A Machine Learning-Based Intelligent Vehicular System (IVS) for Driver’s Diabetes Monitoring in Vehicular Ad-Hoc Networks (VANETs)
Abstract
1. Introduction
1.1. Motivation
1.2. Research Questions
- a.
- How does the vehicle detect diabetic patients?
- b.
- How is genetic testing valuable in the diabetes monitoring of drivers?
- c.
- How do vehicles take appropriate measures upon detecting hypo/hyperglycemia?
2. Related Work
Discussion
3. Proposed Methodology
- (a)
- Machine learning-based diabetes prediction using genetic information;
- (b)
- Intelligent vehicular system (IVS) framework for real-time data processing.
3.1. Machine Learning-Based Diabetes Prediction Using Genetic Information
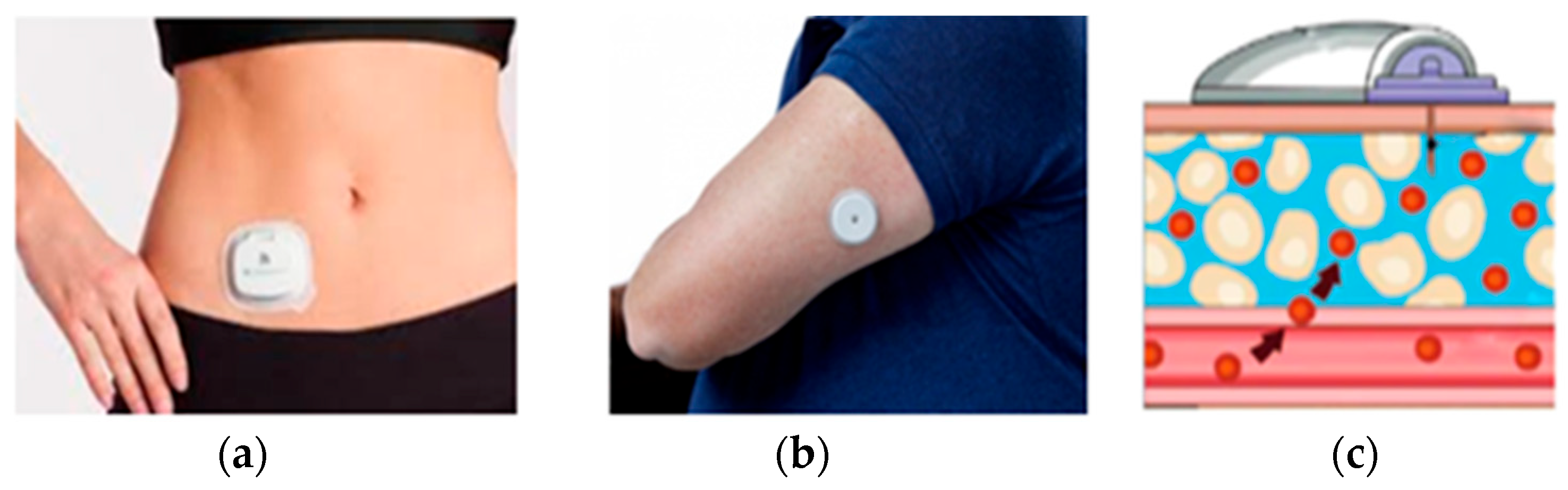
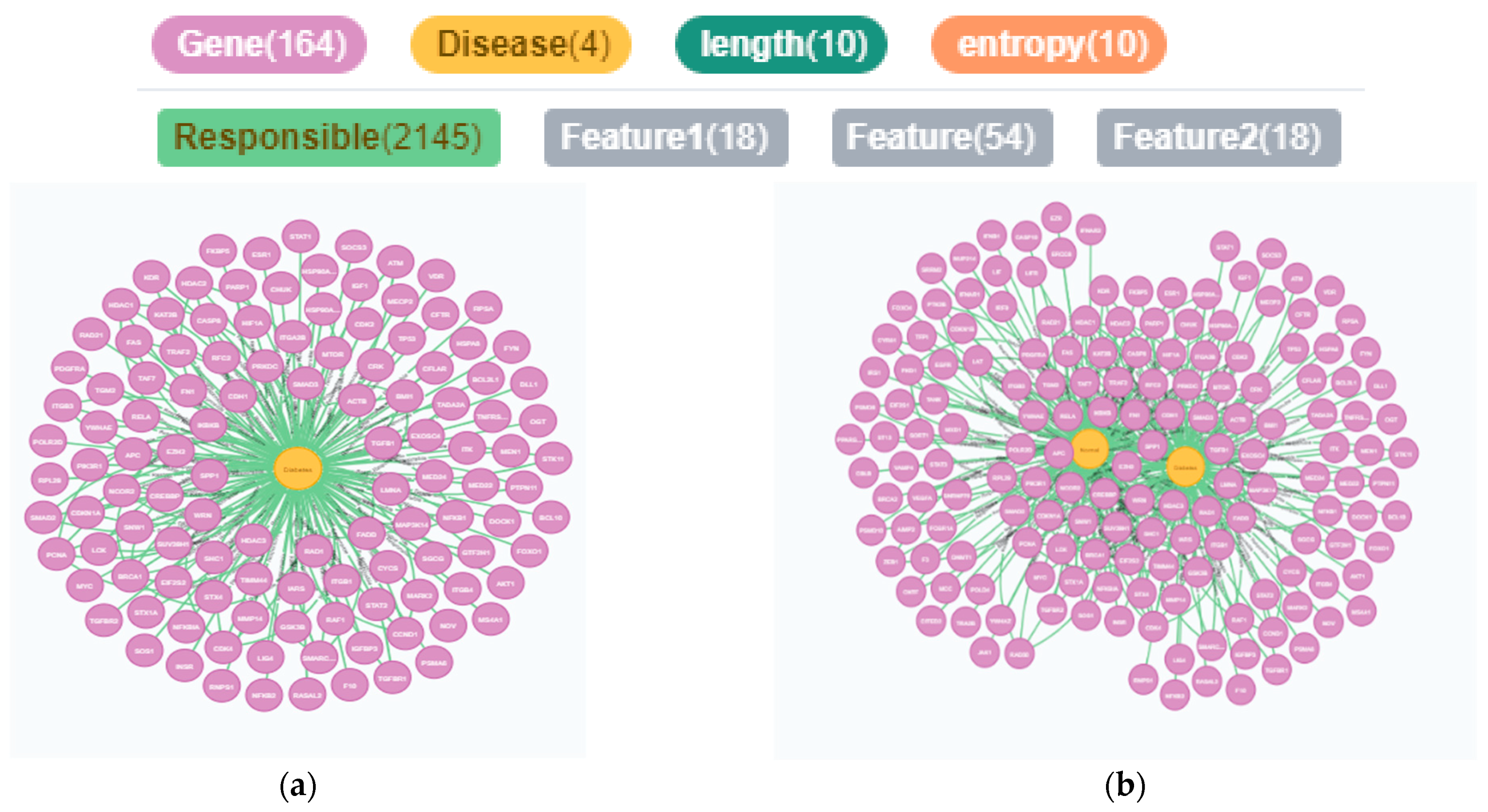
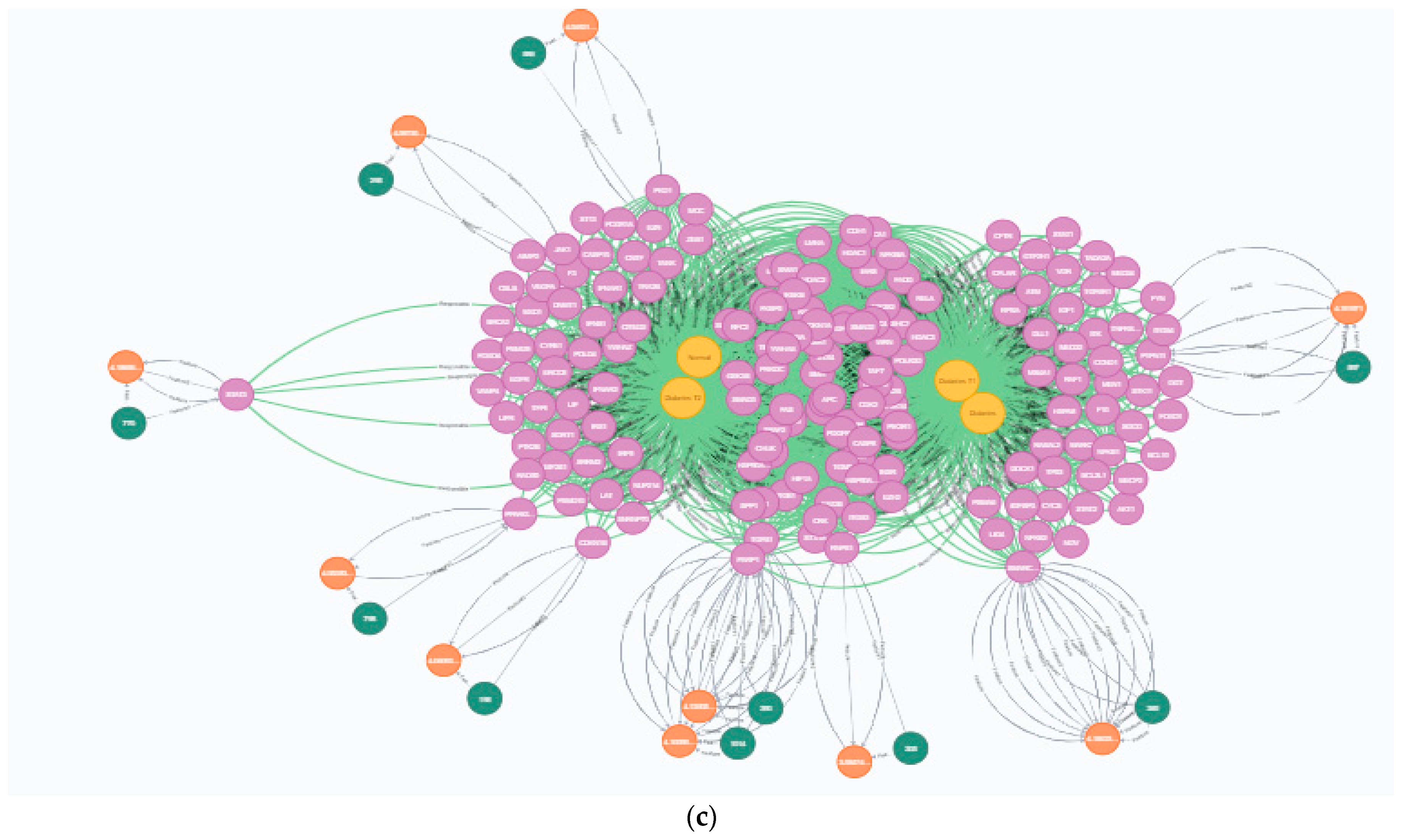
3.1.1. Data Acquisition
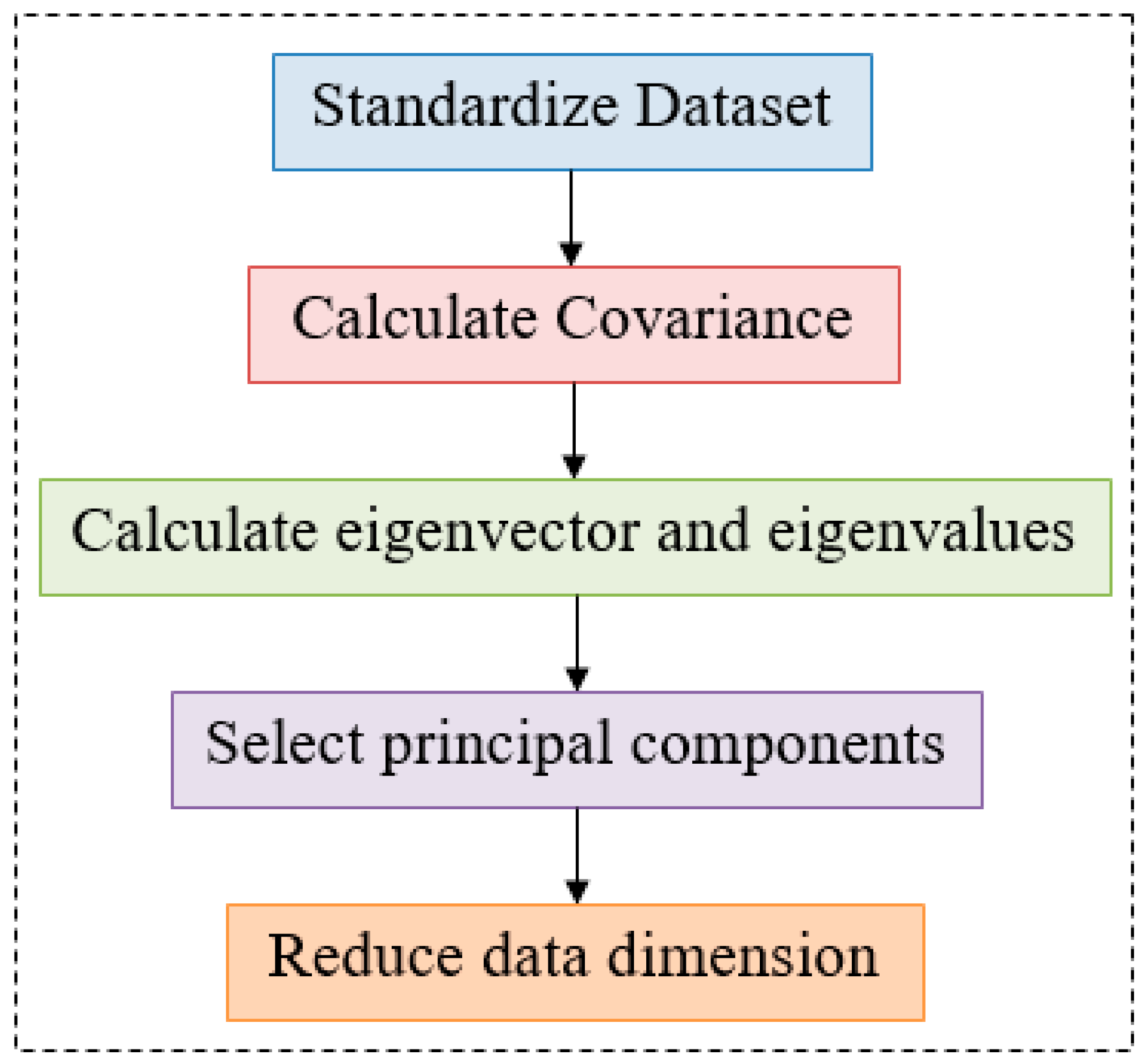
3.1.2. Feature Extraction
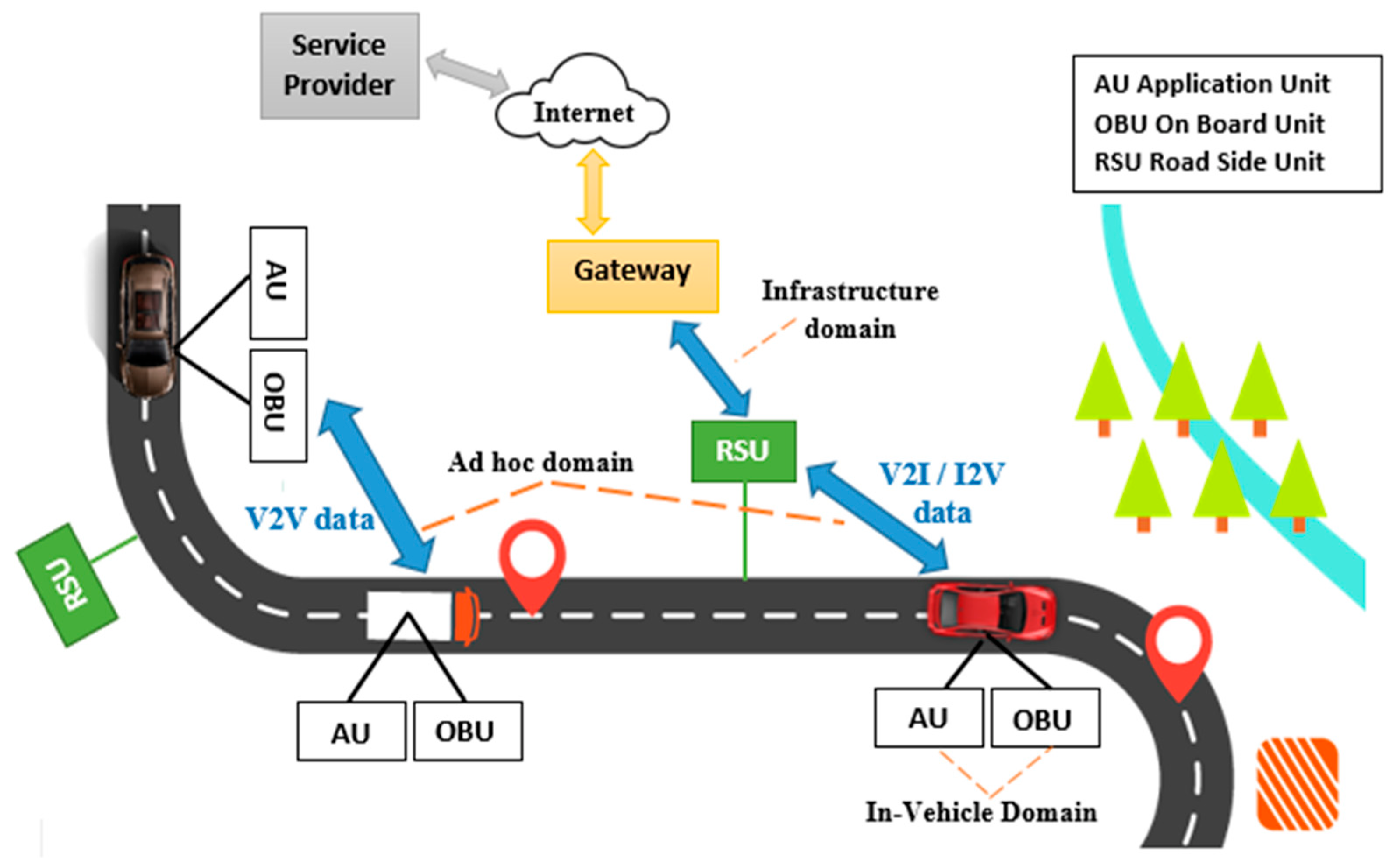
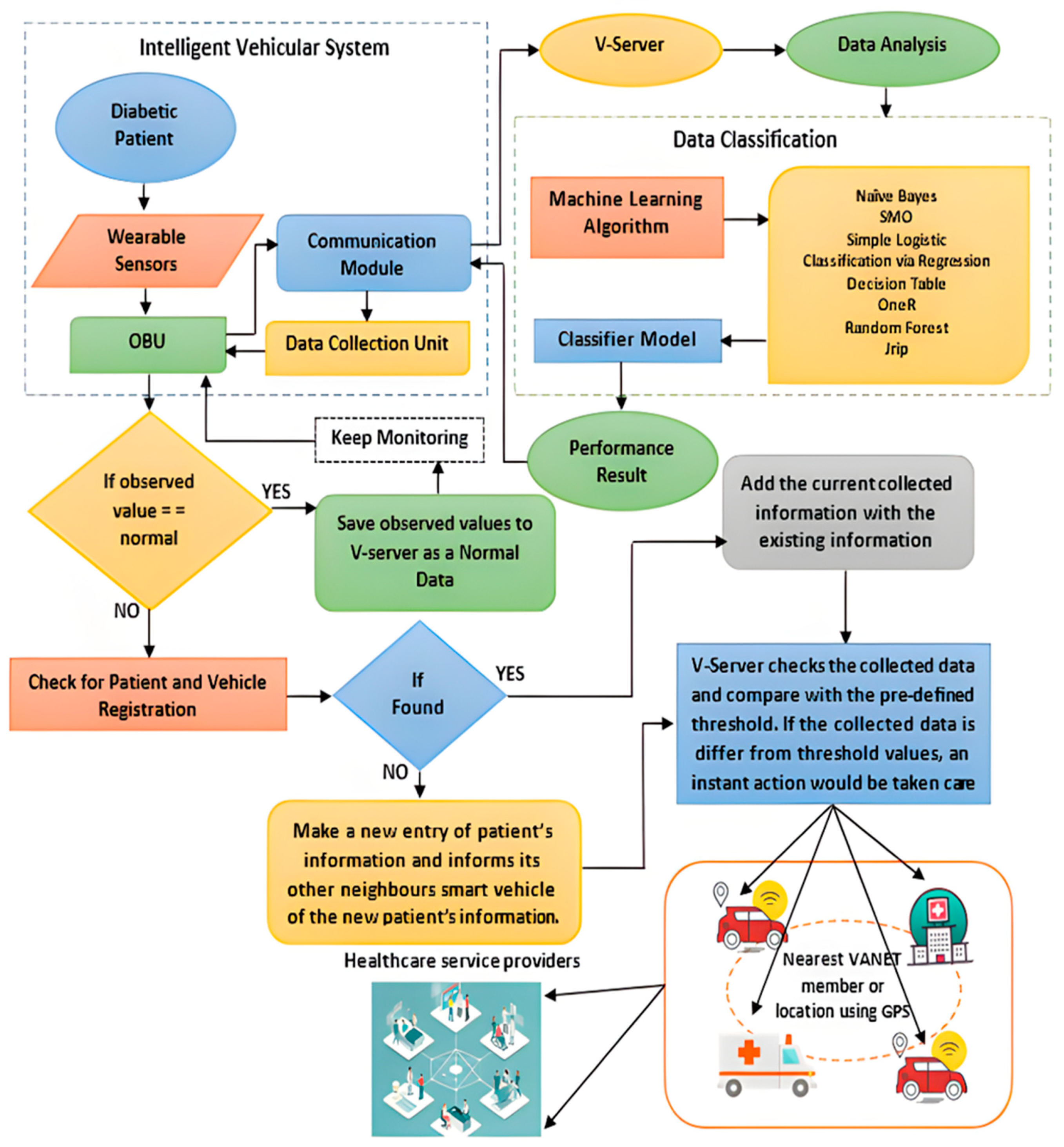
3.2. Intelligent Vehicular System (IVS) Framework for Real-Time Data Processing
4. Experiments and Results
4.1. Experimental Environment
Dataset Description
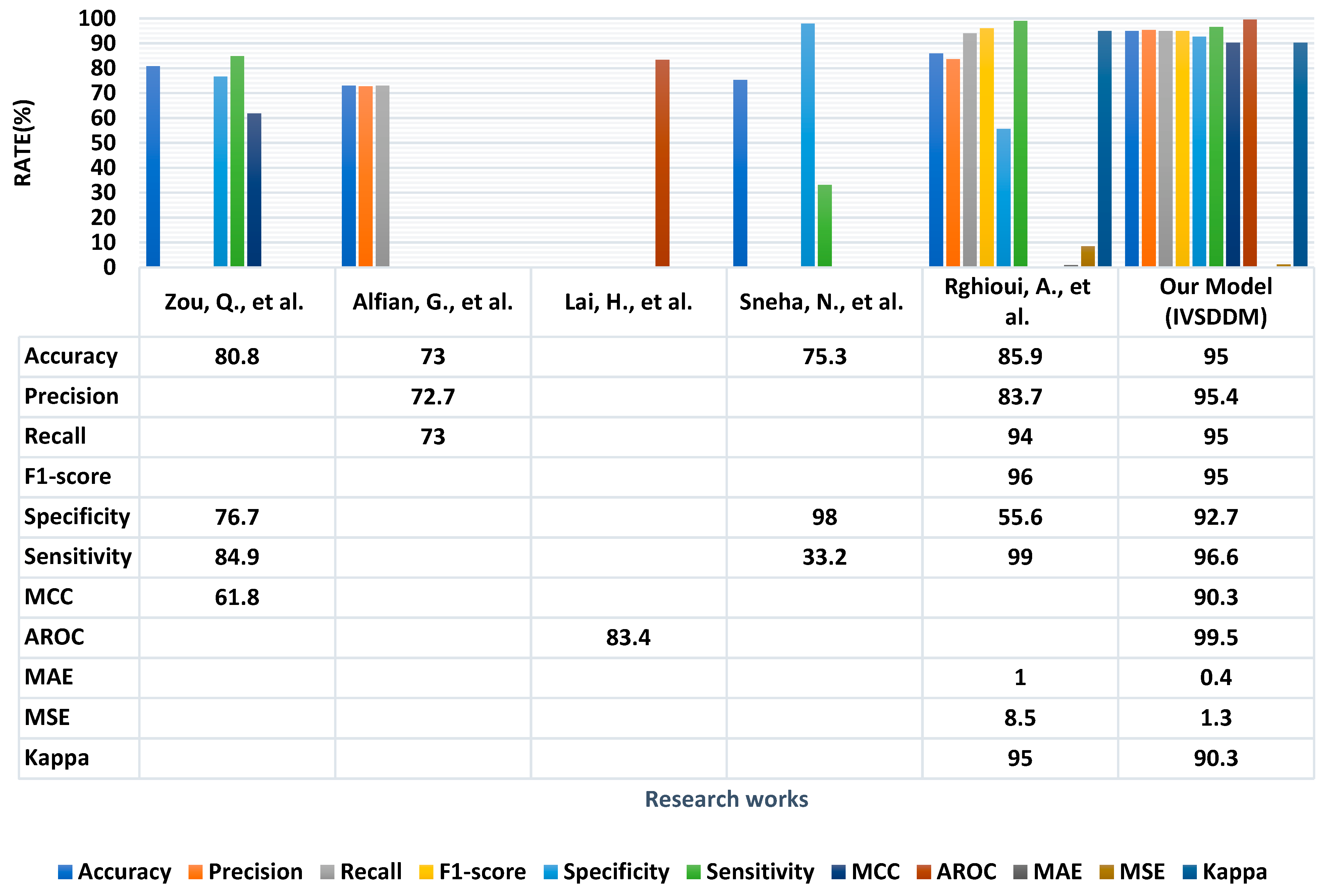
4.2. Performance Evaluation Parameter
4.3. Comparison to Previous Work
5. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- King, H.; Aubert, R.E.; Herman, W.H. Global burden of diabetes, 1995–2025: Prevalence, numerical estimates, and projections. Diabetes Care 1998, 21, 1414–1431. [Google Scholar] [CrossRef]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef]
- Zou, Q.; Qu, K.; Luo, Y.; Yin, D.; Ju, Y.; Tang, H. Predicting diabetes mellitus with machine learning techniques. Front. Genet. 2018, 515, 00515. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Foster, J.R. A history of blood glucose meters and their role in self-monitoring of diabetes mellitus. Br. J. Biomed. Sci. 2012, 69, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.K.; Potts, R.O.; Ackerman, N.R.; Fermi, S.J.; Tamada, J.A.; Chase, H.P. Correlation of fingerstick blood glucose measurements with GlucoWatch biographer glucose results in young subjects with type 1 diabetes. Diabetes Care 1999, 22, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Petrosyan, L.; Ghazaryan, Z.; Muradyan, G.; Aghajanova, E.; Brabece, M.; Žďárská, D.J.; Halčiakova, K.; Polák, J.; Frier, B.M.; Brož, J. Limited knowledge of safe driving practice among drivers with diabetes in Armenia: Association with greater risk of motor vehicle accidents. J. Diabetes Mellit. 2019, 9, 14. [Google Scholar] [CrossRef]
- Keten, A. Diabetes and driving safety. Accid. Anal. Prev. 2021, 149, 105854. [Google Scholar] [CrossRef] [PubMed]
- Potter, K.; Virtanen, H.; Stewart, F.; Luca, P.; Ho, J.; Nettel-Aguirre, A.; Pacaud, D. Exploring knowledge and safety practices for driving in youth with type 1 diabetes. Can. J. Diabetes 2020, 44, 169–174. [Google Scholar] [CrossRef]
- Cooper, C.; Franklin, D.; Ros, M.; Safaei, F.; Abolhasan, M. A comparative survey of VANET clustering techniques. IEEE Commun. Surv. Tutor. 2016, 19, 657–681. [Google Scholar] [CrossRef]
- Da Cunha, F.D.; Boukerche, A.; Villas, L.; Viana, A.C.; Loureiro, A.A. Data Communication in VANETs: A Survey, Challenges and Applications. Ph.D. Thesis, University of Bordeaux, Bordeaux, France, INRIA Saclay, Bordeaux, France, 2014. [Google Scholar]
- Shah, S.S.; Malik, A.W.; Rahman, A.U.; Iqbal, S.; Khan, S.U. Time barrier-based emergency message dissemination in vehicular ad-hoc networks. IEEE Access 2019, 7, 16494–16503. [Google Scholar] [CrossRef]
- Kavakiotis, I.; Tsave, O.; Salifoglou, A.; Maglaveras, N.; Vlahavas, I.; Chouvarda, I. Machine learning and data mining methods in diabetes research. Comput. Struct. Biotechnol. J. 2017, 15, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Rghioui, A.; Lloret, J.; Sendra, S.; Oumnad, A. A smart architecture for diabetic patient monitoring using machine learning algorithms. Healthcare 2020, 8, 348. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, J.; Zhou, J.; Hao, Y.; Zhang, J.; Youn, C.H. 5G-smart diabetes: Toward personalized diabetes diagnosis with healthcare big data clouds. IEEE Commun. Mag. 2018, 56, 16–23. [Google Scholar] [CrossRef]
- Noshadi, H.; Giordano, E.; Hagopian, H.; Pau, G.; Gerla, M.; Sarrafzadeh, M. Remote medical monitoring through vehicular ad hoc network. In Proceedings of the 2008 IEEE 68th Vehicular Technology Conference, Calgary, AB, Canada, 21–24 September 2008; pp. 1–5. [Google Scholar]
- Songer, T.J.; Dorsey, R.R. High risk characteristics for motor vehicle crashes in persons with diabetes by age. Annu. Proc. Assoc. Adv. Automot. Med. 2006, 50, 335. [Google Scholar]
- Merickel, J.; High, R.; Smith, L.; Wichman, C.; Frankel, E.; Smits, K.; Drincic, A.; Desouza, C.; Gunaratne, P.; Ebe, K.; et al. Driving safety and real-time glucose monitoring in insulin-dependent diabetes. Int. J. Automot. Eng. 2019, 10, 34–40. [Google Scholar] [CrossRef]
- Chang, Y.H.; Hou, W.H.; Wu, K.F.; Li, C.Y.; Hsu, I.L. Risk of motorcycle collisions among patients with type 2 diabetes: A population-based cohort study with age and sex stratifications in Taiwan. Acta Diabetol. 2022, 59, 1625–1634. [Google Scholar] [CrossRef]
- Alfian, G.; Syafrudin, M.; Ijaz, M.F.; Syaekhoni, M.A.; Fitriyani, N.L.; Rhee, J. A personalized healthcare monitoring system for diabetic patients by utilizing BLE-based sensors and real-time data processing. Sensors 2018, 18, 2183. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Huang, H.; Keshavjee, K.; Guergachi, A.; Gao, X. Predictive models for diabetes mellitus using machine learning techniques. BMC Endocr. Disord. 2019, 19, 101. [Google Scholar] [CrossRef] [PubMed]
- Sneha, N.; Gangil, T. Analysis of diabetes mellitus for early prediction using optimal features selection. J. Big Data 2019, 6, 13. [Google Scholar] [CrossRef]
- Sikandar, A.; Anwar, W.; Bajwa, U.I.; Wang, X.; Sikandar, M.; Yao, L.; Jiang, Z.L.; Chunkai, Z. Decision tree based approaches for detecting protein complex in protein protein interaction network (PPI) via link and sequence analysis. IEEE Access 2018, 6, 22108–22120. [Google Scholar] [CrossRef]
- Mabrouk, M.S. A study of the potential of EIIP mapping method in exon prediction using the frequency domain techniques. Am. J. Biomed. Eng. 2012, 2, 17–22. [Google Scholar] [CrossRef]
- Li, T.; Li, Q.; Zhu, S.; Ogihara, M. A survey on wavelet applications in data mining. ACM SIGKDD Explor. Newsl. 2002, 4, 49–68. [Google Scholar] [CrossRef]
- Polat, K.; Güneş, S. An expert system approach based on principal component analysis and adaptive neuro-fuzzy inference system to diagnosis of diabetes disease. Digit. Signal Process. 2007, 17, 702–710. [Google Scholar] [CrossRef]
- You, Y.; Cai, H.; Chen, J. Low rank representation and its application in bioinformatics. Curr. Bioinform. 2018, 13, 508–517. [Google Scholar] [CrossRef]
- Khan Academy. Available online: http://www.khanacademy.org/math/statistics-probability/summarizing-quantitative-data/variance-standard-deviation-population/a/calculating-standard-deviation-step-by-step/ (accessed on 12 August 2022).
- Corporate Finance Institute. Available online: http://www.corporatefinanceinstitute.com/resources/knowledge/finance/covariance/ (accessed on 12 August 2022).
- Math is Fun. Available online: http://www.mathsisfun.com/algebra/eigenvalue/ (accessed on 15 August 2022).
- Ur Rehman, S.; Khan, M.A.; Zia, T.A.; Zheng, L. Vehicular ad-hoc networks (VANETs)-an overview and challenges. J. Wirel. Netw. Commun. 2013, 3, 29–38. [Google Scholar]
- Hamdi, M.M.; Audah, L.; Rashid, S.A.; Mohammed, A.H.; Alani, S.; Mustafa, A.S. A review of applications, characteristics and challenges in vehicular ad hoc networks (VANETs). In Proceedings of the 2020 International Congress on Human-Computer Interaction, Optimization and Robotic Applications (HORA), Istanbul, Turkey, 26–27 June 2020; pp. 1–7. [Google Scholar]
- Wikipedia.org/wiki/Mean_squared_error. Available online: https://en.wikipedia.org/wiki/Mean_squared_error (accessed on 15 October 2022.).
- Statisticshowto.com/absolute-error. Available online: https://www.statisticshowto.com/absolute-error/ (accessed on 17 October 2022.).
- Towardsdatascience.com/cohens-kappa-9786ceceab58. Available online: https://towardsdatascience.com/cohens-kappa-9786ceceab58 (accessed on 25 October 2022).

| Publication | Compared Algorithms | Parameters | Best Accuracy |
|---|---|---|---|
| Zou Quan et al. [3] | RF, J48, Neural Networks | Accuracy, Sensitivity, Specificity, MCC | Random Forest Accuracy = 80.84% |
| Rghioui A., et al. [13] | Naïve Bayes, J48, SMO, ZeroR, OneR, Simple Logistic, Random Forest | Accuracy, Precision, Sensitivity, Specificity, Recall, F-measure | SMO Accuracy = 99.66% |
| Alfian G., et al. [19] | Random Forest, Naïve Bayes, SVM, Logistic Regression, Multilayer Perceptron | Precision, Recall, Accuracy | Multilayer Perceptron Accuracy = 77.08% |
| Lai H. et al. [20] | Logistic Regression, Gradient Boosting Machine, Random Forest, RPART | AROC, Sensitivity | Logistic Regression Sensitivity = 73.4% |
| N. Sneha, et al. [21] | Decision Tree, Naïve Bayes, Support Vector Machine, Random Forest, KNN | Accuracy, Sensitivity, Specificity | Naïve Bayes Accuracy = 82.30% |
| Name | Alanine | Arginine | Asparagine | Aspartic Acid | Cysteine | Glutamine | Glutamic acid | Glycine | Histidine | Isoleucine | Leucine | Lysine | Methionine | Phenylalanine | Proline | Serine | Threonine | Tryptophan | Tyrosine | Valine |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | A | R | N | D | C | Q | E | G | H | I | L | K | M | F | P | S | T | W | Y | V |
| EIIP | 0.0373 | 0.0959 | 0.0036 | 0.1263 | 0.0829 | 0.0761 | 0.0058 | 0.0050 | 0.0242 | 0 | 0 | 0.0371 | 0.0823 | 0.0946 | 0.0198 | 0.0829 | 0.0941 | 0.0548 | 0.0516 | 0.0057 |
| Total number of instances (Genes) | 1030 |
| List of attributes omitted (Features) | 104 |
| Parameters | Values |
|---|---|
| Simulator Used | NS-2.34 |
| Network Area Range | Highway of 1400 m × 1400 m |
| Total Simulation Time | 350 ms |
| Nodes Density | 10, 20, 30, 40, 50, 60, 70, 80 |
| Transmission Range Among Vehicles | 260 m |
| Number of Vehicles | 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100 |
| Number of RSUs | 10 (1 per 10 vehicles) |
| Network Connectivity | Wi-Fi |
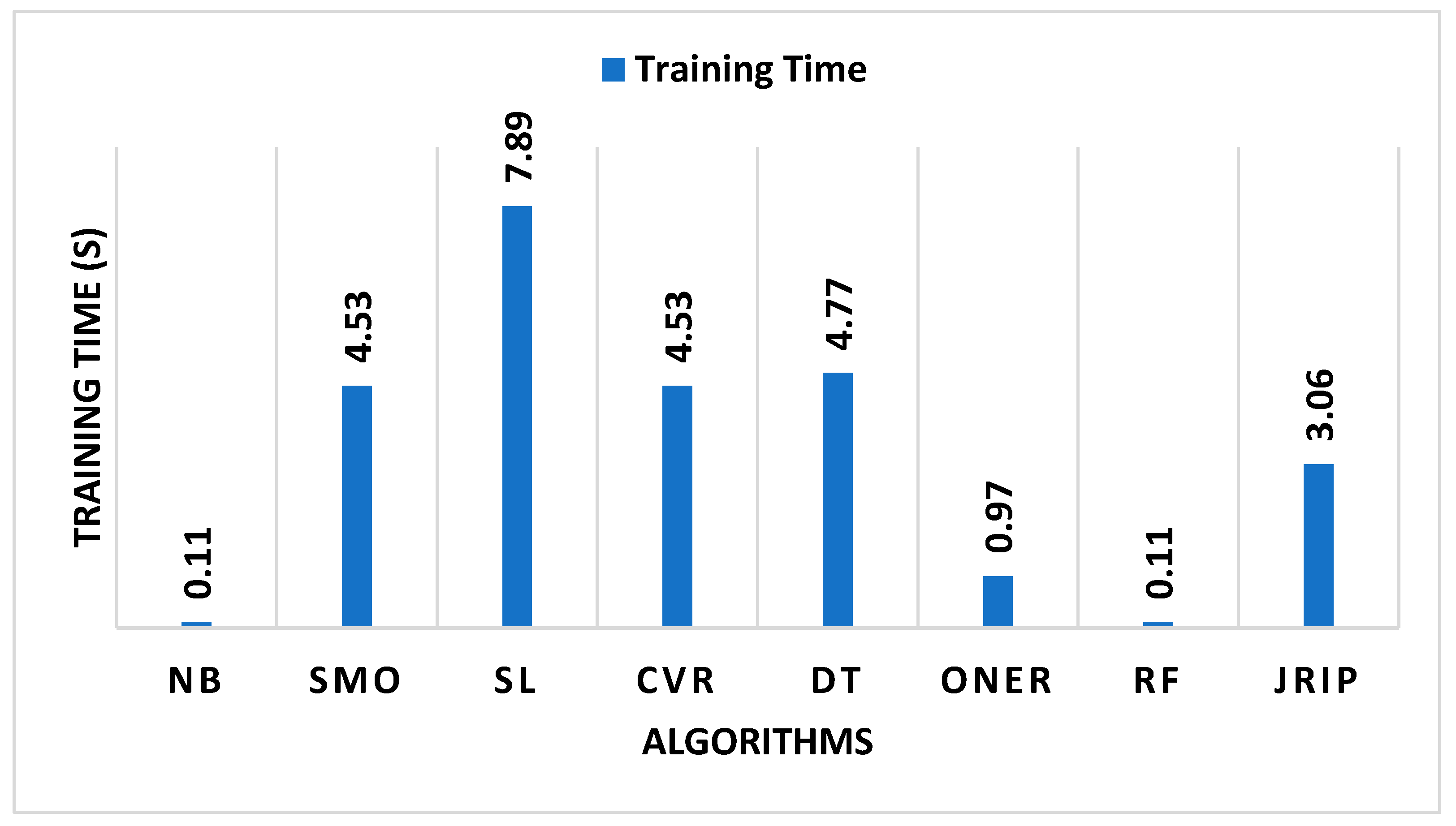
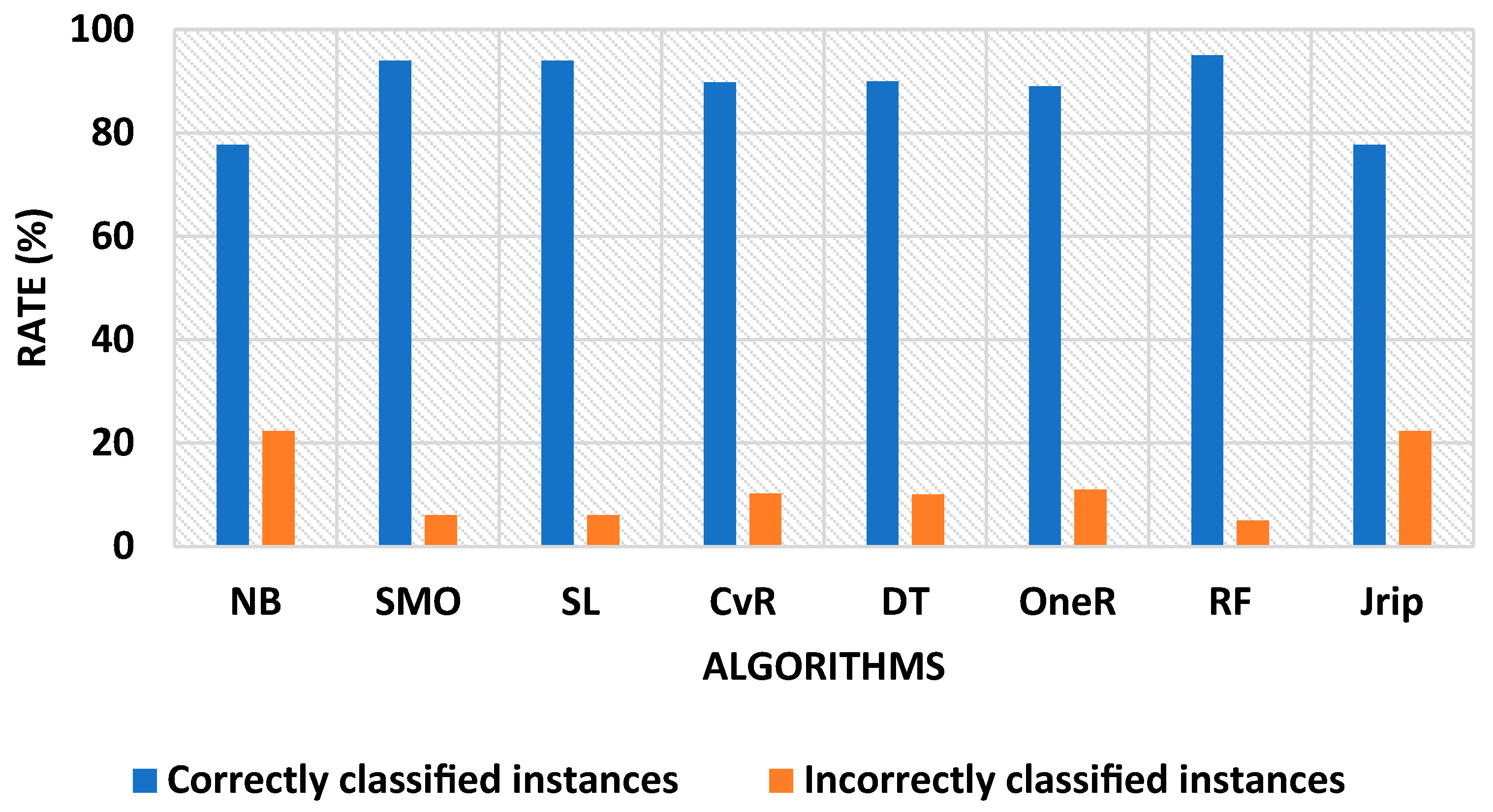
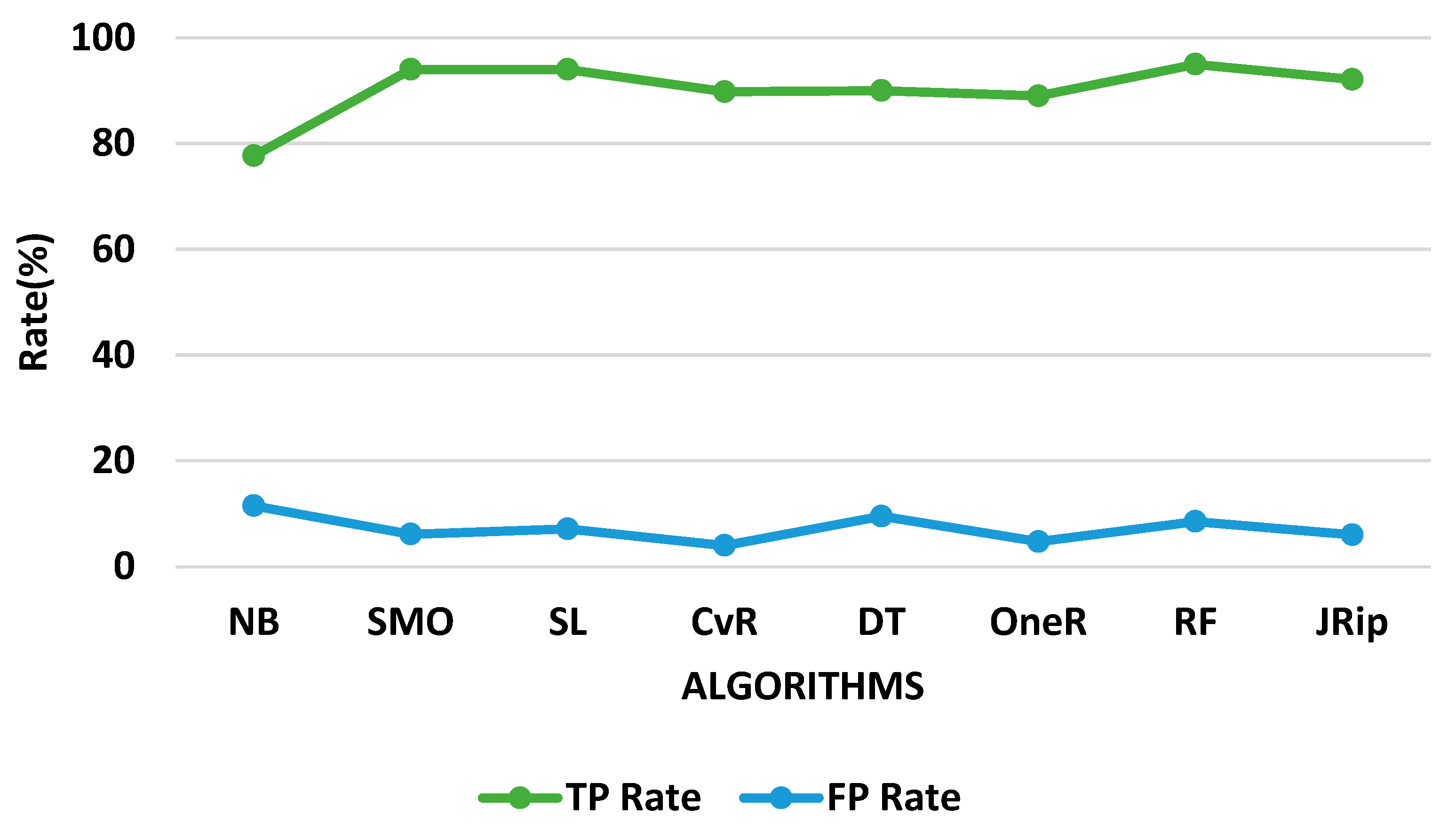
| Algorithms | Training Time (s) | Correctly Classified Instances (%) | Incorrectly Classified Instances (%) | FP Rate (%) | TP Rate (%) |
|---|---|---|---|---|---|
| Naïve Bayes | 0.11 | 77.6699 | 22.3301 | 11.5 | 77.7 |
| SMO | 4.53 | 93.9806 | 6.0194 | 6.1 | 94.0 |
| Simple Logistic | 7.89 | 93.9806 | 6.0194 | 7.1 | 94.0 |
| Classification via Regression | 4.53 | 89.8058 | 10.1942 | 4.0 | 89.8 |
| Decision Table | 4.77 | 90.0 | 10.0 | 9.5 | 90.0 |
| OneR | 0.97 | 89.0291 | 10.9709 | 4.7 | 89.0 |
| Random Forest | 0.11 | 95.0485 | 4.9515 | 8.5 | 95.0 |
| JRip | 3.06 | 77.6699 | 22.3301 | 6.0 | 92.1 |
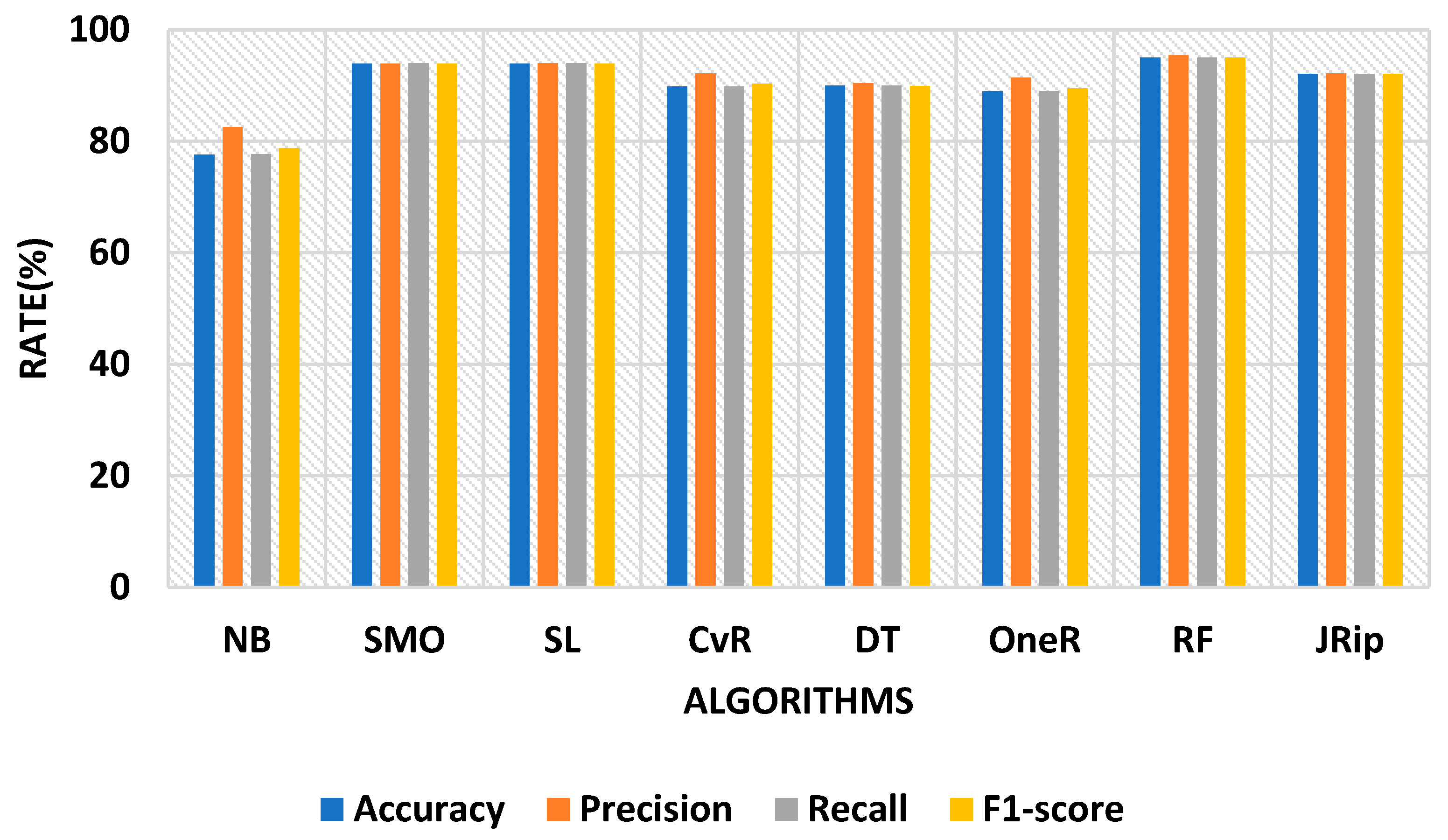
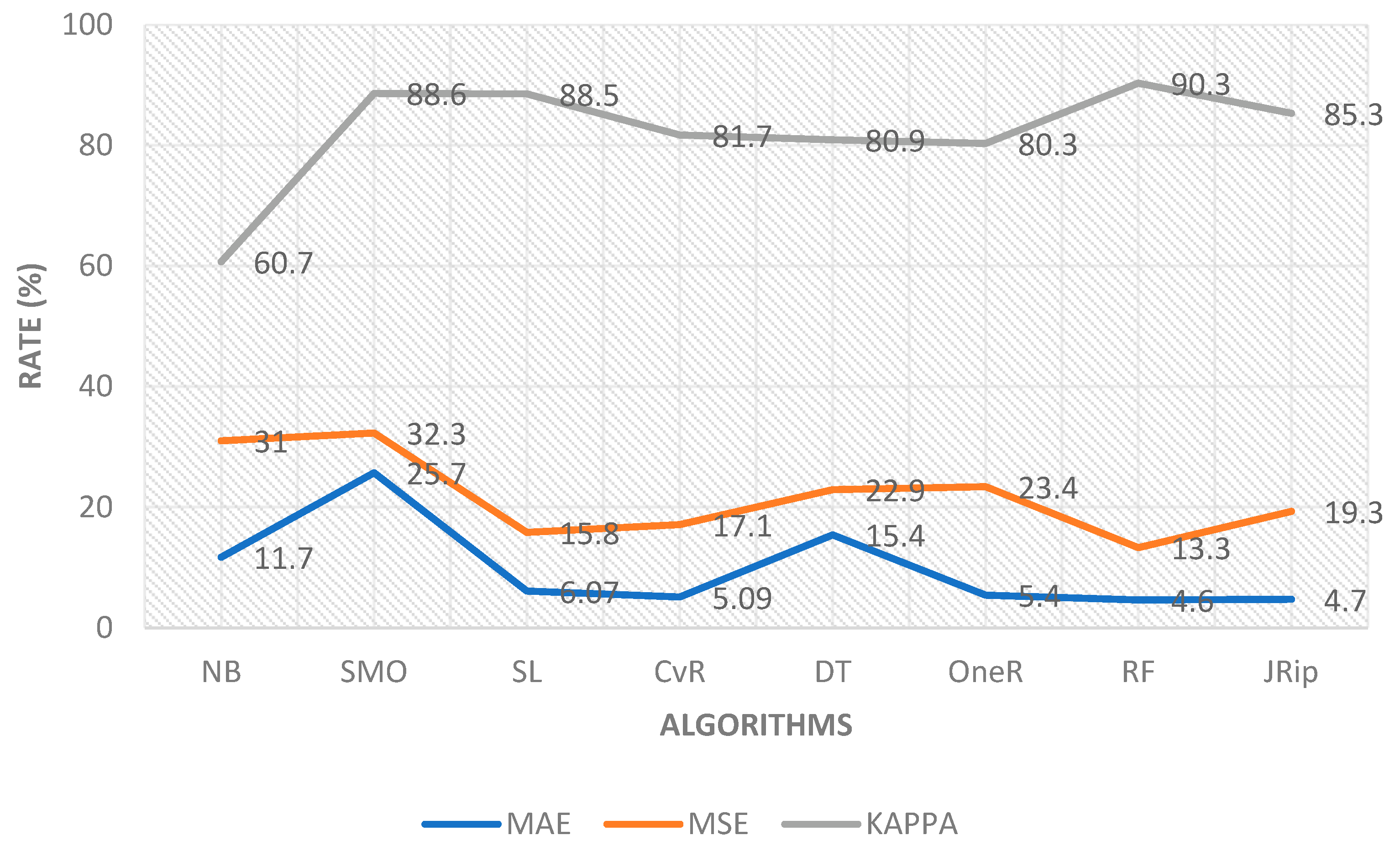
| Algorithms | Accuracy (%) | Precision (%) | Recall (%) | F-Measure (%) | MSE | MAE | KAPPA |
|---|---|---|---|---|---|---|---|
| Naïve Bayes | 77.6 | 82.5 | 77.7 | 78.8 | 31.0 | 11.7 | 60.7 |
| SMO | 93.9 | 93.9 | 94.0 | 93.9 | 32.3 | 25.7 | 88.6 |
| Simple Logistic | 93.9 | 94.0 | 94.0 | 93.9 | 15.8 | 6.07 | 88.5 |
| CvR | 89.8 | 92.2 | 89.8 | 90.3 | 17.1 | 5.09 | 81.7 |
| Decision Table | 90.0 | 90.4 | 90.0 | 89.9 | 22.9 | 15.4 | 80.9 |
| OneR | 89.0 | 91.4 | 89.0 | 89.5 | 23.4 | 05.4 | 80.3 |
| Random Forest | 95.0 | 95.4 | 95.0 | 95.0 | 13.3 | 04.6 | 90.3 |
| JRip | 92.1 | 92.2 | 92.1 | 92.1 | 19.3 | 04.7 | 85.3 |
| Algorithms | Sensitivity (%) | Specificity (%) | MCC (%) | AROC (%) |
|---|---|---|---|---|
| Naïve Bayes | 83.9 | 69.8 | 64.5 | 91.5 |
| SMO | 95.9 | 91.2 | 88.7 | 94.1 |
| Simple Logistic | 95.9 | 91.2 | 88.6 | 97.8 |
| Classification via Regression | 92.9 | 85.4 | 83.5 | 98.1 |
| Decision Table | 93.1 | 85.7 | 82.0 | 96.0 |
| OneR | 92.4 | 84.3 | 82.1 | 92.2 |
| Random Forest | 96.6 | 92.7 | 90.3 | 99.5 |
| JRip | 94.6 | 88.6 | 86.0 | 93.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohail, R.; Saeed, Y.; Ali, A.; Alkanhel, R.; Jamil, H.; Muthanna, A.; Akbar, H. A Machine Learning-Based Intelligent Vehicular System (IVS) for Driver’s Diabetes Monitoring in Vehicular Ad-Hoc Networks (VANETs). Appl. Sci. 2023, 13, 3326. https://doi.org/10.3390/app13053326
Sohail R, Saeed Y, Ali A, Alkanhel R, Jamil H, Muthanna A, Akbar H. A Machine Learning-Based Intelligent Vehicular System (IVS) for Driver’s Diabetes Monitoring in Vehicular Ad-Hoc Networks (VANETs). Applied Sciences. 2023; 13(5):3326. https://doi.org/10.3390/app13053326
Chicago/Turabian StyleSohail, Rafiya, Yousaf Saeed, Abid Ali, Reem Alkanhel, Harun Jamil, Ammar Muthanna, and Habib Akbar. 2023. "A Machine Learning-Based Intelligent Vehicular System (IVS) for Driver’s Diabetes Monitoring in Vehicular Ad-Hoc Networks (VANETs)" Applied Sciences 13, no. 5: 3326. https://doi.org/10.3390/app13053326
APA StyleSohail, R., Saeed, Y., Ali, A., Alkanhel, R., Jamil, H., Muthanna, A., & Akbar, H. (2023). A Machine Learning-Based Intelligent Vehicular System (IVS) for Driver’s Diabetes Monitoring in Vehicular Ad-Hoc Networks (VANETs). Applied Sciences, 13(5), 3326. https://doi.org/10.3390/app13053326