Sex-Based Differences in Bronchial Asthma: What Are the Mechanisms behind Them?
Abstract
1. Introduction
2. Sex- or Gender-Based Differences in Asthma?
3. Endotypes and Phenotypes of Bronchial Asthma
4. Sex-Based Differences in Different Life Periods
4.1. Childhood
4.2. Adolescence
4.3. Adulthood
5. Factors Contributing to Gender-Based Differences in Asthma
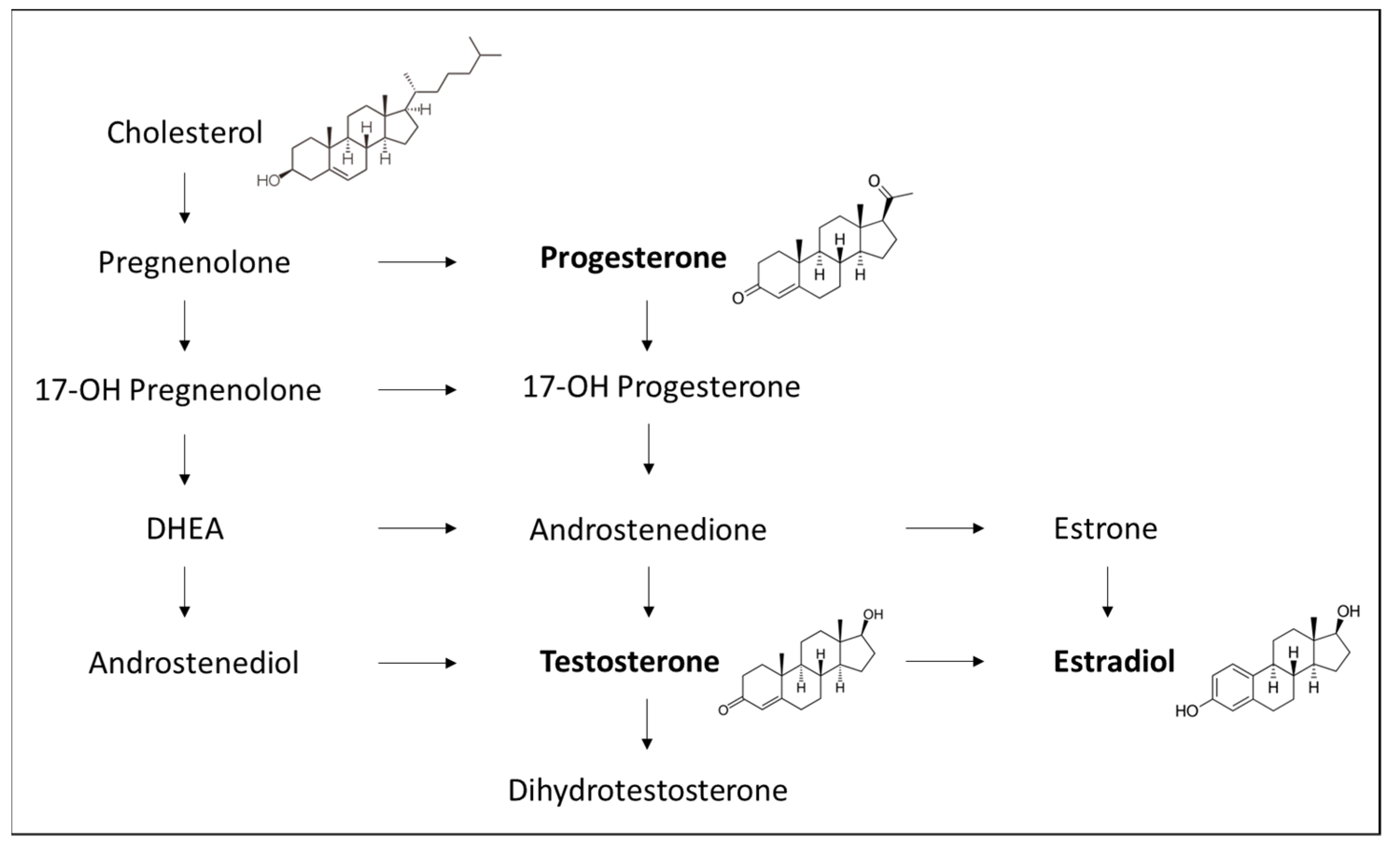
5.1. Sex Hormones
5.1.1. Estrogens
5.1.2. Progesterone
5.1.3. Testosterone
5.2. Anatomical and Physiological Differences in the Lungs
5.3. Obesity and Lifestyle
5.4. Environmental Factors and Smoking
5.5. Chronic Stress
5.6. Genetically Conditioned Factors
5.6.1. Sex-Specific Genes, Epigenetic Changes, and miRNA
5.6.2. Susceptibility to Several Diseases in Relation to Bronchial Asthma
6. Sex-Related Differences in Responses to Therapies Given for Asthma
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Schleich, F.; Demarche, S.; Louis, R. Biomarkers in the Management of Difficult Asthma. Curr. Top. Med. Chem. 2016, 16, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, M.E.; Lee, F.E.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Palova, R.; Adamcakova, J.; Mokra, D.; Mokry, J. Bronchial asthma: Current trends in treatment. Acta Med. Martiniana 2020, 20, 9–17. [Google Scholar] [CrossRef]
- Plevkova, J.; Brozmanova, M.; Harsanyiova, J.; Sterusky, M.; Honetschlager, J.; Buday, T. Various aspects of sex and gender bias in biomedical research. Physiol. Res. 2020, 69, S367–S378. [Google Scholar] [CrossRef] [PubMed]
- LoMauro, A.; Aliverti, A. Sex differences in respiratory function. Breathe 2018, 14, 131–140. [Google Scholar] [CrossRef]
- Clayton, J.A.; Tannenbaum, C. Reporting Sex, Gender, or Both in Clinical Research? JAMA 2016, 316, 1863–1864. [Google Scholar] [CrossRef]
- Tliba, O.; Panettieri, R.A., Jr. Paucigranulocytic asthma: Uncoupling of airway obstruction from inflammation. J. Allergy Clin. Immunol. 2019, 143, 1287–1294. [Google Scholar] [CrossRef]
- Pembrey, L.; Brooks, C.; Mpairwe, H.; Figueiredo, C.A.; Oviedo, A.Y.; Chico, M.; Ali, H.; Nambuya, I.; Tumwesige, P.; Robertson, S.; et al. WASP Study Group. Asthma inflammatory phenotypes on four continents: Most asthma is non-eosinophilic. Int. J. Epidemiol. 2022, dyac173. [Google Scholar] [CrossRef]
- Svenningsen, S.; Nair, P. Asthma Endotypes and an Overview of Targeted Therapy for Asthma. Front. Med. 2017, 4, 158. [Google Scholar] [CrossRef]
- Esteban-Gorgojo, I.; Antolín-Amérigo, D.; Domínguez-Ortega, J.; Quirce, S. Non-eosinophilic asthma: Current perspectives. J. Asthma Allergy. 2018, 11, 267–281. [Google Scholar] [CrossRef]
- Muneswarao, J.; Hassali, M.A.; Ibrahim, B.; Saini, B.; Ali, I.A.H.; Verma, A.K. It is time to change the way we manage mild asthma: An update in GINA 2019. Respir. Res. 2019, 20, 183. [Google Scholar] [CrossRef] [PubMed]
- Hinks, T.S.C.; Levine, S.J.; Brusselle, G.G. Treatment options in type-2 low asthma. Eur. Respir. J. 2021, 57, 2000528. [Google Scholar] [CrossRef]
- Ramadan, A.A.; Gaffin, J.M.; Israel, E.; Phipatanakul, W. Asthma and Corticosteroid Responses in Childhood and Adult Asthma. Clin. Chest Med. 2019, 40, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Chipps, B.E.; Beasley, R.; Panettieri, R.A., Jr.; Israel, E.; Cooper, M.; Dunsire, L.; Jeynes-Ellis, A.; Johnsson, E.; Rees, R.; et al. Albuterol-Budesonide Fixed-Dose Combination Rescue Inhaler for Asthma. N. Engl. J. Med. 2022, 386, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Mahemuti, G.; Zhang, H.; Li, J.; Tieliwaerdi, N.; Ren, L. Efficacy and side effects of intravenous theophylline in acute asthma: A systematic review and meta-analysis. Drug Des. Dev. Ther. 2018, 12, 99–120. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Tamaoki, J.; Nagase, H.; Yamaguchi, M.; Horiguchi, T.; Hozawa, S.; Ichinose, M.; Iwanaga, T.; Kondo, R.; Nagata, M.; et al. Japanese guidelines for adult asthma 2020. Allergol. Int. 2020, 69, 519–548. [Google Scholar] [CrossRef]
- Navinés-Ferrer, A.; Serrano-Candelas, E.; Molina-Molina, G.J.; Martín, M. IgE-Related Chronic Diseases and Anti-IgE-Based Treatments. J. Immunol. Res. 2016, 2016, 8163803. [Google Scholar] [CrossRef]
- McGregor, M.C.; Krings, J.G.; Nair, P.; Castro, M. Role of Biologics in Asthma. Am. J. Respir. Crit. Care Med. 2019, 199, 433–445. [Google Scholar] [CrossRef]
- Bakakos, A.; Rovina, N.; Bakakos, P. Treatment Challenges in Severe Eosinophilic Asthma: Differential Response to Anti-IL-5 and Anti-IL-5R Therapy. Int. J. Mol. Sci. 2021, 22, 3969. [Google Scholar] [CrossRef]
- Farne, H.A.; Wilson, A.; Milan, S.; Banchoff, E.; Yang, F.; Powell, C.V. Anti-IL-5 therapies for asthma. Cochrane Database Syst. Rev. 2022, 7, CD010834. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.; Dávila, I.J.; Domínguez-Ortega, J.; Severe Asthma Group (SEAIC). Clinical Recommendations for the Management of Biological Treatments in Severe Asthma Patients: A Consensus Statement. J. Investig. Allergol. Clin. Immunol. 2021, 31, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Ragnoli, B.; Morjaria, J.; Pignatti, P.; Montuschi, P.; Barbieri, M.; Mondini, L.; Ruggero, L.; Trotta, L.; Malerba, M. Dupilumab and tezepelumab in severe refractory asthma: New opportunities. Ther. Adv. Chronic Dis. 2022, 13, 20406223221097327. [Google Scholar] [CrossRef] [PubMed]
- Marone, G.; Spadaro, G.; Braile, M.; Poto, R.; Criscuolo, G.; Pahima, H.; Loffredo, S.; Levi-Schaffer, F.; Varricchi, G. Tezepelumab: A novel biological therapy for the treatment of severe uncontrolled asthma. Expert Opin. Investig. Drugs. 2019, 28, 931–940. [Google Scholar] [CrossRef]
- Chauhan, B.F.; Jeyaraman, M.M.; Singh Mann, A.; Lys, J.; Abou-Setta, A.M.; Zarychanski, R.; Ducharme, F.M. Addition of anti-leukotriene agents to inhaled corticosteroids for adults and adolescents with persistent asthma. Cochrane Database Syst. Rev. 2017, 3, CD010347. [Google Scholar] [CrossRef]
- Bruno, F.; Spaziano, G.; Liparulo, A.; Roviezzo, F.; Nabavi, S.M.; Sureda, A.; Filosa, R.; D’Agostino, B. Recent advances in the search for novel 5-lipoxygenase inhibitors for the treatment of asthma. Eur. J. Med. Chem. 2018, 153, 65–72. [Google Scholar] [CrossRef]
- Moran, A.; Pavord, I.D. Anti-IL-4/IL-13 for the treatment of asthma: The story so far. Expert Opin. Biol. Ther. 2020, 20, 283–294. [Google Scholar] [CrossRef]
- Braithwaite, I.E.; Cai, F.; Tom, J.A.; Galanter, J.M.; Owen, R.P.; Zhu, R.; Williams, M.; McGregor, A.G.; Eliahu, A.; Durk, M.R.; et al. Inhaled JAK inhibitor GDC-0214 reduces exhaled nitric oxide in patients with mild asthma: A randomized, controlled, proof-of-activity trial. J. Allergy Clin. Immunol. 2021, 148, 783–789. [Google Scholar] [CrossRef]
- Georas, S.N.; Donohue, P.; Connolly, M.; Wechsler, M.E. JAK inhibitors for asthma. J. Allergy Clin. Immunol. 2021, 148, 953–963. [Google Scholar] [CrossRef]
- Sunata, K.; Kabata, H.; Kuno, T.; Takagi, H.; So, M.; Masaki, K.; Fukunaga, K. The effect of statins for asthma. A systematic review and meta-analysis. J. Asthma 2022, 59, 801–810. [Google Scholar] [CrossRef]
- Alabed, M.; Elemam, N.M.; Ramakrishnan, R.K.; Sharif-Askari, N.S.; Kashour, T.; Hamid, Q.; Halwani, R. Therapeutic effect of statins on airway remodeling during asthma. Expert Rev. Respir. Med. 2022, 16, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Hiles, S.A.; McDonald, V.M.; Guilhermino, M.; Brusselle, G.G.; Gibson, P.G. Does maintenance azithromycin reduce asthma exacerbations? An individual participant data meta-analysis. Eur. Respir. J. 2019, 54, 1901381. [Google Scholar] [CrossRef] [PubMed]
- Gonem, S.; Berair, R.; Singapuri, A.; Hartley, R.; Laurencin, M.F.M.; Bacher, G.; Holzhauer, B.; Bourne, M.; Mistry, V.; Pavord, I.D.; et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: A single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir. Med. 2016, 4, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.G.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; Peters, M.J.; Marks, G.B.; Baraket, M.; et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): A randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Kew, K.M.; Dahri, K. Long-acting muscarinic antagonists (LAMA) added to combination long-acting beta2-agonists and inhaled corticosteroids (LABA/ICS) versus LABA/ICS for adults with asthma. Cochrane Database Syst. Rev. 2016, 2016, CD011721. [Google Scholar] [CrossRef]
- Meng, J.F.; Li, H.; Luo, M.J.; Li, H.B. Efficacy of tiotropium in treating patients with moderate-to-severe asthma: A meta-analysis and systematic review based on 14 randomized controlled trials. Medicine 2019, 98, e16637. [Google Scholar] [CrossRef]
- Meltzer, E.O.; Chervinsky, P.; Busse, W.; Ohta, K.; Bardin, P.; Bredenbröker, D.; Bateman, E.D. Roflumilast for asthma: Efficacy findings in placebo-controlled studies. Pulm. Pharmacol. Ther. 2015, 35, S20–S27. [Google Scholar] [CrossRef]
- Al-Sajee, D.; Yin, X.; Gauvreau, G.M. An evaluation of roflumilast and PDE4 inhibitors with a focus on the treatment of asthma. Expert Opin. Pharmacother. 2019, 20, 609–620. [Google Scholar] [CrossRef]
- O’Byrne, P.M.; Metev, H.; Puu, M.; Richter, K.; Keen, C.; Uddin, M.; Larsson, B.; Cullberg, M.; Nair, P. Efficacy and safety of a CXCR2 antagonist, AZD5069, in patients with uncontrolled persistent asthma: A randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2016, 4, 797–806. [Google Scholar] [CrossRef]
- Watz, H.; Uddin, M.; Pedersen, F.; Kirsten, A.; Goldmann, T.; Stellmacher, F.; Groth, E.; Larsson, B.; Böttcher, G.; Malmgren, A.; et al. Effects of the CXCR2 antagonist AZD5069 on lung neutrophil recruitment in asthma. Pulm. Pharmacol. Ther. 2017, 45, 121–123. [Google Scholar] [CrossRef]
- Berry, M.A.; Hargadon, B.; Shelley, M.; Parker, D.; Shaw, D.E.; Green, R.H.; Bradding, P.; Brightling, C.E.; Wardlaw, A.J.; Pavord, I.D. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N. Engl. J. Med. 2006, 354, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Morjaria, J.B.; Chauhan, A.J.; Babu, K.S.; Polosa, R.; Davies, D.E.; Holgate, S.T. The role of a soluble TNFalpha receptor fusion protein (etanercept) in corticosteroid refractory asthma: A double blind, randomised, placebo controlled trial. Thorax 2008, 63, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Holgate, S.; Kerwin, E.; Chon, Y.; Feng, J.; Lin, J.; Lin, S.L. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am. J. Respir. Crit. Care Med. 2013, 188, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Hynes, G.M.; Hinks, T.S.C. The role of interleukin-17 in asthma: A protective response? ERJ Open Res. 2020, 6, 00364-2019. [Google Scholar] [CrossRef]
- Trivedi, A.; Pavord, I.D.; Castro, M. Bronchial thermoplasty and biological therapy as targeted treatments for severe uncontrolled asthma. Lancet Respir. Med. 2016, 4, 585–592. [Google Scholar] [CrossRef]
- Goorsenberg, A.W.M.; d’Hooghe, J.N.S.; Srikanthan, K.; Ten Hacken, N.H.T.; Weersink, E.J.M.; Roelofs, J.J.T.H.; Kemp, S.V.; Bel, E.H.; Shah, P.L.; Annema, J.T.; et al. Bronchial Thermoplasty Induced Airway Smooth Muscle Reduction and Clinical Response in Severe Asthma. The TASMA Randomized Trial. Am. J. Respir. Crit. Care Med. 2021, 203, 175–184. [Google Scholar] [CrossRef]
- Paivandy, A.; Pejler, G. Novel Strategies to Target Mast Cells in Disease. J. Innate Immun. 2021, 13, 131–147. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Bjermer, L. Mast Cell-Mediated Orchestration of the Immune Responses in Human Allergic Asthma: Current Insights. Clin. Rev. Allergy Immunol. 2019, 56, 234–247. [Google Scholar] [CrossRef]
- Shah, R.; Newcomb, D.C. Sex Bias in Asthma Prevalence and Pathogenesis. Front. Immunol. 2018, 9, 2997. [Google Scholar] [CrossRef]
- Chowdhury, N.U.; Guntur, V.P.; Newcomb, D.C.; Wechsler, M.E. Sex and gender in asthma. Eur. Respir. Rev. 2021, 30, 210067. [Google Scholar] [CrossRef]
- Duijts, L. Fetal and infant origins of asthma. Eur. J. Epidemiol. 2012, 27, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Canoy, D.; Pekkanen, J.; Elliott, P.; Pouta, A.; Laitinen, J.; Hartikainen, A.L.; Zitting, P.; Patel, S.; Little, M.P.; Järvelin, M.R. Early growth and adult respiratory function in men and women followed from the fetal period to adulthood. Thorax 2007, 62, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Hancox, R.J.; Poulton, R.; Greene, J.M.; McLachlan, C.R.; Pearce, M.S.; Sears, M.R. Associations between birth weight, early childhood weight gain and adult lung function. Thorax 2009, 64, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Jones, M. Effect of preterm birth on airway function and lung growth. Paediatr. Respir. Rev. 2009, 10, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Fanaroff, A.A.; Wright, L.L.; Stevenson, D.K.; Shankaran, S.; Donovan, E.F.; Ehrenkranz, R.A.; Younes, N.; Korones, S.B.; Stoll, B.J.; Tyson, J.E.; et al. Very-low-birth-weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, May 1991 through December 1992. Am. J. Obstet. Gynecol. 1995, 173, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Ingemarsson, I. Gender aspects of preterm birth. BJOG 2003, 110, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Manktelow, B.N.; Lal, M.K.; Field, D.J.; Sinha, S.K. Antenatal corticosteroids and neonatal outcomes according to gestational age: A cohort study. Arch. Dis. Child. Fetal Neonatal Ed. 2010, 95, F95–F98. [Google Scholar] [CrossRef] [PubMed]
- Gortner, L.; Shen, J.; Tutdibi, E. Sexual dimorphism of neonatal lung development. Klin. Padiatr. 2013, 225, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Carey, M.A.; Card, J.W.; Voltz, J.W.; Arbes, S.J., Jr.; Germolec, D.R.; Korach, K.S.; Zeldin, D.C. It’s all about sex: Gender, lung development and lung disease. Trends Endocrinol. Metab. 2007, 18, 308–313. [Google Scholar] [CrossRef]
- Pei, L.; Chen, G.; Mi, J.; Zhang, T.; Song, X.; Chen, J.; Ji, Y.; Li, C.; Zheng, X. Low birth weight and lung function in adulthood: Retrospective cohort study in China, 1948-1996. Pediatrics 2010, 125, e899–e905. [Google Scholar] [CrossRef]
- Sonnenschein-van der Voort, A.M.; Arends, L.R.; de Jongste, J.C.; Annesi-Maesano, I.; Arshad, S.H.; Barros, H.; Basterrechea, M.; Bisgaard, H.; Chatzi, L.; Corpeleijn, E.; et al. Preterm birth, infant weight gain, and childhood asthma risk: A meta-analysis of 147,000 European children. J. Allergy Clin. Immunol. 2014, 133, 1317–1329. [Google Scholar] [CrossRef]
- Matheson, M.C.; D Olhaberriague, A.L.; Burgess, J.A.; Giles, G.G.; Hopper, J.L.; Johns, D.P.; Abramson, M.J.; Walters, E.H.; Dharmage, S.C. Preterm birth and low birth weight continue to increase the risk of asthma from age 7 to 43. J. Asthma 2017, 54, 616–623. [Google Scholar] [CrossRef]
- Takata, N.; Tanaka, K.; Nagata, C.; Arakawa, M.; Miyake, Y. Preterm birth is associated with higher prevalence of wheeze and asthma in a selected population of Japanese children aged three years. Allergol. Immunopathol. 2019, 47, 425–430. [Google Scholar] [CrossRef]
- Castro-Rodriguez, J.A.; Forno, E.; Rodriguez-Martinez, C.E.; Celedón, J.C. Risk and Protective Factors for Childhood Asthma: What Is the Evidence? J. Allergy Clin. Immunol. Pract. 2016, 4, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Lux, A.L.; Henderson, A.J.; Pocock, S.J. Wheeze associated with prenatal tobacco smoke exposure: A prospective, longitudinal study. ALSPAC study team. Arch. Dis. Child. 2000, 83, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Lannerö, E.; Wickman, M.; Pershagen, G.; Nordvall, L. Maternal smoking during pregnancy increases the risk of recurrent wheezing during the first years of life (BAMSE). Respir. Res. 2006, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Håberg, S.E.; Stigum, H.; Nystad, W.; Nafstad, P. Effects of pre- and postnatal exposure to parental smoking on early childhood respiratory health. Am. J. Epidemiol. 2007, 166, 679–686. [Google Scholar] [CrossRef]
- Forno, E.; Young, O.M.; Kumar, R.; Simhan, H.; Celedón, J.C. Maternal obesity in pregnancy, gestational weight gain, and risk of childhood asthma. Pediatrics 2014, 134, e535–e546. [Google Scholar] [CrossRef]
- Zhao, D.; Su, H.; Cheng, J.; Wang, X.; Xie, M.; Li, K.; Wen, L.; Yang, H. Prenatal antibiotic use and risk of childhood wheeze/asthma: A meta-analysis. Pediatr. Allergy Immunol. 2015, 26, 756–764. [Google Scholar] [CrossRef]
- Hasunuma, H.; Yoda, Y.; Tokuda, N.; Taniguchi, N.; Takeshima, Y.; Shima, M.; Japan Environment and Children’s Study (JECS) Group. Effects of early-life exposure to dust mite allergen and endotoxin on the development of asthma and wheezing: The Japan Environment and Children’s Study. Clin. Transl. Allergy 2021, 11, e12071. [Google Scholar] [CrossRef]
- Jensen, M.E.; Murphy, V.E.; Gibson, P.G.; Mattes, J.; Camargo, C.A., Jr. Vitamin D status in pregnant women with asthma and its association with adverse respiratory outcomes during infancy. J. Matern. Fetal Neonatal Med. 2019, 32, 1820–1825. [Google Scholar] [CrossRef]
- Hayatbakhsh, M.R.; Sadasivam, S.; Mamun, A.A.; Najman, J.M.; Williams, G.M.; O’Callaghan, M.J. Maternal smoking during and after pregnancy and lung function in early adulthood: A prospective study. Thorax 2009, 64, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Duijts, L.; Jaddoe, V.W.V.; van der Valk, R.J.P.; Henderson, J.A.; Hofman, A.; Raat, H.; Steegers, E.A.P.; Moll, H.A.; de Jongste, J.C. Fetal exposure to maternal and paternal smoking and the risks of wheezing in preschool children. Chest 2012, 141, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Wijga, A.; Tabak, C.; Postma, D.S.; Kerkhof, M.; Wieringa, M.H.; Hoekstra, M.O.; Brunekreef, B.; de Jongste, J.C.; Smit, H.A. Sex differences in asthma during the first 8 years of life: The Prevention and Incidence of Asthma and Mite Allergy (PIAMA) birth cohort study. J. Allergy Clin. Immunol. 2011, 127, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Freishtat, R.J.; Gordish-Dressman, H.; Teach, S.J.; Resca, L.; Hoffman, E.P.; Wang, Z. Natural progression of childhood asthma symptoms and strong influence of sex and puberty. Ann. Am. Thorac. Soc. 2014, 11, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Fuseini, H.; Newcomb, D.C. Mechanisms Driving Gender Differences in Asthma. Curr. Allergy Asthma Rep. 2017, 17, 19. [Google Scholar] [CrossRef]
- Naeem, A.; Silveyra, P. Sex Differences in Paediatric and Adult Asthma. Eur. Med. J. (Chelmsf). 2019, 4, 27–35. [Google Scholar] [CrossRef]
- Uekert, S.J.; Akan, G.; Evans, M.D.; Li, Z.; Roberg, K.; Tisler, C.; Dasilva, D.; Anderson, E.; Gangnon, R.; Allen, D.B.; et al. Sex-related differences in immune development and the expression of atopy in early childhood. J. Allergy Clin. Immunol. 2006, 118, 1375–1381. [Google Scholar] [CrossRef]
- Mohammad, H.R.; Belgrave, D.; Kopec Harding, K.; Murray, C.S.; Simpson, A.; Custovic, A. Age, sex and the association between skin test responses and IgE titres with asthma. Pediatr. Allergy Immunol. 2016, 27, 313–319. [Google Scholar] [CrossRef]
- Bibi, H.; Shoseyov, D.; Feigenbaum, D.; Genis, M.; Friger, M.; Peled, R.; Sharff, S. The relationship between asthma and obesity in children: Is it real or a case of over diagnosis? J. Asthma 2004, 41, 403–410. [Google Scholar] [CrossRef]
- Ulrik, C.S.; Lophaven, S.N.; Andersen, Z.J.; Sørensen, T.I.; Baker, J.L. BMI at school age and incident asthma admissions in early adulthood: A prospective study of 310,211 children. Clin. Epidemiol. 2018, 10, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rodriguez, J.A.; Holberg, C.J.; Morgan, W.J.; Wright, A.L.; Martinez, F.D. Increased incidence of asthmalike symptoms in girls who become overweight or obese during the school years. Am. J. Respir. Crit. Care Med. 2001, 163, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Ekström, S.; Magnusson, J.; Kull, I.; Andersson, N.; Bottai, M.; Besharat Pour, M.; Melén, E.; Bergström, A. Body mass index development and asthma throughout childhood. Am. J. Epidemiol. 2017, 186, 255–263. [Google Scholar] [CrossRef]
- Chen, Y.; Stewart, P.; Johansen, H.; McRae, L.; Taylor, G. Sex difference in hospitalization due to asthma in relation to age. J. Clin. Epidemiol. 2003, 56, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Schatz, M.; Camargo, C.A., Jr. The relationship of sex to asthma prevalence, health care utilization, and medications in a large managed care organization. Ann. Allergy Asthma Immunol. 2003, 91, 553–558. [Google Scholar] [CrossRef] [PubMed]
- de Marco, R.; Locatelli, F.; Sunyer, J.; Burney, P. Differences in incidence of reported asthma related to age in men and women. A retrospective analysis of the data of the European Respiratory Health Survey. Am. J. Respir. Crit. Care Med. 2000, 162, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Fagan, J.K.; Scheff, P.A.; Hryhorczuk, D.; Ramakrishnan, V.; Ross, M.; Persky, V. Prevalence of asthma and other allergic diseases in an adolescent population: Association with gender and race. Ann. Allergy Asthma Immunol. 2001, 86, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, O.; Scherzer, H.H.; DeGraff, A.C., Jr. Morbidity in asthma in relation to the menstrual cycle. J. Allergy Clin. Immunol. 1986, 77, 87–94. [Google Scholar] [CrossRef]
- Shames, R.S.; Heilbron, D.C.; Janson, S.L.; Kishiyama, J.L.; Au, D.S.; Adelman, D.C. Clinical differences among women with and without self-reported perimenstrual asthma. Ann. Allergy Asthma Immunol. 1998, 81, 65–72. [Google Scholar] [CrossRef]
- Rao, C.K.; Moore, C.G.; Bleecker, E.; Busse, W.W.; Calhoun, W.; Castro, M.; Chung, K.F.; Erzurum, S.C.; Israel, E.; Curran-Everett, D.; et al. Characteristics of perimenstrual asthma and its relation to asthma severity and control: Data from the Severe Asthma Research Program. Chest 2013, 143, 984–992. [Google Scholar] [CrossRef]
- Pauli, B.D.; Reid, R.L.; Munt, P.W.; Wigle, R.D.; Forkert, L. Influence of the menstrual cycle on airway function in asthmatic and normal subjects. Am. Rev. Respir. Dis. 1989, 140, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Farha, S.; Asosingh, K.; Laskowski, D.; Hammel, J.; Dweik, R.A.; Wiedemann, H.P.; Erzurum, S.C. Effects of the menstrual cycle on lung function variables in women with asthma. Am. J. Respir. Crit. Care Med. 2009, 180, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Eid, R.C.; Palumbo, M.L.; Cahill, K.N. Perimenstrual Asthma in Aspirin-Exacerbated Respiratory Disease. J. Allergy Clin. Immunol. Pract. 2020, 8, 573–578.e4. [Google Scholar] [CrossRef] [PubMed]
- Macsali, F.; Real, F.G.; Omenaas, E.R.; Bjorge, L.; Janson, C.; Franklin, K.; Svanes, C. Oral contraception, body mass index, and asthma: A cross-sectional Nordic-Baltic population survey. J. Allergy Clin. Immunol. 2009, 123, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Juniper, E.F.; Kline, P.A.; Roberts, R.S.; Hargreave, F.E.; Daniel, E.E. Airway responsiveness to methacholine during the natural menstrual cycle and the effect of oral contraceptives. Am. Rev. Respir. Dis. 1987, 135, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Nwaru, B.I.; Sheikh, A. Hormonal contraceptives and asthma in women of reproductive age: Analysis of data from serial national Scottish Health Surveys. J. R. Soc. Med. 2015, 108, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Schatz, M.; Harden, K.; Forsythe, A.; Chilingar, L.; Hoffman, C.; Sperling, W.; Zeiger, R.S. The course of asthma during pregnancy, post partum, and with successive pregnancies: A prospective analysis. J. Allergy Clin. Immunol. 1988, 81, 509–517. [Google Scholar] [CrossRef]
- Belanger, K.; Hellenbrand, M.E.; Holford, T.R.; Bracken, M. Effect of pregnancy on maternal asthma symptoms and medication use. Obs. Gynecol. 2010, 115, 559–567. [Google Scholar] [CrossRef]
- Juniper, E.F.; Daniel, E.E.; Roberts, R.S.; Kline, P.A.; Hargreave, F.E.; Newhouse, M.T. Improvement in airway responsiveness and asthma severity during pregnancy. A prospective study. Am. Rev. Respir. Dis. 1989, 140, 924–931. [Google Scholar] [CrossRef]
- Schatz, M.; Dombrowski, M.P.; Wise, R.; Thom, E.A.; Landon, M.; Mabie, W.; Newman, R.B.; Hauth, J.C.; Lindheimer, M.; Caritis, S.N.; et al. Asthma morbidity during pregnancy can be predicted by severity classification. J. Allergy Clin. Immunol. 2003, 112, 283–288. [Google Scholar] [CrossRef]
- Troisi, R.J.; Speizer, F.E.; Willett, W.C.; Trichopoulos, D.; Rosner, B. Menopause, postmenopausal estrogen preparations, and the risk of adultonset asthma: A prospective cohort study. Am. J. Respir Crit. Care Med. 1995, 152, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Gómez Real, F.; Svanes, C.; Bjornsson, E.H.; Franklin, K.A.; Gislason, D.; Gislason, T.; Gulsvik, A.; Janson, C.; Jögi, R.; Kiserud, T.; et al. Hormone replacement therapy, body mass index and asthma in perimenopausal women: A cross sectional survey. Thorax 2006, 61, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Real, F.G.; Svanes, C.; Omenaas, E.R.; Antò, J.M.; Plana, E.; Jarvis, D.; Janson, C.; Neukirch, F.; Zemp, E.; Dratva, J.; et al. Lung function, respiratory symptoms, and the menopausal transition. J. Allergy Clin. Immunol. 2008, 121, 72–80.e3. [Google Scholar] [CrossRef] [PubMed]
- Triebner, K.; Johannessen, A.; Puggini, L.; Benediktsdóttir, B.; Bertelsen, R.J.; Bifulco, E.; Dharmage, S.C.; Dratva, J.; Franklin, K.A.; Gíslason, T.; et al. Menopause as a predictor of new-onset asthma: A longitudinal Northern European population study. J. Allergy Clin. Immunol. 2016, 137, 50–57. [Google Scholar] [CrossRef]
- Scioscia, G.; Carpagnano, G.E.; Lacedonia, D.; Soccio, P.; Quarato, C.M.I.; Trabace, L.; Fuso, P.; Barbaro, M.P.F. The Role of Airways 17β-Estradiol as a Biomarker of Severity in Postmenopausal Asthma: A Pilot Study. J. Clin. Med. 2020, 9, 2037. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Shah, S.A.; Tibble, H.; Pillinger, R.; McLean, S.; Ryan, D.; Critchley, H.; Hawrylowicz, C.M.; Simpson, C.R.; Soyiri, I.N.; et al. Hormone Replacement Therapy and Risk of Severe Asthma Exacerbation in Perimenopausal and Postmenopausal Women: 17-Year National Cohort Study. J. Allergy Clin. Immunol. Pract. 2021, 9, 2751–2760.e1. [Google Scholar] [CrossRef]
- Shah, S.A.; Tibble, H.; Pillinger, R.; McLean, S.; Ryan, D.; Critchley, H.; Price, D.; Hawrylowicz, C.M.; Simpson, C.R.; Soyiri, I.N.; et al. Hormone replacement therapy and asthma onset in menopausal women: National cohort study. J. Allergy Clin. Immunol. 2021, 147, 1662–1670. [Google Scholar] [CrossRef]
- Chen, Y.; Dales, R.; Tang, M.; Krewski, D. Obesity may increase the incidence of asthma in women but not in men: Longitudinal observations from the Canadian national population health surveys. Am. J. Epidemiol. 2002, 155, 191–197. [Google Scholar] [CrossRef]
- Loerbroks, A.; Apfelbacher, C.J.; Amelang, M.; Stürmer, T. Obesity and adult asthma: Potential effect modification by gender, but not by hay fever. Ann. Epidemiol. 2008, 18, 283–289. [Google Scholar] [CrossRef]
- Sutherland, E.R.; Goleva, E.; King, T.S.; Lehman, E.; Stevens, A.D.; Jackson, L.P.; Stream, A.R.; Fahy, J.V.; Leung, D.Y.; Asthma Clinical Research Network. Cluster analysis of obesity and asthma phenotypes. PLoS ONE 2012, 7, e36631. [Google Scholar] [CrossRef]
- Scott, H.A.; Gibson, P.G.; Garg, M.L.; Upham, J.W.; Wood, L.G. Sex hormones and systemic inflammation are modulators of the obese-asthma phenotype. Allergy 2016, 71, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Pramanik, J.; Mahata, B. Revisiting steroidogenesis and its role in immune regulation with the advanced tools and technologies. Genes Immun. 2021, 22, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Ambhore, N.S.; Kalidhindi, R.S.R.; Sathish, V. Sex-Steroid Signaling in Lung Diseases and Inflammation. Adv. Exp. Med. Biol. 2021, 1303, 243–273. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Lopez, F.R.; Larrad-Mur, L.; Kallen, A.; Chedraui, P.; Taylor, H.S. Gender differences in cardiovascular disease: Hormonal and biochemical influences. Reprod. Sci. 2010, 17, 511–531. [Google Scholar] [CrossRef]
- Shufelt, C.L.; Pacheco, C.; Tweet, M.S.; Miller, V.M. Sex-Specific Physiology and Cardiovascular Disease. Adv. Exp. Med. Biol. 2018, 1065, 433–454. [Google Scholar] [CrossRef]
- Stanhewicz, A.E.; Wenner, M.M.; Stachenfeld, N.S. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1569–H1588. [Google Scholar] [CrossRef]
- Gaignard, P.; Fréchou, M.; Liere, P.; Thérond, P.; Schumacher, M.; Slama, A.; Guennoun, R. Sex differences in brain mitochondrial metabolism: Influence of endogenous steroids and stroke. J. Neuroendocrinol. 2018, 30, e12497. [Google Scholar] [CrossRef]
- Rainville, J.R.; Tsyglakova, M.; Hodes, G.E. Deciphering sex differences in the immune system and depression. Front. Neuroendocrinol. 2018, 50, 67–90. [Google Scholar] [CrossRef]
- Rubinow, D.R.; Schmidt, P.J. Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology 2019, 44, 111–128. [Google Scholar] [CrossRef]
- Palmisano, B.T.; Zhu, L.; Stafford, J.M. Role of Estrogens in the Regulation of Liver Lipid Metabolism. Adv. Exp. Med. Biol. 2017, 1043, 227–256. [Google Scholar] [CrossRef]
- Palmisano, B.T.; Zhu, L.; Eckel, R.H.; Stafford, J.M. Sex differences in lipid and lipoprotein metabolism. Mol. Metab. 2018, 15, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.F.; Montagner, A.; Gourdy, P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Reyes-García, J.; Montaño, L.M.; Carbajal-García, A.; Wang, Y.X. Sex Hormones and Lung Inflammation. Adv. Exp. Med. Biol. 2021, 1304, 259–321. [Google Scholar] [CrossRef] [PubMed]
- Plevkova, J.; Buday, T.; Kavalcikova-Bogdanova, N.; Ioan, I.; Demoulin-Alexikova, S. Sex differences in cough reflex. Respir. Physiol. Neurobiol. 2017, 245, 122–129. [Google Scholar] [CrossRef]
- Miller, W.L. Steroidogenesis: Unanswered Questions. Trends Endocrinol. Metab. 2017, 28, 771–793. [Google Scholar] [CrossRef]
- Cholesterol. Available online: https://en.wikipedia.org/wiki/Cholesterol (accessed on 5 January 2023).
- Progesterone. Available online: https://en.wikipedia.org/wiki/Progesterone (accessed on 5 January 2023).
- Testosterone. Available online: https://en.wikipedia.org/wiki/Testosterone (accessed on 5 January 2023).
- Estradiol. Available online: https://en.wikipedia.org/wiki/Estradiol (accessed on 5 January 2023).
- Nelson, L.R.; Bulun, S.E. Estrogen production and action. J. Am. Acad. Dermatol. 2001, 45, S116–S124. [Google Scholar] [CrossRef]
- Katzenellenbogen, B.S. Estrogen receptors: Bioactivities and interactions with cell signaling pathways. Biol. Reprod. 1996, 54, 287–293. [Google Scholar] [CrossRef]
- Thakur, M.K.; Paramanik, V. Role of steroid hormone coregulators in health and disease. Horm. Res. 2009, 71, 194–200. [Google Scholar] [CrossRef]
- Samavat, H.; Kurzer, M.S. Estrogen metabolism and breast cancer. Cancer Lett. 2015, 356, 231–243. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef]
- Klinge, C.M. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001, 29, 2905–2919. [Google Scholar] [CrossRef] [PubMed]
- Göttlicher, M.; Heck, S.; Herrlich, P. Transcriptional cross-talk, the second mode of steroid hormone receptor action. J. Mol. Med. 1998, 76, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Lösel, R.; Wehling, M. Nongenomic actions of steroid hormones. Nat. Rev. Mol. Cell. Biol. 2003, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Björnström, L.; Sjöberg, M. Mechanisms of estrogen receptor signaling: Convergence of genomic and nongenomic actions on target genes. Mol. Endocrinol. 2005, 19, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Vrtačnik, P.; Ostanek, B.; Mencej-Bedrač, S.; Marc, J. The many faces of estrogen signaling. Biochem. Med. 2014, 24, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Shang, Y. Estrogen and cancer. Annu. Rev. Physiol. 2013, 75, 225–240. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Shughrue, P.J.; Merchenthaler, I.; Gustafsson, J.A. The estrogen receptor beta subtype: A novel mediator of estrogen action in neuroendocrine systems. Front. Neuroendocrinol. 1998, 19, 253–286. [Google Scholar] [CrossRef]
- Hall, J.M.; McDonnell, D.P. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 1999, 140, 5566–5578. [Google Scholar] [CrossRef]
- Ivanova, M.M.; Mazhawidza, W.; Dougherty, S.M.; Minna, J.D.; Klinge, C.M. Activity and intracellular location of estrogen receptors alpha and beta in human bronchial epithelial cells. Mol. Cell. Endocrinol. 2009, 305, 12–21. [Google Scholar] [CrossRef]
- Chiarella, S.E.; Cardet, J.C.; Prakash, Y.S. Sex, Cells, and Asthma. Mayo Clin. Proc. 2021, 96, 1955–1969. [Google Scholar] [CrossRef]
- Townsend, E.A.; Meuchel, L.W.; Thompson, M.A.; Pabelick, C.M.; Prakash, Y.S. Estrogen increases nitric-oxide production in human bronchial epithelium. J. Pharmacol. Exp. Ther. 2011, 339, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.C.; Moreno, S.; Robertson, L.; Robinson, S.; Gant, K.; Bryant, A.J.; Sabo-Attwood, T. Transforming growth factor beta1 targets estrogen receptor signaling in bronchial epithelial cells. Respir. Res. 2018, 19, 160. [Google Scholar] [CrossRef] [PubMed]
- Tam, A.; Wadsworth, S.; Dorscheid, D.; Man, S.F.; Sin, D.D. Estradiol increases mucus synthesis in bronchial epithelial cells. PLoS ONE 2014, 9, e100633. [Google Scholar] [CrossRef] [PubMed]
- Degano, B.; Prévost, M.C.; Berger, P.; Molimard, M.; Pontier, S.; Rami, J.; Escamilla, R. Estradiol decreases the acetylcholine-elicited airway reactivity in ovariectomized rats through an increase in epithelial acetylcholinesterase activity. Am. J. Respir. Crit. Care Med. 2001, 164, 1849–1854. [Google Scholar] [CrossRef] [PubMed]
- Card, J.W.; Carey, M.A.; Bradbury, J.A.; DeGraff, L.M.; Morgan, D.L.; Moorman, M.P.; Flake, G.P.; Zeldin, D.C. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J. Immunol. 2006, 177, 621–630. [Google Scholar] [CrossRef]
- Dimitropoulou, C.; White, R.E.; Ownby, D.R.; Catravas, J.D. Estrogen reduces carbachol-induced constriction of asthmatic airways by stimulating large-conductance voltage and calcium-dependent potassium channels. Am. J. Respir. Cell. Mol. Biol. 2005, 32, 239–247. [Google Scholar] [CrossRef]
- Matsubara, S.; Swasey, C.H.; Loader, J.E.; Dakhama, A.; Joetham, A.; Ohnishi, H.; Balhorn, A.; Miyahara, N.; Takeda, K.; Gelfand, E.W. Estrogen determines sex differences in airway responsiveness after allergen exposure. Am. J. Respir. Cell. Mol. Biol. 2008, 38, 501–508. [Google Scholar] [CrossRef]
- Hayashi, T.; Adachi, Y.; Hasegawa, K.; Morimoto, M. Less sensitivity for late airway inflammation in males than females in BALB/c mice. Scand. J. Immunol. 2003, 57, 562–567. [Google Scholar] [CrossRef]
- Corteling, R.; Trifilieff, A. Gender comparison in a murine model of allergen-driven airway inflammation and the response to budesonide treatment. BMC. Pharmacol. 2004, 4, 4. [Google Scholar] [CrossRef]
- Melgert, B.N.; Postma, D.S.; Kuipers, I.; Geerlings, M.; Luinge, M.A.; van der Strate, B.W.; Kerstjens, H.A.; Timens, W.; Hylkema, M.N. Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin. Exp. Allergy 2005, 35, 1496–1503. [Google Scholar] [CrossRef]
- Paharkova-Vatchkova, V.; Maldonado, R.; Kovats, S. Estrogen preferentially promotes the differentiation of CD11c+ CD11b(intermediate) dendritic cells from bone marrow precursors. J. Immunol. 2004, 172, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Cephus, J.Y.; Stier, M.T.; Fuseini, H.; Yung, J.A.; Toki, S.; Bloodworth, M.H.; Zhou, W.; Goleniewska, K.; Zhang, J.; Garon, S.L.; et al. Testosterone Attenuates Group 2 Innate Lymphoid Cell-Mediated Airway Inflammation. Cell. Rep. 2017, 21, 2487–2499. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Tanabe, M.; Ito, W.; Ueki, S.; Konnno, Y.; Chihara, M.; Itoga, M.; Kobayashi, Y.; Moritoki, Y.; Kayaba, H.; et al. Gender difference in allergic airway remodelling and immunoglobulin production in mouse model of asthma. Respirology 2013, 18, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Maret, A.; Coudert, J.D.; Garidou, L.; Foucras, G.; Gourdy, P.; Krust, A.; Dupont, S.; Chambon, P.; Druet, P.; Bayard, F.; et al. Estradiol enhances primary antigen-specific CD4 T cell responses and Th1 development in vivo. Essential role of estrogen receptor alpha expression in hematopoietic cells. Eur. J. Immunol. 2003, 33, 512–521. [Google Scholar] [CrossRef]
- Conneely, O.M.; Mulac-Jericevic, B.; Lydon, J.P. Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids 2003, 68, 771–778. [Google Scholar] [CrossRef]
- Giangrande, P.H.; McDonnell, D.P. The A and B isoforms of the human progesterone receptor: Two functionally different transcription factors encoded by a single gene. Recent Prog. Horm. Res. 1999, 54, 291–313. [Google Scholar]
- Piette, P.C.M. The pharmacodynamics and safety of progesterone. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 69, 13–29. [Google Scholar] [CrossRef]
- Asavasupreechar, T.; Saito, R.; Miki, Y.; Edwards, D.P.; Boonyaratanakornkit, V.; Sasano, H. Systemic distribution of progesterone receptor subtypes in human tissues. J. Steroid. Biochem. Mol. Biol. 2020, 199, 105599. [Google Scholar] [CrossRef]
- Hellings, P.W.; Vandekerckhove, P.; Claeys, R.; Billen, J.; Kasran, A.; Ceuppens, J.L. Progesterone increases airway eosinophilia and hyper-responsiveness in a murine model of allergic asthma. Clin. Exp. Allergy 2003, 33, 1457–1463. [Google Scholar] [CrossRef]
- Mitchell, V.L.; Gershwin, L.J. Progesterone and environmental tobacco smoke act synergistically to exacerbate the development of allergic asthma in a mouse model. Clin. Exp. Allergy 2007, 37, 276–286. [Google Scholar] [CrossRef]
- Jain, R.; Ray, J.M.; Pan, J.H.; Brody, S.L. Sex hormone-dependent regulation of cilia beat frequency in airway epithelium. Am. J. Respir. Cell. Mol. Biol. 2012, 46, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Saltzman, A.; Yeh, S.; Young, W.; Keller, E.; Lee, H.J.; Wang, C.; Mizokami, A. Androgen receptor: An overview. Crit. Rev. Eukaryot. Gene Expr. 1995, 5, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Mikkonen, L.; Pihlajamaa, P.; Sahu, B.; Zhang, F.P.; Jänne, O.A. Androgen receptor and androgen-dependent gene expression in lung. Mol. Cell. Endocrinol. 2010, 317, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Fuseini, H.; Yung, J.A.; Cephus, J.Y.; Zhang, J.; Goleniewska, K.; Polosukhin, V.V.; Peebles, R.S., Jr.; Newcomb, D.C. Testosterone Decreases House Dust Mite-Induced Type 2 and IL-17A-Mediated Airway Inflammation. J. Immunol. 2018, 201, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Suto, W.; Kai, Y.; Chiba, Y. Mechanisms underlying the pathogenesis of hyper-contractility of bronchial smooth muscle in allergic asthma. J. Smooth Muscle Res. 2017, 53, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Card, J.W.; Voltz, J.W.; Ferguson, C.D.; Carey, M.A.; DeGraff, L.M.; Peddada, S.D.; Morgan, D.L.; Zeldin, D.C. Male sex hormones promote vagally mediated reflex airway responsiveness to cholinergic stimulation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L908–L914. [Google Scholar] [CrossRef]
- Bulkhi, A.A.; Shepard, K.V., 2nd; Casale, T.B.; Cardet, J.C. Elevated Testosterone Is Associated with Decreased Likelihood of Current Asthma Regardless of Sex. J. Allergy Clin. Immunol. Pract. 2020, 8, 3029–3035.e4. [Google Scholar] [CrossRef]
- Zein, J.G.; McManus, J.M.; Sharifi, N.; Erzurum, S.C.; Marozkina, N.; Lahm, T.; Giddings, O.; Davis, M.D.; DeBoer, M.D.; Comhair, S.A.; et al. Benefits of Airway Androgen Receptor Expression in Human Asthma. Am. J. Respir. Crit. Care Med. 2021, 204, 285–293. [Google Scholar] [CrossRef]
- McManus, J.M.; Gaston, B.; Zein, J.; Sharifi, N. Association Between Asthma and Reduced Androgen Receptor Expression in Airways. J. Endocr. Soc. 2022, 6, bvac047. [Google Scholar] [CrossRef]
- Weinstein, R.E.; Lobocki, C.A.; Gravett, S.; Hum, H.; Negrich, R.; Herbst, J.; Greenberg, D.; Pieper, D.R. Decreased adrenal sex steroid levels in the absence of glucocorticoid suppression in postmenopausal asthmatic women. J. Allergy Clin. Immunol. 1996, 97, 1–8. [Google Scholar] [CrossRef]
- Montaño, L.M.; Flores-Soto, E.; Sommer, B.; Solís-Chagoyán, H.; Perusquía, M. Androgens are effective bronchodilators with anti-inflammatory properties: A potential alternative for asthma therapy. Steroids 2020, 153, 108509. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xiang, X.; Ji, X.; Wang, W.; Luo, M.; Luo, S.; Li, K.; Gong, S.; Liu, S.; Ma, L.; et al. Effects and mechanism of dehydroepiandrosterone on epithelial-mesenchymal transition in bronchial epithelial cells. Exp. Lung Res. 2014, 40, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Koziol-White, C.J.; Goncharova, E.A.; Cao, G.; Johnson, M.; Krymskaya, V.P.; Panettieri, R.A., Jr. DHEA-S inhibits human neutrophil and human airway smooth muscle migration. Biochim. Biophys. Acta 2012, 1822, 1638–1642. [Google Scholar] [CrossRef] [PubMed]
- Marozkina, N.; Zein, J.; DeBoer, M.D.; Logan, L.; Veri, L.; Ross, K.; Gaston, B. Dehydroepiandrosterone Supplementation May Benefit Women with Asthma Who Have Low Androgen Levels: A Pilot Study. Pulm. Ther. 2019, 5, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Ekpruke, C.D.; Silveyra, P. Sex Differences in Airway Remodeling and Inflammation: Clinical and Biological Factors. Front. Allergy. 2022, 3, 875295. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Özuygur Ermis, S.S.; Rådinger, M.; Bossios, A.; Kankaanranta, H.; Nwaru, B. Sex Disparities in Asthma Development and Clinical Outcomes: Implications for Treatment Strategies. J. Asthma Allergy 2022, 15, 231–247. [Google Scholar] [CrossRef]
- Jenkins, C.R.; Boulet, L.P.; Lavoie, K.L.; Raherison-Semjen, C.; Singh, D. Personalized Treatment of Asthma: The Importance of Sex and Gender Differences. J. Allergy Clin. Immunol. Pract. 2022, 10, 963–971. [Google Scholar] [CrossRef]
- Torday, J.S.; Nielsen, H.C. The sex difference in fetal lung surfactant production. Exp. Lung. Res. 1987, 12, 1–19. [Google Scholar] [CrossRef]
- Laube, M.; Thome, U.H. Y It Matters-Sex Differences in Fetal Lung Development. Biomolecules 2022, 12, 437. [Google Scholar] [CrossRef]
- Esmailpour, N.; Högger, P.; Rabe, K.F.; Heitmann, U.; Nakashima, M.; Rohdewald, P. Distribution of inhaled fluticasone propionate between human lung tissue and serum in vivo. Eur. Respir. J. 1997, 10, 1496–1499. [Google Scholar] [CrossRef]
- Wu, W.; Bleecker, E.; Moore, W.; Busse, W.W.; Castro, M.; Chung, K.F.; Calhoun, W.J.; Erzurum, S.; Gaston, B.; Israel, E.; et al. Unsupervised phenotyping of Severe Asthma Research Program participants using expanded lung data. J. Allergy Clin. Immunol. 2014, 133, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Holguin, F.; Bleecker, E.R.; Busse, W.W.; Calhoun, W.J.; Castro, M.; Erzurum, S.C.; Fitzpatrick, A.M.; Gaston, B.; Israel, E.; Jarjour, N.N.; et al. Obesity and asthma: An association modified by age of asthma onset. J. Allergy Clin. Immunol. 2011, 127, 1486–1493.e2. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.; Farzan, S. Clinical implications of the obese-asthma phenotypes. Immunol. Allergy Clin. North Am. 2014, 34, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Grace, J.; Wang, B.R.; Lugogo, N. The Effects of Obesity in Asthma. Curr. Allergy Asthma Rep. 2019, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Cowan, D.C. Obesity, Inflammation, and Severe Asthma: An Update. Curr. Allergy Asthma Rep. 2021, 21, 46. [Google Scholar] [CrossRef] [PubMed]
- Wulster-Radcliffe, M.C.; Ajuwon, K.M.; Wang, J.; Christian, J.A.; Spurlock, M.E. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem. Biophys. Res. Commun. 2004, 316, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Ali Assad, N.; Sood, A. Leptin, adiponectin and pulmonary diseases. Biochimie 2012, 94, 2180–2189. [Google Scholar] [CrossRef]
- Messinis, I.E.; Papageorgiou, I.; Milingos, S.; Asprodini, E.; Kollios, G.; Seferiadis, K. Oestradiol plus progesterone treatment increases serum leptin concentrations in normal women. Hum. Reprod. 2001, 16, 1827–1832. [Google Scholar] [CrossRef]
- Kennedy, A.; Gettys, T.W.; Watson, P.; Wallace, P.; Ganaway, E.; Pan, Q.; Garvey, W.T. The metabolic significance of leptin in humans: Gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J. Clin. Endocrinol. Metab. 1997, 82, 1293–1300. [Google Scholar] [CrossRef]
- Peters-Golden, M.; Swern, A.; Bird, S.S.; Hustad, C.M.; Grant, E.; Edelman, J.M. Influence of body mass index on the response to asthma controller agents. Eur. Respir. J. 2006, 27, 495–503. [Google Scholar] [CrossRef]
- Sutherland, E.R.; Goleva, E.; Strand, M.; Beuther, D.A.; Leung, D.Y. Body mass and glucocorticoid response in asthma. Am. J. Respir. Crit. Care Med. 2008, 178, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Nzekwu, M.M. The effects of body mass index on lung volumes. Chest 2006, 130, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.E.; Pratley, R.E.; Forgione, P.M.; Kaminsky, D.A.; Whittaker-Leclair, L.A.; Griffes, L.A.; Garudathri, J.; Raymond, D.; Poynter, M.E.; Bunn, J.Y.; et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J. Allergy Clin. Immunol. 2011, 128, 508–515.e2. [Google Scholar] [CrossRef]
- Chapman, D.G.; Irvin, C.G.; Kaminsky, D.A.; Forgione, P.M.; Bates, J.H.; Dixon, A.E. Influence of distinct asthma phenotypes on lung function following weight loss in the obese. Respirology 2014, 19, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Lodge, C.J.; Lowe, A.J.; Dharmage, S.C.; Cassim, R.; Tan, D.; Russell, M.A. Are adults with asthma less physically active? A systematic review and meta-analysis. J. Asthma 2021, 58, 1426–1443. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S. Sex-dependent differences in voluntary physical activity. J. Neurosci. Res. 2017, 95, 279–290. [Google Scholar] [CrossRef]
- Moscato, G.; Apfelbacher, C.; Brockow, K.; Eberle, C.; Genuneit, J.; Mortz, C.G.; Quecchia, C.; Quirce, S.; Siracusa, A.; Tarlo, S.M.; et al. Gender and occupational allergy: Report from the task force of the EAACI Environmental and Occupational Allergy Interest Group. Allergy 2020, 75, 2753–2763. [Google Scholar] [CrossRef]
- Langhammer, A.; Johnsen, R.; Holmen, J.; Gulsvik, A.; Bjermer, L. Cigarette smoking gives more respiratory symptoms among women than among men. The Nord-Trondelag Health Study (HUNT). J. Epidemiol. Community Health 2000, 54, 917–922. [Google Scholar] [CrossRef]
- Ben-Zaken Cohen, S.; Paré, P.D.; Man, S.F.; Sin, D.D. The growing burden of chronic obstructive pulmonary disease and lung cancer in women: Examining sex differences in cigarette smoke metabolism. Am. J. Respir. Crit. Care Med. 2007, 176, 113–120. [Google Scholar] [CrossRef]
- Sathish, V.; Freeman, M.R.; Long, E.; Thompson, M.A.; Pabelick, C.M.; Prakash, Y.S. Cigarette Smoke and Estrogen Signaling in Human Airway Smooth Muscle. Cell. Physiol. Biochem. 2015, 36, 1101–1115. [Google Scholar] [CrossRef]
- Lazarus, S.C.; Chinchilli, V.M.; Rollings, N.J.; Boushey, H.A.; Cherniack, R.; Craig, T.J.; Deykin, A.; DiMango, E.; Fish, J.E.; Ford, J.G.; et al. National Heart Lung and Blood Institute’s Asthma Clinical Research Network. Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am. J. Respir. Crit. Care Med. 2007, 175, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, R.; Livingston, E.; McMahon, A.D.; Thomson, L.; Borland, W.; Thomson, N.C. Cigarette smoking impairs the therapeutic response to oral corticosteroids in chronic asthma. Am. J. Respir. Crit. Care Med. 2003, 168, 1308–1311. [Google Scholar] [CrossRef] [PubMed]
- Ricciardolo, F.L.; Di Stefano, A. Corticosteroid resistance in smokers with asthma. Am. J. Respir. Crit. Care Med. 2004, 169, 1252. [Google Scholar] [CrossRef] [PubMed]
- Goleva, E.; Li, L.B.; Eves, P.T.; Strand, M.J.; Martin, R.J.; Leung, D.Y. Increased glucocorticoid receptor beta alters steroid response in glucocorticoid-insensitive asthma. Am. J. Respir. Crit. Care Med. 2006, 173, 607–616. [Google Scholar] [CrossRef]
- Thomson, N.C.; Chaudhuri, R. Asthma in smokers: Challenges and opportunities. Curr. Opin. Pulm. Med. 2009, 15, 39–45. [Google Scholar] [CrossRef]
- Dijkstra, A.; Vonk, J.M.; Jongepier, H.; Koppelman, G.H.; Schouten, J.P.; ten Hacken, N.H.; Timens, W.; Postma, D.S. Lung function decline in asthma: Association with inhaled corticosteroids, smoking and sex. Thorax 2006, 61, 105–110. [Google Scholar] [CrossRef]
- Palumbo, M.L.; Prochnik, A.; Wald, M.R.; Genaro, A.M. Chronic Stress and Glucocorticoid Receptor Resistance in Asthma. Clin. Ther. 2020, 42, 993–1006. [Google Scholar] [CrossRef]
- Chen, E.; Miller, G.E. Stress and inflammation in exacerbations of asthma. Brain Behav. Immun. 2007, 21, 993–999. [Google Scholar] [CrossRef]
- Loerbroks, A.; Gadinger, M.C.; Bosch, J.A.; Stürmer, T.; Amelang, M. Work-related stress, inability to relax after work and risk of adult asthma: A population-based cohort study. Allergy 2010, 65, 1298–1305. [Google Scholar] [CrossRef]
- Plourde, A.; Lavoie, K.L.; Raddatz, C.; Bacon, S.L. Effects of acute psychological stress induced in laboratory on physiological responses in asthma populations: A systematic review. Respir. Med. 2017, 127, 21–32. [Google Scholar] [CrossRef]
- Miyasaka, T.; Dobashi-Okuyama, K.; Takahashi, T.; Takayanagi, M.; Ohno, I. The interplay between neuroendocrine activity and psychological stress-induced exacerbation of allergic asthma. Allergol. Int. 2018, 67, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Duan, X.H.; Wu, J.F.; Liu, B.J.; Luo, Q.L.; Jin, H.L.; Du, Y.J.; Zhang, H.Y.; Cao, Y.X.; Dong, J.C. Impact of psychosocial stress on airway inflammation and its mechanism in a murine model of allergic asthma. Chin. Med. J. 2013, 126, 325–334. [Google Scholar] [PubMed]
- Haczku, A.; Panettieri, R. A Jr. Social stress and asthma: The role of corticosteroid insensitivity. J. Allergy Clin. Immunol. 2010, 125, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Eng, A.; Mannetje, A.; Pearce, N.; Douwes, J. Work-related stress and asthma: Results from a workforce survey in New Zealand. J. Asthma 2011, 48, 783–789. [Google Scholar] [CrossRef]
- Runeson-Broberg, R.; Norbäck, D. Work-related psychosocial stress as a risk factor for asthma, allergy, and respiratory infections in the Swedish workforce. Psychol. Rep. 2014, 114, 377–389. [Google Scholar] [CrossRef]
- Forster, F.; Weinmann, T.; Gerlich, J.; Schlotz, W.; Weinmayr, G.; Genuneit, J.; Windstetter, D.; Vogelberg, C.; von Mutius, E.; Nowak, D.; et al. Work-related stress and incident asthma and rhinitis: Results from the SOLAR study. Int. Arch. Occup. Environ. Health 2019, 92, 673–681. [Google Scholar] [CrossRef]
- Loerbroks, A.; Ding, H.; Han, W.; Wang, H.; Wu, J.P.; Yang, L.; Angerer, P.; Li, J. Work stress, family stress and asthma: A cross-sectional study among women in China. Int. Arch. Occup. Environ. Health 2017, 90, 349–356. [Google Scholar] [CrossRef]
- Colombo, D.; Zagni, E.; Ferri, F.; Canonica, G.W.; PROXIMA study centers. Gender differences in asthma perception and its impact on quality of life: A post hoc analysis of the PROXIMA (Patient Reported Outcomes and Xolair® In the Management of Asthma) study. Allergy Asthma Clin. Immunol. 2019, 15, 65. [Google Scholar] [CrossRef]
- Gautam, Y.; Afanador, Y.; Abebe, T.; López, J.E.; Mersha, T.B. Genome-wide analysis revealed sex-specific gene expression in asthmatics. Hum. Mol. Genet. 2019, 28, 2600–2614. [Google Scholar] [CrossRef]
- Espuela-Ortiz, A.; Herrera-Luis, E.; Lorenzo-Díaz, F.; Hu, D.; Eng, C.; Villar, J.; Rodriguez-Santana, J.R.; Burchard, E.G.; Pino-Yanes, M. Role of Sex on the Genetic Susceptibility to Childhood Asthma in Latinos and African Americans. J. Pers. Med. 2021, 11, 1140. [Google Scholar] [CrossRef]
- Myers, R.A.; Scott, N.M.; Gauderman, W.J.; Qiu, W.; Mathias, R.A.; Romieu, I.; Levin, A.M.; Pino-Yanes, M.; Graves, P.E.; Villarreal, A.B.; et al. Genome-wide interaction studies reveal sex-specific asthma risk alleles. Hum. Mol. Genet. 2014, 23, 5251–5259. [Google Scholar] [CrossRef] [PubMed]
- Santillan, A.A.; Camargo, C.A., Jr.; Ramirez-Rivera, A.; Delgado-Enciso, I.; Rojas-Martinez, A.; Cantu-Diaz, F.; Barrera-Saldaña, H.A. Association between beta2-adrenoceptor polymorphisms and asthma diagnosis among Mexican adults. J. Allergy Clin. Immunol. 2003, 112, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Hunninghake, G.M.; Soto-Quirós, M.E.; Avila, L.; Kim, H.P.; Lasky-Su, J.; Rafaels, N.; Ruczinski, I.; Beaty, T.H.; Mathias, R.A.; Barnes, K.C.; et al. TSLP polymorphisms are associated with asthma in a sex-specific fashion. Allergy 2010, 65, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Karaaslan, C.; Yavuz, T.S.; Cosgun, E.; Kalayci, O.; Sackesen, C. The genetic variants of thymic stromal lymphopoietin protein in children with asthma and allergic rhinitis. Int. Arch. Allergy Immunol. 2014, 163, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Syamsu; Yusuf, I.; Budu; Patellongi, I. The effect of polymorphism of the beta-2 adrenergic receptor on the response to beta-2 agonist in bronchial asthma patients. Acta Med. Indones. 2007, 39, 8–12. [Google Scholar]
- Labuda, M.; Laberge, S.; Brière, J.; Bérubé, D.; Krajinovic, M. RGS5 gene and therapeutic response to short acting bronchodilators in paediatric asthma patients. Pediatr. Pulmonol. 2013, 48, 970–975. [Google Scholar] [CrossRef]
- Pua, H.H.; Ansel, K.M. MicroRNA regulation of allergic inflammation and asthma. Curr. Opin. Immunol. 2015, 36, 101–118. [Google Scholar] [CrossRef]
- Kho, A.T.; Sharma, S.; Davis, J.S.; Spina, J.; Howard, D.; McEnroy, K.; Moore, K.; Sylvia, J.; Qiu, W. Circulating MicroRNAs: Association with Lung Function in Asthma. PLoS ONE 2016, 11, e0157998. [Google Scholar] [CrossRef]
- Weidner, J.; Bartel, S.; Kılıç, A.; Zissler, U.M.; Renz, H.; Schwarze, J.; Schmidt-Weber, C.B.; Maes, T.; Rebane, A.; Krauss-Etschmann, S.; et al. Spotlight on microRNAs in allergy and asthma. Allergy 2021, 76, 1661–1678. [Google Scholar] [CrossRef]
- Weidner, J.; Ekerljung, L.; Malmhäll, C.; Miron, N.; Rådinger, M. Circulating microRNAs correlate to clinical parameters in individuals with allergic and non-allergic asthma. Respir. Res. 2020, 21, 107. [Google Scholar] [CrossRef]
- Li, J.; Panganiban, R.; Kho, A.T.; McGeachie, M.J.; Farnam, L.; Chase, R.P.; Weiss, S.T.; Lu, Q.; Tantisira, K.G. Circulating MicroRNAs and Treatment Response in Childhood Asthma. Am. J. Respir. Crit. Care Med. 2020, 202, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, H.; Klein, S.L. Sex Differences in Immunity to Viral Infections. Front. Immunol. 2021, 12, 720952. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.P.; Brouwer, M.C.; van de Beek, D. Sex and Gender Differences in Bacterial Infections. Infect. Immun. 2022, 90, e0028322. [Google Scholar] [CrossRef] [PubMed]
- Ngo, S.T.; Steyn, F.J.; McCombe, P.A. Gender differences in autoimmune disease. Front. Neuroendocrinol. 2014, 35, 347–369. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, A.; Anticoli, S.; D’Ambrosio, A.; Giordani, L.; Viora, M. The influence of sex and gender on immunity, infection and vaccination. Ann. Ist. Super. Sanita 2016, 52, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Viveiros, A.; Rasmuson, J.; Vu, J.; Mulvagh, S.L.; Yip, C.Y.Y.; Norris, C.M.; Oudit, G.Y. Sex differences in COVID-19: Candidate pathways, genetics of ACE2, and sex hormones. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H296–H304. [Google Scholar] [CrossRef] [PubMed]
- Chanana, N.; Palmo, T.; Sharma, K.; Kumar, R.; Graham, B.B.; Pasha, Q. Sex-derived attributes contributing to SARS-CoV-2 mortality. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E562–E567. [Google Scholar] [CrossRef]
- Wark, P.A.B.; Pathinayake, P.S.; Kaiko, G.; Nichol, K.; Ali, A.; Chen, L.; Sutanto, E.N.; Garratt, L.W.; Sohal, S.S.; Lu, W.; et al. ACE2 expression is elevated in airway epithelial cells from older and male healthy individuals but reduced in asthma. Respirology 2021, 26, 442–451. [Google Scholar] [CrossRef]
- Ferastraoaru, D.; Hudes, G.; Jerschow, E.; Jariwala, S.; Karagic, M.; de Vos, G.; Rosenstreich, D.; Ramesh, M. Eosinophilia in Asthma Patients Is Protective Against Severe COVID-19 Illness. J. Allergy Clin. Immunol. Pract. 2021, 9, 1152–1162.e3. [Google Scholar] [CrossRef]
- Lipworth, B.; Chan, R.; RuiWen Kuo, C. Type 2 Asthma Inflammation and COVID-19: A Double Edged Sword. J. Allergy Clin. Immunol. Pract. 2021, 9, 1163–1165. [Google Scholar] [CrossRef]
- Camiolo, M.; Gauthier, M.; Kaminski, N.; Ray, A.; Wenzel, S.E. Expression of SARS-CoV-2 receptor ACE2 and coincident host response signature varies by asthma inflammatory phenotype. J. Allergy Clin. Immunol. 2020, 146, 315–324.e7. [Google Scholar] [CrossRef] [PubMed]
- Rogliani, P.; Cavalli, F.; Ritondo, B.L.; Cazzola, M.; Calzetta, L. Sex differences in adult asthma and COPD therapy: A systematic review. Respir. Res. 2022, 23, 222. [Google Scholar] [CrossRef] [PubMed]
- Pace, S.; Pergola, C.; Dehm, F.; Rossi, A.; Gerstmeier, J.; Troisi, F.; Pein, H.; Schaible, A.M.; Weinigel, C.; Rummler, S.; et al. Androgen-mediated sex bias impairs efficiency of leukotriene biosynthesis inhibitors in males. J. Clin. Investig. 2017, 127, 3167–3176. [Google Scholar] [CrossRef] [PubMed]
- Nerpin, E.; Ferreira, D.S.; Weyler, J.; Schlunnsen, V.; Jogi, R.; Raherison Semjen, C.; Gislasson, T.; Demoly, P.; Heinrich, J.; Nowak, D.; et al. Bronchodilator response and lung function decline: Associations with exhaled nitric oxide with regard to sex and smoking status. World Allergy Organ. J. 2021, 14, 100544. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.J.; Mohamed, M.H.; Self, T.H.; Eberle, L.V.; Johnson, J.A. Importance of beta(2)adrenergic receptor genotype, gender and race on albuterol-evoked bronchodilation in asthmatics. Pulm. Pharmacol. Ther. 2000, 13, 127–134. [Google Scholar] [CrossRef]
- Harvey, E.S.; Langton, D.; Katelaris, C.; Stevens, S.; Farah, C.S.; Gillman, A.; Harrington, J.; Hew, M.; Kritikos, V.; Radhakrishna, N.; et al. Mepolizumab effectiveness and identification of super-responders in severe asthma. Eur. Respir. J. 2020, 55, 1902420. [Google Scholar] [CrossRef]
- Kerstjens, H.A.; Moroni-Zentgraf, P.; Tashkin, D.P.; Dahl, R.; Paggiaro, P.; Vandewalker, M.; Schmidt, H.; Engel, M.; Bateman, E.D. Tiotropium improves lung function, exacerbation rate, and asthma control, independent of baseline characteristics including age, degree of airway obstruction, and allergic status. Respir. Med. 2016, 117, 198–206. [Google Scholar] [CrossRef]
- Bousquet, J.; Cabrera, P.; Berkman, N.; Buhl, R.; Holgate, S.; Wenzel, S.; Fox, H.; Hedgecock, S.; Blogg, M.; Cioppa, G.D. The effect of treatment with omalizumab, an anti-IgE antibody, on asthma exacerbations and emergency medical visits in patients with severe persistent asthma. Allergy 2005, 60, 302–308. [Google Scholar] [CrossRef]

| Eosinophilic Asthma | Non-Eosinophilic Asthma | Mixed Granulocytic Asthma | |||
|---|---|---|---|---|---|
| Allergic | Non-allergic | Paucigranulocytic | Neutrophilic | ||
| Triggering agents | Allergens | Pollutants, microbes | Pollutants, oxidative stress | Pollutants, oxidative stress, microbes, obesity | |
| Involvement of eosinophils | ++ | ++ | − | − | + |
| Sputum eosinophils | >3% | >3% | <3% | <3% | >3% |
| Blood eosinophils | + | + | − | − | − |
| Atopy | + | − | − | − | − |
| Involvement of neutrophils | − | − | − | ++ | + |
| Sputum neutrophils | <61% | <61% | <61% | ≥61% | ≥61% |
| Epithelial damage | ++ | ++ | + | ++ | ++ |
| Mucus | + | + | +/− | ++ | ++ |
| Reticular membrane basement thickening | ++ | ++ | +/− | + | + |
| Airway smooth muscle mass | ++ | ++ | + | + | + |
| Eosinophilic Asthma | Neutrophilic Asthma | Paucigranulocytic Asthma | |
|---|---|---|---|
| FeNO | ++ | +/− | + |
| AHR | ++ | +/− | + |
| Endotype-specific changes in biochemical biomarkers | ↑ serum periostin ↑ serum IgE (in atopy) ↑ sputum supernatant ECP, EDN, EPO, eotaxin-2, IL-5, IL-13, GM-CSF, osteopontin, angiopoietin-I, Grx1; ↑ MMP-9 | ↑ serum CRP and IL-6 ↑ sputum supernatant IL-8, IL-17, MPO, NE, TNFα, CXCR1, and CXCR2 ↓ sputum gal-3 ↑ gal-3BP and IL-1β ↓ gal-3/gal-3BP and IL-1Ra/IL-1β | ↑ Grx1 |
| Classified Drugs | Examples of Drugs Used | References |
|---|---|---|
| Eosinophilic asthma | ||
| Currently available treatments: | ||
| Inhaled corticosteroids (ICSs) | Budesonide, fluticasone | [12,14,15] |
| Oral corticosteroids (OCSs) | Prednisolone | [12,14] |
| Bronchodilators (SABA, LABA, SAMA, LAMA) | Salbutamol, formoterol, ipratropium, tiotropium | [12,15] |
| Xanthine derivatives | Theophylline | [16,17] |
| Anti-IgE | Omalizumab | [18,19] |
| Anti-IL-5/IL-5R | Mepolizumab, benralizumab | [20,21] |
| Anti-IL-4R | Dupilumab | [22,23] |
| Anti-TSLP | Tezepelumab | [23,24] |
| Anti-leukotrienes | Montelukast | [25,26] |
| Prospective treatments: | ||
| Anti-IL-13 | Tralokinumab | [27] |
| Tyrosine kinase inhibitors | Dasatinib | [28,29] |
| Statins | Simvastatin, atorvastatin | [30,31] |
| Macrolide antibiotics | Azithromycin | [32] |
| PGD2 receptor 2 antagonist | Fevipiprant | [33] |
| Neutrophilic asthma | ||
| Currently available treatments: | ||
| Macrolide antibiotics | Azithromycin | [32,34] |
| Bronchodilators (LAMA) | Tiotropium | [35,36] |
| Xanthine derivatives | Theophylline | [16,17] |
| Prospective treatments: | ||
| PDE4 inhibitors | Roflumilast | [37,38] |
| Statins | Simvastatin, atorvastatin | [30,31] |
| Tyrosine kinase inhibitors | Dasatinib | [28,29] |
| CXCR2 antagonists | AZD5069 | [39,40] |
| Anti-TNFα | Etanercept | [41,42] |
| Anti-IL-17 | Brodalumab | [43,44] |
| Paucigranulocytic asthma | ||
| Prospective treatments: | ||
| Bronchial thermoplasty | [45,46] | |
| Bronchodilators (LAMA) | Tiotropium | [35,36] |
| Mast-cell directed therapy | [47,48] |
| Males | Females | |
|---|---|---|
| Lung maturation in neonates | Delayed compared to female neonates | |
| Lung dysanapsis | Retarded AW growth in boys | Smaller AWs vs. lungs in adult women |
| Excitability for cough | ↓ | ↑ |
| Clinical course of asthma | ↑ severity and hospital admissions | |
| Type 2 inflammation | ↓ | ↑ |
| Non-type 2 inflammation | ↓ | ↑ |
| General immune response | ↓ | ↑ |
| Effectiveness of response to viral infections, including COVID-19 | ↓ | ↑ |
| Effectiveness of response to bacterial infections | ↓ | ↑ |
| Relation to smoking | ↑ exposure to smoke | ↑ vulnerability to smoke |
| Occupation-associated asthma | ↑ exposure to organic dust, diesel, flour/bakery products, wood/wood-component dust | ↑ exposure to inorganic dust, hair products, ozone |
| Obesity-associated asthma | ↑ risk in boys | ↑ risk in adolescent and adult women |
| Stress-associated asthma | ↑ risk | ↑ risk |
| Sex-specific genes associated with asthma | ↑ FBXL7, ITPR3, ALOX15; ↓ RAD51B | ↓ HLA_DQA1 |
| Sex-specific alleles associated with asthma risk | 5q31.1, 10q26.1 | 2q23.3, 2q34, 6q27, 17213.3 |
| Response to corticosteroids | ↑ | ↓ |
| Response to bronchodilators | ↓ | ↑ |
| Response to anti-leukotrienes | ↓ | ↑ |
| Response to anti-IgE | ↑ | ↓ |
| Response to anti-IL-5 | ↓ | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokra, D.; Barosova, R.; Mokry, J. Sex-Based Differences in Bronchial Asthma: What Are the Mechanisms behind Them? Appl. Sci. 2023, 13, 2694. https://doi.org/10.3390/app13042694
Mokra D, Barosova R, Mokry J. Sex-Based Differences in Bronchial Asthma: What Are the Mechanisms behind Them? Applied Sciences. 2023; 13(4):2694. https://doi.org/10.3390/app13042694
Chicago/Turabian StyleMokra, Daniela, Romana Barosova, and Juraj Mokry. 2023. "Sex-Based Differences in Bronchial Asthma: What Are the Mechanisms behind Them?" Applied Sciences 13, no. 4: 2694. https://doi.org/10.3390/app13042694
APA StyleMokra, D., Barosova, R., & Mokry, J. (2023). Sex-Based Differences in Bronchial Asthma: What Are the Mechanisms behind Them? Applied Sciences, 13(4), 2694. https://doi.org/10.3390/app13042694