Method to Calculate Melanopic Light Reaching the Retina Depending on the Optical Density of an Aging Crystalline Lens
Abstract
1. Introduction
2. Background
2.1. Technical Features
2.2. Theoretical Melanopic Considerations in Lighting for Elderly Individuals
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spitschan, M. Melanopsin Contributions to Non-Visual and Visual Function. Curr. Opin. Behav. Sci. 2019, 30, 67–72. [Google Scholar] [CrossRef] [PubMed]
- NPR-CEN/TR 16791:2017; Quantifying Irradiance for Eye-Mediated Non-Image-Forming Effects of Light in Humans. European Committee for Standardization (CEN): Brussels, Belgium, 2017.
- Pokorny, J.; Smith, V.C.; Lutze, M. Aging of the Human Lens. Appl. Opt. 1987, 26, 1437. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Pokorny, J.; Smith, V.C. Optical Density of the Human Lens. J. Opt. Soc. Am. A 1997, 14, 953. [Google Scholar] [CrossRef]
- Van de Kraats, J.; Van Norren, D. Optical Density of the Aging Human Ocular Media in the Visible and the UV. JOSA A 2007, 24, 1842–1857. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.J.; Peirson, S.N.; Berson, D.M.; Brown, T.M.; Cooper, H.M.; Czeisler, C.A.; Figueiro, M.G.; Gamlin, P.D.; Lockley, S.W.; O’Hagan, J.B.; et al. Measuring and Using Light in the Melanopsin Age. Trends Neurosci. 2014, 37, 1–9. [Google Scholar] [CrossRef]
- Bailes, H.J.; Lucas, R.J. Human Melanopsin Forms a Pigment Maximally Sensitive to Blue Light (Λmax ≈ 479 nm) Supporting Activation of Gq/11 and Gi/o Signalling Cascades. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122987. [Google Scholar] [CrossRef]
- Chellappa, S.L. Individual Differences in Light Sensitivity Affect Sleep and Circadian Rhythms. Sleep 2021, 44, zsaa214. [Google Scholar] [CrossRef]
- Najjar, R.P.; Chiquet, C.; Teikari, P.; Cornut, P.-L.; Claustrat, B.; Denis, P.; Cooper, H.M.; Gronfier, C. Aging of Non-Visual Spectral Sensitivity to Light in Humans: Compensatory Mechanisms? PLoS ONE 2014, 9, e85837. [Google Scholar] [CrossRef]
- Eto, T.; Ohashi, M.; Nagata, K.; Shin, N.; Motomura, Y.; Higuchi, S. Crystalline Lens Transmittance Spectra and Pupil Sizes as Factors Affecting Light-induced Melatonin Suppression in Children and Adults. Ophthalmic Physiol. Opt. 2021, 41, 900–910. [Google Scholar] [CrossRef]
- Schlangen, L.J.; Price, L.L. The Lighting Environment, Its Metrology, and Non-Visual Responses. Front. Neurol. 2021, 12, 624861. [Google Scholar] [CrossRef]
- Esquiva, G.; Lax, P.; Pérez-Santonja, J.J.; García-Fernández, J.M.; Cuenca, N. Loss of Melanopsin-Expressing Ganglion Cell Subtypes and Dendritic Degeneration in the Aging Human Retina. Front. Aging Neurosci. 2017, 9, 79. [Google Scholar] [CrossRef]
- CIE S 026/E:2018; CIE System for Metrology of Optical Radiation for IpRGC-Influenced Responses to Light. CIE Central Bureau: Vienna, Austria, 2018.
- CIE. Position Statement on Non-Visual Effects of Light—Recommending Proper Light at the Proper Time, 2nd ed.; CIE: Vienna, Austria, 2019. [Google Scholar]
- Brown, T.M.; Brainard, G.C.; Cajochen, C.; Czeisler, C.A.; Hanifin, J.P.; Lockley, S.W.; Lucas, R.J.; Münch, M.; O’Hagan, J.B.; Peirson, S.N.; et al. Recommendations for Daytime, Evening, and Nighttime Indoor Light Exposure to Best Support Physiology, Sleep, and Wakefulness in Healthy Adults. PLoS Biol. 2022, 20, e3001571. [Google Scholar] [CrossRef] [PubMed]
- Giménez, M.C.; Stefani, O.; Cajochen, C.; Lang, D.; Deuring, G.; Schlangen, L.J.M. Predicting Melatonin Suppression by Light in Humans: Unifying Photoreceptor-Based Equivalent Daylight Illuminances, Spectral Composition, Timing and Duration of Light Exposure. J. Pineal Res. 2022, 72, e12786. [Google Scholar] [CrossRef]
- Turner, P.L.; Mainster, M.A. Circadian Photoreception: Ageing and the Eye’s Important Role in Systemic Health. Br. J. Ophthalmol. 2008, 92, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Kessel, L.; Lundeman, J.H.; Herbst, K.; Andersen, T.V.; Larsen, M. Age-Related Changes in the Transmission Properties of the Human Lens and Their Relevance to Circadian Entrainment. J. Cataract Refract. Surg. 2010, 36, 308–312. [Google Scholar] [CrossRef]
- Charman, W.N. Age, Lens Transmittance, and the Possible Effects of Light on Melatonin Suppression. Ophthalmic Physiol. Opt. 2003, 23, 181–187. [Google Scholar] [CrossRef]
- Houser, K.W.; Esposito, T. Human-Centric Lighting: Foundational Considerations and a Five-Step Design Process. Front. Neurol. 2021, 12, 630553. [Google Scholar] [CrossRef] [PubMed]
- Gkaintatzi-Masouti, M.; van Duijnhoven, J.; Aarts, M.P.J. Simulations of non-image-forming effects of light in building design: A literature review. Light. Res. Technol. 2022, 14771535221142812. [Google Scholar] [CrossRef]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by Retinal Ganglion Cells That Set the Circadian Clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef]
- Hattar, S.; Liao, H.-W.; Takao, M.; Berson, D.M.; Yau, K.-W. Melanopsin-Containing Retinal Ganglion Cells: Architecture, Projections, and Intrinsic Photosensitivity. Science 2002, 295, 1065–1070. [Google Scholar] [CrossRef]
- Provencio, I.; Jiang, G.; De Grip, W.J.; Hayes, W.P.; Rollag, M.D. Melanopsin: An Opsin in Melanophores, Brain, and Eye. Proc. Natl. Acad. Sci. USA 1998, 95, 340–345. [Google Scholar] [CrossRef]
- Provencio, I.; Rodriguez, I.R.; Jiang, G.; Hayes, W.P.; Moreira, E.F.; Rollag, M.D. A Novel Human Opsin in the Inner Retina. J. Neurosci. 2000, 20, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Wyszecki, G.; Stiles, W.S. Color Science: Concepts and Methods, Quantitative Data and Formulae; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Sánchez-Cano, A.; Aporta, J. Optimization of Lighting Projects Including Photopic and Circadian Criteria: A Simplified Action Protocol. Appl. Sci. 2020, 10, 8068. [Google Scholar] [CrossRef]
- Esposito, T.; Houser, K. Correlated Color Temperature Is Not a Suitable Proxy for the Biological Potency of Light. Sci. Rep. 2022, 12, 20223. [Google Scholar] [CrossRef] [PubMed]
- The WELL Building Standard. In Feature 54: Circadian Lighting Design; International WELL Building Institute: New York, NY, USA, 2020.
- Vetter, C.; Pattison, P.M.; Houser, K.; Herf, M.; Phillips, A.J.K.; Wright, K.P.; Skene, D.J.; Brainard, G.C.; Boivin, D.B.; Glickman, G. A Review of Human Physiological Responses to Light: Implications for the Development of Integrative Lighting Solutions. LEUKOS 2022, 18, 387–414. [Google Scholar] [CrossRef]
- Spitschan, M.; Santhi, N. Individual Differences and Diversity in Human Physiological Responses to Light. eBioMedicine 2022, 75, 103640. [Google Scholar] [CrossRef] [PubMed]
- CEN/TC 169-Light and Lighting; EN 12464-1:2021; Lighting of work places—Part 1: Indoor work places. European Committee for Standardization: Brussels, Belgium, 2011.
- CIBSE. Literature Review on Circadian Lighting; CIBSE: London, UK, 2017. [Google Scholar]
- Underwriters Laboratories (UL). Design Guideline for Promoting Circadian Entrainment with Light for Day-Active People; DG 24480; Underwriters Laboratories: Northbrook, IL, USA, 2020. [Google Scholar]
- CIE TN 011:2020; What to Document and Report in Studies of ipRGC-Influenced Responses to Light. CIE Central Bureau: Vienna, Austria, 2020. [CrossRef]
- Spitschan, M.; Stefani, O.; Blattner, P.; Gronfier, C.; Lockley, S.W.; Lucas, R.J. How to Report Light Exposure in Human Chronobiology and Sleep Research Experiments. Clocks Sleep 2019, 1, 280–289. [Google Scholar] [CrossRef]
- Rukmini, A.V.; Milea, D.; Aung, T.; Gooley, J.J. Pupillary Responses to Short-Wavelength Light Are Preserved in Aging. Sci. Rep. 2017, 7, 43832. [Google Scholar] [CrossRef]
- Rukmini, A.V.; Milea, D.; Gooley, J.J. Chromatic Pupillometry Methods for Assessing Photoreceptor Health in Retinal and Optic Nerve Diseases. Front. Neurol. 2019, 10, 76. [Google Scholar] [CrossRef]
- Eto, T.; Teikari, P.; Najjar, R.P.; Nishimura, Y.; Motomura, Y.; Kuze, M.; Higuchi, S. A Purkinje Image-Based System for an Assessment of the Density and Transmittance Spectra of the Human Crystalline Lens In Vivo. Sci. Rep. 2020, 10, 16445. [Google Scholar] [CrossRef]
- Yamakawa, M.; Tsujimura, S.; Okajima, K. A Quantitative Analysis of the Contribution of Melanopsin to Brightness Perception. Sci. Rep. 2019, 9, 7568. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.B.; Yellott, J.I. A Unified Formula for Light-Adapted Pupil Size. J. Vis. 2012, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Spitschan, M. Time-Varying Light Exposure in Chronobiology and Sleep Research Experiments. Front. Neurol. 2021, 12, 654158. [Google Scholar] [CrossRef] [PubMed]
- Diakite-Kortlever, A.; Knoop, M. Non-Image Forming Potential in Urban Settings—An Approach Considering Orientation-Dependent Spectral Properties of Daylight. Energy Build. 2022, 265, 112080. [Google Scholar] [CrossRef]
- Ezpeleta, S.; Orduna-Hospital, E.; Aporta, J.; Luesma, M.J.; Pinilla, I.; Sánchez-Cano, A. Evaluation of Visual and Nonvisual Levels of Daylight from Spectral Power Distributions Considering Orientation and Seasonality. Appl. Sci. 2021, 11, 5996. [Google Scholar] [CrossRef]
- Diakite-Kortlever, A.; Weber, N.; Knoop, M. Reconstruction of Daylight Spectral Power Distribution Based on Correlated Color Temperature: A Comparative Study between the CIE Approach and Localized Procedures in Assessing Non-Image Forming Effects. LEUKOS 2022, 19, 118–145. [Google Scholar] [CrossRef]
- Kompier, M.E.; Smolders, K.C.; Kramer, R.P.; van Marken Lichtenbelt, W.; de Kort, Y.A. Contrasting Dynamic Light Scenarios in an Operational Office: Effects on Visual Experience, Alertness, Cognitive Performance, and Sleep. Build. Environ. 2022, 212, 108844. [Google Scholar] [CrossRef]
- CIE 170-1:2006; Fundamental Chromaticity Diagram with Physiological Axes-Part 1. CIE Central Bureau: Vienna, Austria, 2006.
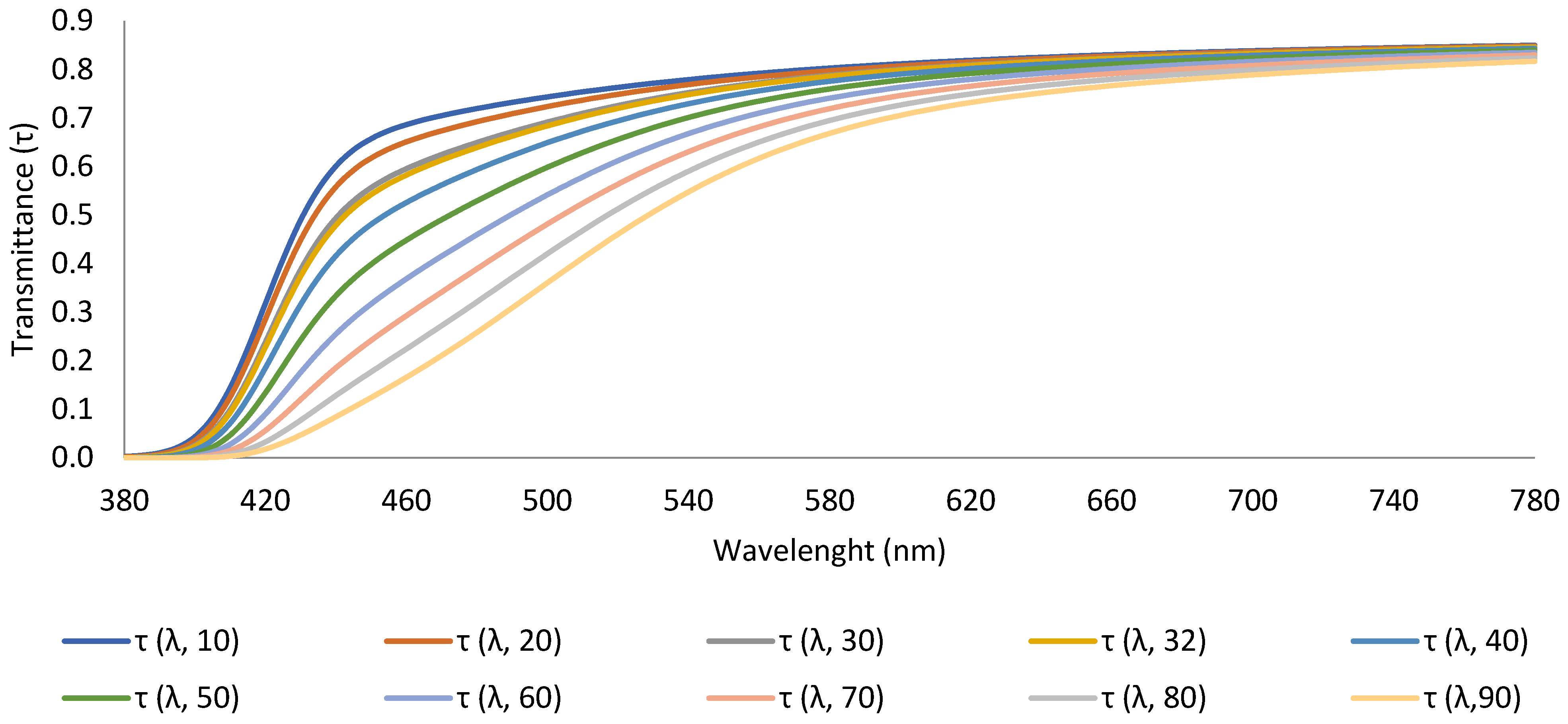

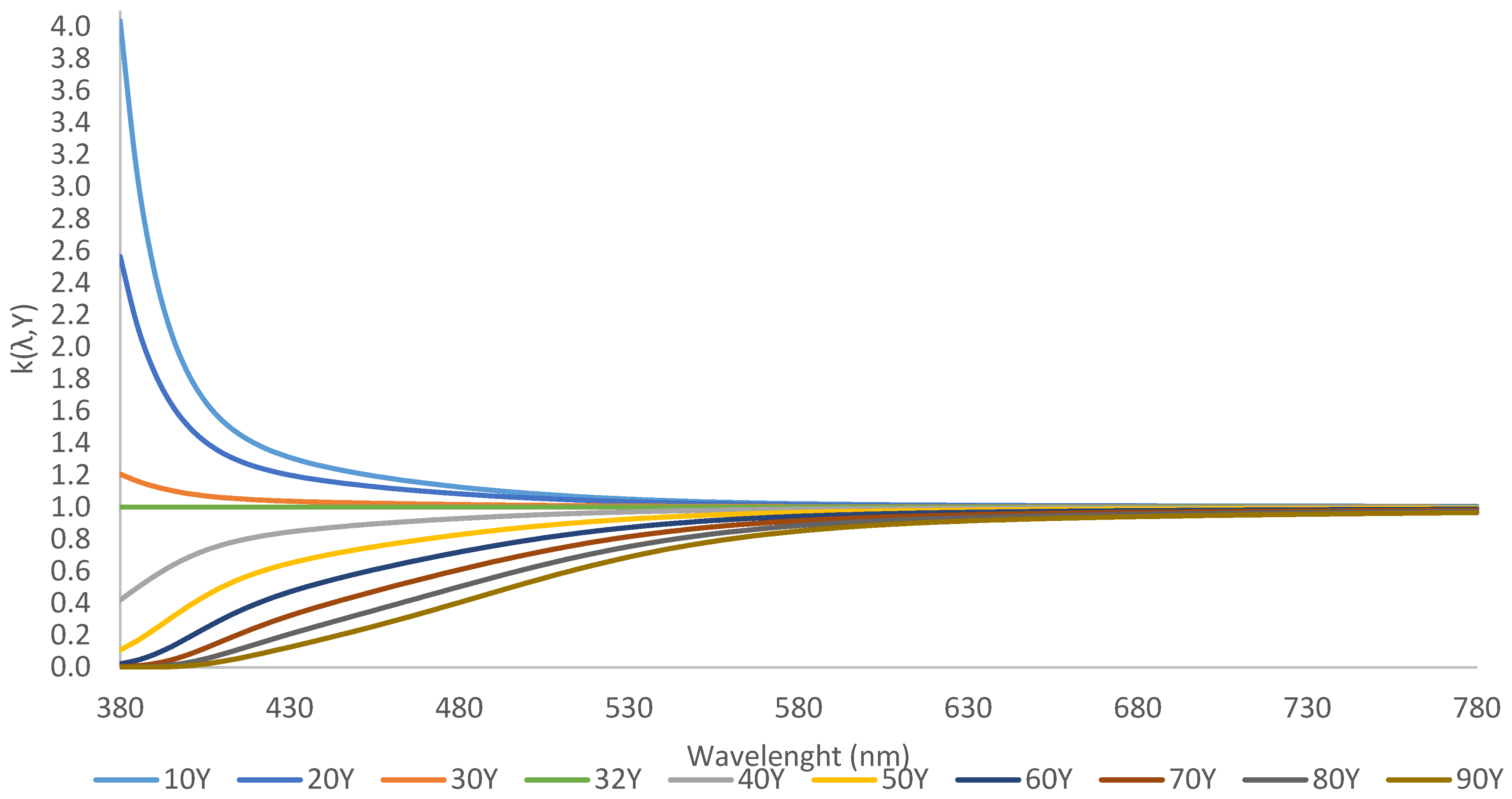
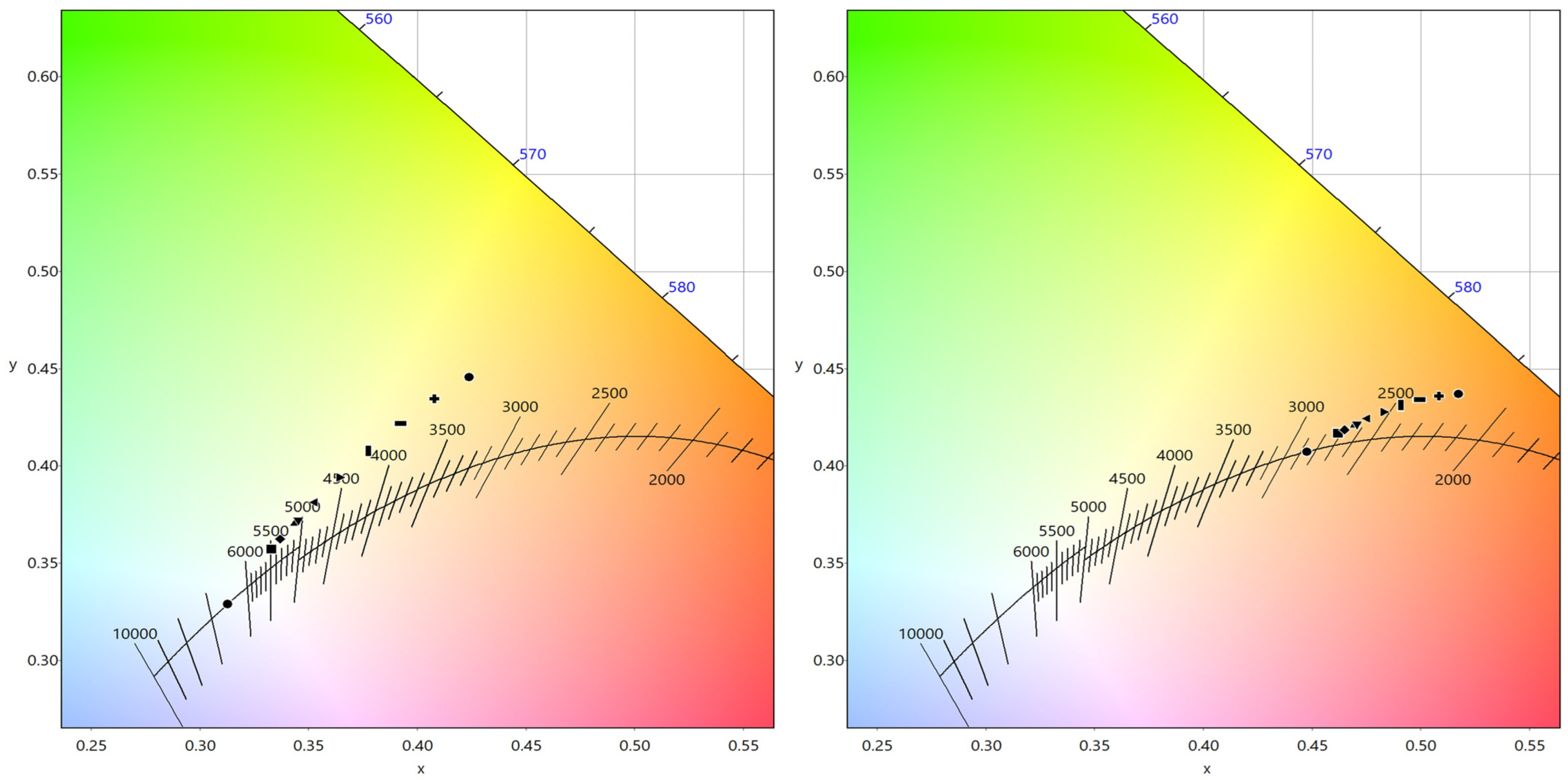
| CCT (K) | CRI | R9 | x | y | |
|---|---|---|---|---|---|
| Standard illuminant D65: | 6498 | 100 | 100 | 0.3128 | 0.3291 |
| Standard illuminant D65+Lens_10Years | 5493 | 96 | 87 | 0.3327 | 0.3574 |
| Standard illuminant D65+Lens_20Years | 5340 | 96 | 84 | 0.3368 | 0.3625 |
| Standard illuminant D65+Lens_30Years | 5112 | 95 | 80 | 0.3435 | 0.3707 |
| Standard illuminant D65+Lens_32Years | 5061 | 95 | 79 | 0.3452 | 0.3727 |
| Standard illuminant D65+Lens_40Years | 4842 | 93 | 76 | 0.3528 | 0.3815 |
| Standard illuminant D65+Lens_50Years | 4556 | 92 | 72 | 0.3642 | 0.3941 |
| Standard illuminant D65+Lens_60Years | 4274 | 91 | 68 | 0.3775 | 0.4078 |
| Standard illuminant D65+Lens_70Years | 4007 | 89 | 64 | 0.3923 | 0.4216 |
| Standard illuminant D65+Lens_80Years | 3760 | 88 | 60 | 0.4079 | 0.4345 |
| Standard illuminant D65+Lens_90Years | 3534 | 87 | 57 | 0.4239 | 0.4457 |
| Standard illuminant A: | 2856 | 100 | 100 | 0.4475 | 0.4075 |
| Standard illuminant A+Lens_10Years | 2719 | 99 | 97 | 0.4620 | 0.4169 |
| Standard illuminant A+Lens_20Years | 2694 | 98 | 96 | 0.4648 | 0.4186 |
| Standard illuminant A+Lens_30Years | 2652 | 98 | 94 | 0.4694 | 0.4212 |
| Standard illuminant A+Lens_32Years | 2643 | 98 | 94 | 0.4705 | 0.4218 |
| Standard illuminant A+Lens_40Years | 2599 | 97 | 92 | 0.4754 | 0.4244 |
| Standard illuminant A+Lens_50Years | 2535 | 96 | 89 | 0.4827 | 0.4279 |
| Standard illuminant A+Lens_60Years | 2464 | 95 | 86 | 0.4908 | 0.4312 |
| Standard illuminant A+Lens_70Years | 2389 | 94 | 83 | 0.4995 | 0.4340 |
| Standard illuminant A+Lens_80Years | 2311 | 93 | 80 | 0.5084 | 0.4359 |
| Standard illuminant A+Lens_90Years | 2231 | 92 | 77 | 0.5173 | 0.4370 |
| Standard Illuminant D65 | Standard Illuminant A | |||||
|---|---|---|---|---|---|---|
| MAFtrans | EDI lux | EML lux | MAFtrans | EDI lux | EML lux | |
| Without age considerations | 0.906 | 200 | 221 | 0.449 | 99 | 109 |
| 10 Years | 0.923 | 204 | 225 | 0.448 | 99 | 109 |
| 20 Years | 0.917 | 202 | 224 | 0.448 | 99 | 109 |
| 30 Years | 0.908 | 200 | 221 | 0.449 | 99 | 109 |
| 32 Years | 0.906 | 200 | 221 | 0.449 | 99 | 109 |
| 40 Years | 0.897 | 198 | 219 | 0.451 | 100 | 110 |
| 50 Years | 0.883 | 195 | 215 | 0.452 | 100 | 110 |
| 60 Years | 0.866 | 191 | 211 | 0.454 | 100 | 111 |
| 70 Years | 0.847 | 187 | 207 | 0.455 | 100 | 111 |
| 80 Years | 0.827 | 183 | 202 | 0.456 | 101 | 111 |
| 90 Years | 0.806 | 178 | 196 | 0.457 | 101 | 111 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Cano, A.; Orduna-Hospital, E.; Fernández-Espinosa, G.; Aporta, J. Method to Calculate Melanopic Light Reaching the Retina Depending on the Optical Density of an Aging Crystalline Lens. Appl. Sci. 2023, 13, 2569. https://doi.org/10.3390/app13042569
Sanchez-Cano A, Orduna-Hospital E, Fernández-Espinosa G, Aporta J. Method to Calculate Melanopic Light Reaching the Retina Depending on the Optical Density of an Aging Crystalline Lens. Applied Sciences. 2023; 13(4):2569. https://doi.org/10.3390/app13042569
Chicago/Turabian StyleSanchez-Cano, Ana, Elvira Orduna-Hospital, Guisela Fernández-Espinosa, and Justiniano Aporta. 2023. "Method to Calculate Melanopic Light Reaching the Retina Depending on the Optical Density of an Aging Crystalline Lens" Applied Sciences 13, no. 4: 2569. https://doi.org/10.3390/app13042569
APA StyleSanchez-Cano, A., Orduna-Hospital, E., Fernández-Espinosa, G., & Aporta, J. (2023). Method to Calculate Melanopic Light Reaching the Retina Depending on the Optical Density of an Aging Crystalline Lens. Applied Sciences, 13(4), 2569. https://doi.org/10.3390/app13042569








