The Emergence of Antibiotics Resistance Genes, Bacteria, and Micropollutants in Grey Wastewater
Abstract
1. Introduction
1.1. Development of Resistance
1.2. Prevalence of Antibiotic-Resistant Bacteria and Antibiotic-Resistance Genes in GW
2. Emerging Pollutants
2.1. Cross-Resistance
2.2. Ecotoxicological Effects of EMPs
3. Irrigation with Treated GW
4. Irrigation Spiked with EMPs
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- UN-Water. Summary Progress Update 2021: SDG 6-Water and Sanitation for All; UN-Water: Geneva, Switzerland, 2021. [Google Scholar]
- Gross, A.; Maimon, A.; Alfiya, Y.; Friedler, E. Greywater Reuse; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar] [CrossRef]
- Gorgich, M.; Mata, T.M.; Martins, A.; Caetano, N.S.; Formigo, N. Application of domestic greywater for irrigating agricultural products: A brief study. Energy Rep. 2019, 18, 811–817. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Merayo, N.; Prinsen, P.; Luque, R.; Blanco, A.; Zhao, M. A review on greywater reuse: Quality, risks, barriers and global scenarios. Rev. Environ. Sci. Biotechnol. 2019, 18, 77–99. [Google Scholar] [CrossRef]
- Al-Hamaiedeh, H.; Bino, M. Effect of treated grey water reuse in irrigation on soil and plants. Desalination 2010, 256, 115–119. [Google Scholar] [CrossRef]
- Gross, A.; Shmueli, O.; Ronen, Z.; Raveh, E. Recycled vertical flow constructed wetland (RVFCW)-A novel method of recycling greywater for irrigation in small communities and households. Chemosphere 2007, 66, 916–923. [Google Scholar] [CrossRef]
- James, D.; Ganjian, E.; Surendran, S.; Ifelebuegu, A.; James, D.T.K.; Surendran, S.; Ifelebuegu, A.O.; Ganjian, E.; Kinuthia, J. Grey Water Reclamation for Urban Non-Potable Reuse-Challenges and Solutions. 2016. Available online: https://www.researchgate.net/publication/315705246 (accessed on 16 November 2022).
- Davies, J.; Davies, M. Origins and evolution of antibiotic resistance. Microbiología 2010, 12, 9–16. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-A review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Gatica, J.; Cytryn, E. Impact of treated wastewater irrigation on antibiotic resistance in the soil microbiome. Environ. Sci. Pollut. Res. 2013, 20, 3529–3538. [Google Scholar] [CrossRef]
- Tanwar, J.; Das, S.; Fatima, Z.; Hameed, S. Multidrug resistance: An emerging crisis. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 541340. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Langsrud, S.; Sundheim, G.; Holck, A.L. Cross-resistance to antibiotics of Escherichia coli adapted to benzalkonium chloride or exposed to stress-inducers. J. Appl. Microbiol. 2004, 96, 201–208. [Google Scholar] [CrossRef]
- Zgurskaya, H.I.; López, C.A.; Gnanakaran, S. Permeability Barrier of Gram-Negative Cell Envelopes and Approaches to Bypass It. ACS Infect. Dis. 2016, 1, 512–522. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 2019, 17, 441–448. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 464–472. [Google Scholar] [CrossRef]
- Thomas, C.M.; Nielsen, K.M. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 2005, 3, 711–721. [Google Scholar] [CrossRef]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef]
- Levin-Reisman, I.; Ronin, I.; Gefen, O.; Braniss, I.; Shoresh, N.; Balaban, N.Q. Antibiotic tolerance facilitates the evolution of resistance. Science 2017, 355, 826–830. [Google Scholar] [CrossRef]
- Hendriksen, R.S.; Munk, P.; Njage, P.; van Bunnik, B.; McNally, L.; Lukjancenko, O.; Röder, T.; Nieuwenhuijse, D.; Pedersen, S.K.; Kjeldgaard, J.; et al. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat. Commun. 2019, 10, 1124. [Google Scholar] [CrossRef]
- Casanova, L.M.; Little, V.; Frye, R.J.; Gerba, C.P. A survey of the microbial quality of recycled household graywater. J. Am. Water Resour. Assoc. 2001, 37, 1313–1319. [Google Scholar] [CrossRef]
- Shi, K.W.; Wang, C.W.; Jiang, S.C. Quantitative microbial risk assessment of Greywater on-site reuse. Sci. Total Environ. 2018, 635, 1507–1519. [Google Scholar] [CrossRef]
- Porob, S.; Craddock, H.A.; Motro, Y.; Sagi, O.; Gdalevich, M.; Ezery, Z.; Davidovitch, N.; Ronen, Z.; Moran-Gilad, J. Quantification and characterization of antimicrobial resistance in greywater discharged to the environment. Water 2020, 12, 1460. [Google Scholar] [CrossRef]
- Ottoson, J.; Stenstrom, T. Faecal contamination of greywater and associated microbial risks. Water Res. 2003, 37, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Acheamfour, C.L.; Parveen, C.L.; Hashem, S. Levels of Salmonella enterica and Listeria monocytogenes in Alternative Irrigation Water Vary Based on Water Source on the Eastern Shore of Maryland. Microbiol. Spectr. 2021, 9, e00669-21. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Torres, M.; Betancourt, W.Q.; Sharma, M.; Micallef, S.A.; Gerba, C.; Sapkota, A.R.; Sapkota, A.; Parveen, S.; Hashem, F.; et al. Incidence of fecal indicator and pathogenic bacteria in reclaimed and return flow waters in Arizona, USA. Environ. Res. 2019, 170, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Malayil, L.; Ramachandran, P.; Chattopadhyay, S.; Allard, S.M.; Bui, A.; Butron, J.; Callahan, M.T.; Craddock, H.A.; Murray, R.; East, C.; et al. Variations in Bacterial Communities and Antibiotic Resistance Genes Across Diverse Recycled and Surface Water Irrigation Sources in the Mid-Atlantic and Southwest United States: A CONSERVE Two-Year Field Study. Environ. Sci. Technol. 2022, 56, 15019–15033. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef]
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.N.; et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wang, T.; Xu, N.; Lu, T.; Hong, W.; Penuelas, J.; Gillings, M.; Wang, M.; Gao, W.; et al. Assessment of global health risk of antibiotic resistance genes. Nat. Commun. 2022, 13, 1553. [Google Scholar] [CrossRef]
- Pazda, M.; Kumirska, J.; Stepnowski, P.; Mulkiewicz, E. Antibiotic resistance genes identified in wastewater treatment plant systems—A review. Sci. Total Environ. 2019, 697, 134023. [Google Scholar] [CrossRef]
- Szczepanowski, R.; Linke, B.; Krahn, I.; Gartemann, K.H.; Gützkow, T.; Eichler, W.; Pühler, A.; Schlüter, A. Detection of 140 clinically relevant antibiotic-resistance genes in the plasmid metagenome of wastewater treatment plant bacteria showing reduced susceptibility to selected antibiotics. Microbiology 2009, 155, 2306–2319. [Google Scholar] [CrossRef]
- Christou, A.; Agüera, A.; Bayona, J.M.; Cytryn, E.; Fotopoulos, V.; Lambropoulou, D.; Manaia, C.M.; Michael, C.; Revitt, M.; Schröder, P.; et al. The potential implications of reclaimed wastewater reuse for irrigation on the agricultural environment: The knowns and unknowns of the fate of antibiotics and antibiotic resistant bacteria and resistance genes—A review. Water Res. 2017, 123, 448–467. [Google Scholar] [CrossRef]
- Randall, L.P.; Cooles, S.W.; Osborn, M.K.; Piddock, L.J.V.; Woodward, M.J. Antibiotic resistance genes, integrons and multiple antibiotic resistance in thirty-five serotypes of Salmonella enterica isolated from humans and animals in the UK. J. Antimicrob. Chemother. 2004, 53, 208–216. [Google Scholar] [CrossRef]
- Hayatgheib, N.; Calvez, S.; Fournel, C.; Pineau, L.; Pouliquen, H.; Moreau, E. Antimicrobial susceptibility profiles and resistance genes in genus aeromonas spp. Isolated from the environment and rainbow trout of two fish farms in France. Microorganisms 2021, 9, 1201. [Google Scholar] [CrossRef]
- Troiano, E.; Beneduce, L.; Gross, A.; Ronen, Z. Antibiotic-resistant bacteria in greywater and greywater-irrigated soils. Front. Microbiol. 2018, 9, 2666. [Google Scholar] [CrossRef]
- Craddock, H.A.; Chattopadhyay, S.; Rjoub, Y.; Rosen, D.; Greif, J.; Lipchin, C.; Mongodin, E.F.; Sapkota, A.R. Antibiotic-resistant Escherichia coli and Klebsiella spp. in greywater reuse systems and pond water used for agricultural irrigation in the West Bank, Palestinian Territories. Environ. Res. 2020, 188, 109777. [Google Scholar] [CrossRef]
- Yomoda, S.; Okubo, T.; Takahashi, A.; Murakami, M.; Iyobe, S. Presence of Pseudomonas putida strains harboring plasmids bearing the metallo-β-lactamase gene blaIMP in a hospital in Japan. J. Clin. Microbiol. 2003, 41, 4246–4251. [Google Scholar] [CrossRef]
- Guo, J.; Li, J.; Chen, H.; Bond, P.L.; Yuan, Z. Metagenomic analysis reveals wastewater treatment plants as hotspots of antibiotic resistance genes and mobile genetic elements. Water Res. 2017, 123, 468–478. [Google Scholar] [CrossRef]
- ECHA. Efficacy—Assessment and Evaluation. In Guidance on the Biocidal Products Regulation; European Chemicals Agency: Helsinki, Finland, 2017; Volume 2. [Google Scholar]
- Leal, L.H.; Vieno, N.; Temmink, H.; Zeeman, G.; Buisman, C.J.N. Occurrence of xenobiotics in gray water and removal in three biological treatment systems. Environ. Sci. Technol. 2010, 44, 6835–6842. [Google Scholar] [CrossRef]
- Pereira, B.M.P.; Tagkopoulos, I. Benzalkonium chlorides: Uses, regulatory status, and microbial resistance. Appl. Environ. Microbiol. 2019, 85, e00377-19. [Google Scholar] [CrossRef]
- Pal, C.; Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Co-occurrence of resistance genes to antibiotics, biocides and metals reveals novel insights into their co-selection potential. BMC Genom. 2015, 16, 964. [Google Scholar] [CrossRef]
- Giuliano, C.A.; Rybak, M.J. Efficacy of triclosan as an antimicrobial hand soap and its potential impact on antimicrobial resistance: A focused review. Pharmacotherapy 2015, 35, 328–336. [Google Scholar] [CrossRef]
- Goudarzi, M.; Navidinia, M. Overview perspective of bacterial strategies of resistance to biocides and antibiotics. Arch. Clin. Infect. Dis. 2019, 14, e65744. [Google Scholar] [CrossRef]
- Romero, J.L.; Grande Burgos, M.J.; Pérez-Pulido, R.; Gálvez, A.; Lucas, R. Resistance to Antibiotics, Biocides, Preservatives and Metals in Bacteria Isolated from Seafoods: Co-Selection of Strains Resistant or Tolerant to Different Classes of Compounds. Front. Microbiol. 2017, 8, 1650. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tan, L.; Zhang, L.; Tian, W.; Ma, L.A. Review of the Distribution of Antibiotics in Water in Different Regions of China and Current Antibiotic Degradation Pathways. Front. Environ. Sci. 2021, 9, 692298. [Google Scholar] [CrossRef]
- Russell, A.D. Antibiotic and Biocide Resistance in Bacteria: Introduction. Available online: https://academic.oup.com/jambio/article/92/s1/1S/6721470 (accessed on 23 January 2023).
- Lu, J.; Wang, Y.; Li, J.; Mao, L.; Nguyen, S.H.; Duarte, T.; Coin, L.; Bond, P.; Yuan, Z.; Guo, J. Triclosan at environmentally relevant concentrations promotes horizontal transfer of multidrug resistance genes within and across bacterial genera. Environ. Int. 2018, 121, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Mavri, A.; Smole Možina, S. Development of antimicrobial resistance in Campylobacter jejuni and Campylobacter coli adapted to biocides. Int. J. Food Microbiol. 2013, 160, 304–312. [Google Scholar] [CrossRef]
- Alderton, I.; Palmer, B.R.; Heinemann, J.A.; Pattis, I.; Weaver, L.; Gutiérrez-Ginés, M.J.; Horswell, J.; Tremblay, L.A. The role of emerging organic contaminants in the development of antimicrobial resistance. Emerg. Contam. 2021, 7, 160–171. [Google Scholar] [CrossRef]
- Cameron, A.; Barbieri, R.; Read, R.; Church, D.; Adator, E.H.; Zaheer, R.; McAllister, T.A. Functional screening for triclosan resistance in a wastewater metagenome and isolates of Escherichia coli and Enterococcus spp. From a large Canadian healthcare region. PLoS ONE 2019, 14, 1144. [Google Scholar] [CrossRef]
- Liu, J.L.; Wong, M.H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef]
- Singer, H.; Müller, S.; Tixier, C.; Pillonel, L. Triclosan: Occurrence and fate of a widely used biocide in the aquatic environment: Field measurements in wastewater treatment plants, surface waters, and lake sediments. Environ. Sci. Technol. 2002, 36, 4998–5004. [Google Scholar] [CrossRef]
- Kumar, K.S.; Priya, S.M.; Peck, A.M.; Sajwan, K.S. Mass loadings of triclosan and triclocarbon from four wastewater treatment plants to three rivers and landfill in Savannah, Georgia, USA. Arch. Environ. Contam. Toxicol. 2010, 58, 275–285. [Google Scholar] [CrossRef]
- Schweizer, H.P. Triclosan: A widely used biocide and its link to antibiotics. FEMS Microbiol. Lett. 2001, 202, 1–7. [Google Scholar] [CrossRef]
- Halden, R.U. On the need and speed of regulating triclosan and triclocarban in the United States. Environ. Sci. Technol. 2014, 48, 3603–3611. [Google Scholar] [CrossRef]
- Birošová, L.; Mikulášová, M. Development of triclosan and antibiotic resistance in Salmonella enterica serovar Typhimurium. J. Med. Microbiol. 2009, 58, 436–441. [Google Scholar] [CrossRef]
- McMurry, L.M.; Oethinger, M.; Levy, S.B. Triclosan targets lipid synthesis. Nature 1998, 394, 531–532. [Google Scholar] [CrossRef]
- Fahimipour, A.K.; ben Maamar, S.; McFarland, A.G.; Blaustein, R.A.; Chen, J.; Glawe, A.J.; Kline, J.; Green, J.L.; Halden, R.U.; van den Wymelenberg, K.; et al. Antimicrobial Chemicals Associate with Microbial Function and Antibiotic Resistance Indoors. mSystems 2018, 3, e00200-18. [Google Scholar] [CrossRef]
- Lu, J.; Jin, M.; Nguyen, S.H.; Mao, L.; Li, J.; Coin, L.J.M.; Yuan, Z.; Guo, J. Non-antibiotic antimicrobial triclosan induces multiple antibiotic resistance through genetic mutation. Environ. Int. 2018, 118, 257–265. [Google Scholar] [CrossRef]
- Rana, P.; Ghouse, S.M.; Akunuri, R.; Madhavi, Y.; Chopra, S.; Nanduri, S. FabI (enoyl acyl carrier protein reductase)—A potential broad spectrum therapeutic target and its inhibitors. Eur. J. Med. Chem. 2020, 208, 15. [Google Scholar] [CrossRef]
- Zhu, L.; Bi, H.; Ma, J.; Hu, Z.; Zhang, W.; Cronan, J.E.; Wang, H. The two functional enoyl-acyl carrier protein reductases of Enterococcus faecalis do not mediate triclosan resistance. mBio 2013, 4, e00613-13. [Google Scholar] [CrossRef]
- Li, M.; He, Y.; Sun, J.; Li, J.; Bai, J.; Zhang, C. Chronic Exposure to an Environmentally Relevant Triclosan Concentration Induces Persistent Triclosan Resistance but Reversible Antibiotic Tolerance in Escherichia coli. Environ. Sci. Technol. 2019, 53, 3277–3286. [Google Scholar] [CrossRef]
- Sanchez, P.; Moreno, E.; Martinez, J.L. The biocide triclosan selects Stenotrophomonas maltophilia mutants that overproduce the SmeDEF multidrug efflux pump. Antimicrob. Agents Chemother. 2005, 49, 781–782. [Google Scholar] [CrossRef]
- Karatzas, K.A.G.; Webber, M.A.; Jorgensen, F.; Woodward, M.J.; Piddock, L.J.V.; Humphrey, T.J. Prolonged treatment of Salmonella enterica serovar Typhimurium with commercial disinfectants selects for multiple antibiotic resistance, increased efflux and reduced invasiveness. J. Antimicrob. Chemother. 2007, 60, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, E.M.; Hickey, R.; Hsu, T.; Betancourt Román, C.M.; Chen, J.; Schwager, R.; Kline, J.; Brown, G.Z.; Halden, R.U.; Huttenhower, C.; et al. Antimicrobial Chemicals Are Associated with Elevated Antibiotic Resistance Genes in the Indoor Dust Microbiome. Environ. Sci. Technol. 2016, 50, 9807–9815. [Google Scholar] [CrossRef] [PubMed]
- Valkova, N.; Lépine, F.; Valeanu, L.; Dupont, M.; Labrie, L.; Bisaillon, J.G.; Beaudet, R.; Shareck, F.; Villemur, R. Hydrolysis of 4-Hydroxybenzoic Acid Esters (Parabens) and Their Aerobic Transformation into Phenol by the Resistant Enterobacter cloacae Strain EM. Appl. Environ. Microbiol. 2001, 67, 2404–2409. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.; Jabłońska, E.; Ratajczak-Wrona, W. Controversy around parabens: Alternative strategies for preservative use in cosmetics and personal care products. Environ. Res. 2021, 198, 110488. [Google Scholar] [CrossRef] [PubMed]
- Bredin, J.; Davin-Régli, A.; Pagès, J.M. Propyl paraben induces potassium efflux in Escherichia coli. J. Antimicrob. Chemother. 2005, 55, 1013–1015. [Google Scholar] [CrossRef]
- Bolujoko, N.B.; Unuabonah, E.I.; Alfred, M.O.; Ogunlaja, A.; Ogunlaja, O.O.; Omorogie, M.O.; Olukanni, O.D. Toxicity and removal of parabens from water: A critical review. Sci. Total Environ. 2021, 792, 148092. [Google Scholar] [CrossRef]
- Soni, M.G.; Carabin, I.G.; Burdock, G.A. Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem. Toxicol. 2005, 43, 985–1015. [Google Scholar] [CrossRef]
- Selvaraj, K.K.; Sivakumar, S.; Sampath, S.; Shanmugam, G.; Sundaresan, U.; Ramaswamy, B.R. Paraben resistance in bacteria from sewage treatment plant effluents in India. Water Sci. Technol. 2013, 68, 2067–2073. [Google Scholar] [CrossRef]
- Blanco, P.; Hernando-Amado, S.; Reales-Calderon, J.A.; Corona, F.; Lira, F.; Alcalde-Rico, M.; Bernardini, A.; Sanchez, M.B.; Martinez, J.L. Bacterial multidrug efflux pumps: Much more than antibiotic resistance determinants. Microorganisms 2016, 4, 14. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Q.; Wang, Y.; Deng, C.; Yu, C.P. Comparative studies of aerobic and anaerobic biodegradation of methylparaben and propylparaben in activated sludge. Ecotoxicol. Environ. Saf. 2017, 138, 25–31. [Google Scholar] [CrossRef]
- Rimkus, G.G. Polycyclic Musk Fragrances in the Aquatic Environment. 1999. Available online: www.elsevier.com/locate/toxlet (accessed on 5 January 2023).
- Clara, M.; Gans, O.; Windhofer, G.; Krenn, U.; Hartl, W.; Braun, K.; Scharf, S.; Scheffknecht, C. Occurrence of polycyclic musks in wastewater and receiving water bodies and fate during wastewater treatment. Chemosphere 2011, 82, 1116–1123. [Google Scholar] [CrossRef]
- Alfiya, Y.; Gross, A.; Sklarz, M.; Friedler, E. Reliability of on-site greywater treatment systems in Mediterranean and arid environments—A case study. Water Sci. Technol. 2013, 67, 1389–1395. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Bach, H.; Bach, H. Antimicrobial and anti-inflammatory activities of commercial aromatizing fragrances. Future Sci. OA 2021, 7, FSO704. [Google Scholar] [CrossRef]
- Ding, T.; Li, W.; Cai, M.; Jia, X.; Yang, M.; Yang, B.; Li, J. Algal toxicity, accumulation and metabolic pathways of galaxolide. J. Hazard. Mater. 2020, 384, 121360. [Google Scholar] [CrossRef]
- Lozano, C.; Matallana-Surget, S.; Givens, J.; Nouet, S.; Arbuckle, L.; Lambert, Z.; Lebaron, P. Toxicity of UV filters on marine bacteria: Combined effects with damaging solar radiation. Sci. Total Environ. 2020, 722, 137803. [Google Scholar] [CrossRef]
- Lozano, C.; Lebaron, P.; Matallana-Surget, S. Shedding light on the bacterial resistance to toxic UV filters: A comparative genomic study. PeerJ 2021, 9, e12278. [Google Scholar] [CrossRef]
- Hora, P.I.; Pati, S.G.; McNamara, P.J.; Arnold, W.A. Increased Use of Quaternary Ammonium Compounds during the SARS-CoV-2 Pandemic and Beyond: Consideration of Environmental Implications. Environ. Sci. Technol. Lett. 2020, 7, 622–631. [Google Scholar] [CrossRef]
- Kucken, D.; Feucht, H.-H.; Kaulfers, P.-M. Association of qacE and qacE Δ 1 with multiple resistance to antibiotics and antiseptics in clinical isolates of Gram-negative bacteria. FEMS Microbiol. Lett. 2000, 183, 95–98. [Google Scholar] [CrossRef]
- Burata, O.E.; Yeh, T.J.; Macdonald, C.B.; Stockbridge, R.B. Still rocking in the structural era: A molecular overview of the small multidrug resistance (SMR) transporter family. J. Biol. Chem. 2022, 298, 102482. [Google Scholar] [CrossRef]
- Zou, L.; Meng, J.; McDermott, P.F.; Wang, F.; Yang, Q.; Cao, G.; Hoffmann, M.; Zhao, S. Presence of disinfectant resistance genes in Escherichia coli isolated from retail meats in the USA. J. Antimicrob. Chemother. 2014, 69, 2644–2649. [Google Scholar] [CrossRef] [PubMed]
- Hegstad, K.; Langsrud, S.; Lunestad, B.T.; Scheie, A.A.; Sunde, M.; Yazdankhah, S.P. Does the Wide Use of Quaternary Ammonium Compounds Enhance the Selection and Spread of Antimicrobial Resistance and Thus Threaten Our Health? Microb. Drug Resist. 2010, 16, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Gaze, W.H.; Abdouslam, N.; Hawkey, P.M.; Wellington, E.M.H. Incidence of class 1 integrons in a quaternary ammonium compound-polluted environment. Antimicrob. Agents Chemother. 2005, 49, 1802–1807. [Google Scholar] [CrossRef] [PubMed]
- Barber, O.W.; Hartmann, E.M. Benzalkonium chloride: A systematic review of its environmental entry through wastewater treatment, potential impact, and mitigation strategies. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2691–2719. [Google Scholar] [CrossRef]
- Kim, M.; Weigand, M.R.; Oh, S.; Hatt, J.K.; Krishnan, R.; Tezel, U.; Pavlostathis, S.G.; Konstantinidis, K.T. Widely Used Benzalkonium Chloride Disinfectants Can Promote Antibiotic Resistance. Appl. Environ. Microbiol. 2018, 84, e01201-18. [Google Scholar] [CrossRef]
- Guérin, A.; Bridier, A.; le Grandois, P.; Sévellec, Y.; Palma, F.; Félix, B.; Roussel, S.; Soumet, C.; Karpíšková, R.; Pomelio, F.; et al. Exposure to quaternary ammonium compounds selects resistance to ciprofloxacin in listeria monocytogenes. Pathogens 2021, 10, 220. [Google Scholar] [CrossRef]
- Jiang, X.; Yu, T.; Xu, P.; Xu, X.; Ji, S.; Gao, W.; Shi, L. Role of Efflux Pumps in the in vitro Development of Ciprofloxacin Resistance in Listeria monocytogenes. Front. Microbiol. 2018, 9, 2350. [Google Scholar] [CrossRef]
- Bay, D.C.; Stremick, C.A.; Slipski, C.J.; Turner, R.J. Secondary multidrug efflux pump mutants alter Escherichia coli biofilm growth in the presence of cationic antimicrobial compounds. Res. Microbiol. 2017, 168, 208–221. [Google Scholar] [CrossRef]
- Khan, R.; Lee, M.H.; Joo, H.; Jung, Y.H.; Ahmad, S.; Choi, J.; Lee, S.W. Triclosan resistance in a bacterial fish pathogen, Aeromonas salmonicida subsp. salmonicida, is mediated by an enoyl reductase, FabV. J. Microbiol. Biotechnol. 2015, 25, 511–520. [Google Scholar] [CrossRef]
- Carey, D.E.; McNamara, P.J. The impact of triclosan on the spread of antibiotic resistance in the environment. Front. Microbiol. 2014, 5, 780. [Google Scholar] [CrossRef]
- Condell, O.; Sheridan, A.; Power, K.A.; Bonilla-Santiago, R.; Sergeant, K.; Renaut, J.; Burgess, C.; Fanning, S.; Nally, J.E. Comparative proteomic analysis of Salmonella tolerance to the biocide active agent triclosan. J. Proteom. 2012, 75, 4505–4519. [Google Scholar] [CrossRef]
- Yazdankhah, S.P.; Scheie, A.A.; Høiby, E.A.; Lunestad, B.-T.; Heir, E.; Fotland, T.Ø.; Naterstad, K.; Kruse, H. Triclosan and Antimicrobial Resistance in Bacteria: An Overview. 2006. Available online: www.liebertpub.com (accessed on 12 January 2023).
- Lozano, C.; Lee, C.; Wattiez, R.; Lebaron, P.; Matallana-Surget, S. Unraveling the molecular effects of oxybenzone on the proteome of an environmentally relevant marine bacterium. Sci. Total Environ. 2021, 793, 148431. [Google Scholar] [CrossRef]
- Bondurant, S.; McKinney, T.; Bondurant, L.; Fitzpatrick, L. Evaluation of a benzalkonium chloride hand sanitizer in reducing transient Staphylococcus aureus bacterial skin contamination in health care workers. Am. J. Infect. Control 2020, 48, 522–526. [Google Scholar] [CrossRef]
- Fazlara, A.; Ekhtelat, M. The Disinfectant Effects of Benzalkonium Chloride on Some Important Foodborne Pathogens. J. Agric. Environ. Sci. 2012, 12, 23–29. [Google Scholar]
- Gravel, J.; Paradis-Bleau, C.; Schmitzer, A.R. Adaptation of a bacterial membrane permeabilization assay for quantitative evaluation of benzalkonium chloride as a membrane-disrupting agent. Medchemcomm 2017, 8, 1408–1413. [Google Scholar] [CrossRef]
- Tandukar, M.; Oh, S.; Tezel, U.; Konstantinidis, K.T.; Pavlostathis, S.G. Long-term exposure to benzalkonium chloride disinfectants results in change of microbial community structure and increased antimicrobial resistance. Environ. Sci. Technol. 2013, 47, 9730–9738. [Google Scholar] [CrossRef]
- Zhou, Y.; Anwar, M.N.; Guo, B.; Huang, W.; Liu, Y. Response of antibiotic resistance genes and microbial niches to dissolved oxygen in an oxygen-based membrane biofilm reactor during greywater treatment. Sci. Total Environ. 2022, 833, 155062. [Google Scholar] [CrossRef]
- Singer, A.C.; Shaw, H.; Rhodes, V.; Hart, A. Review of antimicrobial resistance in the environment and its relevance to environmental regulators. Front. Microbiol. 2016, 7, 1728. [Google Scholar] [CrossRef]
- Nesme, J.; Simonet, P. The soil resistome: A critical review on antibiotic resistance origins, ecology and dissemination potential in telluric bacteria. Environ. Microbiol. 2015, 17, 913–930. [Google Scholar] [CrossRef]
- Fahrenfeld, N.; Ma, Y.; O’Brien, M.; Pruden, A. Reclaimed water as a reservoir of antibiotic resistance genes: Distribution system and irrigation implications. Front. Microbiol. 2013, 4, 130. [Google Scholar] [CrossRef]
- Han, X.M.; Hu, H.W.; Shi, X.Z.; Wang, J.T.; Han, L.L.; Chen, D.; He, J.Z. Impacts of reclaimed water irrigation on soil antibiotic resistome in urban parks of Victoria, Australia. Environ. Pollut. 2016, 211, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Kampouris, I.D.; Klümper, U.; Agrawal, S.; Orschler, L.; Cacace, D.; Kunze, S.; Berendonk, T.U. Treated wastewater irrigation promotes the spread of antibiotic resistance into subsoil pore-water. Environ. Int. 2021, 146, 106190. [Google Scholar] [CrossRef] [PubMed]
- Pepper, I.L.; Brooks, J.P.; Gerba, C.P. Antibiotic Resistant Bacteria in Municipal Wastes: Is There Reason for Concern? Environ. Sci. Technol. 2018, 52, 3949–3959. [Google Scholar] [CrossRef] [PubMed]
- Oved, T.; Shaviv, A.; Goldrath, T.; Mandelbaum, R.T.; Minz, D. Influence of Effluent Irrigation on Community Composition and Function of Ammonia-Oxidizing Bacteria in Soil. Appl. Environ. Microbiol. 2001, 67, 3426–3433. [Google Scholar] [CrossRef] [PubMed]
- Ndour, N.Y.B.; Baudoin, E.; Guissé, A.; Seck, M.; Khouma, M.; Brauman, A. Impact of irrigation water quality on soil nitrifying and total bacterial communities. Biol. Fertil. Soils 2008, 44, 797–803. [Google Scholar] [CrossRef]
- Hidri, Y.; Bouziri, L.; Maron, P.A.; Anane, M.; Jedidi, N.; Hassan, A.; Ranjard, L. Soil DNA evidence for altered microbial diversity after long-term application of municipal wastewater. Agron. Sustain. Dev. 2010, 30, 423–431. [Google Scholar] [CrossRef]
- Orlofsky, E.; Bernstein, N.; Sacks, M.; Vonshak, A.; Benami, M.; Kundu, A.; Maki, M.; Smith, W.; Wuertz, S.; Shapiro, K.; et al. Comparable levels of microbial contamination in soil and on tomato crops after drip irrigation with treated wastewater or potable water. Agric. Ecosyst. Environ. 2016, 215, 140–150. [Google Scholar] [CrossRef]
- Negreanu, Y.; Pasternak, Z.; Jurkevitch, E.; Cytryn, E. Impact of treated wastewater irrigation on antibiotic resistance in agricultural soils. Environ. Sci. Technol. 2012, 46, 4800–4808. [Google Scholar] [CrossRef]
- Benami, M.; Gross, A.; Herzberg, M.; Orlofsky, E.; Vonshak, A.; Gillor, O. Assessment of pathogenic bacteria in treated graywater and irrigated soils. Sci. Total Environ. 2013, 458, 298–302. [Google Scholar] [CrossRef]
- Chen, C.; Li, J.; Chen, P.; Ding, R.; Zhang, P.; Li, X. Occurrence of antibiotics and antibiotic resistances in soils from wastewater irrigation areas in Beijing and Tianjin, China. Environ. Pollut. 2014, 193, 94–101. [Google Scholar] [CrossRef]
- McLain, J.E.; Williams, C.F. Sustainability of water reclamation: Long-term recharge with reclaimed wastewater does not enhance antibiotic resistance in sediment bacteria. Sustainability 2014, 6, 1313–1327. [Google Scholar] [CrossRef]
- Ibekwe, A.M.; Gonzalez-Rubio, A.; Suarez, D.L. Impact of treated wastewater for irrigation on soil microbial communities. Sci. Total Environ. 2018, 622, 1603–1610. [Google Scholar] [CrossRef]
- Cerqueira, F.; Matamoros, V.; Bayona, J.; Piña, B. Antibiotic resistance genes distribution in microbiomes from the soil-plant-fruit continuum in commercial Lycopersicon esculentum fields under different agricultural practices. Sci. Total Environ. 2019, 652, 660–670. [Google Scholar] [CrossRef]
- Marano, R.B.M.; Zolti, A.; Jurkevitch, E.; Cytryn, E. Antibiotic resistance and class 1 integron gene dynamics along effluent, reclaimed wastewater irrigated soil, crop continua: Elucidating potential risks and ecological constraints. Water Res. 2019, 164, 114906. [Google Scholar] [CrossRef]
- Marano, R.B.M.; Gupta, C.L.; Cozer, T.; Jurkevitch, E.; Cytryn, E. Hidden Resistome: Enrichment Reveals the Presence of Clinically Relevant Antibiotic Resistance Determinants in Treated Wastewater-Irrigated Soils. Environ. Sci. Technol. 2021, 55, 6814–6827. [Google Scholar] [CrossRef]
- Becerra-Castro, C.; Lopes, A.R.; Vaz-Moreira, I.; Silva, E.F.; Manaia, C.M.; Nunes, O.C. Wastewater reuse in irrigation: A microbiological perspective on implications in soil fertility and human and environmental health. Environ. Int. 2015, 75, 117–135. [Google Scholar] [CrossRef]
- Shi, X.; Xia, Y.; Wei, W.; Ni, B.J. Accelerated spread of antibiotic resistance genes (ARGs) induced by non-antibiotic conditions: Roles and mechanisms. Water Res. 2022, 224, 119060. [Google Scholar] [CrossRef]
- Burdon, F.J.; Bai, Y.; Reyes, M.; Tamminen, M.; Staudacher, P.; Mangold, S.; Singer, H.; Räsänen, K.; Joss, A.; Tiegs, S.D.; et al. Stream microbial communities and ecosystem functioning show complex responses to multiple stressors in wastewater. Glob. Chang. Biol. 2020, 26, 6363–6382. [Google Scholar] [CrossRef]
- Chen, J.; Ying, G.G.; Wei, X.D.; Liu, Y.S.; Liu, S.S.; Hu, L.X.; He, L.Y.; Chen, Z.F.; Chen, F.R.; Yang, Y.Q. Removal of antibiotics and antibiotic resistance genes from domestic sewage by constructed wetlands: Effect of flow configuration and plant species. Sci. Total Environ. 2016, 571, 974–982. [Google Scholar] [CrossRef]
- Qin, Q.; Chen, X.; Zhuang, J. The fate and impact of pharmaceuticals and personal care products in agricultural soils irrigated with reclaimed water. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1379–1408. [Google Scholar] [CrossRef]
- Barancheshme, F.; Munir, M. Strategies to combat antibiotic resistance in the wastewater treatment plants. Front. Microbiol. 2018, 8, 2603. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.J.S.; Ontiveros-Valencia, A.; Ilhan, Z.E.; Zhou, Y.; Miranda, E.; Maldonado, J.; Krajmalnik-Brown, R.; Rittmann, B.E. Enhancing biodegradation of C16-alkyl quaternary ammonium compounds using an oxygen-based membrane biofilm reactor. Water Res. 2017, 123, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Ma, M.; Hu, Y.; Yu, W.; Liu, H.; Bao, Z. The fate and impacts of pharmaceuticals and personal care products and microbes in agricultural soils with long term irrigation with reclaimed water. Agric. Water Manag. 2021, 251, 106862. [Google Scholar] [CrossRef]
- Asaf, S.; Numan, M.; Khan, A.L.; Al-Harrasi, A. Sphingomonas: From diversity and genomics to functional role in environmental remediation and plant growth. Crit. Rev. Biotechnol. 2020, 40, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Lange, M.; Niedziałkowska, K.; Bernat, P.; Lisowska, K. In vitro study of the ecotoxicological risk of methylisothiazolinone and chloroxylenol towards soil bacteria. Sci. Rep. 2022, 12, 19068. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Hemkemeyer, M.; Schwalb, S.A.; Heinze, S.; Joergensen, R.G.; Wichern, F. Functions of elements in soil microorganisms. Microbiol. Res. 2021, 252, 126832. [Google Scholar] [CrossRef]
- Harrow, D.I.; Felker, J.M.; Baker, K.H. Impacts of Triclosan in Greywater on Soil Microorganisms. Appl. Environ. Soil Sci. 2011, 2011, 646750. [Google Scholar] [CrossRef]
- Liu, F.; Ying, G.G.; Yang, L.H.; Zhou, Q.X. Terrestrial ecotoxicological effects of the antimicrobial agent triclosan. Ecotoxicol. Environ. Saf. 2009, 72, 86–92. [Google Scholar] [CrossRef]
- Gallego, S.; Montemurro, N.; Béguet, J.; Rouard, N.; Philippot, L.; Pérez, S.; Martin-Laurent, F. Ecotoxicological risk assessment of wastewater irrigation on soil microorganisms: Fate and impact of wastewater-borne micropollutants in lettuce-soil system. Ecotoxicol. Environ. Saf. 2021, 223, 111981. [Google Scholar] [CrossRef]
- Waller, N.J.; Kookana, R.S. Effect of triclosan on microbial activity in Australian soils. Environ. Toxicol. Chem. 2009, 28, 65–70. [Google Scholar] [CrossRef]
- Izabel-Shen, D.; Li, S.; Luo, T.; Wang, J.; Li, Y.; Sun, Q.; Yu, C.-P.; Hu, A. Repeated introduction of micropollutants enhances microbial succession despite stable degradation patterns. ISME Commun. 2022, 2, 48. [Google Scholar] [CrossRef]
- Zaayman, M.; Siggins, A.; Horne, D.; Lowe, H.; Horswell, J. Investigation of triclosan contamination on microbial biomass and other soil health indicators. FEMS Microbiol. Lett. 2017, 364, fnx163. [Google Scholar] [CrossRef]
- Cha, J.; Cupples, A.M. Detection of the antimicrobials triclocarban and triclosan in agricultural soils following land application of municipal biosolids. Water Res. 2009, 43, 2522–2530. [Google Scholar] [CrossRef]
- Yin, Y.; Wu, H.; Jiang, Z.; Jiang, J.; Lu, Z. Degradation of Triclosan in the Water Environment by Microorganisms: A Review. Microorganisms 2022, 10, 1713. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Microbiological effects of sublethal levels of antibiotics. Nat. Rev. Microbiol. 2014, 12, 465–478. [Google Scholar] [CrossRef]
- Noman, E.A.; Radin Mohamed, R.M.S.; Al-Gheethi, A.A.; Al-shaibani, M.M.; Al-Wrafy, F.A.; Al-Maqtari, Q.A.; Vo, D.V.N. Antibiotics and antibiotic-resistant bacteria in greywater: Challenges of the current treatment situation and predictions of future scenario. Environ. Res. 2022, 212, 113380. [Google Scholar] [CrossRef]
- Anand, U.; Reddy, B.; Singh, V.K.; Singh, A.K.; Kesari, K.K.; Tripathi, P.; Kumar, P.; Tripathi, V.; Simal-Gandara, J. Potential environmental and human health risks caused by antibiotic-resistant bacteria (ARB), antibiotic resistance genes (ARGs) and emerging contaminants (ECs) from municipal solid waste (MSW) landfill. Antibiotics 2021, 10, 374. [Google Scholar] [CrossRef]
- Piepersberg, W.; Heinzel, P.; Mansouri, K.; Mönnighoff, U.; Pissowotzki, K. Evolution of Antibiotic Resistance and Production Genes in Streptomycetes; Baumberg, S., Krügel, H., Noack, D., Eds.; Federation of European Microbiological Societies Symposium Series; Springer: Boston, MA, USA, 1991. [Google Scholar]
- Alcalde-Rico, M.; Olivares-Pacheco, J.; Halliday, N.; Cámara, M.; Martínez, J.L. The impaired quorum sensing response of Pseudomonas aeruginosa MexAB-OprM efflux pump overexpressing mutants is not due to non-physiological efflux of 3-oxo-C12-HSL. Environ. Microbiol. 2020, 22, 5167–5188. [Google Scholar] [CrossRef]
- Ruppé, É.; Woerther, P.L.; Barbier, F. Mechanisms of antimicrobial resistance in Gram-negative bacilli. Ann. Intensive Care 2015, 5, 61. [Google Scholar] [CrossRef]
- Meroueh, S.O.; Minasov, G.; Lee, W.; Shoichet, B.K.; Mobashery, S. Structural aspects for evolution β-lactamases from penicillin-binding proteins. J. Am. Chem. Soc. 2003, 125, 9612–9618. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Sa, P.; Nchez, Â.; Marto Ânez, J.Â.L. Environmental selection of antibiotic resistance genes. Environ. Microbiol. 2001, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stanton, I.C.; Tipper, H.J.; Chau, K.; Klümper, U.; Subirats, J.; Murray, A.K. Does Environmental Exposure to Pharmaceutical and Personal Care Product Residues Result in the Selection of Antimicrobial-Resistant Microorganisms, and is this Important in Terms of Human Health Outcomes? Environ. Toxicol. Chem. 2022. [Google Scholar] [CrossRef] [PubMed]
- Slobodiuk, S.; Niven, C.; Arthur, G.; Thakur, S.; Ercumen, A. Does irrigation with treated and untreated wastewater increase antimicrobial resistance in soil and water: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 1046. [Google Scholar] [CrossRef]
- Mutuku, C.; Gazdag, Z.; Melegh, S. Occurrence of antibiotics and bacterial resistance genes in wastewater: Resistance mechanisms and antimicrobial resistance control approaches. World J. Microbiol. Biotechnol. 2022, 38, 1461–1466. [Google Scholar] [CrossRef]
| Compound | Molecular Formula | Chemical Structure | Molecular Weight (g/mol) |
|---|---|---|---|
| Tonalide | C18H26O |  | 258.4 |
| Galaxolide | C18H26O | 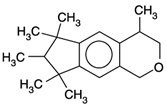 | 258.4 |
| Oxybenzone | C14H12O3 | 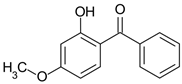 | 228.2 |
| Octocrylene | C24H27NO2 | 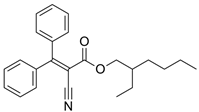 | 361.5 |
| Methylparaben | C8H8O3 | 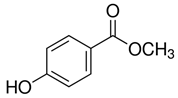 | 152.2 |
| Propylparaben | C10H12O3 | 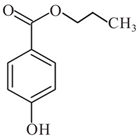 | 180.2 |
| Triclosan | C12H7Cl3O2 |  | 289.5 |
| Benzalkonium chloride | C22H42CINO | 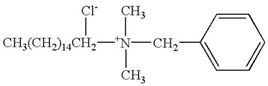 | 372.0 |
| Active Compound | Disinfectant Working Mechanism | Bacterial Adaptation to Disinfectant | References |
|---|---|---|---|
| Triclosan |
|
| [61,95,96,97,98] |
| Methyl- and propylparaben |
|
| [69,70,71] |
| Tonalide and Galaxolide |
|
| [80,81] |
| Oxybenzone and octocrylene |
|
| [82,83,99] |
| Benzalkonium chlorides |
|
| [100,101,102,103] |
| Research | Location | Findings | Reference |
|---|---|---|---|
| Field irrigated with TWW or fertilizer-amended water | Israel |
| [111] |
| Fields subjected to short wastewater irrigation regime for more than 15 years | Senegal |
| [112] |
| short- and long-term irrigation with municipal wastewater | Tunisia |
| [113] |
| Field irrigated with TWW or potable water. | Israel |
| [114] |
| Fields irrigated with either TWW or freshwater | Israel |
| [115] |
| Household gardens irrigated with either treated GW or freshwater | Israel |
| [116] |
| Five fields irrigated with TWW directly or from rivers that receive effluent, compared to fields without irrigation | China |
| [117] |
| Soil from water storage basins irrigated either with TWW or groundwater | USA |
| [118] |
| Soil columns in plastic containers irrigated with TWW and synthetic freshwater | USA |
| [119] |
| Household gardens irrigated with either treated GW or freshwater | Israel |
| [36] |
| The field was irrigated with 92% TWW from 10 wastewater treatment plants, compared to the field with groundwater irrigation | Spain |
| [120] |
| Two experimental plots irrigated with either freshwater or TWW | Israel |
| [121] |
| Field irrigated with either TWW or freshwater | Germany |
| [109] |
| Soil microcosms with either freshwater or TWW | Israel |
| [122] |
| Micropollutant Exposure | Methodology | Effect Exposure | Reference |
|---|---|---|---|
| Triclosan | Soil microcosms irrigated with synthetic GW, supplemented with 2.0 μg/mL TCS |
| [135] |
| Triclosan | Biolog ECO plates were used with soil from a rice paddy. The soil was spiked with six different concentrations (0, 0.1, 1, 10, 30, 50 mg/kg), sealed inside a plastic bag, incubated at 28 °C in the dark, and read every 24 h over 7 days. |
| [136] |
| A mixture of 14 pharmaceuticals | Irrigation with wastewater spiked at 10 and 100 μg/L in a controlled greenhouse experiment |
| [137] |
| Triclosan | Soil exposed to TCS at concentrations of 0, 1, 5, 10, 50, and 100 mg/kg of soil |
| [138] |
| Triclosan | Freshwater microbes exposed to TCS, alone or together with three different micropollutants |
| [139] |
| PPCPs | Soil irrigated long-term with wastewater compared to soil irrigation with groundwater |
| [130] |
| Triclosan | Soil microcosms exposed to different concentrations of TCS |
| [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itzhari, D.; Ronen, Z. The Emergence of Antibiotics Resistance Genes, Bacteria, and Micropollutants in Grey Wastewater. Appl. Sci. 2023, 13, 2322. https://doi.org/10.3390/app13042322
Itzhari D, Ronen Z. The Emergence of Antibiotics Resistance Genes, Bacteria, and Micropollutants in Grey Wastewater. Applied Sciences. 2023; 13(4):2322. https://doi.org/10.3390/app13042322
Chicago/Turabian StyleItzhari, Daniella, and Zeev Ronen. 2023. "The Emergence of Antibiotics Resistance Genes, Bacteria, and Micropollutants in Grey Wastewater" Applied Sciences 13, no. 4: 2322. https://doi.org/10.3390/app13042322
APA StyleItzhari, D., & Ronen, Z. (2023). The Emergence of Antibiotics Resistance Genes, Bacteria, and Micropollutants in Grey Wastewater. Applied Sciences, 13(4), 2322. https://doi.org/10.3390/app13042322







