Synthesis of New 2D-π-2A Chromophores Based on Tetraphenyl Fulvene and Investigation of Their Optical Properties
Abstract
Featured Application
Abstract
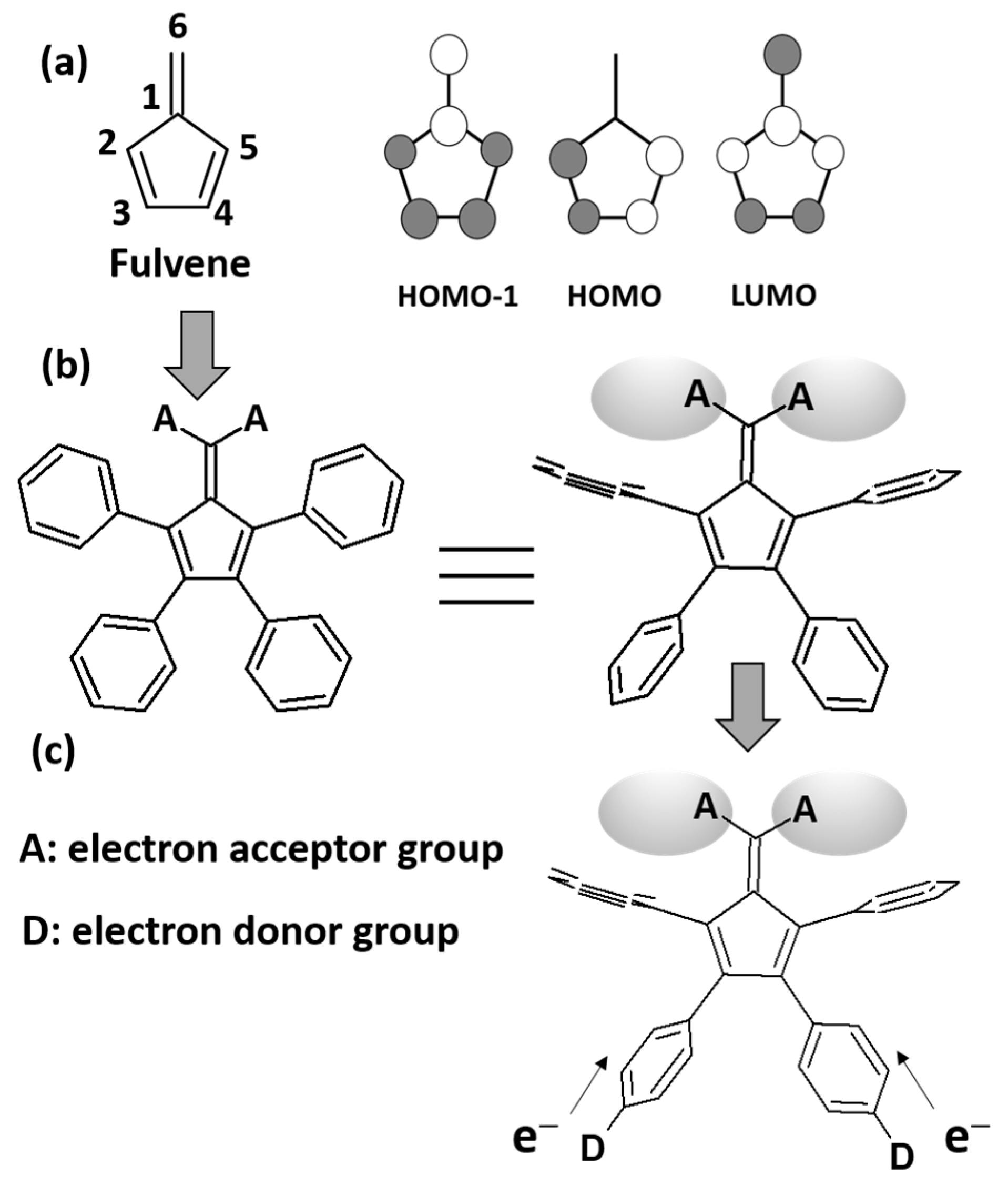
1. Introduction
2. Materials and Methods
3. Results
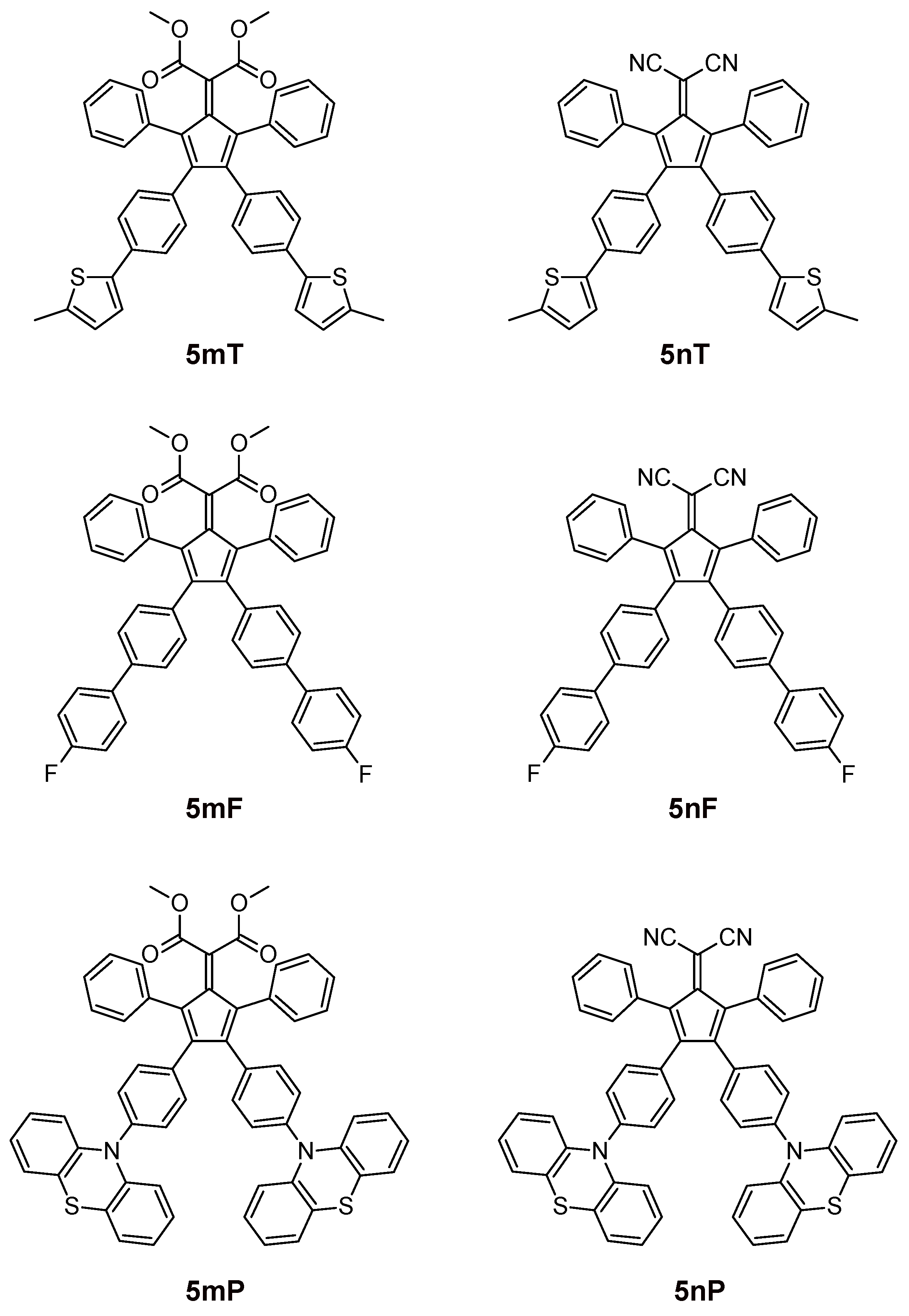
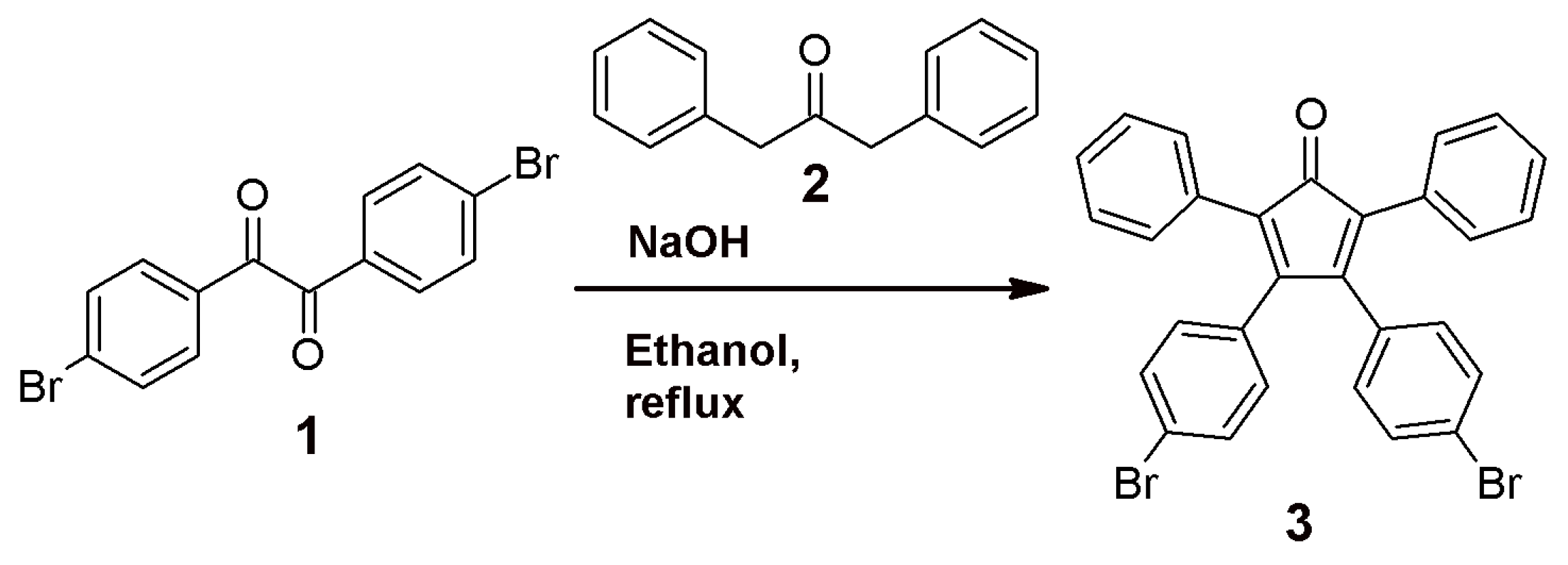
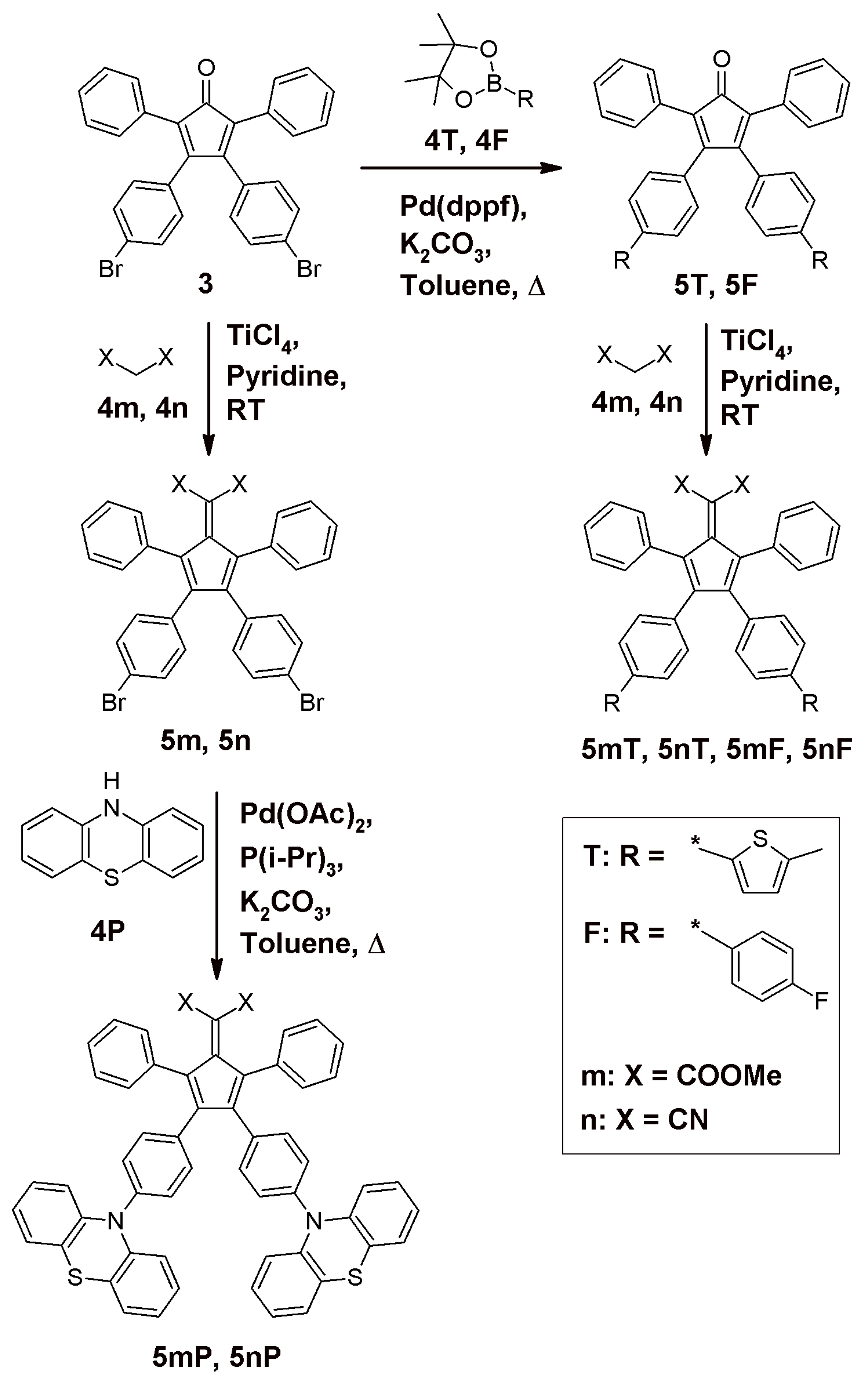
3.1. Synthesis
3.2. Optical Properties
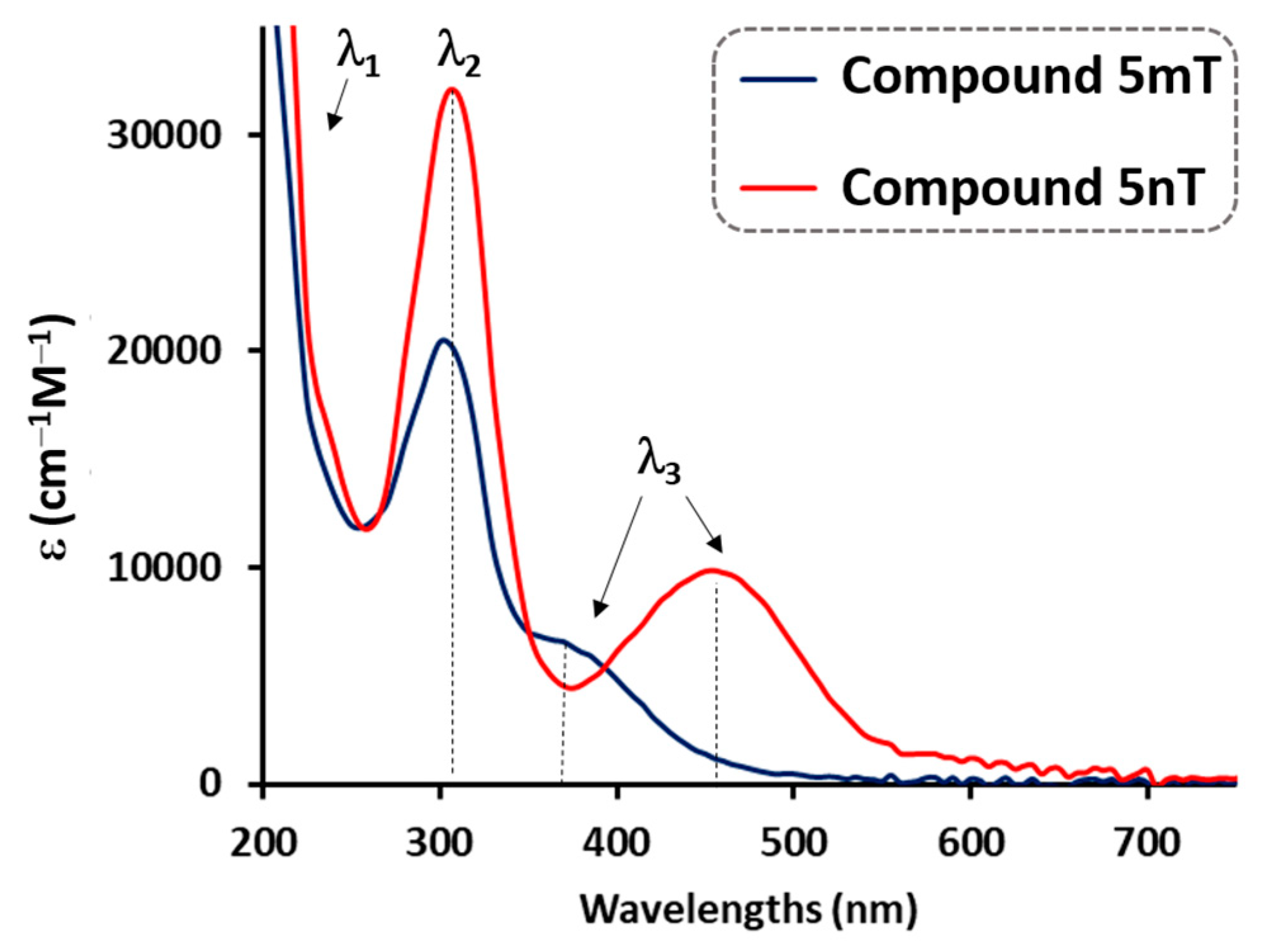
3.2.1. UV–Vis Absorption Spectra
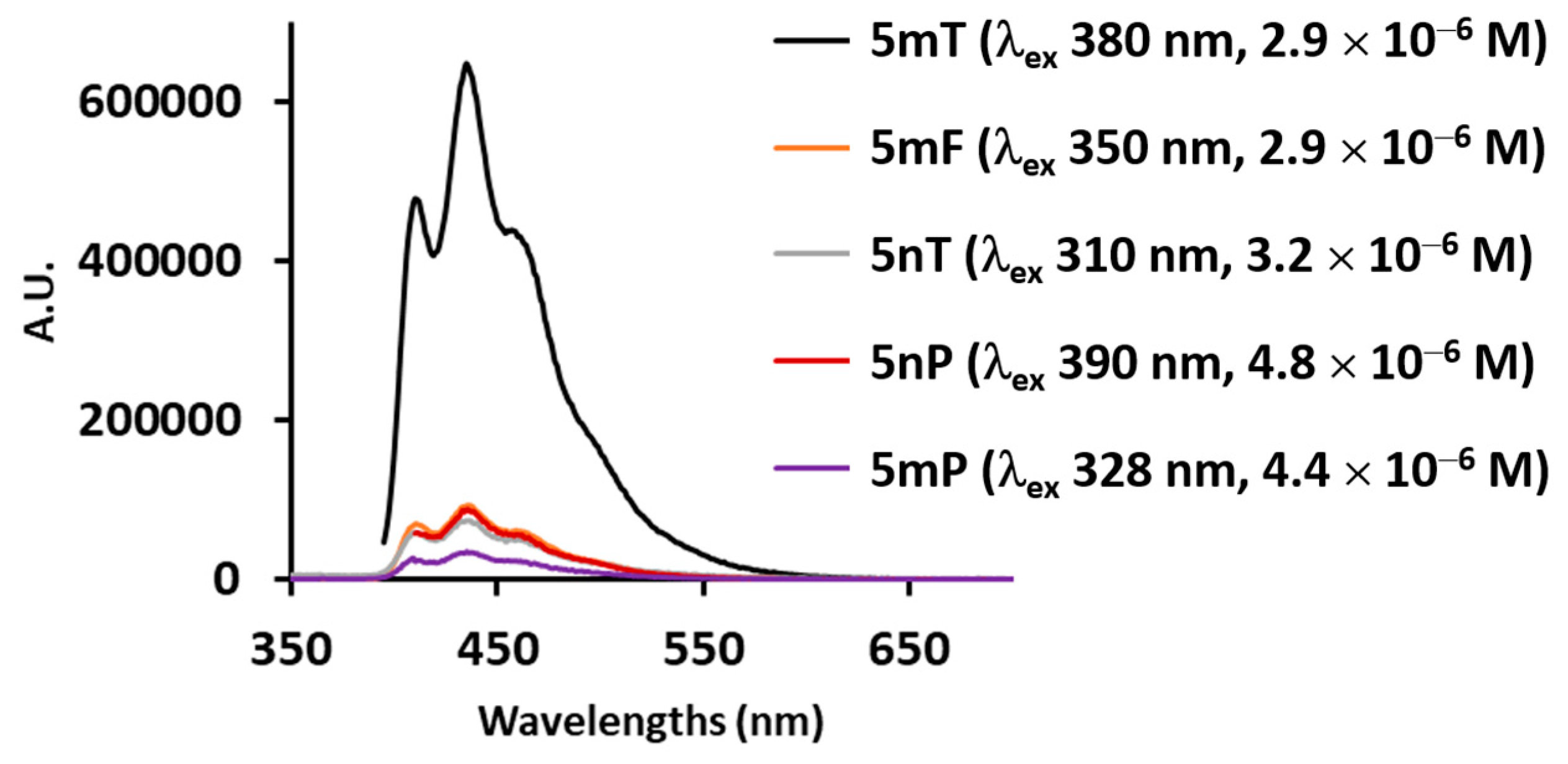
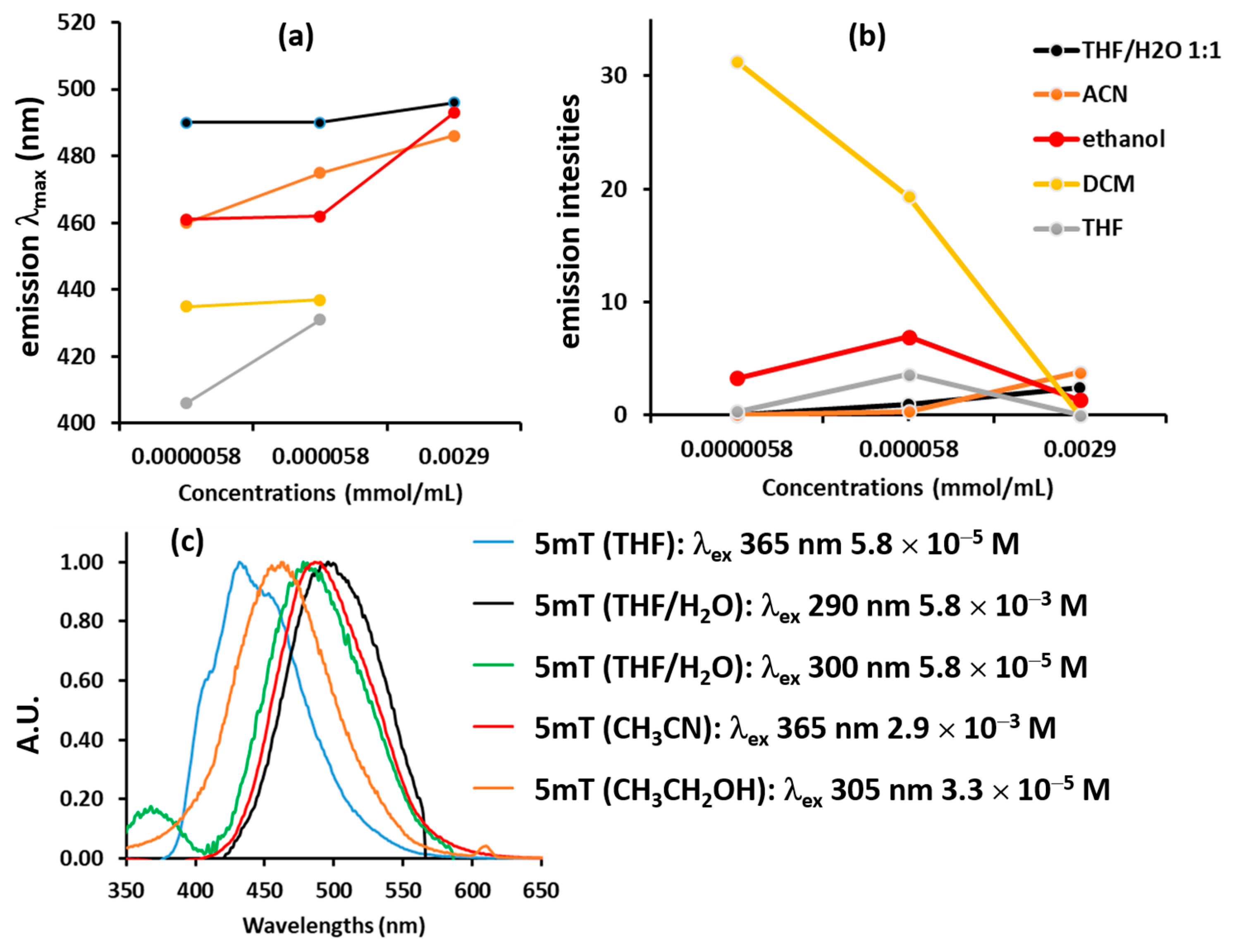
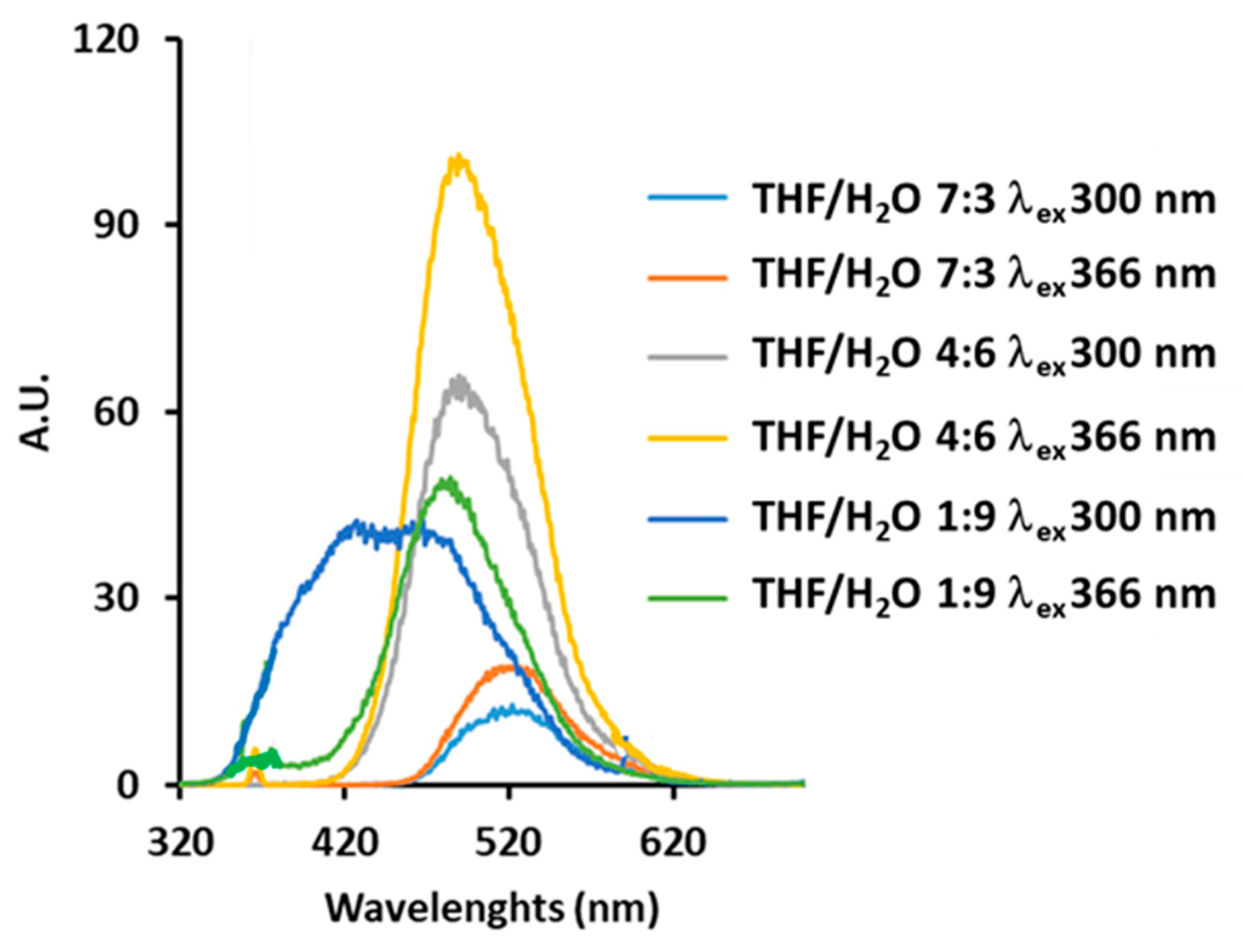
3.2.2. Fluorescence Spectra
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duan, L.; Hou, L.; Lee, T.W.; Qiao, J.; Zhang, D.; Dong, G.; Wang, L.; Qiu, Y. Solution processable small molecules for organic light-emitting diodes. J. Mater. Chem. 2010, 20, 6392–6407. [Google Scholar] [CrossRef]
- Tian, X.; Murfin, L.C.; Wu, L.; Lewis, S.E.; James, T.D. Fluorescent small organic probes for biosensing. Chem. Sci. 2021, 12, 3406–3426. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Matsubara, Y.; Ochi, T.; Wakamiya, T.; Yoshida, Z.I. How the π conjugation length affects the fluorescence emission efficiency. J. Am. Chem. Soc. 2008, 130, 16442. [Google Scholar] [CrossRef]
- Milián-Medina, B.; Gierschner, J. π-Conjugation. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 513–524. [Google Scholar] [CrossRef]
- Itoh, T. Fluorescence and phosphorescence from higher excited states of organic molecules. Chem. Rev. 2012, 112, 4541–4568. [Google Scholar] [CrossRef]
- Lichtman, J.W.; Conchello, J.A. Fluorescence microscopy. Nat. Methods 2005, 2, 910–919. [Google Scholar] [CrossRef]
- Forrest, S.R.; Thompson, M.E. Introduction: Organic electronics and optoelectronics. Chem. Rev. 2007, 107, 923–925. [Google Scholar] [CrossRef]
- Mitschke, U.; Bäuerle, P. The electroluminescence of organic materials. J. Mater. Chem. 2000, 10, 1471–1507. [Google Scholar] [CrossRef]
- Xiao, L.; Chen, Z.; Qu, B.; Luo, J.; Kong, S.; Gong, Q.; Kido, J. Recent progresses on materials for electrophosphorescent organic light-emitting devices. Adv. Mater. 2011, 23, 926–952. [Google Scholar] [CrossRef] [PubMed]
- Thejo Kalyani, N.; Dhoble, S.J. Organic light emitting diodes: Energy saving lighting technology—A review. Renew. Sustain. Energy Rev. 2012, 16, 2696–2723. [Google Scholar] [CrossRef]
- Yu, T.; Liu, L.; Xie, Z.; Ma, Y. Progress in small-molecule luminescent materials for organic light-emitting diodes. Sci. China Chem. 2015, 58, 907–915. [Google Scholar] [CrossRef]
- Tang, C.W.; Vanslyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Ma, D.; Wang, G.; Hu, Y.; Zhang, Y.; Wang, L.; Jing, X.; Wang, F.; Lee, C.S.; Lee, S.T. A dinuclear aluminum 8-hydroxyquinoline complex with high electron mobility for organic light-emitting diodes. Appl. Phys. Lett. 2003, 82, 1296–1298. [Google Scholar] [CrossRef]
- Burroughes, J.H.; Bradley, D.D.C.; Brown, A.R.; Marks, R.N.; Mackay, K.; Friend, R.H.; Burns, P.L.; Holmes, A.B. Light-emitting diodes based on conjugated polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z. Molecular conformation and packing: Their critical roles in the emission performance of mechanochromic fluorescence materials. Mater. Chem. Front. 2017, 1, 2174–2194. [Google Scholar] [CrossRef]
- Qian, G.; Zhong, Z.; Luo, M.; Yu, D.; Zhang, Z.; Wang, Z.Y.; Ma, D. Simple and efficient near-infrared organic chromophores for light-emitting diodes with single electroluminescent emission above 1000 nm. Adv. Mater. 2009, 21, 111–116. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.; Dong, H.; Meng, L.; Jiang, L.; Jiang, L.; Wang, Y.; Yu, J.; Sun, Y.; Hu, W.; et al. High mobility emissive organic semiconductor. Nat. Commun. 2015, 6, 10032. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Tang, B.Z.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 381, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Li, K.; Liu, B.; Tang, B.Z. Bioprobes based on AIE fluorogens. Acc. Chem. Res. 2013, 46, 2441–2453. [Google Scholar] [CrossRef]
- La, D.D.; Bhosale, S.V.; Jones, L.A.; Bhosale, S.V. Tetraphenylethylene-Based AIE-Active Probes for Sensing Applications. ACS Appl. Mater. Interfaces 2018, 10, 12189–12216. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Law, C.C.W.; Lam, J.W.Y.; Dong, Y.; Lo, S.M.F.; Williams, I.D.; Zhu, D.; Tang, B.Z. Restricted Intramolecular Rotation of 1, 1-Substituted 2, 3, 4, 5-Tetraphenylsiloles. Chem. Mater. 2003, 79, 1535–1546. [Google Scholar] [CrossRef]
- Ren, Y.; Dong, Y.; Lam, J.W.Y.; Tang, B.Z.; Wong, K.S. Studies on the aggregation-induced emission of silole film and crystal by time-resolved fluorescence technique. Chem. Phys. Lett. 2005, 402, 468–473. [Google Scholar] [CrossRef]
- Tang, B.Z.; Zhan, X.; Yu, G.; Sze Lee, P.P.; Liu, Y.; Zhu, D. Efficient blue emission from siloles. J. Mater. Chem. 2001, 11, 2974–2978. [Google Scholar] [CrossRef]
- Yu, M.; Huang, R.; Guo, J.; Zhao, Z.; Tang, B.Z. Promising applications of aggregation-induced emission luminogens in organic optoelectronic devices. Photonix 2020, 1, 11. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 29, 4332–4353. [Google Scholar] [CrossRef]
- Nitti, A.; Villafiorita-Monteleone, F.; Pacini, A.; Botta, C.; Virgili, T.; Forni, A.; Cariati, E.; Boiocchi, M.; Pasini, D. Structure–activity relationship for the solid state emission of a new family of “push–pull” π-extended chromophores. Faraday Discuss. 2017, 196, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, R. Aggregation induced emission: Concluding Remarks. Faraday Discuss. 2017, 196, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Lam, J.W.Y.; Dong, Y.; Tang, B.Z.; Wong, K.S. Enhanced Emission Efficiency and Excited State Lifetime Due to Restricted Intramolecular Motion in Silole Aggregates. J. Phys. Chem. B 2005, 109, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.Z.; Gong, Y.; Chen, S.; Shen, X.Y.; Lam, J.W.Y.; Lu, P.; Lu, Y.; Wang, Z.; Hu, R.; Xie, N.; et al. Efficient solid emitters with aggregation-induced emission and intramolecular charge transfer characteristics: Molecular design, synthesis, photophysical behaviors, and OLED application. Chem. Mater. 2012, 24, 1518–1528. [Google Scholar] [CrossRef]
- Wells, J.M.; Parsons, Z.; Jelly, R.; Payne, A.D.; Lewis, S.W. Negative result: 6-(N,N-dimethylamino)fulvene as a reagent for the detection of latent fingermarks on paper surfaces. Forensic Sci. Int. Rep. 2019, 1, 100005. [Google Scholar] [CrossRef]
- Finke, A.D.; Diederich, F. 6,6-Dicyanopentafulvenes: Teaching an old dog new tricks. Chem. Rec. 2015, 15, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Finke, A.D.; Dumele, O.; Zalibera, M.; Confortin, D.; Cias, P.; Jayamurugan, G.; Gisselbrecht, J.-P.; Boudon, C.; Schweizer, W.B.; Gescheidt, G.; et al. 6,6-Dicyanopentafulvenes: Electronic structure and regioselectivity in [2 + 2] cycloaddition-retroelectrocyclization reactions. J. Am. Chem. Soc. 2012, 134, 18139–18146. [Google Scholar] [CrossRef] [PubMed]
- Finke, A.D.; Jahn, B.O.; Saithalavi, A.; Dahlstrand, C.; Nauroozi, D.; Haberland, S.; Gisselbrecht, J.P.; Boudon, C.; Mijangos, E.; Schweizer, W.B.; et al. The 6,6-dicyanopentafulvene core: A template for the design of electron-acceptor compounds. Chem.—A Eur. J. 2015, 21, 8168–8176. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Rho, J.; Yoon, S.E.; Seok, J.H.; Kim, H.; Min, J.; Yoon, W.; Lee, S.; Yun, H.; Kwon, O.P.; et al. Full Color Tunable Aggregation-Induced Emission Luminogen for Bioimaging Based on an Indolizine Molecular Framework. Bioconjug. Chem. 2020, 31, 2522–2532. [Google Scholar] [CrossRef] [PubMed]
- Peloquin, A.J.; Stone, R.L.; Avila, S.E.; Rudico, E.R.; Horn, C.B.; Gardner, K.A.; Ball, D.W.; Johnson, J.E.B.; Iacono, S.T.; Balaich, G.J. Synthesis of 1, 3-diphenyl-6-alkyl/aryl-substituted fulvene chromophores: Observation of π–π interactions in a 6-pyrene-substituted 1, 3-diphenylfulvene. J. Org. Chem. 2012, 77, 6371–6376. [Google Scholar] [CrossRef]
- Wang, Z.; Shao, H.; Ye, J.; Tang, L.; Lu, P. Dibenzosuberenylidene-Ended Fluorophores: Rapid and Efficient Synthesis, Characterization, and Aggregation-Induced Emissions. J. Phys. Chem. B 2005, 109, 19627–19633. [Google Scholar] [CrossRef] [PubMed]
- Ooyama, Y.; Sagisaka, R.; Enoki, T.; Tsunoji, N.; Ohshita, J. Tetraphenylethene-and diphenyldibenzofulvene-anthracene-based fluorescence sensors possessing photo-induced electron transfer and aggregation-induced emission enhancement characteristics for detection of water. New J. Chem. 2018, 42, 13339–13350. [Google Scholar] [CrossRef]
- Gopikrishna, P.; Iyer, P.K. Monosubstituted dibenzofulvene-based luminogens: Aggregation-induced emission enhancement and dual-state emission. J. Phys. Chem. C 2016, 120, 26556–26568. [Google Scholar] [CrossRef]
- Crocker, R.D.; Zhang, B.; Pace, D.P.; Wong, W.W.H.; Nguyen, T.V. Tetrabenzo[5.7]fulvalene: A forgotten aggregation induced-emission luminogen. Chem. Commun. 2019, 55, 11591–11594. [Google Scholar] [CrossRef]
- Li, Q.; Blancafort, L. A conical intersection model to explain aggregation induced emission in diphenyl dibenzofulvene. Chem. Commun. 2013, 49, 5966–5968. [Google Scholar] [CrossRef]
- Tran, T.; Prlj, A.; Lin, K.H.; Hollas, D.; Corminboeuf, C. Mechanisms of fluorescence quenching in prototypical aggregation-induced emission systems: Excited state dynamics with TD-DFTB. Phys. Chem. Chem. Phys. 2019, 21, 9026–9035. [Google Scholar] [CrossRef]
- Kleinpeter, E.; Fettke, A. Quantification of the (anti)aromaticity of fulvenes subject to ring size. Tetrahedron Lett. 2008, 49, 2776–2781. [Google Scholar] [CrossRef]
- Deeb, O.; Cogan, S.; Zilberg, S. The nature of the S1/S0conical intersection of fulvene. Chem. Phys. 2006, 325, 251–256. [Google Scholar] [CrossRef]
- Najafian, K.; Schleyer, P.V.R.; Tidwell, T.T. Aromaticity and antiaromaticity in fulvenes, ketocyclopolyenes, fulvenones, and diazocyclopolyenes. Org. Biomol. Chem. 2003, 1, 3410–3417. [Google Scholar] [CrossRef]
- Krygowski, T.M.; Oziminski, W.P.; Palusiak, M.; Fowler, P.W.; McKenzie, A.D. Aromaticity of substituted fulvene derivatives: Substituent-dependent ring currents. Phys. Chem. Chem. Phys. 2010, 12, 10740–10745. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.; Ottosson, H.; Kilså, K. Influence of excited state aromaticity in the lowest excited singlet states of fulvene derivatives. Phys. Chem. Chem. Phys. 2011, 13, 12912–12919. [Google Scholar] [CrossRef]
- Coluccini, C.; Anusha, P.T.; Chen, H.-Y.T.; Liao, S.-L.; Ko, Y.K.; Yabushita, A.; Luo, C.W.; Ng, Y.M.; Khung, Y.L. Tuning of the Electro-Optical Properties of Tetraphenylcyclopentadienone via Substitution of Oxygen with Sterically-Hindered Electron Withdrawing Groups. Sci. Rep. 2019, 9, 12762. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Tao, Q.; Gao, Z.; Liu, D. Synthesis and properties of novel spirobifluorene-cored dendrimers. Dye. Pigment. 2012, 94, 136–142. [Google Scholar] [CrossRef]
- Boiani, M.; Baschieri, A.; Cesari, C.; Mazzoni, R.; Stagni, S.; Zacchini, S.; Sambri, L. A new tetraarylcyclopentadienone based low molecular weight gelator: Synthesis, self-assembly properties and anion recognition. New J. Chem. 2012, 36, 1469–1478. [Google Scholar] [CrossRef]
- Andrew, T.L.; Cox, J.R.; Swager, T.M. Synthesis, Reactivity and Electronic Properties of 6, 6-Dicyanofulvenes. Org. Lett. 2010, 12, 5302–5305. [Google Scholar]
- Nath, S.; Pal, H.; Palit, D.K.; Sapre, A.V.; Mittal, J.P. Steady-state and time-resolved studies on photoinduced interaction of phenothiazine and 10-methylphenothiazine with chloroalkanes. J. Phys. Chem. A 1998, 102, 5822–5830. [Google Scholar] [CrossRef]
- Ohki, S. Dielectric constant and refractive index. J. Theor. Biol. 1968, 19, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Manohara, S.R.; Kumar, V.U.; Shivakumaraiah; Gerward, L. Estimation of ground and excited-state dipole moments of 1, 2-diazines by solvatochromic method and quantum-chemical calculation. J. Mol. Liq. 2013, 181, 97–104. [Google Scholar] [CrossRef]
- Mathapati, G.B.; Ingalagondi, P.K.; Patil, O.; Hanagodimath, S.M.; Scholars, R. Estimation of ground and excited state dipole moments of newly synthesized coumarin molecule. Int. J. Res. Anal. Rev. 2018, 5, 1061–1065. [Google Scholar]
- Espinar-Barranco, L.; Meazza, M.; Linares-Perez, A.; Rios, R.; Paredes, J.M.; Crovetto, L. Synthesis, photophysics, and solvatochromic studies of an aggregated-induced-emission luminogen useful in bioimaging. Sensors 2019, 19, 4932. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Maka, V.K.; Moorthy, J.N. Remarkable influence of ‘phane effect’ on the excited-state properties of cofacially oriented coumarins. Phys. Chem. Chem. Phys. 2017, 19, 4758–4767. [Google Scholar] [CrossRef]
- Divac, V.M.; Šakić, D.; Weitner, T.; Gabričević, M. Solvent effects on the absorption and fluorescence spectra of Zaleplon: Determination of ground and excited state dipole moments. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2019, 212, 356–362. [Google Scholar] [CrossRef]
- Duereh, A.; Anantpinijwatna, A.; Latcharote, P. Prediction of solvatochromic polarity parameters for aqueous mixed-solvent systems. Appl. Sci. 2020, 10, 8480. [Google Scholar] [CrossRef]
- Marcus, Y. The use of chemical probes for the characterization of solvent mixtures. Part 2. Aqueous mixtures. J. Chem. Soc. Perkin Trans. 2 1994, 8, 1751–1758. [Google Scholar] [CrossRef]
- Zhu, H.; Li, M.; Hu, J.; Wang, X.; Jie, J.; Guo, Q.; Chen, C.; Xia, A. Ultrafast Investigation of Intramolecular Charge Transfer and Solvation Dynamics of Tetrahydro[5]-helicene-Based Imide Derivatives. Sci. Rep. 2016, 6, 24313. [Google Scholar] [CrossRef] [PubMed]
- Chanmungkalakul, S.; Ervithayasuporn, V.; Boonkitti, P.; Phuekphong, A.; Prigyai, N.; Kladsomboon, S.; Kiatkamjornwong, S. Anion identification using silsesquioxane cages. Chem. Sci. 2018, 9, 7753–7765. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, T.; Dhokale, B.; Misra, R. Effect of the cyano group on solid state photophysical behavior of tetraphenylethene substituted benzothiadiazoles. J. Mater. Chem. C 2015, 3, 9063–9068. [Google Scholar] [CrossRef]
- Loginov, S.V. Electron loss in microsecond megaampere plasma opening switches. IEEE Trans. Plasma Sci. 2011, 39, 3386–3390. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR chemical shifts of trace impurities: Common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
| λabs = nm (e) 1 | λex = nm 2 | λem = nm (Φdil.) 3 | ||||
|---|---|---|---|---|---|---|
| Solvent | CH2Cl2 | CH3CN | CH2Cl2 | CH3CN | CH2Cl2 | CH3CN |
| 5mT | 305 (30) 385 (10) | 300 (20) 365 (7) | 385 | 365 | 435 (0.53) | 465 (0.0024) |
| 5nT | 310 (37) 480 (13) | 305 (32) 460 (10) | 310 | 305 | 435 (0.018) | 435 (0.0010) |
| 5mF | 260 (28) 350 (9) | 260 (27) 335 (9) | 350 | 365 | 435 (0.076) | 435 (0.0012) |
| 5nF | 275 (19) 425 (9) | 270 (24) 410 (11) | - | - | - | - |
| 5mP | 254 (210) 328 (50) | 254 (98) 328 (20) | 328 | 328 | 435 (0.010) | 435 (0.0020) |
| 5nP | 254 (110) 348 (20) 500 (9) | 254 (66) 390 (10) 476 (5) | 348 | 390 | 435 (0.056) | 435 (0.0018) |
| Solvent | d1 | n1 | λabs nm 2 | λem nm 3 | I 4 |
|---|---|---|---|---|---|
| CH2Cl2 | 9.08 | 1.424 | 305 (30) 385 (10) | 435–437 | 0–31 |
| THF | 7.60 | 1.407 | 300 (25) 376 (10) | 431–463 | 0–3.6 |
| CH3CH2OH | 24.55 | 1.363 | 305 (23) 359 (10) | 461–493 | 1.4–6.9 |
| CH3CN | 37.5 | 1.344 | 300 (20) 360 (7) | 460–486 | 0.08–3.80 |
| THF/H2O 1:1 | 41.21 | 1.38 | 300 (25) 376 (10) | 490–496 | 0–2.5 |
| Conc. 1 | a (Å) 2 | μg 3 | μe 3 | Δμ 4 | Δμ 5 | Δμ 6 | Δμ 7 |
|---|---|---|---|---|---|---|---|
| 10−6 M | 5.33 | 6.67 | 6.91 | 23.87 | 13.58 | 5.64 | 6.09 |
| 10−5 M | 5.33 | 6.77 | 8.10 | 26.15 | 14.87 | 3.98 | 6.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, Y.M.; Coluccini, C. Synthesis of New 2D-π-2A Chromophores Based on Tetraphenyl Fulvene and Investigation of Their Optical Properties. Appl. Sci. 2023, 13, 2976. https://doi.org/10.3390/app13052976
Ng YM, Coluccini C. Synthesis of New 2D-π-2A Chromophores Based on Tetraphenyl Fulvene and Investigation of Their Optical Properties. Applied Sciences. 2023; 13(5):2976. https://doi.org/10.3390/app13052976
Chicago/Turabian StyleNg, Yoke Mooi, and Carmine Coluccini. 2023. "Synthesis of New 2D-π-2A Chromophores Based on Tetraphenyl Fulvene and Investigation of Their Optical Properties" Applied Sciences 13, no. 5: 2976. https://doi.org/10.3390/app13052976
APA StyleNg, Y. M., & Coluccini, C. (2023). Synthesis of New 2D-π-2A Chromophores Based on Tetraphenyl Fulvene and Investigation of Their Optical Properties. Applied Sciences, 13(5), 2976. https://doi.org/10.3390/app13052976






