Abstract
The characteristics of elderberries and their flowers as well as selected properties of chlorogenic acid related to phenolic compounds are described. The aim of this study was to optimize the content of chlorogenic acid in ethanol extracts of elderberry flowers using the response surface methodology (RSM). The experiment was carried out with four research variants: fresh raw material treated with warm ethanol (50 °C) or cold ethanol (25 °C) and the same procedure for raw material that was frozen for a month and then dried. For each variant, nine methods of obtaining extracts were prepared using three different ethanol concentrations by volume (40, 68 or 95%) and three different extraction times (7, 20 or 30 days). Higher contents of chlorogenic acid were found in variants treated with warm ethanol (106.3 to 384.8 µg/cm3) in comparison to the samples treated with cold ethanol (60.77 to 298.3 µg/cm3). Optimization models of the response surface showed that with small losses of efficiency, the extraction process can be carried out for up to 20 days (instead of 30) with the use of ethanol with a concentration of approx. 68% (instead of 95%). The optimization of the efficiency of technological processes in the processing of herbs can be supported by the use of the response surface methodology.
1. Introduction
Elderberries are shrubs or small trees from the drill family that grow up to several meters. Their branches are filled with a white core, and their trunks are covered with gray or brown bark. The leaves on short petioles are large, composed of 5–7 opposite elliptical leaves with finely serrated edges. Elderberries bloom from May to June. Small flowers are creamy white in color; set on long stalks and are collected in large, flat inflorescences in the shape of umbels, i.e., they consist of flowers growing on stalks of the same length. They have a characteristic and intense smell. The fruit is a purple-black drupe with 3–6 seeds [1].
Elderberry flowers are hermaphrodites collected in large and flat umbel-shaped inflorescences with diameters of up to 15–20 cm. Single flowers are white or cream and small (about 5 mm in diameter), do not have nectaries and are characterized by short, greenish calyxes with five tepals and a yellowish or greyish-white crown composed of five roundish petals. The anthers are light yellow and elliptical, and the stamens are arranged between the petals. The flowers are characterized by an intoxicating, insipid smell and a slimy, sweet taste that attracts pollinating insects [2].
The chemical compositions of individual elements of elderberry (Sambucus nigra L.) are diverse and depend, among others, on the variety, the degree of maturity, the climatic conditions and the place where the raw material was obtained. In the case of flowers, an important factor affecting the contents of active ingredients is the flowering period in which the harvest was made [3]. Elderberry flowers are classified as herbal raw materials with high contents of bioactive ingredients.
Elderberry flowers are a rich source of flavonoids (1.5–4.0%). Their main representatives in this raw material include, e.g., quercetin, astralagine, rutin and hyperoside. These are compounds that, due to the presence of many hydroxyl groups in the molecule, have a strong antioxidant effect, protecting the cell against oxidative stress because, among other effects, they are able to capture reactive oxygen species (ROS), chelate and reduce transition metal ions, e.g., iron, thus preventing the formation of a reactive oxidative radical in the cell. In particular, quercetin and rutin prevent the oxidation of vitamin C, increasing its absorption from the digestive tract and stabilizing the ascorbic acid molecule. In addition, rutin, together with vitamin C, improves elasticity and strengthens blood vessels [4].
The contents of bioactive components in plant extracts are significantly affected by the type of extracting agent, its temperature and the method of preparing the raw material for extraction.
When writing about biologically active substances found in plant materials, in particular herbal ones, they can be defined as components containing medicinal and nutritional substances that are usually present in plants in small amounts [5]. Biologically active substances include phenolic compounds, including flavonoids, phenolic acids and tannins. Flavonoids are one of the most important groups of polyphenols, which due to their diverse chemical structures, perform many functions in plants [6]. The second group of phenolic compounds distinguished by their bioactive properties are phenolic acids [7].
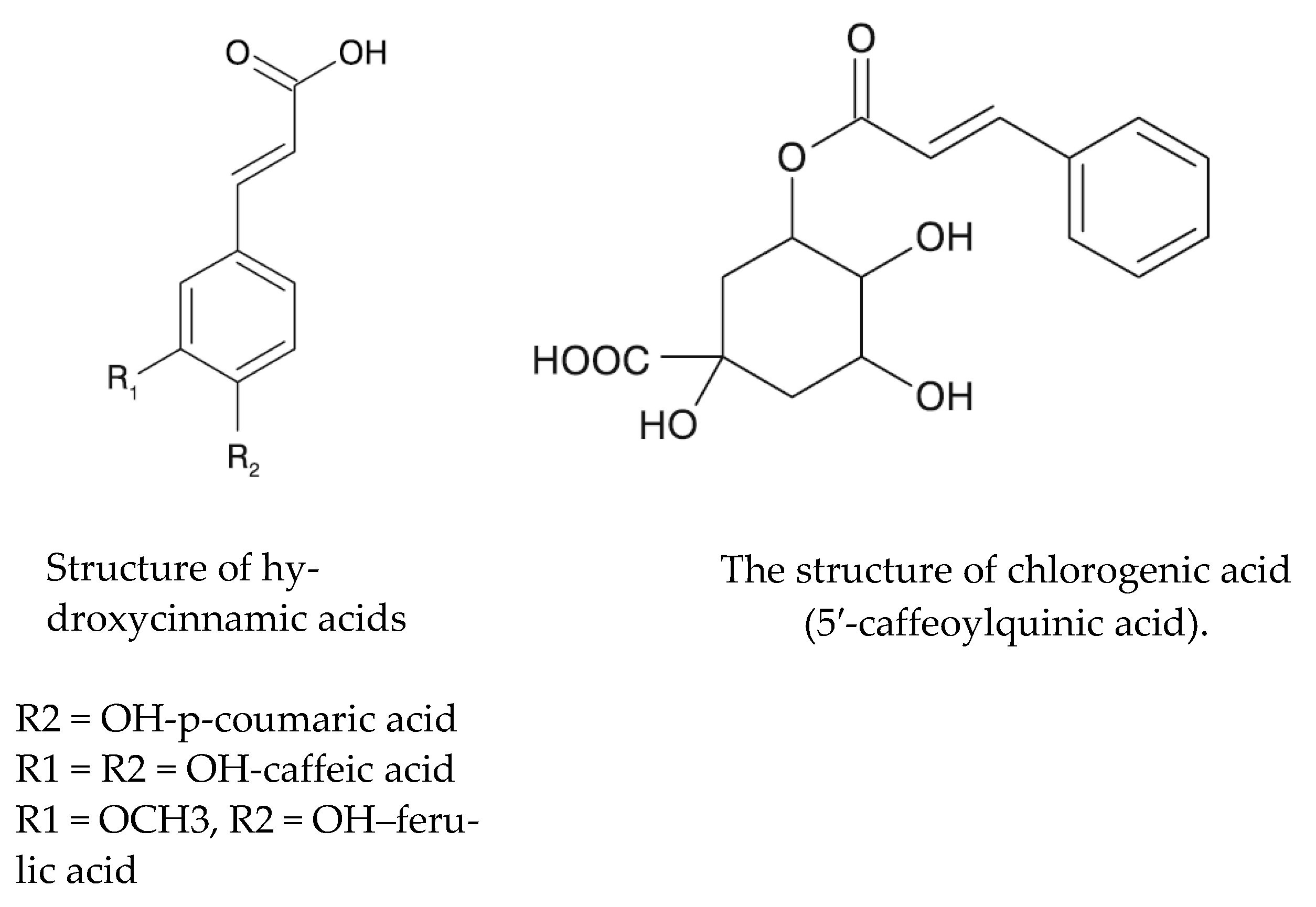
Phenolic compounds can be divided into simple phenols (phenol, thymol, carvacrol, eugenol, myristicin, anethole, guaiacol and hydroquinone), alcoholphenols, aldehydehydephenols (anisaldehyde and vanillin), phenolic acids (salicylic acid and caffeic acid), depsides (chlorogenic acid and cynarin) and phenolic glycosides (arbutin) [8]. Caffeic acid is one of the most common phenolic compounds found in most flowering plants, especially in the form of a depside called chlorogenic acid (Figure 1). In turn, the latter compound is formed as a result of combining the carboxyl group of caffeic acid with the phenolic group of quinic acid. It is found in large amounts in coffee (Coffea robusta), cocoa seeds, chokeberry and blueberry fruits, mulberry fruit and leaves, and herbal spices [6] as well as in elderberry flowers. It belongs to a group of strong antioxidants. It increases the sensitivity of cells to insulin, probably reduces the risk of diabetes and has choleretic properties [8].
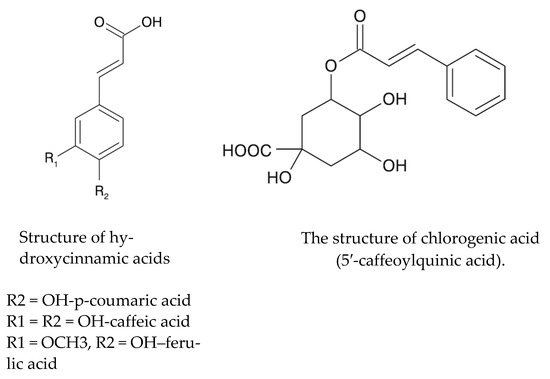
Figure 1.
Structures of hydroxycinnamic acids and chlorogenic acid [6].
Chlorogenic acid is mainly known for its anticarcinogenic, antioxidant and anti-inflammatory properties [9]. Many epidemiologic studies have also focused on chlorogenic acid in the context of its health benefits [10,11]. It has been proposed to have benefits in obesity [12], type 2 diabetes (T2DM) [13], stroke [14] and Alzheimer’s disease [15] and on endothelial function as well as blood pressure [16,17]. Another study revealed the adverse effects of CGA, including headache, diarrhea and complications in higher doses for a person with a sensitive stomach [18]. However, no comprehensive investigation has been conducted regarding the side effects of chlorogenic acid.
Chlorogenic acid, as mentioned, plays an important role in the prevention of cancer. Experiments conducted on animals proved the high level of effectiveness of chlorogenic acid as a means of protecting liver cells against poisoning with carbon tetrachloride and cobalt and cadmium isotopes [19]. In the same study, it was shown that caffeic, chlorogenic, ferulic, ellagic and gallic acids have the ability to block carcinogenic compounds formed by metabolic transformations of some carcinogenic substances, e.g., 4-nitroquinoline-1-oxides. According to other authors [20], chlorogenic, chicoric, caffeic and cynarin acids can be used as agents against skin damage caused by UV-A and UV-B radiation, which is the result of an action preventing collagen degradation.
It is worth considering that in such a complex system as the various biologically active compounds present in the plant (herbal) raw material, it is not possible to clearly indicate a substance with a specific effect (at a specific concentration) without taking into account other substances present in various quantities. Of course, this also applies to the extraction methods and their efficiency in relation to the individual components. For example, in studies [21] in which only the concentrations of two acids, caffeic and chlorogenic acid, were taken into account, it was found that at the level of 2.8 × 10−4 mol/dm3 they showed similar antioxidant activities, while the use of acids with concentrations above 10 × 10−4 mol/dm3 showed a higher antioxidant efficiency of caffeic acid than chlorogenic acid during the oxidation of triacylglycerols in sunflower oil. A similar dependence of the higher antioxidant efficiency of caffeic acid compared to chlorogenic acid was confirmed by other researchers in relation to sunflower oil [22] and corn oil [23]. A more detailed explanation of the mechanisms related to the antioxidant properties of selected phenolic acids can be found in a paper by Parus [6].
The above examples indicate that the biological activities of raw plant materials depend on many factors (the raw material, environmental factors, mutual quantitative relationships of individual chemical compounds, epigenetic reactions of plants to stressors, etc.), and therefore a very strict and precise determination of all the health-promoting properties of plants is very difficult, if not impossible, at the moment. Nevertheless, an attempt to build mathematical models based on a smaller amount of input data may be justified, as they gives an opportunity to select narrowed technological ranges during the processing of herbal raw material in order to obtain the optimal amount of the selected biologically active substance (in terms of the efficiency or energy consumption of the process). One such optimization method that can be used in herbal processing seems to be the response surface methodology, which was used in the experimental part of this work in relation to ethanolic extracts in terms of the content of chlorogenic acid obtained from dried elderberry flowers. Thus, the aim of this work was to optimize the content of chlorogenic acid in ethanol extracts of elderberry flowers using the response surface methodology.
2. Materials and Methods
The research material was elderberry flowers collected on the sunny days of 17–18 June 2021 in Przemyśl, Poland.
The green, thick elements of the flower canopy were cut off immediately after harvesting, and the raw material was divided into two test variants. In the first series, the raw material, after 24 h of storage at room temperature, was dried in a “Niewiadów” food dryer (type 972.04) via air circulation at a temperature of 54 °C (±1 °C) for 3 h. In the second series, fresh raw material, after being sealed in plastic containers, was frozen at −18 °C for 30 days, and then, after thawing for one day at 4 °C, it was also dried in the same way as in the first series.
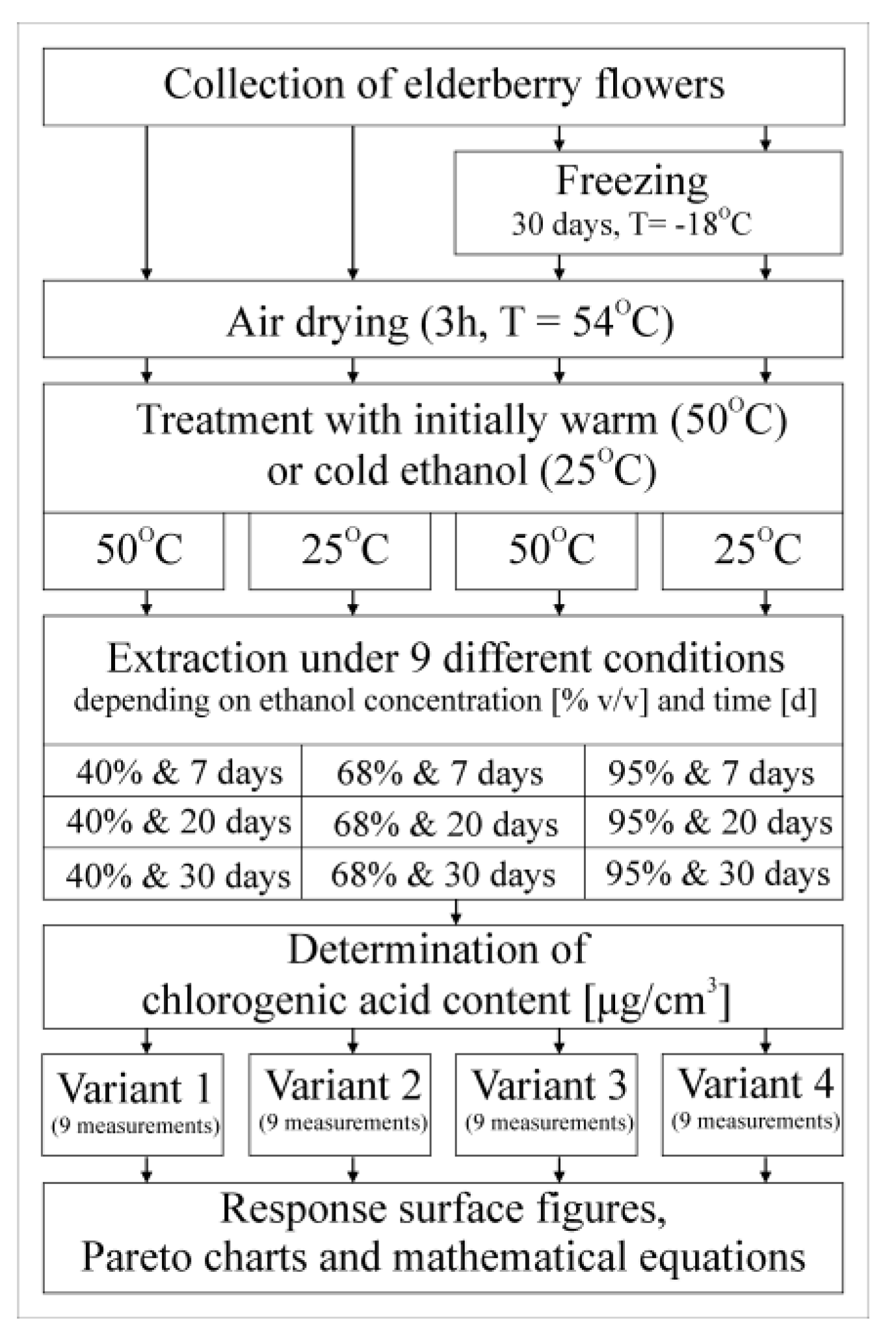
In order to prepare ethanol extracts, approx. 1 g of the dried raw material was weighed (with a few green elements) and placed in 99 cm3 of an ethanol solution with three different volume concentrations: 40, 68 and 95%. In order to verify the effectiveness of chlorogenic acid extraction depending on the initial temperature of the ethanol solutions, it was decided to additionally heat them to 50 °C (hot extraction). A parallel variant was extracted with appropriate but not heated ethanol solutions, the temperature of which in summer conditions was 25 °C (cold extraction). The setup of the experiment is shown in Figure 2.
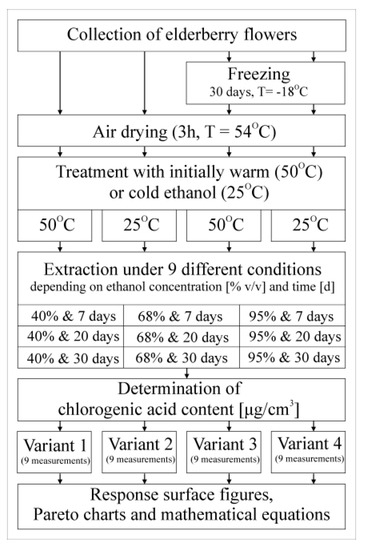
Figure 2.
Experimental design.
The LC-MS/MS method was used to identify and quantify the chlorogenic acid contained in the analyzed samples. A ten-fold dilution of the extract was analyzed on a Shimadzu 8045 LC-MS apparatus using a Kinetex column (2.6 µm C18 100A, 100 × 3 mm, Phenomenex, Aschaffenburg, Germany). The separation took place at 35 °C, and phase A (water with 0.1% formic acid) and phase B (methanol with 0.1% formic acid) were used. The flow rate was 0.3 mL/min. The analyses were carried out in MRM mode with positive ionization, and the mass spectrometer was operated with the following parameters: a nebulizing gas flow of 3 L/min, a heating gas flow of 10 L/min, an interface temperature of 300 °C, a desolvation temperature of 526 °C and a drying gas flow 10 L/min [24].
The 36 results of the determinations of chlorogenic acid content were first divided into 4 test variants with 9 results each:
Variant 1: fresh raw material and initial heat extraction.
Variant 2: fresh raw material and initial cold extraction.
Variant 3: frozen raw material and initial heat extraction.
Variant 4: frozen raw material and initial cold extraction.
The results were subjected to statistical calculations and visualization using the Statistica 13.3 program. The focus was on a process optimization method called the response surface methodology (RSM). Each graph of this type was accompanied by a Pareto diagram, thanks to which it was known which components of the input data had a statistically significant impact on the final optimization model shown in the main figure as the response surface. The main charts should be interpreted as the projected values of individual determinants according to the adopted mathematical model in terms of the volatility factors shown in the figures. Response surface models based on empirical measurements are very useful tools for the design and technological forecasting of the characteristics of a specific product, but they cannot be the starting point for any extrapolation attempts going beyond the accepted ranges of input variability [25].
3. Results and Discussion
The contents of chlorogenic acid in different variants of the experiment are presented in Table 1. These results were treated as input data and were used to develop RSM models (response surfaces). The variables were the ethanol volume concentration (40, 68 or 95%) and extraction time (7, 20 or 30 days). The research variants were differentiated by the type of extraction, i.e., depending on the initial temperature of ethanol during the pouring of elderberry flowers (heat extraction at 50 °C and cold extraction at 25 °C) as well as the drying of the raw material from a fresh or frozen state.

Table 1.
Chlorogenic acid contents (μg/cm3) in ethanol extracts, depending on the research variant: input data for RSM model optimization.
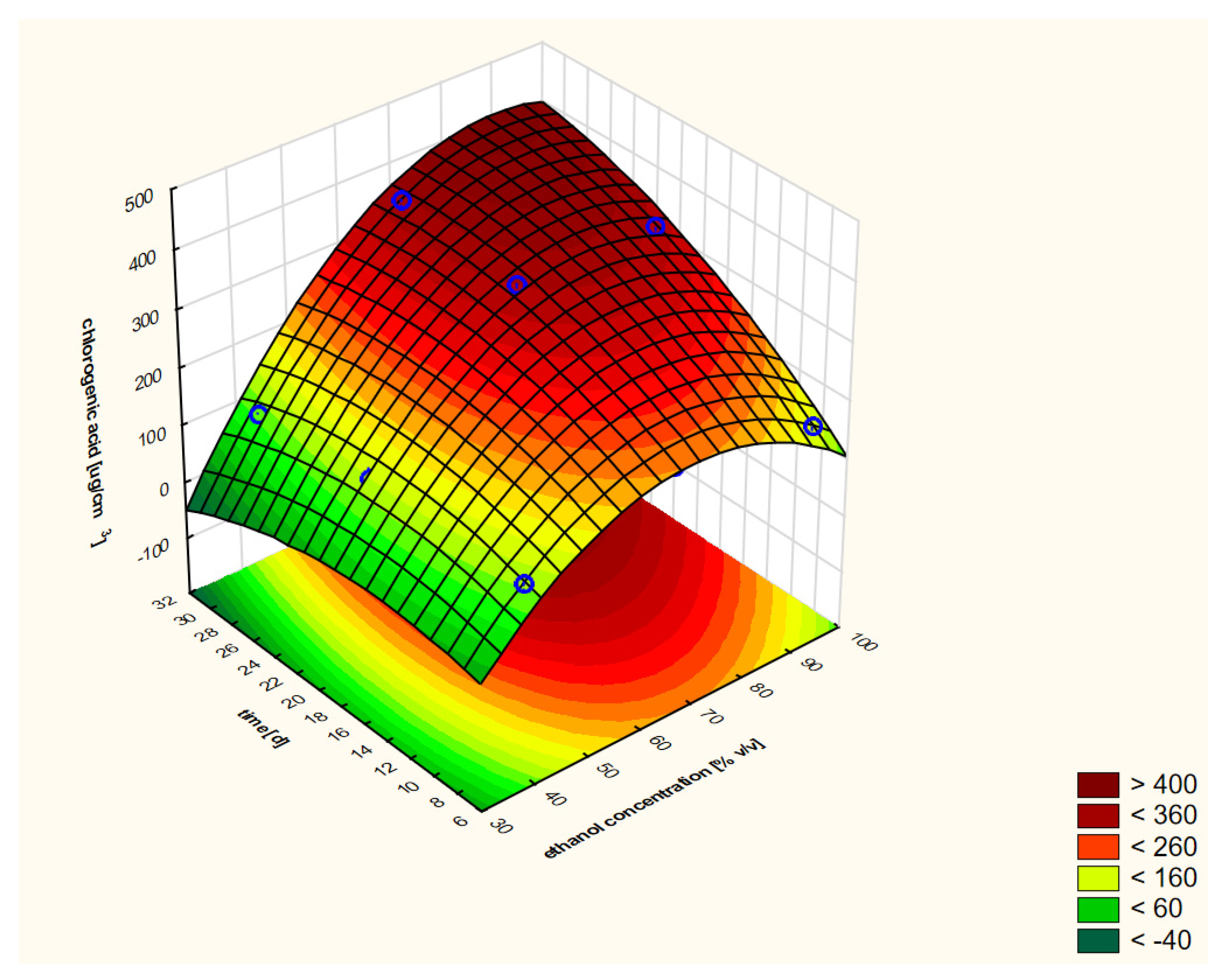
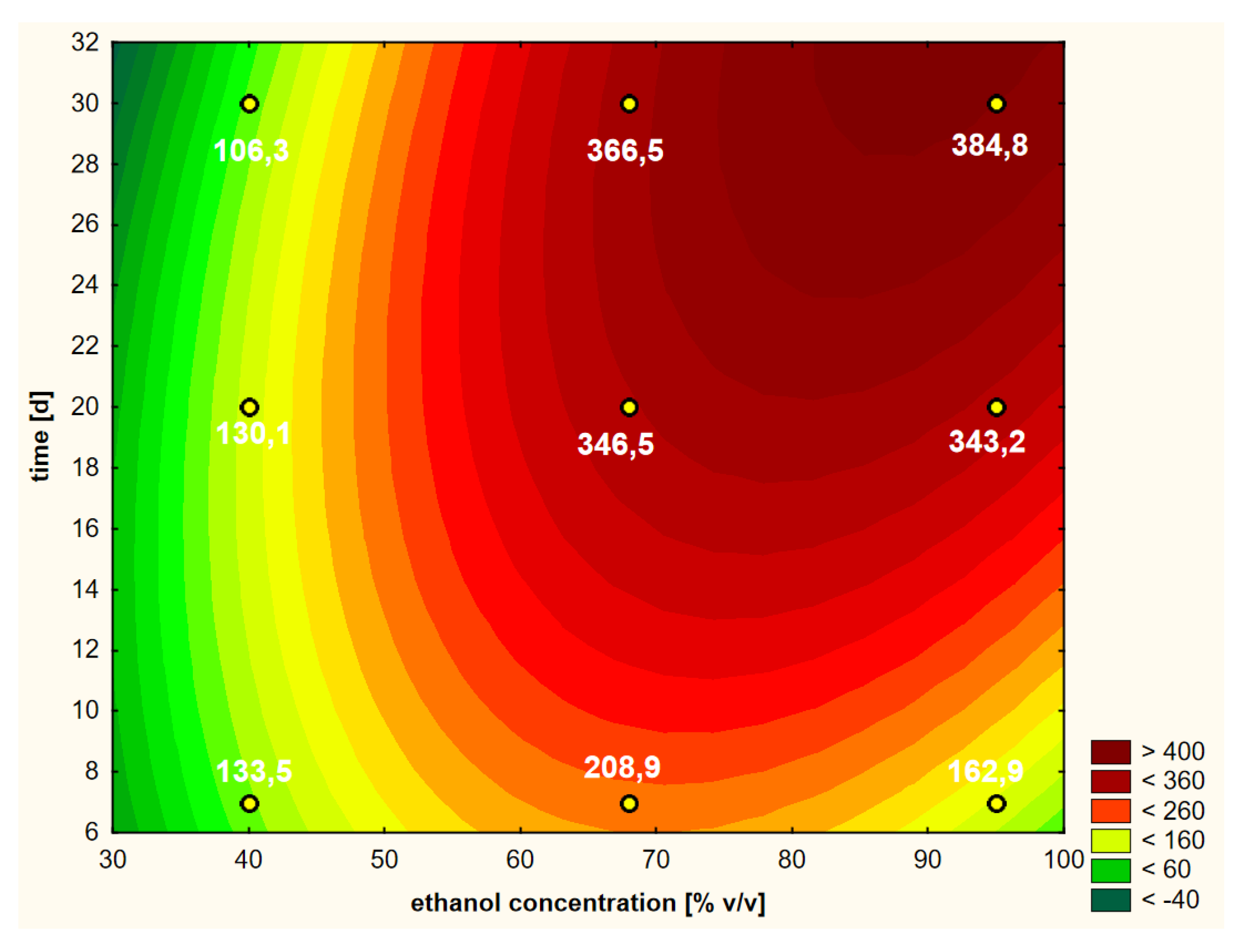
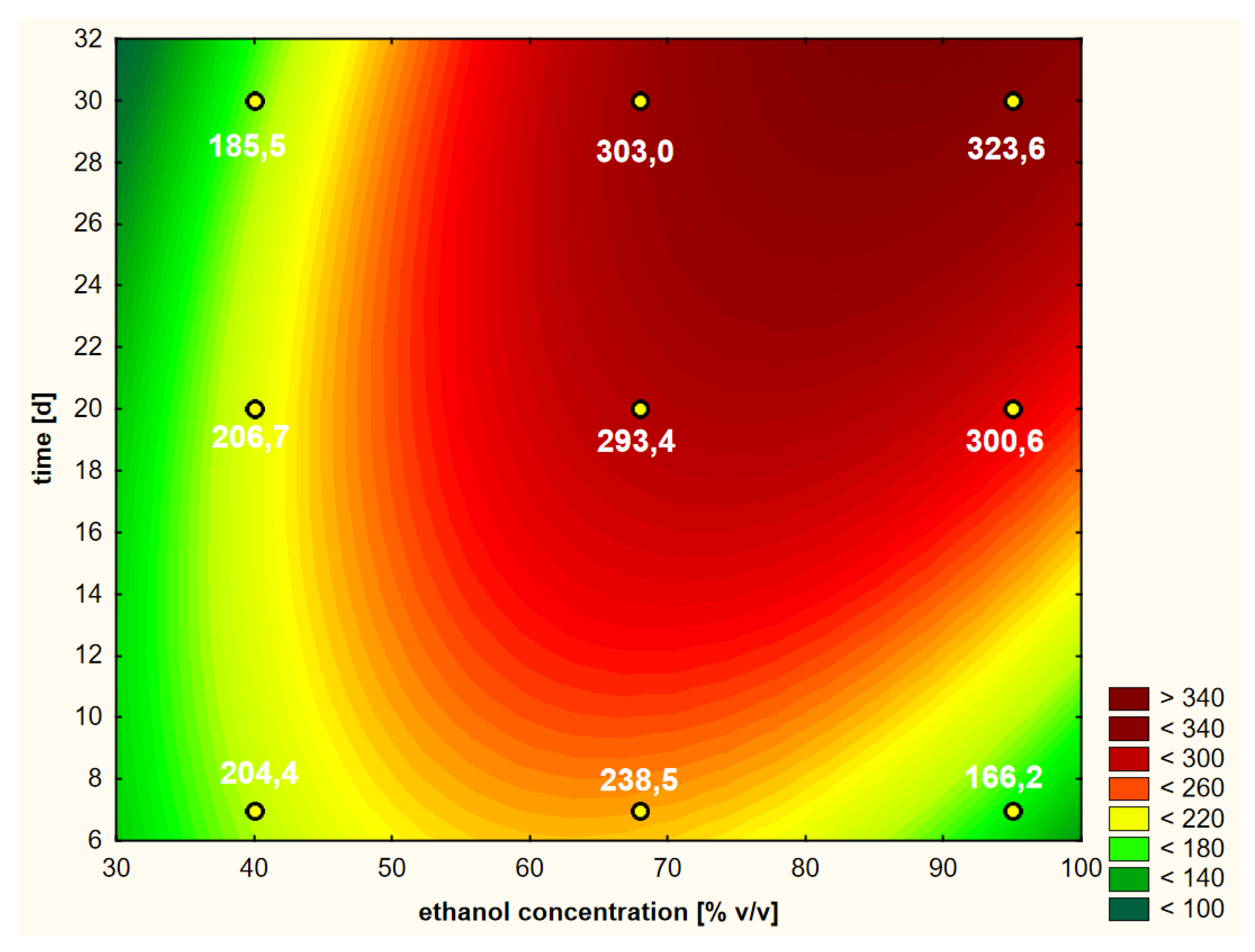
On the basis of the performed RSM analysis, the response curve presented in Figure 3 (as a 3D model) and in Figure 4 (in 2D) was obtained in the case of the first variant.
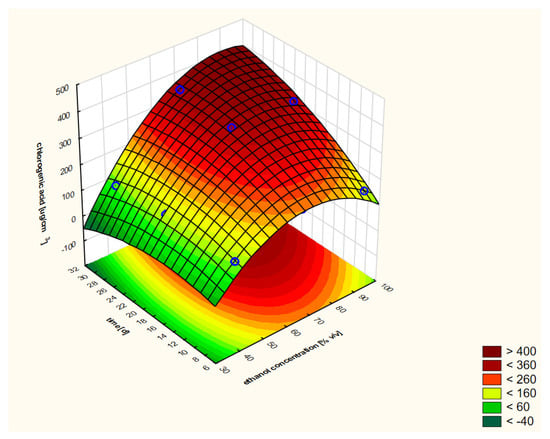
Figure 3.
Model of the response surface (3D) regarding the contents of chlorogenic acid in ethanol extracts of elderberry flowers obtained from fresh raw material that was initially treated with warm ethanol at 50 °C. The 3D model is shown only once for a better understanding of the RSM concept.
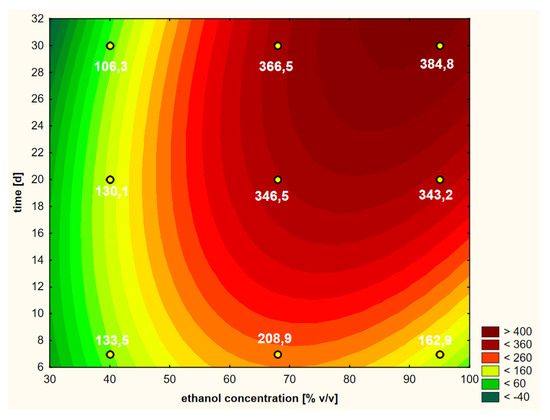
Figure 4.
Model of the response surface (2D) regarding the contents of chlorogenic acid in ethanol extracts of elderberry flowers obtained from fresh raw material that was initially treated with warm ethanol at 50 °C.
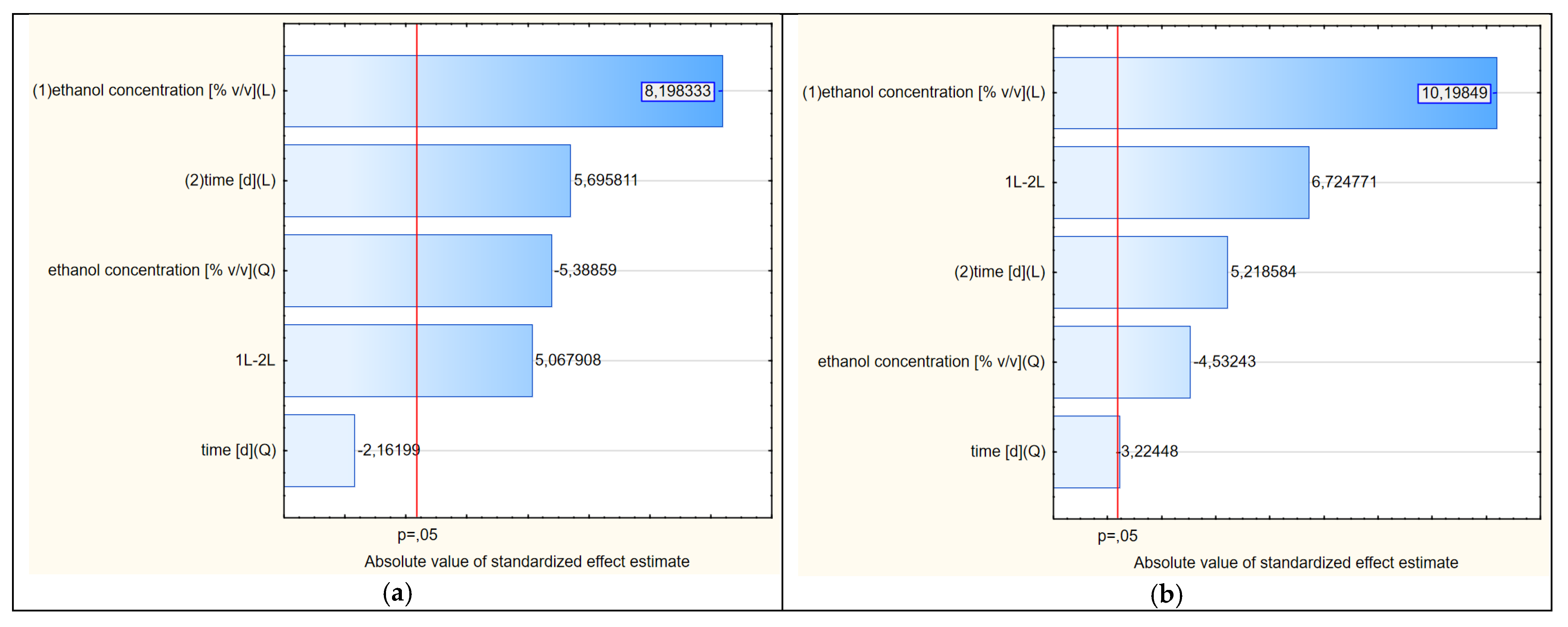
The analysis of the response surface makes sense when the variables of the model, i.e., in our case, the concentration of ethanol and the extraction time, significantly affect the obtained shape of the curves. It does not have to prove the influence of both factors; at least one is enough. In addition, in RSM analysis, the most common model is not linear but quadratic. Therefore, thanks to this method, it is possible to unambiguously determine whether the linear component (L) or the square component (Q) have statistically significant effects on the model. For the first variable, its linear and square components were analyzed. The same was true for the second variable. Therefore, we obtained information about four components. The fifth and final component that may affect the RSM model was the interaction of the linear components of the principal factors (LxL). The significance of the impacts of these five components were analyzed using a Pareto chart, as presented in Figure 5a.
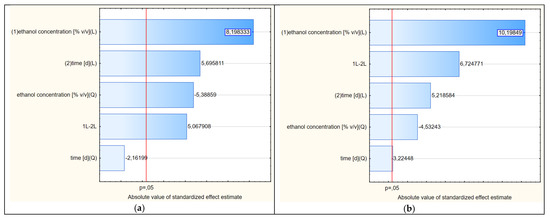
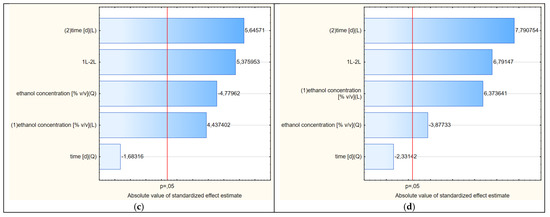
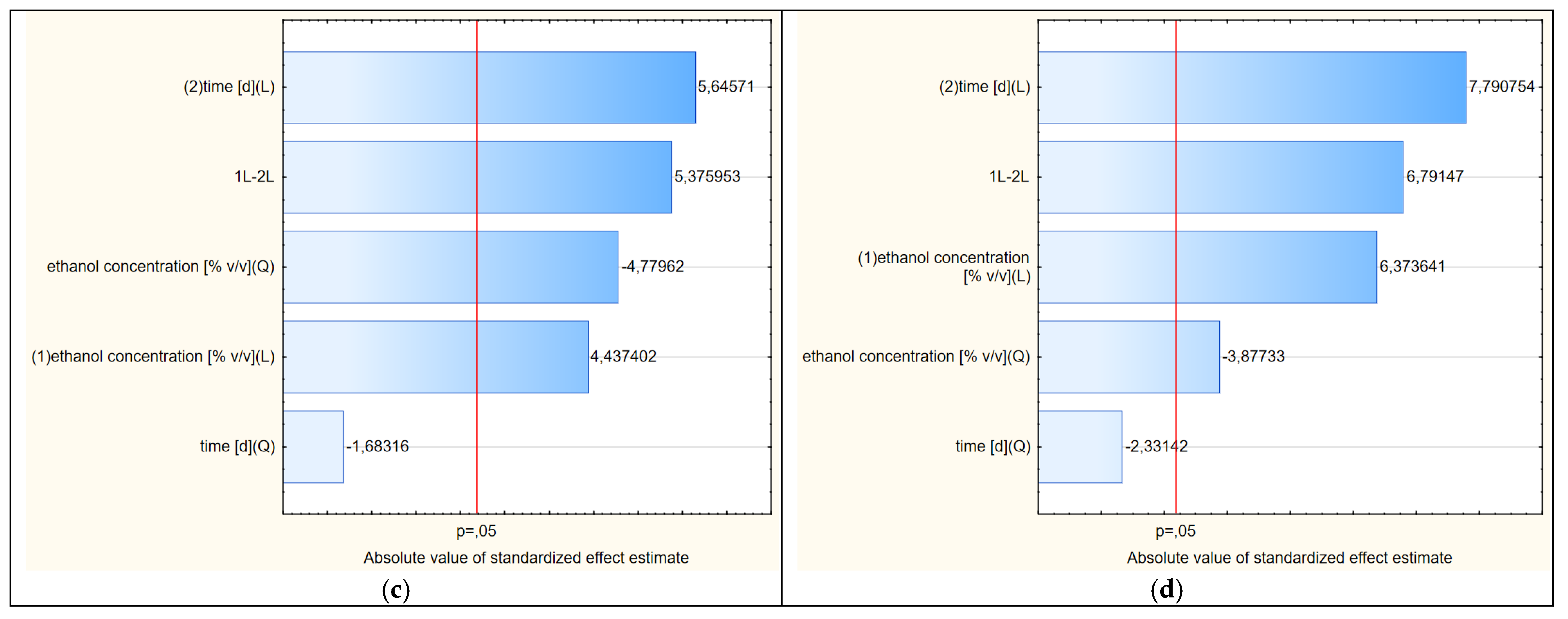
Figure 5.
(a–d) Pareto charts showing the statistical significance of the factors of variability affecting the shapes of the response surfaces (2D) shown in Figures 4, 6, 8 and 9, respectively.
Due to the fact that at least one of the five analyzed factors of variability had an absolute value higher than the minimum (at p = 0.05), it can be assumed that the determined response curves in Figure 4 are useful for further analysis. In the analyzed case, as many as four variables had significant impacts on the shapes of the curves in the RSM model. The concentration of ethanol (linear component L) had the greatest influence on the model. To a lesser but still statistically significant extent, the model was also influenced by the extraction time (linear component), the ethanol concentration (as a squared component) and the interaction of extraction time and ethanol concentration (linear components L × L). The extraction time as a square component had no statistical effect.
Looking holistically at the obtained response surface (Figure 4), i.e., in the full ranges of variability of the two variability factors (i.e., ethanol concentration and extraction time), the main and strongest factor was the alcohol concentration in linear terms. It can be assumed that the higher the concentration of ethanol, the better the extraction efficiency of chlorogenic acid. If we want to analyze the individual areas of volatility a bit more carefully, it is worth focusing on the ranges that interest us. The graph in Figure 4 is similar in its distribution of curves in the range from 40% to approximately 60% ethanol content. There are clearly dominant lines running parallel to the OY axis, i.e., the axis on which the extraction time is shown. This means that in this range, the concentration of ethanol has the main impact and the greatest impact on the content of chlorogenic acid; the higher it is, the higher the content of acid in the extract. In the second range of ethanol concentration, from about 60% to 95%, an increasing influence of the extraction time can be clearly seen, which is manifested by the shape of the croissant curves. Moreover, the square component (Q) of the ethanol concentration, as well as the interaction of extraction time and ethanol concentration, influenced this characteristic shape of the response surface.
A practical conclusion resulting from the shape of the curves in Figure 4 is the indication of a point or a narrow area for which the extraction efficiency can be optimized with a minimum of time and raw material expenditure. For example, at about the twentieth day of extraction with 68% ethanol, the process can be stopped if its efficiency is at a satisfactory level. It is about 346 μg/cm3 here, i.e., slightly lower than after 30 days of extraction and similar to the level with the use of 95% ethanol for the same time. On the basis of the obtained response areas, it is easier to decide on the shortest possible extraction time and the lowest concentration of ethanol to use in order to obtain a product with a satisfactory content of the active substance in the extract, which in this case, is chlorogenic acid.
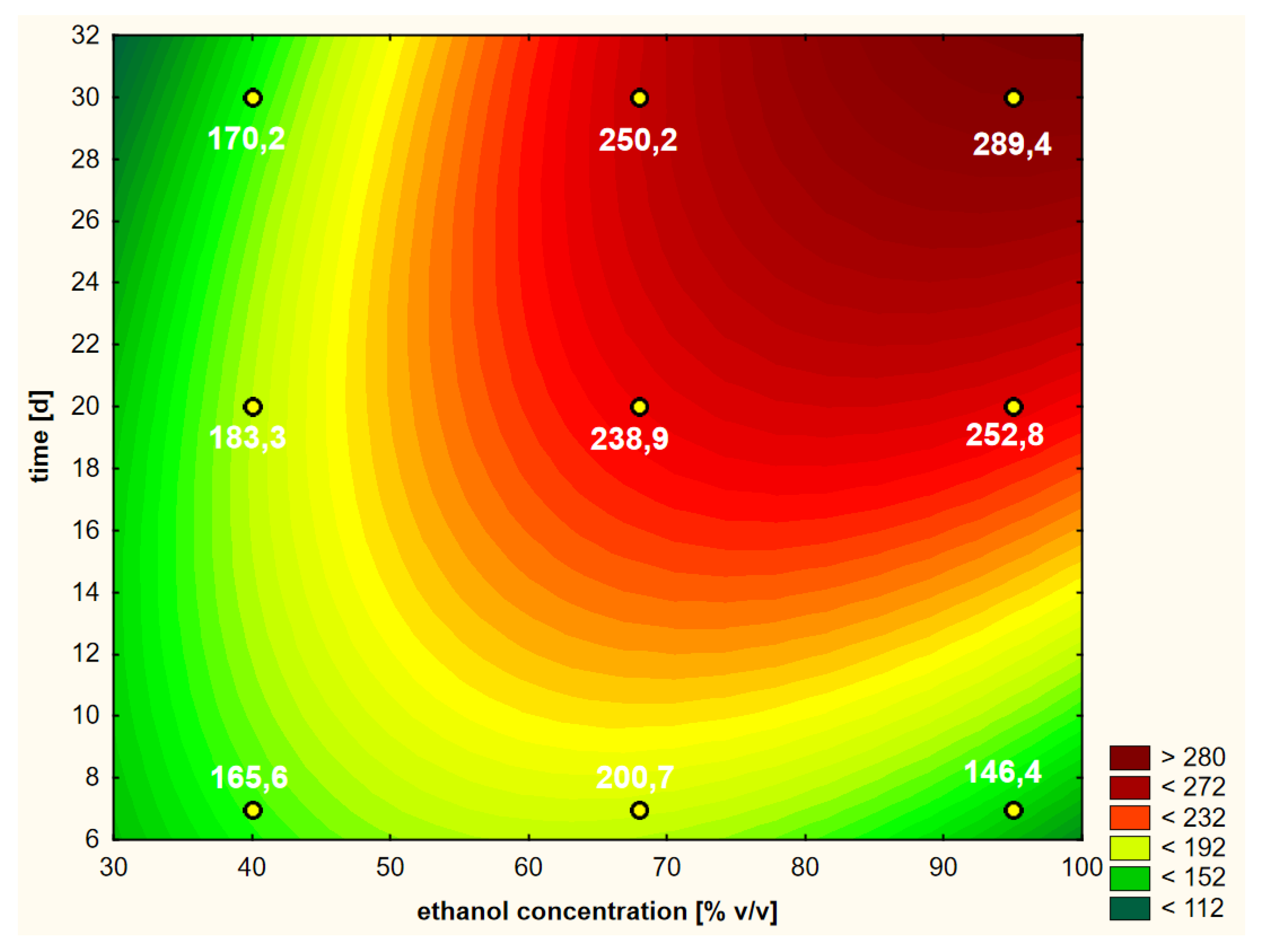
Suggestions of one of the authors indicated that the efficiency of the extraction of biologically active substances with ethanol can be increased if it is warm when added to the herbal raw material [26]. In order to empirically verify this statement, the first variant of the experiment, in which warm ethanol was used at the beginning (heated to 50 °C), was compared with the second variant, in which cold ethanol was used in summer conditions (25 °C). For this reason, the first variant (discussed above) was implemented “hot” and the second was implemented “cold”. The shape of the response surface of this second variant is shown in Figure 6.
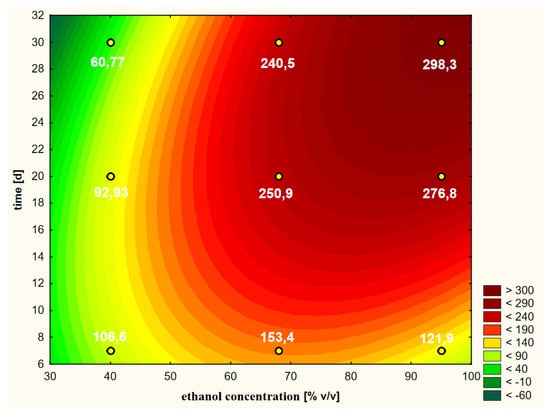
Figure 6.
Model of the response surface regarding the contents of chlorogenic acid in ethanol extracts of elderberry flowers obtained from fresh raw material that was initially treated with cold ethanol at a temperature of 25 °C.
The Pareto analysis (Figure 5b) showed that all variability factors had significant impacts on the shapes of the curves shown in Figure 6. As in the previous variant, the linear aspect of the ethanol concentration had the greatest impact. The second factor of variability in terms of the strength of the impact on the RSM model was the interaction between the two main variables (LxL), and the third was the extraction time (L). The other two quadratic aspects of concentration and time were less significant, but the shapes of the curves show some similarity to the RSM model referring to the first variant.
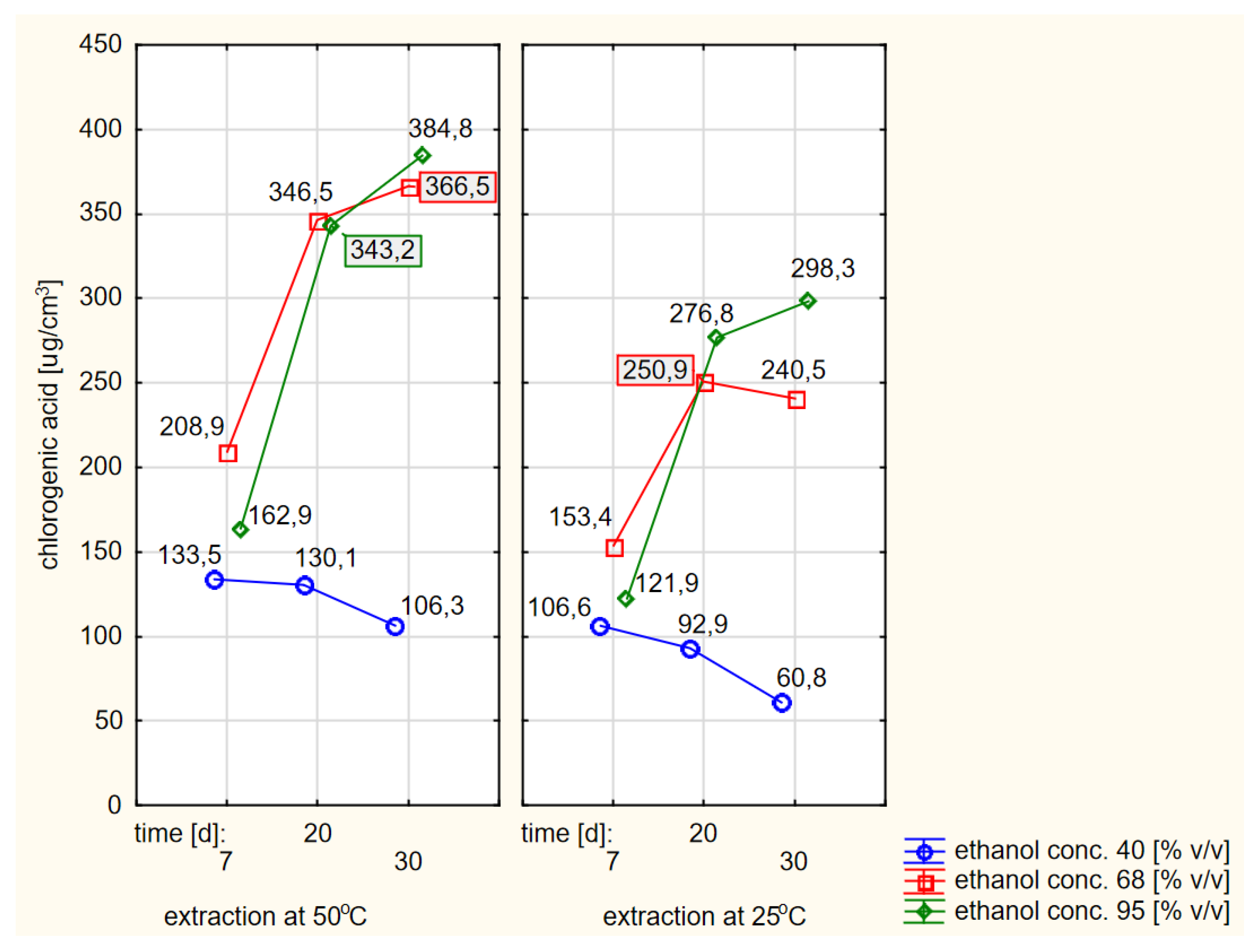
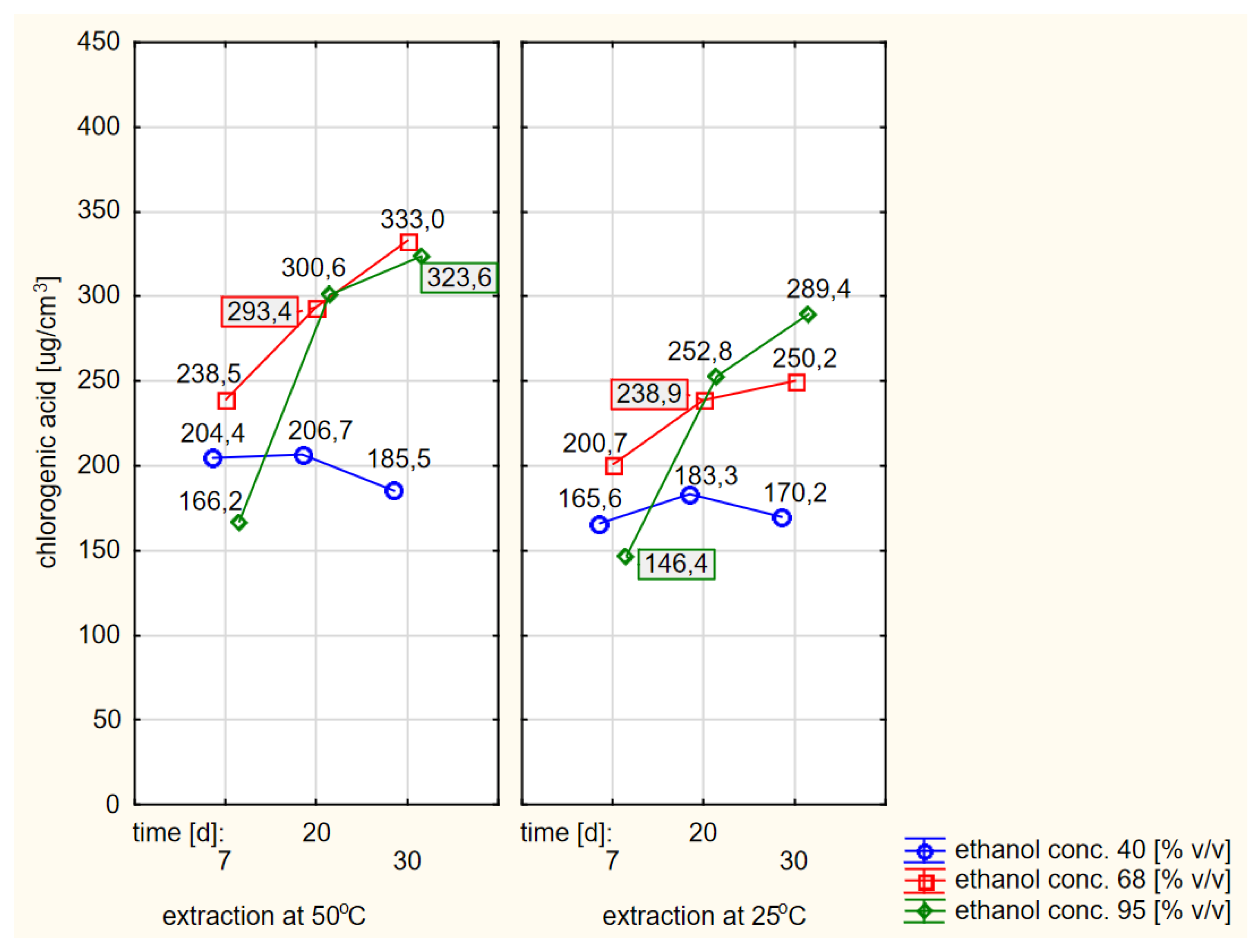
When comparing the absolute values of the contents of chlorogenic acid in extracts obtained by flooding dried elderberry flowers with warm or cold ethanol, a better effect (i.e., a higher concentration) could be found in the case of using warm ethanol. The differences were statistically significant. When grouping the relevant data, for the sake of the clarity of the work, only a simple compilation of individual results was chosen, as shown in Figure 7.
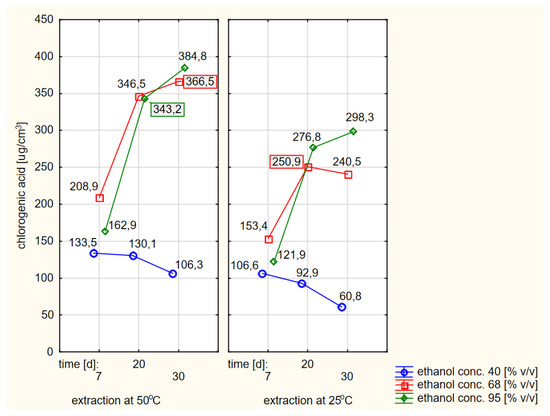
Figure 7.
Profiles of chlorogenic acid values in ethanol extracts for the dried raw material obtained from fresh elderberry flowers, presented separately for the hot and cold extraction variants.
The analysis of the data shown in Figure 7 confirmed the conclusions of the RSM models that the extraction efficiency was lowest when using 40% ethanol and was significantly higher at higher concentrations of ethanol with extraction times of at least twenty days, especially if the ethanol was preheated at the beginning.
In order to check how the freezing process can affect the extraction efficiency of the same raw material in comparison to non-frozen raw material, the results of variants 3 and 4 were analyzed. Raw material that was frozen and thawed after a month was subjected to identical experiments. Figure 8 shows the response surface curves in variant 3, i.e., after freezing elderberry flowers and performing hot ethanol extraction.
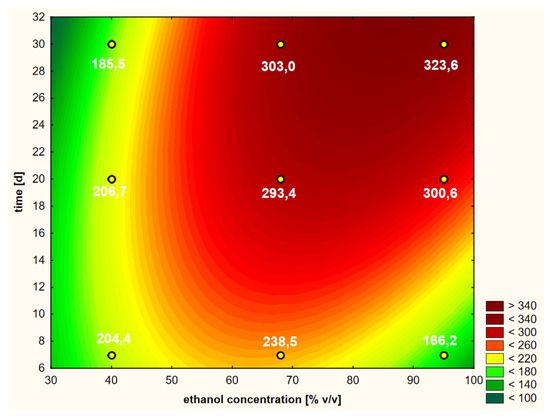
Figure 8.
Model of the response surface regarding the contents of chlorogenic acid in ethanol extracts of elderberry flowers obtained from previously frozen raw material that was initially treated with warm ethanol at 50 °C.
The shapes of the curves in Figure 8 were statistically significantly affected by four variability factors (Figure 5c), of which the extraction time in linear terms was the most important. The concentration of ethanol, in contrast to freshly dried raw material, was less significant. The shapes of the curves were nevertheless similar to those of variants 1 and 2.
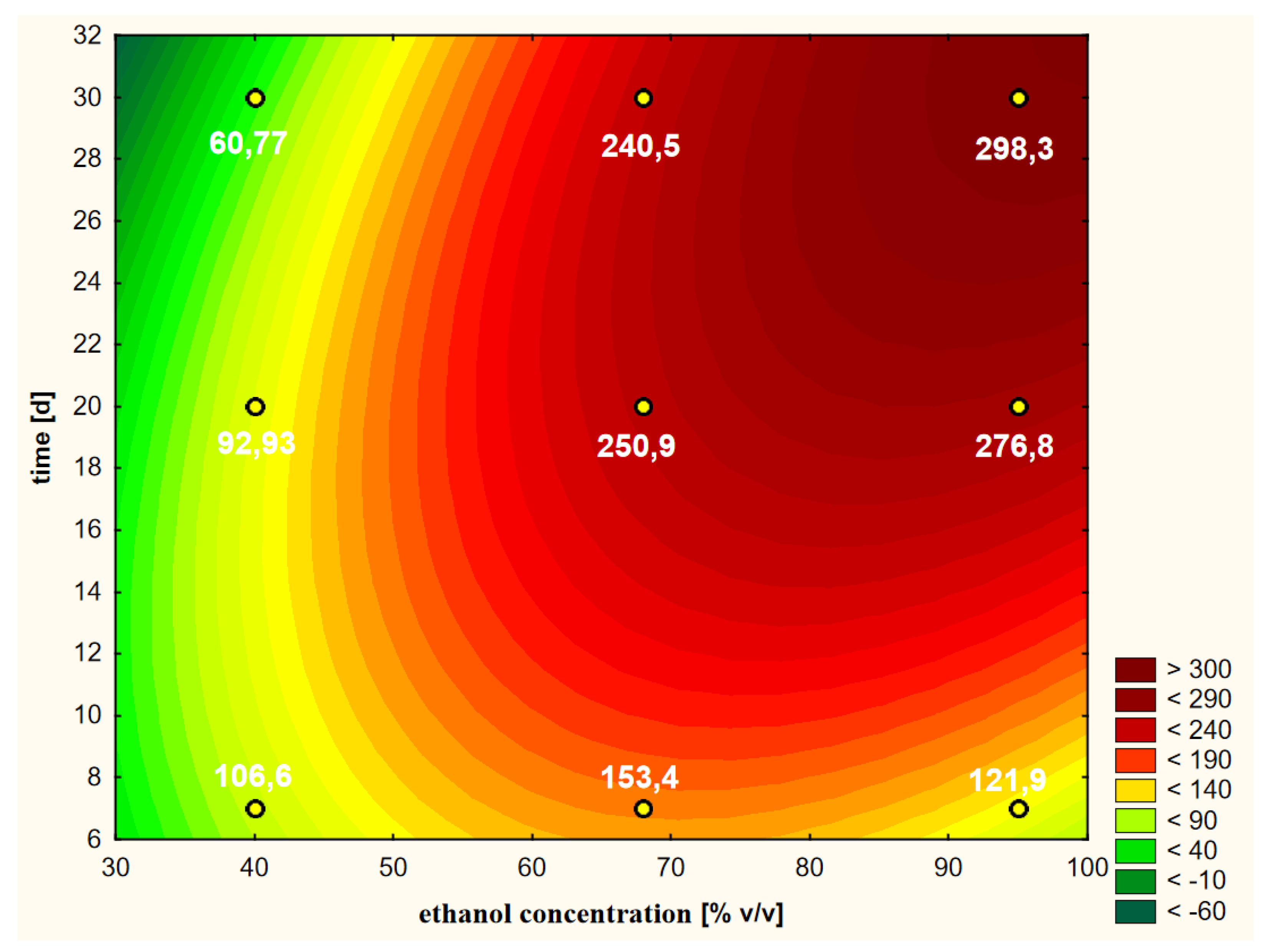
A graphical representation of the RSM model in relation to the last, i.e., the fourth variant, in which the thawed raw material treated with cold ethanol was used, is shown in Figure 9. The Pareto diagram shown in Figure 5d confirmed the similarity of this variant to the third variant in terms of the significance and the order of the variability factors influencing the final course of the RSM model curves. Moreover, the extraction time in linear terms turned out to be the most important factor, with the ethanol concentration having less importance in shaping the curves. Although the distribution of the curves in variant 4 was similar to the other three variants, the contents of chlorogenic acid were lower than in variant 3, where warm ethanol was initially used, which can also be compared in Figure 10. This plot also shows that the extraction efficiency in relation to chlorogenic acid was the least differentiated in this variant, i.e., the profiles (for 40, 68 and 95%) were the closest to each other and somewhat flattened.
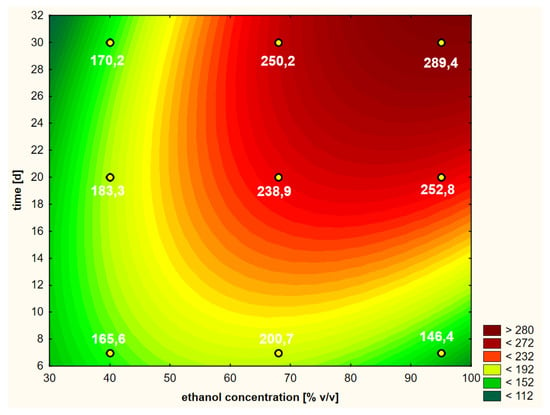
Figure 9.
Model of the response surface concerning the contents of chlorogenic acid in ethanol extracts of elderberry flowers obtained from previously frozen raw material that was initially treated with cold ethanol at a temperature of 25 °C.
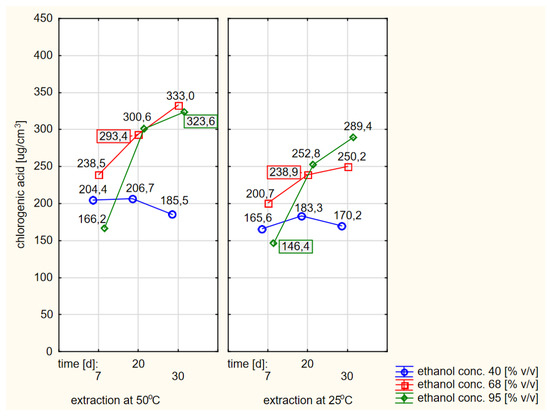
Figure 10.
Profiles of chlorogenic acid values in ethanol extracts for the dried raw material obtained from previously frozen elderberry flowers, presented separately for the hot and cold extraction variants.
The freezing of elderberry flowers and their subsequent drying had an impact on the effectiveness of chlorogenic acid extraction compared to the tests in which fresh raw material was dried. The subsequent use of 68 or 95% ethanol and previously frozen samples was usually less effective than using fresh material. An inverse relationship was found for the use of ethanol with a concentration of 40% (Figure 7 and Figure 10), i.e., the contents of chlorogenic acid were higher in the variants where elderberry flowers were previously frozen compared to the variants with fresh raw material.
Table 2 presents mathematical formulas that allow a precisely calculated (but estimated according to the RSM model) content of chlorogenic acid to be obtained for any selected value of ethanol concentration (from 40 to 95%) and extraction time (from 7 to 30 days). Approximate values for any ranges of these two variables can also be read on all response surface plots included in this work. The formulas given below apply separately to each of the four variants of the experiment.

Table 2.
Formulas for Calculating the Content of Chlorogenic Acid (μg/cm3) in ethanol extracts, depending on the research variant.
It is difficult to directly relate the methodology proposed in this paper to other papers dealing with the properties of ethanol extracts of elderberry flowers, as such works probably do not exist. The authors previously used the response surface method for process optimization, but this was in relation to raw egg material [27], not herbal material. It seems, however, that this work indicates the practical usefulness of this methodology, which may result in its more frequent use in the future in relation to research on other herbal raw materials in the context of optimizing the chemical composition of the finished herbal product, depending on the methods and technological parameters used.
4. Conclusions
The conducted research showed that the use of the RSM method to assess the effectiveness of chlorogenic acid extraction is very effective. Statistical analyses confirmed the higher effectiveness of chlorogenic acid extraction in the variants in which warm ethanol (at a temperature of 50 °C) was used with dried elderberry flowers compared to the variants in which cold ethanol (at a temperature of 25 °C) was used.
The freezing of elderberry flowers and their subsequent drying caused a relatively slight decrease in the effectiveness of chlorogenic acid extraction compared to freshly dried raw material but only in the case of using 68% or 95% ethanol. In the case of samples obtained with the use of 40% ethanol, the efficiency of the extraction after freezing elderberry flowers was higher.
In all four analyzed variants of the experiment, significant impacts of variability factors, i.e., the ethanol concentration and extraction time in linear or square terms as well as their interaction, on the response surface methodology models were found.
In the case of freshly dried raw material, the concentration of ethanol had the greatest impact on the optimization response surface models, while the extraction time had the greatest impact for previously frozen raw material.
The response surface models indicated the possibility of shortening the extraction time (to 20 days instead of 30) and using ethanol with a lower concentration (approx. 68% instead of higher concentrations, even up to 95%) with the acceptance of only a slightly lower yield (i.e., the content of chlorogenic acid), especially in the case of frozen raw material.
Based on the obtained results, it can be concluded that the optimization of the efficiency of the technological processes in the processing of herbs can be supported by the use of advanced computational techniques such as response surface analysis.
Author Contributions
Conceptualization, M.O. and A.N.-O.; methodology, M.O. and A.N.-O.; software, M.O.; validation, M.O., A.N.-O. and D.M.; formal analysis, M.O. and A.N.-O.; investigation, M.O., A.N.-O. and D.M.; resources, M.O. and D.M.; data curation, M.O.; writing—original draft preparation, M.O.; writing—review and editing, A.N.-O. and M.O.; visualization, M.O.; supervision, M.O. and A.N.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are archived on the server of the Wrocław University of Environmental and Life Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cis, J.; Nowak, G. Niezwykłe skarby natury [Extraordinary Treasures of Nature]. In Ziołowe Królestwo. Ziołolecznictwo—Kosmetyka—Uprawa Ziół [Herbal Kingdom. Herbal Medicine—Cosmetics—Cultivation of Herbs]; Publishing House Publicat: Poznań, Poland, 2009; p. 35. [Google Scholar]
- Gudej, J.; Owczarek, A. Roślinne Surowce Lecznicze—Badania Makroskopowo-Mikroskopowe [Medicinal Plant Raw Materials—Macroscopic Microscopic Research]; Uniwersytet Medyczny w Łodzi. Katedra i Zakład Farmakognozji: Łódź, Poland, 2012. [Google Scholar]
- Waszkiewicz-Robak, B.; Biller, E. Właściwości Prozdrowotne Czarnego Bzu [Health-Promoting Properties of Elderberry]. Probl. Hig. I Epidemiol. 2018, 99, 217–224. [Google Scholar]
- Ostrowska, J.; Skrzydlewska, E. Aktywność Biologiczna Flawonoidów [Biological Activity of Flavonoids]. Postępy Fitoter. 2005, 3–4, 71–79. [Google Scholar]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113 (Suppl. 9B), 71S–88S. [Google Scholar] [CrossRef] [PubMed]
- Parus, A. Przeciwutleniające i Farmakologiczne Właściwości Kwasów Fenolowych [Antioxidant and Pharmacological Properties of Phenolic Acids]. Postępy Fitoter. 2013, 1, 48–53. [Google Scholar]
- Harborne, J.B.; Baxter, H.; Moss, G.P. Phytochemical Dictionary: Handbook of Bioactive Compounds from Plant, 2nd ed.; Taylor and Francis: London, UK, 1999. [Google Scholar]
- Kusznierewicz, B. Związki fenolowe [Phenolic Compounds]. In Chemia Żywności [Food Chemistry]; Sikorski, Z.E., Staroszczyk, H., Eds.; Publishing House PWN: Amsterdam, The Netherlands, 2019; Volume 2, pp. 62–71. [Google Scholar]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.D.; Almeida, M.C.; Lopes, N.P.; De Souza, G.E.P. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol. Pharm. Bull. 2006, 29, 2236. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Suzuki, O.; Igarashi, K. Protective effects of chlorogenic acid on paraquat-induced oxidative stress in rats. Biosci. Biotechnol. Biochem. 1996, 60, 765–801. [Google Scholar] [CrossRef]
- Thom, E. The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long-term in overweight and obese people. J. Int. Med. Res. 2007, 35, 900–908. [Google Scholar] [CrossRef]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: Glycemic effects of chlorogenic acid and caffeine. Am. J. Clin. Nutr. 2003, 78, 728–733. [Google Scholar] [CrossRef]
- Lapchak, P.A. The phenylpropanoid micronutrient chlorogenic acid improves clinical rating scores in rabbits following multiple infarct ischemic strokes: Synergism with tissue plasminogen activator. Exp. Neurol. 2007, 205, 407–413. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Lee, H.-K.; Kim, J.-A.; Hong, S.-I.; Kim, H.-C.; Jo, T.-H.; Park, Y.-I.; Lee, C.-K.; Kim, Y.-B.; Lee, S.-Y. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Kagawa, D.; Ochiai, R.; Tokimitsu, I.; Saito, I. Green coffee bean extract and its metabolites have a hypotensive effect in spontaneously hypertensive rats. Hypertens Res. 2002, 25, 99–107. [Google Scholar] [CrossRef]
- Suzuki, A.; Fujii, A.; Yamamoto, N.; Yamamoto, M.; Ohminami, H.; Kameyama, A.; Shibuya, Y.; Nishizawa, Y.; Tokimitsu, I.; Saito, I. Improvement of hypertension and vascular dysfunction by hydroxyhydroquinone-free coffee in a genetic model of hypertension. FEBS Lett. 2006, 580, 2317–2322. [Google Scholar] [CrossRef]
- Onakpoya, I.; Terry, R.; Ernst, E. The use of green coffee extract as a weight loss supplement: A systematic review and meta-analysis of randomised clinical trials. Gastroenterol. Res. Pract. 2010, 2011, 382852. [Google Scholar] [CrossRef] [PubMed]
- Lamer-Zarawska, E.; Oszmiański, J. Rola Niektórych Substancji Roślinnych w Profilaktyce Przeciwnowotworowej [The Role of Some Plant Substances in Anticancer Prophylaxis]. Wiadomości Zielar. 1998, 5, 1–4. [Google Scholar]
- Gawlik-Dziki, U. Fenolokwasy Jako Bioaktywne Składniki Żywności [Phenolic Acids as Bioactive Food Ingredients]. Żywność Nauka Technol. Jakość. 2004, 41, 29–40. [Google Scholar]
- Marinova, E.M.; Toneva, A.; Yanishlieva, N. Comparison of the antioxidative properties of caffeic and chlorogenic acids. Food Chem. 2009, 114, 1498–1502. [Google Scholar] [CrossRef]
- Medina, I.; Gallardo, J.M.; Gonzalez, M.J.; Lois, S.; Hedges, N. Effect of molecular structure of phenolic families as hydroxycinnamic acids and catechins on their antioxidant effectiveness in minced fish muscle. J. Agric. Food Chem. 2007, 55, 3889–3895. [Google Scholar] [CrossRef]
- Chen, J.H.; Ho, C.T. Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. J. Agric. Food Chem. 1997, 45, 2374–2378. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J. Content of bioactive compounds and antioxidant capacity in skin tissues of pear. J. Funct. Foods 2016, 23, 40–51. [Google Scholar] [CrossRef]
- Pietraszek, J. Dobór Planu Doświadczenia i Analiza Wyników w Badaniach Technicznych [Selection of the Experiment Plan and Analysis of the Results in Technical Research]. 2013, pp. 73–85. Available online: https://media.statsoft.pl/_old_dnn/downloads/dobor_planu_doswiadczenia.pdf (accessed on 31 January 2023).
- Nowak, Z.T. Apteka Natury ma Leki na Wirusy [Nature’s Pharmacy Has Cures for Viruses]; Publishing House AA: Kraków, Poland, 2020; p. 72. [Google Scholar]
- Oziembłowski, M.; Nawirska-Olszańska, A.; Maksimowski, D.; Trenka, M.; Break, A.; Kulig, D.; Miernik, A. The effect of Concentrated Microwave Field (CMF) on selected physical and rheological properties of liquid egg products. Appl. Sci. 2021, 11, 1832. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).