Obtaining Polysaccharide-Based Fabrics with Improved Moisture Sorption and Dye Adsorption Properties
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Jute Fabric Treatment with Chitosan
2.3. Fabrics’ Surface Chemistry Characterization
2.4. Determination of the Moisture Sorption
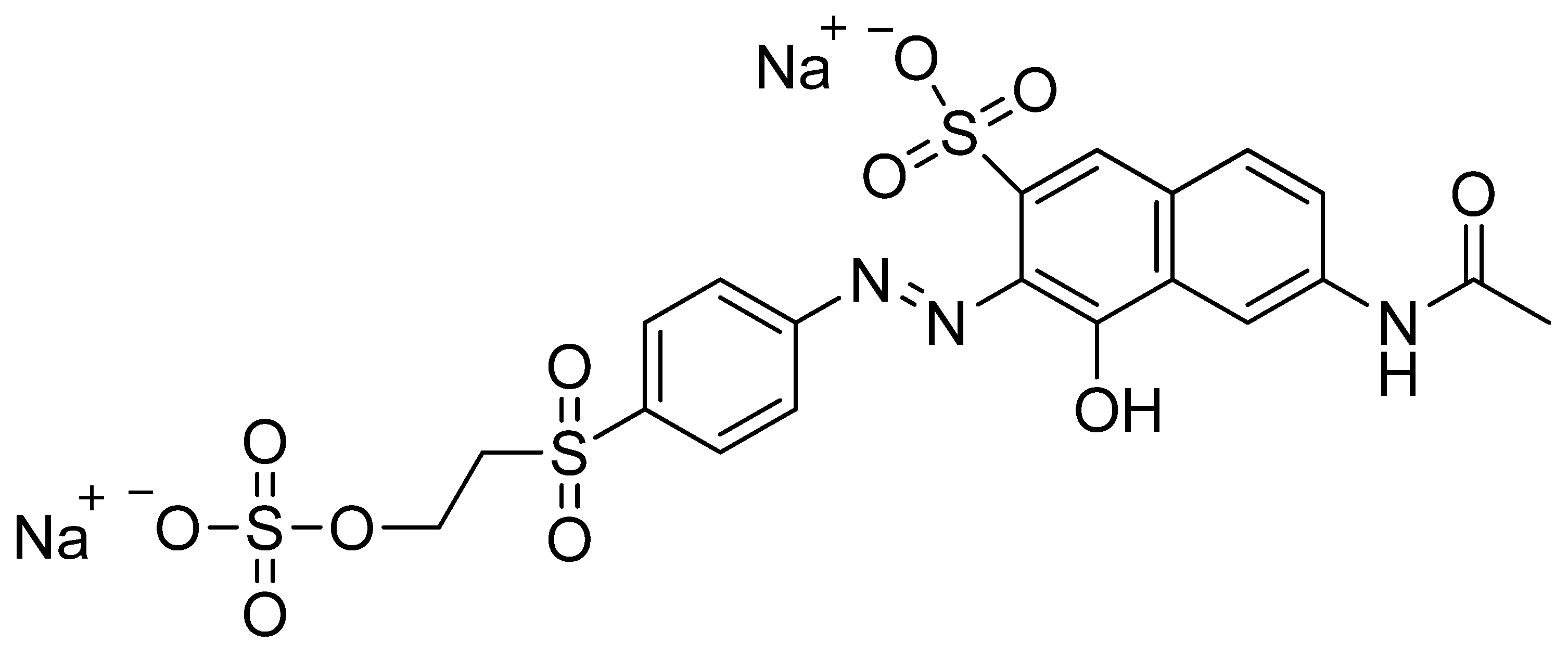
2.5. Dye Adsorption Experiments
3. Results and Discussion
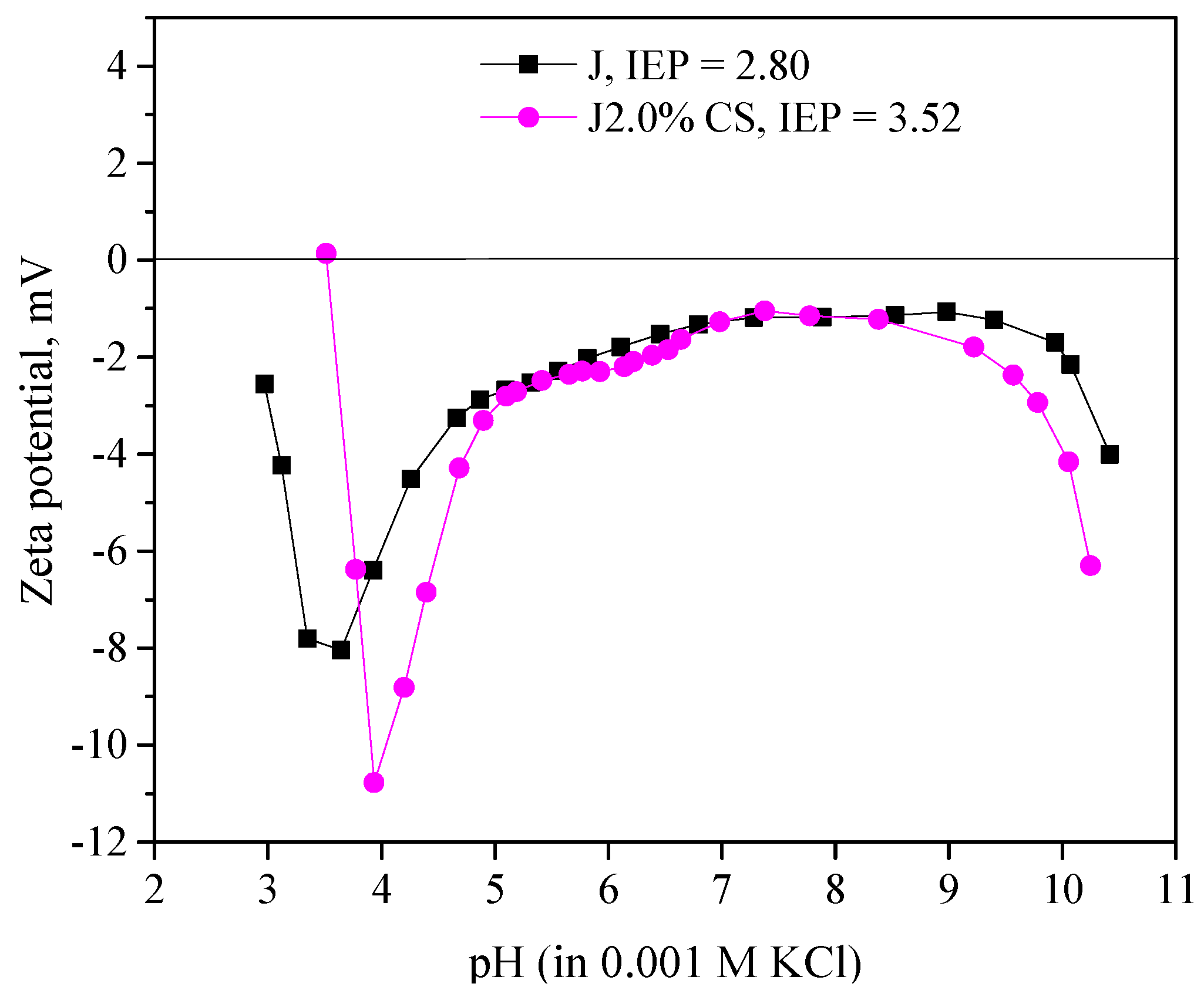
3.1. Characterization of Raw and Chitosan Treated Fabrics’ Surface Chemistry
3.2. Moisture Sorption
3.3. Adsorption of Reactive Orange 16 (RO 16) as an Indicator for Fabric Sorption Properties
3.3.1. Optimization of the Initial Solution’s pH to Achieve Maximum RO 16 Removal
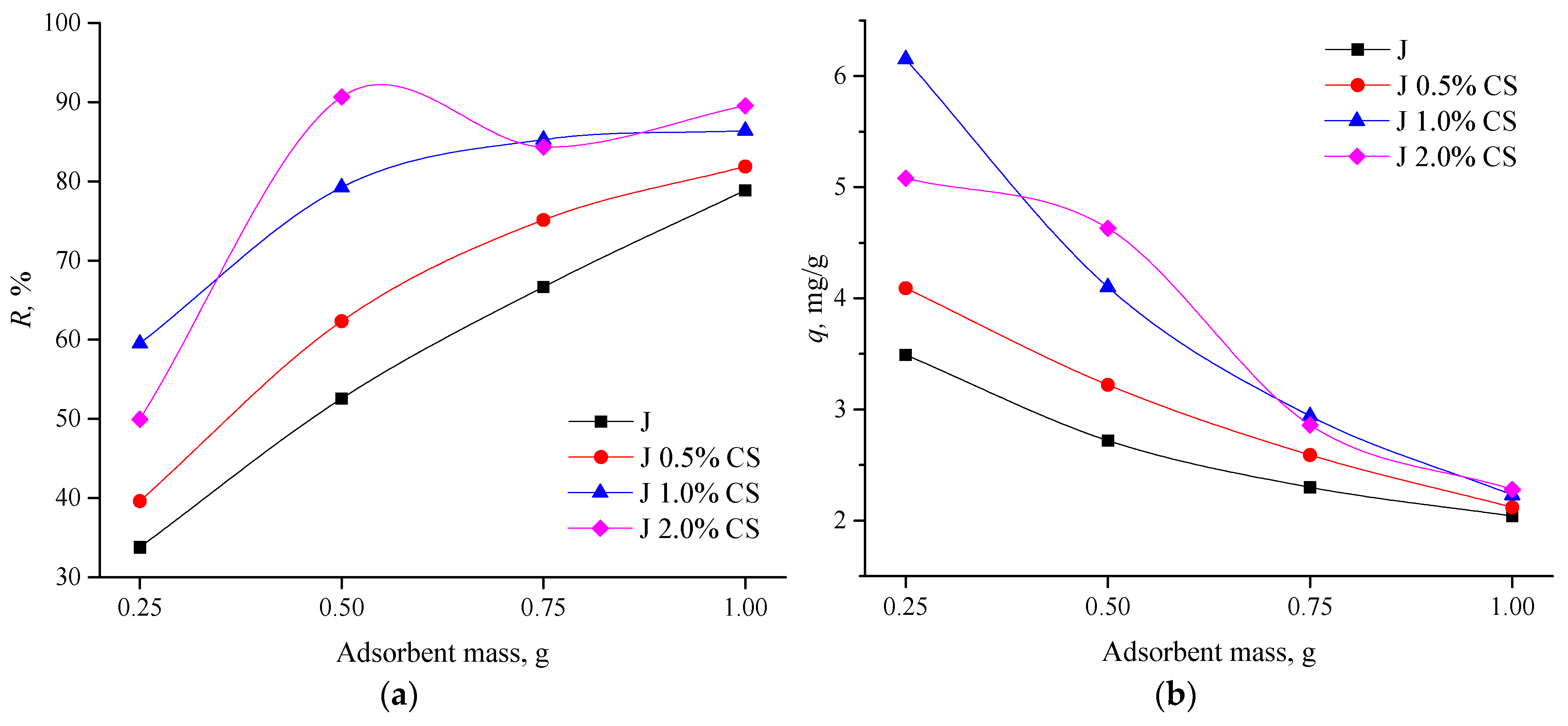
3.3.2. Influence of Adsorbent Mass on Dye Removal and Fabrics’ Adsorption Potential for RO 16
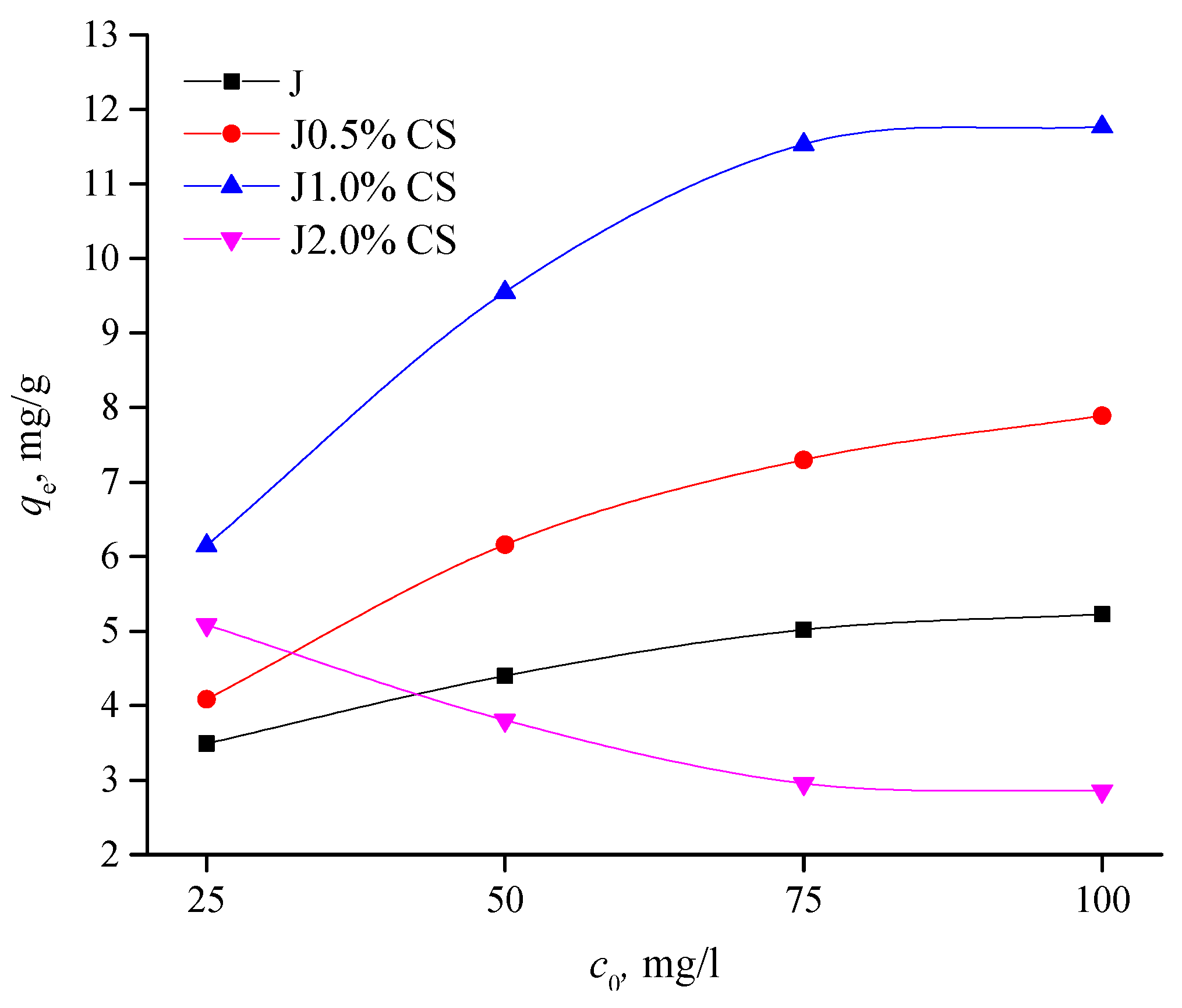
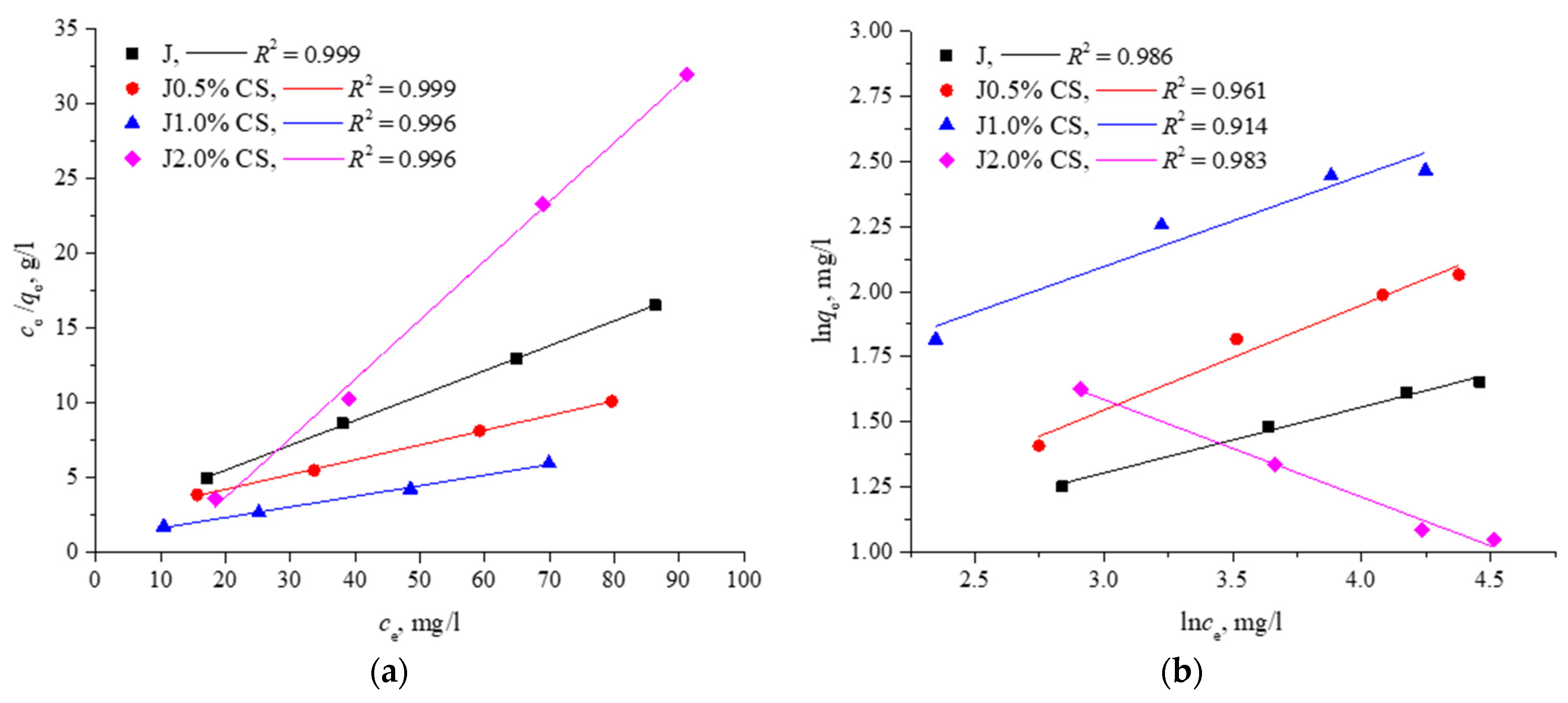
3.3.3. Isotherm Experiments
3.3.4. Thermodynamic Studies
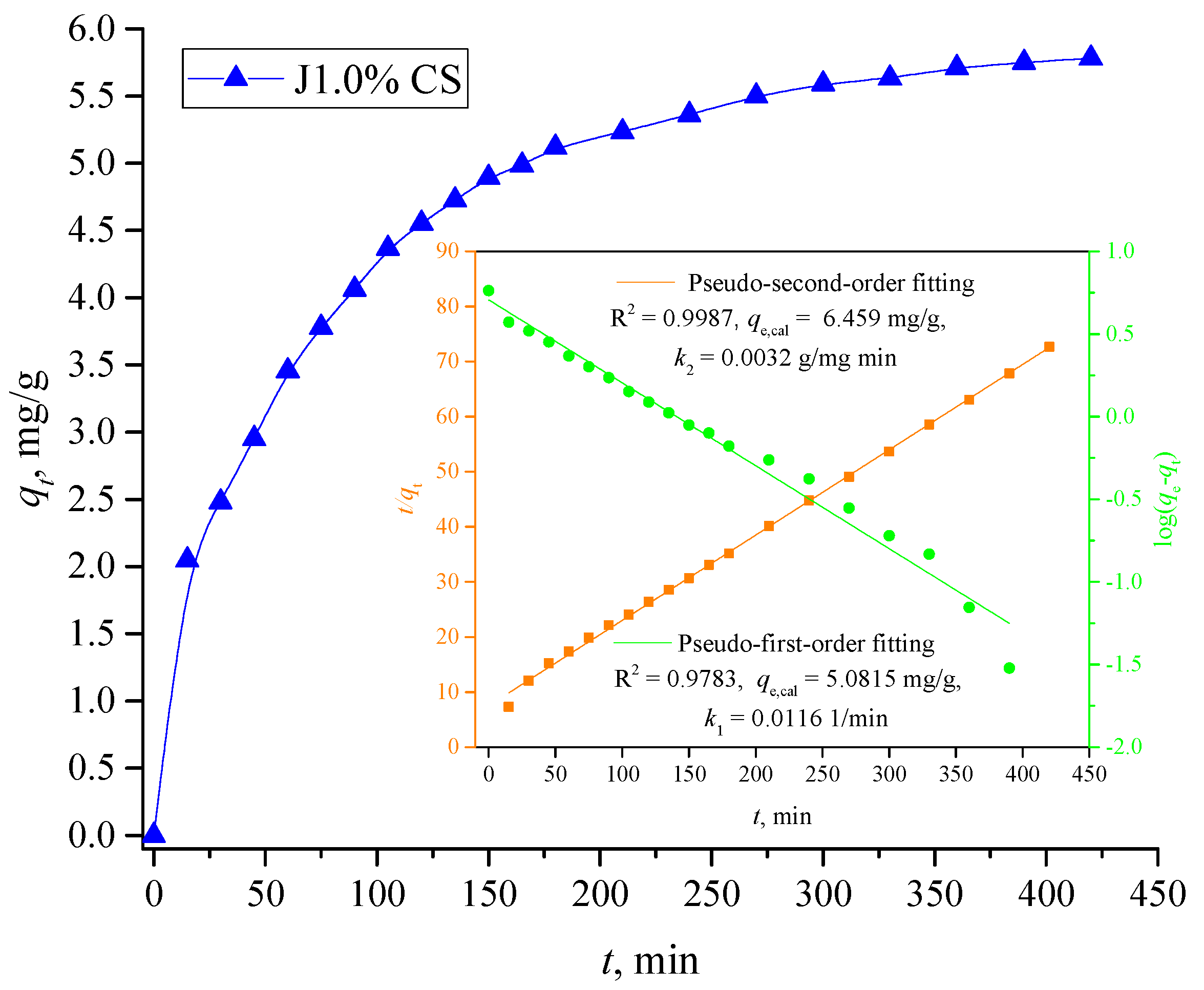
3.3.5. Kinetic Experiments
- (i)
- rapid dye adsorption during the first 2 h, adsorption process shows 79% of the equilibrium adsorption capacity of J1.0% CS which is ascribed to a high number of free available sites capable of binding dye molecules;
- (ii)
- a slower rate of dye adsorption from 2 to 4.5 h reaching about 95% of the adsorbent equilibrium adsorption capacity, an increased competition of dye molecules present in the aqueous phase occurred as a consequence of the fewer available binding sites on the adsorbent surface;
- (iii)
- dye adsorption becomes negligible in the last stage from 4.5 to 7 h, Δqt is 4.8%, so it can be stated that equilibrium was reached after 4.5 h of contact time.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reynolds, J.F.; Stafford Smith, D.M.; Lambin, E.F.; Turner, B.L.; Mortimore, M.; Batterbury, S.P.J.; Downing, T.E.; Dowlatabadi, H.; Fernández, R.J.; Herrick, J.E.; et al. Global desertification: Building a science for dryland development. Science 2007, 316, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Chamizo, S.; Mugnai, G.; Rossi, F.; Certini, G.; De Philippis, R. Cyanobacteria inoculation improves soil stability and fertility on different textured soils: Gaining insights for applicability in soil restoration. Front. Environ. Sci. 2018, 6, 49. [Google Scholar] [CrossRef]
- Rossi, F.; Li, H.; Liu, Y.; De Philippis, R. Cyanobacterial inoculation (cyanobacterisation): Perspectives for the development of a standardized multifunctional technology for soil fertilization and desertification reversal. Earth-Sci. Rev. 2017, 171, 28–43. [Google Scholar] [CrossRef]
- Antoninka, A.; Faist, A.; Rodriguez-Caballero, E.; Young, K.E.; Chaudhary, V.B.; Condon, L.A.; Pyke, D.A. Biological soil crusts in ecological restoration: Emerging research and perspectives. Restor. Ecol. 2020, 28, S3–S8. [Google Scholar] [CrossRef]
- Roncero-Ramos, B.; Muñoz-Martín, M.A.; Cantón, Y.; Chamizo, S.; Rodríguez-Caballero, E.; Mateo, P. Land degradation effects on composition of pioneering soil communities: An alternative successional sequence for dryland cyanobacterial biocrusts. Soil Biol. Biochem. 2020, 146, 107824. [Google Scholar] [CrossRef]
- Wu, Y.; Rao, B.; Wu, P.; Liu, Y.; Li, G.; Li, D. Development of artificially induced biological soil crusts in fields and their effects on top soil. Plant Soil 2013, 370, 115–124. [Google Scholar] [CrossRef]
- Belnap, J. Recovery rates of cryptobiotic crusts: Inoculant use and assessment methods. Great Basin Natur. 1993, 53, 89–95. [Google Scholar]
- Svirčev, Z.; Marković, S.B.; Stevens, T.; Codd, G.A.; Smalley, I.; Simeunović, J.; Obreht, I.; Dulić, T.; Pantelić, D.; Hambach, U. Importance of biological loess crusts for loess formation in semi-arid environments. Quat. Int. 2013, 296, 206–215. [Google Scholar] [CrossRef]
- Svirčev, Z.; Dulić, T.; Obreht, I.; Codd, G.A.; Lehmkuhl, F.; Marković, S.B.; Hambach, U.; Meriluoto, J. Cyanobacteria and loess—An underestimated interaction. Plant Soil 2019, 439, 293–308. [Google Scholar] [CrossRef]
- Ivanovska, A.; Asanović, K.; Jankoska, M.; Pavlović, S.; Poparić, G.; Kostić, M. Alkali treated jute fabrics suitable for the production of inexpensive technical textiles. Fiber. Polym. 2022, 23, 2306–2315. [Google Scholar] [CrossRef]
- Korica, M.; Peršin, Z.; Trifunović, S.; Mihajlovski, K.; Nikolić, T.; Maletić, S.; Fras Zemljič, L.; Kostić, M.M. Influence of different pretreatments on the antibacterial properties of chitosan functionalized viscose fabric: TEMPO oxidation and coating with TEMPO oxidized cellulose nanofibrils. Materials 2019, 12, 3144. [Google Scholar] [CrossRef]
- Kramar, A.D.; Ilic-Tomic, T.R.; Lađarević, J.M.; Nikodinovic-Runic, J.B.; Kostic, M.M. Halochromic cellulose textile obtained via dyeing with biocolorant isolated from Streptomyces sp. strain NP4. Cellulose 2021, 28, 8771–8784. [Google Scholar] [CrossRef]
- Ivanovska, A.M.; Kostić, M.M. Electrokinetic properties of chemically modified jute fabrics. J. Serb. Chem. Soc. 2020, 85, 1621–1627. [Google Scholar] [CrossRef]
- Koblyakov, A. Laboratory Practice in the Study of Textile Materials, 1st ed.; Mir Publishers: Moscow, Russia, 1989; pp. 192–200. [Google Scholar]
- El-Shafei, A.M.; Adel, A.M.; Ibrahim, A.A.; Al-Shemy, M.T. Dual functional jute fabric biocomposite with chitosan and phosphorylated nano-cellulose (antimicrobial and thermal stability). Int. J. Biol. Macromol. 2019, 124, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kwon, G.-J.; Hwang, K.; Kim, D.-Y. Cellulose-chitosan antibacterial composite films prepared from LiBr solution. Polymers 2018, 10, 1058. [Google Scholar] [CrossRef]
- Ivanovska, A.; Asanovic, K.; Jankoska, M.; Mihajlovski, K.; Pavun, L.; Kostic, M. Multifunctional jute fabrics obtained by different chemical modifications. Cellulose 2020, 27, 8485–8502. [Google Scholar] [CrossRef]
- Kim, U.-J.; Lee, Y.R.; Kang, T.H.; Choi, J.W.; Kimura, S.; Wada, M. Protein adsorption of dialdehyde cellulose-crosslinked chitosan with high amino group contents. Carbohyd. Polym. 2017, 163, 34–42. [Google Scholar] [CrossRef]
- Ivanovska, A.; Milošević, M.; Obradović, B.; Svirčev, Z.; Kostić, M. Plasma treatment as a sustainable method for enhancing the wettability of jute fabrics. Sustainability 2023, 15, 2125. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Bakri, M.K.B.; Jayamani, E.; Hamdan, S.; Rahman, M.R.; Soon, K.H.; Kakar, A. Fundamental study on the effect of alkaline treatment on natural fibers structures and behaviors. ARPN J. Eng. Appl. Sci. 2016, 11, 8759–8763. [Google Scholar]
- Ivanovska, A.; Veljović, S.; Dojčinović, B.; Tadić, N.; Mihajlovski, K.; Natić, M.; Kostić, M. A strategy to revalue a wood waste for simultaneous cadmium removal and wastewater disinfection. Adsorpt. Sci. Technol. 2021, 2021, 3552300. [Google Scholar] [CrossRef]
- Ahuja, D.; Kaushik, A.; Chauhan, G.S. Fractionation and physicochemical characterization of lignin from waste jute bags: Effect of process parameters on yield and thermal degradation. Int. J. Biol. Macromol. 2017, 97, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ming, R.; Yang, G.; Li, Y.; Li, Q.; Shao, H. Influence of alkali treatment on flax fiber for use as reinforcements in polylactide stereocomplex composites. Polym. Eng. Sci. 2015, 55, 2553–2558. [Google Scholar] [CrossRef]
- Ivanovska, A.; Cerovic, D.; Maletic, S.; Jankovic Castvan, I.; Asanovic, K.; Kostic, M. Influence of the alkali treatment on the sorption and dielectric properties of woven jute fabric. Cellulose 2019, 26, 5133–5146. [Google Scholar] [CrossRef]
- Korica, M.; Peršin, Z.; Fras Zemljič, L.; Mihajlovski, K.; Dojčinović, B.; Trifunović, S.; Vesel, A.; Nikolić, T.; Kostić, M.M. Chitosan nanoparticles functionalized viscose fabrics as potentially durable antibacterial medical textiles. Materials 2021, 14, 3762. [Google Scholar] [CrossRef]
- Kramar, A.; Ivanovska, A.; Kostić, M. Regenerated cellulose fiber functionalization by two-step oxidation using sodium periodate and sodium chlorite—Impact on the structure and sorption properties. Fibers Polym. 2021, 22, 2177–2186. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of chitosan—A challenge for pharmaceutical and biomedical applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Shah, J.A.; Butt, T.A.; Mirza, C.R.; Shaikh, A.J.; Khan, M.S.; Arshad, M.; Riaz, N.; Haroon, H.; Gardazi, S.M.H.; Yaqoob, K.; et al. Phosphoric acid activated carbon from Melia azedarach waste sawdust for adsorptive removal of reactive orange 16: Equilibrium modelling and thermodynamic analysis. Molecules 2020, 25, 2118. [Google Scholar] [CrossRef] [PubMed]
- Kheradmand, A.; Negarestani, M.; Mollahosseini, A.; Shayesteh, H.; Farimaniraad, H. Low-cost treated lignocellulosic biomass waste supported with FeCl3/Zn(NO3)2 for water decolorization. Sci. Rep. 2022, 12, 16442. [Google Scholar] [CrossRef]
- Ong, S.T.; Lee, C.K.; Zainal, Z. Removal of basic and reactive dyes using ethylenediamine modified rice hull. Bioresour. Technol. 2007, 98, 2792–2799. [Google Scholar] [CrossRef]
- Suteu, D.; Malutan, T.; Bilba, D. Agricultural waste corn cob as a sorbent for removing reactive dye orange 16: Equilibrium and kinetic study. Cell. Chem. Technol. 2011, 45, 413–420. [Google Scholar]
- Ghosh, R.K.; Ray, D.P.; Tewari, A.; Das, I. Removal of textile dyes from water by jute stick activated carbon: Process optimization and isotherm studies. Int. J. Environ. Sci. Technol. 2021, 18, 2747–2764. [Google Scholar] [CrossRef]
- Rehman, S.; Tariq, M.; Shah, J.A.; Ahmad, R.; Shahzad, M.; Abbasi, A.M.; Ullah, A.; Ismail, B.; Bilal, M. Simultaneous physisorption and chemisorption of reactive orange 16 onto hemp stalks activated carbon: Proof from isotherm modeling. Biointerface Res. Appl. Chem. 2017, 7, 2021–2029. [Google Scholar]
- Thivya, J.; Vijayaraghavan, J. Single and binary sorption of reactive dyes onto red seaweed-derived biochar: Multi-component isotherm and modeling. Desalin. Water Treat. 2019, 156, 87–95. [Google Scholar] [CrossRef]
- Ivanovska, A.; Lađarević, J.; Pavun, L.; Dojčinović, B.; Cvijetić, I.; Mijin, D.; Kostić, M. Obtaining jute fabrics with enhanced sorption properties and “closing the loop” of their lifecycle. Ind. Crop. Prod. 2021, 171, 113913. [Google Scholar] [CrossRef]
- Kheradmand, A.; Negarestani, M.; Kazemi, S.; Shayesteh, H.; Javanshir, S.; Ghiasinejad, H. Adsorption behavior of rhamnolipid modified magnetic Co/Al layered double hydroxide for the removal of cationic and anionic dyes. Sci. Rep. 2022, 12, 14623. [Google Scholar] [CrossRef]
- Won, S.W.; Choi, S.B.; Yun, Y.-S. Performance and mechanism in binding of Reactive Orange 16 to various types of sludge. Biochem. Eng. J. 2006, 28, 208–214. [Google Scholar] [CrossRef]
- Malek, N.N.A.; Jawad, A.H.; Ismail, K.; Razuan, R.; ALOthman, Z.A. Fly ash modified magnetic chitosan-polyvinyl alcohol blend for reactive orange 16 dye removal: Adsorption parametric optimization. Int. J. Biol. Macromol. 2021, 189, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Abdulhameed, A.S.; Mohammad, A.-T.; Jawad, A.H. Application of response surface methodology for enhanced synthesis of chitosan tripolyphosphate/TiO2 nanocomposite and adsorption of reactive orange 16 dye. J. Clean. Prod. 2019, 232, 43–56. [Google Scholar] [CrossRef]
- Jawad, A.H.; Malek, N.N.A.; Abdulhameed, A.S.; Razuan, R. Synthesis of magnetic chitosan-fly ash/Fe3O4 composite for adsorption of reactive orange 16 dye: Optimization by Box–Behnken design. J. Polym. Environ. 2020, 28, 1068–1082. [Google Scholar] [CrossRef]
- Roy, A.; Adhikari, B.; Majumder, S.B. Equilibrium, kinetic, and thermodynamic studies of azo dye adsorption from aqueous solution by chemically modified lignocellulosic jute fiber. Ind. Eng. Chem. Res. 2013, 52, 6502–6512. [Google Scholar] [CrossRef]
- Imessaoudene, A.; Cheikh, S.; Bollinger, J.-C.; Belkhiri, L.; Tiri, A.; Bouzaza, A.; El Jery, A.; Assadi, A.; Amrane, A.; Mouni, L. Zeolite waste characterization and use as low-cost, ecofriendly, and sustainable material for malachite green and methylene blue dyes removal: Box–Behnken design, kinetics, and thermodynamics. Appl. Sci. 2022, 12, 7587. [Google Scholar] [CrossRef]
- Rizzi, V.; Prasetyanto, E.A.; Chen, P.; Gubitosa, J.; Fini, P.; Agostiano, A.; De Cola, L.; Cosma, P. Amino grafted MCM-41 as highly efficient and reversible ecofriendly adsorbent material for the Direct Blue removal from wastewater. J. Mol. Liq. 2019, 273, 435–446. [Google Scholar] [CrossRef]
- Srikantan, C.; Suraishkumar, G.K.; Srivastava, S. A synergistic effect of physicochemical parameters on dye removal and concomitant antioxidant production in sunflower hairy roots. Int. J. Environ. Sci. Technol. 2021, 18, 3379–3394. [Google Scholar] [CrossRef]
- Ali, A.M.A.; Karthikeyan, R.K.; Selvan, M.S.; Rai, M.K.; Priyadharshini, M.; Maheswari, N.; Sree, G.J.; Padmanaban, V.; Singh, R.S. Removal of Reactive Orange 16 by adsorption onto activated carbon prepared from rice husk ash: Statistical modelling and adsorption kinetics. Sep. Sci. Technol. 2020, 55, 26–34. [Google Scholar] [CrossRef]
- Ouakouak, A.; Abdelhamid, M.; Thouraya, B.; Chahinez, H.-O.; Hocine, G.; Hamdi, N.; Syafiuddin, A.; Boopathy, R. Development of a novel adsorbent prepared from dredging sediment for effective removal of dye in aqueous solutions. Appl. Sci. 2021, 11, 10722. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, X.; Liu, X.; Fang, K.; Gong, J.; Si, J.; Gao, W.; Liu, D. In situ generated UiO-66/cotton fabric easily recyclable for reactive dye adsorption. Langmuir 2022, 38, 12095–12102. [Google Scholar] [CrossRef]
- Ivanovska, A.; Branković, I.; Lađarević, J.; Pavun, L.; Kostic, M. Oxidized jute as a valuable adsorbent for Congo Red from an aqueous solution. J. Eng. Fiber. Fabr. 2022, 17, 15589250221101380. [Google Scholar] [CrossRef]
- Lee, C.K.; Ong, S.T.; Zainal, Z. Ethylenediamine modified rice hull as a sorbent for the removal of Basic Blue 3 and Reactive Orange 16. Int. J. Environ. Pollut. 2008, 34, 246–260. [Google Scholar] [CrossRef]
- Mansouri, F.E.; Farissi, H.E.; Zerrouk, M.H.; Cacciola, F.; Bakkali, C.; Brigui, J.; Lovillo, M.P.; Esteves da Silva, J.C.G. Dye removal from colored textile wastewater using seeds and biochar of barley (Hordeum vulgare L.). Appl. Sci. 2021, 11, 5125. [Google Scholar] [CrossRef]
- Rizzi, V.; D’Agostino, F.; Fini, P.; Semeraro, P.; Cosma, P. An interesting environmental friendly cleanup: The excellent potential of olive pomace for disperse blue adsorption/desorption from wastewater. Dyes Pigments 2017, 140, 480–490. [Google Scholar] [CrossRef]
- Abualnaja, K.M.; Alprol, A.E.; Ashour, M.; Mansour, A.T. Influencing multi-walled carbon nanotubes for the removal of ismate violet 2r dye from wastewater: Isotherm, kinetics, and thermodynamic studies. Appl. Sci. 2021, 11, 4786. [Google Scholar] [CrossRef]

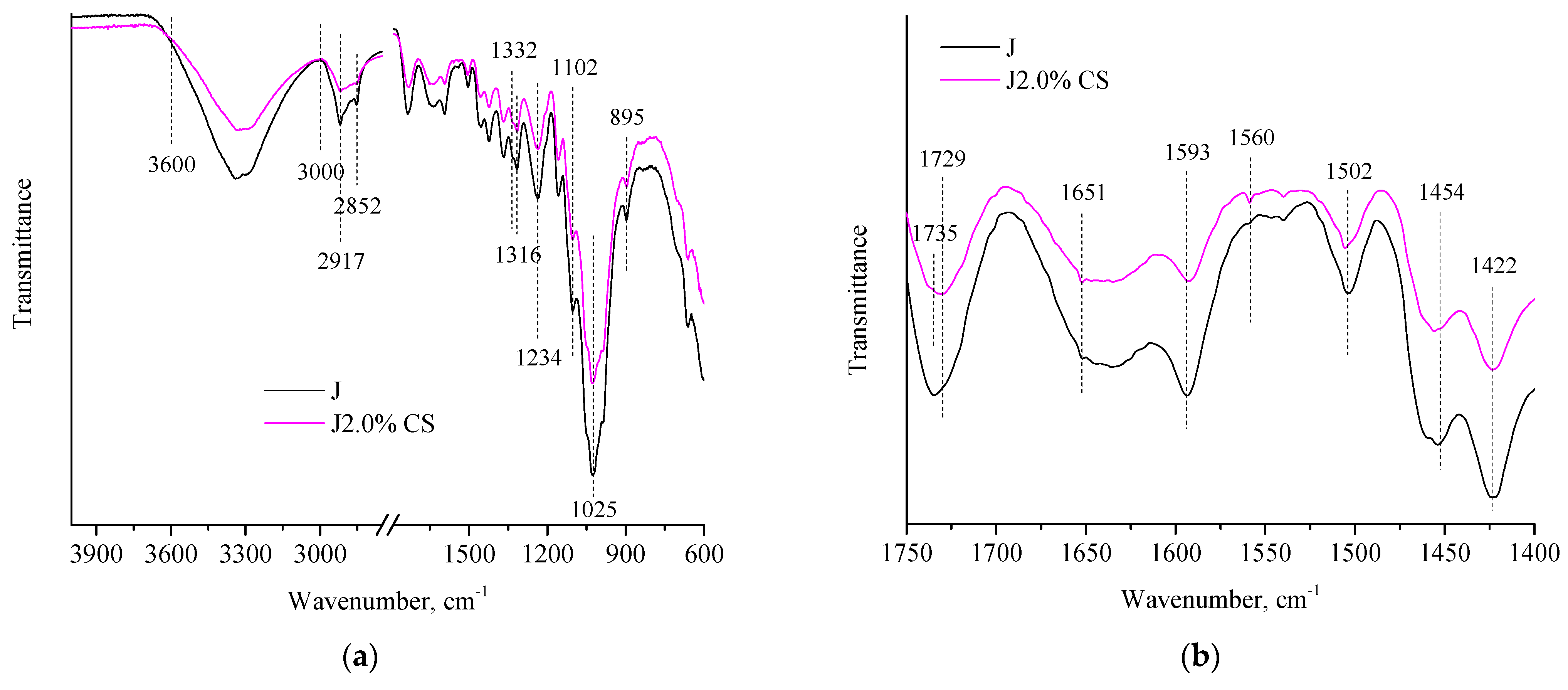



| Wavenumber, cm−1 | Interpretation | Origin |
|---|---|---|
| 2917, 2852 | C–H stretching vibration of CH2 and CH groups | cellulose and hemicelluloses |
| 1332, 1316, 1102 | fingerprint region | polysaccharides, especially cellulose |
| 1025 | C–O stretching bond structure | alcohol’s functional group |
| 1593, 1422 | aromatic skeletal vibrations and ring breathing with C–O stretching | lignin |
| 1502, 1454 | aromatic ring C = C of the phenylpropane group | |
| 1234 | ring breathing of guaiacyl “G” | |
| 895 | β-glycosidic linkages between the glycosidic units | cellulose |
| Adsorption Isotherm | Isotherm Parameters | Adsorbents | |||
|---|---|---|---|---|---|
| J | J0.5% CS | J1.0% CS | J2.0% CS | ||
| Langmuir | KL | 0.008 | 0.044 | 0.080 | −0.093 |
| qm | 5.996 | 10.128 | 14.075 | 2.525 | |
| Freundlich | Kf | 1.719 | 1.394 | 2.838 | 15.035 |
| 1/n | 0.254 | 0.404 | 0.351 | −0.375 |
| Fabric | Temperature, K | Thermodynamic Parameters | ||
|---|---|---|---|---|
| ΔG°, kJ/mol | ΔH°, kJ/mol | ΔS°, J/mol K | ||
| J | 298.15 | −10.17 | −17.80 | −25.81 |
| 308.15 | −9.73 | |||
| 318.15 | −9.59 | |||
| 328.15 | −9.37 | |||
| J0.5% CS | 298.15 | −11.39 | −17.16 | −19.40 |
| 308.15 | −11.12 | |||
| 318.15 | −11.02 | |||
| 328.15 | −10.78 | |||
| J1.0% CS | 298.15 | −12.71 | −16.43 | −12.58 |
| 308.15 | −12.47 | |||
| 318.15 | −12.52 | |||
| 328.15 | −12.27 | |||
| J2.0% CS | 298.15 | −8.53 | 32.14 | 136.52 |
| 308.15 | −9.75 | |||
| 318.15 | −11.82 | |||
| 328.15 | −12.37 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanovska, A.; Milošević, M.; Lađarević, J.; Pavun, L.; Svirčev, Z.; Kostić, M.; Meriluoto, J. Obtaining Polysaccharide-Based Fabrics with Improved Moisture Sorption and Dye Adsorption Properties. Appl. Sci. 2023, 13, 2512. https://doi.org/10.3390/app13042512
Ivanovska A, Milošević M, Lađarević J, Pavun L, Svirčev Z, Kostić M, Meriluoto J. Obtaining Polysaccharide-Based Fabrics with Improved Moisture Sorption and Dye Adsorption Properties. Applied Sciences. 2023; 13(4):2512. https://doi.org/10.3390/app13042512
Chicago/Turabian StyleIvanovska, Aleksandra, Marija Milošević, Jelena Lađarević, Leposava Pavun, Zorica Svirčev, Mirjana Kostić, and Jussi Meriluoto. 2023. "Obtaining Polysaccharide-Based Fabrics with Improved Moisture Sorption and Dye Adsorption Properties" Applied Sciences 13, no. 4: 2512. https://doi.org/10.3390/app13042512
APA StyleIvanovska, A., Milošević, M., Lađarević, J., Pavun, L., Svirčev, Z., Kostić, M., & Meriluoto, J. (2023). Obtaining Polysaccharide-Based Fabrics with Improved Moisture Sorption and Dye Adsorption Properties. Applied Sciences, 13(4), 2512. https://doi.org/10.3390/app13042512











