Kombucha fermentation and its antimicrobial activity. J. Agric. Food Chem. 2000, 48, 2589–2594. [Google Scholar] [CrossRef] [PubMed]
- Queiroz Santos, V.A.; Nascimento, C.G.; Schmidt, C.A.P.; Mantovani, D.; Dekker, R.F.H.; da Cunha, M.A.A. Solid-state fermentation of soybean okara: Isoflavones biotransformation, antioxidant activity and enhancement of nutritional quality. LWT 2018, 92, 509–515. [Google Scholar] [CrossRef]
- Parisutham, V.; Chandran, S.-P.; Mukhopadhyay, A.; Lee, S.K.; Keasling, J.D. Intracellular cellobiose metabolism and its applications in lignocellulose-based biorefineries. Bioresour. Technol. 2017, 239, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Duvnjak, Z.; Turcott, G.; Duan, Z.D. Production and consumption of sorbitol and fructose by Saccharomyces cerevisiae ATCC 36859. J. Chem. Technol. Biotechnol. 1991, 52, 527–537. [Google Scholar] [CrossRef]
- Liu, D.; Tang, W.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Monosaccharide composition analysis of polysaccharides from natural sources: Hydrolysis condition and detection method development. Food Hydrocoll. 2021, 116, 106641. [Google Scholar] [CrossRef]
- Martins, A.M.T.B.S.; Cordeiro, C.A.A.; Freire, A.M.J.P. In situ analysis of methylglyoxal metabolism in Saccharomyces cerevisiae. FEBS Lett. 2001, 499, 41–44. [Google Scholar] [CrossRef]
- Abbott, D.A.; Zelle, R.M.; Pronk, J.T.; van Maris, A.J.A. Metabolic engineering of Saccharomyces cerevisiae for production of carboxylic acids: Current status and challenges. FEMS Yeast Res. 2009, 9, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Kalliopi, R.; Paola, D.; Simone, G.; Fabrizio, T.; Rosanna, T.; Sandra, T.; Giovanna, S.; Luca, R.; Luca, C. Candida zemplinina Can Reduce Acetic Acid Produced by Saccharomyces cerevisiae in Sweet Wine Fermentations. Appl. Environ. Microbiol. 2012, 78, 1987–1994. [Google Scholar] [CrossRef]
- Coutinho, I.D.; Baker, J.M.; Ward, J.L.; Beale, M.H.; Creste, S.; Cavalheiro, A.J. Metabolite Profiling of Sugarcane Genotypes and Identification of Flavonoid Glycosides and Phenolic Acids. J. Agric. Food Chem. 2016, 64, 4198–4206. [Google Scholar] [CrossRef] [PubMed]
- Deseo, M.A.; Elkins, A.; Rochfort, S.; Kitchen, B. Antioxidant activity and polyphenol composition of sugarcane molasses extract. Food Chem. 2020, 314, 126180. [Google Scholar] [CrossRef]
- Zheng, R.; Su, S.; Zhou, H.; Yan, H.; Ye, J.; Zhao, Z.; You, L.; Fu, X. Antioxidant/antihyperglycemic activity of phenolics from sugarcane (Saccharum officinarum L.) bagasse and identification by UHPLC-HR-TOFMS. Ind. Crops Prod. 2017, 101, 104–114. [Google Scholar] [CrossRef]
- Sanchiz, A.; Pedrosa, M.M.; Guillamón, E.; Arribas, C.; Cabellos, B.; Linacero, R.; Cuadrado, C. Influence of boiling and autoclave processing on the phenolic content, antioxidant activity and functional properties of pistachio, cashew and chestnut flours. LWT 2019, 105, 250–256. [Google Scholar] [CrossRef]
- Goulas, V.; Papoti, V.T.; Exarchou, V.; Tsimidou, M.Z.; Gerothanassis, I.P. Contribution of Flavonoids to the Overall Radical Scavenging Activity of Olive (Olea europaea L.) Leaf Polar Extracts. J. Agric. Food Chem. 2010, 58, 3303–3308. [Google Scholar] [CrossRef]
- Greeff, J.; Joubert, J.; Malan, S.F.; van Dyk, S. Antioxidant properties of 4-quinolones and structurally related flavones. Bioorg. Med. Chem. 2012, 20, 809–818. [Google Scholar] [CrossRef]
- Campos, L.; Granell, P.; Tárraga, S.; López-Gresa, P.; Conejero, V.; Bellés, J.M.; Rodrigo, I.; Lisón, P. Salicylic acid and gentisic acid induce RNA silencing-related genes and plant resistance to RNA pathogens. Plant Physiol. Biochem. 2014, 77, 35–43. [Google Scholar] [CrossRef]
- Mechchate, H.; Es-safi, I.; Mohamed Al kamaly, O.; Bousta, D. Insight into Gentisic Acid Antidiabetic Potential Using In Vitro and In Silico Approaches. Molecules 2021, 26, 1932. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Elahi, F.; Daliri, E.B.-M.; Han, S.-I.; Oh, D.-H. Impact of thermal treatment and fermentation by lactic acid bacteria on sorghum metabolite changes, their antioxidant and antidiabetic activities. Food Biosci. 2022, 45, 101502. [Google Scholar] [CrossRef]
- Kiyota, E.; Mazzafera, P.; Sawaya, A.C.H.F. Analysis of Soluble Lignin in Sugarcane by Ultrahigh Performance Liquid Chromatography–Tandem Mass Spectrometry with a Do-It-Yourself Oligomer Database. Anal. Chem. 2012, 84, 7015–7020. [Google Scholar] [CrossRef]
- Kilani-Jaziri, S.; Mokdad-Bzeouich, I.; Krifa, M.; Nasr, N.; Ghedira, K.; Chekir-Ghedira, L. Immunomodulatory and cellular anti-oxidant activities of caffeic, ferulic, and p-coumaric phenolic acids: A structure–activity relationship study. Drug Chem. Toxicol. 2017, 40, 416–424. [Google Scholar] [CrossRef]
- Adeboye, P.T.; Bettiga, M.; Aldaeus, F.; Larsson, P.T.; Olsson, L. Catabolism of coniferyl aldehyde, ferulic acid and p-coumaric acid by Saccharomyces cerevisiae yields less toxic products. Microb. Cell Fact. 2015, 14, 149. [Google Scholar] [CrossRef] [PubMed]
- Vanbeneden, N.; Gils, F.; Delvaux, F.; Delvaux, F.R. Formation of 4-vinyl and 4-ethyl derivatives from hydroxycinnamic acids: Occurrence of volatile phenolic flavour compounds in beer and distribution of Pad1-activity among brewing yeasts. Food Chem. 2008, 107, 221–230. [Google Scholar] [CrossRef]
- Yazawa, K.; Kurokawa, M.; Obuchi, M.; Li, Y.; Yamada, R.; Sadanari, H.; Matsubara, K.; Watanabe, K.; Koketsu, M.; Tuchida, Y.; et al. Anti-Influenza Virus Activity of Tricin, 4′,5,7-trihydroxy-3′,5′-dimethoxyflavone. Antivir. Chem. Chemother. 2011, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.G.; Gao, S.; Lee, J.K.; Chan, Y.-Y.; Wong, E.C.; Zheng, T.; Li, X.-X.; Shaw, P.-C.; Simmonds, M.S.J.; Lau, C.B. A Natural Flavone Tricin from Grains Can Alleviate Tumor Growth and Lung Metastasis in Colorectal Tumor Mice. Molecules 2020, 25, 3730. [Google Scholar] [CrossRef]
- Oyama, T.; Yasui, Y.; Sugie, S.; Koketsu, M.; Watanabe, K.; Tanaka, T. Dietary tricin suppresses inflammation-related colon carcinogenesis in male Crj: CD-1 mice. Cancer Prev. Res. 2009, 2, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Imm, J.Y. Antiobesity Effect of Tricin, a Methylated Cereal Flavone, in High-Fat-Diet-Induced Obese Mice. J. Agric. Food Chem. 2018, 66, 9989–9994. [Google Scholar] [CrossRef]
- Ji, J.; Yang, X.; Flavel, M.; Shields, Z.P.-I.; Kitchen, B. Antioxidant and Anti-Diabetic Functions of a Polyphenol-Rich Sugarcane Extract. J. Am. Coll. Nutr. 2019, 38, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Lauberte, L.; Telysheva, G.; Cravotto, G.; Andersone, A.; Janceva, S.; Dizhbite, T.; Arshanitsa, A.; Jurkjane, V.; Vevere, L.; Grillo, G.; et al. Lignin–Derived antioxidants as value-added products obtained under cavitation treatments of the wheat straw processing for sugar production. J. Clean. Prod. 2021, 303, 126369. [Google Scholar] [CrossRef]
- Shang, Y.-F.; Cha, K.H.; Lee, E.H.; Pan, C.-H.; Um, B.-H. Optimization, bio accessibility of tricin and anti-oxidative activity of extract from black bamboo leaves. Free Radic. Antioxid. 2016, 6, 64–71. [Google Scholar] [CrossRef]
- Li, X.X.; Chen, S.G.; Yue, G.G.L.; Kwok, H.F.; Lee, J.K.M.; Zheng, T.; Shaw, P.C.; Simmonds, M.S.J.; Lau, C.B.S. Natural flavone tricin exerted anti-inflammatory activity in macrophage via NF-κB pathway and ameliorated acute colitis in mice. Phytomedicine 2021, 90, 153625. [Google Scholar] [CrossRef] [PubMed]
- Shalini, V.; Jayalekshmi, A.; Helen, A. Mechanism of anti-inflammatory effect of tricin, a flavonoid isolated from Njavara rice bran in LPS induced hPBMCs and carrageenan induced rats. Mol. Immunol. 2015, 66, 229–239. [Google Scholar] [CrossRef]
- Solyanik, G.I.; Zulphigarov, O.S.; Prokhorova, I.V.; Pyaskovskaya, O.N.; Kolesnik, D.L.; Atamanyuk, V.P. A Comparative Study on Pharmacokinetics of Tricin, a Flavone from Gramineous Plants with Antiviral Activity. J. Biosci. Med. 2021, 9, 76–91. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Datta, S.; Timson, D.J.; Annapure, U.S. Antioxidant properties and global metabolite screening of the probiotic yeast Saccharomyces cerevisiae var. boulardii. J. Sci. Food Agric. 2017, 97, 3039–3049. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Wang, Y.; Wang, X.; Ren, Y.; Yue, T.; Wang, Z.; Gao, Z. Study on the nutritional characteristics and antioxidant activity of dealcoholized sequentially fermented apple juice with Saccharomyces cerevisiae and Lactobacillus plantarum fermentation. Food Chem. 2021, 363, 130351. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ma, Y.; Chen, L.; Zhao, C.; Maubois, J.; Jiang, T.; Li, H.; He, S. Antioxidant activity of two yeasts and their attenuation effect on 4-nitroquinoline 1-oxide induced in vitro lipid peroxidation. Int. J. Food Sci. Technol. 2010, 45, 555–561. [Google Scholar] [CrossRef]
- Hassan, H.M.M. Antioxidant and immunostimulating activities of yeast (Saccharomyces cerevisiae) autolysates. World Appl. Sci. J. 2011, 15, 1110–1119. [Google Scholar]
- Aditi, S.; Sarbjit, S.K.; Om, P.S. Screening of indigenous yeast isolates obtained from traditional fermented foods of Western Himalayas for probiotic attributes. J. Yeast Fungal Res. 2011, 2, 117–126. [Google Scholar]
- Takalloo, Z.; Nikkhah, M.; Nemati, R.; Jalilian, N.; Sajedi, R.H. Autolysis, plasmolysis and enzymatic hydrolysis of baker’s yeast (Saccharomyces cerevisiae): A comparative study. World J. Microbiol. Biotechnol. 2020, 36, 68. [Google Scholar] [CrossRef]
- Đorđević, T.M.; Šiler-Marinković, S.S.; Dimitrijević-Branković, S.I. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010, 119, 957–963. [Google Scholar] [CrossRef]
- Osman, A.; El-Gazzar, N.; Almanaa, T.N.; El-Hadary, A.; Sitohy, M. Lipolytic postbiotic from Lactobacillus paracasei manages metabolic syndrome in albino wistar rats. Molecules 2021, 26, 472. [Google Scholar] [CrossRef] [PubMed]
- Anvar, S.A.; Rahimyan, D.; Golestan, L.; Shojaee, A.; Pourahmad, R. Butter fortified with spray-dried encapsulated Ferulago angulata extract nanoemulsion and postbiotic metabolite of Lactiplantibacillus plantarum subsp. plantarum improves its physicochemical, microbiological and sensory properties. Int. J. Dairy Technol. 2023. [Google Scholar] [CrossRef]
- Watson, E.-J.; Giles, J.; Scherer, B.L.; Blatchford, P. Human faecal collection methods demonstrate a bias in microbiome composition by cell wall structure. Sci. Rep. 2019, 9, 16831. [Google Scholar] [CrossRef]
- Oku, T.; Nakamura, S. Fructooligosaccharide: Metabolism through gut microbiota and prebiotic effect. Food Nutr. J. 2017, 2, 1–10. [Google Scholar] [CrossRef]
- Tornero-Martínez, A.; Cruz-Ortiz, R.; Jaramillo-Flores, M.E.; Osorio-Díaz, P.; Ávila-Reyes, S.V.; Alvarado-Jasso, G.M.; Mora-Escobedo, R. In vitro fermentation of polysaccharides from Aloe vera and the evaluation of antioxidant activity and production of short chain fatty acids. Molecules 2019, 24, 3605. [Google Scholar] [CrossRef]
- Peredo-Lovillo, A.; Romero-Luna, H.E.; Jiménez-Fernández, M. Health promoting microbial metabolites produced by gut microbiota after prebiotics metabolism. Food Res. Int. 2020, 136, 109473. [Google Scholar] [CrossRef]
- Wong, J.M.W.; De Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Hague, A.; Elder, D.J.E.; Hicks, D.J.; Paraskeva, C. Apoptosis in colorectal tumour cells: Induction by the short chain fatty acids butyrate, propionate and acetate and by the bile salt deoxycholate. Int. J. Cancer 1995, 60, 400–406. [Google Scholar] [CrossRef] [PubMed]
- McRorie Jr, J.W.; McKeown, N.M. Understanding the physics of functional fibers in the gastrointestinal tract: An evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.R.; Hoyles, L.; Flint, H.J.; Dumas, M.-E. Colonic bacterial metabolites and human health. Curr. Opin. Microbiol. 2013, 16, 246–254. [Google Scholar] [CrossRef]
- Fehlbaum, S.; Prudence, K.; Kieboom, J.; Heerikhuisen, M.; Van den Broek, T.; Schuren, F.H.J.; Steinert, R.E.; Raederstorff, D. In vitro fermentation of selected prebiotics and their effects on the composition and activity of the adult gut microbiota. Int. J. Mol. Sci. 2018, 19, 3097. [Google Scholar] [CrossRef]
- Rodrigues, D.; Walton, G.; Sousa, S.; Rocha-Santos, T.A.P.; Duarte, A.C.; Freitas, A.C.; Gomes, A.M.P. In vitro fermentation and prebiotic potential of selected extracts from seaweeds and mushrooms. LWT 2016, 73, 131–139. [Google Scholar] [CrossRef]
- Broekaert, W.F.; Courtin, C.M.; Verbeke, K.; Van de Wiele, T.; Verstraete, W.; Delcour, J.A. Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides. Crit. Rev. Food Sci. Nutr. 2011, 51, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef]
- Vazquez, M.J.; Alonso, J.L.; Domınguez, H.; Parajo, J.C. Xylooligosaccharides: Manufacture and applications. Trends Food Sci. Technol. 2000, 11, 387–393. [Google Scholar] [CrossRef]
- Scott, E.; De Paepe, K.; Van de Wiele, T. Postbiotics and Their Health Modulatory Biomolecules. Biomolecules 2022, 12, 1640. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Duncan, S.H.; Sheridan, P.O.; Walker, A.W.; Flint, H.J. Microbial lactate utilisation and the stability of the gut microbiome. Gut Microbiome 2022, 3, e3. [Google Scholar] [CrossRef]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef]
- Gänzle, M.G. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Karnaouri, A.; Matsakas, L.; Krikigianni, E.; Rova, U.; Christakopoulos, P. Valorization of waste forest biomass toward the production of cello-oligosaccharides with potential prebiotic activity by utilizing customized enzyme cocktails. Biotechnol. Biofuels 2019, 12, 285. [Google Scholar] [CrossRef]
- Ávila, P.F.; Silva, M.F.; Martins, M.; Goldbeck, R. Cello-oligosaccharides production from lignocellulosic biomass and their emerging prebiotic applications. World J. Microbiol. Biotechnol. 2021, 37, 73. [Google Scholar] [CrossRef] [PubMed]
- Gwiazdowska, D.; Juś, K.; Jasnowska-Małecka, J.; Kluczyńska, K. The impact of polyphenols on Bifidobacterium growth. Acta Biochim. Pol. 2015, 62, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A. The inhibitory effect of polyphenols on human gut microbiota. J. Physiol. Pharmacol. 2012, 63, 497–503. [Google Scholar]
- Molan, A.-L.; Liu, Z.; Kruger, M. The ability of blackcurrant extracts to positively modulate key markers of gastrointestinal function in rats. World J. Microbiol. Biotechnol. 2010, 26, 1735–1743. [Google Scholar] [CrossRef]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef]
- Nunes, A.R.; Gonçalves, A.C.; Alves, G.; Falcão, A.; Garcia-Viguera, C.; Moreno, D.A.; Silva, L.R. Valorisation of Prunus avium L. By-Products: Phenolic Composition and Effect on Caco-2 Cells Viability. Foods 2021, 10, 1185. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G.; Ng, S.C. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017, 152, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Edwards, A.; Martín-Pérez, L.; Jiménez-Aspee, F.; Theoduloz, C.; Schmeda-Hirschmann, G.; Larrosa, M. Anti-inflammatory effect of polyphenols from Chilean currants (Ribes magellanicum and R. punctatum) after in vitro gastrointestinal digestion on Caco-2 cells: Anti-inflammatory activity of in vitro digested Chilean currants. J. Funct. Foods 2019, 59, 329–336. [Google Scholar] [CrossRef]
- Martins, I.M.; Macedo, G.A.; Macedo, J.A. Biotransformed grape pomace as a potential source of anti-inflammatory polyphenolics: Effects in Caco-2 cells. Food Biosci. 2020, 35, 100607. [Google Scholar] [CrossRef]
- Prakash, M.D.; Stojanovska, L.; Feehan, J.; Nurgali, K.; Donald, E.L.; Plebanski, M.; Flavel, M.; Kitchen, B.; Apostolopoulos, V. Anti-cancer effects of polyphenol-rich sugarcane extract. PLoS ONE 2021, 16, e0247492. [Google Scholar] [CrossRef]
- Corral, L.G.; Haslett, P.A.J.; Muller, G.W.; Chen, R.; Wong, L.-M.; Ocampo, C.J.; Patterson, R.T.; Stirling, D.I.; Kaplan, G. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-α. J. Immunol. 1999, 163, 380–386. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.J.; Kim, T.-W.; Kim, S.; Kim, J.H.; Park, J.-T.; Lee, N.H.; Choi, Y.-S.; Kang, M.-C.; Song, K.-M. Immune-enhancing effects of polysaccharide extract of by-products of Korean liquor fermented by Saccharomyces cerevisiae. Int. J. Biol. Macromol. 2021, 188, 245–252. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, Y.; Li, H. Advances in research on immunoregulation of macrophages by plant polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Alarcon De La Lastra, C.; Villegas, I. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol. Nutr. Food Res. 2005, 49, 405–430. [Google Scholar] [CrossRef]
- Chuang, C.-C.; McIntosh, M.K. Potential mechanisms by which polyphenol-rich grapes prevent obesity-mediated inflammation and metabolic diseases. Annu. Rev. Nutr. 2011, 31, 155–176. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Guo, Y.; Zhao, J.; Xu, X.; Wang, N.; Liu, Q. Ferulic Acid Ameliorates Lipopolysaccharide-Induced Barrier Dysfunction via MicroRNA-200c-3p-Mediated Activation of PI3K/AKT Pathway in Caco-2 Cells. Front. Pharmacol. 2020, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Romier-Crouzet, B.; Van De Walle, J.; During, A.; Joly, A.; Rousseau, C.; Henry, O.; Larondelle, Y.; Schneider, Y.-J. Inhibition of inflammatory mediators by polyphenolic plant extracts in human intestinal Caco-2 cells. Food Chem. Toxicol. 2009, 47, 1221–1230. [Google Scholar] [CrossRef]
- Batista, V.L.; De Jesus, L.C.L.; Tavares, L.M.; Barroso, F.L.A.; Fernandes, L.J.d.S.; Freitas, A.d.S.; Americo, M.F.; Drumond, M.M.; Mancha-Agresti, P.; Ferreira, E. Paraprobiotics and Postbiotics of Lactobacillus delbrueckii CIDCA 133 Mitigate 5-FU-Induced Intestinal Inflammation. Microorganisms 2022, 10, 1418. [Google Scholar] [CrossRef] [PubMed]
| Analysis | Non-Fermented Straw Extract | Fermented Straw Extract | |
|---|---|---|---|
| Total phenolics | 12.2 ± 0.2 b | 16.2 ± 0.3 a | |
| Total protein | 326.0 ± 1.0 a | 332.0 ± 4.0 a | |
| Sugars | Glucose | 41.5 ± 2.6 a | ND |
| Cellobiose | 12.6 ± 1.0 a | ND | |
| Organic acids | Lactic acid | ND | 9.8 ± 0.1 a |
| Acetic acid | 80.7 ± 0.2 b | 194.2 ± 35.5 a | |
| Proposed Name | Formula-H | m/z Theoretical Mass [M − H]− | m/z Measured Mass [M − H]− | Error (ppm) | MS/MS Fragments (m/z) |
|---|---|---|---|---|---|
| Hydroxybenzoic acids | |||||
| Protocatechuic acid | C7H5O4 | 153.0184 | 153.0193 | 3.3 | 109, 153 |
| 2,5-Dihydroxybenzoic acid isomer 1 | C7H5O4 | 153.0184 | 153.0193 | 3.2 | 109, 153 |
| 2,5-Dihydroxybenzoic acid isomer 2 | C7H5O4 | 153.0181 | 153.0193 | 3.3 | 65, 109 |
| 4-Hydroxybenzoic acid | C7H5O3 | 137.0235 | 137.0244 | 4.6 | 137 |
| 3,4-Dihydroxybenzaldehyde | C7H5O3 | 137.0233 | 137.0244 | 4.5 | 93, 137 |
| 4-Hydroxybenzaldehyde | C7H5O2 | 121.0285 | 121.0295 | 3.0 | 121 |
| Hydroxycinnamic acids | |||||
| 5-O-Feruloylquinic acid | C17H19O9 | 367.1021 | 367.1035 | 3.8 | 134, 193 |
| trans-3-Feruloylquinic acid | C17H19O9 | 367.1023 | 367.1030 | 3.5 | 173 |
| Ferulic acid | C10H9O4 | 193.0391 | 193.0506 | 4.3 | 134, 161, 193 |
| p-Coumaric acid | C9H7O3 | 163.0401 | 163.0401 | −1.0 | 119 |
| Flavonoids | |||||
| Isovitexin 2″-O-arabinoside | C26H27O14 | 563.1401 | 563.1406 | 1.9 | 353, 443 |
| Luteolin-8-C-glucoside isomer 1 | C21H19O11 | 447.0920 | 447.0933 | 2.9 | 327, 357 |
| Luteolin-8-C-glucoside isomer 2 | C21H19O11 | 447.0920 | 447.0933 | 2.9 | 327, 357 |
| Tricin-O-neohesperoside isomer 1 | C29H33O16 | 637.1772 | 637.1774 | −3.9 | 329 |
| Tricin-O-neohesperoside isomer 2 | C29H33O16 | 637.1775 | 637.1638 | −0.1 | 329 |
| Tricin-7-O-glucoside | C25H31O10 | 491.1919 | 491.1823 | 0.7 | 329 |
| Tricin-7-O-rhamnosyl-glucuronide | C29H31O17 | 651.1570 | 651.1567 | −0.4 | 329 |
| Proposed Name | Concentration (µg/g Dry Extract) | |
|---|---|---|
| Non-Fermented Straw Extract | Fermented Straw Extract | |
| Protocatechuic acid | 2.75 ± 0.55 b | 3.81 ± 0.59 a |
| 2,5-Dihydroxybenzoic acid isomer 1 | 0.93 ± 1.82 a | NQ |
| 2,5-Dihydroxybenzoic acid isomer 2 | 24.56 ± 4.35 a | 20.58 ± 0.28 a |
| 4-Hydroxybenzoic acid | 1.97 ± 0.10 a | 1.38 ± 0.02 a |
| 3,4-Dihydroxybenzaldehyde | 7.17 ± 0.21 b | 24.82 ± 0.04 a |
| 4-Hydroxybenzaldehyde | 17.71 ± 0.17 a | 0.70 ± 0.05 b |
| 5-O-Feruloylquinic acid | 8.39 ± 0.05 a | 8.99 ± 0.68 a |
| trans-3-Feruloylquinic acid | 17.07 ± 0.28 a | 17.19 ± 0.68 a |
| Ferulic acid | 110.36 ± 0.01 a | 42.61 ± 0.38 b |
| p-Coumaric acid | 12.18 ± 0.74 a | 11.16 ± 0.09 a |
| Isovitexin 2″-O-arabinoside | NQ | NQ |
| Luteolin-8-C-glucoside isomer 1 | NQ | NQ |
| Luteolin-8-C-glucoside isomer 2 | NQ | NQ |
| Tricin-O-neohesperoside isomer 1 | 0.65 ± 0.01 a | 0.61 ± 0.01 a |
| Tricin-O-neohesperoside isomer 2 | 0.60 ± 0.00 a | 0.61 ± 0.01 a |
| Tricin-7-O-glucoside | 1.57 ± 0.02 a | 0.75 ± 0.01 b |
| Tricin-7-O-rhamnosyl-glucuronide | 1.27 ± 0.02 b | 1.38 ± 0.01 a |
| Analysis | Non-Fermented Straw Extract | Fermented Straw Extract | |
|---|---|---|---|
| Antioxidant activity | ABTS IC50 (mg/mL) | 7.34 ± 0.34 a | 2.62 ± 0.98 b |
| DPPH IC50 (mg/mL) | 13.08 ± 0.34 a | 6.85 ± 0.24 b | |
| ORAC (μmol TE/g) | 171.21 ± 11.27 b | 302.23 ± 19.49 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, A.L.S.; Seara, M.; Carvalho, M.J.; de Carvalho, N.M.; Costa, E.M.; Silva, S.; Duarte, M.; Pintado, M.; Oliveira, C.; Madureira, A.R. Production of Sustainable Postbiotics from Sugarcane Straw for Potential Food Applications. Appl. Sci. 2023, 13, 3391. https://doi.org/10.3390/app13063391
Oliveira ALS, Seara M, Carvalho MJ, de Carvalho NM, Costa EM, Silva S, Duarte M, Pintado M, Oliveira C, Madureira AR. Production of Sustainable Postbiotics from Sugarcane Straw for Potential Food Applications. Applied Sciences. 2023; 13(6):3391. https://doi.org/10.3390/app13063391
Chicago/Turabian StyleOliveira, Ana L. S., Marta Seara, Maria João Carvalho, Nelson Mota de Carvalho, Eduardo M. Costa, Sara Silva, Marco Duarte, Manuela Pintado, Carla Oliveira, and Ana Raquel Madureira. 2023. "Production of Sustainable Postbiotics from Sugarcane Straw for Potential Food Applications" Applied Sciences 13, no. 6: 3391. https://doi.org/10.3390/app13063391
APA StyleOliveira, A. L. S., Seara, M., Carvalho, M. J., de Carvalho, N. M., Costa, E. M., Silva, S., Duarte, M., Pintado, M., Oliveira, C., & Madureira, A. R. (2023). Production of Sustainable Postbiotics from Sugarcane Straw for Potential Food Applications. Applied Sciences, 13(6), 3391. https://doi.org/10.3390/app13063391












