Comparative Study of the Antibacterial Activity, Total Phenolic and Total Flavonoid Content of Nine Hypericum Species Grown in Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Extracts
2.3. Quantification of Total Phenolic Content
2.4. Quantification of Total Flavonoid Content
2.5. Antibacterial Activity
2.6. Statistical Analysis
3. Results
3.1. Total Phenolic and Total Flavonoid Content
3.2. Antimicrobial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2011; Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net); ECDC: Stockholm, Sweden, 2012. [Google Scholar]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A brief review of phenolic compounds identified from plants: Their extraction, analysis, and biological activity. Nat. Prod. Commun. 2022, 17, 1934578X211069721. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 29, 162750. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Future J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative structure–activity relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Ecevit, K.; Barros, A.A.; Silva, J.M.; Reis, R.L. Preventing Microbial Infections with Natural Phenolic Compounds. Future Pharmacol. 2022, 2, 460–498. [Google Scholar] [CrossRef]
- Robson, N.K.B. Studies in the genus Hypericum L. (Hypericaceae) 5(1). Sections 10. Olympia to 15/16 Crossophyllum. Phytotaxa 2010, 4, 5–126. [Google Scholar] [CrossRef]
- Crockett, S.L.; Robson, N.K. Taxonomy and Chemotaxonomy of the Genus Hypericum. Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 1–13. [Google Scholar]
- Linden, M.; Wurzendorf, K.; Ploch, M.; Schaefer, M. Self medication with St. John’s wort in depressive disorders: An observational study in community pharmacies. J. Affect. Disord. 2008, 107, 205–210. [Google Scholar] [CrossRef]
- Lewis, S.; Willis, K.; Kokanovic, R.; Pirotta, M. ‘I’m managing myself’: How and why people use St John’s wort as a strategy to manage their mental health risk. Health Risk Soc. 2015, 17, 439–457. [Google Scholar] [CrossRef]
- Solati, K.; Karimi, M.; Rafieian-Kopaei, M.; Abbasi, N.; Abbaszadeh, S.; Bahmani, M. Phytotherapy for wound healing: The most important herbal plants in wound healing based on Iranian ethnobotanical documents. Mini. Rev. Med. Chem. 2021, 21, 500–519. [Google Scholar] [CrossRef]
- Gunther, R. Dioscorides, ‘De Materia Medica’, The Greek Herbal of Dioscordes; Hafner Publishing Co.: New York, NY, USA, 1968. [Google Scholar]
- Öztürk, N.; Tunçel, M.; Potoğlu-Erkara, İ. Phenolic compounds and antioxidant activities of some Hypericum species: A comparative study with H. perforatum. Pharm. Biol. 2009, 47, 120–127. [Google Scholar] [CrossRef]
- Babotă, M.; Frumuzachi, O.; Mocan, A.; Tămaș, M.; Dias, M.I.; Pinela, J.; Stojković, D.; Soković, M.; Bădărău, A.S.; Crișan, G.; et al. Unravelling Phytochemical and Bioactive Potential of Three Hypericum Species from Romanian Spontaneous Flora: H. alpigenum, H. perforatum and H. rochelii. Plants 2022, 11, 2773. [Google Scholar] [CrossRef]
- Tanaka, N.; Kashiwada, Y. Characteristic metabolites of Hypericum plants: Their chemical structures and biological activities. J. Nat. Med. 2021, 75, 423–433. [Google Scholar] [CrossRef]
- Oliveira, A.I.; Pinho, C.; Sarmento, B.; Dias, A.C. Neuroprotective Activity of Hypericum perforatum and Its Major Components. Front. Plant Sci. 2016, 7, 1004. [Google Scholar] [CrossRef]
- Saddiqe, Z.; Naeem, I.; Maimoona, A. A review of the antibacterial activity of Hypericum perforatum L. J. Ethnopharmacol. 2010, 131, 511–521. [Google Scholar] [CrossRef]
- Ilieva, Y.; Marinov, T.; Trayanov, I.; Kaleva, M.; Zaharieva, M.M.; Yocheva, L.; Kokanova-Nedialkova, Z.; Najdenski, H.; Nedialkov, P. Outstanding Antibacterial Activity of Hypericum rochelii-Comparison of the Antimicrobial Effects of Extracts and Fractions from Four Hypericum Species Growing in Bulgaria with a Focus on Prenylated Phloroglucinols. Life 2023, 13, 274. [Google Scholar] [CrossRef]
- Dall’Agnol, R.; Ferraz, A.; Bernardi, A.P.; Albring, D.; Nör, C.; Sarmento, L.; Lamb, L.; Hass, M.; von Poser, G.; Schapoval, E.E. Antimicrobial activity of some Hypericum species. Phytomedicine 2003, 10, 511–516. [Google Scholar] [CrossRef]
- Kakouri, E.; Kanakis, C.; Trigas, P.; Tarantilis, P.A. Characterization of the chemical composition of Drimianumidica plant parts using high-resolution mass spectrometry: Study of their total phenolic content and antioxidant activity. Anal. Bioanal. Chem. 2019, 411, 3135–3150. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia 7th Edition (7.5). Ref. 07/2008:1874. Available online: https://file.wuxuwang.com/yaopinbz/EP6/EP6.2_01__114.pdf (accessed on 30 January 2023).
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Charalambous, D.; Eliades, N.-G.H.; Christoforou, M.; Kakouri, E.; Kanakis, C.; Tarantilis, P.A.; Pantelidou, M. Chemical Characterization, Antioxidant and Antimicrobial Properties of Different Types of Tissue of Cedrusbrevifolia Henry Extracts. Molecules 2022, 27, 2717. [Google Scholar] [CrossRef] [PubMed]
- Santos-Buelga, C.; García-Viguera, C.; Tomás-Barberán, F.A. On-line identification of flavonoids by HPLC coupled to diode array detection. In Methods in Polyphenol Analysis, 1st ed.; Santos-Buelga, C., Williamson, G., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2003; pp. 92–127. [Google Scholar]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B.; Mabry, T.J.; Markham, K.R.; Thomas, M.B. The ultraviolet spectra of flavones and flavonols. In The Systematic Identification of Flavonoids, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1970; pp. 41–164. [Google Scholar]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B.J. Thorough Study of Reactivity of Various Compound Classes toward the Folin-Ciocalteu Reagent. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Mammen, D.; Daniel, M. A critical evaluation on the reliability of two aluminum chloride chelation methods for quantification of flavonoids. Food Chem. 2012, 135, 1365–1368. [Google Scholar] [CrossRef]
- Hollman, P.C. Absorption, bioavailability, and metabolism of flavonoids. Pharm. Biol. 2004, 42 (Suppl. S1), 74–83. [Google Scholar] [CrossRef]
- Aligiannis, N.; Kalpoutzakis, E.; Mitaku, S.; Chinou, I.B. Composition and antimicrobial activity of the essential oils of two Origanum species. J. Agric. Food Chem. 2001, 49, 4168–4170. [Google Scholar] [CrossRef]
- Fabry, W.; Okemo, P.O.; Ansorg, R. Antibacterial activity of East African medicinal plants. J. Ethnopharmacol. 1998, 60, 79–84. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Shah, S.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Li, F.S.; Weng, J.K. Demystifying traditional herbal medicine with modern approach. Nat. Plants 2017, 3, 17109. [Google Scholar] [CrossRef] [PubMed]
- Alahmad, A.; Alghoraibi, I.; Zein, R.; Kraft, S.; Dräger, G.; Walter, J.G.; Scheper, T. Identification of Major Constituents of Hypericum perforatum L. Extracts in Syria by Development of a Rapid, Simple, and Reproducible HPLC-ESI-Q-TOF MS Analysis and Their Antioxidant Activities. ACS Omega 2022, 7, 13475–13493. [Google Scholar] [CrossRef]
- Bridi, H.; de Carvalho Meirelles, G.; von Poser, G.L. Structural diversity and biological activities of phloroglucinol derivatives from Hypericum species. Phytochemistry 2018, 155, 203–232. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Meirelles, G.; Bridi, H.; Rates, S.M.K.; von Poser, G.L. Southern brazilian Hypericum species, promising sources of bioactive metabolites. Stud. Nat. Prod. Chem. 2018, 59, 491–507. [Google Scholar]
- Liu, J.; Du, C.; Beaman, H.T.; Monroe, M.B.B. Characterization of phenolic acid antimicrobial and antioxidant structure–property relationships. Pharmaceutics 2020, 12, 419. [Google Scholar] [CrossRef]
- Saddiqe, Z.; Naeem, I.; Hellio, C.; Patel, A.V.; Abbas, G. Phytochemical profile, antioxidant and antibacterial activity of four Hypericum species from the UK. S. Afr. J. Bot. 2020, 133, 45–53. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. St John’s wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2001, 53, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Ion, V.; Ielciu, I.; Cârje, A.G.; Muntean, D.L.; Crişan, G.; Păltinean, R. Hypericum spp.—An Overview of the Extraction Methods and Analysis of Compounds. Separations 2022, 9, 17. [Google Scholar] [CrossRef]
- Rusalepp, L.; Raal, A.; Puessa, T.; Maeeorg, U. Comparison of chemical composition of Hypericum perforatum and H. maculatum in Estonia. Biochem. Syst. Ecol. 2017, 73, 41–46. [Google Scholar] [CrossRef]
- Daskalaki, A.; Grafakou, M.E.; Barda, C.; Kypriotakis, Z.; Heilmann, J.; Skaltsa, H. Secondary metabolites from Hypericum trichocaulon Boiss. & Heldr., growing wild in the island of crete. Biochem. Syst. Ecol. 2021, 97, 104294. [Google Scholar]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liang, S.; Zhang, M.; Wang, Z.; Wang, Z.; Ren, X. The effect of chlorogenic acid on Bacillus subtilis based on metabolomics. Molecules 2020, 25, 4038. [Google Scholar] [CrossRef]
- Cecchini, C.; Cresci, A.; Coman, M.M.; Ricciutelli, M.; Sagratini, G.; Vittori, S.; Lucarini, D.; Maggi, F. Antimicrobial activity of seven hypericum entities from central Italy. Planta Med. 2007, 73, 564–566. [Google Scholar] [CrossRef]
- Avato, P.; Raffo, F.; Guglielmi, G.; Vitali, C.; Rosato, A. Extracts from St John’s Wort and their antimicrobial activity. Phytother. Res. 2004, 18, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Radulović, N.; Stankov-Jovanović, V.; Stojanović, G.; Šmelcerović, A.; Spiteller, M.; Asakawa, Y. Screening of in vitro antimicrobial and antioxidant activity of nine Hypericum species from the Balkans. Food Chem. 2007, 103, 15–21. [Google Scholar] [CrossRef]
- Gagetti, P.; Bonofiglio, L.; García Gabarrot, G.; Kaufman, S.; Mollerach, M.; Vigliarolo, L.; Specht, M.; Toresani, I.; Lopardo, H.A. Resistencia a los β-lactámicosenenterococos. Rev. Argent. Microbiol. 2019, 51, 179–183. [Google Scholar] [PubMed]
- Feßler, A.T.; Scholtzek, A.D.; Schug, A.R.; Kohn, B.; Weingart, C.; Hanke, D.; Schink, A.-K.; Bethe, A.; Lübke-Becker, A.; Schwarz, S. Antimicrobial and Biocide Resistance among Canine and Feline Enterococcus faecalis, Enterococcus faecium, Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii Isolates from Diagnostic Submissions. Antibiotics 2022, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Del Monte, D.; De Martino, L.; Marandino, A.; Fratianni, F.; Nazzaro, F.; De Feo, V. Phenolic content, antimicrobial and antioxidant activities of Hypericum perfoliatum L. Ind. Crops Prod. 2015, 74, 342–347. [Google Scholar] [CrossRef]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef]
- Kızıl, G.; Kızıl, M.; Yavuz, M.; Emen, S.; Hakimoğlu, F. Antioxidant Activities of Ethanol Extracts of Hypericum triquetrifolium. and Hypericum scabroides. Pharm. Biol. 2008, 46, 231–242. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Nedialkov, P.; Kitanov, G. Radical scavenging and antioxidant activities of methanolic extracts from Hypericum species growing in Bulgaria. Pharmacogn. Mag. 2010, 6, 74. [Google Scholar] [CrossRef] [PubMed]
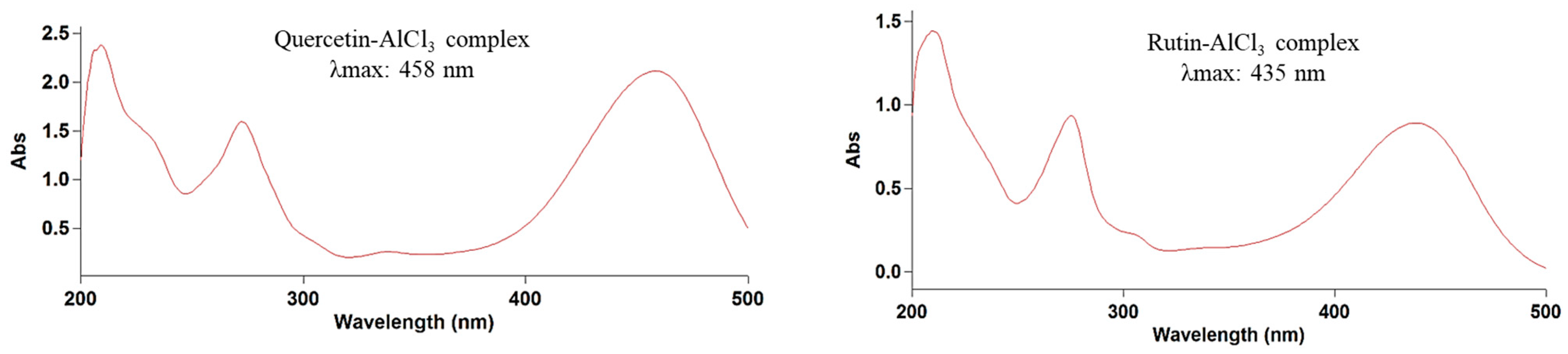
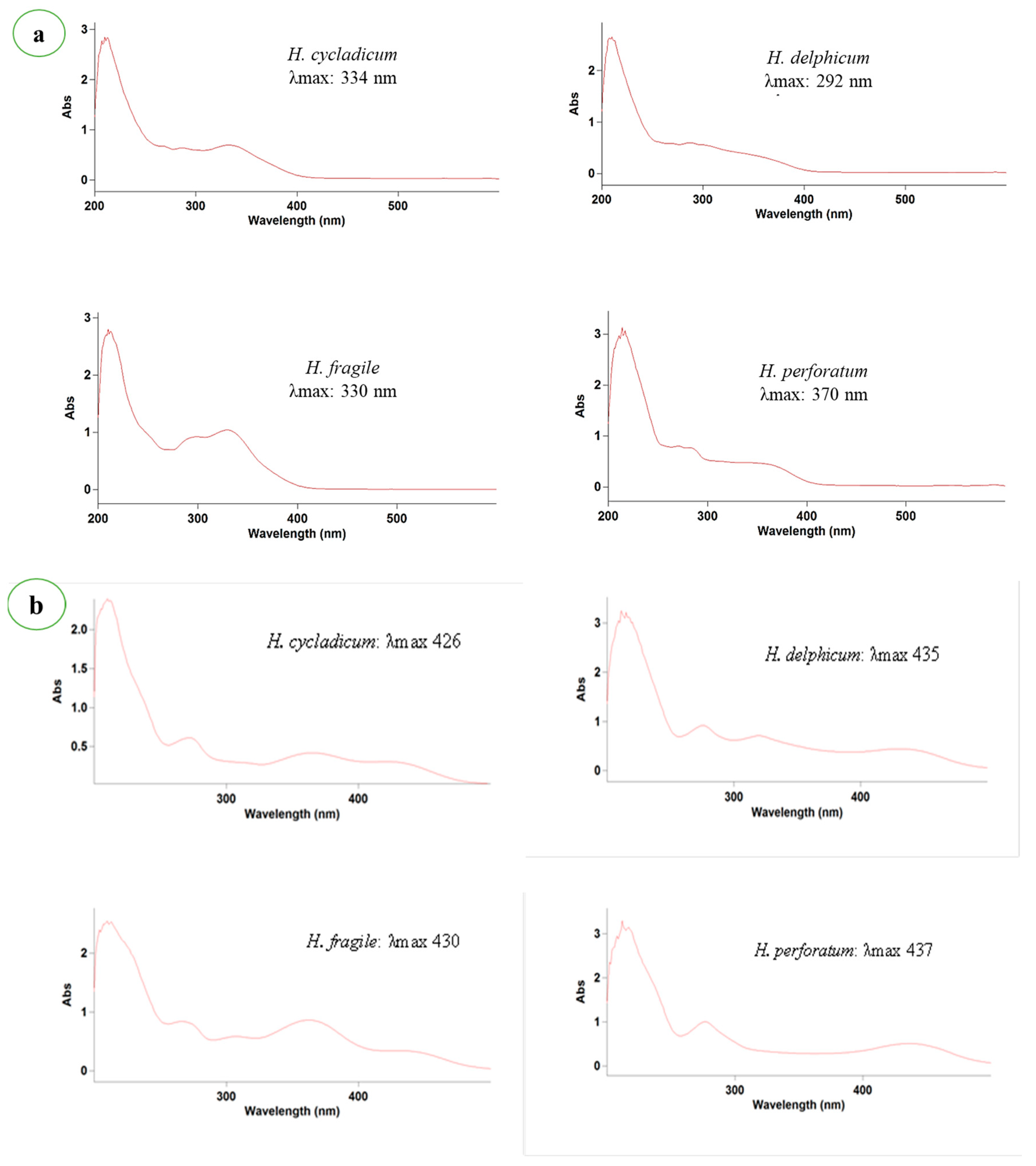
| Taxon | Section | Collection Site | Collection Date | Latitude | Longitude | Elevation (m) | Habitat | Voucher Number |
|---|---|---|---|---|---|---|---|---|
| H. cycladicum | Drosocarpium | Andros Island | 12 May2019 | 37°54′ | 24°52′ | 30 | Phrygana | 12285 |
| H. delphicum | Adenosepalum | Evvia Island | 14 June2019 | 38°53′ | 23°52′ | 1000 | Forest clearings | 12281 |
| H. fragile | Taeniocarpium | Evvia Island | 15 June 2019 | 38°32′ | 24°01′ | 420 | Cliffs | 12283 |
| H. olympicum | Olympia | Evvia Island | 14 June 2019 | 38°36′ | 23°51′ | 890 | Rocky slopes | 12288 |
| H. perfoliatum | Drosocarpium | Mt. Parnon | 1 June 2019 | 37°15′ | 22°39′ | 1050 | Forest | 12282 |
| H. perforatum | Hypericum | Andros Island | 8 June 2019 | 37°50′ | 24°53′ | 560 | Rocky slopes | 12280 |
| H. rumeliacum subsp. apollinis | Drosocarpium | Mt. Parnassos | 27 May 2019 | 38°33′ | 22°34′ | 1760 | Rocky slopes | 12286 |
| H. tetrapterum | Hypericum | Evvia Island | 14 June 2019 | 38°36′ | 23°51′ | 910 | Wet places | 12287 |
| H. vesiculosum | Drosocarpium | Mt. Chelmos | 5 June 2022 | 38°05′ | 22°10′ | 910 | Woodland | 12284 |
| Species | TPC ± SD | TFC ± SD |
|---|---|---|
| mg GAE/g dry plant material | mg RE/g dry plant material | |
| H. perfoliatum | 59.31 ± 16.47 a,* | 0.21 ± 0.14 A |
| H. rumeliacum subsp. apollinis | 54.87 ± 2.73 a | 1.18 ± 0.22 B |
| H. vesiculosum | 26.92 ± 10.32 b | 0.30 ± 0.12 C |
| H. cycladicum | 54.59 ± 8.55 a | 1.34 ± 0.05 D |
| H. perforatum | 86 ± 13.34 c | 0.76 ± 0.11 E |
| H. tetrapterum | 51.26 ± 21.36 a | 1.58 ± 0.08 F |
| H. fragile | 39.72 ± 6.05 d | 0.54 ± 0.12 G |
| H. olympicum | 32.63 ± 17.42 b | 0.64 ± 0.09 H |
| H. delphicum | 54.09 ± 4.29 a | 0.6 ± 0.17 I |
| Hypericum Species Extracts | E. coli | S. enteritidis | S. aureus * | E. faecalis ** | ||||
|---|---|---|---|---|---|---|---|---|
| MIC 2 (mg/mL) ± SD | MBC 3 (mg/mL) ± SD | MIC 2 (mg/mL) ± SD | MBC 3 (mg/mL) ± SD | MIC 2 (mg/mL) ± SD | MBC 3 (mg/mL) ± SD | MIC 2 (mg/mL) ± SD | MBC 3 (mg/mL) ± SD | |
| H. perfoliatum | - | - | - | - | 1.25 ± 0.02 a | 1.25 ± 0.02 A | 1.25 ± 0.02 a | 1.25 ± 0.02 A |
| H. rumeliacum subsp. apollinis | - | - | - | - | 1.25 ± 0.02 a | 1.25 ± 0.02 A | 1.25 ± 0.02 a | 5.00 ± 0.04 B |
| H. vesiculosum | - | - | - | - | 1.25 ± 0.02 a | 2.50 ± 0.03 B | 1.25 ± 0.04 a | 5.00 ± 0.05 B |
| H. cycladicum | - | - | - | - | 0.31 ± 0.04 b | 1.25 ± 0.04 A | 0.6 ± 0.05 b | 1.25 ± 0.02 A |
| H. perforatum | - | - | - | - | 0.06 ± 0.01 c | 0.51 ± 0.06 C | 0.13 ± 0.02 c | 0.51 ± 0.05 C |
| H. tetrapterum | - | - | - | - | 1.25 ± 0.03 a | 1.25 ± 0.03 A | 1.25 ± 0.05 a | 2.50 ± 0.24 D |
| H. fragile | - | - | - | - | 1.75 ± 0.05 d | 1.75 ± 0.05 D | 3.50 ± 0.06 d | 5.00 ± 0.06 B |
| H. olympicum | - | - | - | - | 0.31 ± 0.05 b | 1.25 ± 0.04 A | 0.63 ± 0.04 b | 2.50 ± 0.05 D |
| H. delphicum | - | - | - | - | 0.16 ± 0.02 e | 0.63 ± 0.05 E | 0.31 ± 0.03 e | 0.63 ± 0.03 E |
| Amp 1 | 0.03 ± 0.002 | 0.03 ± 0.002 | 0.02 ± 0.001 | 0.02 ± 0.001 | NA | NA | NA | NA |
| Gen 1 | NA | NA | NA | NA | 0.02 ± 00.01 | 0.02 ± 0.001 | 0.02 ± 0.003 | 0.02 ± 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakouri, E.; Daferera, D.; Trigas, P.; Charalambous, D.; Pantelidou, M.; Tarantilis, P.A.; Kanakis, C.D. Comparative Study of the Antibacterial Activity, Total Phenolic and Total Flavonoid Content of Nine Hypericum Species Grown in Greece. Appl. Sci. 2023, 13, 3305. https://doi.org/10.3390/app13053305
Kakouri E, Daferera D, Trigas P, Charalambous D, Pantelidou M, Tarantilis PA, Kanakis CD. Comparative Study of the Antibacterial Activity, Total Phenolic and Total Flavonoid Content of Nine Hypericum Species Grown in Greece. Applied Sciences. 2023; 13(5):3305. https://doi.org/10.3390/app13053305
Chicago/Turabian StyleKakouri, Eleni, Dimitra Daferera, Panayiotis Trigas, Despina Charalambous, Maria Pantelidou, Petros A. Tarantilis, and Charalabos D. Kanakis. 2023. "Comparative Study of the Antibacterial Activity, Total Phenolic and Total Flavonoid Content of Nine Hypericum Species Grown in Greece" Applied Sciences 13, no. 5: 3305. https://doi.org/10.3390/app13053305
APA StyleKakouri, E., Daferera, D., Trigas, P., Charalambous, D., Pantelidou, M., Tarantilis, P. A., & Kanakis, C. D. (2023). Comparative Study of the Antibacterial Activity, Total Phenolic and Total Flavonoid Content of Nine Hypericum Species Grown in Greece. Applied Sciences, 13(5), 3305. https://doi.org/10.3390/app13053305









