Abstract
The world is facing the global challenges of insecurity, poverty and climate change, which can impede food availability, production and nutritional security. Due to these factors, the production and availability of crop species such as legumes, pulses and cereals are declining, while some are gradually becoming extinct, which affects consumption. To meet global food demands, efforts should be geared towards promoting the cultivation and utilization of underexploited and neglected crops, which have the potential to improve food and nutrition security. However, the exploitation and utilization of crops mostly depend on existing knowledge. Therefore, this review gives an overview of the current knowledge regarding lima beans (Phaseolus lunatus L.), an underutilized legume that can serve as a promising potential food crop. While there are some studies on lima beans, they cannot compare to the abundance of studies on other legumes. It is essential to exploit the nutritional and health properties of this crop, as well as to explore processing techniques such as cooking, soaking, fermentation and germination for transforming them into other food forms. Despite the dearth of information on this crop compared to other legumes, there is a case for the promotion of lima beans, especially where there are incessant food shortages, as they will allow for dietary diversity. This is vital considering the vulnerability of world food systems, coupled with an ever-growing population, necessitating a focus on other neglected crops to improve food security.
1. Introduction
Legumes, belonging to the Leguminosae family, also known as Fabaceae, play a significant role in food and nutrition worldwide. The Leguminosae family is large, containing more than 18,000 species of shrubs, herbs, trees, and climbers, of which only a few are used in the human diet. Legumes are also known to be the second-most significant source of food after cereals [1]. Legumes are essential sources of nutrients such as minerals, carbohydrates, vitamins, and dietary fiber, and serve as excellent sources of inexpensive plant proteins, in comparison to animal products [2]. Despite increasing interest in these crops, their application and cultivation is relatively small. According to Popoola et al. [3], legumes are grouped into minor and major species. The minor legumes are considered to be neglected, underutilized, and less exploited, for example, the lima bean, winged bean, marama bean, rice bean and Bambara groundnut. The major legumes such as cowpea, common bean, soybean, chickpea, groundnut and others are known for their well-recognized agronomic practices, cultivation, utilization and domestication [3].
Underutilized and neglected legumes could contribute to enhancing livelihoods in various ways, by diversifying production systems, improving nutrition, guaranteeing food security, establishing new markets, stabilizing ecosystems and providing replacements for crop survival under stress conditions [4]. However, several legumes have been underutilized. One such underutilized legume are lima beans (Phaseolus lunatus L.). Lima beans, also known as ‘butter beans’, are green or white, medium-to-large sized beans [5]. Considering the current challenges to food security we are facing as a planet, exploring the cultivation, domestication and use of an underutilized legume such as the lima bean could help to ameliorate the current global over-dependence on other legumes such as soybeans and common beans. Such an over-dependence can have a negative ecological, nutritional, economic and agronomic impact. As few scientific reviews exist on lima bean utilization [6,7,8,9], this review provides updated information on this crop as a vital, yet underutilized legume crop for improving food and nutrition security. Available literature was sourced from major databases (Scopus, Web of Science and Google Scholar). In addition to providing up-to-date information, this review also describes the nutritional, anti-nutritional and health composition of the crop, an overview of its microstructure, the impact of food processing on these constituents and the utilization of the lima bean for food use.
2. Taxonomy, Origin and Description of Lima Bean
According to Temegne et al. [10], the taxonomic description of the lima bean is as follows: Domain: Eukaryota, Kingdom: Plantae, Phylum: Spermatophyta, Subphylum: Angiospermae, Class: Dicotyledonae, Order: Fabales, Family: Fabaceae, Genus: Phaseolus, Species: Phaseolus lunatus. The genus Phaseolus consists of 50 species, of which only five {Phaseolus lunatus L., (lima bean), Phaseolus acutifolius A. Gray (tepary bean), Phaseolus dumosus Macfad (year bean), Phaseolus vulgaris L. (common bean), and Phaseolus coccineus L. (scarlet runner bean)} have been domesticated [11]. The lima bean is the second-most agronomically and economically-significant legume species for humans in tropical regions after the common bean [12]. According to Bonita et al. [9], the origin and taxonomy of the lima bean can be confusing. It appears to have originated in the tropical area of Central and South America [13], and after the discovery of the Americas by the Europeans, it rapidly spread into Asia, Europe and Africa as new varieties were selected or evolved [14]. Figure 1 shows the current geographical distribution and concentration of this crop, where it is particularly found in South America, Africa, Asia and Australia. Other geographical records of this crop include Aldabra, the Caribbean, Central America and Comoros Island [15]. Wild forms are only found in other parts of the world [10]. Indigenous names for lima beans include amaijalero (Uganda), bakla, kaisam bali-pati, loba, lobia (India), bonchi, bonchi-kai, dambala, dara-dambala, potu-bonchi (Sri-Lanka), frijol, frijol de lima, frijol de media luna, frijol de monte, frijol de rat, frijolillo (Nicaragua, Mexico), gros pois (Marituis), guaracara (Venezuela), Haricot De Lima (French), haricot de Madagascar, kabaro, kalamaka, konoka (Madagascar), kratok, kacang kara, kekara (Indonesia), haricot de sieva, mange-tout (Rwanda), hereboontjies (South Africa), papala (Nigeria), and tubabu soso (Senegal) [15]. Other common names include Burma bean, broad bean, butter bean, Carolina bean, civet bean, common bean, dwarf bean, frash bean, French bean, garden bean, green bean, haba bean, Hibbert bean, sugar bean, pallar beans, Madagascar bean and guffin bean [15,16].
The lima plant has a hood shape of twinning keels and typical flowers, and it is an annual to perennial growing plant, which can reach about 600 cm in height [10,17]. The crop tolerates warm temperate climates (16–27 °C), a precipitation range of 800–1500 mm and tends to prefer well-drained soil with a pH > 6 [18,19,20]. The increasing growing habits of wild lima bean species show more variation, with protracted flowering periods as well as the production of larger pods than the domesticated varieties [21]. Their roots are thin and the plants can be up to 2 m in length, while the leaves are trifoliated with egg-shaped leaflets of about 1–11 cm and 3–20 cm in width and height, respectively. [10,22]. The fruits are dehiscent pods (oblong-falcate, large flat with a crescent moon-shape) and can measure up to 12 cm in height, containing between two and four seeds [20,22,23].
The sprouts and young pods of the lima bean may be consumed, but the crops are mostly grown commercially for their seeds [24]. The matured green seeds are utilized as a vegetable for canning, freezing, or fresh market use [14,25]. Seeds vary in terms of color, eye appearance, size, and shape, while their size is usually smaller than that of the common bean (Phaseolus vulgaris) [17,26]. The average weight of the seeds is around 146 g, with a thickness of 0.72 cm, a width of 0.8–1.50 cm and a length of 1–15 cm, depending on the cultivar [22,27]. The weight of the seeds is higher than those of other legumes such as Phaseolus vulgaris L. [28], Phaseolus coccineus L. var. (purple scarlet runner) [29] and Vicia fabae var. (broad bean) [30]. The shapes of the seeds tend to be spherical, curved, or kidney-like. Lima seeds are mostly green or cream in color, although some varieties are speckled and mottled red, black, purple, white, dark or light brown (Figure 2). Lima bean seeds tend to have a remarkably starchy flavor [16].
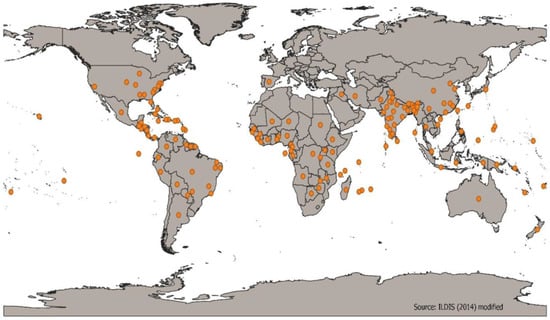
Figure 1.
Distribution of lima bean. (Source: [31,32,33,34]).

Figure 2.
(A) Lima bean pods and leaves (B) Flower (C) Lima bean seeds and pods [10] (D) Lima bean seeds variability [35].
Three morphotypes have been distinguished in Phaseolus lunatus, namely, the Big lima, which has a big flat seed, the Sieva, which has a medium flat seed, and the Potato, which has small, rounded seeds [36]. Botanical varieties of lima beans identified are the var. lunatus (domesticated types) and var. silvester (wild types); however, it has been posited that only the Phaseolus lunatus species name should be retained, as the domesticated and wild groups cannot be strictly differentiated [37]. Domesticated and wild lima beans have been discovered in a wide range of climatic conditions [18,38]. According to genomic data, the wild types are structured into three major gene pools, namely, one Andean (AI) and two Mesoamerican (MI and MII), as well as gene pools with one domestication that occurs in each of them [17,39]. While AI is constrained to southern Ecuador and northern Peru, where these species actually originated, and MI mainly occurs in central-western Mexico, MII is widely more distributed, from southern Mexico and Central America to tropical South America [39,40]. Lima bean seeds are usually planted between April and June and harvested about 5 months later, then stored in silos or, in a kitchen-crop setting, tied and hung up in a kitchen to be dried by smoke [41]. Lima beans adapt well in poor soils, and in areas such as tropical lowland rainforests, where most crops struggle, they tolerate both drought and heat conditions [25,42,43]. They are grown from sea level to above 2000 m elevation. Ideal temperatures range from 16 to 27 °C, and the bean is frost intolerant [25].
3. Nutritional, Anti-Nutritional and Health Beneficial Constituents of Lima Beans
Lima beans have a good nutritional profile and are an excellent source of proteins, amino acids (AA), minerals, dietary fiber and B-complex vitamins (folate, B6 and niacin). An advantage the seeds have over other legumes is their fat-free protein quality [44], There is, however, variation within species in terms of nutritional composition [45,46], which can be attributed to fluctuations in climate and growing stages [36]. Its nutritional composition, such as fat, protein, carbohydrate and ash, ranges from 0.21 to 3.12%, 8.61 to 26.02%, 50.44 to 77.39% and 2.35 to 6.12%, respectively [45,46,47,48] (Table 1). The lima bean hulls also have a higher protein content than most cereals [49], which means that lima beans are a good source of dietary protein that can be used to complement other food crops such as cereals.

Table 1.
Nutritional composition (%) of lima bean reported in literature.
In general, lima seeds have two folds of added protein compared to cereals [9,67] and are rich in lysine, an essential amino acid (AA). As reflected in Table 2, lysine levels can range between 4.2 and 101.4 g/100 g. Other notable essential amino acids (which have to be sourced from food as the body cannot produce them) of benefit to humans present in lima beans are phenylalanine (2.8–128.3 g/100 g), leucine (1.42–156.7 g/100 g), valine (0.81–98.3 g/100 g), threonine (0.84–102.7 g/100 g), isoleucine (0.77–90.8 g/100 g) and histidine (0.09–62.4 g/100 g) (Table 2). These AAs are important biological components needed in the human body for biosynthesis, neurotransmission and other metabolic activities, and studies indicate that lima beans are a good source of these components. The amino acid (AA) levels of lima beans are similar to that of cowpea and soybean crops [68]. Levels of essential Aas, such as phenylalanine, arginine and leucine, are higher than the FAO recommended daily allowances (RDA) in lima seeds (Table 2), with the exception of methionine, which is reported to occur in low levels [46,48]. Palupi et al. [27], however, reported an increase in methionine and concluded that methionine is part of the limiting Aas of pulse proteins. This is not unexpected, as low levels of methionine are a common trend in legumes [48]. The few available studies conducted on the AA composition of lima beans (summarized in Table 2) suggest that more research efforts should be geared toward this.

Table 2.
Amino acid composition (g/100 g) of lima bean reported in literature.
As shown in Table 1, lima beans are low in fat but contain substantial amounts of linoleic acids [72]. They are also free from cholesterol, which is crucial in terms of dietary recommendations. Lima beans have moderately high carbohydrate levels (50.44–77.39%, Table 1), and so the bean could be used to produce starch and its byproducts. As documented in previous studies [27,45,53], starch is the major carbohydrate component of lima beans, constituting between 37 and 41.96%. As observed on scanning electron microscopy, the starch granules are reported to have oval, heterogeneous and spherical shapes (Figure 3A,B) [73,74], with small pores near the granule’s equatorial region (Figure 3A). However, a study by Okekunle et al. [74] indicated that lima bean starch showed no evidence of pores or fissures in the different varieties investigated (Figure 3B).
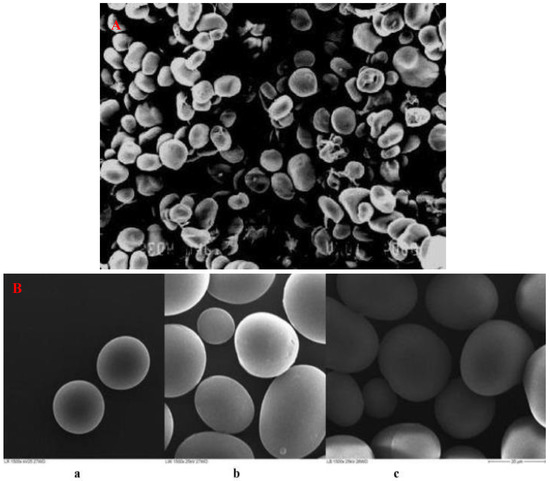
Figure 3.
(A): Scanning electron microscope of lima bean starch at 200× magnification [73]. (B): Scanning electron microscope of lima bean starches at 1500× magnification (a) red native starch (b) white native starch (c) brown native starch [74].
Lima beans are a source of dietary fiber, as the grain consists of insoluble and soluble dietary fractions. Like other legumes, the seeds are rich in vitamins including niacin, riboflavin and thiamin [75]. The varying levels of minerals in the lima bean (Table 3) show high levels of calcium, iron, zinc, phosphorus and potassium. These are minerals known to assist with muscle movement, keeping the nervous system healthy, and building strong bones and teeth. High levels of potassium and phosphorus in lima beans, with levels up to 1181.7 and 4080 mg/100 g, respectively, suggest that the lima bean is a food source that can provide the body with these essential food components.

Table 3.
Mineral composition (mg/100 g) of lima beans reported in literature.
Generally, legumes are consumed with their hulls, which conserves the micronutrient content, unlike most cereals which are polished before being consumed [79]. However, high levels of minerals do not necessarily mean a corresponding high bioavailability [80]. Regrettably, despite a high nutritional content, the lima bean also contains antinutrients such as lectins (particularly phytohaemagglutinins), tannins, trypsin inhibitors, thioglucosides, oxalate and cyanide-producing glucosides, which inhibit the absorption and utilization of essential minerals and decrease the nutritive component of foods, protein digestibility and can cause deleterious effects.
Also known as anti-nutritional factors (ANFs), antinutrients are found in all legumes and act as metabolic functions, while the consequences in the body can be negative, positive, or both [78,81]. According to Gemede and Ratta [82], protease inhibitors reduce the digestion of protein, phytates reduce the absorption of iron and calcium, phytohaemagglutinins prevent the absorption and digestion of end products in the small intestine, oxalates encourage the formation of kidney stones and reduce the absorption of calcium, while cyanide inhibits respiration. Maturity stage, cultivars, agroecology, and environmental conditions are some of the parameters that affect ANF content. Anti-nutritional factors, such as phytohaemagglutinin, trypsin inhibitor, tannins, oxalate and phytic acids have been reported to be present in lima beans (Table 4) [46,54,83]. Levels of phytic acids, tannins, trypsin inhibitor, oxalate and hydrogen cyanide reported in lima beans range from 0.02 to 47.2 mg/g, 0.01 to 78.9 mg/g, 3.4 to 184.1 Tiu/mL, 0.01 to 5.42 mg/g and 0.01 to 5.68 mg/g (Table 4) [46,52,54,83,84]. Consuming raw or inadequately processed lima beans, as with any other legume, is believed to cause countless detrimental effects. Elimination or deactivation of ANFs will enhance the nutritional quality of lima beans and increase their acceptance and application as a valuable food [48]. Most of these ANFs can be reduced or removed completely by using different processing methods such as fermentation, dehulling, cooking and soaking.

Table 4.
Anti-nutritional factors (mg/g) of lima beans reported in literature.
The nutritional composition of dry beans such as lima beans can provide health benefits in a way that few other foods can match [80]. Lima beans can be used to maintain the function of the digestive system, including in diverticulosis and irritable bowel syndrome [87]. It also contains insoluble fiber, which prevents constipation and improves stool bulk. Lima beans have also been reported to promote cardiovascular health, control blood sugar levels due to its low glycemic index, manage blood glucose and reduce cholesterol, as it is rich in fibre [61,88,89]. According to Ezeagu and Ibegbu [46], lima beans are reported to contain trace amounts of isoflavones (daidzein and genisten) which can assist in preventing breast and colon cancer. Studies have also shown that lima bean seeds have lunatusin (a 7 kDa peptide) which has shown inhibited proliferation in the breast cancer cell line MCF-7 [90]; lectins in lima beans have also been described to have anticancer activities [9]. Lima beans also help to replenish iron stores [54]. This can be beneficial to children, adolescents and women, especially during a menstrual period, when many women are iron deficient. A diet high in lima beans can assist with menstrual iron deficiency, as iron is an integral element of hemoglobin, as well as a part of the main enzyme system for energy metabolism and production [54]. Studies have also indicated the presence of gastroprotective, antimicrobial, antioxidant, antiviral and antihypertensive activities in lima beans [91,92,93,94].
4. Impacts of Certain Processing Methods on the Nutritional, Anti-Nutritional Profile and Techno-Functional Properties of the Lima Bean
Prior to consumption, the lima bean has to undergo food processing to improve its palatability and make it edible. Lima beans can also be utilized as a functional ingredient to enhance the dietary value of other foods. As with other legumes, lima beans can be prepared using different processing methods [95]. Some of these traditional processing methods (such as cooking, soaking, fermentation, and germination) will be briefly discussed with regard to their impact on the nutritional composition, ANFs and the techno-functional properties of the lima bean.
4.1. Soaking
According to Uwaegbute and Nnanyelugo [96], soaking reduces the levels of surface contaminants and toxic substances through the leaching of these undesirable constituents into the soaking medium. Soaking the seeds before cooking can also be used to moisten and soften the cotyledon and lower the cooking time. During the soaking process, water disperses into the starch granules and protein fractions, which enables the subsequent gelatinization of starch and protein denaturation during further processes, softening the bean texture [97]. The conditions to be considered when soaking lima bean seeds are temperature, time, pH, and type of media/solution (water is a common medium).
Soaking lima bean seeds in water for 24 h has been reported to cause a reduction in phytates, tannins, oxalates, saponins and trypsin inhibitors [58]. The loss in phytase may be attributed to the leaching of phytase ions into the soaking water due to variances in the chemical potential, which controls the diffusion rate [98,99]. Tannin reduction during soaking, on the other hand, has been linked to the leaching of polyphenols into the soaked bean water [100]. However, a study by Adebayo [56] on the effect of soaking time (12–48 h) on the proximate compositions of lima beans, showed no significant differences in the protein and ash of soaked and unsoaked lima beans. All the reported minerals (potassium, sodium, calcium, phosphorus and magnesium) in the study increased upon soaking, and this might be equated to the reduction in the ANFs with an increase in soaking time [56].
4.2. Dehulling
The hull or seed coat of legumes has a bitter taste and is indigestible, and so legumes are sometimes dehulled of their seed coat to improve the taste. Dehulling is also used to reduce cooking time, as the seed coat impedes water uptake during cooking [101]. A comparison of the nutritional composition of undehulled and dehulled lima seed flours showed that carbohydrates, crude fiber, ash and protein contents were lower in the dehulled sample, while crude fat was higher in the undehulled sample [41]. The authors of this study concluded that the obtained values for fat were acceptable because lima beans have a low-fat content [41]. They also found that functional properties (oil absorption, foam and emulsion capacity) were higher in the dehulled lima bean, while bulk density and water absorption capacity were lower in the dehulled sample [41].
According to another study, the protein content of the dehulled lima bean is lower than that of the undehulled sample. This could be attributed to the numerous distributions of protein within some legumes, as proteins in legumes can be spread across the endosperm, hulls, or bran [102]. A further study found that the fiber and fat content of undehulled lima bean samples were higher than those of a dehulled Christmas lima bean sample [102]. This indicates that dehulling lima bean seeds could reduce the fat content. The reduction in fat during dehulling can be linked to the leaching out of oil or fat molecules from the cotyledons [103].
The ash and carbohydrate contents of undehulled lima beans appear to have no significant difference from dehulled lima beans [103]. In a study, dehulled samples had lower viscosity, swelling index, and oil and water absorption capacity than the undehulled samples. Low water absorption capacity could be due to the lower availability of polar amino acids [104]. The low oil absorption capacity may be attributed to the large proportion of polar (hydrophilic) amino acids on the protein molecule’s surface [104,105]. Dehulling increased the gelation capacity of dehulled samples in studies, and this may be linked to the higher protein content reported in dehulled samples as well as seed coat removal [103].
4.3. Cooking, Roasting and Autoclaving
Cooking is one of the oldest methods used for making food edible prior to consumption. Generally, legumes are subjected to three different types of cooking, namely, pressure-cooking, ordinary cooking and microwave cooking. Nutrients, convenience, texture, flavor, safety, and cost are factors that consumers take into consideration when choosing food for cooking at home, with flavor being the most significant factor [106,107,108]. The effects of cooking on nutritional composition and ANF have been reported to lower the iron, copper and zinc content of lima beans [109]. Egbe and Akinyele [83] investigated the effect of cooking time (60–160 min) on the ANFs of lima beans and reported that the conventional method of preparing meals, which includes discarding and changing the cooking water several times, may decrease the level of ANFs, particularly HCN, and increase the nutrient digestibility of this legume. Cooking was also reported to have reduced the protein, ash, fiber and fat contents in lima beans, but increased the carbohydrate content [59].
A study comparing different heat treatments (boiling, roasting and autoclaving) determined that the crude protein, ash, fiber and fat contents of lima bean flours were significantly lower in boiled and autoclaved samples compared to roasted samples [60]. Tests on the ANFs of the flours showed that levels of trypsin inhibitors, saponins, oxalates, alkaloids, tannins and phytates were reduced significantly during autoclaving and boiling treatments compared to the roasting sample. Likewise, the saponin content was moderately higher in boiled samples compared to roasted and autoclaved samples [60]. Functional properties such as the solubility index, foam capacity, bulk density, swelling capacity, and water and oil absorption capacity were higher in roasted flours than in boiled and autoclaved samples [76]. Flours with a better water absorption capacity would be more appropriate for use in the preparation of baby or complementary foods [76]. In a similar manner, flours with a good foam capacity are required for the preparation of salad dressings and whipped cream [76]. A study on the effect of different processing methods (roasting cooking, soaking, germination, autoclaving and decortication) showed that all the processing methods were effective in reducing antinutrients and improving the nutritional value of lima beans, thus increasing its benefit in foods and food formulations [58].
4.4. Fermentation
Fermentation is a traditional food processing technique that improves the nutritional content of foods through the biosynthesis of essential amino acids, and vitamins, and through antinutrient degradation [63,110,111]. A decline in the protein content of lima bean flour, however, was reported by N’zi et al. [63], with authors attributing this to an increase in the catabolism of the protein matrix by microorganisms during fermentation. The same authors ascribed fat reduction during the fermentation of the bean to the hydrolysis of lipids by lipases, which are produced by fermenting microorganisms [63]. The fermentation of lima beans also has a decreasing effect on iron, copper, calcium and phosphorus, possibly linked to the reduction in phytate, tannins and HCN [54,63].
In a study, ground lima beans were fermented with starter cultures (Rhizopus stolonifera, Saccharomyces cerevisiae and Aspergillus fumigatus), and their nutritional and anti-nutritional compositions were investigated [84]. Significant reductions were seen in all the anti-nutritional factors (oxalate, saponin, phytic acid, tannin, and cyanide) of the starter culture fermented samples over the naturally fermented samples [84]. Significant reductions in anti-nutrients in the starter culture-fermented samples indicate that the fungi Rhizopus stolonifera, Saccharomyces cerevisiae and Aspergillus fumigatus were liable for these reductions [84]. Variations in the mineral contents of the starter cultures and naturally fermented samples were observed, but these were significantly lower than in the raw samples. The starter culture-fermented samples contained high fat, ash and protein contents, and low carbohydrate and crude fiber, over the naturally fermented samples [84].
The reported decrease in carbohydrate and crude fiber contents of starter culture-fermented samples over the naturally fermented samples could be attributed to the secretion of extracellular enzymes, for example, hemicellulases ligninases and cellulases by the fungi [112] These fungi are able to hydrolyze carbohydrates and crude fiber into simple sugars, while the organisms could be used as a carbon source to convert them to other macromolecules such as fats and proteins [113]. The substantial quantity of ash content in the starter culture fermented sample over the naturally fermented sample could indicate that these fungi might have played unique roles through hydrolytic or biosynthetic mechanisms to enhance the inorganic mineral elements in the samples [84].
4.5. Germination
Germination has been defined as a controlled bioprocessing step, normally adopted to alter inherent food grain composition for desirable characteristics, including the biosynthesis of health-promoting compounds [114]. During this process, the inherent physical attributes of protein, including its composition, structure, conformation and amino acid sequence, are affected and have been linked to an increase in the protein content of the germinated lima bean, noted in a study conducted by Adebayo and Okoli [59]. The changes or mechanisms occurring during the fermentation or germination of lima beans have been determined to be similar, with slight modifications in their mechanisms.
According to Farinde et al. [16], a reduction in fat, protein, fiber and ash was observed in lima beans after germination, with an increase in the carbohydrate content. However, compared to other food processing methods (roasting, fermentation, cooking), germination produces the lowest carbohydrate content [16]. The high carbohydrate content in the processed lima bean samples could be due to processing methods that soften the cellulose, causing starch granules to break down and make the starch readily available [115]. The lower carbohydrate content in germinated samples might be due to the breaking down of carbohydrates in the seed into simple sugars as the embryo is the bean’s major energy source for growth [16].
The minerals zinc, sodium, phosphorous, iron, calcium, potassium and magnesium increase with germination [16]. Likewise, all the processing methods discussed, including germination, significantly reduce the antinutrient content in lima beans. Tannins and other polyphenols in legumes may be lessened during germination, and this could be a result of the formation of polyphenol complexes with proteins, and gradual oligosaccharide degradation [116]. Germination is reported to have the lowest reduction effect (among tested processing methods) on trypsin inhibitors in the lima bean. This gives an indication that heat treatment, specifically moist heat, is more efficient at reducing trypsin inhibitors to acceptable minimal levels [16]. It should be noted that these processing methods can be combined or used singly during the preparation of lima beans.
4.6. Drying
Matured lima beans are usually processed into dried form through an open sun drying method [117]. Although the sun drying method is easy and cheap, it can be subjected to several environmental disruptions such as rain, dust, birds, insects and so on [117]. Lima beans also reduce in quality due to solar radiation [117]. To overcome such disadvantages, a controlled, closed environment should be used. Some food industries use mechanical dryers powered by either excessive electrical energy or fossil fuels to dry lima beans, which has resulted in the development of solar dryers.
The drying behavior of lima beans was investigated in a study comparing recirculatory, vacuum and tray dryers [118]. Drying behavior is a function of temperature and time. The beans, when dried in tray, recirculatory and vacuum dryers, took 220, 265 and 300 min, respectively, to dry in ranges of 40 to 80 °C. Drying at 80 °C using recirculatory dryers was determined to be the most appropriate method for drying lima beans [118].
5. Utilization of Lima Bean
In comparison to other legumes, lima beans are an underutilized crop, and this could perhaps be associated with a relatively low awareness of the nutritional and health potentials of the crop and the best preparation methods. Lima beans are commercially grown for their tender green pods, which are used as green vegetables, and are used dried as a pulse, and, due to their subtle flavor, have broad applications in different dishes [119]. Conventionally, the beans are usually cooked before consumption and can be eaten fresh, fried, boiled, canned, frozen, baked, dried, or roasted [7,120]. Dishes include salads, casseroles, soups and stews [119] (Figure 4).

Figure 4.
Foods from lima beans (A) Canned lima bean [5] (B) Lima beans dip [121] (C) Baked lima beans [122].
The bean also has medicinal applications [123] and could be eaten in combination with other crops such as roots, tubers and cereals [109,124]. It can be mixed with cereals such as sorghum to produce bread [125], with maize to produce blend extrudate [126] and can be processed into flour [56]. Lima beans can be used as a thickener in soups [25], to produce biscuits [48], moi-moi [54], succotash [119], protein isolate [51] and protein fractions. These are promising food ingredients, particularly once their functionalities have been determined [127]. Native or oxidized starches can also be produced from lima beans, and these might be of great assistance in the food industries in meeting an increasing demand for starch [74]. Lima beans show promising potential in the fortification of traditional weaning and adult foods [124]. The bean has also been used as a substitute during the production of ‘daddawa’ (a Nigerian fermented condiment) [86,128].
According to Lim [25], lima bean leaves and young pods are sometimes consumed in Malawi and Ghana as they have a bitter taste. The Yoruba tribe in Nigeria processes the seeds into cakes, porridge and puddings, while the Japanese cook the dry seeds as “nimame” (a boiled bean sweetened with sugar). In Java, lima beans are regularly eaten as a side dish with rice, and the young pods are cooked or steamed [25]. Lima beans also have other uses apart from their utilization in food. The leaves and stems can be turned into hay or silage, it may be planted as a green manure and cover crop and the crop is sometimes used as fodder. However, use in its raw form may lead to hydrogen cyanide poisoning [25]. The lima seeds are difficult to cook, particularly after they have been stored for a long-time, and contain toxic elements known as cyanogenic glycosides, which are the major limitations of cultivation and utilization. However, the commercialization of agricultural products such as lima beans is an important process in increasing food security and improving nutrition and crop productivity and reducing poverty [129,130]. The commercialization of lima beans into value-added products will further improve the livelihood of farmers and assist household consumers and food industries in dietary diversification.
6. Conclusions
A review of the overall nutritional quality and the anti-nutritional factors from various studies on lima beans shows that there is an untapped potential regarding its domestication and utilization. Lima beans have been found to be a good source of protein, fiber and carbohydrates, and provide energy, essential minerals and balanced essential amino acids which could be useful during food formulation. Lima beans also have certain antinutrients that could affect the body, either positively or negatively. The potential health benefits encourage the promotion of lima beans in our diet to take advantage of its components, which are nutritious and promote health. However, it is recommended that for the successful utilization of this bean, further detailed analyses of its overall components should be considered, including the techno-functional properties, in order to increase our depth of understanding. Studies should also be geared toward the domestication, development, application, processing and preservation of lima beans, particularly into value-added products that would boost the economy and help solve food insecurity challenges. Further research using in vivo studies could uncover more beneficial effects of lima beans for human health.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Maphosa, Y.; Jideani, V.A. The Role of Legumes in Human Nutrition. In Functional Food—Improve Health through Adequate Food; Hueda, M.C., Ed.; Intech Open: London, UK, 2017; pp. 103–121. [Google Scholar]
- Annor, G.A.; Ma, Z.; Irene, J.; Boye, J.I. Food Processing: Principles and Applications, 2nd ed.; Wiley and Sons: Hoboken, NJ, USA, 2014; pp. 305–337. [Google Scholar]
- Popoola, J.; Ojuederie, O.; Omonhinmin, C.; Adegbite, A. Neglected and underutilized legume crops: Improvement and future prospects. Improvement and Future Prospects. In Recent Advances in Grain Crops Research; Shah, F., Khan, Z., Iqbal, A., Turan, M., Olgun, M., Eds.; Intech Open: London, UK, 2019; pp. 1–12. [Google Scholar]
- Ogwu, M.C.; Osawaru, M.E.; Aiwansoba, R.O.; Iroh, R.N. Status and prospects of vegetables in Africa. In Proceedings of the Joint Biodiversity Conservation Conference of Nigeria Tropical Biology Association and Nigeria Chapter of Society for Conservation Biology on MDGs to SDGs: Toward Sustainable Biodiversity Conservation in Nigeria; Borokini, I.T., Babalola, F.D., Eds.; University of Ilorin: Ilorin, Nigeria, 2016; pp. 47–57. [Google Scholar]
- Featherstone, S. Canning of vegetables. In A Complete Course in Canning and Related Processes, 14th ed.; Volume 3, Processing Procedures for Canned Food Products; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2016; pp. 17–187. [Google Scholar]
- Nwokolo, E. Lima bean (Phaseolus lunatus L.). In Food and Feed from Legumes and Oilseeds; Nwokolo, E., Smartt, J., Eds.; Springer: New York, NY, USA, 1996; pp. 144–158. [Google Scholar]
- Heuzé, V.; Tran, G.; Sauvant, D.; Bastianelli, D.; Lebas, F. Lima Bean (Phaseolus lunatus). Feedipedia, a Programme by INRA, CIRAD, AFZ and FAO. 2015. Available online: http://www.feedipedia.org/node/267 (accessed on 12 September 2022).
- Baudoin, J.P. Genetic Resources, Domestication and Evolution of the Lima Bean Phaseolus lunatus. In Genetic Resources of Phaseolus Beans; Gepts, P., Ed.; Kluwer Academic Publishers: Boston, MA, USA, 1988. [Google Scholar]
- Bonita, L.C.; Shantibala Devi, G.A.; Singh, B.C.H. Lima bean (Phaseolus lunatus L.) a health perspective. Int. J. Sci. Technol. Res. 2020, 9, 5638–5649. [Google Scholar]
- Temegne, N.C.; Tsoata, E.; Ngome, A.F.E.; Tonfack, L.B.; Agendia, A.P.; Youmbi, E. Lima bean. In The Beans and the Peas—From Orphan to Mainstream Crops; Pratap, A., Gupta, S., Eds.; Woodhead Publishing: Duxford, UK, 2021; pp. 133–152. [Google Scholar]
- Bitocchi, E.; Rau, D.; Bellucci, E.; Rodriguez, M.; Murgia, M.L.; Gioia, T.; Santo, D.; Nanni, L.; Attene, G.; Papa, R. Beans (Phaseolus ssp.) as a model for understanding crop evolution. Front. Plant Sci. 2017, 8, 722. [Google Scholar] [CrossRef] [PubMed]
- Gepts, P. The contribution of genetic and genomic approaches to plant domestication studies. Curr. Opin. Plant Bio. 2014, 18, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Mackie, W.W. Origin, dispersal and variability in the lima bean. Hilgardia 1943, 15, 1–29. [Google Scholar] [CrossRef]
- Davis, D.W. Lima Bean. The University of Minnesota Digital Conservancy. 1987, pp. 103–112. Available online: https://hdl.handle.net/11299/202618 (accessed on 30 August 2022).
- ILDIS. International Legume Database and Information Service. 2023. Available online: https://ildis.org/cgi-bin/Araneus.pl (accessed on 9 January 2022).
- Farinde, E.O.; Olanipekun, O.T.; Olasupo, R.B. Nutritional composition and antinutrients content of raw and processed lima bean (Phaseolus lunatus). Ann. Food Sci. Technol. 2018, 19, 250–264. [Google Scholar]
- Bria, E.J.; Suharyanto, E.; Purnomo. Variability and intra-specific classification of lima bean (Phaseolus lunatus L.) from Timor Island based on morphological characters. J. Trop. Biodiv. Biotech. 2019, 4, 62–71. [Google Scholar] [CrossRef]
- Baudoin, J.P.; Rocha, O.; Degreef, J.; Maquet, A.; Guarino, L. Ecogeography, Demography, Diversity and Conservation of Phaseolus lunatus L. In the Central Valley of Costa Rica; Systematic and Ecogeographic Studies on Crop Genepools 12; International Plant Genetic Resources Institute: Rome, Italy, 2004. [Google Scholar]
- Long, R.; Temple, S.; Meyer, R.; Schwankl, L.; Godfrey, L.; Canevari, M.; Roberts, P. Lima Production in California; ANR Publication: Davis, CA, USA, 2014; p. 24. [Google Scholar]
- Ecocrop. Ecocrop Database. FAO, Rome. 2011. Available online: http://ecocrop.fao.org/ecocrop/srv/en/home (accessed on 10 August 2022).
- Zoro Bi, I.; Maquet, A.; Baudoin, J.P. Population genetic structure of wild Phaseolus lunatus (Fabaceae) with special references to population sizes. Am. J. Bot. 2003, 90, 897–904. [Google Scholar] [CrossRef]
- Baudoin, J.P. Phaseolus lunatus L. In PROTA 1: Cereals and Pulses/Céréales et Légumes Secs. [CD-Rom]; Brink, M., Belay, G., Eds.; PROTA: Wageningen, The Netherlands, 2006. [Google Scholar]
- Dohle, S. Development of Resources for Lima Bean (Phaseolus lunatus) Breeding and Genetics Research. Ph.D. Thesis, University of California, Los Angeles, CA, USA, 2017; p. 99. [Google Scholar]
- Nabhan, G.; Weber, C.W.; Berry, J.W. Variation in composition of Hopi Indian beans. Ecol. Food Nutr. 1985, 16, 135–152. [Google Scholar] [CrossRef]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Fruits; Springer: Berlin/Heidelberg, Germany, 2012; Volume 2, pp. 804–814. [Google Scholar]
- Beyra, A.; Artiles, G.R. Revision taxonomica de los generos Phaseolus Y Vigna (Leguminosae-papilionoideae) En Cuba. An. Jardín Botánico Madr. 2004, 61, 135–154. [Google Scholar]
- Palupi, H.T.; Estiasih, T.; Yunianta; Sutrisno, A. Physicochemical and protein characterization of lima bean (Phaseolus lunatus L.) seed. Food Res. 2022, 6, 168–177. [Google Scholar] [CrossRef]
- Wani, I.A.; Sogi, D.S.; Wani, A.A.; Gill, B.S. Physical and cooking characteristics of some Indian kidney bean (Phaseolus vulgaris L.) cultivars. J. Saudi Soc. Agric. Sci. 2017, 16, 7–15. [Google Scholar] [CrossRef]
- Corzo-Ríos, L.J.; Sánchez-Chino, X.M.; Cardador-Martínez, A.; Martínez-Herrera, J.; Jiménez-Martínez, C. Effect of cooking on nutritional and non-nutritional compounds in two species of Phaseolus (P. vulgaris and P. coccineus) cultivated in Mexico. Int. J. Gastr. Food Sci. 2020, 20, 100206. [Google Scholar] [CrossRef]
- Zhong, L.; Fang, Z.; Wahlqvist, M.L.; Wu, G.; Hodgson, J.M.; Johnson, S.K. Seed coats of pulses as a food ingredient: Characterization, processing, and applications. Trends Food Sci. Technol. 2018, 80, 35–42. [Google Scholar] [CrossRef]
- ILDIS, International Legume Database and Information Service. School of Plant Sciences, University of Reading: Reading, UK, 2014. Available online: http://www.ildis.org/ (accessed on 10 August 2022).
- Tsoata, E.; Temegne, C.N.; Youmbi, E. Analysis of early biochemical criterion to screen four Fabaceae plants for their tolerance to drought stress. Int. J. Curr. Res. 2017, 9, 44568–44575. [Google Scholar]
- Tsoata, E.; Temegne, C.N.; Youmbi, E. Analysis of early growth criterion to screen four Fabaceae plants for their tolerance to drought stress. RJLBPCS 2017, 2, 94–109. [Google Scholar]
- CABI. Invasive Species Compendium—Phaseolus lunatus (Lima Bean). 2019. Available online: https://www.cabi.org/isc/datasheet/40620 (accessed on 10 August 2022).
- Rulkens, T. Available online: https://commons.wikimedia.org/wiki/File:Phaseolus_lunatus-seeds(5563413427).jpg (accessed on 10 August 2022). CC BY-SA 2.0 https://creativecommons.org/licenses/by-sa/2.0 via Wiki-Media Commons. Created: 26 March 2011. (No Changes Made to This Image).
- Piergiovanni, A.R.; Sparvoli, F.; Zaccardelli, M. ‘Fagiolo a Formel la’, an Italian lima bean ecotype: Biochemical and nutritional characterisation of dry and processed seeds. J. Sci. Food Agric. 2012, 92, 2387–2393. [Google Scholar] [CrossRef]
- Debouck, D.G. Systematics and morphology. In Common Beans: Research for Crop Improvement; Van Schoonhoven, A., Voysest, O., Eds.; CAB: Oxon, UK, 1991; pp. 55–118. [Google Scholar]
- Cerda-Hurtado, I.M.; Mayek-Pérez, N.; Hernández-Delgado, S.; Muruaga-Martínez, J.S.; Reyes-Lara, M.A.; Reyes-Valdés, M.H.; González-Prieto, J.M. Climatic adaptation and ecological descriptors of wild beans from Mexico. Ecol. Evol. 2018, 8, 6492–6504. [Google Scholar] [CrossRef]
- Chacón-Sánchez, M.I.; Martínez-Castillo, J. Testing domestication scenarios of Lima bean (Phaseolus lunatus L.) in mesoamerica: Insights from genome-wide genetic markers. Front. Plant Sci. 2017, 8, 1551. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Serrano, M.L.; Hernández-Torres, J.; Castillo-Villamizar, G.; Debouck, D.G.; Chacón Sánchez, M.I. Gene pools in wild Lima bean (Phaseolus lunatus L.) from the Americas: Evidences for an Andean origin and past migrations. Mol. Phylo. Evol. 2010, 54, 76–87. [Google Scholar] [CrossRef]
- Ibeabuchi, J.C.; Okafor, D.C.; Ahaotu, N.N.; Eluchie, C.N.; Agunwah, I.J.; Chukwu, M.N.; Amandikwa, C. Effect of dehulling on proximate composition and functional properties of lima bean (Phaseolus lunatus) grown in Enugu state. J. Food Res. 2019, 8, 116–121. [Google Scholar] [CrossRef]
- Akinmutimi, A.H. Effect of potash-cooked Lima bean (Phaseolus lunatus) on broiler starter diet. Niger. Agric. J. 2001, 32, 109–118. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Blair, M.W.; Acosta-Gallegos, J.A. Phenotyping common beans for adaptation to drought. Front. Phys. 2013, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Rafael Larco, H. Los Mochicas; Museo Arqueologico Rafael Larco Herrera: Lima, Peru, 2001; ISBN 9972-9341-0-1. [Google Scholar]
- Granito, M.; Brito, Y.; Torres, A. Chemical composition, antioxidant capacity and functionality of raw and processed Phaseolus lunatus. J. Sci. Food Agric. 2007, 87, 2801–2809. [Google Scholar] [CrossRef]
- Ezeagu, I.E.; Ibegbu, M.D. Biochemical composition and nutritional potential of Ukpa: A variety of tropical lima beans (Phaseolus lunatus) from Nigeria a short report. Pol. J. Food Nutr. Sci. 2010, 60, 231–235. [Google Scholar]
- Seidu, K.T.; Osundahunsi, O.F.; Osamudiamen, P.M. Nutrients assessment of some lima bean varieties grown in southwest Nigeria. Int. Food Res. J. 2018, 25, 848–853. [Google Scholar]
- El-Gohery, S.S. Effect of different treatments on nutritional value of lima bean (Phaseolus lunatus) and its utilization in biscuit manufacture. Food Nutr. Sci. 2021, 12, 372–391. [Google Scholar]
- Seidu, K.T.; Osundahunsi, O.F.; Olaleye, M.T.; Oluwalana, I.B. Amino acid composition, mineral contents and protein solubility of some lima bean (Phaseolus lunatus L. Walp) seeds coat. Food Res. Int. 2015, 73, 130–134. [Google Scholar] [CrossRef]
- Giami, S.Y. Quality attributes of three new improved lines of Nigerian lima beans (Phaseolus lunatus L. Walp.). Plant Foods Hum. Nutr. 2001, 56, 325–333. [Google Scholar] [CrossRef]
- Chel-Guerrero, L.; Pérez-Flores, V.; Betancur-Ancona, D.; Dávila-Ortiz, G. Functional properties of flours and protein isolates from Phaseolus lunatus and Canavalia ensiformis seeds. J. Agric. Food Chem. 2002, 50, 584–591. [Google Scholar] [CrossRef]
- Fasoyiro, S.B.; Ajibade, S.R.; Omole, A.J.; Adeniyan, O.N.; Farinde, E.O. Proximate, minerals and antinutritional factors of some underutilized grain legumes in south-western Nigeria. Nutr. Food Sci. 2006, 36, 18–23. [Google Scholar] [CrossRef]
- Bello-Perez, L.A.; Sayago-Ayerdi, S.G.; Chavez-Murillo, C.E.; Agama-Acevedo, E.; Tovar, J. Proximal composition and in vitro digestibility of starch in Lima bean (Phaseolus lunatus) varieties. J. Food Sci. Agric. 2007, 87, 2570–2575. [Google Scholar] [CrossRef]
- Obiakor, P.N. Effects of Processing Techniques on Chemical, Functional and Microbial Properties of Two Varieties of Lima Bean (Phaseolus lunatus) and Maize (Zea mays) Flours and Sensory Properties of Their Products. Ph.D. Thesis, University of Nigeria, Nsukka, Nigeria, 2009. [Google Scholar]
- Iheanacho, K.M.E. Comparative studies of the nutritional composition of soy bean (Glycine max) and lima bean (Phaseolus lunatus). Sci. Afr. 2010, 9, 29–35. [Google Scholar]
- Adebayo, S.F. Effect of soaking time on the proximate, mineral compositions and anti-nutritional factors of lima bean. Food Sci. Qual. Manag. 2014, 27, 1–3. [Google Scholar]
- Yellavila, S.B.; Agbenorhevi, J.K.; Asibuo, J.Y.; Sampson, G.O. Proximate composition, minerals content and functional properties of five lima bean accessions. J. Food Secur. 2015, 3, 69–94. [Google Scholar]
- Jayalaxmi, B.; Vijayalakshmi, D.; Usha, R.; Revanna, M.L.; Chandru, R.; Ramanjini Gowda, P.H. Effect of different processing methods on proximate, mineral and antinutrient content of lima bean (Phaseolus lunatus) seeds. Legume Res. Int. J. 2016, 39, 543–549. [Google Scholar] [CrossRef]
- Adebayo, S.F.; Okoli, E.C. Effect of germination and cooking on the nutrient and poly-phenol content of lima bean (Phaseolus lunatus). Food Sci. Qual. Manag. 2017, 65, 1–4. [Google Scholar]
- Oraka, C.O.; Okoye, J.I. Effect of heat processing treatments on the nutrient and anti-nutrient contents of lima bean (Phaseolus lunatus) flour. Int. J. Food Sci. Nutr. 2017, 2, 13–17. [Google Scholar]
- Chukwunyere, E.O.; Abtew, W.G. Proximate analysis of thirteen (13) lima bean (Phaseolus lunatus) accessions in Ethiopia. ISABB J. Food Agric. Sci. 2018, 8, 7CA133259681. [Google Scholar]
- Gemede, H.F.; Birhanu, E. Nutritional, antinutritional and phenolic properties of lima bean (Phaseolus lunatus) accessions: Underutilized legume in Ethiopia. Acta Univ. Cibiniensis Ser. E Food Technol. 2020, 24, 195–204. [Google Scholar] [CrossRef]
- N’zi, K.P.; Martial-Didier, A.K.; N’guessan, K.F.; Attchelouwa, K.C.; Kablan, T. Effect of spontaneous fermentation time on physicochemical, nutrient, anti-nutrient and microbiological composition of Lima Bean (Phaseolus lunatus) flour. J. Appl. Biosci. 2021, 162, 16707–16725. [Google Scholar]
- Ogungbemi, K.; Ilesanmi, F.F.; Ayangbemi, B.T.; Oyewole, S.O.; Olaleye-Oriwo, P.I. Influence of different processing methods on the chemical analysis, nutritional and phytochemical composition of lima beans (Phaseolus lunatus); an underutilized edible crop. Alex. Sci. Exch. J. 2022, 43, 161–167. [Google Scholar] [CrossRef]
- Sahasakul, Y.; Aursalung, A.; Thangsiri, S.; Wongchang, P.; Sangkasa-ad, P.; Wongpia, A.; Polpanit, A.; Inthachat, W.; Temviriyanukul, P.; Suttisansanee, U. Nutritional compositions, phenolic contents, and antioxidant potentials of ten original lineage beans in Thailand. Foods 2022, 11, 2062. [Google Scholar] [CrossRef]
- Oke, M.O.; Sobowale, S.S.; Ogunlakin, G.O. Evaluation of the effect of processing methods on the nutritional and anti-nutritional compositions of two under-utilized Nigerian grain legumes. Pak. J. Biol. Sci. 2013, 16, 2015–2020. [Google Scholar] [CrossRef] [PubMed]
- Kathirvel, P.; Kumudha, P. A comparative study on the chemical composition of wild and cultivated germplasm of Phaseolus lunatus L. Int. J. Appl. Biol. Pharm. Technol. 2011, 2, 296–305. [Google Scholar]
- Aletor, V.A.; Aladetimi, O.O. Compositional evaluation of some cowpea varieties and some underutilized edible legumes in Nigeria. Die Nahr. 1989, 33, 999–1007. [Google Scholar] [CrossRef]
- Domínguez-Magaña, M. Aislamiento de Biopeptidos con Actividad Inhibitoria de la Enzima Convertidora de Angiotensina-I a Partir de Hidrolizados de P. lunatus. Ph.D. Thesis, Autonomous University of Yucatan, Merida, Yucatan, Mexico, 2009. [Google Scholar]
- Palupi, H.T.; Estiasih, T.; Yunianta; Sutrisno, A. Characterization of nutritional and functional properties of Lima bean flour (Phaseolus Lunatus L.). Int. Conf. Green Agro-Ind. Bioecon. IOP Conf. Ser. Earth Environ. Sci. 2021, 924, 012033. [Google Scholar] [CrossRef]
- FAO/WHO (Food and Agriculture Organization/World Health Organization). Protein Quality Evaluation: Report of Joint FAO/WHO Expert Consultation; FAO Food and Nutrition Paper No. 5; FAO: Rome, Italy, 1991. [Google Scholar]
- Osagie, A.U.; Okoye, W.I.; Oluwayose, B.O.; Dawodu, O.A. Chemical quality parameters and fatty acid composition of oils of some underexploited tropical seeds. Niger. J. Appl. Sci. 1986, 4, 151–162. [Google Scholar]
- Betancur-Ancona, D.; Gallegos-Tintore, S.; Chel-Guerrero, L. Wet fractionation of Phaseolus lunatus seeds: Partial characterization of starch and protein. J. Sci. Food Agric. 2004, 84, 1193–1201. [Google Scholar] [CrossRef]
- Okekunle, M.O.; Adebowale, K.O.; Olu-Owolabi, B.I.; Lamprecht, A. Physicochemical, morphological and thermal properties of oxidized starches from Lima bean (Phaseolus lunatus). Sci. Afr. 2020, 8, e00432. [Google Scholar] [CrossRef]
- Sathe, S.K.; Deshpande, S.S.; Salunke, D.K. Dry beans of Phaseolus: Chemical composition: Carbohydrates, fibre, minerals, vitamins and lipids. Crit. Rev. Food Sci. Nutr. 1984, 21, 41–93. [Google Scholar] [CrossRef]
- Oraka, C.O.; Okoye, J.I. Effect of heat processing treatments on the chemical composition and functional properties of lima bean (Phaseolus lunatus) flour. Am. J. Food Sci. Nutr. 2017, 1, 14–24. [Google Scholar]
- Adeparusi, E.O. Effect of processing on the nutrients and anti-nutrients of lima bean (Phaseolus lunatus L.) flour. Nahr. Food 2001, 45, 94–96. [Google Scholar] [CrossRef]
- Bolade, M.K.; Agarry, I.E.; Bolade, O.O. Impact of trona-aided boiling on the phytochemical constituents and beneficial micronutrients of lima bean (Phaseolus lunatus L.). Afr. J. Biotech. 2017, 16, 2062–2071. [Google Scholar]
- Broughton, W.J.; Hernández, G.; Blair, M.; Beebe, S.; Gepts, P.; Vanderleyden, J. Beans (Phaseolus spp.) model food legumes. Plant Soil 2003, 252, 55–128. [Google Scholar] [CrossRef]
- Geil, P.B.; Anderson, J.W. Nutrition and health implications of dry beans: A review. J. Am. Coll. Nutr. 1994, 13, 549–558. [Google Scholar] [CrossRef]
- Awulachew, M.T. A review of anti-nutritional factors in plant based foods. Adv. Nutr. Food Sci. 2022, 7, 223–236. [Google Scholar]
- Gemede, H.F.; Ratta, N. Anti-nutritional factors in plant foods: Potential health benefits and adverse effects. Int. J. Nutr. Food Sci. 2014, 3, 284–289. [Google Scholar] [CrossRef]
- Egbe, I.A.; Akinyele, I.O. Effect of cooking on the antinutritional factors of lima beans (Phaseolus lunatus). Food Chem. 1990, 35, 81–87. [Google Scholar] [CrossRef]
- Adegbehingbe, K.T. Effect of fermentation on nutrient composition and anti-nutrient contents of ground Lima bean seeds fermented with Aspergillus fumigatus, Rhizopus stolonifer and Saccharomyces cerevisiae. Int. J. Adv. Res. 2014, 2, 1208–1215. [Google Scholar]
- Adegbehingbe, K.T.; Daramola, O.B. Fermentation of single and mixed substrates of lima bean seeds (Phaseolus lunatus) and African locust bean seeds (Parkia biglobosa). Ife J. Sci. 2019, 21, 333–343. [Google Scholar] [CrossRef]
- Adeniran, H.A.; Farinde, E.O.; Obatolu, V.A. Effect of heat treatment and fermentation on anti-nutrients content of lima bean (Phaseolus lunatus) during production of daddawa analogue. Ann. Res. Rev. Biol. 2013, 3, 256–266. [Google Scholar]
- Ensminger, A.H.; Ensiminger, M.K. Food for Health: A Nutrition Encyclopedia; Pagus Press: Clovis, CA, USA, 1986; pp. 19–21. [Google Scholar]
- Oboh, H.A.; Omofoma, C.O. The effects of heat-treated lima bean (Phaseolus lunatus) on plasma lipids in hypercholesterolaemic rats. Pak. J. Nutr. 2008, 7, 636–639. [Google Scholar] [CrossRef]
- Garden-Robinson, J.; McNeal, K. All about Beans Nutrition, Health Benefits, Preparation and Use in Menus; North Dakota State University Publication: Fargo, ND, USA, 2019; pp. 1–8. Available online: www.ag.ndsu.edu/publications/food-nutrition/all-about-beans-nutrition-health-benefits-preparation-and-use-in-menus/fn1643 (accessed on 14 September 2022).
- Wong, J.H.; Ng, T.B. Lunatusin, a trypsin-stable antimicrobial peptide from lima beans (Phaseolus lunatus L.). Peptides 2005, 26, 2086–2092. [Google Scholar] [CrossRef]
- Torruco-Uco, J.; Chel-Guerrero, L.; Martínez-Ayala, A.; Dávila-Ortíz, G.; Betancur-Ancona, D. Angiotensin-I converting enzyme inhibitory and antioxidant activities of protein hydrolysates from Phaseolus lunatus and Phaseolus vulgaris seeds. LWT—Food Sci. Technol. 2009, 42, 1597–1604. [Google Scholar] [CrossRef]
- Marimuthu, K.; Nagaraj, N.; Ravi, D. GC MS analysis of phytochemicals, fatty acids and antimicrobial potency of dry Christmas lima beans. Int. J. Pharm. Sci. Rev. Res. 2014, 27, 63–66. [Google Scholar]
- Agostini Costa, T.S.; Teodoro, A.P.; Alves, R.B.; Braga, L.R.; Ribeiro, I.F.; Silva, J.P.; Quintana, L.Q.; Burle, M.L. Total phenolics, flavonoids, tannins and antioxidant activity of lima beans conserved in a Brazilian Genebank. Ciênc. Rural 2015, 45, 335–341. [Google Scholar] [CrossRef]
- Lacerda, E.; do Nascimento, R.R.; de Lacerda, E.S.; Pinto, J.T.; Rizzi, L.D.; Bezerra, M.M.; Pinto, I.R.; Filho, S.M.P.; Pinto, V.P.T.; Filho, G.C.; et al. Lectin from seeds of a Brazilian lima bean variety (Phaseolus lunatus L. var. cascavel) presents antioxidant, antitumour and gastroprotective activities. Int. J. Biol. Macromol. 2017, 95, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Y.; Ahmad, A. Impact of processing on nutritional and antinutritional factors of legumes: A review. Ann. Food Sci. Technol. 2018, 19, 199–215. [Google Scholar]
- Uwaegbute, A.C.; Nnanyelugo, D.C. Nutritive value and biological evaluation of processed cowpea diets compared with local weaning foods in Nigeria. Nutr. Rep. Int. 1989, 36, 119–129. [Google Scholar]
- Siddiq, M.; Uebersax, M.A. Dry Beans and Pulses Production, Processing, and Nutrition; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Deshpande, S.; Cheryan, M. Effects of phytic acid, divalent cations, and their interactions on α-amylase activity. J. Food Sci. 1984, 49, 516–519. [Google Scholar] [CrossRef]
- Vidal Valverde, C.; Frias, J.; Estrella, I.; Gorospe, M.J.; Ruiz, R.; Bacon, J. Effect of processing on some antinutritional factors of lentils. J. Agric. Food Chem. 1994, 42, 2291–2295. [Google Scholar] [CrossRef]
- Uzogara, S.G.; Moorton, I.D.; Daniel, J.W. Changes in some anti-nutrients in cowpea (Vigna unguiculata) processed with “akanwa” alkaline salt. Plant Foods Hum. Nutr. 1990, 40, 249–258. [Google Scholar] [CrossRef]
- Wang, N.; Hatcher, D.W.; Toews, R.; Gawalko, E.J. Influence of cooking and dehulling on nutritional composition of several varieties of lentils (Lens culinaris). LWT—Food Sci. Technol. 2009, 42, 842–848. [Google Scholar] [CrossRef]
- Ibeabuchi, J.C.; Okafor, D.C.; Peter-Ikechukwu, A.; Agunwah, I.M.; Eluchie, C.N.; Ofoedu, C.E.; Nwatu, N.P. Comparative study on the proximate composition, functional and sensory properties of three varieties of beans Phaseolus lunatus, Phaseolus vulgaris and Vigna umbellata. Int. J. Adv. Eng. Technol. Mang. Appl. Sci. 2017, 5, 1–23. [Google Scholar]
- Ihediohanma, N.C.; Ofoedu, C.E.; Ojimba, N.C.; Adedokun, A.O. Comparative effect of milling methods on the proximate composition and functional properties of cowpea (Vigna uniquiculata). Int. J. Life Sci. 2014, 3, 170–177. [Google Scholar]
- Kuntz, J.; Irwin, D. hydration of macromolecules. III. Hydration of polypeptides. J. Am. Chem. Soc. 2007, 93, 514–516. [Google Scholar] [CrossRef]
- Adeleke, R.; Odedeji, J.O. Functional Properties of Wheat and Sweet Potato Flour Blends; Department of Food Technology, Osun State Polytechnic: Iree, Nigeria, 2010. [Google Scholar]
- Fabbri, A.D.T.; Crosby, G.A. A review of the impact of preparation and cooking on the nutritional quality of vegetables and legumes. Int. J. Gastr. Food Sci. 2016, 3, 2–11. [Google Scholar] [CrossRef]
- Azarnia, S.; Boye, J.I.; Warkentin, T.; Malcolmson, L.; Sabik, H.; Bellido, A.S. Volatile flavour profile changes in selected field pea cultivars as affected by crop year and processing. Food Chem. 2011, 124, 326–335. [Google Scholar] [CrossRef]
- Yoo, K.S.; Lee, E.J.; Patil, B.S. Changes in flavor precursors, pungency, and sugar content in short-day onion bulbs during 5-month storage at various temperatures or in controlled atmosphere. J. Food Sci. 2012, 77, C216–C221. [Google Scholar] [CrossRef]
- Apata, D.F.; Ologhobo, A.D. Biochemical evaluation of some Nigerian legume seeds. Food Chem. 1994, 49, 333–338. [Google Scholar] [CrossRef]
- Nwosu, J.N. Effect of soaking, blanching and cooking on the anti-nutritional properties of asparagus bean (Vigna sesquipedis) flour. Sci. Nat. 2020, 8, 163–167. [Google Scholar]
- Adebo, J.A.; Njobeh, P.B.; Gbashi, S.; Oyedeji, A.B.; Ogundele, O.M.; Oyeyinka, S.A.; Adebo, O.A. Fermentation of cereals and legumes: Impact on nutritional constituents and nutrient bioavailability. Fermentation 2022, 8, 63. [Google Scholar] [CrossRef]
- Oboh, G.; Elusiyan, C.A. Changes in the nutrient and anti-nutrient content of micro-fungi fermented cassava flour produced from low- and medium-cyanide variety of cassava tubers. Afr. J. Biotechnol. 2007, 6, 2150–2157. [Google Scholar] [CrossRef]
- Oboh, G.; Akindahunsi, A.A. Biochemical changes in cassava products (flour & gari) subjected to Saccharomyces cerevisiae solid media fermentation. Food Chem. 2003, 82, 599–602. [Google Scholar]
- Ohanenye, I.C.; Tsopmo, A.; Ejike, C.E.C.C.; Udenigwe, C.C. Germination as a bioprocess for enhancing the quality and nutritional prospects of legume proteins. Trends Food Sci. Technol. 2020, 101, 213–222. [Google Scholar] [CrossRef]
- Agiang, M.A.; Umoh, I.B.; Essien, A.I.; Eteng, M.U. Nutrient changes and antinutrient contents of beniseed and beniseed soup during cooking using a Nigerian traditional method. Pak. J. Biol. Sci. 2010, 13, 1011–1015. [Google Scholar] [CrossRef]
- Camacho, L.; Sierra, C.; Campos, R.; Guzman, M.D. Nutritional changes caused by germination of legumes commonly eaten in Chile. Arch. Lat. Nutr. 1992, 42, 283–290. [Google Scholar]
- Mani, P.; Thirumalai Natesan, V. Experimental investigation of drying characteristics of lima beans with passive and active mode greenhouse solar dryers. J. Food Process Eng. 2021, 44, e13667. [Google Scholar] [CrossRef]
- Mohite, A.M.; Sharma, N. Drying behaviour and engineering properties of lima beans. Agric. Eng. Int. CIGR J. 2018, 20, 180–185. [Google Scholar]
- Sood, S.; Gupta, N. Lima bean. In Vegetable Crops Science; Rana, M.K., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 701–713. [Google Scholar]
- Alves, A.U.; de Oliveira, A.P.; Alves, A.U.; Dornelas, C.S.M.; Alves, E.U.; Cardoso, E.A.; de Oliveira, A.N.P.; da Cruz, I.S. Lima beans production and economic revenue as function of organic and mineral fertilization. Hortic. Brasi. 2008, 26, 251–254. [Google Scholar] [CrossRef]
- Kato, M. Available online: https://commons.wikimedia.org/wiki/File:Lima_Bean_Dip.jpg (accessed on 10 August 2022). CC BY 2.0 https://creativecommons.org/licenses/by/2.0, via Wikimedia Commons. Created: 31 December 2009. (No Changes Made to This Image).
- Doll, J. Available online: https://commons.wikimedia.org/wiki/File:Baked_lima_beans_(28309216747).jpg (accessed on 10 August 2022). CC BY 2.0 https://creativecommons.org/licenses/by/2.0, via Wikimedia Commons. Created: 21 June 2010. (No Changes Made to This Image).
- Hasan, M.M.; Saleem, Z.M.; Ahmed, S. Phaseolus lunatus Linn: Botany, medicinal uses, phytochemistry and pharmacology. World J. Pharm. Sci. 2016, 5, 87–93. [Google Scholar]
- Adegbehingbe, K.T. Microbiological analyses and nutrient composition of sorghum co-fermented with Lima bean seeds. Curr. Res. Microb. Biotech. 2014, 2, 431–437. [Google Scholar]
- Sobowale, S.S.; Animashaun, O.H.; Omosebi, O.M. Influence of traditional and back-slopping steeping methods on some quality attributes of lima bean-sorghum composite flour and its bread making potential. J. Food Proces. Preser. 2021, 45, e15030. [Google Scholar] [CrossRef]
- Pérez-Navarrete, C.; González, R.; Chel-Guerrero, L.; Betancur-Ancona, D. Effect of extrusion on nutritional quality of maize and Lima bean flour blends. J. Sci. Food Agric. 2006, 86, 2477–2484. [Google Scholar] [CrossRef]
- Chel-Guerrero, L.; Gallegos-Tintoré, S.; Martínez-Ayala, A.; Castellanos-Ruelas, A.; Betancur-Ancona, D. Functional properties of proteins from lima bean (Phaseolus lunatus L.) seeds. Food Sci. Technol. Int. 2011, 17, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Farinde, E.O.; Adeniran, H.A.; Abiose, S.H. Proximate composition, mineral content and sensory assessment of an iru analogue produced from lima bean (Phaseolus lunatus). Ife J. Technol. 2011, 20, 1–6. [Google Scholar]
- Läpple, D.; Hennessy, T.; Newman, C. Quantifying the economic return to participatory extension programmes in Ireland: An endogenous switching regression analysis. J. Agric. Econ. 2013, 64, 467–482. [Google Scholar] [CrossRef]
- Muriithi, B.W.; Matz, J.A. Welfare effects of vegetable commercialization: Evidence from smallholder producers in Kenya. Food Policy 2015, 50, 80–91. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).